Abstract
Rationale and Objectives
Hyposmia/anosmia is common among patients with coronavirus disease-2019 (COVID-19). Various imaging modalities have been used to assess olfactory dysfunction in COVID-19. In this systematic review, we sought to categorize and summarize the imaging data in COVID-19-induced anosmia.
Material and Methods
Eligible articles were included after a comprehensive review using online databases including Google scholar, Scopus, PubMed, Web of science and Elsevier. Duplicate results, conference abstracts, reviews, and studies in languages other than English were excluded.
Results
In total, 305 patients undergoing MRI/functional MRI (177), CT of paranasal sinuses (129), and PET/CT or PET/MRI scans (14) were included. Out of a total of 218 findings reported on MRI, 80 were reported on early (≤ 1 month) and 85 on late (>1 month) imaging in relation to the onset of anosmia. Overall, OB morphology and T2-weighted or FLAIR signal intensity were normal in 68/218 (31.2%), while partial or complete opacification of OC was observed in 60/218 (27.5%). T2 hyperintensity in OB was detected in 11/80 (13.75%) and 18/85 (21.17%) on early and late imaging, respectively. Moreover, OB atrophy was reported in 1/80 (1.25%) on early and in 9/85 (10.58%) on late imaging. Last, among a total of 129 CT scans included, paranasal sinuses were evalualted in 88 (68.21%), which were reported as normal in most cases (77/88, [87.5%]).
Conclusion
In this systematic review, normal morphology and T2/FLAIR signal intensity in OB and OC obstruction were the most common findings in COVID-19-induced anosmia, while paranasal sinuses were normal in most cases. OC obstruction is the likely mechanism for olfactory dysfunction in COVID-19. Abnormalities in OB signal intensity and OB atrophy suggest that central mechanisms may also play a role in late stage in COVID-19-induced anosmia.
Key Words: computed tomography, magnetic resonance imaging, positron emission tomography, anosmia, olfactory dysfunction, COVID-19
INTRODUCTION
Hyposmia/anosmia is relatively common among patients with coronavirus disease-2019 (COVID-19), both as an isolated symptom or concomitant with other systemic or respiratory symptoms (1,2). Olfactory dysfunction may present as an alteration in the intensity of perceived odor or in odor quality. While hyposmia/anosmia is a frequent symptom associated with COVID-19, it is usually transient, and spontaneously resolves within a few weeks (1,2).
The first step in the olfactory perception is activation by odorants of sensory neurons that are located in the olfactory epithelium. This sensory epithelium is located in the olfactory clefts (OC), which are two narrow passages at the upper part of the nasal cavities (3). The olfactory receptor neurons originating from the OC cross the cribriform plate to reach the olfactory bulb (OB) (3). The exact pathophysiology of COVID-19-induced anosmia is not fully understood (1, 2, 3). Various imaging modalities have been used to assess the olfactory dysfunction in COVID-19, mainly by imaging OB and OC, such as with magnetic resonance imaging (MRI) (4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28), computed tomography (CT) of paranasal sinuses (29, 30, 31, 32), or with functional modalities such as positron emission tomography (PET) of brain (15,22,33). These studies have reported a variety of findings, which are at times divergent.
In the present report, we sought to systematically review the imaging studies that have investigated COVID-19-induced olfactory dysfunction and to categorize the data from these studies, including based on the timing of the imaging in relation to the onset of olfactory dysfunction in the course of COVID-19.
MATERIALS AND METHODS
Study Selection and Eligibility Criteria
We identified studies that have used imaging modalities such as brain CT, MRI, or PET in patients with COVID-19, confirmed with reverse transcription-polymerase chain reaction, and a clinical presentation with anosmia or olfactory dysfunction. Relevant studies were searched using MEDLINE (PubMed), Web of Science, Embase (Elsevier), Scopus, and Google Scholar databases (search was undertaken on May 26, 2021). The following medical subject headings and keywords were used: “Coronavirus,” “COVID-19,” “SARS-CoV-19,” “2019-CoV-19,” “radiolog*,” “radiograph*,” “Anosmia,” “Hyposmia,” “Olfactory Bulb,” “Tomography, X-ray Computed,” “CT,” “MRI,” and “PET.” The full list of the keywords that were used for search in the PubMed is provided in the Supplementary Appendix. Duplicates, conference abstracts, reviews, and studies in languages other than English were excluded.
Data Collection and Analysis
The following data from the eligible studies were collected by two independent investigators and were subsequently cross-checked. Disagreements between the investigators were resolved by a third investigator after discussions and reaching consensus. The following information were collected: first author's name, country where the study was conducted, study type, clinical characteristics of participants, clinical presentation of the olfactory dysfunction, findings on brain imaging, and clinical outcomes.
RESULTS
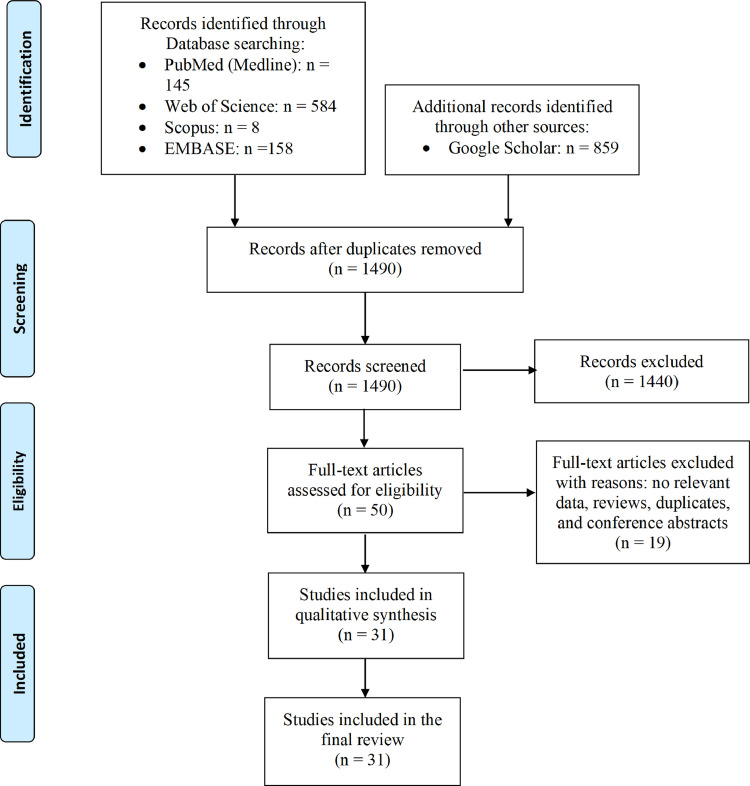
After excluding duplicate search results, titles and abstracts of 1490 studies were assessed, and 50 eligible studies were assessed by full-text review. Thirty-one studies, including seven cohort studies, six case series/reports, two case-control studies, one cross-sectional study, and 15 commentaries, letters to the editor, and short reports were included in the final analysis. The PRISMA flow diagram for study selection is shown in Figure 1 .
Figure 1.
Flow diagram of the study selection process. (Color version of figure is available online.)
Clinical Characteristics of the Study Participants
Types of the studies and clinical characteristics of the patients are summarized in Table 1 . In total, 305 patients were included in the studies. Of these participants, 161 (52.8%) were female, 137 (44.9%) male, and gender was not specified in 7 (2.3%). Age of the participants ranged between 13 and 84 years. Olfactory disorder was self-reported in 157 (51.5%) and by objective olfactory tests in 148 (48.5%). 259 (84.9%) patients had olfactory dysfunction (hyposmia/anosmia). Concomitant taste dysfunction was seen in 98 (32.1%) cases. The most common general symptoms were fever (141, 46.2%), cough (128, 41.9%), myalgia (120, 39.3%), headache (111, 36.3%), dyspnea (98, 32.1%), asthenia/fatigue (38, 12.4%), and gastrointestinal symptoms (21, 6.8%). Other clinical findings (rhinorrhea, coriza, nasal obstruction and facial pain) were less frequently reported.
Table 1.
Design of the Imaging Studies and Ear, Nose, Throat and Neurological Symptoms in Patients with Olfactory Dysfunction Secondary to COVID-19
| First Author (Ref) | Country | Study Design | Pariticipants | ENT and Neurological Symptoms (n) | Reporting ofAnosmia (n) | Resolution of Symptoms |
|---|---|---|---|---|---|---|
| Politi et al.(4) | Italy | Case report (n=1) | Female (25 yo) | Anosmia, dysgeusia | Self- reported | Full recovery |
| Aragao et al.(5) | Brazil | Case series (n=5) | Not reported | Anosmia, motor deficit (1) | Self- reported | Not reported |
| Chiu et al.(6) | USA | Case report (n=1) | Female (19 yo) | Anosmia | Self- reported | Not reported |
| Correa et al.(7) | Brazil | Case report (n=1) | Female (41 yo) | Anosmia, ageusia | Self- reported | Persistent anosmia |
| Eliezer et al.(8) | France | Prospective case-control (n=20) | 10 males, 10 females (21-53 yo) | Sudden-onset olfactory dysfunction (18), progressive olfactory dysfunction (2) |
Olfactory test | Full recovery (4) Partial recovery (4) Not reported (12) |
| Hatipoglu et al.(9) | Turkey | Case series (n=3) | 2 males (13 yo) 1 female (13 yo) | Anosmia | Self- reported | Full recovery (2) Persistent anosmia (1) |
| Kandemirli et al.(10) | Turkey | Prospective non-controlled (n=23) | 9 males, 14 females (median age: 29 yo) |
Anosmia (23) (sudden-onset [4], progressive [19]), sinonasal symptoms (7) | Olfactory test | Not reported |
| Lechien et al.(29) | Belgium | Prospective non-controlled (n=16) | 8 males, 8 females (36±10.1 yo) | Anosmia (16), nasal obstruction (6) | Olfactory test | Not reported |
| Schonegger et al.(11) | Austria | Case series (n=5) | 5 females (41-57 yo) | Anosmia (3), hyposmia (1), hypogeusia (2), ageusia (1) | Olfactory test | Persistent anosmia (2) Partial recovery (1) Full recovery (2) |
| Strauss et al.(12) | USA | Retrospective case-control (n=24) | 9 males, 15 females (58 ± 15 yo) |
Anosmia (8), hyposmia (3) phantosmia (2), paresthesia (1), altered mental status (9), status epilepticus (1), ataxia and dysarthria(1) |
Self-reported | Not reported |
| Wang et al.(13) | USA | Case report (n=1) | Male (17 yo) | Anosmia, ageusia, epistaxis | Olfactory test | Not reported |
| Zhang et al.(34) | USA | Case report (n=1) | Female (60 yo) | Anosmia, dysgeusia | Self- reported | Not reported |
| Safavi Naeinia et al.(30) | Iran | Prospective non-controlled (n=49) | 27 males, 22 females (45 ± 12.2 yo) | Anosmia/hyposmia (49), dysgeusia (37), nasal obstruction (16), sore throat (22,) rhinorrhea (9) | Self- reported | Not reported |
| Chetrit et al.(14) | France | Case series (n=23) | 9 males, 14 females (39 ± 17.1 yo) | Anosmia (19), no anosmia (4, used as "controls") | Olfactory test | Not reported |
| Yousefi-Koma et al.(15) | Iran | Case report (n=1) | Female (28 yo) | Anosmia followed by persistent parosmia | Self- reported | Not reported |
| Tsivgoulis et al.(16) | Greece | Case series (n=8) | 2 males, 6 females (19-53 yo) | Anosmia (1), hyposmia (6), ageusia (7) |
Olfactory test | Not reported |
| Karimi-Galougahi et al.(33) | Iran | Case report (n=1) | Female (27 yo) | Anosmia | Self- reported | Not reported |
| Li et al.(17) | Taiwan | Case report (n=1) | Male (21 yo) | Anosmia | Self- reported | Partial recovery |
| Laurendon et al.(18) | France | Case report (n=1) | Male (27 yo) | Anosmia, dysgeusia | Self- reported | Full recovery |
| Liang et al.(19) | Taiwan | Case report (n=1) | Female (16 yo) | Hyposmia, rhinorrhea | Self-reported | Persistent hyposmia |
| Yildiz et al.(31) | Turkey | Prospective non-controlled (n=40) | 20 males, 20 females (58±20.57 yo) | Anosmia (5), mild hyposmia (13), moderate hyposmia (13), severe hyposmia (9) | Olfactory test | Not reported |
| Theodorou et al.(20) | Greece | Case report (n=1) | Female (68 yo) | Anosmia | Self-reported | Not reported |
| Melegari et al.(21) | Italy | Case report (n=1) | Female (31 yo) | Anosmia, ageusia | Self-reported | Persistent anosmia |
| Niesen et al.(22) | Belgium | Prospective non-controlled (n=12) | 2 males, 10 females (23-60 yo) | Anosmia (7), hyposmia (5), dysgeusia (11) |
Olfactory test |
Full recovery (5) Persistent hyposmia (7) |
| Jalessi et al.(32) | Iran | Prospective non-controlled (n=22) | 13 males, 9 females (52 ± 11.7 yo) | Anosmia/hyposmia (22), dysgeusia (15), sore throat (8), rhinorrhea (5) | Self-reported | Full recovery (21) Partial recovery (1) |
| Ismail et al.(24) | Kuwait | Case report (n=1) | Female (25 yo) | Anosmia, ageusia | Self-reported | Partial recovery |
| Eliezer et al.(23) | France | Case report (n=1) | Female (40 yo) | Anosmia | Self-reported | Not reported |
| Karimi-Galougahi et al.(25) | Iran | Case report (n=1) | Male (27 yo) | Anosmia | Self-reported | Not reported |
| Letterio et al.(26) | Italy | Case series (n=4) | 2 females (25, 39 yo), sex/age not reported(2) | Anosmia (4), dysgeusia (1) | Self-reported | Full recovery |
| Hajjij et al.(27) | Morocco | Case report (n=1) | Male (21 yo) | Anosmia | Self-reported | Persistent anosmia |
| Aragao et al.(28) | Brazil | Retrospective study (n=35) | 14 females, 21 males (25-84 yo) | Anosmia (7) | Self –reported | Not reported |
| Total (n=305) | Self-reported anosmia: 157, Anosmia based on olfactory tests: 148 | |||||
ENT, ear, nose, and throat; Ref, reference.
In total, 14 studies (74 of 305 patients, 24.3%) reported the outcome of the olfactory symptoms. In 28 of 74 (37.8%) cases with reported outcomes, hyposmia/anosmia persisted at the time of reporting, while in 46 of 74 (62.2%) hyposmia/anosmia was partially or completely resolved.
Imaging Modalities
Findings using various imaging modalities are summarized in Table 2 . In total, 305 patients undergoing a total of 320 imaging studies were included. Imaging modalities used to assess changes in the olfactory tract in COVID-19-induced anosmia included MRI/functional MRI (n=177), CT of the paranasal sinuses and brain (n=129), and PET/CT or PET/MRI scans (n=14).
Table 2.
Findings on Various Imaging Modalities in Patients with Olfactory Dysfunction Secondary to COVID-19
| First Author (Ref) | MRI (n) | PNS CT (n) | PET/CT, PET/MRI (n) |
|---|---|---|---|
| Politi et al.(4) | OB and right Hyperintensity in the rectus gyrus cortex, subtle hyperintensity of OBs |
||
| Aragao et al.(5) | Hypointensity in OB (1), enhancement in OB (4) | ||
| Chiu et al.(6) | Volume reduction in OB | ||
| Correa et al.(7) | Bilateral enhancement of OB | ||
| Eliezer et al.(8) | Normal olfactory cleft (1), olfactory cleft obstruction (19) (bilateral: 17, right: 1, left: 1) | ||
| Hatipoglu et al.(9) | Normal OB morphology and signal intensity (3) | ||
| Kandemirli et al.(10) | -Olfactory cleft opacification (17) (partial [16], total [1]) -OB morphology: normal (8), mild irregularity with preserved J shape (2), contour lobulations (5), rectangular shape (8) -OB signal intensity: normal (2), diffusely increased signal (9), hyperintense foci (16), hyperintense foci with halo (5), microhemorrhages (4) -Olfactory tract signal abnormality (7), olfactory cortex signal abnormality (5), olfactory nerve morphology: normal (13), evident clumping (8), thinning with scarcity (2) | Olfactory cleft opacification: partial (16), total (1), normal olfactory cleft (6) Normal ethmoid air cells and nasal cavity (no opacification) in all cases (23) |
|
| Lechien et al.(29) | Olfactory cleft pacification (9) (total [3], partial [6]), Normal (7) | ||
| Schonegger et al.(11) | Slight hyperintensity of the left caudate and parahippocampus (1), normal OB morphology and signal intensity (5) | ||
| Strauss et al.(12) | Intraneural hyperintensity (4), normal olfactory recess (12), Normal OB morphology and signal intensity (12), hypersignal orbitofrontal and entorhinal cortex (1) | ||
| Wang et al.(13) | Normal morphology of OB and olfactory sulcus | ||
| Zhang et al.(34) | Stable white matter changes | ||
| Safavi Naeinia et al.(30) | Septal deviation (24), concha bullosa (27), no significant changes on PNS CT (49) | ||
| Chetrit et al.(14) | Normal OB morphology and signal intensity (14) (4 normosmic and 10 anosmic patients) Olfactory cleft obstruction (10) (1 normosmic and 9 anosmic patients) |
||
| Yousefi-Koma et al.(15) | Normal OB volume and signal intensity | Hypometabolism in left insula, inferior frontal gyrus, hippocampus, amygdal (1) | |
| Tsivgoulis et al.(16) | Mild to moderate OB atrophy (7), Olfactory mucosal thickening (4), Olfactory mucosal enhancement (1) | ||
| Karimi-Galougahi et al.(33) | Hypometabolism in the left orbitofrontal cortex (1) | ||
| Li et al.(17) | Bilateral linear hyperintensities within olfactory nerves (suggestive of bilateral olfactory neuropathy), smaller OB on the right side |
||
| Laurendon et al.(18) | Hyperintensity and significant enlargement of OB, mild edema in the olfactory cleft, normal olfactory pathway and cortical projection | ||
| Liang et al.(19) | Smaller OB on the right-side, olfactory tract hyperitensity | ||
| Yildiz et al.(31) | Abnormal finding: drainage disorder or soft tissue thickening in olfactory region (13) | ||
| Theodorou et al.(20) | Hyperintensity in olfactory sulci, high-signal intensity infarcts in olfactory tract | ||
| Melegari et al.(21) | Persistent hyperintensity of olfactory nucleus | ||
| Niesen et al.(22) | Complete olfactory cleft obstruction (6), partial olfactory cleft obstruction (2), normal olfactory cleft (4) | Olfactory cleft obliteration (6), OB asymmetry (3). Glucose metabolism alteration in olfactory core and higher brain networks (12) | |
| Jalessi et al.(32) | Obstruction of olfactory cleft (kissing mucosal swelling) (1) | ||
| Ismail et al.(24) | Normal OB and sulci BOLD map: inactivation of orbitofrontal cortex with strong BOLD signal within right piriform cortex and uncus |
||
| Eliezer et al.(23) | Bilateral obstruction of the olfactory cleft, normal OB morphology and signal intensity | ||
| Karimi-Galougahi et al.(25) | Normal olfactory bulb and signal intensity | ||
| Letterio et al.(26) | Bilateral hyperintensity in OB (1), similar but less obvious hyperintensity of OB (1), normal OB volume and signal intensity (2) | ||
| Hajjij et al.(27) | Normal olfactory bulb and signal intensity | ||
| Aragao et al.(28) | "OB injury" (enhancement or microbleeding) (12) |
BOLD, Blood oxygen level dependent; CT, Computed tomography; MRI, Magnetic resonance imaging; NL, Normal, OB: Olfactory bulb; OC, Olfactory cleft; OM, Olfactory mucosa; PET/CT, Positron emission tomography/ Computed tomography; PNS CT, Paranasal sinus computed tomography.
Olfactory bulb or cortical hyperintensity were reported on 2D or 3D fluid-attenuated inversion recovery or T2-weighted images. Enhancement was reported based on T1-weighted images following administration of gadolinium-based contrasts.
MRI
A summary of the findings on MRI in anosmia of COVID-19 is provided in Tables 2 and 3 . In total, 218 MRI findings were reported. Abnormalities on MRI can be divided into the following categories: changes in the morphology and signal intensity in OB, OC, or cortex, and abnormalities on functional MRI. With regard to the timing of imaging in relation to the onset of olfactory symptoms, the studies can be categorized to early imaging (≤1 month since the onset of olfactory dysfunction) and late imaging (> 1 month). Of the total of 218 MRI findings, 80 were detected in the early and 85 in the late phase, while the timing was not specified in the remainder (n=53) (Table 4 ).
Table 3.
Findings on MRI in Patients with Olfactory Dysfunction Secondary to COVID-19
| Ref | NLOB | OB Enhancement | Hypersignal OB/Tract | Hyposignal OB/Microbleed | OBAtrophy | NLOC | OC Oblitertaion | OM Enhancement | Hypersignal Cortex |
|---|---|---|---|---|---|---|---|---|---|
| 4 | - | - | 1 | - | 1 | - | - | - | 1 |
| 5 | - | 4 | - | 1 | - | - | - | - | - |
| 6 | - | - | - | - | 1 | - | - | - | - |
| 7 | - | 1 | - | - | - | - | - | - | - |
| 8 | - | 1 | - | - | - | 1 | 19 | - | - |
| 9 | 3 | - | - | - | - | - | - | - | - |
| 10 | 23 | - | 17 | 4 | - | - | 17 | - | 5 |
| 11 | 5 | - | - | - | - | - | - | - | 1 |
| 12 | 12 | - | 4 | - | - | - | - | - | 1 |
| 13 | 1 | - | - | - | - | - | - | - | - |
| 14 | 14 | - | - | - | - | - | 10 | - | - |
| 15 | 1 | - | - | - | - | - | - | - | - |
| 16 | - | - | - | - | 7 | - | 4 | 1 | - |
| 17 | - | - | 1 | - | - | - | - | - | - |
| 18 | - | - | 1 | - | - | - | 1 | - | - |
| 19 | - | - | 1 | - | 1 | - | - | - | - |
| 20 | - | - | 1 | - | - | - | - | - | - |
| 21 | - | - | 1 | - | - | - | - | - | - |
| 22 | 3 | - | - | - | - | 4 | 8 | - | - |
| 23 | 1 | - | - | - | - | - | 1 | - | - |
| 24 | 1 | - | - | - | - | - | - | - | - |
| 25 | 1 | - | - | - | - | - | - | - | - |
| 26 | 2 | - | 2 | - | - | - | - | - | 2 |
| 27 | 1 | - | - | - | - | - | - | - | - |
| 28 | - | 12 | - | 12 | - | - | - | - | - |
| Total n=218 |
68 31.2% |
18 8.25% |
29 13.3% |
17 7.8% |
10 4.6% |
5 2.3% |
60 27.5% |
1 0.45% |
10 4.6% |
NL, Normal; NR, not reported; OB, Olfactory bulb; OC, Olfactory cleft; OM, Olfactory mucosa.
Olfactory bulb or cortical hyperintensity were reported on 2D or 3D fluid-attenuated inversion recovery or T2-weighted images. Enhancement was reported based on T1-weighted images following administration of gadolinium-based contrasts.
Table 4.
Findings on MRI of Olfactory Tract and Central Pathways in Anosmia Secondary to COVID-19 Based on the Timing of Imaging
| MRI ≤ 1 Month From Onset of Anosmia; (n, %)(Total n=80) | MRI > 1 Month After Onset of Anosmia;(n, %) (Total n=85) | Timing Not Reported; (n, %)(Total n=53) | |||
|---|---|---|---|---|---|
| OC obliteration | 28 (35%) | Normal OB | 26 (30.58%) | OB enhancement | 16 (30.18%) |
| Normal OB | 28 (35%) | OC obliteration | 22 (25.9%) | Normal OB | 14 (26.4%) |
| Hypersignal OB/tract | 11 (13.75%) | Hypersignal OB/tract | 18 (21.17%) | Hyposignal (microbleed) OB | 13 (24.52%) |
| Normal OC | 5 (6.25%) | OB atrophy | 9 (10.58%) | OC obliteration | 10 (18.9%) |
| Hypersignal cortex | 5 (6.25%) | Hypersignal cortex | 5 (5.9%) | Hypersignal OB/tract | 0 (0%) |
| OB enhancement | 2 (2.5%) | Hyposignal (microbleed) OB | 4 (4.70%) | Normal OC | 0 (0%) |
| OB atrophy | 1 (1.25%) | OM enhancement | 1 (1.17%) | Hypersignal cortex | 0 (0%) |
| Hyposignal (microbleed) OB | 0 (0%) | Normal OC | 0 (0.0%) | OB atrophy | 0 (0%) |
| OM enhancement | 0 (0%) | OB enhancement | 0 (0.0%) | OM enhancement | 0 (0%) |
OB, Olfactory bulb; OC, Olfactory cleft; OM, Olfactory mucosa.
Findings are listed in the order of their frequencies.
Morphology and Signal Intensity of OB
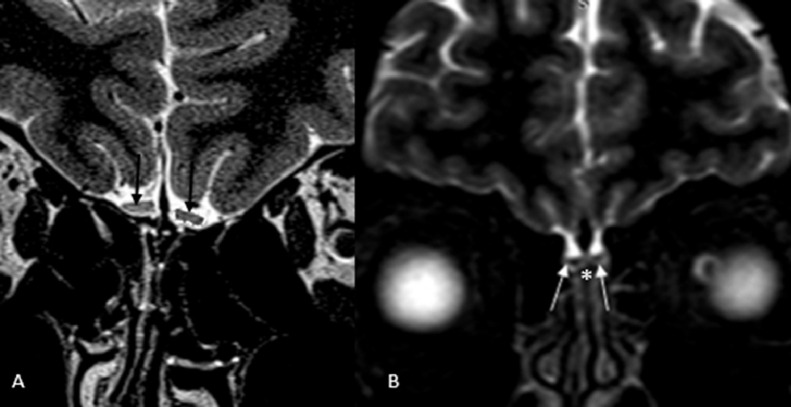
Of the total of 218 findings on MRI, both morphology and signal intensity of OB were normal in 68/218 (31.2%) (Fig 2 ), which were the most common overall MRI finding (9, 10, 11, 12, 13, 14, 15,22, 23, 24, 25, 26, 27). OB morphology and signal was normal in 28/80 (35%) in the early and in 26/85 (30.6%) on late imaging, similarly constituting the most common findings on both early and late imaging.
Figure 2.
A. Coronal T2-weighted magnetic resonance imaging (MRI) of olfactory bulb in a patient with COVID-19-induced anosmia shows rectangular shape of both olfactory bulbs (arrow). Adapted from Kandemirli et al (10) with permission. B. Coronal nonenhanced T2-weighted MRI of a young man with sudden-onset anosmia shows normal olfactory bulb morphology and signal intensity (arrow). Olfactory cleft is also shown (*) with no evidence of mucosal thickening or obliteration. Adapted from Galougahi et al (25) with permission.
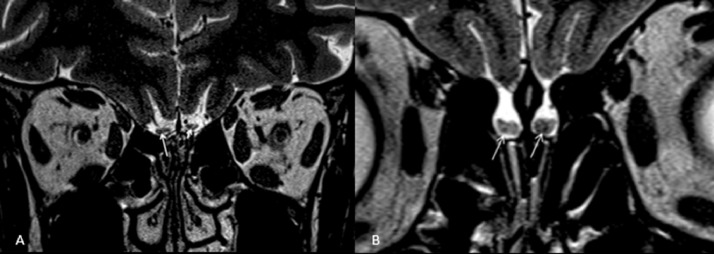
Other findings on MRI included OB enhancement following gadolinium injection in 18/218 (8.25%) (5,7,8,28) and T2 hyperintensity in the OB/tract in 29/218 (13.30%) (4,10,12,17, 18, 19, 20, 21,26) (Fig 3 a), while hypointense focus, consistent with microhemorrhage/methemoglobin deposition (Fig 3b), was identified in 17/218 (7.8%) (5,10,28). T2 hyperintensity in OB was detected in 11/80 (13.75%) and 18/85 (21.17%) during the early and late imaging, respectively. Moreover, a reduction in the OB volume was detected in 10/218 (4.6%) (4,6,16,19). OB atrophy was reported in one case (1/80 [1.25%]) on early imaging and in none on late imaging (9/85 [10.58%]) (Table 4).
Figure 3.
(a) Hyperintense foci are noted within both olfactory bulbs of a patient with COVID-19-induced anosmia (arrows). (b) Coronal T2-weighted image at the level of olfactory bulbs revealed bilateral scattered foci of hypointensity (arrows). Both images are adapted from Kandemirli et al (10) with permission.
Morphology and Signal Intensity of OC
Partial or complete obliteration of OC was the second most common finding on MRI, and was observed in 60/218 (27.5%) (8,10,14,16,18,22,23). OC was normal in 5/218 (2.3%) (8,22). OC obliteration was detected in 28/80 (35%) on early imaging and in 22/85 (25.9%) on late imaging. No enhancement in the mucosal lining of OC was detected on early imaging, while this change was only detected in one case (1/85 [1.17%]) in the late phase (16).
Morphology and Signal Intensity of Cortex
Studies assessing central olfactory centers were scarce, with signal alteration in the olfactory cortices reported in 10/218 [4.6%]) (4,10,11,12,26). Hyperintensity in the orbitofrontal and entorhinal cortices (12), in the right rectus gyrus (4) and in the left caudate and parahippocampus (11) have been reported. T2 hyperintensity in cortex both on early and late imaging were reported in 6.3% and 5.9% cases, respectively. Bilateral inferior frontal lobe hypodensity involving the straight gyrus was reported in one patient (20).
Changes on Functional MRI
Only one report on the use of functional MRI in anosmia secondary to COVID-19 has been published, where strong blood oxygen level-dependent signal within the piriform and right uncal cortices was detected (24).
CT of Paranasal Sinuses
Findings on CT of paranasal sinuses can be categorized into two main groups: changes in the paranasal sinuses (opacification of the sinuses) or in OC (partial or complete opacification). Among a total of 129 CT scans included in the present review, paranasal sinuses were evaluated and reported in 88 of 129 (68.21%) (10,29,30). In the majority of these scans (77/88 [87.5%]), paranasal sinuses were normal (10,30), with opacification of the paranasal sinuses only observed in 11/88 [12.5%]) (10,30). Moreover, out of the total 129 CT scans, OC was normal in 100/129 (77.5%), while in 29/129 (22.5%) complete or partial opacification of OC was reported (10,29, 30, 31, 32).
PET/CT
Few studies using PET/CT for assessment of olfactory dysfunction in COVID-19 have been performed. Reduction in fluorodeoxyglucose uptake in the left insula, left inferior frontal gyrus, left hippocampus and left amigdala compared with the contralateral side were identified in one patient (15). Additionally, hypometabolic activity of the left orbitofrontal cortex was detected in a patient who underwent PET/CT for evaluation of COVID-19-induced olfactory dysfunction (33). Alterantions in glucose metabolism in the olfactory tract and higher brain networks have also been reported in 12 patients with anosmia secondary to COVID-19 (22).
Imaging Data in Case-Control Studies
There was heterogeneity in what has been defined as “control” in the three case-control studies included in the current review. Some studies included normosmic individuals without COVID-19 as the control group (8), whereas others included normosmic patients with COVID-19 as controls (14). In one study, patients with olfactory dysfunction but without COVID-19 were included as controls for imaging evaluation of neurological manifestations of COVID-19 (12).
Elezier et al. (8) prospectively compared 20 anosmic patients with COVID-19 with 20 age- and sex-matched normosmic individuals without COVID-19 on MRI, which was performed at baseline and 1 month later. There was OC opacification in 19 of 20 cases and in none of the controls at baseline (p<0.001). On repeat MRI, OC opacification was resolved in 12 of 19 and persisted in seven of 12 patients, with the resolution of OC opacification correlating with improved olfaction as assessed by olfactory tests. There was no difference in the OB volume between cases and controls at baseline (p=0.33), and no significant changes in the OB volume were noted in the cases on follow-up MRI (p=0.53). Chetrit et al. (14) prospectively compared 19 patients with COVID-19 and olfactory dysfunction with four patients with COVID-19 and no olfactory dysfunction as controls. There was OC opacification in nine of 19 cases and one of four controls (p value not reported). The OC opacification in one control patient with COVID-19 but without olfactory dysfunction was attributed to initial inflammatory reaction of the nasal mucosa to mild-to-moderate COVID-19. There was higher T2-weighted fluid attenuated inversion recovery (T2 FLAIR) signal intensity in the OB in the cases compared with controls (p<0.001). Last, Strauss et al. (12) retrospectively compared 12 patients with COVID-19 and neurological symptoms with 12 age-matched controls without COVID-19 who had undergone MRI for evaluation of olfactory dysfunction. T2 FLAIR signal intensity in OB was higher in patients with COVID-19 compared to patients with olfactory dysfunction without COVID-19 (p=0.003). Four of 12 patients with COVID-19 demonstrated T2 signal hyperintensity on 3D T2 FLAIR compared to none of the 12 controls with olfactory dysfunction without COVID-19 (p=0.028). OB volume was similar between the two groups (p value not reported).
DISCUSSION
In this systematic review, opacification of OC together with normal OB morphology and signal intensity were the most common imaging findings in patients with olfactory dysfunction secondary to COVID-19, while paranasal sinuses were normal in most cases. OC opacification was detected in most anosmic patients with COVID-19 compared to the normal OC in normosmic controls, with resolution of OC opacification correlating with improved olfaction. Taken together, these data suggest obstruction of OC, which likely results in mixed conductive and peripheral sensory-neural anosmia, as the most likely mechanism for olfactory dysfunction in COVID-19. An increase in the frequency of abnormalities in signal intensity and morphology of OB on late versus early imaging suggests that central mechanisms for anosmia may also play a role, especially in the late phase - a postulate that is supported by functional abnormalities detected on PET and functional MRI studies.
The exact pathogenesis of the olfactory dysfunction in COVID-19 is not fully elucidated. Possible mechanisms include neurotropism and invasion of the OB by the virus, inflammatory changes, and impairment of the olfactory epithelium via angiotensin converting enzyme two receptors that are expressed by the non-neural olfactory supporting cells (35). Persistent olfactory dysfunction after sinonasal symptoms resolve suggests possible injury to the olfactory stem cells and impairment of the supporting cells (36).
Post-viral ansomia due to upper respiratory tract infections can occur in up to 40% of cases, which is often secondary to diffuse sinonasal mucosal thickening. Nevertheles, sudden, transient olfactory dysfunction is common in COVID-19 (1,4,8,12,23,24,26,29,39). Lack of concomitant sinonasal symptoms (i.e., nasal obstruction and mucosal congestion) in majority of the cases (37) indicates that diffuse sinonasal mucosal thickening may not be a contributing mechanism in most cases of COVID-19-induced anosmia. Consistent with this, CT of paranasal sinuses was normal in most patients with COVID-19-induced anosmia, with no mucosal thickening or obstruction detected (10,29, 30, 31, 32).
Similarly, On MRI, OB and OC were normal in most cases with COVID-19-induced anosmia. Nevertheless, infection of the olfactory epithelial support cells (Bowman and sustentacular cells) plays a key role in ansomia of COVID-19 (38). SARS-CoV-2 utilizes angiotensin converting enzyme 2 and transmembrane serine protease 2 to enter the respiratory epithelial cells, which act as a viral reservoir for COVID-19 (35). The localized respiratory epithelial involvement and inflammation in the OC may appear as hyperintense mucosal thickening and obstruction, which indeed was the second most common abnormality on MRI in the present review. It is postulated that patients with obstruction in the OC may develop more severe olfactory dysfunction compared with patients with normal OC (29).
Inflammation of the OB with blood brain barrier breakdown can result in edema in OB, which appears as hypersignal intensity on T2-weighted images. Prolonged inflammation of the olfactory tract can be detected as thickening and clumped appearance of the olfactory nerve filia on MRI (5,7,8). Additionally, microbleeding secondary to microvascular injury and microthrombosis by SARS-Cov-2 may appear as foci of hyposignal intensity within the OB (5,10,28).
Overall, timing of the imaging studies during the course of COVID-19-induced olfactory dysfunction appears to be important in detection of abnormalities on imaging. While imaging of OB in most cases is normal within the early days after the onset of olfactory dysucntion, a decrease in the OB volume, detected as thinning or loss of normal oval shape and asymmetry compared with the contralateral OB (19), is more frequently detected at later stages. Additionally, it is plausible that persistently low activity in the afferent olfactory neurons may result in further reduction in the OB volume. Indeed, it takes several weeks for structural changes in the OB to appear in the course of COVID-19-induced anosmia. This observation might suggest impairment in the olfactory stem cells in addition to damage to the epithelial support cells (36), which in combination may lead to olfactory dysfunction and atrophy of OB.
Finally, decreased metabolic activity of the orbitofrontal cortex on PET-CT (33) may be a result of direct viral neurotropism through the olfactory pathways (37) as suggested by cortical hyperintensity on FLAIR sequence on MRI (11,20). Additionally, deafferentation process and functional reorganization secondary to absent sensory stimulation of the olfactory pathways (22,39) may also contribute to the observed hypometabolism on PET-CT.
Limitations: there was significant heterogeneity in the reported studies, which included case reports, case series, retrospective studies as well as prospective controlled and non-controlled studies. Due to the paucity of well-designed prospective studies, case reports and case series reporting the imaging findings in anosmia of COVID-19 constitute a significant proportion of the available data and were therefore included in the present review. Other limitations include incomplete reporting of demographics and timing of the imaging studies in relation to the onset of olfactory dysfunction and a lack of systemic follow-up in some studies.
CONCLUSION
This systematic review suggests that focal mucosal thickening and obstruction of OC plays a dominant mechanistic role in COVID-19-induced olfactory dysfunction. Hyperintensity in the OB, OB atrophy, cortical hyperintensity, and hypometabolic cortical activity indicate a central mechanism at later stages, likely due to the direct neurotropism of SARS-CoV-2. Further studies are needed to elucidate the exact pathogenesis and the full spectrum of imaging manifestations during the evolution of olfactory dysfunction secondary to COVID-19.
Author Contributions
Study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of the final version of the submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, P.K., F.Y., S.H.; and manuscript editing, all authors.
Footnotes
Supplementary material associated with this article can be found in the online version at 10.1016/j.acra.2021.08.010.
Appendix. Search Strategy
PubMed Keywords
("coronavirus 2" [Title/Abstract] OR "coronavirus 2"[Mesh] OR "coronavirus infections"[Title/Abstract] OR "coronavirus infections"[Mesh] OR "COVID-19"[Title/Abstract] OR "COVID-19"[Mesh] OR "coronavirus"[Title/Abstract] OR "coronavirus"[Mesh] OR "2019-nCoV"[Title/Abstract] OR "COVID-2019"[Title/Abstract] OR "COVID19"[Title/Abstract] OR "nCoV"[Title/Abstract] OR "coronavirus disease 2019"[Title/Abstract] OR "2019 novel coronavirus"[Title/Abstract] OR "severe acute respiratory syndrome"[Title/Abstract] OR "SARS-CoV-19"[Title/Abstract] OR "SARS-CoV-2"[Title/Abstract] OR "2019-CoV-19"[Title/Abstract] OR "SARS-CoV"[Title/Abstract] OR "2019nCoV"[Title/Abstract] OR "coronavirinae"[Title/Abstract] OR "2019 Novel Coronavirus Infection"[Title/Abstract] OR "2019 nCoV Infection"[Title/Abstract] OR "Bat coronavirus"[Title/Abstract] OR "betacoronavirus*"[Title/Abstract] OR "coronavirus Infection Disease 2019"[Title/Abstract] OR "covid*"[Title/Abstract] OR "Novel Coronavirus Pneumonia"[Title/Abstract] OR "Wuhan virus"[Title/Abstract]) AND (Radiology[Mesh] OR Radiology[Title/Abstract] OR "Tomography, X-ray Computed"[Mesh] OR "computed assisted tomography"[Title/Abstract] OR CT[Title/Abstract] OR (computed[Title/Abstract] AND tomograph[Title/Abstract]) OR (x-ray[Title/Abstract] AND computed[Title/Abstract]) OR (imaging[Title/Abstract] AND display[Title/Abstract]) OR "Diagnostic Imaging"[Title/Abstract] OR image*[Title/Abstract] OR imaging[Title/Abstract] OR radiography[Title/Abstract] OR radiolog*[Title/Abstract] OR radiograph*[Title/Abstract] OR MRI[Title/Abstract] OR "Magnetic Resonance Imaging"[Title/Abstract] OR PET[Title/Abstract] OR MRI-PET[Title/Abstract] OR PET/CT[Title/Abstract] OR PET/MR[Title/Abstract] OR PET-CT[Title/Abstract] OR "Positron Emission Tomograph*"[Title/Abstract] OR "FDG PET"[Title/Abstract] OR FDG-PET[Title/Abstract] OR "FDG PET/CT"[Title/Abstract]) AND (Anosmia[Mesh] OR Anosmia[Title/Abstract] OR Olfactory[Title/Abstract] OR "Olfactory Dysfunction"[Title/Abstract] OR "Olfactory Bulb"[Mesh] OR "Olfactory Bulb"[Title/Abstract] OR "Olfactory cortex"[Mesh] OR "Olfactory cortex"[Title/Abstract] OR "Olfactory tract"[Title/Abstract] OR Hyposmia[Title/Abstract] OR Parosmia[Title/Abstract] OR Phantosmia[Title/Abstract] OR "Loss of smell"[Title/Abstract])
Time: 2019 – 2021
Appendix B. Supplementary materials
References
- 1.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet North Am Ed. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strauss S, Lantos J, Heier L, et al. Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. Am J Neuroradiol. 2020;41(10):1882–1887. doi: 10.3174/ajnr.A6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020;77(8):1028–1029. doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 5.MdFVV Aragão, M Leal, Cartaxo Filho O, et al. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. Am J Neuroradiol. 2020;41(9):1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu A, Fischbein N, Wintermark M, et al. COVID-19-induced anosmia associated with olfactory bulb atrophy. Neuroradiology. 2021;63(1):147–148. doi: 10.1007/s00234-020-02554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrêa DG, da Cruz Jr LCH, Lopes FCR, et al. Magnetic resonance imaging features of COVID-19-related cranial nerve lesions. J Neurovirol. 2021;27(1):171–177. doi: 10.1007/s13365-020-00934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliezer M, Hamel A-L, Houdart E, et al. Loss of smell in patients with COVID-19: MRI data reveal a transient edema of the olfactory clefts. Neurology. 2020;95(23):e3145–e3e52. doi: 10.1212/WNL.0000000000010806. [DOI] [PubMed] [Google Scholar]
- 9.Hatipoglu N, Yazici ZM, Palabiyik F, et al. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia in pediatric cases. Int J Pediatr Otorhinolaryngol. 2020;139 doi: 10.1016/j.ijporl.2020.110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandemirli SG, Altundag A, Yildirim D, et al. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad Radiol. 2021;28(1):28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schönegger CM, Gietl S, Heinzle B, et al. Smell and taste disorders in COVID-19 patients: objective testing and magnetic resonance imaging in five cases. SN Compr Clin Med. 2020;2(12):2535–2539. doi: 10.1007/s42399-020-00606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strauss S, Lantos J, Heier L, et al. Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. Am J Neuroradiol. 2020;41(10):1882–1887. doi: 10.3174/ajnr.A6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang E, Ulualp SO, Liu C, et al. Sudden anosmia and ageusia in a child: a COVID-19 case report. Otolaryngol Case Rep. 2021;18 doi: 10.1016/j.xocr.2021.100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chetrit A, Lechien JR, Ammar A, et al. Magnetic resonance imaging of COVID-19 anosmic patients reveals abnormalities of the olfactory bulb: preliminary prospective study. J Infect. 2020;1(5):816–846. doi: 10.1016/j.jinf.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yousefi-Koma A, Haseli S, Bakhshayeshkaram M, et al. Multimodality imaging with PET/CT and MRI reveals hypometabolism in tertiary olfactory cortex in parosmia of COVID-19. Acad Radiol. 2021;28(5):749–751. doi: 10.1016/j.acra.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsivgoulis G, Fragkou PC, Lachanis S, et al. Olfactory bulb and mucosa abnormalities in persistent COVID-19-induced anosmia: a magnetic resonance imaging study. Eur J Neurol. 2021;28(1):e6–e8. doi: 10.1111/ene.14537. [DOI] [PubMed] [Google Scholar]
- 17.Li C-W, Syue L-S, Tsai Y-S, et al. Anosmia and olfactory tract neuropathy in a case of COVID-19. J Microbiol Immunol Infect. 2021;54(1):93–96. doi: 10.1016/j.jmii.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulb edema during COVID-19–related anosmia. Neurology. 2020;95(5):224–225. doi: 10.1212/WNL.0000000000009850. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y-C, Tsai Y-S, Syue L-S, et al. Olfactory bulb atrophy in a case of COVID-19 with hyposmia. Acad Radiol. 2020;21(11):1649–1650. doi: 10.1016/j.acra.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theodorou DJ, Theodorou SJ, Tsaggou V, et al. Anosmia caused by ischaemic olfactory infarction: false alert for COVID-19 infection. QJM. 2021;114(1):50–51. doi: 10.1093/qjmed/hcaa294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melegari G, Rivi V, Zelent G, et al. Mild to severe neurological manifestations of COVID-19: cases reports. Int J Environ Res Public Health. 2021;18(7):3673. doi: 10.3390/ijerph18073673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niesen M, Trotta N, Noel A, et al. Structural and metabolic brain abnormalities in COVID-19 patients with sudden loss of smell. Eur J Nucl Med Mol Imaging. 2021;48(6):1890–1891. doi: 10.1007/s00259-020-05154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eliezer M, Hautefort C, Hamel A-L, et al. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(7):674–675. doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 24.Ismail II, Gad KA. Absent blood oxygen level–dependent functional magnetic resonance imaging activation of the orbitofrontal cortex in a patient with persistent cacosmia and cacogeusia after COVID-19 infection. JAMA Neurol. 2021;78(5):609–610. doi: 10.1001/jamaneurol.2021.0009. [DOI] [PubMed] [Google Scholar]
- 25.Galougahi MK, Ghorbani J, Bakhshayeshkaram M, et al. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia: the first report. Acad Radiol. 2021;27(6):892–893. doi: 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Politi LS, Grimaldi M, Balzarini L. MRI depicts olfactory bulbs and cortical involvement in COVID-19 patients with anosmia. MAGNETOM Flash; 2021;78(1)
- 27.Hajjij A, Benslima N, Aasfara J, et al. An MRI of the olfactory tract in a case of post-COVID-19 persistent anosmia. Integr J Med Sci. 2021;8 doi: 10.15342/ijms.2021.406. [DOI] [Google Scholar]
- 28.MdFVV Aragao, MdC Leal, Andrade PHP, et al. Clinical and radiological profiles of COVID-19 patients with neurological symptomatology: a comparative study. Viruses. 2021;13(5):845. doi: 10.3390/v13050845. 2020;27(6):892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lechien JR, Michel J, Radulesco T, et al. Clinical and radiological evaluations of COVID-19 patients with anosmia: preliminary report. Laryngoscope. 2020;130(11):2526–2531. doi: 10.1002/lary.28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naeini AS, Karimi-Galougahi M, Raad N, et al. Paranasal sinuses computed tomography findings in anosmia of COVID-19. Am J Otolaryngol. 2020;41(6) doi: 10.1016/j.amjoto.2020.102636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yıldız E, Balcı A, Selendili O, Kuzu S. Covid 19 pandemic: paranasal diagnostic imaging in patients with olfactory loss. Research Square, 2020, doi: 10.21203/rs.3.rs-31774/v1. [DOI]
- 32.Jalessi M, Barati M, Rohani M, et al. Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19. Neurol Sci. 2020;41(9):2331–2338. doi: 10.1007/s10072-020-04590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karimi-Galougahi M, Yousefi-Koma A, Bakhshayeshkaram M, et al. 18FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID-19. Acad Radiol. 2020;27(7):1042–1043. doi: 10.1016/j.acra.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q, Shan KS, Abdollahi S, et al. Anosmia and ageusia as the only indicators of Coronavirus Disease 2019 (COVID-19) Cureus. 2020;12(5):e7918. doi: 10.7759/cureus.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bilinska K, Butowt R. Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem Neurosci. 2020;11(15):2152–2155. doi: 10.1021/acschemneuro.0c00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper KW, Brann DH, Farruggia MC, et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020;107(2):219–233. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butowt R, von Bartheld CS. covid19 Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2020 doi: 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng J, Wong LY, Li K, et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2021;589(7843):603–607. doi: 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han AY, Mukdad L, Long JL, et al. Anosmia in COVID-19: mechanisms and significance. Chem Senses. 2020;45(6):4. doi: 10.1093/chemse/bjaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.