Abstract
Objective
To evaluate the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) anti-spike (S) IgG antibody production after vaccination with BNT162b2 and the protection from symptomatic breakthrough infections in health care workers.
Methods
This prospective observational study (RENAISSANCE) had as a primary end point the evaluation of serologic response to BNT162b2 14 days after a second dose. SARS-CoV-2 anti-S IgG antibodies were evaluated with LIAISON SARS-CoV-2 TrimericS IgG assay (DiaSorin S.p.A.), which is able to detect the presence of both binding and neutralizing antibodies for trimeric spike glycoprotein. Participants were recruited from February 1, 2021, to February 22, 2021. Occurrence of vaccine breakthrough infections was assessed by reverse transcription–polymerase chain reaction on symptomatic and contact cases up to June 6, 2021.
Results
Of 2569 staff evaluated, only 4 were nonresponders (0.16%; 95% CI, 0.04% to 0.41%). All 4 nonresponders were severely immunosuppressed and receiving treatment with mycophenolate mofetil or mycophenolic acid. At 14 days after the second dose, 67.5% (1733) of staff had anti-S IgG titers of 2000 BAU/mL or higher; 19.2% (494), between 1500 and 2000 BAU/mL; 9.8% (251), between 1000 and 1500 BAU/mL; and 3.4% (87), 1000 BAU/mL or lower. Women had a higher probability of having higher titers than men (64.5% [1044/1618] vs 58.3% [410/703]; P=.005). This was confirmed after adjustment for age group (odds ratio, 1.275; 95% CI, 1.062 to 1.531; P=.009). Four months after the end of the vaccination program, only 13 participants (0.26%) had experienced a breakthrough SARS-CoV-2 infection, including 1 nonresponder. This was the only participant requiring hospitalization for severe COVID-19.
Conclusion
The vaccination campaign among health care workers at the ASST GOM Niguarda has resulted in a marked serologic response and reduction of incident COVID-19 cases. Yet, the lack of protection should not be overlooked in immunocompromised individuals.
Abbreviations and Acronyms: BAU, binding antibody unit; IMPDH, inosine-5′-monophosphate dehydrogenase; IQR, interquartile range; MPA, mycophenolic acid; RT-PCR, reverse transcription–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VOC, variant of concern
On December 21, 2020, the European Commission granted the first European conditional marketing authorization for the active immunization of adults older than 16 years with the BNT162b2 vaccine (Pfizer-BioNTech). This approval was the result of an unprecedented effort undertaken by companies, regulatory authorities, and academia that enabled the development of vaccines in as short as 10 months. Soon after the approval, vaccination campaigns for health care workers across the United States and Europe began immediately. As of May 10, 2021, the Centers for Disease Control and Prevention COVID-19 vaccination tracker has counted a total of 259,716,989 doses administered in the United States (https://covid.cdc.gov/covid-data-tracker/#vaccinations).
BNT162b2 is a vaccine containing a nucleoside-modified messenger RNA (mRNA) able to encode the full-length severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) glycoprotein, enclosed within a lipid nanoparticle shell with the role of protecting it from degradation and thus promoting delivery to the target cell. In the phase 3 trial that led to market approval, the BNT162b2 vaccine demonstrated 95% clinical efficacy in preventing COVID-19 (95% CI, 90.3 to 97.6; >0.9999 posterior probability of true vaccine efficacy >30%).1
The immunogenicity of BNT162b2 had previously been evaluated in smaller phase 1-2 trials. These demonstrated that for all doses tested, anti-S IgG antibody production was detectable at 21 days after the first dose, with a boost effect from the second dose, leading to a peak antibody response within the next 7 days. A 50% neutralization titer was found to peak at 7 days after the second dose in the population between 18 and 55 years of age; but in the population between 65 and 85 years of age, the peak of production was reached 14 days after the second dose.2 The rapid approval of COVID-19 vaccines, dictated by the impending need, previously had led to a poor representation, in clinical trials, of variability in background and clinical and demographic conditions of the population to which clinical practice studies are called to respond. BNT162b2 clinical trials systematically excluded immunosuppressed individuals and those with a history of autoimmune disease or an active autoimmune disease requiring therapeutic intervention. In addition, the immunogenicity of the BNT162b2 at the approved dose of 30 μg has been evaluated in only 12 participants in the age group of 18 to 55 years and 12 in the age group of 65 to 85 years.2 Data on large populations, more representative of the general population in individual epidemiologic settings, are therefore urgently needed.
The RENAISSANCE study is an observational study that aimed to evaluate the serologic response to the BNT162b2 COVID-19 vaccine in the staff of the main COVID-19 reference hospital of Milan, Italy, at multiple time points after a hospital-wide vaccination campaign. Here we report the efficacy of the BNT162b2 vaccine in promoting anti-S antibody production and conferring protection against symptomatic and severe forms of COVID-19 at day 14 after the second vaccine dose in more than 2500 health care workers.
Methods
Study Design and Participants
RENAISSANCE is a prospective, observational, population-based study of health care workers of ASST Grande Ospedale Metropolitano Niguarda in Milan, Italy. All staff with no history of previous laboratory-confirmed COVID-19 who completed the BNT162b2 vaccine schedule were eligible for inclusion. BNT162b2 has been administered in 2 doses of 30 μg, as described in the summary of product characteristics.3 The primary end point of the study was the evaluation of IgG antibodies against S glycoprotein produced 14 days after the second dose of BNT162b2. The secondary end point was the evaluation of the clinical efficacy of vaccination, testified by the protection from symptomatic COVID-19. No systematic reverse transcription–polymerase chain reaction (RT-PCR) screening was established, but RT-PCR was provided to all symptomatic participants and to those who reported direct contact to COVID-19 cases. Anti-nucleocapsid (N) total Ig seropositivity at day 14 after the second vaccine dose was used to discriminate staff who had a history of unrecognized contact with SARS-CoV-2.
During the clinical visit at the recruitment appointment, each participant received a self-administered, standardized questionnaire covering major chronic health conditions, sociodemographic characteristics, occupational histories, and, for women, reproductive history to be compiled on a volunteer basis. A survey for the self-report of adverse events was also administered at the time of enrollment.
The study was approved by the local ethics committee Milano Area 3, by the national ethics committees for COVID-19 studies Lazzaro Spallanzani–IRCCS, and by the Italian Medicines Agency (AIFA). All participants provided signed informed consent.
Procedures
All eligible hospital staff were invited to participate in the study by email, which provided the indication for an appointment for a visit. Two samples of 3 mL of peripheral venous blood were taken from each participant. Blood samples were stored at room temperature for 2 to 4 hours on collection and then centrifuged to separate plasma that was conserved at +4°C until processing, which occurred within 1 to 5 days. The full study protocol is available online (in Italian).
Laboratory Methods
SARS-CoV-2 anti-S IgG antibody production was evaluated by the LIAISON SARS-CoV-2 TrimericS IgG assay (DiaSorin S.p.A.), a second-generation standardized, automated chemiluminescent assay able to detect the presence of antibodies for trimeric S glycoprotein, including those directed against the receptor-binding domain. The DiaSorin test is quantitative, with optimal sensitivity and specificity for IgG detection within the time interval useful for the study (98.7% and 99.5%, respectively), and consistently correlated with neutralizing activity.4 Results are provided as binding antibody units (BAU) per milliliter.4 According to the manufacturer’s indications, values below 33.8 BAU/mL were reported as negative.
Total Anti-N Ig antibody production was assessed by an electrochemiluminescence immunoassay5 (Elecsys anti–SARS-CoV-2 assay; Roche Diagnostics). According to the manufacturer’s instructions, a signal/cutoff ratio of 1.0 or greater was interpreted as reactive. The test is reported to have a clinical specificity of 99.80% (95% CI, 99.69% to 99.88%) and a sensitivity of 99.5% (95% CI, 97.0% to 100%) in the time range useful for the study.
SARS-CoV-2 RNA detection by real-time RT-PCR on nasopharyngeal swabs was performed with 1 of the following assays, by random choice: GeneFinder COVID-19 Plus RealAmp Kit (OSANG Healthcare) on an InGenius instrument (ELITech Group); AllplexTM 2019-nCoV Assay (Seegene Inc) on a Nimbus instrument (Hamilton); and Xpert Xpress SARS-CoV-2 (Cepheid) on the GeneXpert Instrument System (Cepheid).
Assessment of SARS-CoV-2 spike variants in post-vaccine RT-PCR–positive samples was performed by multiplex real-time PCR (Allplex SARS-CoV-2 Variants Assay; Arrow Diagnostics S.R.L.) and confirmed by next-generation sequencing of the full S gene (Illumina).
Statistical Analyses
Categorical variables were summarized as number and percentage. Age and body mass index were summarized as median and interquartile range (IQR). The proportion of staff who developed an antibody response at day 14 after receiving the second vaccine dose and its 95% CI were calculated by the exact Clopper-Pearson method. The association between SARS-CoV-2 antibody response and demographic and clinical characteristics was evaluated by the χ 2 or Fisher exact test (categorical variables) and Mann-Whitney test (quantitative variables). All tests were 2 sided with an α level of .05.
A multivariable logistic regression on titers above 2000 BAU/mL (yes/no) was implemented, considering age group and sex as independent variables. The adjusted odds ratio and its 95% CI were reported. Stata 16.1 (StataCorp LLC) was used for all analyses.
Results
Study Population
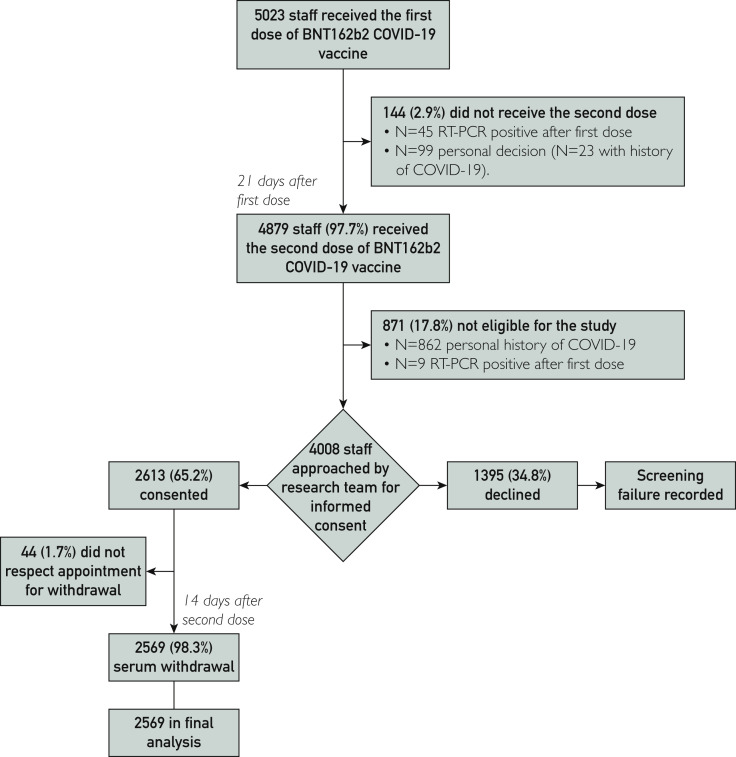
Figure 1 details the study flowchart; 5023 staff received the first dose of BNT162b2 COVID-19 vaccine, and 4879 (97.7%) received the second dose. The reason for not completing the vaccination protocol was COVID-19 diagnosis in 45 of 144 cases (31.3%), whereas it remained undetermined for the others. We sent emails to 4008 eligible staff and invited them to participate in the 14-day screening; 2613 (65.2%) accepted the invitation and between February 1, 2021, and March 1, 2021, presented themselves to the study visit. There were 44 participants (1.7%) who showed up more than 7 days late for their scheduled appointment and were therefore excluded from the analysis. The final study population thus consisted of 2569 staff.
Figure 1.
Flowchart for staff recruitment and enrollment. COVID-19, coronavirus disease 2019; RT-PCR, real-time reverse transcription–polymerase chain reaction.
Anti-N antibody screening revealed an anti-N seroprevalence of 6.3% (95% CI, 5.4% to 7.4%) as 163 participants with no previous history of laboratory-confirmed COVID-19 were found to be anti-N Ig positive, suggesting a history of unrecognized contact with SARS-CoV-2. Table 1 describes the demographic characteristics and job titles of the total population and subpopulations of staff who were anti-N positive and negative. Overall, the median (IQR) age was 48 (36-56) years, and 787 (30.6%) were 55 years of age or older. Female sex accounted for 69.6% of the population (n=1789); median (IQR) body mass index was 23 kg/m2 (21-27 kg/m2). Most of the staff who joined the study were nurses (32.4%; n=832) and medical doctors (23.7%; n=608).
Table 1.
Demographic and Baseline Clinical Characteristics and Job Titles of Staff Who Participated in Serologic Screening After Completing BNT162b2 COVID-19 Vaccinationa,b
| Study population, all | SARS-CoV-2 anti-nucleocapsid serostatus at enrollment |
P value | ||
|---|---|---|---|---|
| Anti-N total Ig negative | Anti-N total Ig positive | |||
| Overall | 2569 | 2406 | 163 | |
| Sex | ||||
| Female | 1789 (69.6) | 1680 (69.8) | 109 (66.9) | .427 |
| Male | 780 (30.4) | 726 (30.2) | 54 (33.1) | |
| Age (y) | 48 (36-56) | 48 (36-56) | 47 (36-56) | .547 |
| Age group | ||||
| 19-30 years | 301 (11.7) | 277 (11.5) | 24 (14.7) | .444 |
| 31-55 years | 1481 (57.6) | 1392 (57.9) | 89 (54.6) | |
| >55 years | 787 (30.6) | 737 (30.6) | 50 (30.7) | |
| Residency in Milan | 1774 (74.9) | 1653 (74.6) | 121 (79.1) | .139 |
| Job title | ||||
| Nurse staff | 832 (32.4) | 769 (32.0) | 63 (38.7) | |
| Medical staff | 608 (23.7) | 569 (23.6) | 39 (23.9) | |
| Other sanitary staff | 467 (18.2) | 437 (18.2) | 30 (18.4) | |
| Administrative staff | 353 (13.7) | 337 (14) | 16 (9.8) | |
| Laboratory staff | 167 (6.5) | 154 (6.4) | 13 (8.0) | |
| Non-sanitary staff | 87 (3.4) | 85 (3.5) | 2 (1.2) | |
| Pharmacy and physics staff | 55 (2.1) | 55 (2.3) | 0 (0.0) | |
| Participated in survey | 1886 | 1771 | 115 | |
| Body mass index (kg/m2) | 23 (21-27) | 23 (21-27) | 23 (21-26) | .895 |
| Body mass index range | ||||
| ≤18.49 kg/m2 | 83 (4.6) | 80 (4.7) | 3 (2.7) | .541 |
| 18.5-24.99 kg/m2 | 1095 (60.5) | 1024 (60.4) | 71 (62.8) | |
| 25-29.99 kg/m2 | 436 (24.1) | 406 (23.9) | 30 (26.6) | |
| 30-34.99 kg/m2 | 144 (8.0) | 136 (8.0) | 8 (7.1) | |
| ≥35 kg/m2 | 51 (2.8) | 50 (3.0) | 1 (0.9) | |
| Female population | ||||
| Currently breastfeeding | 19 (1.5) | 18 (1.6) | 1 (1.4) | .999 |
| Combined oral contraceptive use | 55 (4.3) | 50 (4.1) | 5 (6.9) | .23 |
| Selected drug use | ||||
| Oral corticosteroids | 11 (0.6) | 11 (0.6) | 0 (0.0) | .999 |
| ACEi | 117 (6.2) | 113 (6.4) | 4 (3.5) | .217 |
| Comorbiditiesc | ||||
| At least 1 reported | 428 (22.7) | 402 (22.7) | 26 (22.6) | .982 |
| Cardiovascular disease | 204 (10.8) | 190 (10.7) | 14 (12.2) | .627 |
| Hypertension | 155 (8.2) | 145 (8.2) | 10 (8.7) | .845 |
| Endocrine disease (thyroid or ovary) | 103 (5.5) | 98 (5.5) | 5 (4.3) | .588 |
| Autoimmune disease | 68 (3.6) | 66 (3.7) | 2 (1.7) | .268 |
| Respiratory disease | 60 (3.2) | 54 (3.0) | 6 (5.2) | .199 |
| Diabetes | 30 (1.6) | 29 (1.6) | 1 (0.9) | .999 |
| Hypercholesterolemia | 30 (1.6) | 29 (1.6) | 1 (0.9) | .999 |
| Allergies | 30 (1.6) | 29 (1.6) | 1 (0.9) | .999 |
| Immunosuppression | 27 (1.4) | 27 (1.5) | 0 (0.0) | .406 |
| Arrhythmia | 23 (1.2) | 20 (1.1) | 3 (2.6) | .161 |
| Multiple sclerosis | 6 (0.3) | 5 (0.3) | 1 (0.9) | .314 |
| Coinfection with HIV | 6 (0.3) | 6 (0.3) | 0 (0.0) | .999 |
| Coinfection with hepatitis B virus | 4 (0.2) | 4 (0.2) | 0 (0.0) | .999 |
ACEi, angiotensin-converting enzyme inhibitor; HIV, human immunodeficiency virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Categorical variables are presented as number (percentage). Continuous variables are presented as median (interquartile range). Fisher exact test and χ2 test were used to compare all variables except body mass index, compared by Mann-Whitney test.
Data on comorbidities are for 1886 participants.
Anti-S and Neutralizing IgG Response at Day 14 after Second Vaccine Dose
Of 2569 staff tested, only 4 were anti-S antibody negative (0.16% prevalence; 95% CI, 0.04% to 0.40%) and thus were classified as nonresponders. All of them were anti-N IgG negative, immunocompromised, and receiving mycophenolate (Table 2 ).
Table 2.
Clinical Characteristics of Staff in Whom BNT162b2 Vaccination Failed to Elicit an Anti-Spike Antibody Response
| Nonresponder 1 | Nonresponder 2 | Nonresponder 3 | Nonresponder 4 | |
|---|---|---|---|---|
| Sex | Female | Female | Male | Female |
| Age (y) | 56 | 37 | 58 | 37 |
| Underlying chronic disease or clinical condition | Renal transplant | Myasthenia gravis | Cardiac transplant | Systemic lupus erythematosus |
| Immunosuppressive therapy, current | Cyclosporine 150 mg daily, mycophenolate mofetil 1440 mg daily | Prednisone 20 mg daily, mycophenolate mofetil 1500 mg daily, intravenous immunoglobulins 60 g monthly | Cyclosporine, mycophenolate mofetila | Prednisone 12.5 mg daily, mycophenolic acid 1440 mg daily, colchicine 0.5 mg daily |
| Leukocyte count (109 cells/L) at screening | 9.51 | 6.37 | 9.85 | |
| Lymphocyte count (109 cells/L) at screening | 2.81 | 1.9 | 1.16 |
Additional information regarding drug dose, leukocyte count, and lymphocyte count were not available.
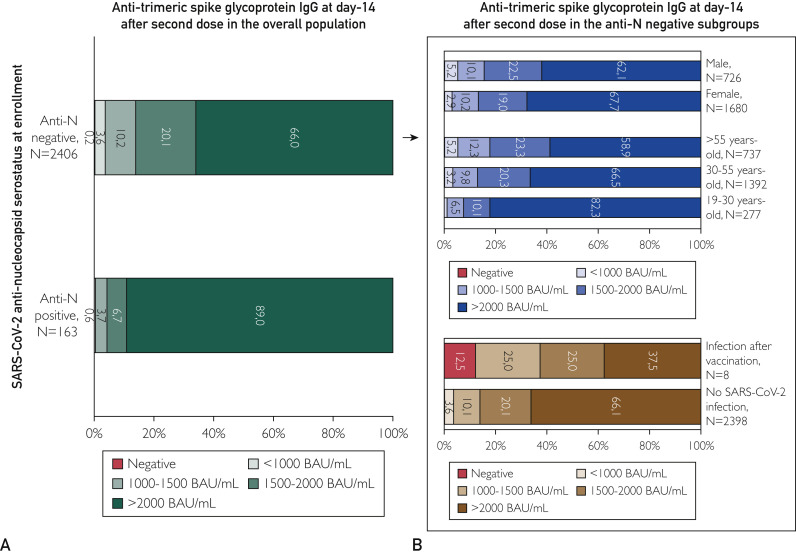
A minority of vaccinated participants (87/2569 [3.4%]) had detectable anti-S IgG but 1000 BAU/mL or less, whereas 67.5% (1733/2569) had titers of 2000 BAU/mL or more. In addition, 9.8% (251/2569) had anti-S IgG titers between 1000 and 1500 BAU/mL, and 19.2% (494/2569) had titers between 1500 and 2000 BAU/mL. As reported in Figure 2 A, the distribution of anti-S IgG titers was correlated with the history of contact with SARS-CoV-2, favoring anti-N–positive participants over anti-N–negative participants in development of higher values. Indeed, the proportion of participants with 2000 BAU/mL or more in anti-N–positive staff significantly outnumbered that of anti-N–negative staff (89.0% [145/163] vs 66.0% [1588/2406], respectively; P<.001).
Figure 2.
Distribution of anti-trimeric spike glycoprotein IgG titers at day 14 after second BNT162b2 vaccine dose. Negative values were those below 33.8 BAU/mL, per the manufacturer’s instructions. The distribution of anti-spike IgG titers is reported separately according to anti-nucleocapsid (anti-N) serostatus (A) and for sex and age in the population of anti-N–negative staff (B). BAU, binding antibody unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In anti-N–negative staff, sex and age were 2 determinant characteristics for anti-S IgG response (Figure 2B). Women had a significantly higher probability than men of having anti-S IgG titer of 2000 BAU/mL or higher (67.7% [1137/1680] vs 62.1% [451/726]; P=.008). In addition, anti-S titers were 2000 BAU/mL or higher in the 82.3% of staff aged 19 to 30 years (228/277) vs 66.5% in staff aged 30 to 50 years (926/1392) and 58.9% in staff aged 55 years and older (434/737; P<.001). Multivariable analysis confirmed the association of sex and anti-S IgG titers of 2000 BAU/mL and higher, even after adjustment for age group (odds ratio, 1.248; 95% CI, 1.039 to 1.499; P=.02; Supplemental Figure, available online at http://www.mayoclinicproceedings.org).
Factors Affecting Anti-S and Neutralizing IgG Response
A subgroup of 1886 staff volunteered to complete a survey on concurrent comorbidities, and 22.7% (428/1886) declared themselves to be affected by at least 1 disease (Supplemental Table 1, available online at http://www.mayoclinicproceedings.org). The most frequently reported were cardiovascular diseases (10.8% [204/1886]) and endocrine system diseases (5.5% [103/1886]). Of these 1886 participants, 3.6% (68) declared an autoimmune disease, whereas 1.4% (27) were immunosuppressed.
The concomitant immunosuppression at the time of vaccination was significantly associated with a different distribution of anti-S IgG titers (Supplemental Table 2, available online at http://www.mayoclinicproceedings.org). Of the 27 participants who reported being immunosuppressed, 4 (14.8%) did not develop anti-S IgG, 4 (14.8%) had a titer below 1000 BAU/mL, 1 (3.7%) had a titer between 1000 and 1500 BAU/mL, 8 (29.6%) had a titer between 1500 and 2000 BAU/mL, and only 10 (37.0%) had a titer greater than 2000 BAU/mL (P<.001). Notably, mycophenolate consumption at the time of vaccination was declared exclusively by the 4 nonresponders.
We found no significant differences in the distribution of anti-S IgG titers according to the concomitant clinical conditions when autoimmune diseases, cardiovascular diseases, hypertension, arrhythmias, hypercholesterolemia, respiratory diseases, allergies, multiple sclerosis, endocrine diseases, diabetes, human immunodeficiency virus seropositivity, and hepatitis B virus infection were taken into account.
Clinical Efficacy of COVID-19 Vaccine in Preventing SARS-CoV-2 Infection
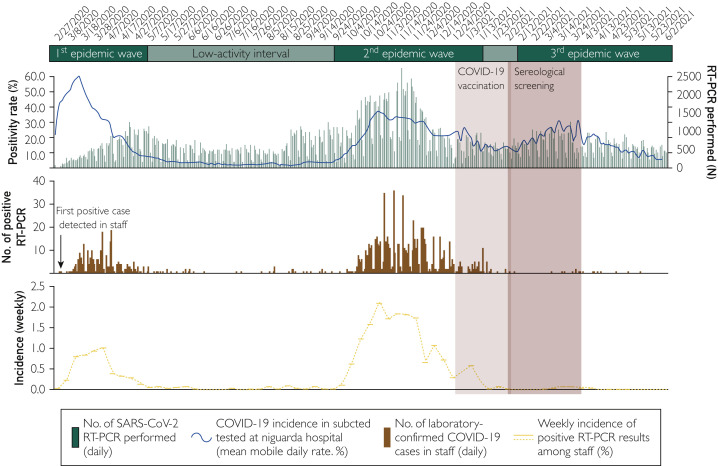
The pandemic epidemiologic context at ASST GOM Niguarda from February 27, 2020 (start of clinical and diagnostic activity against SARS-CoV-2 infection), until June 6, 2021, is shown in Figure 3 . On March 5, 2020, the first case of a health care worker infected with SARS-CoV-2 was diagnosed with RT-PCR, and from that day onward, the incidence of COVID-19 among staff followed the trend of the epidemic waves in the general population. The vaccination program was started on December 29, 2020, when the staff was battling against the second pandemic wave and the rate of new daily RT-PCR positive responses diagnosed at our hospital was steadily above 20%. Vaccination of staff ended on February 2, 2021, just a few days before the beginning of a third epidemic wave, characterized by the spread of new viral variants. Yet, notably, for the first time since the start of the pandemic, the curve of infections among staff did not mirror that of the general population but deviated sharply from it. Indeed, up to June 6, 2021, only 13 of the total 5023 participants vaccinated (0.26%) had a laboratory-confirmed SARS-CoV-2 infection, testified by 2 consecutive positive RT-PCR responses or 1 positive RT-PCR response plus anti-N seroconversion after 14-day screening. Infections occurred a median (IQR) of 62 (54-75) days after the second vaccine dose (Supplemental Table 2). The nasopharyngeal SARS-CoV-2 genomic RNA load ranged from 1195 to more than 107 copies/mL, as assessed through Droplet Digital PCR (Bio-Rad).6
Figure 3.
Daily rate of laboratory-confirmed coronavirus disease 2019 (COVID-19) cases at ASST GOM Niguarda (top), positive real-time reverse transcription–polymerase chain reaction (RT-PCR) results among staff (middle), and weekly incidence of new laboratory-confirmed COVID-19 cases among staff (bottom). The mean mobile daily rate of first-time positive real-time PCR results on nasopharyngeal swabs (red line) was calculated on the total number of swabs processed at ASST GOM Niguarda (dark gray bars) from February 27, 2020, to March 15, 2021. The number of first-time positive RT-PCR results (red boxes) and the median and interquartile range weekly incidence of new laboratory-confirmed COVID-19 cases are reported for staff who participated to the BNT162b2 COVID-19 vaccination program (N=5023). Gray shading indicates the COVID-19 pandemic waves in Lombardy, Italy. Light blue shading shows the duration of the COVID-19 vaccination program. Dark blue shading indicates sampling period for screening. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Notably, the only individual who developed a symptomatic, moderate to severe infection requiring hospitalization was 1 of the 4 nonresponders enrolled in our study. Among the 7 infected participants enrolled in the study who developed a positive anti-S IgG titer, 2 participants had titers between 1000 and 1500 BAU/mL, 2 had titers between 1500 and 2000 BAU/mL, and 3 had titers above 2000 BAU/mL. Despite the small numbers, the distribution of anti-S titers at 14 days was significantly different between those who later became infected with SARS-CoV-2 and the rest of the population (P=.008 by Fisher exact test; Figure 2B), mainly because of the overrepresentation of anti-S negatives and, as expected, a lower presence of participants with anti-S IgG titers above 2000 BAU/mL.
Adverse Effects
Overall, 1924 enrolled participants completed the survey for the self-report of adverse events associated with the first dose of BNT162b2 vaccine; 1881 participants completed the survey relating to the second dose (Table 3 ). A higher percentage of female participants experienced at least 1 adverse effect of both the first dose (male participants, 107/566 [18.9%]; female participants, 442/1340 [32.99%]) and the second dose (male participants, 217/510 [42.55%]; female participants, 739/1173 [32.99%]). The difference was statistically significant (P<.001).
Table 3.
Self-Reported Adverse Effects Associated With First and Second Doses of BNT162b2 Vaccine
| First dose | Second dose | |
|---|---|---|
| Overall | 1924 | 1881 |
| Participants who had at least 1 adverse effect | 549 (28.80) | 956 (54.60) |
| Serious adverse effect | 2 (0.12) | 4 (0.48) |
| Participants who needed to take medication for vaccine adverse effect | 153 (8.22) | 563 (53.72) |
| Pain in injection site | 1107 (57.54) | 1232 (69.84) |
| Fatigue | 371 (19.28) | 772 (43.94) |
| Malaise | 204 (10.60) | 523 (30,09) |
| Headache | 281 (14.60) | 585 (33.58) |
| Joint pain | 207 (10.76) | 535 (30.55) |
| Muscle pain | 381 (19.80) | 706 (40.30) |
| Chills and tremors | 151 (7.85) | 439 (25.22) |
| Fever | 33 (1.72) | 271 (15.59) |
| Diarrhea | 40 (2.08) | 107 (6.69) |
| Nausea | 65 (3.38) | 183 (10.53) |
| Vomiting | 7 (0.36) | 29 (1.67) |
| Abdominal pain | 39 (2.03) | 116 (6.69) |
| Lymph node swelling | 61 (3.17) | 190 (10.94) |
| Insomnia | 87 (4.52) | 184 (10.55) |
Values are reported as number (percentage).
Discussion
Fourteen days after the second dose of BNT162b2 vaccine, 99.8% (2565/2569) of the staff of the largest COVID-19 reference hospital in Milan, Italy, had developed an IgG response against the trimeric (native) form of the SARS-CoV-2 spike protein. This immunologic response adequately protected the hospital staff from the resurgence of COVID-19 epidemics in Milan that caused a third epidemic wave in February-April 2021. As of June 6, 2021, 4 months after the end of the staff vaccination program, only 13 participants had a laboratory-confirmed diagnosis of breakthrough SARS-CoV-2 infection, 0.26% of the overall 2569 fully vaccinated. Even though the breakthrough infections were generally sustained by nasopharyngeal SARS-CoV-2 load of more than 10,000 copies/mL, only 1 patient required hospitalization, with 99.9% BNT162b2 vaccine efficacy in protection from moderate to severe COVID-19.
Our results are in agreement with emerging data from other health care settings that documented a consistent immunologic response to BNT162b2 vaccination7, 8, 9 and rare occurrence of vaccine breakthrough infections.10, 11, 12, 13, 14, 15 Indeed, similar-sized studies in Israel, Spain, and California reported only sporadic cases of asymptomatic or paucisymptomatic breakthrough infections among fully vaccinated individuals tested more than 14 days after a second dose of an mRNA vaccine.12 , 14 , 15
During vaccine rollout and follow-up, Milan has witnessed an alarming increase in viral variants. The B.1.1.7 has been the dominant variant of concern (VOC)16 circulating in the Lombardy region since February 2021 (when our study was initiated) and progressively increased in prevalence from 64% to 89.1% in May 18, 2021 (last epidemiologic update available).17 The second most prevalent VOC in Lombardy is B.1.617 (Indian variant), with 2.5% prevalence, followed by P.1 (Brazilian variant), at 2.1% prevalence. Cases of infection with B.1.351 lineage (South African variant) are still limited (0.8% prevalence).17 This epidemiologic scenario is compatible with our low breakthrough infection rate as the estimated effectiveness of BNT162b2 vaccine against the B.1.1.7 VOC is 89.5% at 14 days or more after the second dose.18 In vitro data support the preservation of neutralizing activity by antibodies developed with BNT162b2 vaccine against B.1.1.719 , 20 while pointing toward a possible reduction of effectiveness against B.1.351 (South African)20 , 21 and P.1 (Brazilian)22 VOCs. Reassuringly, emerging data on the new B.1.617 VOC suggest that the reduction of BNT162b2 efficacy is likely to be modest.23 , 24 While assessment of viral lineages in our vaccine breakthrough infection is ongoing, careful surveillance is mandatory, especially in light of the reduced neutralizing activity of emerging VOCs.
The few cases of breakthrough infections we registered were “enriched” by patients with lower anti-S IgG titers at 14-day follow-up, including 1 of the 4 nonresponders. This was the only patient requiring hospital admission for severe pneumonia. Although we still lack solid data to define an antibody titer “fully protective” because of heterogeneity and continuous evolution of the commercial assay, all 4 participants who did not respond to vaccination share an important immunosuppression, a condition that was associated with lower response rates to BNT162b2 vaccination in our study. Immunosuppressed or immunocompromised individuals have been excluded for protocol from all trials on COVID-19 vaccine approved to date1 , 25, 26, 27 excluding Ad26.COV2.S (NCT04505722). Despite this lack of evidence and because of the high-risk profile, this population has been included in the vaccination plans of every country.
Notably, all 4 nonresponders were receiving high doses of immunosuppressive drugs, including mycophenolate mofetil. Mycophenolate mofetil, a mycophenolic acid (MPA) prodrug, depletes guanosine nucleotides through the inhibition of inosine-5′-monophosphate dehydrogenase (IMPDH), acting preferentially on T and B lymphocytes.1 IMPDH is the rate-limiting enzyme in the de novo synthesis of guanosine nucleotides, and T and B lymphocytes depend on this pathway more than other cell types do. Mycophenolic acid is also a more potent inhibitor of the type II isoform of IMPDH, which is expressed in activated lymphocytes, than of the type I isoform of IMPDH, which is expressed in most other cell types.2 Therefore, MPA exerts a more potent cytostatic effect on lymphocytes than on other cell types. This is the main mechanism by which MPA suppresses the cell-mediated immune response and antibody formation. However, 2 patients were also receiving prednisone and 2 patients cyclosporine,28 which cause T-cell proliferation inhibition and decrease in the total T-cell number.29 Boyarsky et al30 reported the evidence of low response after a single dose of SARS-CoV-2 mRNA vaccine in solid organ transplant patients. Our cohort of vaccinated participants included 2 transplant patients. Neither responded to vaccination after both doses.
Of note, Boyarsky et al30 reported a lower probability for development of a response to vaccination in patients receiving antimetabolite immunosuppressive drugs. This reminds us that the population of immunocompromised patients, which also includes patients with autoimmune diseases receiving immunosuppressive drugs, needs special attention. Collecting data on whether immunosuppressive therapies may or may not have an impact on the response to SARS-CoV-2 vaccines is of vital importance as this is the only way to evaluate whether there is a clinical necessity to establish protocols for the suspension (when possible) of immunosuppressive therapies close to vaccination. Waiting for further data, it appears extremely clear that immunocompromised or immunosuppressed patients should be monitored by assessing the anti-S IgG antibody titer after vaccination.
Age and sex also contributed to the immunologic response to vaccination in our study, consistent with previous literature31 , 32 and clinical trials.1 Individuals older than 55 years were less frequently able to develop antibody titers above 2000 BAU/mL compared with the younger population, even though only 5.2% of them had anti-S IgG titers below 1000 BAU/mL. In addition, regardless of age, we observed a significantly higher probability for women than for men to have the highest anti-trimeric S IgG titer. Although the positive influence of estrogens on the immune response is known,33 , 34 no impact of estroprogestinic therapy on the response to vaccination has been observed.
According to the Centers for Disease Control and Prevention COVID-19 vaccine safety update35 on the first 13.7 million COVID-19 vaccine doses administered in the United States, 79.1% of the adverse effects had been experienced by women, whereas only 61.2% of the vaccines had been received by a woman. As recently highlighted by Vijayasingham et al,36 sex-disaggregated analysis and reporting have been neglected also in COVID-19 vaccine clinical trials. Given the remarkable diversity in the immune response between men and women, data regarding serologic response and adverse events in women should be reported to precisely understand sex-specific characteristics of response to vaccines and also to hypothesize a sex-differentiated dosing regimen.
Of note, although patients with a known history of exposure to SARS-CoV-2 were excluded by protocol, we included in our analysis those individuals who had never been diagnosed with infection but who tested positive for anti-N antibodies at the time of execution of our serologic evaluation. Among these, we recorded a higher prevalence of individuals with antibody titer above 2000 BAU/mL. In line with this, Saadat et al37 reported a higher IgG binding titer against spike trimer in health care workers with previous COVID-19 infection who received a single vaccine dose of BNT162b2 or mRNA-1273. This evidence could constitute a first point of discussion to evaluate the possibility of administering a single dose of vaccine instead of 2 doses in individuals who have already had a previous exposure to SARS-CoV-2.
Conclusion
Vaccination with BNT162b2 induced the development of high titers of neutralizing IgG against spike trimer within 14 days of the second dose. Vaccination successfully protected frontline health care workers from resurgence of COVID-19 pandemics in Milan. However, further assessments are needed on special populations, such as immunocompromised or immunosuppressed individuals, as well as on the duration of the immune response and its effectiveness against circulating variants.
Acknowledgments
RENAISSANCE study group: Daniela Campisi, Valeria Cento, Federica Di Ruscio, Oscar Massimiliano Epis, Diana Fanti, Elisa Matarazzo, Marco Merli, Alice Nava, Silvia Nerini Molteni, Arianna Pani, Massimo Puoti, Marco Merli, Silvia Renica, Francesco Scaglione, Livia Tartaglione, Nicola Ughi, Chiara Vismara, Claudio Rossetti, Fabrizio Colombo, Ruggero Gibilisco, Alberto Zampiero, Stefano Agliardi, Gianluca Gazzaniga, Alice Schianchi, Matteo Maggi, Tommaso Conti, Oscar Gagliardi, Alessandra Romandini, Michele Senatore, Paolo Schenardi, Silvano Rossini, Roberto Crocchiolo, Irene Cuppari, Olga Disoteo, Irene Di Matteo, Stella De Nicola, Valentina Panetta, Mariateresa Pugliano, Elisabetta Volpato, Giuliana Lando, Giorgia Cornacchini.
Arianna Pani and Valeria Cento contributed equally to this article. Silvano Rossini, Massimo Puoti, and Francesco Scaglione share joint senior authorship.
Data Sharing
| Will individual participant data be available (including data dictionaries)? | Yes |
|---|---|
| What data in particular will be shared? | Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) |
| What other documents will be available? | Study protocol (in Italian) |
| When will data be available (start and end dates) | Immediately following publication; no end date |
| With whom? | Investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose and who provide a methodologically sound proposal |
| For what types of analyses? | To achieve aims in the approved proposal |
| By what mechanism will data be made available? | Proposals should be directed to the corresponding author; to gain access, data requestors will need to sign a data access agreement |
Footnotes
For editorial comment, see page 2934
Grant Support: The study was funded through divisional funds from the Clinical Chemistry and Microbiology, Infectious Diseases, and Transfusion Medicine units of ASST GOM Niguarda. ASST GOM Niguarda provided support from the nursing staff and facilities to conduct the study.
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh E.E., Frenck R.W., Jr., Falsey A.R., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Medicines Agency. Comirnaty—EPAR product information. 23/02/2021 Comirnaty-EMEA/H/C/005735-II/0009. https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf. Accessed September 16, 2021.
- 4.DiaSorin S.p.A. LIAISON SARS-CoV-2 TrimericS IgG assay. https://www.diasorin.com/sites/default/files/allegati_prodotti/liaisonr_sars-cov-2_trimerics_igg_assay_m0870004408_a_lr.pdf Accessed March 2021.
- 5.Roche Diagnostics. Elecsys anti-SARS-CoV-2 factsheet. https://www.fda.gov/media/137605/download. Accessed April 2021.
- 6.Alteri C., Cento V., Antonello M., et al. Detection and quantification of SARS-CoV-2 by droplet digital PCR in real-time PCR negative nasopharyngeal swabs from suspected COVID-19 patients. PLoS One. 2020;15(9):e0236311. doi: 10.1371/journal.pone.0236311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaffina S., Alteri C., Ruggiero A., et al. Induction of immune response after SARS-CoV-2 mRNA BNT162b2 vaccination in healthcare workers. J Virus Erad. 2021;7(2):100046. doi: 10.1016/j.jve.2021.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padoan A., Dall'Olmo L., Rocca F.D., et al. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin Chim Acta. 2021;519:60–63. doi: 10.1016/j.cca.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kageyama T., Ikeda I., Tanaka S., et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine in 2,015 healthcare workers ina single tertiary referral hospital in Japan. medRxiv. 2021 doi: 10.1101/2021.06.01.21258188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacisuleyman E., Hale C., Saito Y., et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384(23):2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keehner J., Horton L.E., Pfeffer M.A., et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384(18):1774–1775. doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson K.B., Pinsky B.A., Montez Rath M.E., et al. Post-vaccination SARS-CoV-2 infections and incidence of presumptive B.1.427/B.1.429 variant among healthcare personnel at a northern California academic medical center. Clin Infect Dis. 2021:ciab554. doi: 10.1093/cid/ciab554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porto M.H., Castro B., Diaz Z., Pedroso Y., Ramos M.J., Lecuona M. SARS-CoV-2 infection in properly vaccinated healthcare workers. Int J Infect Dis. 2021 doi: 10.1016/j.ijid.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angel Y., Spitzer A., Henig O., et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457–2465. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention SARS-CoV-2 variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html Accessed September 23, 2021.
- 17.Istituto Superiore di Sanità Prevalenza e distribuzione delle varianti del virus SARS-CoV-2 di interesse per la sanità pubblica in Italia. https://www.iss.it/cov19-cosa-fa-iss-varianti Accessed June 25, 2021.
- 18.Abu-Raddad L.J., Chemaitelly H., Butt A.A., National Study Group for COVID-19 Vaccination Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385(2):187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muik A., Wallisch A.K., Sanger B., et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021;371(6534):1152–1153. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P., Nair M.S., Liu L., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D., Dejnirattisai W., Supasa P., et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–2361.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang P., Casner R.G., Nair M.S., et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29(5):747–751.e4. doi: 10.1016/j.chom.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edara V.V., Lai L., Sahoo M.K., et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. bioRxiv. 2021 doi: 10.1101/2021.05.09.443299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernal J.L., Andrews N., Gower C., et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021 doi: 10.1101/2021.05.22.212576582021. [DOI] [PubMed] [Google Scholar]
- 25.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK [erratum appears in Lancet. 2021;397(10269):98] Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia [erratum appears in Lancet. 2021;397(10275):670] Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess A.D., Colombani P.M., Esa A.H. Cyclosporine and the immune response: basic aspects. Crit Rev Immunol. 1986;6(2):123–149. [PubMed] [Google Scholar]
- 29.Schuyler M.R., Gerblich A., Urda G. Prednisone and T-cell subpopulations. Arch Intern Med. 1984;144(5):973–975. [PubMed] [Google Scholar]
- 30.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lord J.M. The effect of ageing of the immune system on vaccination responses. Hum Vaccin Immunother. 2013;9(6):1364–1367. doi: 10.4161/hv.24696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Resta C., Ferrari D., Vigano M., et al. The gender impact assessment among healthcare workers in the SARS-CoV-2 vaccination—an analysis of serological response and side effects. Vaccines (Basel) 2021;9(5):522. doi: 10.3390/vaccines9050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein S.L., Marriott I., Fish E.N. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flanagan K.L., Fink A.L., Plebanski M., Klein S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention Vaccine Adverse Event Reporting System (VAERS) https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html Accessed April 8, 2021.
- 36.Vijayasingham L., Bischof E., Wolfe J. Gender and COVID-19 Research Agenda-setting Initiative. Sex-disaggregated data in COVID-19 vaccine trials. Lancet. 2021;397(10278):966–967. doi: 10.1016/S0140-6736(21)00384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saadat S., Rikhtegaran Tehrani Z., Logue J., et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325(14):1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.