Abstract
Influenza vaccination is widely advocated to avoid infection with influenza virus, a serious respiratory pathogen, and this was greatly emphasized during the raging COVID-19 epidemic. We conducted a study for baseline Flu specific immunity in a group of health care workers with documented past SARs-CoV-2 infection (designated COVID+) with mild or no symptoms and compared them with a control group that had not been infected with SARS CoV-2 (COVID-). Concurrently, we examined flu and SARS-CoV-2 specific T cell responses using the AIM (activation induced molecules) assay by flow cytometry. All COVID+ and 40% COVID- participants exhibited AIM responses to SARS-CoV-2 peptides, but only COVID+ were positive for SARs-CoV-2 antibody. Influenza HIN1 antigen specific CD4 T cells were found in 92% COVID+ and 76% COVID- participants and exhibited a strong direct correlation with SARS-CoV-2 specific CD4 T cells. This observation suggests that influenza specific T cell immunity may impact immune responses to SARS-CoV-2.
Keywords: Flu and COVID, COVID19, SARS-CoV-2 and Influenza, COVID and T cell immunity, COVID and immune response, SARS-CoV-2 antigen specific immunity
1. Introduction
According to CDC estimates, the 2018–2019 influenza season in the US was associated with over 35 million illnesses, over 16 million medical visits, close to 500,000 hospitalizations, and 34,200 deaths. Global estimates are that infection with Influenza virus leads to approximately 650,000 deaths each year. This topic achieves greater prominence during the COVID19 pandemic as it was unclear how the two respiratory infections with some overlapping symptomology would influence each other. Infections with human circulating “common cold” coronaviruses (HCoVs), such as HCoV-229E, -OC43, -NL63, and -HKU1 cause 15%–30% of milder common colds in adults [1]. Unlike SARS-CoV and influenza viruses that spread from the upper airway to cause a severe lower respiratory tract infection, HCoVs replicate principally in the upper respiratory tract epithelial cells, to cause local respiratory symptoms. It is currently not known whether coinfection with influenza or other HCoVs impact COVID-19 disease outcomes.
Emerging data suggests that SARS-CoV-2 specific CD4 and CD8 T cells are identifiable in a subset of people without evidence of active infection with or antibody directed against SARS-CoV-2 [2], [3], [4]. This immune response against SARS-CoV-2 has been attributed to preexisting immunologic memory against HCOVs that is cross-reactive for SARS-CoV-2 because of partial sequence homology of HCOVs with SARS-CoV-2 [4]. We questioned whether immunity to influenza virus, an unrelated respiratory pathogen, could influence immunity to SARS-CoV-2 through a mechanism such as “trained immunity”. Reports that BCG and MMR vaccination could confer an immunologic benefit to some persons with SARS-CoV-2 supported this concept [5], [6], [7], [8], [9], [10], [11], [12]. In this study, we investigated Flu H1N1 antigen specific T cell responses in conjunction with SARS-CoV-2 specific T cell responses. The study was conducted in participants during June-August 2020 with and without recent documented asymptomatic COVID-19 but prior to the onset of the 2020–2021 flu vaccination season. Two key observations were made. First, in agreement with published reports [2], [3], [4], [13], a significant proportion of COVID Ab negative persons without history or evidence of SARS Cov-2 infection exhibited demonstrable SARS-CoV-2 specific CD4 T cell immune responses. Second, a novel finding was that SARS-CoV-2 specific CD4 T cells were strongly correlated with H1N1 antigen specific CD4 T cells.
2. Materials and methods
2.1. Study groups
We identified participants with prior SARS-CoV-2 infection confirmed by SARS-CoV-2 DNA+ (n = 21) during June 2020 - August 2020. All COVID+ participants were health care workers employed at the University of Miami and are a subset of participants from a larger cohort of COVID immunity study. All participants had mild/moderate symptoms without hospitalization. The median age of COVID+ participants was 34.5 yrs (range: 27–61 yrs) with 52% (11/21) females. A group of SARS-CoV-2 seronegative community participants (n = 33) was 35 yrs (range: 24–77 yrs) with 51.5% (17/33) females and were included as a COVID- group. A summary of the demographic characteristics and influenza vaccination history of the study groups is shown in Table 1 . Antibody response to flu measured as hemagglutination inhibition (HAI) titers to H1N1 flu antigen at the study entry did not differ between the groups but a trend of higher response (P = 0.054) was noted in the COVID+ group (not shown). Peripheral venous blood was collected after obtaining written informed consent and peripheral blood mononuclear cells (PBMC) were separated and cryopreserved in liquid N2. This study was approved by the Institutional Review Boards of the University of Miami.
Table 1.
Summary of the demographic characteristics of the study groups.
| COVID+ | COVID- | |
|---|---|---|
| Number | 21 | 33 |
| Age in Yrs, Median (Range) | 34.5 (27–61) | 35 (24–77) |
| Gender (F/M) | 11/10 | 17/16 |
| Past COVID PCR+ | 21 | 0 |
| Spike IgG + | 18 | 0 |
| Median Spike IgG Titer, Median (range) | 1600 (400–6400) | 0 |
| Flu Vaccinated, n (%) | 21 (95.2) | 14 (42.4) |
| Flu Vaccination History | ||
| 3 or more years yrs, n (%) | 19 (90.5) | 9 (27.2) |
| Never, n (%) | 1 (4.7) | 5 (15.2) |
| Not Reported, n (%) | 0 | 14 (42.4) |
2.2. Antigens used for the stimulation experiments
Megapools specific to SARS-CoV-2spike (CD4S) and non-spike (CD4R), CD8 A and CD8 B were provided as a gift by Dr. Sette, UCSD, La Jolla, CA. Peptide sequences and the effectiveness of these peptides to induce SARS-CoV-2 specific responses have been reported previously by the Sette lab [2], [4] and recently in a collaborative study between the Sette group and our lab [14]. Briefly, SARS-CoV-2 virus-specific CD4 and CD8 peptides were synthesized as crude material, resuspended in DMSO, pooled and sequentially lyophilized. SARS-CoV-2 epitopes were predicted using the protein sequences derived from the SARS-CoV-2 reference (GenBank: MN908947) and IEDB analysis-resource. MPs were generated based on the predicted SARS-CoV-2 epitopes. CD4_R MP corresponds to 221 predicted HLA class II CD4 + T cell epitopes covering all proteins in the viral genome, apart from the spike (S) antigen (n = 221 peptides). For the MP_S, a separate MP containing 253 peptides covering the entire antigen with 15-mer peptides overlapping by 10-residues was used. CD8 SARS-CoV-2 epitope prediction was performed for the top 12 more frequent HLA alleles and the resulting 628 predicted CD8 epitopes were split in two CD8 MPs containing 314 peptides each (CD8-A and CD8-B) [2], [4]. The H1N1 antigen used in this study was a purified, formalin inactivated, whole virus preparation obtained from the Center for Biologics Evaluation and Research (CBER), FDA that has been previously used for investigation of flu specific T cell responses [15], [16]. We and others have reported the use of inactivated, whole virus preparation for flu specific assays to capture the breadth of the antigen specific CD4 and CD8 T cell responses that have included intracellular cytokine secretion (ICS) as a functional readout [15], [16], [17], [18].
2.3. Antigen induced activation molecule (AIM) assay
Thawed PBMC were cultured for 24 h in the presence of 4 different SARS-CoV-2 specific megapools 2 each for CD4 and CD8 T cells at 1 µg/ml, inactivated H1N1 2019–2020 virus antigen at 2 µg/ml or anti-CD3 at 0.01 µg/mL concentration in 96-wells U bottom plates at 1.5 x106 PBMC per well. Anti-CD40 at 1 µg/ml was included in the cultures to prevent the activation induced downregulation of CD40L. A stimulation condition with equimolar amount of DMSO with no antigen was used as a negative control. After stimulation, cells were stained with surface markers CD3, CD4, CD8 along with antigen induced activation molecules (CD69, OX40, CD137), fixed, in 1% paraformaldehyde and acquired on a Cytek Aurora flow cytometer. For COVID+ participants, additional antigen induced activation molecules (CD25, CD40L) were included in the panel to define antigen-specific cells in a broader manner. Data analysis was performed using FlowJo software (FlowJo V10).
2.4. Statistical analyses
All statistical analysis was performed using GraphPad Prism software version 8.4.3. Data were expressed as Mean ± Standard Deviation (SD). Correlation analyses were performed using Spearman, while Kruskal-Wallis test with Dunn’s multiple comparisons was used to compare the data between groups. Antigen specific T cell data have been calculated as background subtracted data or as stimulation index. Background subtracted data were derived by subtracting the percentage of AIM + cells after SARS-CoV-2 or H1N1 antigen stimulation from the DMSO stimulation. Stimulation Index was calculated by dividing the percentage of AIM + cells after SARS-CoV-2 or H1N1 antigen stimulation with the percentage of AIM + cells derived from DMSO condition. When two stimuli were combined for calculating the total SARS-CoV-2 specific responses, the percentage of AIM + cells after SARS-CoV-2 stimulation were added together and the value was then subtracted by twice the value of the percentage of AIM + cells derived from DMSO stimulation. A p value < 0.05 was significant.
3. Results
3.1. COVID-19 seronegative participants exhibited SARS-CoV-2 specific CD4 T cells
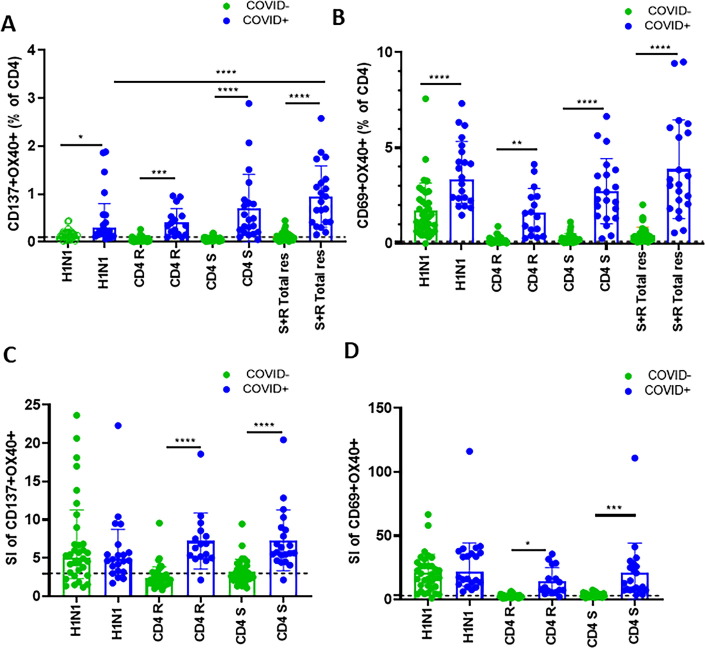
Antigen specific CD4 T cells were identified as CD137+ OX40+ CD4 T cells ( Fig. 1 A) or as CD69+ OX40+ CD4 T cells ( Fig. 1 B). SARS-CoV-2 spike (S), SARS-CoV-2 non-spike (R) and total response calculated by adding the frequencies of spike and non-spike specific CD4 T cells together were present in all (100%) COVID+ participants ( Fig. 1 A, B). In line with recent reports [2], [4], [14] nearly 40% COVID- participants had detectable SARS-CoV-2 specific CD4 T cells above the background levels with a stimulation index ≥ 3 ( Fig. 1 C, D).
Fig. 1.
Higher H1N1 specific and SARS-CoV-2 specific CD4 T cell responses in COVID19+ participants: Thawed PBMC from study participants (COVID19+, n = 21 and COVID-, n = 33) were cultured for 24 hours with H1N1 antigen or with SARS-CoV-2 specific megapools for CD4, non-spike (CD4 R) and spike (CD4 S) peptides. Frequencies of antigen specific activation induced markers (AIM) were determined by flow-cytometry. (A, B): Bar graphs showing background subtracted data derived by subtracting the percentage of AIM + cells after SARS-CoV-2 or H1N1 antigen stimulation from the DMSO stimulation were plotted for each antigen stimulation and compared between groups for A) CD137+ OX40+ and B) CD69+ OX40+ responses. C-D) Bar graphs showing stimulation Index calculated by dividing the percentage of AIM + cells after SARS-CoV-2 or H1N1 antigen stimulation with the percentage of AIM + cells derived from DMSO stimulation were plotted for each antigen stimulation and compared between groups for A) CD137+ OX40+ and B) CD69+ OX40+ responses. Data were expressed as Mean ± Standard Deviation (SD) and Kruskal-Wallis test with Dunn’s multiple comparisons was used for comparing between group. Line with stars indicates difference between 231 time points within a group and between groups and the level significance as *p < 0.05; **p < 0.01; ***p < 0.001 A p value of < 0.05 was considered as significant.
3.2. Influenza virus H1N1 antigen specific CD4 T cells strongly correlate with the SARS-CoV-2 specific CD4 T cell responses
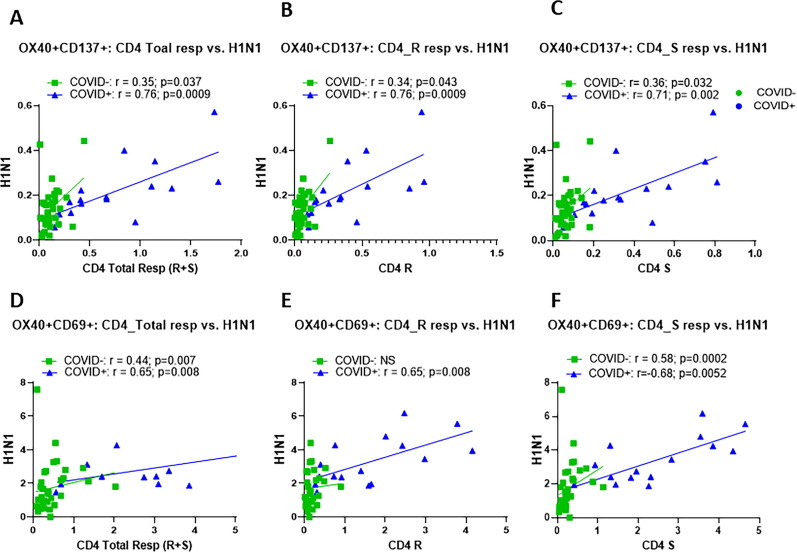
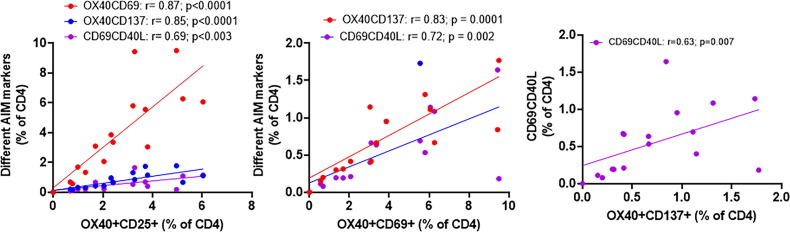
H1N1 specific AIM + CD4 T cells above background levels were detected in 92% COVID+ and 55% of COVID- participants ( Fig. 1 A, B) while SI above 3 for H1N1 response was found in 92% COVID+ and 76% of COVID- participants ( Fig. 1 C, D). A strong direct correlation was observed between H1N1 specific OX40+ CD137+ CD4 T cells with total SARS-CoV-2 specific CD4 T cells ( Fig. 2 A), individual CD4 R ( Fig. 2 B) and CD4 S ( Fig. 2 C) peptide specific CD4 T cells in both COVID+ and COVID- groups. Similar direct correlations were found between H1N1 specific CD69+ OX40+ CD4 T cells and SARS-CoV-2 specific CD69+ OX40+ CD4 T cells in both the groups ( Fig. 2 D–E). In the COVID+ cohort, additional activation induced marker combinations OX40+ CD25+ and CD40L+ CD69+ were also detected on CD4 T cells following antigen stimulation, and they correlated with each other ( Fig. 3 ).
Fig. 2.
Frequencies of H1N1 specific CD4 T cells strongly correlated with the SARS-CoV-2 specific CD4 T cells in COVID+ and seronegative participants: Thawed PBMC from COVID19+ (n = 21) and seronegative (n = 33) were stimulated with H1N1 antigen, SARS-CoV-2 specific CD4 non-spike (CD4 R) and spike (CD4 S) peptides for 24 hours and frequencies of antigen specific activation induced markers (AIM) were measured by flow-cytometry. Correlation between H1N1 specific CD4 T cells and SARS-CoV-2 specific CD4 T cells for A), OX40+ CD137+ total S + R responses; B), OX40+ CD137+ R response; C), OX40+ CD137+ S response; D), OX40+ CD69+ R response total S + R responses; E), OX40+ CD69+ R response; F), OX40+ CD69+ S response. For correlation analyses, non-parametric spearman correlation was used. A p value of < 0.05 was considered as significant.
Fig 3.
AIM phenotypes identified by different markers in COVID19+ participants correlate with each other: Total SARS-CoV-2 specific CD4 T cell responses were calculated for OX40+ CD25+, OX40+ CD137+, OX40+ CD69+ and CD40L+ CD69 + phenotypes and correlated with each other For correlation analyses, non-parametric spearman correlation was used. A p value of < 0.05 was considered as significant.
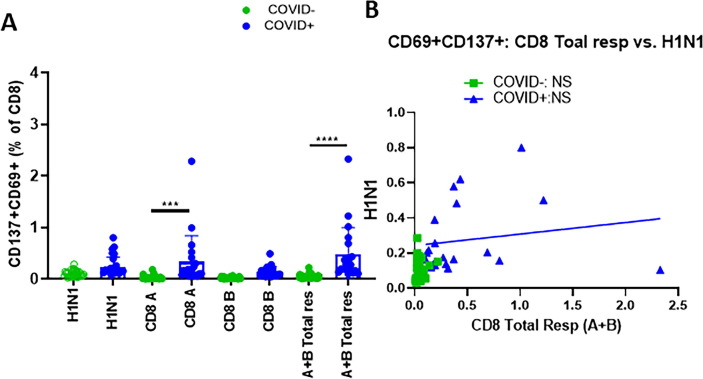
3.3. Frequencies of H1N1 specific CD8 T cells did not correlate with the SARS-CoV-2 specific CD8 T cell responses
We next analyzed the relationship between H1N1 specific CD8 T cells and SARS-CoV-2 specific CD8 T cells in this cohort based on the co-expression of CD69 and CD137 [14]. As expected, SARS-CoV-2 specific CD8 T cell responses were significantly higher in the COVID+ group compared to the COVID- group. SARS-CoV-2 specific CD8 T cells above the background levels were less frequent in the seronegative participants with 9% response and stronger in the COVID+ participants with 95% response. Stimulation index above 3 for SARS-CoV-2 specific CD8 T cells was found in 9% seronegative and 66% in COVID+ participants. Frequencies of H1N1 specific CD8 T cell response were not significantly different between COVID+ and COVID- participants although a trend of higher frequencies was noted in COVID+ participants ( Fig. 4 A). H1N1 specific CD8 T cell responses above background levels were seen in 42% COVID- and 95% COVID+ participants while SI of CD8 T cell response above 3 was noticed in 79% of COVID- and 95% COVID+ participants. Correlation analysis did not show any association between H1N1 specific CD8 T cell responses with SARS-CoV-2 specific CD8 T cell responses in either COVID+ or COVID- participants ( Fig. 4 B).
Fig. 4.
H1N1 specific CD8 T cell response did not differ between study groups and did not correlate with SARS-CoV-2specific CD8 responses: Thawed PBMC from COVID19+ (n = 21) and seronegative (n = 33) were stimulated with H1N1 antigen, SARS-CoV-2specific CD8 megapools A and B for 24 h and frequencies of antigen specific activation induced markers (AIM) on CD8 T cells were identified as CD137+ CD69+ cells by flow-cytometry. A), Bar graphs showing means ± SD of the frequencies of CD137+ CD69+ CD8 T cells; B), Correlation between H1N1 specific CD8 T cells and SARS-CoV-2specific CD8 T cells. Kruskal-Wallis test with Dunn’s multiple comparisons was used for comparing between group. For correlation analyses, non-parametric spearman correlation was used. A p value of < 0.05 was considered as significant.
Taken together, our data support a strong association of antigen specific CD4 T cell responses for Flu and SARS-CoV-2 that was evident in both COVID+ and COVID- participants with CD4 T cell responses to SARS-CoV-2 in 40 % of the COVID- groups. Although CD8 T cell responses were higher in COVID + participants, the CD8 T cell response did not show any association with Flu response in either group.
4. Discussion
The goal of this study was to investigate if immune responses to H1N1 influenza virus antigen were also associated with SARS-CoV-2 cellular immune responses that were detected in people without serologic evidence of prior CoV-2 infection as compared to those with documented prior SARS-CoV-2 infection (PCR confirmed and IgG Ab positive). We have recently reported the finding that SARS-CoV-2 specific CD4 T cell immunity is detectable in >50% of a group of high-risk healthcare workers who did not have prior SARS-CoV-2 infection and were seronegative while 96 % of people with past documented COVID-19 who were also seropositive had strong CD4 T cell immunity [14]. As this study was conducted during June-August 2020, prior to the 2020–2021 influenza vaccination period we investigated the same participants for pre-existing influenza memory responses and serology. A strong relationship was observed between CD4 T cell activation induced markers in response to influenza virus H1N1 antigen and SARS-CoV-2 antigens. We contend that pre-existing immunity to influenza may affect the immunity to SARS-CoV-2.
There is growing consensus that pre-existing immunity against other respiratory pathogens may enhance the COVID-19 specific immunity through a mechanism entailing cross reactive antigens. This concept is supported by the fact that more than 90% of the human population is seropositive for at least three of the HCoVs [13] and the reported T cell reactivity thus far was highest against a pool of SARS-CoV-2 spike peptides that had significant homology of homology to HCoVs [13], [19]. Findings from this study point to a relationship of immunity against influenza with immunity against SARS-CoV-2. A strong relationship of Flu H1N1 specific CD4 T cell responses with the SARS-CoV-2 specific CD4 T cell response was observed that was absent for the CD8 T cell compartment. Three different combinations of AIM markers on CD4 T cells signifying memory cell activation showed similar correlations solidifying the consistency of this observation. The underlying mechanism for the observed association of cellular CD4 T cell responses against flu and SARS-CoV-2 is not known but several possibilities exist. There is data supporting cross reactivity of immunity between Flu and coronavirus [20] due to the similarity in their viral envelope glycoprotein hemagglutinin-esterases (HE) that mediate virion attachment, receptor destruction, and membrane fusion [21]. The most plausible explanation for the T cell immunity to Flu is that of repeated past vaccinations, which is of particular relevance in this participant group comprised of health care workers. 95% of our COVID + participants were vaccinated with seasonal flu vaccine in the 2019–2020 influenza season, prior to becoming in infected with SARS-CoV-2, implying pre-existing H1N1 specific memory T cells in this cohort. Further studies are needed to understand the direct effect of the immune responses associated with SARS-CoV-2 infection on the flu specific T cell responses in our study participants. A previous study reported an increased non-specific reactivity of PBMC of COVID-19 recovered patients to unrelated antigenic stimulation [22]. However, higher non-specific activation of T cells was not evident in our participants as we did not find significant differences in the activation status of the CD4 and CD8 T cells at baseline or after 24 h of H1N1 stimulation measured as frequencies of HLA-DR+ CD38+ cells (not shown). We also did not find any significant alterations in the frequencies of CD4 or CD8 T cells or their maturations subsets between the study groups (not shown). Moreover, the association of SARS-CoV2 specific response was noted only for flu H1N1 antigen but not for CMV peptide- or generalized (anti-CD3+CD28) stimulation (not shown). Although less likely, the possibility exists that the SARS COV-2 infection induced T cell immunity may have enhanced immune responses to Influenza.
We favor the contention that flu specific immunity may also induce bystander immunity analogous to “trained immunity” that can non-specifically augment T cell responses against SARS-CoV-2. The concept of trained immunity is that the long-term functional reprogramming of innate immune cells evoked by exogenous or endogenous stimulation leads to an altered response towards a second heterologous challenge [23]. Epidemiological data supports the concept that live vaccines such as the BCG, measles, smallpox and oral polio have beneficial, non-specific protective effects against infections other than the target diseases [23]. BCG vaccination led to protection against microorganisms in models of controlled human infection, such as yellow fever or malaria, and this was associated with an augmented proinflammatory activity of monocytes [24], [25]. Although not supported by the current data, it is possible that seasonal Flu vaccination or Flu infection results in augmented innate immune response against COVID19 through trained innate immune cells that further enhances the adaptive arm of immunity and increases both the Flu and COVID specific T cell responses. If this is true, then individuals who received prior Flu vaccinations might also show mild disease severity because of Flu vaccine-induced bystander immune responses could act against SARS-CoV-2. Although trained immunity is more relevant to live vaccines, the relationship of a similar mechanism playing a role for Flu vaccination or Flu infection in relation to COVID19 could underlie the observed relationship between H1N1 and CoV 2 immune responses.
Our study is limited by number of study participants, reliance only on AIM markers for immune assessment and use of whole inactivated H1N1 virus as antigen for CD8 T cells that may not capture all specific CD8 epitopes.
5. Conclusions
We speculate a possible benefit of the seasonal flu vaccination as being favorable for SARS-CoV2 specific T cell immunity. Regardless of the basis for the augmentation of T cell responses directed against SARs-CoV-2, flu specific immunity may influence the immune response to COVID-19 in this study population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank Dr. Alessandro Sette and Dr. Daniela Weiskopf from La Jolla Institute for Immunology for providing SARS-CoV-2 specific peptides. We thank the Clinical Research Center at the University of Miami for the blood draw. We also thank Margaret Roach, Elizabeth Varghese, Maria Pallin and Dan Kvistad for their assistance with sample processing and laboratory experiments and to participants of this study.
Funding
This work was supported by National Institutes of Health Grant: R01AI108472 (to S.P.) and AG068110 (to SP and S Pallikkuth) and P30AI073961 (to S.P).
References
- 1.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipsitch M., Grad Y.H., Sette A., Crotty S. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat Rev Immunol. 2020;20(11):709–713. doi: 10.1038/s41577-020-00460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M., et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anbarasu A., Ramaiah S., Livingstone P. Vaccine repurposing approach for preventing COVID 19: can MMR vaccines reduce morbidity and mortality? Hum Vaccin Immunother. 2020;16(9):2217–2218. doi: 10.1080/21645515.2020.1773141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashford J.W., Gold J.E., Huenergardt M.A., Katz R.B.A., Strand S.E., Bolanos J., et al. MMR Vaccination: A Potential Strategy to Reduce Severity and Mortality of COVID-19 Illness. Am J Med. 2021;134(2):153–155. doi: 10.1016/j.amjmed.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold JE, Baumgartl WH, Okyay RA, Licht WE, Fidel PL, Jr., Noverr MC, et al. Analysis of Measles-Mumps-Rubella (MMR) Titers of Recovered COVID-19 Patients Could an Unrelated Live Attenuated Vaccine Serve as a Preventive Measure To Dampen Septic Inflammation Associated with COVID-19 Infection? mBio. 2020;11. [DOI] [PMC free article] [PubMed]
- 8.Madan M., Pahuja S., Mohan A., Pandey R.M., Madan K., Hadda V., et al. TB infection and BCG vaccination: are we protected from COVID-19? Public Health. 2020;185:91–92. doi: 10.1016/j.puhe.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urashima M., Otani K., Hasegawa Y., Akutsu T. BCG Vaccination and Mortality of COVID-19 across 173 Countries: An Ecological Study. Int J Environ Res Public Health. 2020;17(15):5589. doi: 10.3390/ijerph17155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis N., Sparrow A., Ghebreyesus T.A., Netea M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395(10236):1545–1546. doi: 10.1016/S0140-6736(20)31025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escobar L.E., Molina-Cruz A., Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) Proc Natl Acad Sci U S A. 2020;117(30):17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netea M.G., Giamarellos-Bourboulis E.J., Domínguez-Andrés J., Curtis N., van Crevel R., van de Veerdonk F.L., et al. Trained Immunity: a Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell. 2020;181(5):969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sette A., Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol. 2020;20(8):457–458. doi: 10.1038/s41577-020-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Silva Antunes R, Pallikkuth S, Williams E, Esther DY, Mateus J, Quiambao L, et al. Differential T cell reactivity to endemic coronaviruses and SARS-CoV-2 in community and health care workers. J Infect Dis. 2021. [DOI] [PMC free article] [PubMed]
- 15.George V.K., Pallikkuth S., Parmigiani A., Alcaide M., Fischl M., Arheart K.L., et al. HIV infection Worsens Age-Associated Defects in Antibody Responses to Influenza Vaccine. J Infect Dis. 2015;211(12):1959–1968. doi: 10.1093/infdis/jiu840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallikkuth S, de Armas LR, Rinaldi S, George VK, Pan L, Arheart KL, et al. Dysfunctional peripheral T follicular helper cells dominate in people with impaired influenza vaccine responses: Results from the FLORAH study. PLoS Biol. 2019;17:e3000257. [DOI] [PMC free article] [PubMed]
- 17.Tapia-Calle G., Born P.A., Koutsoumpli G., Gonzalez-Rodriguez M.I., Hinrichs W.L.J., Huckriede A.L.W. A PBMC-Based System to Assess Human T Cell Responses to Influenza Vaccine Candidates In Vitro. Vaccines (Basel) 2019;7(4):181. doi: 10.3390/vaccines7040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.L’Huillier A.G., Ferreira V.H., Hirzel C., Nellimarla S., Ku T., Natori Y., et al. T-cell responses following Natural Influenza Infection or Vaccination in Solid Organ Transplant Recipients. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-67172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 20.Zheng J., Perlman S. Immune responses in influenza A virus and human coronavirus infections: an ongoing battle between the virus and host. Curr Opin Virol. 2018;28:43–52. doi: 10.1016/j.coviro.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Q., Langereis M.A., van Vliet A.L.W., Huizinga E.G., de Groot R.J. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc Natl Acad Sci U S A. 2008;105(26):9065–9069. doi: 10.1073/pnas.0800502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuwa H.A., Shaw T.N., Knight S.B., Wemyss K., McClure F.A., Pearmain L., et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med (N Y) 2021 doi: 10.1016/j.medj.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.-Y., Oosting M., et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23(1):89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Walk J., de Bree L.C.J., Graumans W., Stoter R., van Gemert G.-J., van de Vegte-Bolmer M., et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-08659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]