Summary
Background
Cost-effectiveness data for cancer treatment are needed from sub-Saharan Africa, where diffuse large B-cell lymphoma (DLBCL) is a common, curable cancer. In high-income countries, the standard of care for DLBCL is R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemoimmunotherapy. Rituximab is often not available in sub-Saharan Africa due to perceived unaffordability, and treatment with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) is common. We aimed to evaluate the cost-effectiveness of treatment in Malawi, comparing best supportive care, CHOP, or R-CHOP in patients with DLBCL.
Methods
For this cost-effectiveness analysis, we used published Malawi microcosting data, clinical data from a prospective cohort treated with CHOP, and clinical trial data evaluating R-CHOP. We used a decision-tree model to calculate costs per disability-adjusted life-year (DALY) averted from the health system perspective for the treatment of patients with DLBCL with best supportive care, CHOP, or R-CHOP, running the model on a per-patient basis and a Malawi population-level basis. We used the WHO definitions of cost-effective (three times the GDP per capita of the country) and extremely cost-effective (equal to the GDP per capita of the country) as willingness-to-pay thresholds for Malawi.
Findings
On a per-patient level, compared with best supportive care, CHOP was estimated to avert a mean 7.4 DALYs at an incremental cost of US$1384, for an incremental cost-effectiveness ratio (ICER) of $189 per DALY averted, which is substantially lower than the willingness-to-pay threshold (extremely cost-effective). Compared with CHOP, R-CHOP was estimated to avert 2.8 DALYs at an incremental cost of $3324, resulting in an ICER of $1204 per DALY averted, which is slightly higher than the cost-effective willingness-to-pay threshold. In probabilistic sensitivity analyses, CHOP remained cost-effective for DLBCL treatment in more than 99% of simulations, whereas R-CHOP was lower than the threshold in 46% of simulations.
Interpretation
We estimated CHOP to be cost-effective for DLBCL treatment in Malawi, and that the addition of rituximab might be cost-effective. Despite upfront costs, DLBCL treatment is probably a prudent investment relative to other accepted health interventions in sub-Saharan Africa.
Introduction
The cost-effectiveness of cancer treatment in sub-Saharan Africa broadly, and in Malawi specifically, has not been widely assessed but is an essential consideration for health systems with scarce resources facing many competing priorities. The 2017–18 government health budget for Malawi was US$170 million (about $9 per person), and external donors are estimated to contribute approximately another $350 million (about $18 per person) annually to health expenditures.1 Therefore, cancer treatment costs are often considered prohibitive in the absence of a rigorous clinical and economic evaluation within sub-Saharan Africa to guide decision making.
An effort to evaluate the cost-effectiveness of paediatric cancer care across health systems has begun in the past few years,2 and previous studies have investigated the cost-effectiveness of interventions for specific childhood cancers3 and breast cancer.4 However, to our knowledge, no economic evaluations have been done in sub-Saharan Africa among adults with lymphoma, which is notable for being a common, curable cancer throughout the region.
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma subtype worldwide and in sub-Saharan Africa. DLBCL is highly associated with HIV5 and is a particularly common cancer in countries with high HIV burden such as Malawi, where HIV prevalence among adults is approximately 9%.6 DLBCL is curable in sub-Saharan Africa with use of the generic chemotherapy medicines cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).7,8 In high-income countries, absolute improvements in overall survival of 10–20% are achieved by adding rituximab to CHOP (R-CHOP).9–12 Rituximab is an anti-CD20 monoclonal antibody with commercially available biosimilars, but for which rigorous clinical and economic evaluations in sub-Saharan Africa are needed. We previously published data from the Kamuzu Central Hospital (KCH) Lymphoma Study in Lilongwe, Malawi, including a microcosting analysis13 and prospectively recorded outcomes14 of DLBCL treatment with CHOP. Additionally, we completed a phase 2 clinical trial of R-CHOP for DLBCL in Malawi, published this year.12 Drawing on these available data sources for model inputs, we aimed to develop a decision-tree model to analyse the cost-effectiveness of DLBCL treatment, comparing best supportive care, CHOP, or R-CHOP in patients with DLBCL in Malawi.
Methods
Model structure
Whenever possible, the model and results we report adhere to recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine.15 For this cost-effectiveness analysis, we developed a three-strategy decision-tree model to predict outcomes and costs of treating adult patients with DLBCL with best supportive care (ie, symptom-directed palliative treatment without chemotherapy or other curative-intent treatment), CHOP, or R-CHOP (figure 1). For CHOP and R-CHOP, the first chance node separated patients who achieved remission (hereafter referred to as the remission group) from those who had refractory disease (refractory group) or had treatment-related mortality (treatment-related mortality group). In the remission group, for both CHOP and R-CHOP strategies, the second chance node separated patients who relapsed after remission (hereafter referred to as the relapse group) and those who maintained remission at 2 years. Previous studies have shown that patients with DLBCL have life expectancy similar to age-matched and sex-matched controls after achieving 2-year progression-free survival,16,17 and thus we hereafter refer to this group as the DLBCL-free group. For the base-case analysis, all patients were assumed to enter palliative care at the time of relapse, because there are effectively no curative-intent therapies available in Malawi after relapse.
Figure 1: Decision-tree model comparing R-CHOP with CHOP with best supportive care for treatment of diffuse large B-cell lymphoma in Malawi.
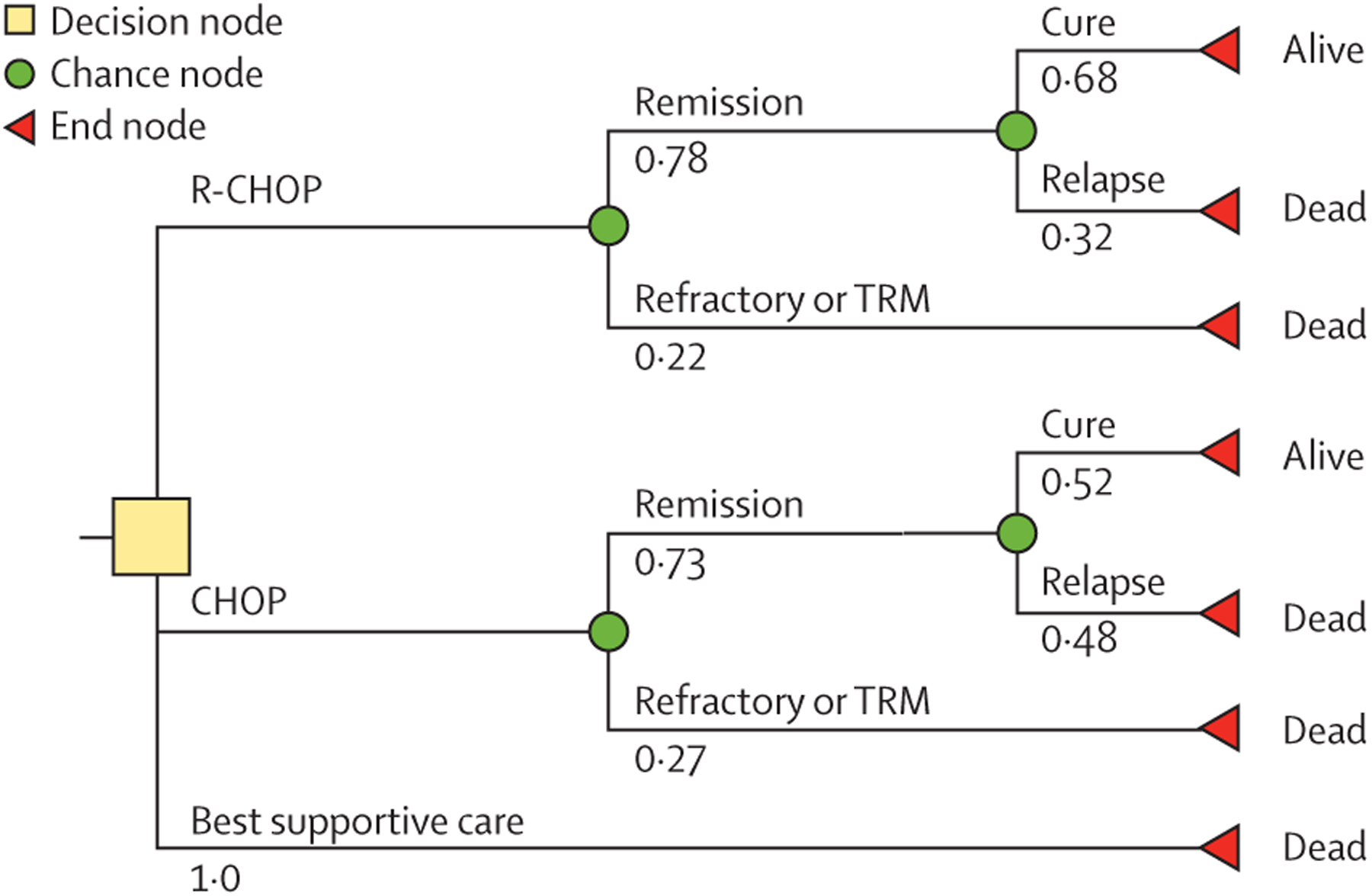
Base-case probabilities are shown for each scenario. CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone. R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. TRM=treatment-related mortality.
We calculated the incremental cost-effectiveness ratio (ICER) of cost per disability-adjusted life year (DALY) averted. DALYs are commonly used in evaluations in low-income and middle-income countries (LMICs) to compare cost-effectiveness across different health interventions. DALYs are calculated as the sum of the years of life lost due to premature mortality and the years of healthy life lost due to disability. One DALY represents the loss of the equivalent of 1 year of full health. We used disability weights assigned by the Global Burden of Disease study for non-Hodgkin lymphoma.18 All costs were initially collected in Malawian kwacha and then converted to 2017 US dollars. Costs collected in past years were inflation-adjusted to 2017 US dollars by use of the Malawi gross domestic product (GDP) deflator from the World Bank.19 Costs and outcomes reflect a health-systems perspective, including overhead and capital, as previously described.13 In the base-case scenario, we discounted costs and outcomes by 3% annually, and we report the base-case model outcome as a deterministic value from the base-case values of each input. We used the WHO definitions of cost-effective (three times the GDP per capita of the country, or $1014 in 2017) and extremely cost-effective (equal to the GDP per capita of the country, or $338 in 2017) as willingness-to-pay thresholds for Malawi.20
Population-level analysis and budget impact analysis
The model was run on a per-patient basis and a Malawi population-level basis. To estimate DLBCL burden in Malawi, we used data from the most recent Global Cancer Incidence, Mortality and Prevalence (GLOBOCAN) publication (from 2020), available online at the Global Cancer Observatory. The source data are from the Malawi National Cancer Registry, last updated in 2010. There were 1164 estimated new cases of non-Hodgkin lymphoma in Malawi in 2010. DLBCL constituted 54% of all non-Hodgkin lymphoma diagnoses in the KCH pathology laboratory in 2012–20, giving an estimated 629 new cases of DLBCL in Malawi in 2010.21 We then calculated the annual budget impact by multiplying the per-patient costs by the anticipated annual number of incident DLBCL cases.
Intervention treatments
CHOP treatment was administered as described previously in the KCH Lymphoma study, a prospective, observational cohort study that has been continuously enrolling all consenting patients with newly diagnosed lymphoproliferative disorders since 2013.14 Briefly, 21-day cycles were administered. Each cycle consisted of cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1.4 mg/m2 (maximum 2 mg) on day 1 and prednisone 60 mg/m2 on days 1–5. A median of six cycles (IQR 4–6) of CHOP were administered, and patients were followed up for toxicity and health outcomes including death or DLBCL progression.
R-CHOP treatment was administered as described for CHOP, but with the addition of rituximab to each cycle (375 mg/m2) on day 1. This treatment was administered in the context of a single arm, phase 2 clinical trial, and patients were likewise followed up for toxicity and health outcomes including death or DLBCL progression.12
Health outcomes
All clinical outcome inputs for the model were derived from previous publications (table 1).12,14 In the CHOP cohort, of 74 participants who initiated chemotherapy, nine (12%) had treatment-related mortality, 11 (15%) were refractory, and 54 (73%) achieved remission. 2-year progression-free survival among patients who achieved remission was 52% (95% CI 38–67). This is equivalent to the 38% progression-free survival previously reported among all treated patients.14 In the R-CHOP cohort, of 37 participants, four (11%) had treatment-related mortality, four (11%) were refractory, and 29 (78%) achieved remission. 2-year progression-free survival among patients who achieved remission was 68% (49–83). This is equivalent to the 53% progression-free survival reported elsewhere among all treated patients.12 The mean age of patients with DLBCL in Malawi is 47 years (SD 13). According to the 2019 UN life table,23 the life expectancy of an individual aged 46–50 years in Malawi is 29 years.
Table 1:
Input values from clinical and microcosting data of a prospective cohort of patients with diffuse large B-cell lymphoma in Malawi
| Base case | Ranges for DSA | |
|---|---|---|
| Outcome probabilities | ||
| Refractory or TRM, CHOP12,14 | 0.27 | 0.20–0.34 |
| Refractory or TRM, R-CHOP12,14 | 0.22 | 0.10–0.38 |
| Cure, CHOP12,14 | 0.52 | 0.39–0.68 |
| Cure, R-CHOP12,14 | 0.68 | 0.49–0.83 |
| Outcome durations, years | ||
| Time in chemotherapy, CHOP or R-CHOP12,14 | 0.35 | 0.25–0.50 |
| Time to progression, CHOP or R-CHOP12,14 | 0.30 | 0.20–0.40 |
| Time to progression, second-line chemotherapy22 | 0.30 | NA |
| Life expectancy, terminal phase (refractory, relapse, or best supportive care)* | 0.25 | 0.05–0.50 |
| Life expectancy, cure17,23 | 29 | 20–30 |
| DALY weights | ||
| Treatment phase (CHOP or R-CHOP)18 | 0.29 | 0.19–0.40 |
| Terminal phase (refractory, relapse, or no chemotherapy)18 | 0.54 | 0.38–0.69 |
| Controlled phase (remission or cure)18 | 0.05 | 0.03–0.07 |
| Complication probabilities | ||
| Febrile neutropenia, CHOP14,24,25 | 0.20 | 0.17–0.34 |
| Febrile neutropenia, R-CHOP14,24,25 | 0.34 | 0.20–0.52 |
| Blood transfusion, CHOP14,24,25 | 0.11 | 0.10–0.18 |
| Blood transfusion, R-CHOP14,24,25 | 0.11 | 0.03–0.25 |
| Hospitalisation (other grade 3–4 adverse event), CHOP14,24,25 | 0.19 | 0.07–0.24 |
| Hospitalisation (other grade 3–4 adverse event), R-CHOP14,24,25 | 0.32 | 0.18–0.50 |
| Costs (2017 US$) | ||
| Diagnosis13 | 392 | 196–784 |
| CHOP chemotherapy (six cycles) and 2-year surveillance13 | 1321 | 661–2642 |
| Rituximab13 | 3690 | 1845–7380 |
| Transfusion13 | 42 | 21–84 |
| Neutropenic fever13 | 236 | 118–472 |
| Other hospitalisation13 | 210 | 105–420 |
| Palliative care13 | 335 | 168–670 |
| Second-line chemotherapy with either GEMOX or EPIC13 | 2886 | NA |
Complication costs were applied to the appropriate treatment phase weighted for the respective probabilities of occurrence. CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone. DALY=disability adjusted life-year. DSA=deterministic sensitivity analysis. EPIC=etoposide, prednisone, ifosfamide, mesna, and cisplatin. GEMOX=gemcitabine and oxaliplatin. NA=not applicable. TRM=treatment-related mortality.
Expert opinion (SG).
DALY weights with their respective ranges were taken from the weights for non-Hodgkin lymphoma in the Global Burden of Disease study (table 1) for the disease states of interest: treatment phase (applied to all time under treatment with either CHOP or R-CHOP), controlled phase (applied to all time in remission and cure), and terminal phase (applied to all time in best supportive care or relapse). These weights were then applied across the average time spent in each state. We did not add additional disutility for episodes of febrile neutropenia or other complications of chemotherapy for two reasons: first, this is already captured in the disutility of the DALY weight for treatment phase; and second, these episodes occurred over a very short time period of less than 1 week compared with the lifetime time period of this study, and thus would not meaningfully contribute to total DALYs over a lifetime.
Costs
Costs for diagnosis, treatment, complications, and 2 years of surveillance care have been previously described.13 Costs and probabilities for specific events (eg, chemotherapy treatment and neutropenic fever event) are shown in table 1. Probabilities of specific events (eg, neutropenic fever) were taken from our previously published studies for CHOP and R-CHOP. Complication costs were applied to the appropriate treatment phase on a weighted basis from their respective probabilities. For example, for patients treated with CHOP, neutropenic fever probability was 0.2 and cost per neutropenic fever event was $236. Therefore, in the base-case scenario, each patient in the CHOP group was attributed 0.2 × $236=$47.
Deterministic and probabilistic sensitivity analyses
We did extensive, one-way sensitivity analysis to determine which parameters had the greatest effect on model results when other variables remained constant. The variables assessed included costs (ie, CHOP, complications, and end-of-life care), probability of outcomes, and the discount rate. Ranges and distributions of probabilities were derived from their 95% CIs from the primary data.14 Ranges for DALYs were assigned by the Global Burden of Disease study.26 Given that cost inputs were based on single-institution microcosting, we used a wide range of potential costs, from 50–200% of the base-case value. Discount rate for costs and outcomes varied from 0% to 6%, as recommended by the Second Panel on Cost-Effectiveness Analysis.15 Ranges of values used are shown in table 1.
We also completed two additional deterministic sensitivity analyses. In the base-case analysis, we assumed that palliative care was not given, to be more conservative in the ICER analysis of CHOP versus no chemotherapy. However, for the first deterministic sensitivity analysis, we included the palliative care costs in the best supportive care pathway. For the second deterministic sensitivity analysis, we assessed the costs and outcomes if patients were treated with a second-line chemotherapy regimen after CHOP or R-CHOP. High-dose therapy with autologous stem-cell rescue and other curative-intent second-line approaches are not available in Malawi, thus salvage regimens after relapse are palliative in nature. However, many patients are offered second-line chemotherapy for palliation. EPIC (etoposide, prednisone, ifosfamide, mesna, and cisplatin) or GEMOX (gemcitabine and oxaliplatin) are frequently used at KCH. Median time to progression after second-line EPIC treatment in Malawi is 4.5 months.22 We do not have published data on outcomes from second-line GEMOX treatment in Malawi, but previously published data from high-income countries suggest outcomes similar to those of EPIC.27 Costs were derived from previously published microcosting data.13 For second-line treatment, the costs of these two regimens were averaged, including costs related to treatment-related complications. This yielded a second-line treatment cost of $2886, which was added to the decision-tree model for patients who relapsed after CHOP or R-CHOP.
Finally, we did probabilistic sensitivity analyses simulating variable inputs from across their distributions over 1000 simulations of the decision-tree analysis model. Distributions used for these probabilistic sensitivity analyses were β for probabilities, γ for costs, and normal for all others (appendix p 1).
All analyses were done with open-source R statistical software, by use of the dampack library from the Decision Analysis in R for Technologies in Health workgroup.28
Role of the funding source
The funders of the study had no role in data collection, data analysis, data interpretation, or writing of the report.
Results
Regarding patient outcomes, the mean life expectancy for each patient receiving CHOP was 11.6 years (table 2). The mean DALYs averted for each patient was 7.4. For R-CHOP, mean life expectancy was 11.5 years and DALYs averted were 10.8. For best supportive care, the mean life expectancy was 0.2 years and the mean DALYs averted were 0.1.
Table 2:
Base-case analysis of decision-tree model for treatment of diffuse large B-cell lymphoma in Malawi under three scenarios: best supportive care, CHOP chemotherapy, and R-CHOP chemoimmunotherapy
| Best supportive care | CHOP | R-CHOP | |
|---|---|---|---|
| Outcome per patient | |||
| Total costs | $392 | $1776 | $5100 |
| Deaths | 1 | 0.62 | 0.47 |
| Years of life lost | 28.8 | 17.4 | 13.1 |
| DALYs | 28.9 | 21.6 | 18.8 |
| Whole population (Malawi), annually | |||
| Total costs | $246 568 | $1 117 104 | $3 207 900 |
| Deaths | 629 | 390 | 296 |
| Years of life lost | 18 115 | 10 945 | 8240 |
| DALYs | 18 178 | 13 587 | 11 825 |
| Incremental cost-effectiveness ratio | |||
| Per death averted | .. | $3642 | $22 160 |
| Per life-year gained | .. | $122 | $770 |
| Per DALY averted | .. | $189 | $1204 |
Dara are shown on a per-person basis and on a population-level basis for Malawi on the basis of an incidence of 629 cases of diffuse large B-cell lymphoma annually. All costs are shown in 2017 US$. Incremental cost-effectiveness ratios shown are CHOP versus best supportive care in the CHOP column and R-CHOP versus CHOP in the R-CHOP column. CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone. DALY=disability adjusted life-year.
R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
The mean cost in the CHOP group—for diagnosis, CHOP chemotherapy, complications, and 2 years of follow-up—was $1776 per patient, whereas cost per patient in the no chemotherapy group was $392 (table 2). The ICER in the CHOP group compared with that of no chemotherapy was $189 per DALY averted, $3642 per death averted, and $122 per life-year gained. An ICER of $189 per DALY averted is considered an extremely cost-effective intervention according to the WHO definition.
The mean cost in the R-CHOP group—for diagnosis, R-CHOP chemotherapy, complications, and 2 years of follow-up—was $5100 per patient (table 2). The ICER in the R-CHOP group compared with that in CHOP was $1204 per DALY averted, $22 160 per death averted, and $770 per life-year gained. An ICER of $1204 per DALY averted is slightly higher than the WHO definition for a cost-effective intervention.
We did one-way sensitivity analysis on all input parameters to assess which one had the greatest effect on model results when other variables remained constant (figure 2). The ICER for costs per DALY averted for CHOP compared with no chemotherapy was most sensitive to changes in treatment costs and probability of long-term cure after CHOP treatment. The ICER for costs per DALY averted for R-CHOP compared with CHOP was most sensitive to the probability of long-term cure after R-CHOP or CHOP treatment, probability of death before completing R-CHOP or CHOP treatment, and the cost of rituximab. When calculating the ICERs for both scenarios with no discounting for DALYs, the change in the ICER was lower than 1%.
Figure 2: Tornado plots of deterministic, one-way sensitivity analysis of ICER, cost per DALY averted, for CHOP versus best supportive care (A) and R-CHOP versus CHOP (B).
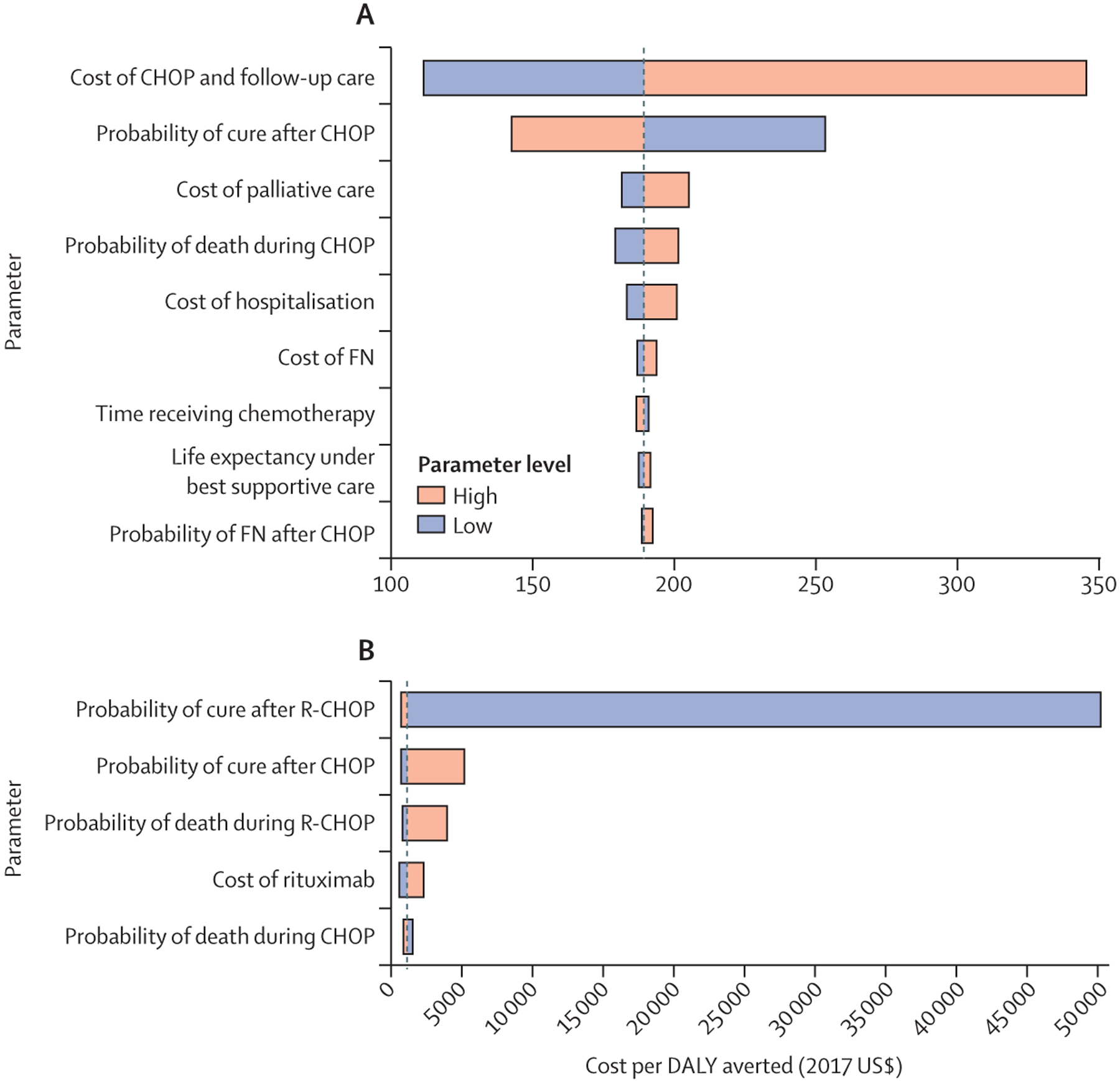
Sensitivity analysis was done on all input parameters, with input values as shown in table 1. Results are only shown for those parameters that resulted in a change higher than 1% in ICER. Parameters are displayed in descending order of variation in ICER by extremes of parameter limits. CHOP=cyclophosphamide, doxorubicin, vincristine, prednisone. DALY=disability-adjusted life-year. FN=febrile neutropenia. ICER=incremental cost-effectiveness analysis. R-CHOP=rituximab plus CHOP.
We also did two specific sensitivity analyses of interest. First, we included palliative care costs in the no chemotherapy pathway. In this scenario, the average cost of the no chemotherapy group increases to $727 with no effect on other groups, as palliative care costs were already included in other groups before death. Under these assumptions, the ICER of CHOP compared with palliative care was $149 per DALY averted, $2766 per death averted, and $95 per life-year gained. This ICER of $149 per DALY averted was substantially lower than the willingness-to-pay threshold (extremely cost-effective). Additionally, we observed no change in findings related to R-CHOP versus CHOP. Second, we assessed the costs and outcomes if patients were treated with a second-line chemotherapy regimen. In this scenario, including the costs of first-line and second-line chemotherapy (second-line therapy attributed to patients who were refractory or relapsed) across the decision-tree model, total costs were estimated to be $2810 per patient for CHOP and $5820 per patient for R-CHOP. DALYs averted were essentially unchanged, at 7.5 per patient for CHOP and 10.3 per patient for R-CHOP. This yielded an ICER for CHOP including second-line chemotherapy compared with no chemotherapy of $329 per DALY averted, which was higher than that in the base-case scenario. The ICER for R-CHOP compared with CHOP when including second-line chemotherapy was $1059 per DALY averted, which was lower than that in the base-case scenario.
In the probabilistic sensitivity analysis of ICERs of costs per DALY averted, the ICER for CHOP compared with no chemotherapy remained lower than the willingness-to-pay threshold of Malawi in more than 99% of simulations (figure 3; absolute values of DALYs and costs on both per patient and population level are shown in the appendix p 2). The ICER for R-CHOP versus CHOP was lower than the threshold in 46% of simulations, higher than the threshold but still with an advantage in DALYs averted for R-CHOP in 49% of simulations, and was dominated (ie, CHOP averted more DALYs than R-CHOP) in 5% of simulations. We found no simulations in which CHOP was more costly than R-CHOP. On the basis of probabilistic sensitivity analyses, we created a willingness-to-pay cost-effectiveness acceptability curve (figure 4). At a threshold lower than $500, no chemotherapy was likely to be the preferred approach; from $500 to $1000, CHOP was likely to be preferred; and for thresholds higher than $1000, R-CHOP was likely to be preferred.
Figure 3: Probabilistic sensitivity analysis ICER planes of CHOP versus no chemotherapy (A) and R-CHOP versus CHOP (B).
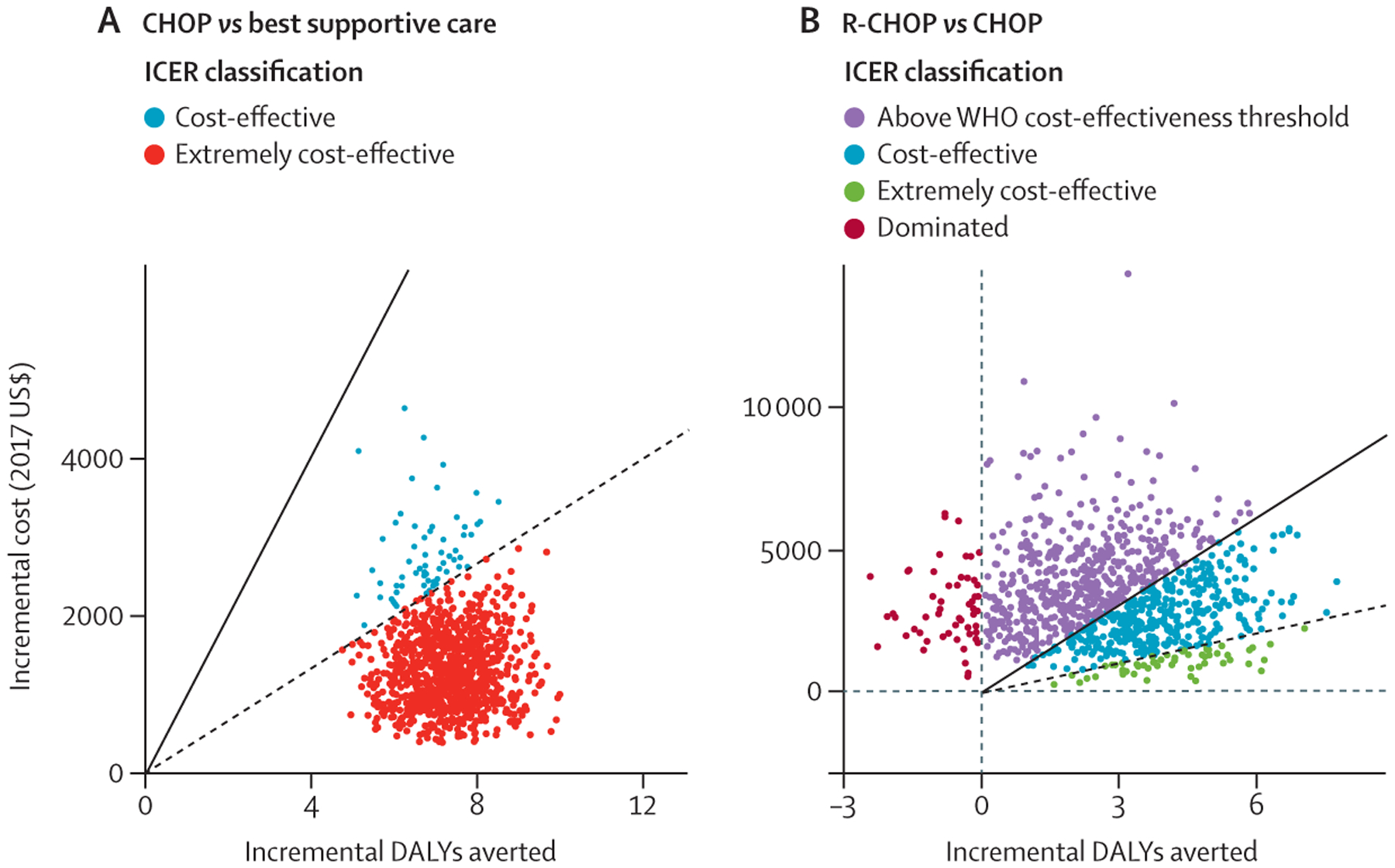
1000 simulations are shown. The solid line shows willingness-to-pay thresholds for costs per DALY averted of three times the GDP per capita of Malawi ($1014 in 2017) and the dashed line is the GDP per capita of Malawi ($338 in 2017). The ICER classifications are as follows: “cost-effective” shows those simulations in which the ICER was lower than three times the GDP per capita (A,B), “extremely cost-effective” shows those simulations in which the ICER was lower than the GDP per capita (A, B), “dominated” designates that the intervention (in this case R-CHOP) was both more expensive and less effective in those simulations (B), and “above WHO cost-effectiveness threshold” shows the simulations in which R-CHOP was more effective than CHOP, but was not lower than the willingness-to-pay threshold of three times the GDP per capita (B). CHOP=cyclophosphamide, doxorubicin, vincristine, prednisone. DALY=disability-adjusted life-year. GDP=gross domestic product. ICER=incremental cost-effectiveness analysis. R-CHOP=rituximab plus CHOP.
Figure 4: Willingness-to-pay cost-effectiveness acceptability curves for treatment of diffuse large B cell lymphoma in Malawi.
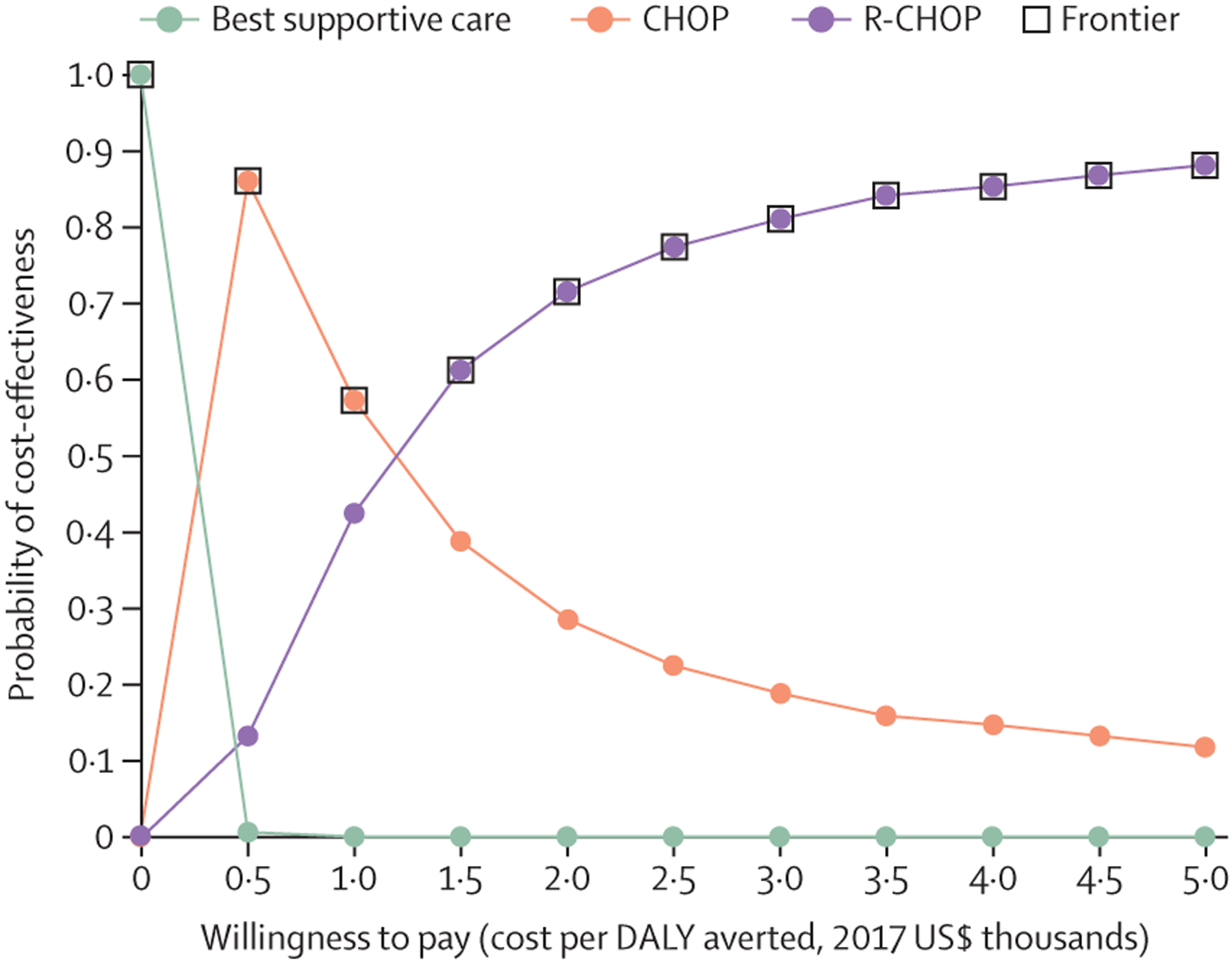
For a willingness-to-pay threshold lower than $500, no chemotherapy is preferred; from $500 to $1000, CHOP is preferred; and higher than $1000, R-CHOP is preferred. CHOP=cyclophosphamide, doxorubicin, vincristine, prednisone. DALY=disability-adjusted life-year. R-CHOP=rituximab plus CHOP.
On a population level, the estimated annual cost for Malawi was $246 568 for no chemotherapy, $1 118 362 for CHOP, and $3 207 900 for R-CHOP, with estimated DALYs of 18 178 for no chemotherapy, 13 649 for CHOP, and 11 825 for R-CHOP (table 2). The incremental cost was estimated to be $871 794 for CHOP over no chemotherapy and $2 089 538 for R-CHOP over CHOP. For CHOP over no chemotherapy, we estimated 239 incremental deaths averted and 4529 DALYs averted; for R-CHOP over CHOP, we estimated 94 incremental deaths averted and 1824 DALYs averted. The 2017–18 government health budget for Malawi was $170 million, and external donors are estimated to additionally contribute approximately $350 million annually to health expenditures.1 Therefore, the annual incremental outlay for CHOP chemotherapy and associated costs would be 0.6% of the annual Malawi health budget; for R-CHOP compared with CHOP, it would be 1.2% of the annual Malawi health budget. These values would be 0.2% for CHOP chemotherapy and 0.4% for R-CHOP compared with CHOP when total health outlays, including donor health expenditures, were included.
Discussion
To our knowledge, this study is among the first formal cost-effectiveness analyses for cancer treatment among adults in sub-Saharan Africa, and similar data are scarce in LMIC contexts generally. Using prospective data from Malawi, we found that the ICER of costs per DALY averted for DLBCL treatment with CHOP is well under the WHO willingness-to-pay definition of three times the GDP per capita, and remained so under various deterministic and probabilistic sensitivity analyses. We also found that adding rituximab to CHOP had an ICER of cost per DALY averted close to the threshold and was lower than the threshold in approximately half of simulations by probabilistic sensitivity analysis.
The cost-effectiveness of R-CHOP versus CHOP has been compared by Hornberger and colleagues in the USA.29 The study found a total cost of drugs for R-CHOP of $20 583, an ICER of $12 304 per life-year gained, and $19 297 per quality-adjusted life year (QALY) gained (in 2003 US$). Although QALYs and DALYs are not directly comparable, $19 297 per QALY is well within the willingness-to-pay threshold of many high-income countries. There are several contextual differences between high-income countries and Malawi that are likely to influence the cost-effectiveness of DLBCL treatment. These include the use of a lower cost rituximab biosimilar in Malawi, health system factors that affect the relative safety and effectiveness of CHOP and R-CHOP treatment compared with high-income countries, and the absence of effective salvage regimens after first-line treatment failure in Malawi.
There are also several methodological differences between the study by Hornberger and colleagues and our report. First, upfront treatment costs in their model only included chemotherapy costs and neglected the costs of complications, personnel, and supplies, as these were assumed to be similar between CHOP and R-CHOP. The authors also used a 5-year time horizon compared with our lifetime time horizon and note that their shorter time horizon might underestimate the cost-effectiveness of rituximab. They included costs and benefits of salvage chemotherapy in their base-case analysis, whereas we included these only in our sensitivity analysis. As in our study, including the costs of salvage chemotherapy improved R-CHOP cost-effectiveness compared with CHOP, given the lower relapse rate and lower probability of accruing salvage chemotherapy costs with R-CHOP. Finally, their sensitivity analysis varied costs by 10%, with results being highly sensitive to variations in rituximab cost, whereas our study was little influenced by variations in rituximab cost.
A strength of cost-effectiveness research is the use of a common outcome that can be compared across many interventions and settings. To place our findings in context, a review of cost-effectiveness metrics for various health interventions in LMICs has been published in 2017.30 The cost per DALY averted of CHOP in our analysis was similar to other interventions that have received broad support from funders and policy makers worldwide as prudent public health investments, such as preventing transmission of HIV from mothers to infants (about $125 per DALY averted), implementing universal HIV test-and-treat programmes (about $300 per DALY averted), and building trauma centres (about $400 per DALY averted). The cost per DALY averted of R-CHOP in this analysis was less favourable, but similar to other interventions that have received broad multi-sectoral support for LMICs, including aspirin and statins for secondary prevention of coronary artery disease (about $900 per DALY averted), psychosocial care for depression (about $800 per DALY averted), and tobacco regulation (about $700 per DALY averted).
Although we report favourable cost-effectiveness ratios for treatment of patients with DLBCL, the total cost and budget impact are also important for policy makers and addressing these issues might require flexible and creative funding models. Although cancer registration and burden estimates in sub-Saharan Africa have clear limitations, using GLOBOCAN data, we estimated that it would cost approximately an incremental $1 million annually to treat all cases of DLBCL in Malawi with CHOP to save 252 lives, and an additional $2 million annually to add rituximab and save an additional 100 lives. These amounts would constitute 0.6% (for CHOP) and 1.2% (for R-CHOP) of the Malawi health budget and 0.2% (for CHOP) and 0.4% (for R-CHOP) of total health expenditures including external donor outlays. For context, Botswana supplies rituximab for lymphoma treatment at a cost of $541 000 annually for an estimated 105 cases of lymphoma annually, or approximately $5410 per patient, similar to the price we determined for R-CHOP care in Malawi.31
This study has several strengths. First, we used prospective data collected in Malawi to parameterise the model from a large cohort of patients with lymphoma treated at a tertiary care facility under real-world conditions. Second, these outcome data are directly matched with robust microcosting data collected in the same facility. Third, we did extensive and robust sensitivity analysis, and our findings were largely insensitive to changes in many parameters.
This study also has some limitations. First, because we relied on data collected from a single tertiary care facility, the generalisability of these findings to other settings or countries might be limited. Along these lines, the microcosting analysis used here was done in the context of a clinical research collaboration between a US academic institution and the Malawi Ministry of Health, which might affect the total costs. However, treatment costs solely within public sector facilities of the Malawi Ministry of Health without external collaborators are typically lower than what we estimated in this study, such that DLBCL treatment might be more cost-effective if clinical outcomes remain similar. Furthermore, this analysis was done from a health systems perspective, and societal impacts of additional years of gainful employment were not considered. However, inclusion of these additional benefits would probably further improve the observed cost-effectiveness ratios. Finally, limitations regarding cancer incidence data from Malawi make budget impact analysis imprecise. We relied on international reference estimates from GLOBOCAN, but we acknowledge the limitations of cancer registries throughout sub-Saharan Africa that have been extensively reviewed elsewhere.32
Further studies are needed to compare the costs of cancer care across diverse sub-Saharan Africa settings. Additionally, as cancer care becomes less centralised, more patients might be diagnosed with cancer and receive treatment, but the costs per patient might increase substantially with less centralised care. Implementation studies are needed to identify the costs and cost-effectiveness of implementing cancer treatment in central specialised facilities versus smaller peripheral health centres, to optimally balance trade-offs between access and equity versus quality and efficiency.
In conclusion, our study shows that curative-intent treatment of DLBCL among adults in Malawi is cost-effective according to current WHO recommendations, convincingly so for CHOP and possibly for R-CHOP. Our findings suggest that DLBCL treatment is a prudent investment relative to other accepted health interventions in LMICs.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed from inception to Dec 30, 2020, with no language restrictions using the following search terms: “(“Cost-effectiveness” OR “Cost-utility”) AND “Africa” AND (“cancer” OR “lymphoma”)”. Including studies across the entire cancer control continuum in sub-Saharan Africa, the search resulted in 234 studies. On review, only 41 articles were original research, including economic analyses. Of these, the vast majority (31) focused on screening or prevention and only ten examined a component of the cost-effectiveness of treatment. To our knowledge, there are no studies assessing the cost-effectiveness of treatment for diffuse large B-cell lymphoma (DLBCL) in sub-Saharan Africa, which is a common, curable cancer in this region.
Added value of this study
We used published cost and outcomes data from prospective studies of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) treatment for DLBCL in adult patients at a national referral hospital in Malawi for our cost-effectiveness analyses. We estimated that the incremental cost-effectiveness ratio (ICER) of treatment of DLBCL with CHOP versus best supportive care was substantially lower than the willingness-to-pay threshold of three times the GDP per capita of Malawi ($1014 in 2017 US$) in the base-case scenario and in more than 99% of simulations in probabilistic sensitivity analysis. The ICER for R-CHOP versus CHOP was estimated to be slightly higher than the willingness-to-pay threshold and lower than that in 46% of simulations.
Implications of all the available evidence
Curative intent treatment for DLBCL is probably a prudent investment compared with other accepted public health interventions in sub-Saharan Africa, particularly for CHOP and possibly for R-CHOP. Further economic evaluations are needed to support an investment case for cancer treatment in sub-Saharan Africa.
Acknowledgments
Funding for this study was provided by NIH (grants K01TW011470, U54CA190152, D43TW009340, UM1CA121947, P30CA233709, R25CA057711, K01TW009488, and T32CA11633911). We want to express appreciation for all our study participants for volunteering to provide essential data regarding lymphoma treatment in Malawi. We want to recognise the assistance of Robin Kajasiche and Deborah Demster Kamwendo in collecting cost data for this study. This work was completed while SG was employed at the University of North Carolina at Chapel Hill. The opinions expressed in this article are the authors own and do not reflect the view of the NIH, the US Department of Health and Human Services, or the US Government.
Declaration of interests
SG and MSP report grants from National Institutes of Health (NIH) during the conduct of the study. SW reports grants from the Pfizer Foundation, outside the submitted work. All other authors report no competing interests.
Footnotes
Data sharing
Deidentified analytical datasets and analysis code are stored in a password-protected repository and are available upon request to the corresponding author for reproducibility.
For the Global Cancer Observatory see https://gco.iarc.fr/today
See Online for appendix
References
- 1.UNICEF. 2017/18 Malawi health budget brief. 2018. https://www.unicef.org/malawi/media/411/file/Health_Budget_Brief_2018_2019.pdf (accessed Nov 30, 2020).
- 2.Renner L, Shah S, Bhakta N, Denburg A, Horton S, Gupta S. Evidence from Ghana indicates that childhood cancer treatment in sub-Saharan Africa is very cost effective: a report from the Childhood Cancer 2030 Network. J Glob Oncol 2018; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denburg AE, Laher N, Mutyaba I, et al. The cost effectiveness of treating Burkitt lymphoma in Uganda. Cancer 2019; 125: 1918–28. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg GM, Lauer JA, Zelle S, Baeten S, Baltussen R. Cost effectiveness of strategies to combat breast, cervical, and colorectal cancer in sub-Saharan Africa and South East Asia: mathematical modelling study. BMJ 2012; 344: e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimani SM, Painschab MS, Horner M-J, et al. Epidemiology of haematological malignancies in people living with HIV. Lancet HIV 2020; 7: e641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. Malawi country factsheet. 2019. http://www.unaids.org/en/regionscountries/countries/malawi (accessed Nov 30, 2020).
- 7.de Witt P, Maartens DJ, Uldrick TS, Sissolak G. Treatment outcomes in AIDS-related diffuse large B-cell lymphoma in the setting roll out of combination antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2013; 64: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milligan MG, Bigger E, Abramson JS, et al. Impact of HIV infection on the clinical presentation and survival of non-Hodgkin lymphoma: a prospective observational study from Botswana. J Glob Oncol 2018; 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan LD, Lee JY, Ambinder RF, et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood 2005; 106: 1538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 2005; 23: 5027–33. [DOI] [PubMed] [Google Scholar]
- 11.Boué F, Gabarre J, Gisselbrecht C, et al. Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin’s lymphoma. J Clin Oncol 2006; 24: 4123–28. [DOI] [PubMed] [Google Scholar]
- 12.Kimani S, Painschab M, Kaimila B, et al. Safety and efficacy of rituximab in patients with diffuse large B-cell lymphoma in Malawi: a prospective, single-arm, non-randomised phase 1/2 clinical trial. Lancet Glob Health 2021; published online May 19. 10.1016/S2214-109X(21)00181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Painschab MS, Kohler RE, Kasonkanji E, et al. Microcosting analysis of diffuse large B-cell lymphoma treatment in Malawi. J Glob Oncol 2019; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Painschab MS, Kasonkanji E, Zuze T, et al. Mature outcomes and prognostic indices in diffuse large B-cell lymphoma in Malawi: a prospective cohort. Br J Haematol 2019; 184: 364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016; 316: 1093–103. [DOI] [PubMed] [Google Scholar]
- 16.Shi Q, Schmitz N, Ou F-S, et al. Progression-free survival as a surrogate end point for overall survival in first-line diffuse large B-cell lymphoma: an indiviaul patient-level analysis of multiple randomized trials (SEAL). J Clin Oncol 2018; 36: 2593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobsen LH, Bøgsted M, Brown PN, et al. Minimal loss of lifetime for patients with diffuse large B-cell lymphoma in remission and event free 24 months after treatment: a Danish population-based study. J Clin Oncol 2017; 35: 778–84. [DOI] [PubMed] [Google Scholar]
- 18.Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health 2015; 3: e712–23. [DOI] [PubMed] [Google Scholar]
- 19.World Bank. Inflation, GDP deflator (annual %)—Malawi. https://data.worldbank.org/indicator/NY.GDP.DEFL.KD.ZG?locations=MW&view=chart (accessed Nov 30, 2020).
- 20.World Bank. GDP per capita (current US$)—Malawi. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=MW (accessed Nov 30, 2020).
- 21.Gopal S, Krysiak R, Liomba NG, et al. Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PLoS One 2013; 8: e70361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaimila B, van der Gronde T, Stanley C, et al. Salvage chemotherapy for adults with relapsed or refractory lymphoma in Malawi. Infect Agent Cancer 2017; 12: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global Health Observatory, WHO. Life tables by country. 2020. https://apps.who.int/gho/data/view.main.60980?lang=en (accessed Nov 30, 2020).
- 24.Morrison VA, Picozzi V, Scott S, et al. The impact of age on delivered dose intensity and hospitalizations for febrile neutropenia in patients with intermediate-grade non-Hodgkin’s lymphoma receiving initial CHOP chemotherapy: a risk factor analysis. Clin Lymphoma 2001; 2: 47–56. [DOI] [PubMed] [Google Scholar]
- 25.Lyman GH, Morrison VA, Dale DC, Crawford J, Delgado DJ, Fridman M. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 2003; 44: 2069–76. [DOI] [PubMed] [Google Scholar]
- 26.Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol 2019; 5: 1749–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López A, Gutiérrez A, Palacios A, et al. GEMOX-R regimen is a highly effective salvage regimen in patients with refractory/relapsing diffuse large-cell lymphoma: a phase II study. Eur J Haematol 2008; 80: 127–32. [DOI] [PubMed] [Google Scholar]
- 28.Jalal H, Pechlivanoglou P, Krijkamp E, Alarid-Escudero F, Enns E, Hunink MGM. An overview of R in health decision sciences. Med Decis Making 2017; 37: 735–46. [DOI] [PubMed] [Google Scholar]
- 29.Hornberger JC, Best JH. Cost utility in the United States of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone for the treatment of elderly patients with diffuse large B-cell lymphoma. Cancer 2005; 103: 1644–51. [DOI] [PubMed] [Google Scholar]
- 30.Horton S, Gelband H, Jamison D, Levin C, Nugent R, Watkins D. Ranking 93 health interventions for low- and middle-income countries by cost-effectiveness. PLoS One 2017; 12: e0182951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martei YM, Chiyapo S, Grover S, et al. Methodology to forecast volume and cost of cancer drugs in low- and middle-income countries. J Glob Oncol 2018; 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


