Abstract
Metaplastic meningioma is a rare World Health Organization Grade I meningioma subtype, accounting for 0.2%-1.6% of all meningiomas. Primary extradural meningiomas represent less than 2% of all meningiomas, with intraosseous meningioma as a subtype of primary extradural meningiomas. Herein, we report the case of a 65-year-old male presenting with headache. His computed tomography scans showed an osteolytic left parietal bone mass, and magnetic resonance imaging revealed hyperintense dots in the mass on T1-weighted images. The mass was then resected and diagnosed on histopathological examination as an intraosseous metaplastic meningioma.
Keywords: Meningioma, Metaplastic, Intraosseous, Calvarial, Imaging
Introduction
Metaplastic meningioma is defined as the presence of focal or widespread mesenchymal components, including osseous, cartilaginous, lipomatous, myxoid, or xanthomatous tissue, singly or in combination. Intraosseous meningioma (IOM) is a subtype of primary extradural meningioma (PEM). Herein, we report a case of intraosseous metaplastic meningioma from both radiological and histopathological points of view. To date and to the best of our knowledge, this is the second report of intraosseous metaplastic meningioma in literature.
Case report
A 65-year-old male with a history of headaches for a few months was referred to our neurosurgery department due to a cranial mass, which was revealed by magnetic resonance imaging (MRI) performed during a previous headache workup. Although previous cranial MRIs had also been taken 4 years prior due to other reasons, there was allegedly no significant change in the lesion's size or appearance between the 2 examinations. The patient's medical history included hypertension, diabetes mellitus, and bronchial asthma, all of which were under medical control. He had no history of malignancy. In addition to mild headache, the patient had no significant neurological abnormalities. There was no visible skull bulge, and a laboratory examination revealed no abnormal readings.
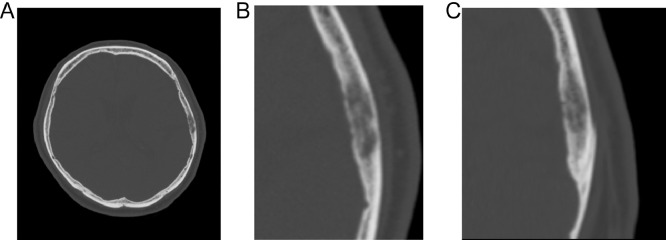
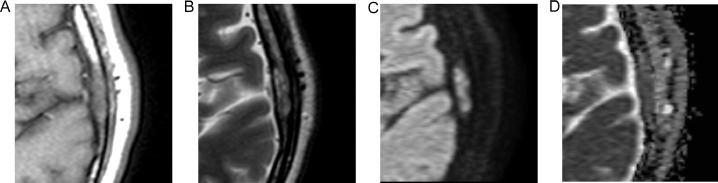
Computed tomography (CT) showed a 19-mm-long, well-defined, left parietal bone mass near the squamosal suture (Fig. 1). The main part of the mass was located in a diploic space, and the trabeculae were sparse but well preserved. There was also a mild bony bulge, although there were no tears in the inner or outer plates. MRI performed at our hospital showed an isointense mass relative to the muscles, which included internal hyperintense dots on T1-weighted images (T1WI) (Fig. 2A). Meanwhile, the mass had heterogeneous intensity on T2-weighted images (Fig. 2B), also showing restricted diffusion (Fig. 2C and D). Due to a history of asthma, contrast-enhanced MRI was not performed. The hyperintense dots on T1WI were thought to be bone marrow fat, and thus we thought that the mass did not destroy the pre-existing structures, such as the bone trabeculae and bone marrow fat. This finding led to the differential diagnosis of osseous hemangioma (venous malformation), hyperplastic bone marrow (red marrow), IOM, Paget disease of bone, and metastatic bone tumor (particularly, intertrabecular type). The mass was suspected to be benign, because there was no increase in size for 4 years and there was no history of malignancy. However, the mass was still resected as per the patient's request.
Fig. 1.
Head computed tomography of a 65-year-old male presenting with headache. Axial (A, B) and coronal (C) images show a 19-mm-long, well-defined, left parietal bone mass near the squamosal suture. The main part of the mass is located in a diploic space, and the trabeculae are sparse but well preserved. There is also a mild bony bulge, although there are no tears in the inner or outer plates.
Fig. 2.
Magnetic resonance images of the mass. Axial T1-weighted image (A) shows the mass is isointense relative to muscles and includes internal hyperintense dots. Axial T2-weighted image (B) shows heterogeneous intensity. Axial diffusion-weighted image (C) and the corresponding apparent diffusion coefficient map (D) show diffusion restriction.
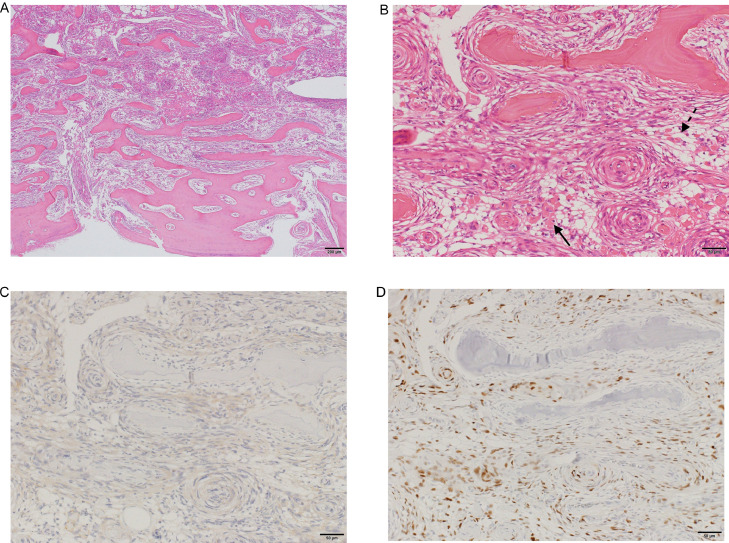
A histopathological examination was then performed after decalcification using Plank-Rychlo fluid, revealing tumor growth in the intertrabecular space and whorl patterns of spindle-shaped cells (Fig. 3A and B). Moreover, the tumor was marked with ossification and lipomatous tissue inclusions, which were different from the pre-existing bone trabeculae and bone marrow fat. Immunohistochemistry showed that the tumor cells expressed epithelial membrane antigen (EMA) (Fig. 3C) and progesterone receptor (Fig. 3D), but they were negative for MIB-1 (Ki-67). Given these findings, the final diagnosis was intraosseous metaplastic meningioma (World Health Organization [WHO] Grade I).
Fig. 3.
Histopathological examination of the mass reveals tumor growth in the intertrabecular space and whorl patterns of spindle-shaped cells (hematoxylin-eosin stain, original magnification, ×4 [A] and ×20 [B]). The tumor is marked with ossification (arrow) and lipomatous tissue (dashed arrow) inclusions, which are different from the pre-existing bone trabeculae and bone marrow fat. Immunohistochemistry shows that the tumor cells express epithelial membrane antigen (C) and progesterone receptor (D), but they are negative for MIB-1 (not shown here). Given these findings, the final diagnosis is intraosseous metaplastic meningioma (World Health Organization Grade I).
Discussion
Meningiomas are classified into 15 histological subtypes according to the 2016 WHO classification of tumors of the central nervous system. Specifically, 9 types (meningothelial, fibrous, transitional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, and metaplastic meningiomas) are classified as WHO Grade I, 3 types (chordoid, clear cell, and atypical meningiomas) as WHO Grade II, and the remaining 3 types (papillary, rhabdoid, and anaplastic meningiomas) as WHO Grade III. Of these 15 subtypes, the 3 most common types are meningothelial, fibrous, and transitional meningiomas. In contrast, metaplastic meningioma is a rare subtype, accounting for 0.2%-1.6% of all meningiomas [1], [2], [3], [4], with a low post-surgical recurrence rate and good prognosis [5]. Metaplastic meningioma is defined as the presence of focal or widespread mesenchymal components, including osseous, cartilaginous, lipomatous, myxoid, or xanthomatous tissue, singly or in combination. To delineate from other subtypes, metaplastic meningioma containing osseous tissue refers only to meningiomas, including tumor cells or peritumoral stromal cells that have transformed into osteoblasts with subsequent bone formation, and is distinguished from other meningiomas including ossification, which is the final stage of psammomatous calcification [6]. Furthermore, metaplastic meningiomas containing lipomatous tissue are not truly “metaplastic,” but rather an accumulation of fat in the tumor cells since these cells express the EMA [7]. The imaging characteristics of metaplastic meningiomas also vary depending on their mesenchymal components [8]. Metaplastic meningiomas containing osseous tissues show areas of bone density on CT, whereas in those containing lipomatous tissues show areas of low density on CT and high intensity on T1WI.
Meningiomas can be divided into primary intradural meningiomas (PIMs) and PEMs, depending on the site of origin. Particularly, the frequency of PEM is noted to be 0.04%-1.6% of all meningiomas [2,9], and this subtype occurs in various organs, such as the calvaria, scalp, orbit, paranasal sinuses, nasopharynx, neck, skin, lung, mediastinum, adrenal gland, paraspinal region, and finger [9].
IOM is a subtype of PEM, generally defined as meningioma which is mainly located in the skull, either infiltrating the dura mater or not [9], [10], [11]. Of note, “en plaque meningioma,” which grows along with the dura mater and invades somewhat into the skull without making a conspicuous intracranial mass, is not called IOM [11]. Some authors say that IOM arises from arachnoid cells that are trapped in the cranial sutures at the time of birth or at traumatic fracture sites [12], while others say that it arises from mesenchymal stem cells [13]. Calvarial meningiomas have been reported to be associated with cranial sutures in many cases, but the frequency of this association varies from 8% to 60% [9,14]. PEM and PIM are also found to occur predominantly during later decades of life, but PEM has a second peak incidence in the second decade [9,10]. Regarding sex predilection, it has been reported that IOM is more common in women [15], but some say that there is little difference between each sex in terms of frequency [9]. Regarding histology, approximately 11%-26% of IOMs have atypical or malignant features [9,16]. In imaging of IOMs, CT scans often show osteosclerotic lesions, which are frequently associated with spiculated borders [17], and MRI shows signals that are similar to that of a PIM, depending on the histological type.
The mass in the present case was pathologically confirmed as IOM with osseous and lipomatous tissue. Moreover, there was ossification in the tumor that seemed to be different from the pre-existing bone trabeculae, without evidence of a transition from the psammoma body to the bone. Therefore, it was thought that the tumor or stromal cells surrounding the tumor had transformed into osteoblasts, leading to bone formation. In addition, there was fat in the tumor that seemed to be different from the pre-existing parietal bone marrow fat. Thus, it was thought that the tumor cells accumulated fat, although the EMA expression of fat-containing cells was unclear due to the difficulty in evaluating the immunostaining of specimens decalcified by Plank-Rychlo fluid. Given all these histopathological and immunostaining findings, a diagnosis of metaplastic meningioma with osseous and lipomatous tissue was made. To date and to the best of our knowledge, this is the second report of intraosseous metaplastic meningioma in literature [18]. This rarity may be due to the difficulty in interpreting ossification and fat in the bone and the fact that its definition is vague. In addition, because most intraosseous metaplastic meningiomas have benign features, there are probably many cases in which the tumor was not resected, and the patient was observed without a definitive diagnosis.
In our case, CT showed a bony density area, and MRI showed internal hyperintense dots on T1WI. We initially believed that the hyperdensity and hyperintensity represented preserved bony trabeculae and bone marrow fat, respectively, although we could not determine whether the process was benign (such as IOM or osseous hemangioma) or malignant (such as intertrabecular metastasis). However, histopathological examination revealed not only pre-existing bone trabeculae and bone marrow fat, but also osseous and lipomatous components of metaplastic meningioma. Therefore, we were able to correlate that the hyperdensity represented preserved bony trabeculae and the osseous component of metaplastic meningioma, whereas the hyperintensity represented preserved bone marrow fat and the lipomatous component. Unfortunately, it was impossible to identify these correlations preoperatively using radiological imaging alone.
Considering the differential diagnoses on imaging, hyperplastic bone marrow is not consistent with osteolytic changes on CT, and Paget disease of bone is atypical in presentation given that our case presents with a single lesion with little change over time. On the contrary, osseous hemangiomas are typically characterized by cavernous and dilated blood vessels with a high signal on T2-weighted images and a "sunburst” appearance of residual trabecular thickening [19], possibly containing fatty components in the trabecular space and present images similar to the present case; thus, in the present case, it would have been difficult to exclude osseous hemangioma.
Conclusion
In the present study, we experienced a case of intraosseous metaplastic meningioma, a rare subtype of meningioma arising from a rare site. Meningiomas can present a variety of imaging findings depending on the histological subtype, especially in cases of metaplastic meningiomas, which can contain various components, such as bone, fat, and cartilage. Thus, when presented with a cranial mass, intraosseous metaplastic meningioma could be considered in the differential diagnosis.
Patient consent
The personal details of the patient are all removed from this article. There is no ethical problem to be declared.
Footnotes
Acknowledgments: We would like to thank Editage (www.editage.com) for English language editing.
Competing interests: The authors have no conflict of interests to declare.
Contributor Information
Yusuke Utsunomiya, Email: yusuke.utsunomiya@gmail.com.
Nobuyuki Mori, Email: nbykmr@osaka-med.jrc.or.jp.
References
- 1.Kim T., Kim J.W., Ji S.Y., Kang H., Kim K.-M., Kim Y.H. Intracranial metaplastic meningioma: clinical and radiological characteristics of 11 cases. J Korean Neurosurg Soc. 2020;63:657–663. doi: 10.3340/jkns.2020.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D.J., Xie Q., Gong Y., Mao Y., Wang Y., Cheng H.X. Histopathological classification and location of consecutively operated meningiomas at a single institution in China from 2001 to 2010. Chin Med J (Engl). 2013;126:488–493. doi: 10.3760/cma.j.issn.0366-6999.20122874. [DOI] [PubMed] [Google Scholar]
- 3.Zouaoui S., Darlix A., Rigau V., Mathieu-Daudé H., Bauchet F., Bessaoud F. Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006–2010. Neurochirurgie. 2018;64:15–21. doi: 10.1016/j.neuchi.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Bhat A.R., Wani M.A., Kirmani A.R., Ramzan A.U. Histological-subtypes and anatomical location correlated in meningeal brain tumors (meningiomas) J Neurosci Rural Pract. 2014;5:244–249. doi: 10.4103/0976-3147.133568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang H., Sun H., Chen H., Gong Y., Mao Y., Xie Q. Clinicopathological analysis of metaplastic meningioma: report of 15 cases in Huashan Hospital. Chinese J Cancer Res. 2013;25:112–118. doi: 10.3978/j.issn.1000-9604.2013.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barresi V., Caffo M., Ieni A., Alafaci C., Tuccari G. Osteoblastic meningiomas: clinico-pathological and immunohistochemical features of an uncommon variant. J Neurooncol. 2011;105:225–232. doi: 10.1007/s11060-011-0588-3. [DOI] [PubMed] [Google Scholar]
- 7.Roncaroli F., Scheithauer B.W., Laeng R.H., Cenacchi G., Abell-Aleff P., Moschopulos M. Lipomatous meningioma: a clinicopathologic study of 18 cases with special reference to the issue of metaplasia. Am J Surg Pathol. 2001;25:769–775. doi: 10.1097/00000478-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Kunimatsu A., Kunimatsu N., Kamiya K., Katsura M., Mori H., Ohtomo K. Variants of meningiomas: a review of imaging findings and clinical features. Jpn J Radiol. 2016;34:459–469. doi: 10.1007/s11604-016-0550-6. [DOI] [PubMed] [Google Scholar]
- 9.Lang F.F., Macdonald O.K., Fuller G.N., DeMonte F. Primary extradural meningiomas: a report on nine cases and review of the CT-era literature. J Neurosurg. 2000;93:940–950. doi: 10.3171/jns.2000.93.6.0940. [DOI] [PubMed] [Google Scholar]
- 10.Tokgoz N., Oner Y.A., Kaymaz M., Ucar M., Yilmaz G., Tali T.E. Primary intraosseous meningioma: CT and MRI appearance. AJNR Am J Neuroradiol. 2005;26:2053–2056. http://www.ncbi.nlm.nih.gov/pubmed/16155159 [PMC free article] [PubMed] [Google Scholar]
- 11.Taori K., Sanyal R., Deshmukh A., Jawale R., Rathod J. Primary intradiploic meningioma. Eur J Radiol Extra. 2006;57:5–8. doi: 10.1016/j.ejrex.2005.10.007. [DOI] [Google Scholar]
- 12.Azar-Kia B., Sarwar M., Marc J.A., Schechter M.M. Intraosseous meningioma. Neuroradiology. 1974;6:246–253. doi: 10.1007/BF00345784. [DOI] [PubMed] [Google Scholar]
- 13.Shuangshoti S., Netsky M.G., Fitz-Hugh G.S. Parapharyngeal meningioma with special reference to cell of origin. Ann Otol Rhinol Laryngol. 1971;80:464–473. doi: 10.1177/000348947108000327. [DOI] [PubMed] [Google Scholar]
- 14.Al-khawaja D., Murali R., Sindler P. Primary calvarial meningioma. J Clin Neurosci. 2007;14:1235–1239. doi: 10.1016/j.jocn.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Butscheidt S., Ernst M., Rolvien T., Hubert J., Zustin J., Amling M., Martens T. Primary intraosseous meningioma: clinical, histological, and differential diagnostic aspects. J Neurosurg. 2019:1–10. doi: 10.3171/2019.3.jns182968. [DOI] [PubMed] [Google Scholar]
- 16.Kwon S.M., Ko Y., Bang S.S. Primary intraosseous osteolytic meningioma: a case report and review of the literature. BMC Neurol. 2019;19:1–6. doi: 10.1186/s12883-019-1392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colas L., Caron S., Cotten A. Skull vault lesions: a review. Am J Roentgenol. 2015;205:840–847. doi: 10.2214/AJR.14.13415. [DOI] [PubMed] [Google Scholar]
- 18.Kim L., Huang C., Morey A.L., Winder M.J. Intraosseous lipomatous meningioma. Case Rep Neurol Med. 2015;2015:1–3. doi: 10.1155/2015/482140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez C.K., Schiffman S.R., Bhatt A.A. Radiological review of skull lesions. Insights Imaging. 2018;9:857–882. doi: 10.1007/s13244-018-0643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]