Abstract
Although the incidence of thyroid carcinoma has increased in recent years, follicular thyroid carcinoma with bone metastasis as the first symptom remains rare. Here, we report a case of occult follicular thyroid carcinoma in a 65-year-old female patient admitted to hospital with cerebrovascular disease. Computed tomography findings suggested a diagnosis of meningioma; however, magnetic resonance imaging results showed multiple skull bone destruction with soft tissue masses on the left side of the skull. After surgical resection, the pathology results revealed skull metastasis of follicular thyroid carcinoma. We present this case not only because of the diagnostic challenge it posed, but also because the patient had multiple skull metastases from follicular thyroid carcinoma.
Keywords: Follicular thyroid carcinoma, Skull metastasis, Computed tomography, Magnetic resonance imaging
Introduction
Follicular thyroid carcinoma (FTC) is the second most common thyroid malignancy after papillary thyroid carcinoma (PTC), accounting for 10%-15% of all primary thyroid cancers [1], [2]. It has the characteristics of a relatively late age of onset and a higher mortality rate than PTC. In the clinic, some patients are diagnosed only after the tumor has metastasized. These neoplasms tend to metastasize hematogenously, with the lung and bones most commonly affected. It can also metastasize to the trachea and mediastinum, soft tissue and skin, liver, and other body parts [3]. Bone metastasis is most likely to occur in the scapula, sternum, ilium, and long bones. While skull metastasis is common in lung, breast, and prostate carcinomas, it is very rare for thyroid carcinomas, occurring in only 2.5%-5.8% of cases [4]. Here, we present a case of FTC with multiple skull metastases located on the left frontotemporal bone and parietal bone.
Case report
Here, we report a case of occult FTC in a 65-year-old female patient. Five hours before admission, the patient developed numbness in her right hand, weakness in her right lower limbs, and was struggling to walk. She was admitted to our hospital with a "cerebrovascular accident." She had no speech, convulsions, and was unconscious. The patient was not able to eat without choking, no 2 stool disorders, and had a good mental state.
Medical history: The patient's medical history included hypertension for 5 years with a maximum recorded blood pressed of 170/100 mm Hg, which was usually treated with oral Ni Fuda tablets: 20 mg in the morning and evening each day. The patient also suffered from cerebral infarction 5 years earlier, with cerebral hemorrhage occurring again during this period. The patient did not regular level 2 prevention. She denied diabetes, hepatitis, tuberculosis, surgery, trauma, drug allergy, and blood transfusion. The patient's vaccination history was unknown.
Physical examination: The patient cooperated with the physical examination which showed a clear mind, clear breathing sounds in both lungs, no dry and wet rales, a normal heart rhythm, no pathological murmurs, no abdominal softness, no tenderness, no rebound pain and muscles, non-palpable liver and spleen, and no edema in either leg. Assessment of the patient's nervous system indicated mental clarity, normal high-level cortex function, no aphasia or dysarthria, bilateral pupils with large perfect circles and a diameter 3.0 mm, a sensitive response to light, sufficient eye movement, no nystagmus or diplopia, and bilateral frontal lines and nose. The patient's lips were symmetrical and the tongue was not biased. The right lower limb muscle strength was 4+, the remaining limb muscle strength was normal, muscle tension was normal, bilateral tendon reflexes were symmetrical, the right upper and lower limbs showed decreased acupuncture sensation, the right Puusepp's sign was positive, Chaddock's sign was bilateral negative, Babinski's sign was bilateral negative, the bilateral finger nose test and heel knee-tibia test results were stable, and meningeal irritation was negative. The patient's NIHSS score was 1 point, there was no dysarthria, assessment of hemiplegic limb function revealed mild motor dysfunction, and the drinking test results were level 1.
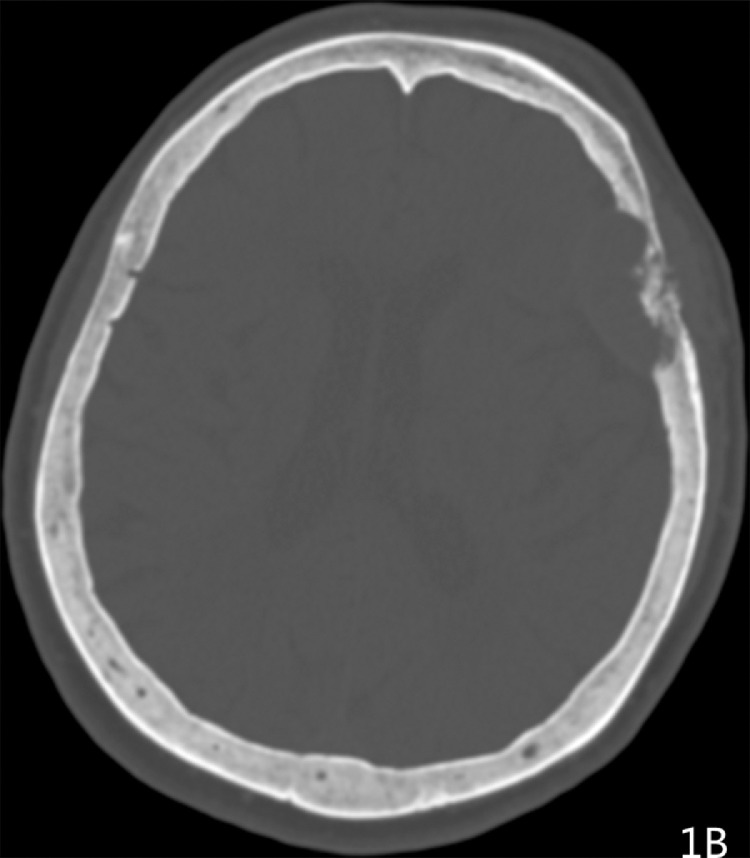
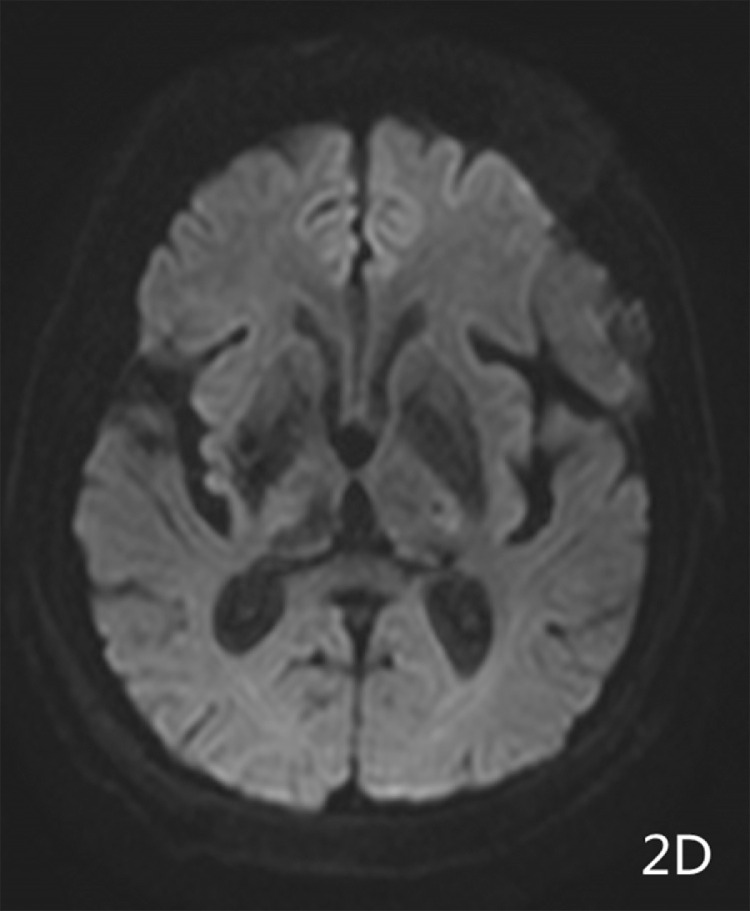
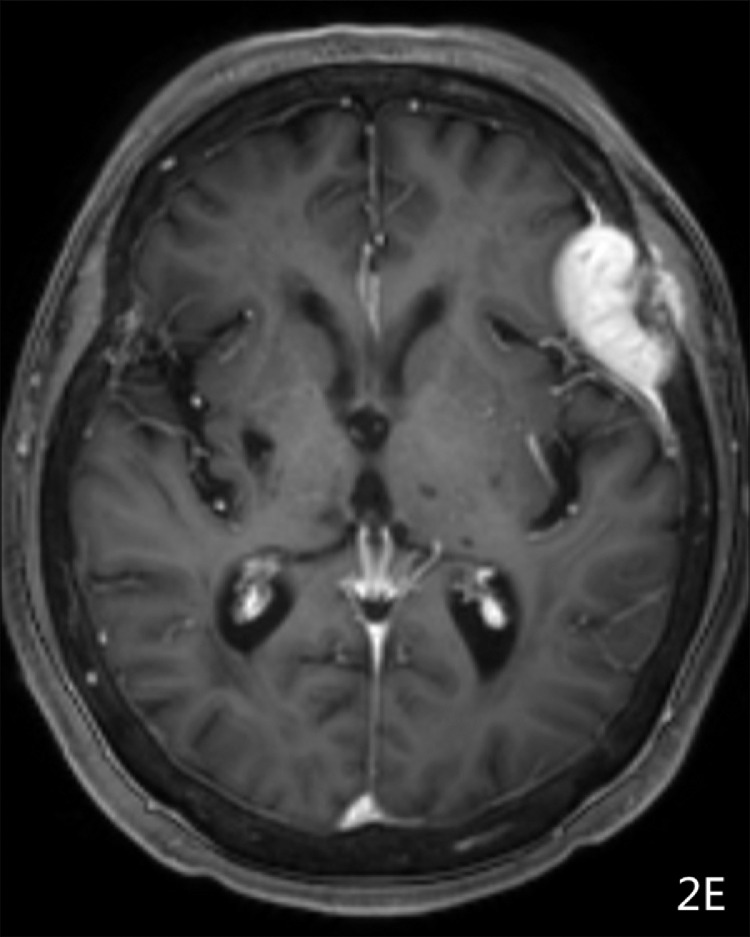
Imaging results: Computed tomography (CT) showed (Fig. 1A-1B) a semicircular, slightly high-density shadow under the left frontotemporal skull plate. The broad base was connected to the skull plate. The outer edge was smooth. The size of the shadow was about 38 × 28 mm and the CT value was about 46 Hu. The adjacent brain mass was compressed on the side of the skull plate. Irregular bone destruction, local skull outer plate destruction, and fusiform soft tissue mass shadows under the adjacent skin were observed. Two round, slightly low-density shadows were seen at the left side of the parietal plate barrier, with a slightly higher density in the middle adjacent to the skull. The shape of the inner plate was regular, and the size of the 2 shadows was 22 × 16 mm and 16 × 12 mm(Fig. 1C). MR showed a semicircular low T1 signal and slightly high T2 signal under the left frontotemporal cranial plate. FLAIR showed a high signal, while DWI showed a similar low signal(Fig. 2A-2D). The outer edge was smooth, showing a short T2 signal dural compression shadow. The adjacent brain mass was compressed, and irregular short T2 signals were observed near the cranial plate. Tumor shadows were also seen under the outer skin of the adjacent cranial plate, while the solid part of the enhanced scan was obviously enhanced(Fig. 2E-2F). The meningeal tail sign was visible and the left parietal plate barrier could be seen. Two areas with low signal with a round shape, poorly defined boundary, and obviously uneven enhancement were observed on the enhanced scan. The larger lesions showed ring-shaped irregularities and obvious enhancement, and had a similar shape as a hamburger.(Fig. 2B-2C)
Fig. 1A.
Head CT scan exhibited an Frontotemporal mass, 3.8 cm × 2.8 cm in size;
Fig. 1B.
With osteolytic destructive features;
Fig. 1C.
Parietal bone with osteolytic destructive,1.6 cm × 1.2 cm in size.
Fig. 2A.
MRI T2WI Axial showed a semicircular slightly high T2 signal under the left frontotemporal cranial plate;
Fig. 2D.
MRI DWI showed a similar low signal;
Fig. 2E.
MRI T1WI Axial enhanced used contrast showed was obviously enhanced;
Fig. 2F.
MRI T1WI Sagittal enhanced used contrast homogeneously by gadolinium, like a sandwich biscuit or hamburger.
Fig. 2B.
MRI T1WI Axial showed a semicircularlow T1 signal;
Fig. 2C.
MRI T2WI Sagittal showed demonstrated an extradural tumor with bone destruction, like a sandwich biscuit or hamburger;
Surgical procedure: The left side enlarged pterion approach was taken. Hemostatic water was subcutaneously injected. The skin was cut in full thickness and the periosteum was separated. After turning over the flap, electrocoagulation was used to stop the bleeding. A mass of about 2 × 2 × 0.5 cm on the top of the forehead was visible. It had no envelope and was fish-like, with clear borders, abundant blood supply, and local skull erosion. An area of the skull measuring about 10 × 11 cm was cut with a milling cutter. Bone wax was used to seal the dead plate and stop bleeding. It could be seen that 3 areas of the skull (the top of the frontal area, the inner plate of the inner and lower areas, and the frontotemporal area) were eroded. A milling cutter was used to mill the eroded skull (the frontal-temporal area was about 4 × 4 cm, the frontal top area was about 3.0 × 3 cm, and the inner and lower skull inner plate of the forehead was about 2.5 × 2.5 cm). Under a microscope, tumor tissue could be seen outside the dura mater where the skull was eroded on the top of the forehead. The dura mater was not damaged. The tumor was carefully removed. The size of the epidural tumor at the fronto-temporal junction was about 4.5 × 4 × 2.5cm and fish-like, with abundant blood supply. The tumor tissue and the dura were bluntly separated, and bleeding was stopped while the tumor was cut. The tumor base and the dura were closely adhered to the dura mater. The surrounding bone was removed with forceps, bone wax was used as a seal to block the bleeding, and the bleeding in the tumor cavity was completely stopped. Intraoperative fast pathological results indicated metastasis. In order to prevent tumor tissue from planting in the brain, while the dura mater was not damaged and the dura mater was not opened.
Pathology results: The results indicated FTC with skull metastasis (Fig. 3). Immunohistochemistry results. The results for the patient were: Tg (+), TTF-1 (+), CK (+), CK20 (-), CDX-2 (-), CD56 (-), and Ki67 (+A high value was about 10%/average value about 2%).
Fig. 3.
Histopathological examination showed the diagnosis of metastatic FTC (HEx40).
Discussion
FTC is second most common subtype of thyroid cancer after PTC, accounting for around 10%-15% of all thyroid cancers. FTC typically occurs in the 40-60 years age group and is generally seen in elderly females [5]. Bone metastasis in FTC most often occurs to the long bones, such as the scapula, sternum, ilium, and long bones. By contrast, skull metastasis in FTC is extremely rare. The literature on skull metastasis in FTC is mostly case reports [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. The extent of the lesions is typically reported to be large and protruding, with the largest one being 20 cm [6]. Most cases are single; there is only 1 other report of multiple skull metastases [10]. Skull metastasis often occurs in the skull base and slope area [1,7,12], but when it occurs in the non-skull base area it is typically the frontal [6,8,11,14] and parietal midline area [9,13].
Comparing the CT and magnetic resonance imaging (MRI) findings in our case with the literature, the lesions typically appear round or oval on CT, are located in the epidural area, and the dura is compressed. Although there is typically no report of brain tissue invasion, it can invade the subcutaneous tissue. The skull is commonly the center of the slightly high-density shadow, which can bulge into the skull and outside the skull, and is hamburger-shaped. The long diameter of the tumor is generally larger than the long diameter of the skull destruction. The tumor boundary is typically clear. Lesions that bulge into the skull are often mistaken as meningiomas [5,13]. In cases of larger tumors, necrosis may occur [9,10,13]. In such cases, enhanced scans show more uniform enhancement, but the necrotic area is not enhanced. The MRI findings are generally similar in shape to the CT findings, with similarly low signal on T1-weighted images, slightly higher signal on T2-weighted images, high signal on FLAIR, and slightly lower signal on DWI. On enhanced scans, there is obviously uniform [7,13] and uneven [10,12] strengthening, but no strengthening of necrosis [9].
It can be difficult to diagnose skull metastasis of FTC before operation. Furthermore, it is difficult to distinguish skull metastasis from nasopharyngeal carcinoma, chordoma, and other metastases. There are many overlaps between epidural metastasis and meningiomas [5,8,13], especially single metastasis, because they all appear as slightly high-density masses on CT. The main difference is that skull metastasis of FTC usually has osteolytic destruction of the skull and the subcutaneous tissue of the skull plate. Whether invasion occurring in a hamburger shape is a characteristic sign needs to be confirmed in a larger number of cases. Notably, meningiomas are rare in osteolytic skull destruction, mostly due to thickening of the cranial plate. In this case, the lesions are multiple and have different shapes. The lesions are larger in the frontotemporal skull plate, the epidural lesions under the skull are larger, and the subcutaneous lesions are smaller. The lesions located in the skull are centered on the skull plate. Destruction and soft tissue masses occurring on the inside and outside of the adjacent cranial plates, which have an appearance like a sandwich cookie or hamburger, are signs that are more indicative of metastases.
Epidural metastasis to the skull in FTC cases is similar to meningiomas in terms of both clinical and imaging characteristics. However, for cases with osteolytic bone destruction and subcutaneous invasion occurring in the shape of a sandwich cookie or hamburger, the possibility of metastasis should be considered first—especially in cases with frequent skull lesions.
Patient consent
Provided that these do not contain any identifying marks and are not accompanied by text that might identify the individual concerned.
Footnotes
Competing interests: The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Contributor Information
Ying Liu, Email: liueagle2012@163.com.
Xuezhi Chen, Email: chenxuezhi666@163.com.
References
- 1.Chan JKC. In: Diagnostic histopathology of tumors. 24th ed. Flethcher C.D.M, editor. Elsevier Saunders; Philadelphia: 2013. Tumours of thyroid ¶thyroid glands; pp. 1177–1272. Volume. [Google Scholar]
- 2.Nawarathna NJ, Kumarasinghe NR, Chandrasekera DN, Senevirathna RJ. Unusual presentation of occult follicular carcinoma of thuroid: as thoracic wall lump. Thyroid Res Pract. 2016;13:36–39. [Google Scholar]
- 3.Battoo AJ, Rasool Z, Sheikh ZA, Haji AG. Follicular thyroid caicinoma presenting as solitary liver metastasis: a case report. J Med case Rep. 2016;10(1):347. doi: 10.1186/s13256-016-1140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Zhao G, Zhang Y, Ding K, Cao H, Yang D, Zhang J, Duan Z, Xin S. Skull matastasis revealing a papillary thyroid carcinoma. Chin J Cancer Res. 2013;25(5):603–607. doi: 10.3978/j.issn.1000-9604.2013.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taziel M, Essadi I, Errihani H. Thyroid carcinoma presenting as a dural metastasis mimicking a meningioma: a case report. N Am J Med Sci. 2011;3(1):39–42. doi: 10.4297/najms.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seema RB, Vishrabdha RP. An unusal presentation of follicular carcinoma of thyroid. JKIMSU. 2019;8(1):86–89. [Google Scholar]
- 7.Chen Z, Qiu QH, Zhang QH, Zhu ZC, Peng Y, Liu H. Skull base metastasis from differentiated thyroid carcinoma: 3 cases report and review of literature. J Clin Otorhinolaryngol Head Neck Surg(China) 2017;31(11):881–884. doi: 10.13201/j.issn.1001-1781.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Shetty A, Chowdappa V, Kasukurti PP. Isolated skull metastasis of follicular carcinoma thyrois in an elderly female: a rare case. J. Clin Diagn Res2017;11(4):ED01-ED02. doi: 10.7860/JCDR/2017/21143.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutomu Y, Fumiko Y, Yuko Y. Large skull metastasis of follicular thyroid carcinoma. BMJ Case Report. 2011 doi: 10.1136/bcr.11.2010.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charandeep SG, Pravin RB, Simran KS, Daljit S, Ravindra KS, Rakesh KG. Massive skull metastasis from follicular thyroid carcinoma-How ignorance can harm your health. MAMC Journal of Medical Sciences. 2017;3:162–165. [Google Scholar]
- 11.Shital K, Ashish P, Sandip S, Bhushan W, Sweta J. Skull metastasis of follicular thyroid carcinoma: a rare case report. WIMJOURNAL. 2016;3(1):52–57. [Google Scholar]
- 12.Akira M, Hideki K, Ryo O. Skull base metastasis from follicular thyroid carcinoma-two case report. Neurol Med Chir. 2010;50:421–425. doi: 10.2176/nmc.50.421. [DOI] [PubMed] [Google Scholar]
- 13.Shen J, Wang S, Zhao X, Shao X, Jiang X, Dai Y, Xu S, Pan X. Skull metastasis from follicular thyroid carcinoma: report of three cases and review of literature. Int J Clin Exp Pathol. 2015;8(11):15285–15293. [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta D, Kumar M, Sen A, Chowdhury JR, Mukhopadhyay M, Mukhopadhyay S, Chowdhury S. Skull metastasis as the presenting feature of mixed medullary and follicular thyroid carcinoma. Notes and Comments, 2014,10(2):299–306.
- 15.Shamim MS, Khuisheed F, Bari ME, Chisti KN, Enam SA. Follicular thyroid carcinoma presenting as solitary skull metastasis: report of two case. J Pak Med Assoc. 2008;58(10):575–577. [PubMed] [Google Scholar]