Abstract
A 68-year-old woman presented with chest pain and loss of consciousness following an anterior wall myocardial infarction. Magnetic resonance imaging of brain showed features of hypoxic brain injury. She subsequently developed memory deficits, drowsiness and behavioral changes. Magnetic resonance imaging of brain done 4 months after ischemic insult showed evidence of delayed posthypoxic leukoencephalopathy also known as Grinker's myelinopathy.
Keywords: Hypoxic brain injury, posthypoxic leukoencephalopathy, anoxic leukoencephalopathy, Grinker's myelinopathy
Introduction
Grinker's myelinopathy is a rare condition affecting the cerebral white matter following a hypoxic insult to the brain. It manifests as a delayed neurological deterioration occurring during the recovery from a period of prolonged hypoxia to the brain.
Case report
A 68-year-old woman presented with chest pain followed by loss of consciousness to the emergency department. She was on treatment for hypertension and dyslipidemia. She had a history of myocardial infarction 6 years prior. Vitals on admission showed blood pressure of 80/62 mm Hg, pulse rate of 120 beats per minute and blood oxygen saturation of 85% on room air. ECG was suggestive of anterior wall MI. She was intubated and put on ventilator support. Vasopressor drugs were administered to maintain systolic blood pressure at adequate levels. MRI of the brain after stabilizing the patient showed T2 hyperintensities (Fig. 1) involving the cortical and deep grey matter structures with reduced diffusivity (Figs. 2 and 3) on diffusion-weighted imaging. The distribution of signal abnormalities to gray matter structures of the brain (Fig. 4) was suggestive of hypoxic brain injury. She underwent stenting of left main coronary artery for a high-grade stenosis. She was discharged after 10 days. At the time of discharge, she was alert and oriented with no neurological deficits. Three weeks after discharge, she developed complaints of drowsiness, frequent falls, behavioral changes, and urinary incontinence. She developed memory deficits and was having difficulty in remembering the name of family members. The symptoms gradually progressed to complete unresponsiveness over a duration of 3 months.
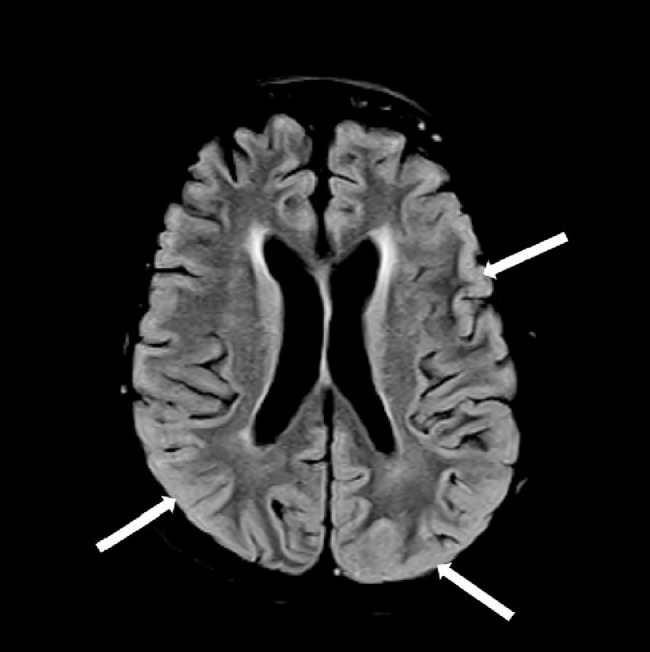
Fig. 1.
T2 FLAIR image showing subtle diffuse hyperintensity of cortex (white arrows).
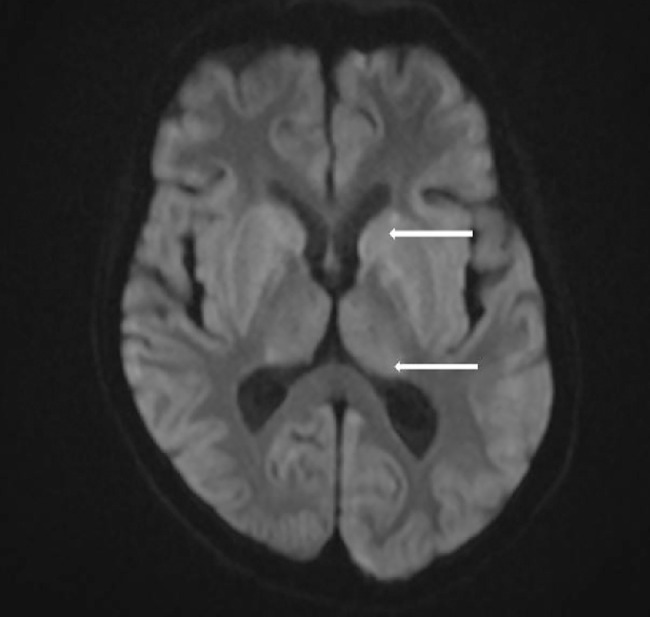
Fig. 2.
Diffusion-weighted imaging showing restricted diffusion (white arrows) of basal ganglia and thalamus.
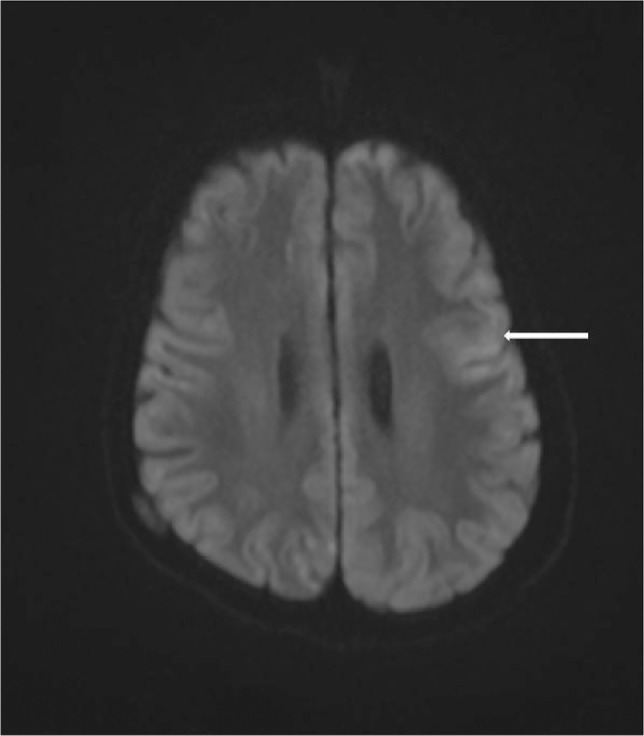
Fig. 3.
Diffusion-weighted imaging showing cortical swelling with restricted diffusion in left frontal lobe (white arrow).
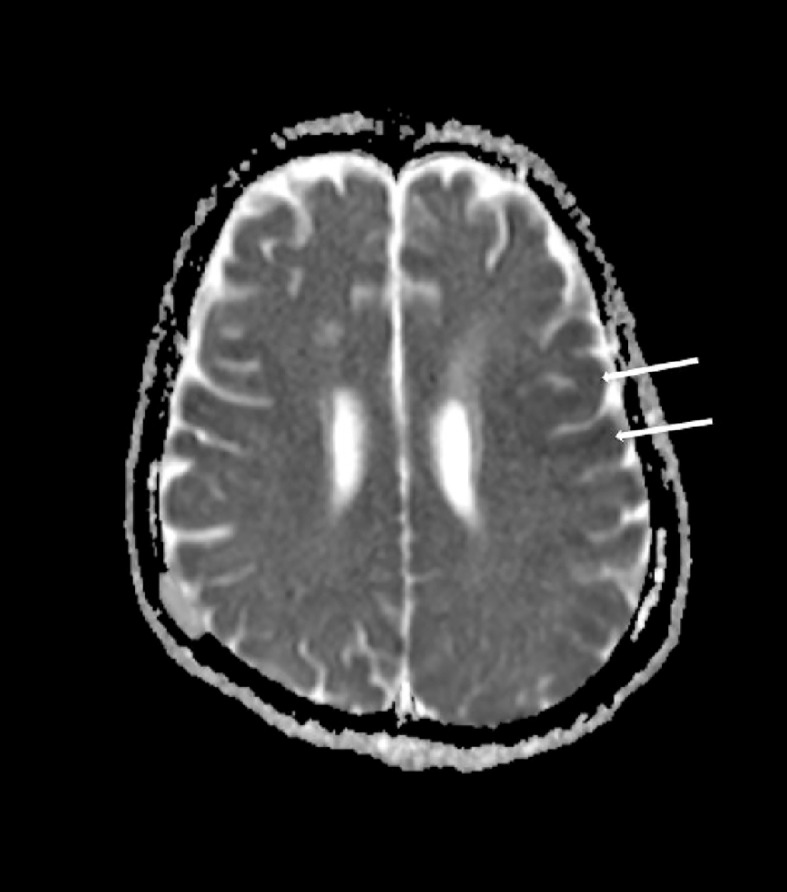
Fig. 4.
ADC map showing areas of restricted diffusion in cortex (white arrows).
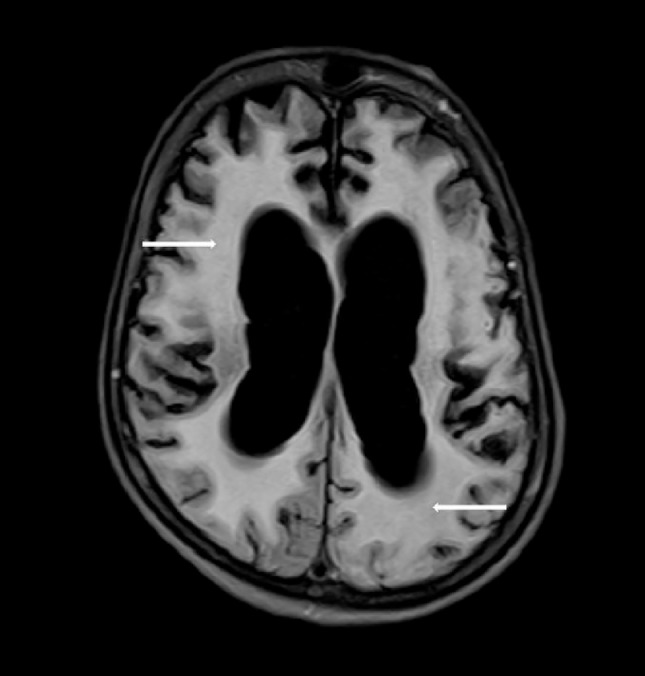
Following multiple episodes of seizures which required hospital admission, an MRI of brain was requested. MRI revealed marked T2 hyperintensity of the supratentorial white matter (Fig. 5) with severe generalized cerebral atrophy in comparison to the scan done 4 months prior. T1-weighted images of brain revealed loss of normal T1 signal of the supratentorial white matter in addition to volume loss (Figs. 6 and 7). She was discharged to a hospice facility and was followed up on outpatient basis. No significant improvement in sensorium or motor functions was noted till her last follow-up visit 5 months after discharge.
Fig. 5.
T2 FLAIR image after four months showing extensive white matter hyperintensity (white arrows) with generalized cerebral atrophy.
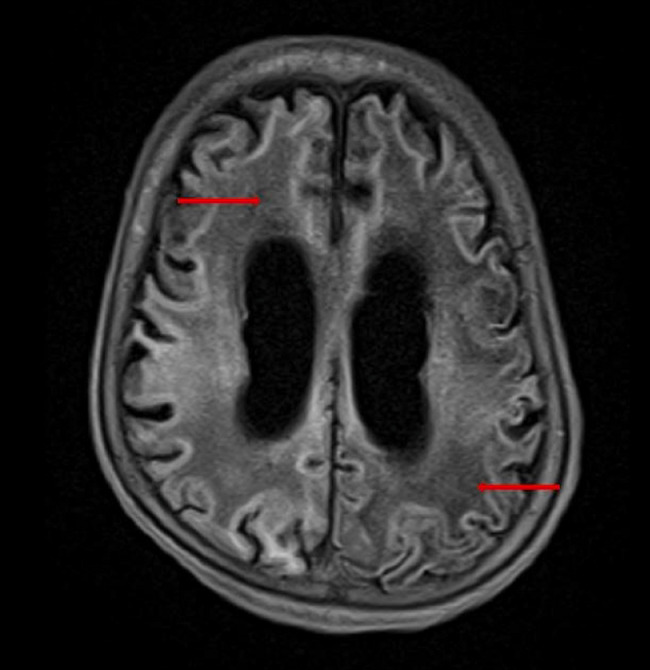
Fig. 6.
T1-weighted image shows abnormal hypointense appearance of white matter (red arrows) suggesting myelin loss.
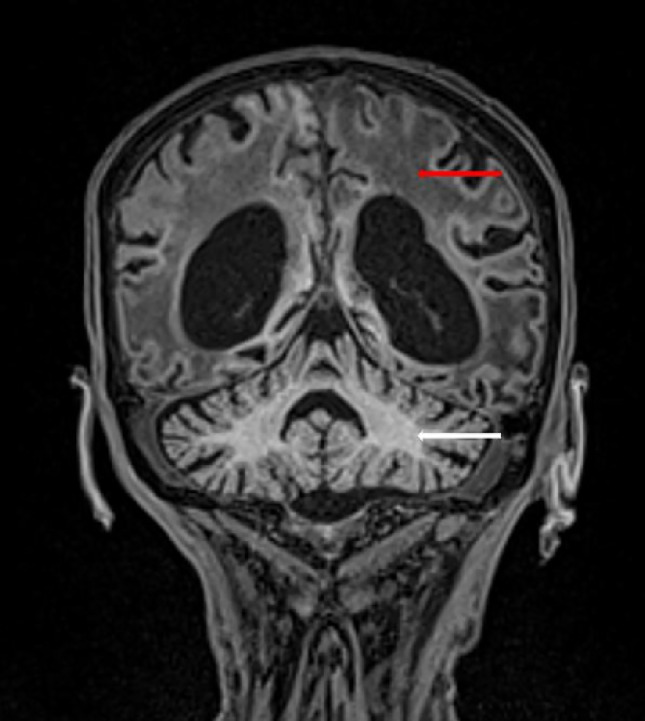
Fig. 7.
Coronal T1-weighted image obtained 4 months after initial ischemic insult showing extensive supratentorial white matter T1 hypointensity (red arrow) and atrophy in comparison to the normal white matter in the cerebellum (white arrow).
Discussion
Grinker's myelinopathy, also known as delayed posthypoxic leukoencephalopathy, is a rarely reported sequalae to hypoxic brain injury [1].
This condition is described as a delayed neurological deterioration occurring in a recovering patient of hypoxic brain injury. The patients may present with complaints similar to parkinsonism or akinetic mutism. The altered behavior, psychomotor retardation may progress to a state of complete unresponsiveness [2,3].
MRI of the brain typically shows diffuse hyperintensity of cerebral white matter with varying degrees of cerebral atrophy depending on the duration from the initial hypoxic event [4]. This pattern of involvement is distinct from acute hypoxic-ischemic injury, which involves the cortical and deep grey matter structures. Mild cases will show sparing of the subcortical U-fibers. The imaging features are often observed in patients suffering from neurocognitive impairments after carbon monoxide poisoning [5].
The cause of delayed white matter changes is considered to be due to the apoptosis of oligodendrocytes responsible for myelin production. The delayed presentation of white matter changes relates to the half-life of myelin-related proteins. Decreased serum arylsulfatase-A levels is noted in some patients of Grinker's myelinopathy, suggesting its role as a predisposing factor [1].
There is no definite treatment for this condition. Majority of cases will follow of a self-limited course with gradual resolution of symptoms [6]. Severity of the index injury and the extent of white matter involvement on MRI aids in prognostication of neurocognitive recovery.
Conclusion
Grinker's myelinopathy is an uncommon sequalae of hypoxic brain injury. Diffuse white matter hyperintensity and cerebral atrophy seen in MRI with the history of an index hypoxic event aids in the diagnosis of this rare entity.
Patient consent
Written informed consent was obtained from the patient's relative and primary caregiver.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Balan S., Gupta K., Balasundaram P., Jadon R. Reversible hypoxic brain injury: the penumbra conundrum of Grinker. BMJ Case Rep. 2019;12(4) doi: 10.1136/bcr-2018-228670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shprecher D.R., Flanigan K.M., Smith A.G., Smith S.M., Schenkenberg T., Steffens J. Clinical and diagnostic features of delayed hypoxic leukoencephalopathy. J Neuropsychiatry Clin Neurosci. 2008;20(4):473–477. doi: 10.1176/jnp.2008.20.4.473. [DOI] [PubMed] [Google Scholar]
- 3.Lee M.S., Marsden C.D. Neurological sequelae following carbon monoxide poisoning clinical course and outcome according to the clinical types and brain computed tomography scan findings. Mov Disord. 1994;9(5):550–558. doi: 10.1002/mds.870090508. [DOI] [PubMed] [Google Scholar]
- 4.Zamora C.A., Nauen D., Hynecek R., Ilica A.T., Izbudak I., Sair H.I. Delayed posthypoxic leukoencephalopathy: a case series and review of the literature. Brain Behav. 2015;5(8):e00364. doi: 10.1002/brb3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazo J., Mukhtar E., Mazo Y., Nagaraj A., Mantello M.T. Delayed brain injury post carbon monoxide poisoning. Radiol Case Rep. 2020;15(10):1845–1848. doi: 10.1016/j.radcr.2020.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapeantong T., Poungvarin N. Delayed encephalopathy and cognitive sequelae after acute carbon monoxide poisoning: report of a case and review of the literature. J Med Assoc Thailand. 2009;92(10):1374–1379. [PubMed] [Google Scholar]