Abstract
Mature Cystic Ovarian Teratoma (MCOTs) are the most common benign ovarian neoplasms, representing around 20% of all ovarian neoplasms with middle age women representing the majority of patients. They usually include two germ cell layers being well differentiated and rarely they demonstrate malignant transformation with squamous cell carcinoma being the most common malignant transformation. In our case, we report an interesting case of a 45 years old lady who was unfortunate to have a MCOT with malignant degeneration and distant metastasis. We concluded that MCOT malignant degeneration is rare, however, if not caught early, mortality can be high.
MCOT, Ovary, Small Bowel, Teratoma, Collision tumor
Introduction
Mature Cystic Ovarian Teratoma (MCOT) is one of the 3 types of Ovarian Teratomas, which is the most common Germ Cell Tumors of the Ovaries [1]. MCOT are a slow growing tumor containing at least two germ cell layers, with most of the tumors containing an ectoderm layer (100-99%), and to a lesser extent a mesodermal (93-73%) and endodermal layers (71-32%) [2], [3].
Malignant transformation of MCOT is uncommon and reported to be around 1-3% of all MCOTs with different histological types of malignant transformations. Squamous cell carcinoma (SCC) represents the majority of these malignant transformations (80%), while Adenocarcinoma, Malignant Melanoma and SCC representing the remainder [4], [5].
Here, we report a case of bilateral MCOT with malignant SCC transformation and subsequent small bowel and abdominal wall metastasis. In addition to an Incidental serous cystadenoma collision tumor.
Case report
45-year-old lady with no significant past medical or surgical history, came to our Emergency Department complaining of abdominal pain and distension for around 2 months with increasing girth, associated with fever, night sweats, vomiting and un-intentional loss of weight.
She underwent a CT scan with IV and oral contrast and showed bilateral Ovarian Teratomas measuring around 6.2 × 5.6 CM on the right side, and 8.5 × 9.7 CM on the left side. Both had small amounts of calcifications, with moderate amounts of soft tissues and fat. However, the right lesion showed a small enhancing soft tissue nodule.
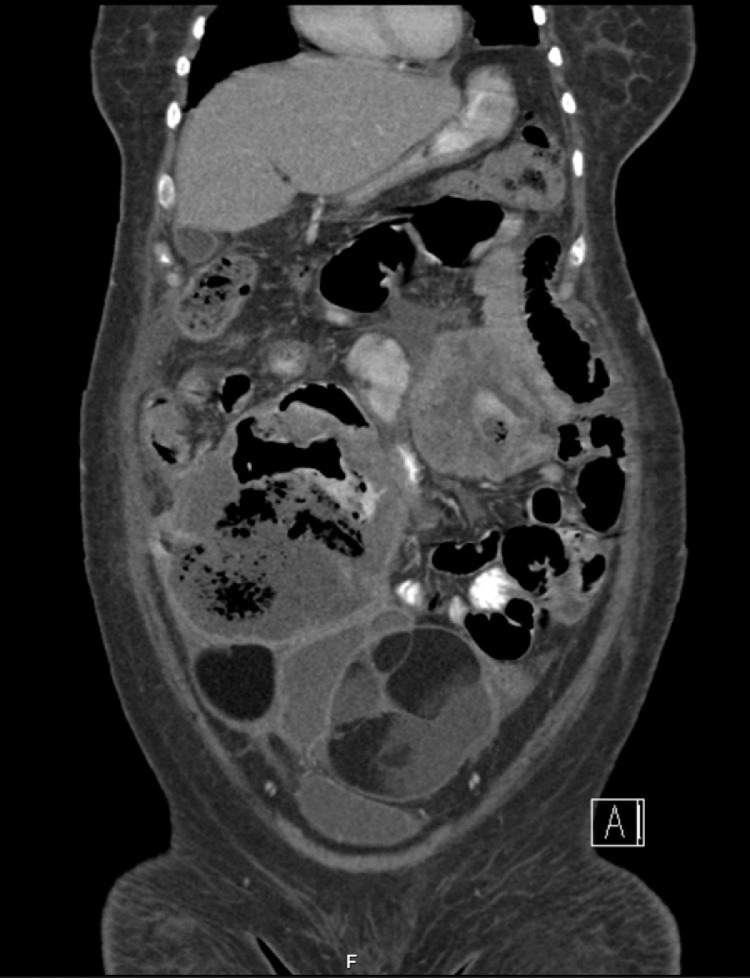
Additionally, there were multiple small bowel loops showing aneurysmal dilation and perforations with mottled material within the affected small bowel loops (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
a) Massively dilated (aneurysmal) and irregularly thickened small bowel loop, with a contained small rupture. (Fig-1b) Another segment of small bowel with similar features. (Fig-1c) Bilateral large will defined adnexal heterogeneous masses with fat, soft tissue and fluid components, in keeping with MCOT. (Fig-1d) Small bowel loop separating the two small bowel lesions.
Fig. 2.
Bilateral adnexal lesions showing different densities of fat and fluid with enhancing septations. The metastatic small bowel segments are seen.
Fig. 3.
Enhancing component noted at the right MCOT proved to be malignant histologically.
In OR, the surgical team did bilateral oophorectomy, they had an incidental finding of a right ovarian cystic mass as well which was resected and sent for histopathology. They resected the dilated and perforated small bowel loops after doing adhesiolysis between the affected bowel loops and the abdominal wall.
After resection, inspection revealed the resected small bowel loops contained a large amount of hair. Since there was no history of past psychiatric illness and no history of hair ingestion, it's most likely secondary to either direct micro-invasion from the MCOT or secondary to metastatic deposits.
Histopathology of the right ovarian teratoma showed a SCC arising from a right ovarian teratoma with metastasis to the abdominal wall and the small bowel Fig. 4.
Fig. 4.
a, d) H&E stained sections showing infiltrative malignant squamous islands. (Fig-2b) the infiltrative malignant squamous islands are seen involving wall of the teratoma. (Fig-2c) as well as the wall of small intestine.
The 2nd right incidentally found Ovarian cystic mass proved to be a Serous Cystadenoma, while the left ovarian biopsy showed a mature ovarian teratoma Fig. 5 .
Fig. 5.
a, b) H&E stained section from right ovarian cyst showing ciliated columnar epithelial lining.
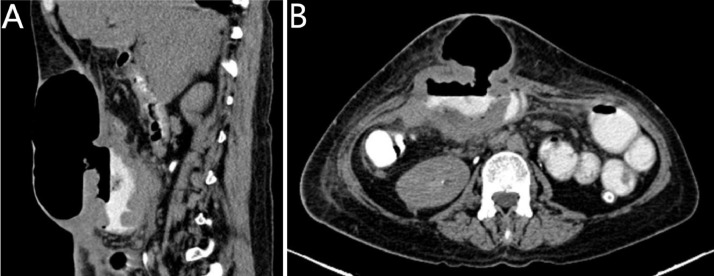
Later on, the patient underwent another CT scan post OP which revealed a small hypodense peripherally enhancing anterior abdominal wall lesion that was mistaken for a postoperative complication in the form of an abscess. However, a later scan showed a marked increase in size of the lesion which started to invade the surrounding structures and caused a similar picture of aneurysmal dilatation of the adjacent small bowel and omental deposit .Fig. 6
Fig. 6.
a) Tumor recurrence involving a small bowel segment post resection. The involved small bowel segment measures approximately 10 cm in length and demonstrate aneurysmal dilatation and marked circumferential wall thickening with associated large fistula extending to the anterior abdominal subcutaneous tissue, where there is a large collection containing air and small amount of contrast material.(Fig-4b) better demonstrated on axial images.
Discussion
MCOT is the most common benign Ovarian neoplasm with 15% percent of cases presenting bilaterally. Patients with malignant degeneration of MCOT were predominantly within their 5th decade. Most patients with malignant degeneration presented with stage I FIGO, while only a minority of patients presented with stage IV 4.4%, the overall 5 years survival rate was 0% [5].
The vast majority of cases of metastasis reported as metastasis to the adjacent pelvic organs. Presentation with a distal metastasis is a rare presentation and associated with high mortality, metastasis with perforation into the bowel has been reported even less mostly due to the complications associated with perforation of a hollow viscus into the peritoneum and the associated increase in mortality [6].
In addition, the majority of Small Bowel metastasis is Adenocarcinoma of the uterus, cervix, colon, lung, breast and melanoma. while SCC represents a minor percentage of small bowel metastasis [12].
Although several studies have reported formations of entero-ovarian fistulas along with small bowel obstruction, no studies have reported distal metastasis of MCOT into the small bowel (jejunum) with further malignant degeneration and perforation of the bowel [7]. Furthermore, omental metastasis is extremely rare in patients with MCOT SCC malignant degeneration [8].
Expected imaging features of metastatic bowel SCC is circumferential wall thickening, obstructive mass and perforation. Instead, Aneurismal dilatation of the metastatic bowels in association with circumferential wall thickening was noted, likely due to extensive lymphatic and neurovascular invasion of the bowel wall [12], [13], [14], [15], [16], [17], [18], [19].
On a further note, Collision tumor is a tumor with two coexistent adjacent tumors, but histologically distinct without histologic admixture in the same tissue or organ. While its relatively rare to have a collision tumor of the ovary, common collision tumors of the ovary that are reported in the literature consists of MCOT and a mucinous cystadenoma or cystadenocarcinoma [9], [10]. In a study done by Singh and colleagues, they reported 4 collision tumors all of which included MCOT as a part of it, while the other histological subtype included Mucinous Cystadenoma for two cases, a Serous Papillary Cystadenoma and Serous Papillary Cystadenocarcinoma. Review of the literature showed that several other collision tumors of the ovary exist that don't include MCOT as part of them [11] ,.
Conclusion
MCOT with SCC malignant degeneration and metastasis to adjacent structures is not uncommon, however distant metastasis to the bowel is extremely uncommon especially when presenting as aneurysmal dilatation. Care must be taken when evaluating MCOT looking for enhancing nodule and/or soft tissue masses, as these can indicate a malignant transformation.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2021.07.073.
Appendix. Supplementary materials
References
- 1.Rha SE, Byun JY, Jung SE, Kim HL, Oh SN, Kim H. Atypical CT and MRI manifestations of mature ovarian cystic teratomas. American Journal of Roentgenology. 2004;183(3):743–750. doi: 10.2214/ajr.183.3.1830743. Sep. [DOI] [PubMed] [Google Scholar]
- 2.Caruso PA, Marsh MR, Minkowitz S, Karten G. An intense clinicopathologic study of 305 teratomas of the ovary. Cancer. 1971;27(2):343–348. doi: 10.1002/1097-0142(197102)27:2<343::aid-cncr2820270215>3.0.co;2-b. Feb. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell WJ, Dockerty MB, Masson JC, Mussey RD. Dermoid cysts of the ovary: their clinical and pathologic significance. American journal of obstetrics and gynecology. 1946;51(2):151–172. doi: 10.1016/s0002-9378(16)39889-1. Feb 1. [DOI] [PubMed] [Google Scholar]
- 4.Rim SY, Kim SM, Choi HS. Malignant transformation of ovarian mature cystic teratoma. International Journal of Gynecologic Cancer. 2006;16(1) doi: 10.1111/j.1525-1438.2006.00285.x. Jan 1. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Zhang Q, Zhang S, Dong R, Sun C, Qiu C. Squamous cell carcinoma transformation in mature cystic teratoma of the ovary: a systematic review. BMC cancer. 2019;19(1):217. doi: 10.1186/s12885-019-5393-y. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitui AH, Fujita R, Sugata F, Kienebuchi M, Suzuki K, Sagawa F. A case of ovarian dermoid cyst with malignant transformation perforated into the rectosigmoid colon and small intestine. Endoscopy. 1983;15(05):331–333. doi: 10.1055/s-2007-1021548. Sep. [DOI] [PubMed] [Google Scholar]
- 7.Esterson YB, Gaballah M, Grimaldi GM, Raj MH, Pellerito JS. Ovarian dermoid cyst complicated by small bowel obstruction, entero-ovarian fistula formation, and malignant degeneration. Clinical Imaging. 2019 Jul 1;56:47–51. doi: 10.1016/j.clinimag.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Mandal S, Badhe BA. Malignant transformation in a mature teratoma with metastatic deposits in the omentum: a case report. Case reports in pathology. 2012;2012 doi: 10.1155/2012/568062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bige O, Demir A, Koyuncuoglu M, Secil M, Ulukus C, Saygili U. Collision tumor: serous cystadenocarcinoma and dermoid cyst in the same ovary. Archives of gynecology and obstetrics. 2009;279(5):767–770. doi: 10.1007/s00404-008-0781-6. May 1. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Kim YJ, Park BK, Cho JY, Kim BH, Byun JY. Collision tumors of the ovary associated with teratoma: clues to the correct preoperative diagnosis. Journal of computer assisted tomography. 1999;23(6):929–933. doi: 10.1097/00004728-199911000-00017. Nov 1. [DOI] [PubMed] [Google Scholar]
- 11.Singh AK, Singh M. Collision tumours of ovary: a very rare case series. Journal of clinical and diagnostic research: JCDR. 2014;8(11):FD14. doi: 10.7860/JCDR/2014/11138.5222. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwivedi R.C., Kazi R., Agrawal N., Chisholm E., Rose S.S., Elmiyeh B. Comprehensive review of small bowel metastasis from head and neck squamous cell carcinoma. Oral oncology. 2010;46(5):330–335. doi: 10.1016/j.oraloncology.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Ono R., Ogino H., Kawachi J., Shimoyama R., Kashiwagi H., Isogai N. Small intestinal metastases from esophageal carcinoma presenting as a perforation: A case report and review of the literature. International journal of surgery case reports. 2018;48:104–108. doi: 10.1016/j.ijscr.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mony S., Patel K., Taunk P. Metastatic Squamous Cell Carcinoma to the Small Bowel Masquerading as Crohn's Disease: A Diagnostic Dilemma: 2654. American Journal of Gastroenterology. 2019;114:S1460. S1461. [Google Scholar]
- 15.Dwivedi R.C., Kazi R., Agrawal N., Chisholm E., Rose S.S., Elmiyeh B. Comprehensive review of small bowel metastasis from head and neck squamous cell carcinoma. Oral oncology. 2010;46(5):330–335. doi: 10.1016/j.oraloncology.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Abdulmajed M., Ghalib A., Mohamed M., Marsh P. Intestinal metastasis from primary epidermoid anal carcinoma in a 34 year old male presented with acute bowel obstruction. Journal of Surgical Case Reports. 2012;2012(2) doi: 10.1093/jscr/2012.2.1. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuridullah R., Kaur P., Estifan E., Sanchez J., Nanavati S., Singhal M. Anal squamous cell carcinoma with metastasis to duodenum causing duodenal stricture and gastric outlet obstruction. AME Case Reports. 2019;3 doi: 10.21037/acr.2019.07.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X., Wang Z., Zhang Z., Liu Y., Huang J. Postoperation of cervical cancer with intestine metastasis: a case report and literature review. World journal of surgical oncology. 2015;14(1):p.2. doi: 10.1186/s12957-015-0759-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di J.Z., Peng J.Y., Wang Z.G. Prevalence, clinicopathological characteristics, treatment, and prognosis of intestinal metastasis of primary lung cancer: a comprehensive review. Surgical Oncology. 2014;23(2):72–80. doi: 10.1016/j.suronc.2014.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.