Highlights
-
•
Optic nerve diameter on MRI correlates with Lumbar puncture opening pressure.
-
•
There is a significant difference in optic nerve diameter on MRI between IIH patients and control patients.
-
•
There is a reverse regression relationship between pituitary height and lumbar puncture opening pressure.
-
•
Receiver operator curve (ROC) analysis showed high accuracy for optic nerve diameter in differentiating between IIH and control groups.
-
•
ROC analysis also showed moderate accuracy for Neck fat thickness and low accuracy for Meckel’s cave diameter and for pituitary height measurement.
Keywords: Idiopathic intracranial hypertension, Brain MRI, Optic nerve diameter, Pituitary height, Meckel's cave diameter, Neck fat thickness
Abstract
Purpose
Aim of this study was to develop quantitative parameters for diagnosing Idiopathic Intracranial Hypertension (IIH) using brain MRI scans.
Methods
This is a case control study with 48 cases and 192 matched controls. Optic nerve diameter (OND), Pituitary height (PH), Meckel’s cave diameter (MCD), and Neck fat thickness (NFT) were measured for both groups. Consequently, means were obtained for the different parameters in both groups with subsequent establishment of best cutoffs using Receiver Operator Curve (ROC) analysis.
Results
For IIH patients the means of OND, PH, MCD, and NFT were 6.2 mm, 3.9 mm, 5 mm, 1.4 cm, respectively while for controls the means were 4.6 mm, 4.5 mm, 4.3 mm, and 0.8 cm with statistical significance between the two groups. ROC analysis showed the cutoff points with best accuracy for the above parameters in diagnosing IIH to be 5.4 mm for OND with sensitivity of 0.77 and specificity of 0.85 representing high accuracy, while for PH a cutoff point of 3 mm showed low accuracy with sensitivity of 0.54 and specificity of 0.7, and a MCD cutoff of 4.5 mm also showed low accuracy with sensitivity of 0.6 and specificity of 0.59, meanwhile a cutoff point of 1.1 cm for NFT was moderately accurate with sensitivity of 0.70 and specificity of 0.81.
Conclusion
Statistical difference in the means for OND, PH, MCD, and NFT between IIH patients and controls is established. Also, we provide cut off points for these parameters to diagnose IIH on brain MRI.
1. Introduction
Idiopathic intracranial hypertension (IIH), formerly known as pseudotumor cerebri, is a syndrome of raised intracranial pressure with no identifiable etiology [1]. IIH typically occurs in young and overweight female patients who develop symptoms and signs of raised intracranial pressure, including headache, visual disturbances, pulsatile tinnitus, and papilledema [2]. The diagnosis of IIH is made by elevated CSF opening pressure without evidence of intracranial mass lesion, hydrocephalus or dural venous thrombosis on neuroimaging along with normal CSF composition [3].
Despite the large evolution of neuroimaging in the last decades, its role has been consistently focusing in excluding any secondary causes of intracranial pressure elevation rather than reaching or excluding the diagnosis of IIH. However, many signs in cross sectional imaging have been reported to be associated with IIH, including flattening of the posterior aspect of the globes, protrusion of the intraocular portion of the optic nerve, vertical tortuosity of the optic nerve, distension of the optic nerve sheaths, enhancement of the optic nerve head, partial empty Sella turcica, slit-like ventricles, tight subarachnoid spaces [2,[4], [5], [6], [7]]. With exception of idiopathic intracranial hypertension without papilledema (IIHWP), a rare form of IIH, these signs are not included in the most widely accepted criteria for diagnosis of IIH which is the modified Dandy’s criteria.
Many of these imaging signs remain subjective, introducing the concern of inter-observer variability, and the specificity and sensitivity are reported with wide range of values in many studies. The aim of this study is to reevaluate the accuracy of some of these imaging signs, provide objective comparison and cutoff values for measurable findings (optic nerve diameter, pituitary height, and Meckel’s cave diameter) and to demonstrate correlative assessment between measurement that can be obtained in MR scan with the value of CSF opening pressure which is the gold standard for diagnosing IIH. Also, the Neck fat thickness was included in the evaluation as imaging indicator of weight status of the patient.
2. Materials and methods
A retrospective, case-control study was conducted at Tertiary University Hospital, and approved by the institutional review board (IRB) with no consent form needed from the patients.
2.1. Cases
Cases were collected using our electronic medical record database search system, using the following keywords; “idiopathic intracranial hypertension”, “IIH” and “pseudotumor cerebri”, between the period of May 2015 to February 2019, the results revealed 79 patients. The medical records for each case were reviewed, and only patients who met the modified Dandy’s criteria of IIH were included and whom medical evaluation documented the results of optic disc examination, CSF opening pressure measurement, and MR scanning. Total of 48 patients of the initial 79 patients were included in the study with 31 patients excluded: 26 patients had no records regarding the opening pressure, and 5 patients had no MR scan. Also, information regarding the medical history, medications, presenting symptoms, weight and treatment were collected from the records.
2.2. Control
Control group was retrieved from our institution MR image archive, by searching for “headache” in the MRI reports (presenting symptoms are included in the reports). Total of 2015 patients were obtained. Initially, subjects from the IIH cases and patients with mass occupying lesions, dural venous thrombosis, hydrocephalus and brain edema were excluded. After age- and gender matching was done, a random sample of 192 subject was obtained with case:control ratio of 1:4.
2.3. MRI technique
MRI brain scans was performed using 3 T (general electrics) and 1.5 T (ingenia, Philips) MRI units using standard head coils. Our routine brain MRI scan includes axial, sagittal and coronal T1, axial and sagittal T2 FSE, axial FLAIR, SWI, DWI, ADC. Post contrast Brain MRI, MRV TOF, post contrast MRV, MRI of the orbit were not consistently performed among cases, therefore, were not included in the assessment.
2.4. Image analysis
A radiologist, blinded to cases and controls groups and to the medical history, reviewed the images and made evaluation of the signs of the IIH and took assigned measurements of the following parameters:
-
1)
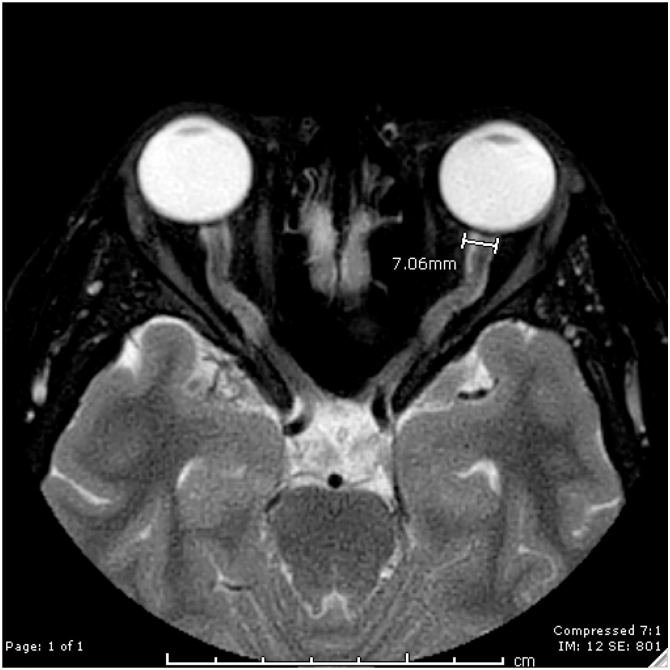
Optic nerve: assessed the presence of vertical tortuosity using sagittal T2WI and assessed distention of the optic sheath using axial T2WI. Transverse diameter of the optic sheath at the left side at its most distal portion were measured and documented in millimeters. (Fig. 1)
-
2)
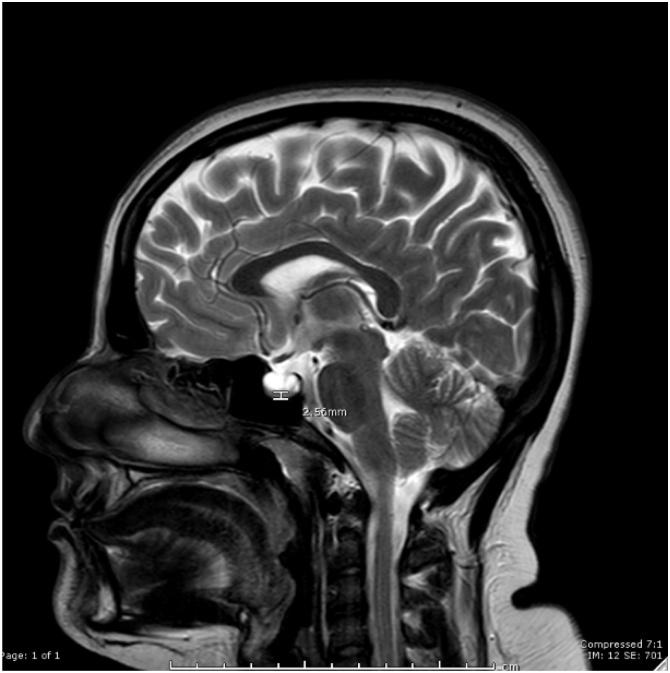
Pituitary height: measurement of the craniocaudal dimensions of the pituitary height was taken at midline using sagittal T2 image and documented in mm. (Fig. 2)
-
3)
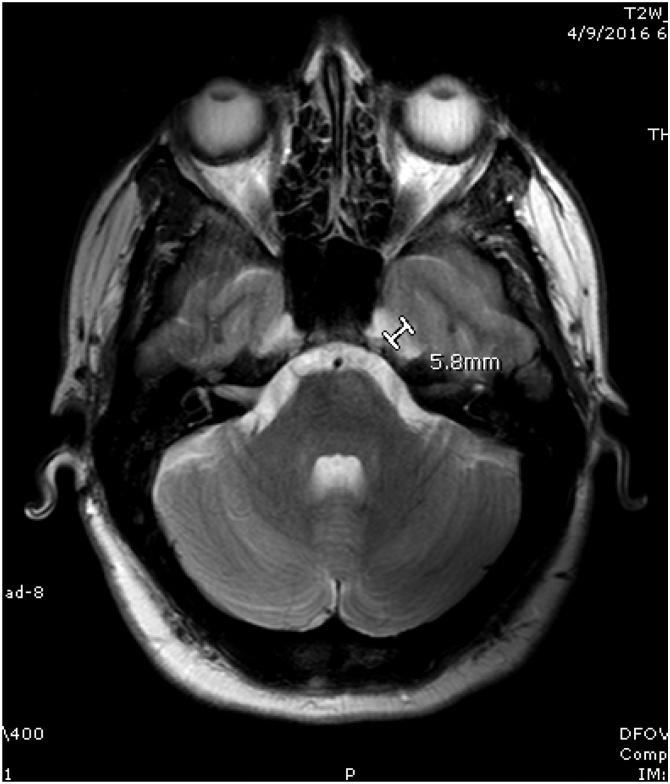
Meckel’s cave: transverse diameter of the Meckel’s cave was taken using axial T2 image and documented in mm. (Fig. 3)
-
4)
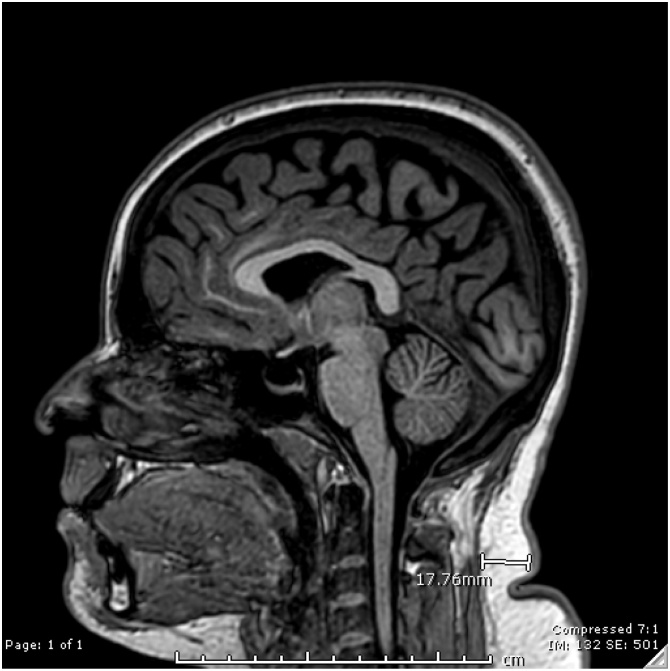
Thickness of the subcutaneous fat of the neck: measurements were taken at the level of C2 using sagittal T1 image and documented in cm. (Fig. 4)
Fig. 1.
Optic Nerve Diameter is shown on this axial T2 image of the orbit for a patient with IIH, as shown the maximal diameter of the optic nerve is 7.1 mm.
Fig. 2.
Pituitary height is shown on this sagittal T2 weighted image for a patient with IIH, as shown the pituitary height is 2.6 mm.
Fig. 3.
Meckel’s cave is shown on this T2 weighted axial brain MRI for a patient with IIH and Meckel’s cave appears widened with a diameter of 5.8 mm.
Fig. 4.
Neck fat thickness is shown on this sagittal T1 weighted MRI of the brain for a patient with IIH, and as shown the neck fat thickness at the level of C2 vertebra measures 1.8 cm.
2.5. Data statistical analysis
Data Statistical analysis was performed using IBM SPSS software version 22. The mean, median and standard deviation were calculated for the cases and controls, and the gender distribution was compared between both groups.
The mean value with T test were performed for both the cases and control on the following variables: optic nerve diameter, pituitary height, Meckel’s cave diameter and the neck fat thickness.
Pearson correlation model analysis was examined for the MR imaging findings in the cases groups and their corresponding opening pressure measurement for the variables of the optic nerve diameter, pituitary height, Meckel’s cave diameter and the neck fat thickness.
Receiver operator curve (ROC) analysis was performed for these variables to set the most accurate cut-off values for the findings that showed statistically significant difference with subsequent calculation of sensitivity and the specificity at these cut-off values.
3. Results
Analysis showed similar distribution of the age the gender between the cases and control groups, with equal value of means and standard deviation for the ages between the cases and control and equal gender distribution, Table (1). Also seen in Table (1) the frequency and grading of papilledema in addition to the frequency of symptoms of presentation.
Table 1.
Demographic, clinical, and MRI quantitative parameters for patients with idiopathic intracranial hypertension and control group.
| Patients | Control | P-value | ||
|---|---|---|---|---|
| Number | 48 | 192 | – | |
| Gender | Female | 40 (83 %) | 160 (83 %) | – |
| Male | 8 (17 %) | 32 (17 %) | – | |
| Age (years) | 36.0, SD= 13.7 | 36.0, SD=13.6 | 1.000 | |
| Opening pressure (cmH2O) | 33, SD = 17 | – | – | |
| Fundoscopic findings | No papilledema | 7 | – | – |
| Grade I | 5 | – | – | |
| Grade II | 18 | – | – | |
| Grade III | 6 | – | – | |
| Grad IV | 9 | – | – | |
| Optic nerve atrophy | 3 | – | – | |
| Symptoms | Headache | 45 (94 %) | – | – |
| Visual symptoms | 36 (75 %) | – | – | |
| Nausea | 9 (19 %) | – | – | |
| Photophobia/phonophobia | 10 (21 %) | – | – | |
| Comorbidities | 13 (27 %) | – | – | |
| Optic sheath diameter (mm) (Mean,Median,SD) | 6.15, 6.2.5 SD=1.4 | 4.58,4.6 SD=0.8 | 0.000 | |
| Pituitary height (mm) (Mean,Median,SD) | 3.92,3.65 SD=1.9 | 4.51, 4.7 SD= 1.6 | 0.030 | |
| Meckel’s cave (mm) (Mean,Median,SD) | 4.97, 4.85 SD = 1.3 | 4.25, 4.2 SD = 1.3 | 0.010 | |
| Neck fat thickness (cm) (Mean,Median,SD) | 1.35, 1.3 SD=0.6 | 0.82, 0.80 SD=0.3 | 0.000 | |
The mean values for optic nerve diameter, pituitary height, Meckel’s cave diameter, and neck fat thickness were compared for the cases and control groups, as demonstrated in Table (1) the results showed statistically significant difference in the means of the optic nerve diameter (case = 6.2 mm, control = 4.6 mm, p value = 0.000), the pituitary height (case = 3.9 mm, control = 4.5 mm, p value = 0.030), the Meckel’s cave diameter (case = 5 mm, Control = 4.3 mm, p value = 0.010), and neck fat thickness (case = 1.4 cm, control = 0.8 cm, p value = 0.000).
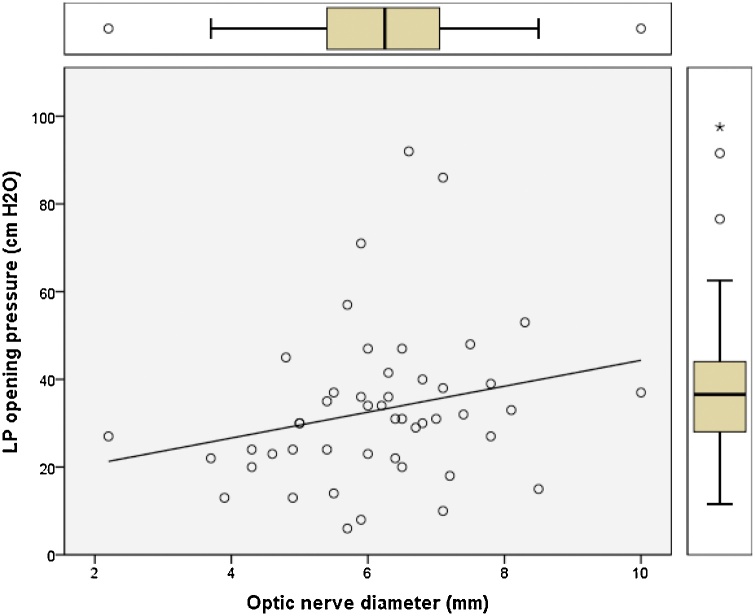
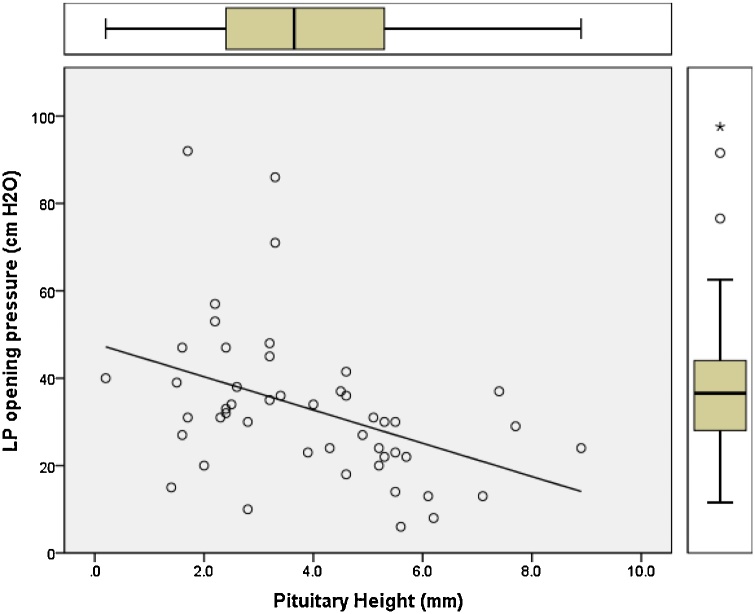
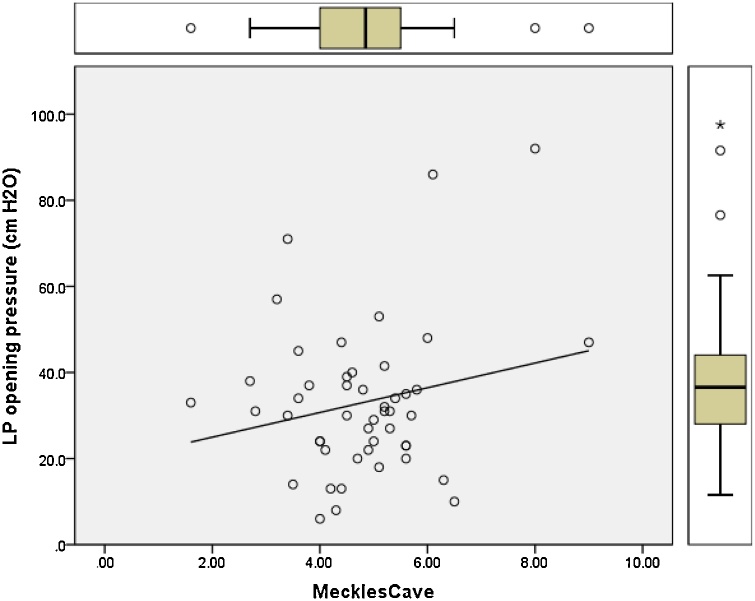
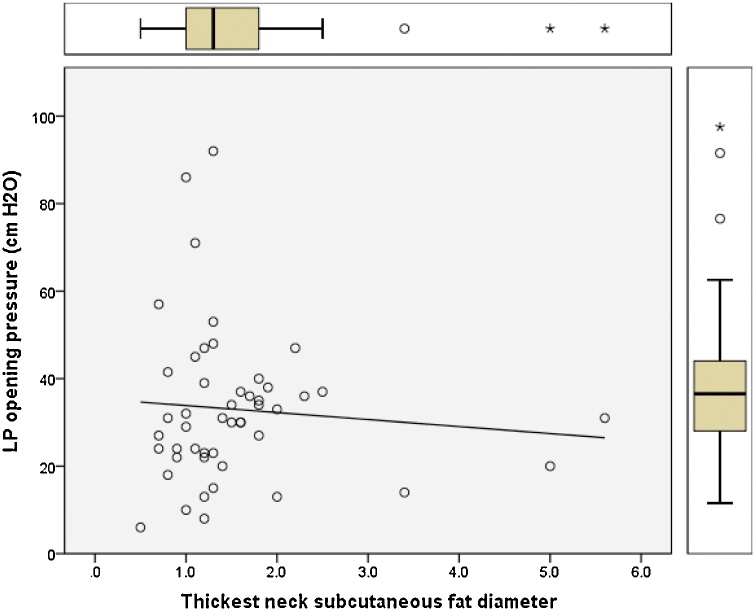
Pearson correlation regression analysis was performed and plotted to model the relation between the opening pressure values measured by lumbar puncture and the changes in optic nerve diameter, pituitary height, Meckel’s cave, and neck fat thickness (Fig. 5, Fig. 6, Fig. 7, Fig. 8). Results showed positive linear regression for the optic nerve diameter (R value = 0.23) and for Meckel’s cave diameter (R value = 0.21). On the other hand, the parameters of pituitary height showed negative (inverse) linear regression (R value= -0.41), while the neck fat thickness showed no significant linear regression relationship (R value = - 0.09).
Fig. 5.
Regression plot of optic nerve diameter versus LP opening pressure for patients diagnosed with IIH showing positive linear relationship (R value = 0.23).
Fig. 6.
Regression plot of Pituitary height versus LP opening pressure for patients with IIH showing negative linear regression relationship (R value = -0.41).
Fig. 7.
Regression plot of Meckel’s cave diameter versus LP opening pressure for patients with IIH showing positive linear relationship (R value = 0.21).
Fig. 8.
Regression plot of neck fat thickness versus LP opening pressure for patients with IIH showing no significant linear relationship (R value = -0.09).
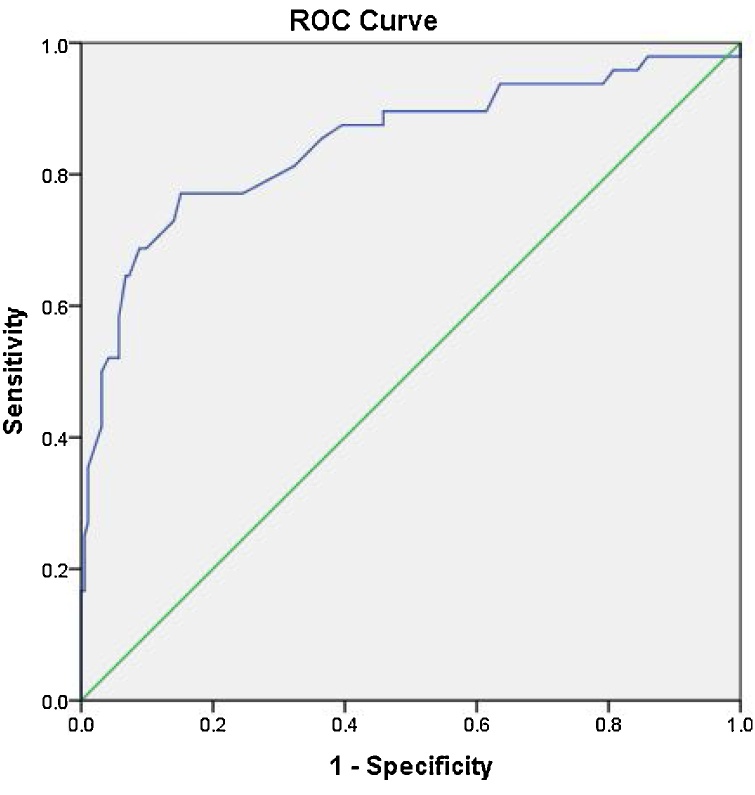
Receiver Operator Curve (ROC) analysis for the optic nerve diameter, pituitary height, Meckel’s cave, and neck fat thickness are shown in the Fig. 9, Fig. 10, Fig. 11, Fig. 12. For the optic nerve diameter, the area under the curve was 0.85. As suggested by the curve, a cut off point at 5.4 mm for the optic nerve diameter could be of diagnostic potential (highest accuracy) with a sensitivity of 77 % and specificity of 85 % (Fig. 9).
Fig. 9.
Receiver operator curve for optic nerve diameter with area under the curve of 0.85, the most accurate cut off value between cases and controls was 5.4 mm with sensitivity of 77 % and specificity of 85 %.
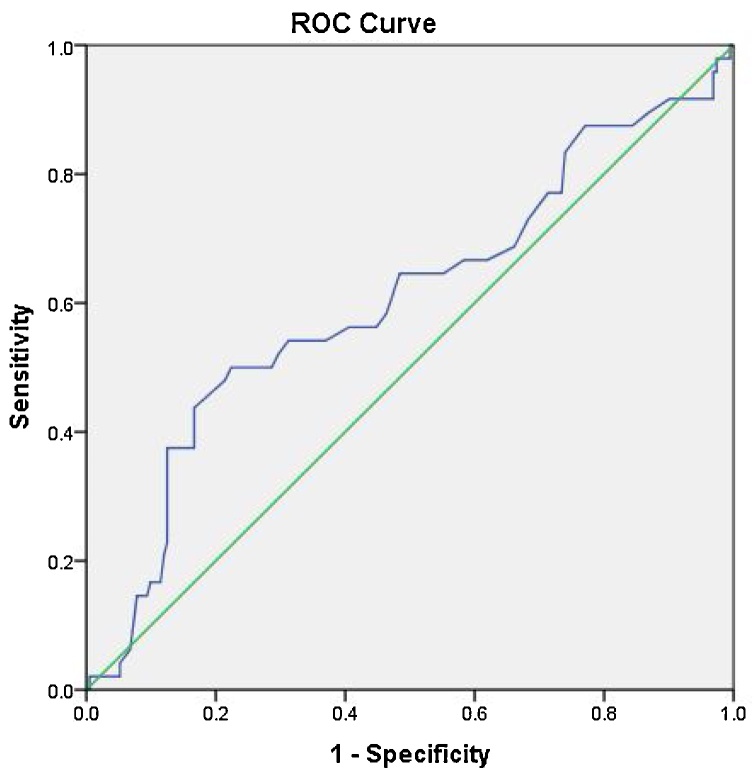
Fig. 10.
Receiver operator curve for pituitary height with area under the curve of 0.6, the most accurate cut off value between cases and controls was 3 mm with sensitivity of 54 % and specificity of 70 %.
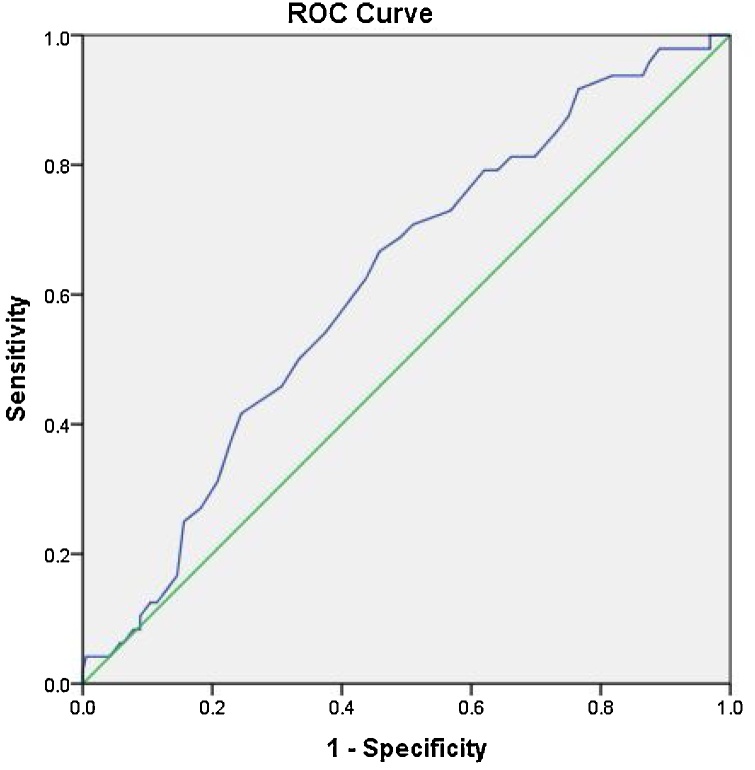
Fig. 11.
Receiver operator curve for Meckel’s cave diameter with area under the curve of 0.62, the most accurate cut off value between cases and controls was 4.5 mm with sensitivity of 60 % and specificity of 59 %.
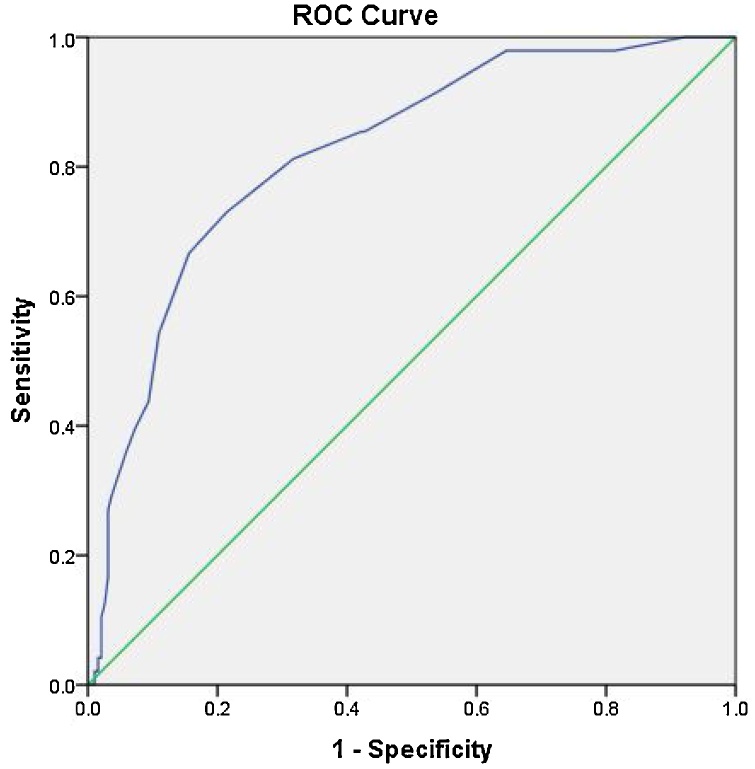
Fig. 12.
Receiver operator curve for Neck fat thickness with area under the curve of 0.82, the most accurate cut off value between cases and controls was 1.1 cm with sensitivity of 70 % and specificity of 81 %.
ROC analysis for the pituitary height showed that the area under the curve was 0.60. As suggested by the curve, cut off point at 3 mm was the most accurate cut-off power, however showing low accuracy with a sensitivity of 54 % and specificity of 70 % (Fig. 10).
ROC analysis Meckel’s cave diameter showed that the area under the curve was 0.62. As suggested by the curve, a cut-off point at 4.5 mm was the most accurate cut-off point, but showed low accuracy with a sensitivity of 60 % and specificity of 59 %.
ROC analysis for the neck fat thickness showed that the area under the curve was 0.82. As suggested by the curve, cut off point at 1.1 cm was the most accurate cut-off point and showed moderate accuracy with a sensitivity of 70 % and specificity of 81 %.
4. Discussion
Idiopathic intracranial hypertension (IIH) is a poorly understood condition, the hallmark of which is elevation of CSF opening pressure without an identifiable intracranial mass or ventriculomegaly [8]. The chronic elevation in intracranial pressure can be associated with structural changes in the orbit, skull base, brain and transverse sinuses identifiable in diagnostic imaging. This has been historically recognized in skull radiographs as demineralization of the pituitary fossa and in ventriculography as small sized ventricles [9]. With advent of new imaging techniques, more radiologic signs have been reported to be associated with IIH with variable sensitivity and specificity rates ranging between 6 %–72 % [9]. Our work focused on the evaluation of the quantitative parameters seen on brain MRI to diagnose IIH with correlation to opening pressure measurements performed, as it is the current gold standard diagnostic test for IIH.
Morphological alteration of the optic nerve sheath reflects accumulation of CSF under elevated pressure as seen in IIH and other causes of increased intracranial pressure. MRI signs associated with this effect include, vertical tortuosity of the optic nerve sheath, distention of the optic nerve, flattening of the posterior sclera and bulging of the optic disc. The latter reflect optic disc edema depicted in ophthalmologic examination. Normal measurement of optic nerve diameter ranges among literatures between values of 3–5.7 mm on studies utilizing MRI [10]. Our control sample of healthy individuals showed similar results with a mean of 4.6 (SD = 0.8). Variations in optic nerve diameter among healthy individuals were shown to be associated with the variation in BMI and eyeball diameter but not the age or gender [11].
While it is agreed that chronic elevation in ICP (Intracranial Pressure) is associated with distention of optic nerve diameter, which was concordant with our results, the objective correlation with the opening pressure and the diameter of the optic nerve were not studied and unlike for ultrasound, there has been no defined upper limit on MRI available for clinical use. Also, our linear regression model showed mild positive linear correlation with the value of the optic nerve diameter relative to the LP opening pressure in patients with confirmed IIH with R value of +0.23. Our ROC analysis suggested that a diameter of 5.4 mm could be of diagnostic potential with good sensitivity and specificity. A study by j. Hoffmann revealed similar upper limit value (5.50 mm) with similar sensitivity and specificity [12].
The “empty sella” is a term used to describe a spectrum of findings related to the bony sella turcica and pituitary gland, ranging from mild superior concavity of the pituitary gland to apparent absence of the gland and CSF expansion of the bony confines of the sella turcica [13]. Although empty sella turcica is the most commonly described imaging sign in the setting IIH, it is more commonly seen as an incidental imaging finding that, per se, requires no further diagnostic evaluation [13]. Our work focused in the measurement of midsagittal CC dimensions of the pituitary gland in patients with IIH compared with control, which showed statistically significant difference in the mean between both groups (3.92 mm for patients with IIH compared with 4.51 mm for control group) rendering it a helpful parameter in the diagnostic settings.
Regression model (Fig. 6) plotted for pituitary height as a product of opening pressure among patients with IIH has demonstrated inverse linear correlation with R value of -0.41, indicating the higher the LP opening pressure the lower the pituitary height, an observation of useful application in predicting the severity of intracranial pressure in patients with confirmed IIH.
Likely due to a similar pathophysiology as the cause of sellar findings, a variety of skull-base meningoceles and encephaloceles can be seen in the setting of IIH, including widening of the diameter of Meckel’s cave. Enlargement of the Meckel cave as well as frank meningoceles in this location can be found in as many as 10 % of IIH patients, but rarely in normal controls [13]. Our data analysis did show significant difference in mean diameter of Meckel’s cave between IIH group and control group (case = 5 mm, control = 4.3 mm, P = 0.01).Regression analysis also showed positive linear regression between LP opening pressure measurements and Meckel’s cave diameter with an R value = 0.21 which indicates the higher the LP opening pressure the wider the Meckel’s cave diameter. ROC analysis suggested the most accurate (although showing low accuracy) cut-off value to be 4.5 mm with sensitivity of 60 % and specificity of 59 %.
BMI is important risk factor for IIH, with a significantly higher incidence rates of IIH in obese patients [14]. Thickness of the subcutaneous fat of the neck obtained from brain scans, a sign indirectly reflecting the weight status of the patient, was shown to be significantly higher in patients with IIH compared with control, with a mean neck fat thickness of 1.4 cm in IIH patients compared 0.8 cm neck fat thickness in the control group.
ROC curve analysis for neck fat thickness showed area under curve of 0.82 with the most accurate cut-off value at 1.1 cm at which the sensitivity is about 70 % and specificity of 81 % indicating moderate accuracy, this represents a useful observation if combined with the other recognized neuroimaging signs is likely to increase the diagnostic accuracy. Regression analysis between neck fat thickness and LP opening pressure showed no significant relationship with an R value of - 0.09.
Findings in MRV, although not included in our analysis, have been extensively studied recently as a diagnostic sign and proposed as etiological factor with therapeutic possibilities targeting the dural sinus veins. In one of the studies, its shown that transverse sinus stenosis was the most consistent cross sectional imaging sign of IIH seen in 94 % of patients. Reversibility of the stenosis after large volume CSF removal or diversion procedures in some patients with IIH suggests that they may simply be a consequence of raised CSF pressure [15]. On the other hand, clinical improvement and reduction of measured intracranial opening pressure have been observed clinical trials following transverse sinus stenting supporting its causative role [16].
Combining multiple radiological signs of IIH have been shown to improve diagnostic accuracy observed in few studies [17]. However, these findings should be used to raise suspicion for the diagnosis of IIH, either excluding or prompting opening pressure measurement by lumbar puncture, and should be interpreted in the clinical context.
IIH is still posing a diagnostic challenge, and scientific efforts have been done in recognizing the neuroimaging role as a noninvasive method in this regard, in part due to its wide utilization as initial diagnostic test, and due to recent advances in its capabilities in the recent years. Ongoing research has gone beyond the structural cross sectional imaging findings to concentrate on the possible mechanism of the disease, in search of more reliable imaging findings associated with IIH [18].
The significance of our work is that it provides objective parameters in diagnosing IIH using brain MRI given the fact that prior studies concentrated on subjective parameters, subsequently our study shows that optic nerve diameter measurements appear reliable in helping diagnose IIH, on the other hand, neck fat thickness is moderately reliable while pituitary height and Meckel’s cave diameter appear weakly reliable. The impact of our work is that it paves the way to use of the noninvasive brain MRI in diagnosing IIH and hopefully develop a scoring system to diagnose IIH incorporating clinical and imaging findings without the need of invasive procedure of lumbar puncture. Further studies are suggested to help confirm our findings.
5. Conclusion
Our study concludes presence of statistically significant difference in means for the parameters of optic nerve diameter, pituitary height, Meckel’s cave diameter, and neck fat thickness between patient’s with IIH when compared to matched control patients on brain MRI scans. Our study also establishes cutoff points for the above parameters to help noninvasively diagnose IIH.
Ethical statement
This Research is a retrospective study that obtained IRB approval with no informed consent needed.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This research work was funded by the Deanship of Research at Jordan University of Science and Technology.
References
- 1.Juhász J., Lindner T., Riedel C., Margraf N.G., Jansen O., Rohr A. Quantitative phase-contrast MR angiography to measure hemodynamic changes in idiopathic intracranial hypertension. AJNR Am. J. Neuroradiol. 2018;39(April (4)):682–686. doi: 10.3174/ajnr.A5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maralani P.J., Hassanlou M., Torres C., Chakraborty S., Kingstone M., Patel V., Zackon D., Bussière M. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin. Radiol. 2012;67(July (7)):656–663. doi: 10.1016/j.crad.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Saindane A.M., Bruce B.B., Riggeal B.D., Newman N.J., Biousse V. Association of MRI findings and visual outcome in idiopathic intracranial hypertension. AJR Am. J. Roentgenol. 2013;201(August (2)):412–418. doi: 10.2214/AJR.12.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris P.P., Black D.F., Port J., Campeau N. Transverse sinus stenosis is the most sensitive MR imaging correlate of idiopathic intracranial hypertension. AJNR Am. J. Neuroradiol. 2017;38(March (3)):471–477. doi: 10.3174/ajnr.A5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bidot S., Saindane A.M., Peragallo J.H., Bruce B.B., Newman N.J., Biousse V. Brain imaging in idiopathic intracranial hypertension. J. Neuroophthalmol. 2015;35(December (4)):400–411. doi: 10.1097/WNO.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 6.Kim D.H., Jun J.S., Kim R. Measurement of the optic nerve sheath diameter with magnetic resonance imaging and its association with eyeball diameter in healthy adults. J. Clin. Neurol. 2018;14(July (3)):345–350. doi: 10.3988/jcn.2018.14.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saindane A.M., Lim P.P., Aiken A., Chen Z., Hudgins P.A. Factors determining the clinical significance of an “empty” sella turcica. AJR Am. J. Roentgenol. 2013;200(May (5)):1125–1131. doi: 10.2214/AJR.12.9013. [DOI] [PubMed] [Google Scholar]
- 8.Morris P., Black D., Port J., Campeau N. Transverse sinus stenosis is the most sensitive MR imaging correlate of idiopathic intracranial hypertension. Am. J. Neuroradiol. 2017;38(3):471–477. doi: 10.3174/ajnr.A5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bidot S., Saindane A., Peragallo J., Bruce B., Newman N., Biousse V. Brain imaging in idiopathic intracranial hypertension. J. Neuro-ophthalmology. 2015;35(4):400–411. doi: 10.3174/ajnr.A5486. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y., Hao D., Tang G., Zhou R., Pang J., Dong C. High-resolution MRI assessment of optic nerve sheath diameter in adults: optic nerve sheath variation and a new diagnostic tool for intracranial hypertension. Acta Radiologica. 2020 doi: 10.1111/j.1399-6576.2011.02432. p. 028418512096671. [DOI] [PubMed] [Google Scholar]
- 11.Kim D., Jun J., Kim R. Measurement of the optic nerve sheath diameter with magnetic resonance imaging and its association with eyeball diameter in healthy adults. J. Clin. Neurol. 2018;14(3) doi: 10.4236/ojrad.2020.103015. p.345.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann J., Huppertz H., Schmidt C., Kunte H., Harms L., Klingebiel R., Wiener E. Morphometric and volumetric MRI changes in idiopathic intracranial hypertension. Cephalalgia. 2013;33(13):1075–1084. doi: 10.2147/JPR.S60633. [DOI] [PubMed] [Google Scholar]
- 13.Holbrook J., Saindane A. Imaging of intracranial pressure disorders. Neurosurgery. 2017;80(3):341–354. doi: 10.1227/NEU.0000000000001362. [DOI] [PubMed] [Google Scholar]
- 14.Boyter E. Idiopathic intracranial hypertension. J. Am. Acad. Physician Assist. 2019;32(5):30–35. doi: 10.1097/01.JAA.0000554732.85914.91. [DOI] [PubMed] [Google Scholar]
- 15.Maralani P., Hassanlou M., Torres C., Chakraborty S., Kingstone M., Patel V., Zackon D., Bussière M. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin. Radiol. 2012;67(7):656–663. doi: 10.1016/j.crad.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Kalyvas A., Neromyliotis E., Koutsarnakis C., Komaitis S., Drosos E., Skandalakis G.P., Pantazi M., Gobin Y.P., Stranjalis G., Patsalides A. A systematic review of surgical treatments of idiopathic intracranial hypertension (IIH) Neurosurg. Rev. 2021;44(April (2)):773–792. doi: 10.1007/s10143-020-01288-1. [DOI] [PubMed] [Google Scholar]
- 17.Bidot S., Saindane A.M., Peragallo J.H., Bruce B.B., Newman N.J., Biousse V. Brain imaging in idiopathic intracranial hypertension. J. Neuroophthalmol. 2015;35(December (4)):400–411. doi: 10.1097/WNO.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 18.Agid R., Farb R.I., Willinsky R.A., Mikulis D.J., Tomlinson G. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48(August (8)):521–527. doi: 10.1007/s00234-006-0095-y. [DOI] [PubMed] [Google Scholar]