Abstract
The medullary thick ascending limb (mTAL) plays a key role in urinary acid and NaCl excretion. NBCn1 and NBCn2 are present in the basolateral mTAL, where NBCn1 promotes NH4+ shunting. IRBIT and L-IRBIT (the IRBITs) are two powerful activators of certain acid-base transporters. Here we use western blotting and immunofluorescence to examine the effects of multiple acid-base and electrolyte disturbances on expression of NBCn1, NBCn2 and the IRBITs in rat kidney. We also use electrophysiology to examine the functional effects of IRBITs on NBCn1 And NBCn2 in Xenopus oocytes. NH4Cl-induced metabolic acidosis (MAc) substantially increases protein expression of NBCn1 and NBCn2 in the outer medulla (OM) of rat kidney. Surprisingly, NaHCO3-induced metabolic alkalosis (MAlk) and high-salt diet (HSD) also increase expression of NBCn1 and NBCn2 (effect of NaHCO3 > HSD). Moreover, all three challenges generally increase OM expression of the IRBITs. In Xenopus oocytes, the IRBITs substantially increase the activities of NBCn1 and NBCn2. We propose that upregulation of basolateral NBCn1 and NBCn2 plus the IRBITs in the mTAL: (1) promotes NH4+ shunting by increasing basolateral HCO3− uptake to neutralize apical NH4+ uptake during MAc; (2) inhibits HCO3− reabsorption during MAlk by opposing HCO3− efflux via the basolateral anion exchanger AE2; and (3) inhibits NaCl reabsorption by mediating (with AE2) net NaCl backflux into the mTAL cell during HSD. Thus, NBCn1, NBCn2 and the IRBITs are at the nexus of the regulatory pathways for multiple renal transport processes.
Keywords: ammonium transport, HCO3- reabsorption, hypertension, metabolic acidosis, metabolic alkalosis, NaCl reabsorption
Introduction
The kidneys play a central role in maintaining systemic acid-base and electrolyte homeostasis. Dysfunctional or inappropriate renal transport of acid-base equivalents or NaCl can cause a range of disorders, such as metabolic acidosis (MAc), contraction alkalosis, and hypertension. The mTAL is a major regulator of both acid-base and NaCl transport.
Ammonium (NH4+) is a major H+ carrier for renal acid excretion. The proximal tubule (PT) epithelium secretes NH3 and H+, thereby generating luminal NH4+. The mTAL reabsorbs the majority of this NH4+ either for recycling (as NH3) to the thin descending limb or short-circuiting to the collecting duct. The apical step of this NH4+ reabsorption involves the Na+-K+-Cl− cotransporter NKCC2 (Garvin et al. 1988; Watts & Good, 1994) and K+ channel ROMK (Weiner & Verlander, 2017). The basolateral-exit step could involve NHE4 (Chambrey et al. 2001; Bourgeois et al. 2010) and NH3-permeable AQP1 (Nakhoul et al. 2001; Musa-Aziz et al. 2009; Cabral & Herrera, 2012).
The mTAL reabsorbs substantial HCO3− (10−15% of filtered load) (Capasso et al. 1991, 1994; Zacchia et al. 2018) and NaCl (~20%) (Hebert & Andreoli, 1984; Fenton & Knepper, 2007). Here, HCO3− reabsorption involves the concerted action of the apical NHE3 and H+-pump (Froissart et al. 1992; Good et al. 2006), as well as the basolateral anion exchanger AE2 (SLC4A2) (Alper et al. 1997; Sun, 1998). In them TAL, NaCl reabsorption involves the apical NKCC2 (Fenton & Knepper, 2007) and NHE3 (Good et al. 2006) plus the basolateral Na+-K+ pump and Cl− channel ClC-K2 or ClC-Kb (Zaika et al. 2016; Hennings et al. 2017). Some reabsorbed Na+ moves via tight junctions.
The mTAL basolateral membrane has Na+/HCO3− cotransport activity (Kikeri et al. 1990; Odgaard et al. 2004), and two identified Na+/HCO3− cotransporters, NBCn1 (SLC4A7) (Vorum et al. 2000; Praetorius et al. 2004) and NBCn2 (SLC4A10) (Wang et al. 2015; Guo et al. 2017). The expression of dual NBCs – NBCn1, which is relatively DIDS-insensitive (Choi et al. 2000), and NBCn2, which is completely inhibited by DIDS (Parker et al. 2008) – would predict a partial sensitivity of the Na+/HCO3− cotransport activity to DIDS. Indeed, based on measurements of intracellular pH (pHi), 400 μM DIDS inhibits only ~70% of Na+/HCO3− cotransport activity in rat mTAL (Kwon et al. 2002).
It has been suggested that NBCn1 facilitates mTAL NH4+ reabsorption by providing cytosolic HCO3− that neutralizes incoming NH4+ to generate NH3 (Praetorius et al. 2004; Weiner & Verlander, 2017). Indeed, NH4Cl-induced MAc greatly upregulates mTAL NBCn1 expression (Odgaard et al. 2004; Praetorius et al. 2004).
The IP3R binding protein released with inositol 1,4,5-trisphosphate (IRBIT) (Ando et al. 2003) stimulates multiple acid-base transporters (Yang et al. 2011), including NBCe1-B (Shirakabe et al. 2006; Lee et al. 2012), NBCn1-A/B (Parker et al.2007b; Hong et al. 2013), and, in a preliminary report, NBCn2-B (Parker et al. 2007a; Parker et al. 2007b). Long-IRBIT (L-IRBIT) is a close homologue of IRBIT (≥90% sequence identity).
Unknowns are: (1) whether MAc enhances mTAL expression of NBCn2, IRBIT, or L-IRBIT; (2) whether metabolic alkalosis (MAlk) or high-salt diet (HSD) impacts mTAL expression of NBCn1, NBCn2, IRBIT or L-IRBIT; (3) which NBCn1 variant(s) is expressed in the mTAL; (4) and whether L-IRBIT stimulates either NBCn1 or NBCn2.
In the present study, we find that two systemic acid-base disturbances (i.e. MA cand MAlk) as well as one additional electrolyte disturbance (i.e. HSD) upregulate – generally by several-fold – the expression of NBCn1 and NBCn2 in rat kidney. In parallel, the three challenges generally increase the expression of IRBIT and L-IRBIT (collectively, ‘IRBITs’), also by several-fold. Finally, the IRBITs markedly stimulate the activities of the renal specific variants of NBCn1 and NBCn2, as expressed in Xenopus oocytes. The parallel and substantial upregulation of not only NBCn1 and NBCn2 but also their powerful activators suggests that these four proteins are at the nexus of regulating acid-base and NaCl transport in the mTAL.
Methods
Ethical approval
In the present study, we use dadult female Sprague–Dawley rats, 8−9 weeks of age, weighing 200–250g, and purchased from the Hubei Provincial Centre for Disease Control (Wuhan, China). The rats were anaesthetized by subcutaneous injection of pentobarbital sodium (2% w/v, 0.5 ml per 100 g bw) and killed for tissue collection. All procedures for the animal experiments have been approved by the Institutional Committee on Animal Care and Use at the Huazhong University of Science and Technology (Animal Study Proposal #2016114). The study conforms to the principles and regulations of The Journal of Physiology (Grundy, 2015).
Animal models
NH4Cl.
The NH4Cl group was fed with water containing 1.5% NH4Cl (0.28 mol l−1) plus 0.5% sucrose. The control group was supplied with water containing 0.5% sucrose. The rats had free access to rodent chow and water.
NaHCO3.
The NaHCO3 groups were supplied with tap water containing 0.28 mol l−1 NaHCO3 for 7 or 14 days. The controls were supplied with plain tap water. The rats had free access to chow and water.
High-salt diet.
The HSD groups were supplied with water containing 0.28moll−1 NaCl for 7 or 14 days. The controls were supplied with plain tap water. The rats had free access to chow and water.
Arterial blood was sampled from the anaesthetized rats, and pH measured with a 3510 bench pH meter (Jenway, Staffordshare, UK). The body weight and arterial blood pH data are summarized in Table 1.
Table 1.
Summary of body weight and arterial blood pH of rats
| Treatment | N | [BW]before/g | [BW]after/g | P value | Arterial pH | P value |
|---|---|---|---|---|---|---|
|
| ||||||
| 7 d NH4Cl | 8 | 225.3 ± 2.66 | 238.3 ± 3.34 | 0.62 | 7.445 ± 0.013 | 0.47 |
| Control | 8 | 225.8 ± 2.69 | 240.4 ± 2.94 | 7.457 ± 0.012 | ||
| 7 d NaHCO3 | 9 | 205.0 ± 3.8 | 223.6 ± 6.4 | 0.20 | 7.462 ± 0.028 | 0.82 |
| Control | 9 | 206.2 ± 3.5 | 234.8 ± 6.1 | 7.455 ± 0.014 | ||
| 14 d NaHCO3 | 8 | 194.1 ± 2.9 | 227.0 ± 6.4 | 0.58 | 7.482 ± 0.018 | 0.53 |
| Control | 8 | 195.6 ± 3.5 | 230.9 ± 3.8 | 7.498 ± 0.019 | ||
| 7 d NaCl | 8 | 206.9 ± 3.3 | 218.0 ± 4.8 | 0.75 | 7.434 ± 0.022 | 0.36 |
| Control | 8 | 209.1 ± 4.8 | 215.4 ± 7.2 | 7.460 ± 0.020 | ||
| 14 d NaCl | 8 | 234.1 ± 2.4 | 263.2 ± 5.3 | 0.87 | 7.436 ± 0.013 | 0.49 |
| Control | 8 | 232.7 ± 3.9 | 262.0 ± 6.3 | 7.454 ± 0.024 | ||
Antibodies
Rabbit polyclonal anti-NBCn1, anti-NBCn2 and anti-L-IRBIT were custom-made and affinity-purified with immunogen by GenScript (Nanjing, CN). Rabbit polyclonal anti-IRBIT was purchased from Cell Signalling Technology (catalogue no. 94248S; Danvers, MA). Anti-NBCn2 was described previously (Guo et al. 2017). Mouse anti-actin was purchased from Beyotime (catalogue no. AA128; Haimen, CN). Mouse anti-α1 was purchased from Abcam (catalogue no. AB7671; Hongkong, CN).
HRP-conjugated goat anti-rabbit IgG and goat anti-mouse IgG were purchased from Beyotime. DyLight549-conjugated goat anti-rabbit IgG was purchased from Abbkine Scientific (catalogue no. A23320–1; Wuhan, China). DyLight488-conjugated goat anti-mouse IgG was purchased from EarthOx (catalogue no. E032210–01; Millbrae, CA, USA).
Quantitative PCR
The relative abundance of the transcripts of Slc4a7 and Slc4a10 in the medulla of rat kidney was analysed by quantitative PCR (qPCR) with ABI StepOnePlus Real-Time PCR Systems using Platinum Quantitative PCR SuperMix-UDG with ROX (Life Technologies). Primers and probes for Slc4a7 are: sense: 5′-CTGTATG TCTCCTGTAATCACTTTTG; anti-sense: 5′-TCCCCA GTATTGTTAGAGGTTGC; probe: FAM-5′-CAAT GAATAGGCAATCCCAGTTAATGATGCTCCA-TAMRA. Primers for Slc4a10 are: sense: 5′-CACTCTTTGGAGC ATCTATGACC; anti-sense: 5′-GCCCGTAAGGA CAAGTATGACAG; probe: FAM-5′-TCTTTTCAAACA CCAGAACAGGTCCCGTACTG-TAMRA. Primers and probes for Actb are: sense: 5′-CCACACGCAGCTC ATTGTAGAAAG; anti-sense: 5′-ACCCTGAAGTACC CCATTGAACAC; probe: FAM-5′-TCTTCTC CATATCGTCCCAGTTGGTGACA-TAMRA. The amplification efficiencies of the primers were greater than 90% and were not significantly different from each other. The assay was started from cDNA preparation with equal amounts (usually 2 μg per 20 μl reaction mixture) of total RNA prepared from rat renal medulla. Three replicates were prepared for each cDNA sample in a specific qPCR assay. H2O was used as a negative control. In the experiments for comparison of the level of Slc4a7 and Slc4a10 transcripts in different samples (treated vs. control), the level of Actb-encoding β-actin was analysed to verify equal loading of cDNA templates. The threshold cycle (CT) was determined by ABI StepOne software v2.3 for evaluating the relative expression level of the target genes.
Western blotting
Protein preparations were prepared as described previously (Guo et al. 2017). Briefly, rat tissue was placed in a pre-cooled buffer (7.5 mM NaH2PO4, 250 mM sucrose, 5 mM EDTA, 5 mM EGTA, pH 7.0) containing 1% protease inhibitor cocktail (Cat#P8340; Sigma-Aldrich, St. Louis, MO) and homogenized. The crude homogenate was centrifuged at 3000 g for 10 min at 4°C. A vial of the supernatant was saved as ‘whole lysate’. The rest of the supernatant was centrifuged at 100,000 g for 60 min at 4°C. The resultant ‘membrane fraction’ was resuspended in a buffer containing 20 mM Tris, 5 mM EDTA, 5% SDS, pH 8.0. The protein preparations were stored in aliquots at −80°C.
Proteins were separated by sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) and blotted onto a PVDF membrane. The blot was blocked with 5% milk in 1 × TBST (1 mM Tris, 150mM NaCl, 0.1% Tween 20, pH 7.4), and then probed with primary antibody. After five washes with 1 × TBST, the blot was incubated with HRP-conjugated secondary antibody, followed by five washes with1×TBST, and then incubated with Super Signal West Pico chemiluminescent reagent (Thermo Scientific, Rockford, IL). The signals were visualized with X-ray film. Densitometric analysis was performed with ImageJ (National Institutes of Health).
Immunofluorescence
Adult rats were fixed by transcardial perfusion with 4% paraformaldehyde in PBS buffer (77.4 mM Na2HPO4, 22.6 mM NaH2PO4, pH 7.4). The kidneys were collected, and stored in PBS containing 0.1% paraformaldehyde plus 0.02% NaN3 until cryo-section (thickness: 8 μm). Sections were incubated at 60°C overnight, rehydrated in 1 × Tris-buffered saline (TBS; 1 mM Tris, 150 mM NaCl, pH 7.4) for 60 min, followed by five washes with 1 × TBS. The section was then incubated at 98°C for 20 min in Improved Citrate Antigen Retrieval Solution (catalogue no. P0083; Beyotime). After five washes with 1 × TBS, the section was blocked for 1 h with Immunol Staining Blocking Buffer (catalogue no. P0102; Beyotime) at room temperature, and then incubated with primary antibody at 4°C overnight. After five washes with 1 × TBS, the section was incubated with DyLight-conjugated secondary antibody at room temperature for 60 min. After three washes with 1 × TBS, the section was counterstained with 4,6-Diamidino-2-Phenylindole (DAPI), washed three times with 1 × TBS, and mounted with Antifade Poly-vinyl Pyrrolidone Medium (cat#P0123; Beyotime). Images were acquired on a Fluo View FV 1000 confocal microscope (Olympus, Tokyo, Japan).
Preparation of oocytes
The vectors for expressing mouse NBCn1-G (tagged with EGFP at amino terminus [Nt]) and rat NBCn2-C (tagged with EGFP at carboxyl terminus [Ct]) have been described previously (Liu et al. 2013a,b). Mouse IRBIT (accession no. AB092504.1) and L-IRBIT (accession no. NM 02 1414.6) were tagged, at Nt, with Myc and HA, respectively. cRNA was prepared with T7 mMessage mMachine kit (cat#AM1344, Life Technologies Corporation). The oocytesofstages V−VIinjected with cRNA were incubated in OR3 medium for 5 days at 18°C prior to western blotting or electrophysiological measurements.
Intracellular pH measurements
For electrophysiology recordings, an oocyte was first perfused with nominally ‘HCO3−-free’ ND96 (in mM: 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 Hepes, pH 7.50, 200 mOsm), and then with a solution containing 1.5% CO2/10 mM HCO3−. The pHi and membrane potential (Vm) of the oocyte were recorded with a H+-selective ‘pHi electrode’ and a ‘Vm electrode’ filled with 3 M KCl, as previously described (Musa-Aziz et al. 2010). The pHi of the oocyte is a linear function of the differential outputs of the pHi and Vm electrodes, acquired by using a Hiz 223 (Warner Instruments, Hamden, CT) dual-channel high-impedance electrometer (World Precision Instruments, Inc., Sarasota, FL) and an OC-275 oocyte clamp (Warner Instrument Corp., Hamden, CT). The differential output of the Vm and bath electrodes represents the membrane potential Vm of the oocyte.
Biotinylation analysis
A group of 10 oocytes were incubated in 3 ml of phosphate buffered saline containing 0.24 mg ml−1 Sulfo-NHS-Biotin (Pierce Cell Surface Isolation Kit, Catalogue # 89881) at 4°C for 2 × 30 min. The biotinylation reaction was stopped by adding ‘quenching solution’. The cells were washed with TBS and disrupted by trituration in 100 μl of lysis buffer with 1% Triton X-100 and protease inhibitor (Roche Applied Biosciences, Indianapolis, IN, USA; catalogue no. 04693159001). After a 10 min centrifugation at 850 g, the lysate was transferred to a Costar Spin-X column (catalogue no. 8163) for another 15 s centrifugation at 21,000 g. 20 μl of the flow-through was saved as ‘total fraction’. The rest was incubated with Neutravidin in a Pierce Spin Column at room temperature for 1 h. After five washes with lysis buffer, the ‘surface proteins’ were eluted by using 80 μl of a 1 × SDS sample buffer containing 50 mM DTT. The proteins were separated on NuPAGE 3−8% Tris-Acetate gel (Invitrogen, catalogue no. 18111371) and blotted onto a PVDF membrane for western blotting analysis. NBCn1 and NBCn2 were probed with anti-EGFP.
Statistical analysis
Quantitative data are presented as means ± standard deviation. Student’s t test was performed with Microsoft Excel. ANOVA analysis was performed with Minitab (Minitab, LLC, http://www.minitab.com). P < 0.05 is considered as statistically significant.
Results
Antibody validation
Figure 1A shows the sequences of the immunizing peptides for the antibodies. By western blotting, anti-NBCn1 specifically recognizes NBCn1 heterologously expressed Xenopus oocytes but not NBCn2 (Fig. 1B), whereas anti-NBCn2 specifically recognizes NBCn2, but not NBCn1 (Fig. 1C). Anti-IRBIT specifically recognizes Myc-IRBIT, but not Myc-L-IRBIT (Fig. 1D), whereas anti-L-IRBIT specifically recognizes Myc-L-IRBIT, but not Myc-IRBIT (Fig. 1E) heterologously expressed Xenopus oocytes. Both Myc-IRBIT and Myc-L-IRBIT can be detected by anti-Myc (Fig. 1F). The data indicate that each antibody is specific for the cognate target molecule.
Figure 1. Validation of antibodies against NBCn1, NBCn2, IRBIT and L-IRBIT.
A, sequences of immunizing peptides. Anti-IRBIT (Cell Signalling Technology) was generated against a peptide surrounding Glu50 of human IRBIT, with no further details available from the source. B, anti-NBCn1 recognizes NBCn1 but not NBCn2. C, anti-NBCn2 recognizes NBCn2, but not NBCn1. D, anti-IRBIT recognizes IRBIT, but not L-IRBIT. E, anti-L-IRBIT recognizes L-IRBIT, but not IRBIT. F, anti-Myc recognizes both IRBIT and L-IRBIT. Mouse NBCn1-G (accession #JQ073566), rat NBCn2-C (accession #AF439855.1), mouse IRBIT (accession no. AB092504.1) and mouse L-IRBIT (accession no. NM_02 1414.6) were each heterologously expressed in Xenopus oocytes. IRBIT and L-IRBIT were tagged with Myc at the amino-termini (Nt).
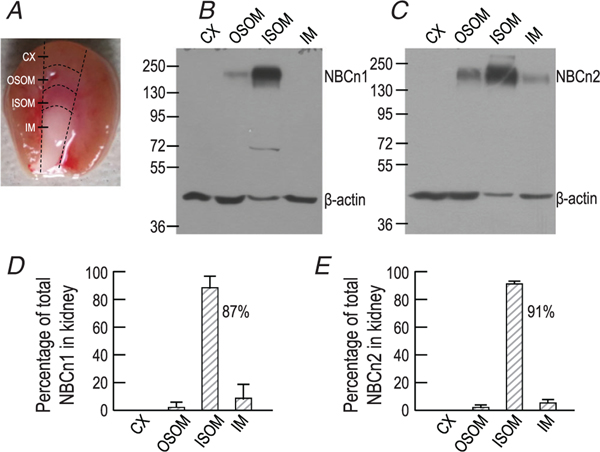
Tissue distribution of NBCn1 and NBCn2 protein in rat kidney
To explore the regional distribution of NBCn1 and NBCn2, we finely dissected the kidney tissues, as exemplified in Fig. 2A, and performed western blotting for NBCn1 (Fig. 2B) and NBCn2 (Fig. 2C). As summarized in Fig. 2D, NBCn1 is predominantly expressed in the inner stripe of outer medulla (ISOM) compared with the other regions of rat kidney (Fig. 2D). The same is true for NBCn2 (Fig. 2E). In a previous study, the apparently high abundance in the inner medulla (IM) of NBCn2 (i.e. MEIK-NBCn2 in Fig.1C of Guo et al. (2017) was likely due to tissue contamination from ISOM and over-exposure of the film, which could eliminate the difference between ISOM and IM.
Figure 2. Fractional distribution of NBCn1 and NBCn2 in cortex (CX), outer stripe of outer medulla (OSOM), inner stripe of outer medulla (ISOM), and inner medulla of rat kidney.
Membrane preparations were prepared from finely dissected CX, OSOM, ISOM and IM of rat kidneys for western blotting. A, diagram for tissue collection. B and C, representative western blots for NBCn1 and NBCn2, respectively. β-actin was probed for loading control. D and E, summary of fractional distribution of NBCn1 and NBCn2 in CX, OSOM, ISOM and IM of rat kidneys. Data were obtained from four independent experiments like those shown in panels B and C.
Effect of NH4Cl or NaHCO3 on renal mRNA levels of Slc4a7 and Slc4a10
By qPCR with total RNA from the rat renal medulla (Fig. 3A), the relative abundance of Slc4a7 is 4.7-fold greater than that of Slc4a10 (Fig. 3B), indicating that the mRNA encoding NBCn1 is dominant over that encoding NBCn2 in the mTAL.
Figure 3. Relative mRNA levels of Slc4a7 and Slc4a10 in renal medulla of control, MAc and MAlk rats.
A, summary to show that threshold cycle (CT) of Slc4a7 is significantly smaller than that of Slc4a10 for qPCR with normal medulla samples. The amplification efficiencies of the primers for Slc4a7 and those for Slc4a10 were not significantly different from each other. Slc4a7 and Slc4a10 were simultaneously analysed for CT collection with duplicates of the same sample. The bars represent the results from three independent experiments. B, summary to show the relative mRNA level of Slc4a7 and Slc4a10. The relative mRNA level was computed based upon the CT data contributed to panel A, by using formula 2ΔCt, where ΔCT represents the difference between the CT of each reaction and the average CT of Slc4a10. The relative abundance of Slc4a7 is significantly greater than that of Slc4a10 in the medulla of control normal rats. C, 7-day NH4Cl-induced MAc significantly increases the abundance of Slc4a7 mRNA, but not Slc4a10 in renal medulla. D, 7-day NaHCO3-induced MAlk has no significant effect on the mRNA abundance of Slc4a7 and Slc4a10. In the experiments for panels C and D, Slc4a7, Scl4a10, and Actb encoding β-actin were analysed for CT collection with duplicates of the same sample. The relative mRNA level was computed by using the formula 2ΔΔCT. Here, ΔCT was first computed by subtracting the CT of Actb from the CT of Slc4a7 or Slc4a10 for a given sample. Each of these ΔCT values was then subtracted by the ΔCT of Slc4a10 of the control to obtain ΔΔCT. Each panel presents the results from three independent experiments.
By qPCR, we also examined the effects of NH4Cl and NaHCO3 in the drinking water on the relative mRNA levels of Slc4a7 and Slc4a10, normalized to the level of Actb, in rat kidney. NH4Cl-induced Mac significantly increases the relative abundance of the Slc4a7 but not Slc4a10 transcript (Fig. 3C), whereas NaHCO3-induced MAlk affects neither (Fig. 3D).
NH4Cl increases whole-kidney protein expression of NBCn1 and NBCn2
MAc increases PT ammoniagenesis and H+ secretion, therebyenhancingtheNH4+ load to the mTAL. Compared with the control, a 7-day NH4Cl treatment substantially increases the abundance of NBCn1 (Fig. 4A) and NBCn2 (Fig. 4B) in membrane preparations of the whole kidney.
Figure 4. NH4Cl, NaHCO3 and HSD increase the expression of NBCn1 and NBCn2 in rat kidney.
Membrane preparations of whole kidney were used for western blotting to examine the effect of NH4Cl, NaHCO3 and NaCl, for a duration of 7 or 14 days, on the expression of NBCn1 and NBCn2. A and B, effect of NH4Cl on NBCn1 and NBCn2. C−F, effects of NaHCO3 on NBCn1 and NBCn2. G−J, effect of NaCl (i.e. high-salt diet [HSD]) on NBCn1 and NBCn2. The numerals in the parentheses indicate the number of rats for each condition. The density of NBC in each lane was normalized to that of actin in the same lane. This ratio of each lane was then normalized to the average of the ratios of the control lanes in the same blot. For statistical comparison, a two-tailed Student’s t test was performed. ‘−’ indicates the controls, whereas ‘+’ indicates rats treated with NH4Cl, NaHCO3 or HSD.
By immunofluorescence, MAc substantially increases NBCn1 expression in the outer medulla (OM) as compared with the control (Fig. 5A1 vs. B1). High-magnification views show that, compared with the control (Fig. 5A2–A4), MAc increases the NBCn1 intensity at the basolateral mTAL, as marked by the α1 subunit of Na+-K+ pump (Fig. 5B2–B4). The same is true for NBCn2 (Fig. 5C1–C4 vs. D1–D4).
Figure 5. Immunofluorescence showing that the expression of NBCn1 and NBCn2 in the basolateral membrane of mTAL is increased by 7-day NH4Cl.
Cryo-sections of the kidney were double-stained with anti-NBCn1 (or anti-NBCn2) and anti-α1 (against the α1 subunit of the Na+-K+ pump, specific marker of the basolateral membrane of mTAL). A1−A4, expression of NBCn1 in outer medulla (OM) of the control rat. B1−B4, expression of NBCn1 in the OM of NH4Cl-treated rat. C1−C4, expression of NBCn2 in the OM of control rat. D1−D4, expression of NBCn2 in the OM of NH4Cl-treated rat. The red shows the expression of NBCn1 or NBCn2. The green shows the expression of α1 of the Na+-K+ pump. The blue in the merge represents the staining of the nuclei by DAPI. The data are representative of at least three independent experiments.
Together, the above data demonstrate that MAc markedly increases the protein expression of NBCn1 and NBCn2, specifically in the mTAL basolateral membrane. The MAc-induced upregulation of NBCn1 protein expression is consistent with our qPCR data (Fig. 3C) and previous studies (Kwon et al. 2002; Odgaard et al. 2004; Praetorius et al. 2004). Note that the novel upregulation of NBCn2 protein contrasts with the lack of effect on Slc4a10 transcript. Thus, MAc presumably upregulates NBCn1 expression, at least in part, at the mRNA level, but NBCn2, exclusively at the post-translational level. Our data extend to NBCn2 observations made earlier on NBCn1, consistent with the idea that NBCn2, like NBCn1, plays an important role in facilitating NH4+ shunting in mTAL.
NaHCO3 increases whole-kidney protein expression of NBCn1 and NBCn2
Because NaHCO3-induced MAlk decreases PT ammoniagenesis and H+ secretion, and thus decreases the NH4+ load to the mTAL, we expected MAlk to downregulate NBCn1 and NBCn2. However, MAlk induced by 7- and 14-day NaHCO3 treatments – like MAc – greatly increases protein expression of NBCn1 (Fig. 4C and D) and NBCn2 (Fig. 4E and F). A previous study reported no effect of NaHCO3 on the protein expression of NBCn1 in rat medulla (Praetorius et al. 2004).
Immunofluorescence shows that MAlk substantially increases NBCn1 levels in the OM (Fig. 6A1 vs. B1). High-magnification views show that, compared with the control (Fig. 6A2–A4), MAlk greatly increases the NBCn1 intensity at the basolateral mTAL (Fig. 6B2–B4). The same is true for NBCn2 (Fig. 6C1–C4 vs. D1–D4).
Figure 6. Immunofluorescence showing that the expression of NBCn1 and NBCn2 in the mTAL of rat kidney is increased by NaHCO3.
The rats were treated with NaHCO3 for 7 days. Cryo-sections of the kidney were double-stained with anti-NBCn1 (or anti-NBCn2) and anti-α1. A1−A4, expression of NBCn1 in the outer medulla (OM) of the control rat. B1−B4, expression of NBCn1 in the OM of NaHCO3-treated rat. C1−C4, expression of NBCn2 in the OM of control rat. D1−D4, expression of NBCn2 in the OM of NaHCO3-treated rat. The red shows the expression of NBCn1 or NBCn2. The green shows the expression of α1 of the Na+-K+ pump. The blue in the merge represents the staining of the nuclei by DAPI. The data are representative of three independent experiments.
Together, the above data demonstrate that MAlk markedly increases the protein expression of NBCn1 and NBCn2, specifically in the mTAL basolateral membrane. Both the NBCn1 and NBCn2 protein-expression data contrast with the above qPCR data showing no effect of MAlk (Fig. 3D). Thus, MAlk must upregulate NBCn1 and NBCn2 post-translationally. Moreover, this stimulatory effect is presumably unrelated to NH4+ shunting, which most probably decreases during MAlk.
NaCl increases whole-kidney protein expression of NBCn1 and NBCn2
Could the NaHCO3-induced upregulation of NBCn1 and NBCn2 be a homeostatic response to the sodium load per se? Indeed, we find that both 7- and 14-day NaCl treatments significantly increase renal levels of both NBCn1 (Fig. 4G and H) and NBCn2 (Fig. 4I and J).
However, after 7 days, the magnitude of changes in NBCn1 and NBCn2 is significantly greater for NaHCO3 than for NaCl (Fig. 4C vs. G and E vs. I; one-way ANOVA), even though the daily Na+ intake of the NaCl group is slightly higher than that of the NaHCO3 group (data not shown). The differences at 14 days do not reach statistical significance. Nevertheless, at least for 7-day NaHCO3, the upregulation of NBCn1 and NBCn2 represents homeostatic responses to both MAlk per se and Na+ load per se.
NH4Cl, NaHCO3 and NaCl specifically increase NBCn1 and NBCn2 protein expression in outer medulla
Because the results shown in Fig. 4 represent data from whole kidney, we performed western blot analyses with finely dissected OM tissue to determine if the Fig. 4 data truly represent changes in the mTAL. As shown in Fig. 7, NH4Cl, NaHCO3 and NaCl – each for a 7-day duration – produce fractional increases in NBCn1 and NBCn2 abundance that are at least as great as in the whole-kidney assays (Fig. 4). Consistent with the aforementioned observations in whole kidney, the change in NBCn1 in the OM is significantly greater for NaHCO3 than for NaCl (Fig. 7C vs. E, one-way ANOVA). The change in NBCn2 in the OM, although it does not reach statistical significance, tends to be greater for NaHCO3 than for NaCl (Fig. 7D vs. F).
Figure 7. NH4Cl, NaHCO3 and HSD increase the expression of NBCn1 and NBCn2 in the OM of rat kidney.
Membrane fractions of the finely dissected OM were used for western blotting. A−B, effect of 7-day NH4Cl on NBCn1 and NBCn2. C−D, effect of 7-day NaHCO3 on NBCn1 and NBCn2. E−F, effect of 7-day NaCl on NBCn1 and NBCn2. All three challenges substantially increase the protein levels of NBCn1 and NBCn2 in the OM to extents that are at least as great as those in the whole-kidney membrane fractions (Fig. 4). The numerals in the parentheses indicate the number of rats for each condition. A two-tailed Student’s t test was performed for statistical comparisons between groups in the same panel. One-way ANOVA followed with the post hoc Fisher’s test was used for comparison between NaHCO3 vs. NaCl across panels. ‘−’ indicates the controls, whereas ‘+’ indicates rats treated with NH4Cl, NaHCO3, or HSD.
Recall that, in control kidneys, the fraction of NBCn1 in ISOM represents ~90% of the total NBCn1 in membrane preparations of whole kidney (Fig. 2). We conclude that nearly all of the increases in NBCn1 in whole kidney (Fig. 4) represent increases in the OM. If NH4Cl, NaHCO3 or NaCl had caused major increases in NBCn1 in other regions of the kidney, the magnitude of changes in the whole kidney would have exceeded those in the finely dissected OM.
The same analysis is true for NBCn2.
Effect of NH4Cl, NaHCO3 and NaCl on renal protein expression of the IRBITs
Whole kidney.
In the membrane fraction, NH4Cl significantly increases the protein expression of IRBIT (Fig. 8A) and L-IRBIT (Fig. 8B). NaHCO3 has no effect on IRBIT (Fig. 8C and D), but significantly increases the expression of L-IRBIT (Fig. 8E and F), particularly at 7 days. Finally, NaCl significantly increases IRBIT (Fig. 8G and H) and L-IRBIT (Fig. 8I and J).
Figure 8. Effect of NH4Cl, NaHCO3 and HSD on expression of the IRBITs in whole rat kidney.
Membrane fractions of whole kidney were used for western blotting to examine the effects of NH4Cl, NaHCO3 and NaCl for durations of 7 or 14 days, on the expression of the IRBITs. A−B, effect of NH4Cl on IRBIT and L-IRBIT. C−F, effects of NaHCO3 on IRBIT and L-IRBIT. G−J, effects of NaCl (i.e. HSD) on IRBIT and L-IRBIT. NH4Cl and NaCl, but not NaHCO3, significantly increase the protein levels of IRBIT. All three challenges significantly increase the levels of L-IRBIT. The numerals in parentheses indicate the number of rat individuals included in each bar. Two-tailed Student’s t tests were performed to examine the statistical significance. ‘−’ indicates the controls, whereas ‘+’ indicates rats treated with NH4Cl, NaHCO3 or NaCl.
In the crude lysate of whole kidney (Fig. 9), the three challenges generally produce patterns similar to those in the membrane fraction. The major differences are that, in the crude lysate, IRBIT is not responsive to NH4Cl (Fig. 9A) or NaCl (Fig. 9G and H), and L-IRBIT is not responsive to NaHCO3 (Fig. 9E and F). Thus, NH4Cl and NaCl promote the translocation of IRBIT from cytosol to membranes – presumably in association with membrane proteins–whereasNaHCO3 promotes an analogous trans location of L-IRBIT.
Figure 9. Effects of NH4Cl, NaHCO3, and HSD on expression of IRBITs in crude lysate of whole kidney.
Crude lysates of whole kidney were used for western blotting to examine the effects of NH4Cl, NaHCO3 and NaCl, for durations of 7 or 14 days, on the expression of the IRBITs. A−B, effect of NH4Cl on IRBIT and L-IRBIT. C−F, effects of NaHCO3 on IRBIT and L-IRBIT. G−J, effects of NaCl (i.e. HSD) on IRBIT and L-IRBIT. The numerals in parenthesis of the bars indicate the number of rat individuals examined. An unpaired two-tailed Student’s t test was performed for statistical analysis. The boxes indicate that the treatments have different effects in whole lysate compared with those observed in membrane preparation in Fig. 8. ‘−’ indicates the controls, whereas ‘+’ indicates the rats treated with NH4Cl, NaHCO3 or NaCl (i.e. high-salt diet [NaCl]).
Finely dissected OM.
In the OM membrane fraction, the three challenges generally produce patterns (Fig. 10) similar to those in the whole-kidney membrane fraction (Fig. 8), except that in the OM membrane fraction, NaHCO3 now produces significant increases in IRBIT protein (Fig. 10C).
Figure 10. Effect of NH4Cl, NaHCO3 and HSD on expression of the IRBITs in the OM of rat kidney.
Membrane fractions of the finely dissected OM were used for western blotting analysis. A−B, effect of 7-day NH4Cl on IRBIT and L-IRBIT. C−D, effect of 7-day NaHCO3 on IRBIT and L-IRBIT. E−F, effect of 7-day NaCl on IRBIT and L-IRBIT. The three challenges generally produce patterns similar to those in membrane preparations of whole kidney in Fig. 8. The exception is that NaHCO3 significantly increases IRBIT in the OM (panel C). The numerals in parentheses indicate the number of rats for each condition. Two-tailed Student’s t tests were performed to examine the statistical significance. ‘−’ indicates the controls, whereas ‘+’ indicates rats treated with NH4Cl, NaHCO3 or NaCl (i.e. high-salt diet [HSD]).
The above data show that MAc, MAlk and NaCl: (1) generally have greater effects on L-IRBIT than on IRBIT; (2) the effects tend to be greater in the OM than whole kidney; and (3) at least some of the challenges promote translocation of IRBIT or L-IRBIT to membranes.
IRBITs stimulate the activities of NBCn1 and NBCn2
Both Slc4a7 and Slc4a10 produce multiple variants (Fig. 11). Under the control of two distinct promoters, Slc4a7 encodes two groups of full-length NBCn1 variants, one starting with MERF (representing the initial four residues) and the other starting with MEAD. Previous studies have shown that MEAD-NBCn1 is expressed in rat kidney (Damkier et al. 2006; Boedtkjer et al. 2008). In the present study, we performed cDNA cloning for NBCn1 from rat kidney and found two MEAD-NBCn1 variants, NBCn1-G (the major one) and NBCn1-I (the minor one; data not shown). As far as NBCn2 is concerned, the rat mTAL expresses NBCn2-C (Wang et al. 2015; Guo et al. 2017).
Figure 11. Diagrams of variants of NBCn1 and NBCn2.
Both Slc4a7 and Slc4a10 are able to express a series of variants due to usage of alternative promoters and alternative splicing of transcripts. The alignment are based on human NBCn1-A (AAD38322.1), mouse NBCn1-B (AFR46591.1), mouse NBCn1-C (AFR46592.1), mouse NBCn1-D (AFR46593.1), human NBCn1-E (ACH61961.1), human NBCn1-F (AAG16773), rat NBCn1-G (AKS30237.1), human NBCn1-H (ACH61958.1), rat NBCn1-I (AKS30236.1), rat NBCn1-J (AKS30238.1), mouse NBCn1-K (ADO51788.1), mouse NBCn1-L (AFB82586.1), mouse NBCn1-M (AFI43934.1), mouse NBCn1-N (AFB82538.1), mouse NBCn1-O (AFI43933.1), mouse NBCn1-P (AFI43932.1) and human NBCn2-A (accession# NP_07 1341), human NBCn2-B (accession# AAQ83632), rat NBCn2-C (accession# AAO59639), mouse NBCn2-D (accession# ADX99207), rat NBCn2-E (accession# JX073715), rat NBCn2-F (accession# JX073716), rat NBCn2-G (accession# JX073717), rat NBCn2-H (accession# JX073718). Here, our cDNA cloning study shows that NBCn1-G and NBCn1-I are expressed in rat kidney, with NBCn1-G being the major variant (data not shown). Previous studies have shown that NBCn2-C is expressed in the mTAL (Liu et al. 2013b; Guo et al. 2017).
To examine whether and to what extent the IRBITs stimulate the major renal variants of NBCn1 and NBCn2, we expressed rat NBCn1-G and NBCn2-C in Xenopus oocytes, each ± IRBIT or L-IRBIT. Physiological assessment by intracellular pH (pHi) measurements of Xenopus oocytes expressing NBCn1-G only shows that the addition of CO2/HCO3− causes a rapid fall of pHi due to CO2 influx, followed by a slower pHi recovery due to HCO3− influx via the NBC (Fig. 12A). However, in oocytes expressing NBCn1-G plus either IRBIT (Fig. 12B) or L-IRBIT (Fig. 12C), the balance of CO2 influx vs. HCO3− influx shifts overwhelmingly in favour of HCO3− influx, so that pHi undergoes an unprecedented increase. The IRBITs also enhance, though less strikingly, the pHi recoveries of NBCn2-C (Fig. 12D–F), but have no effect on H2O-injected oocytes (Fig. 12G–I). Note that, in all cases, the addition of CO2/HCO3− to oocytes expressing NBCn1 or NBCn2 causes small and slow positive shifts in Vm (blue traces), rather than the large and sharp hyperpolarizations characteristic of an electrogenic NBC (Romero et al. 1997). Thus, the co-expression of the IRBITs does not affect the electroneutrality (and thus the stoichiometry) of the NBCs.
Figure 12. IRBITs stimulate the functional activities of NBCn1 and NBCn2 in Xenopus oocytes.
A−C, IRBITs stimulate NBCn1-G. D−F, IRBITs stimulate NBCn2-C. G−I, IRBITs have effect on pHi changes in H2O-injected oocytes. J, summary of early stage rate of pHi change [dpHi/dt]1. K, summary of late stage rate of pHi change [dpHi/dt]2. L, surface and total expression of NBCn1 and NBCn2 in Xenopus oocytes. M, summary showing effects of IRBITs on the surface expression of NBCn1 and NBCn2. N, summary showing effects of IRBITs on total expression of NBCn1 and NBCn2. In panel K, we skip two groups (‘NBCn1 + IRBIT’ and ‘NBCn1 + L-IRBIT’) because the increases in pHi occur at unusually high pHi values that should have greatly slowed the changes. #: significantly different by one-way ANOVA followed with post hoc Fisher’s test (P < 0.05). The numerals in parentheses in panels J & K indicate the number of oocytes included in each bar. The triangle in panel M indicates that the surface level of NBCn2 is significantly lower than that of NBCn1.
Figure 12J summarizes early rates of pHi change – [dpHi/dt]1. The IRBITs flip the direction of the pHi trajectories for NBCn1-G (left panel). Figure 12K summarizes late rates of pHi change – [dpHi/dt]2. Both NBCn1 and NBCn2 in absence of IRBITs cause pHi recovery rates greater than that of H2O-injected controls. Both IRBITs greatly increase pHi recovery rates for NBCn2 (middle panel).
Our analyses of surface and total expression of NBCn1 and NBCn2 (Fig. 12L–N) show that surface expression – probing with an anti-GFP antibody – is substantially lower for NBCn2 than NBCn1. These differences may contribute to the less impressive pHi effects of the IRBITs on NBCn2 (Fig. 12E–F) vs. NBCn1 (Fig. 12B–C). IRBIT, but not L-IRBIT, tends to enhance surface expression of NBCn1 and NBCn2, although the differences do not reach statistical significance.
Discussion
In the present study, we show that: (1) MAc, MAlk, and HSD upregulate expression of NBCn1 and NBCn2 in the mTAL; (2) these effects of Mac and MAlk are specific to the basolateral membrane; (3) the three challenges generally upregulate the expression of IRBIT and L-IRBIT in OM. Of the 12 effects represented by items #1–3 (3 challenges × 2 IRBITs × 2 NBCs) all but one are novel (i.e. the effect of MAc on NBCn1). (4) The IRBITs powerfully stimulate NBCn1 and NBCn2 in Xenopus oocytes. Of the four effects represented by item #4 (2 IRBITs × 2 NBCs), all but one (effect of IRBIT on NBCn1) are novel for a full-length paper. Based on these striking results, we hypothesize that NBCn1 and NBCn2 play pivotal roles in the renal response to diverse acid-base/electrolyte disorders, and that the parallel upregulation of the IRBITs markedly amplify the roles of NBCn1 and NBCn2.
Proposed role of NBCn1 and NBCn2 in promoting mTAL NH4+ reabsorption during MAc
Ammoniagenesis in PT cells, followed by NH3 and H+ secretion, generates luminal NH4+, the majority of which the mTAL reabsorbs for futile recycling to the late PT and descending thin limb of Henle’s loop, or for short-circuiting to the collecting duct and, ultimately, urinary acid excretion. At the mTAL apical membrane (Fig. 13A), NH4+ enters the cell via ROMK or NKCC2. Once inside, NH4+ dissociates into NH3 and H+. The NH3 would exit the mTAL cell via basolateral membrane channels, such as AQP1 (Nakhoul et al. 2001; Musa-Aziz et al. 2009; Cabral & Herrera, 2012). On one hand, the H+ derived from NH4+ influx could be extruded by basolateral NHE4 (Chambrey et al. 2001). On the other hand, this H+ could be titrated by the HCO3− brought into the cell by NBCn1 (Fig. 13A; Praetorius et al. (2004), for review, see Weiner & Verlander, (2017)). Together, the NH3 efflux, Na-H exchange, and Na/HCO3 cotransport would regenerate NH4+ in the interstitial fluid.
Figure 13. Proposed models to show the roles of NBCn1, NBCn2 and the IRBITs in the reabsorption of NH4+, HCO3–, and NaCl by mTAL epithelium.
A, regulation of NH4+ reabsorption. The luminal NH4+ enters the cell via apical NKCC2 and the K+ channel ROMK. NH4+ then dissociates into H+ and NH3. NH3 diffuses into the interstitial space via membrane channels. H+ is either extruded via NHE4 or titrated by intracellular HCO3−. Under MAc, NBCn1 and NBCn2 promote NH4+ reabsorption by providing HCO3− for this titration of intracellular NH4+. B, regulation of HCO3− reabsorption. The luminal HCO3− is titrated to form CO2 by the proton secreted by apical NHE3 and the H+-pump. The CO2 enters into the epithelium to reconstitute HCO3−, which is extruded via basolateral AE2. Under MAlk, NBCn1 and NBCn2 would antagonize the action of the basolateral AE2, increasing [HCO3−]i and pHi, thereby putting a brake on HCO3− reabsorption. C, regulation of NaCl reabsorption. The transepithelial NaCl reabsorption involves NKCC2, NHE3 and the basolateral Na-K pump as well as the Cl− channel ClC-K2. Under HSD, NBCn1 and NBCn2, together with AE2, mediate a net uptake of Na+ and Cl− across the basolateral membrane. The result would be no change in the intracellular [HCO3−] and pHi (indicated by ‘Δ’ with an overlaid ‘/’), but increased intracellular [Na+] and [Cl−], which in turn would inhibit the activity of NKCC2 and put a brake on NaCl reabsorption. Thus, J Na+ would fall, with no change in JHCO3. Under these three conditions, the roles of NBCn1 and NBCn2 in the mTAL are presumably stimulated by their interaction with the IRBITs. For details, see text in the Discussion. Circled ‘+ ‘ indicates effect of stimulation, whereas circled ‘−‘ indicates effect of inhibition.
Metabolic acidosis stimulates the proximal NH4+ secretion. The increased NH4+ delivery to the mTAL lumen leads to increased NH4+ uptake across the apical membrane, and thus an increased NH4+ load to the mTAL cell that causes pHi to fall markedly (Amlal et al. 1994; Watts & Good, 1994). Disruption of NHE4 reduces NH4+ reabsorption by ~50% in the mouse mTAL under conditions of MAc (Bourgeois et al. 2010). MAc also substantially upregulates basolateral NBCn1 in the mTAL, (Kwon et al. 2002; Odgaard et al. 2004; Praetorius et al. 2004). According to Praetorius (2004), the upregulated NBCn1 would increase basolateral HCO3− influx, mitigate the tendency for pHi to fall during MAc, and elevate the [NH3]i/[NH4+]i ratio. The result would be enhanced apicalNH4+ uptake, enhanced basolateral NH3 efflux, and thus increased NH4+ reabsorption.
We now confirm that MAc markedly upregulates basolateral NBCn1 in the mTAL, and further demonstrate a parallel upregulation of NBCn2. Thus, we extend the previous model by suggesting that both NBCn2 and NBCn1 promote apical NH4+ uptake (Fig. 13A) for recycling and shunting. Note that the mouse mTAL appears to express NBCn1 but not NBCn2 (https://cello.shinyapps.io/kidneycellexplorer/). Thus, we would expect the 50% contribution of NHE4 to NH4+ reabsorption in the mTAL of the mouse to be smaller in the rat, which has a third pathway (i.e. NBCn2) to neutralize the MAc-induced intracellular acidosis.
Thus, we make our first proposal, namely, that NBCn1 and NBCn2 facilitate transepithelial NH4+ reabsorption in mTAL. Under MAc, the upregulation of NBCn1 and NBCn2 would stimulate NH4+ recycling as well as shunting from the mTAL to the collecting duct, an appropriate adaptive response to MAc.
Proposed role of NBCn1 and NBCn2 in suppressing mTAL HCO3− reabsorption during MAlk
A surprising observation is that, like NH4Cl-induced MAc, NaHCO3-induced MAlk upregulates NBCn1 and NBCn2 in the mTAL. This upregulation cannot be an adaptive response to proximal ammoniagenesis, which appropriately falls in response to MAlk, thereby lowering urinary acid excretion (Lemieux et al. 1985).
As an adaptive response to MAlk, the kidney downregulates the reabsorption of filtered HCO3− as well as the creation of new HCO3−. The question then arises, whether upregulation of NBCn1 and NBCn2 in the mTAL during MAlk plays a role in lowering acid secretion, which is mainly transepithelial HCO3− reabsorption (JHCO3). Under normal conditions, the mTAL reabsorbs 10−15% of filtered HCO3− (Capassoetal.1991,1994).The classical view (Fig. 13B) is that H+ secretion, via apical NHE3 and H+-pumps, titrate luminal HCO3− to form H2O and CO2. Once inside the mTAL cell, the H2O and CO2 reconstitute H+ and HCO3−. Finally, the HCO3− exits via the basolateral AE2. As one would expect, in rat mTAL, NaHCO3-induced MAlk significantly decreases both the expression and activity of apical NHE3 (Laghmani et al. 1999) and also reduces JHCO3 (Capasso et al. 1994). Surprisingly, however, NaHCO3 increases AE2 expression in rat mTAL (Quentin et al. 2004). Below, we will address why this upregulation of AE2 makes teleological sense. Here we note that, to the extent that NBCn1 and NBCn2 mediateHCO3− influx, they nullify–that is, put a brake on – the AE2-mediated HCO3− efflux and thereby contribute to the observed decrease in JHCO3.
Thus, we make our second proposal, that increased expression of NBCn1 and NBCn2 in mTAL is the appropriate adaptive response to MAlk, counteracting HCO3− reabsorption and thereby increasing urinary HCO3− excretion (Fig. 13B).
Proposed role of NBCn1 and NBCn2 in suppressing mTAL NaCl reabsorption during Na+ loads
In the present study, we find that NaCl (i.e. HSD) enhances NBCn1 and NBCn2 expression. Moreover, NaHCO3 (i.e. MAlk) produces an even greater enhancement of NBCn1 and NBCn2 expression. Thus, the response to MAlk appears to include a component of Na+ load per se. Note, it is impossible to know the extent to which the extra effect of NaHCO3 vs. NaCl is due exclusively to the rise in extracellular [HCO3−] or pH vs. the fall in [Cl−].
In their aforementioned study, Quentin et al observed upregulation of AE2 in response to both NaHCO3 (see above) and NaCl (Quentin et al. 2004). Might NBCn1 and NBCn2, together with AE2, lower transepithelial NaCl reabsorption (JNaCl) in the mTAL?
The mTAL reabsorbs ~20% of filtered NaCl. Here, NaCl reabsorption involves apical NKCC2 and NHE3, and the basolateral Na+-K+ pump and ClC-K2 (Fig. 13C). As an adaptive response to HSD, the kidney downregulates NaCl reabsorption in every relevant nephron segment. Indeed, in mouse kidney, HSD reduces, whereas low-sodium increases, NKCC2 expression (Udwan et al. 2017). NKCC2-mediated Na+ uptake depends on the inward Na+ driving force, established by the basolateral Na+-K+ pump. Indeed, inhibition of the pump by ouabain (Burg & Green, 1973; Rocha & Kokko, 1973) or by removal of peritubular K+ abolishes NaCl absorption by mTAL (Hebert et al. 1981). NKCC2 is also sensitive to intracellular Cl−, being stimulated by lowering [Cl−]i and inhibited by raising [Cl−]i (Russell, 2000; Delpire & Gagnon, 2018).
The coordinated actions of the basolateral NBCn1 and NBCn2 with AE2 would result in the net parallel back-fluxes of Na+ and Cl− from the peritubular fluid into the mTAL cell, which would tend to (a) short-circuit basolateral effluxes of Na+ via the Na+-K+ pump and Cl− via ClC-K2, and (b) increase [Na+]i and [Cl−]i and thereby slow apical NaCl uptake via NKCC2. The increased [Na+]i would also slow apical Na+ uptake via NHE3.
Thus, we make our third proposal, that the increased expression of NBCn1 and NBCn2 in the mTAL is the appropriate adaptive response to whole-body Na+ loads provided by NaHCO3 or NaCl, raising [Na+]i, inhibiting transepithelial NaCl reabsorption, and thereby increasing urinary NaCl excretion (Fig. 13C).
An interesting parallel exists with the PT, where Na+ loading – in the guise of either NaHCO3 or NaCl – downregulates the functional expression (i.e. product of surface abundance and single-molecule activity) of apical NHE3 and basolateral NBCe1 (Amlal et al. 2001). These effects, which would decrease proximal reabsorption of Na+ and HCO3−, are functionally consistent with the upregulation of NBCn1 and NBCn2 that we observe with NaHCO3 and NaCl in the mTAL.
Proposed role of AE2 in suppressing mTAL NaCl reabsorption during Na+ loads
In parallel, we make our fourth proposal, that the increased expression of AE2 in the mTAL is likewise the appropriate adaptive response to the Na+ loads provided by NaHCO3 or NaCl, raising [Cl−]i, inhibiting NKCC2, and thereby increasing urinary NaCl excretion (Fig. 13C). Consistent with the hypothesis that increased AE2 activity acts to lower NaCl reabsorption, we note that HSD increases the expression of AE2 in mTAL (Quentin et al. 2004).
Potential pathological relevance of NBCn1, NBCn2 and AE2 in NaCl reabsorption by mTAL
The proposed synergistic roles of NBCn1 and NBCn2 and AE2 in NaCl reabsorption by the mTAL provides an explanation for multiple previous observations related to hypertension and systemic acid-base/electrolyte disorders. In humans, both SLC4A2 (encoding AE2) and SLC4A7 (encoding NBCn1) are associated with hypertension (Sober et al. 2009; Boedtkjer et al. 2011; Ehret et al. 2011; Wang et al. 2017). SLC4A10 (encoding NBCn2) is associated with dysregulation of plasma osmolality and systemic water balance (Boger et al. 2017). In Slc4a7-null mice, the development of hypertension is attributed to the dysregulation of pHi in vascular smooth muscle cells and endothelia (Boedtkjer et al. 2011). Given the role of NBCn1 in regulating NaCl reabsorption in mTAL, we suggest that the development of NBCn1-associated hypertension includes a renal component.
Proposed roles of the IRBITs in promoting NH4+ shunting, and suppressing HCO3− and NaCl reabsorption
As tested in Xenopus oocytes, both IRBITs powerfully augment the functional expression of both NBCn1 and NBCn2. As shown in Fig. 12M, IRBIT tends to increase the surface expression of NBCn1. Even if this effect were statistically significant, it would be far too small to accountfortheIRBIT-inducedincreaseinNBCn1activity. Moreover, L-IRBIT has no effect on the surface expression of NBCn1 or NBCn2. Thus, these three examples of increased functional expression must largely be due to an increase in the single-molecule activity. On the other hand, the IRBIT-induced stimulation of NBCn2 in oocytes could be due solely to augmentation of surface expression.
The mechanism underlying the stimulation of NBCn1 and NBCn2 by the IRBITs remains to be addressed. Both NBCn1 and NBCn2 have long cationic clusters in their N-termini, consistent with the presence of an auto-inhibitory domain (Lee & Boron, 2018). IRBIT, upon phosphorylation at multiple sites in the PEST domain (Shirakabe et al. 2006; Devogelaere et al. 2007; Lee et al. 2012), binds to and thereby counteracts the auto-inhibition of NBCe1-B (Shirakabe et al. 2006; Lee et al. 2012; Hong et al. 2013; Lee & Boron, 2018). The dephosphorylation of Ser residues in NBCe1-B N-terminal domain may also contribute (Vachel et al. 2018).
In the present study, MAc, MAlk and HSD generally increase expression of the IRBITs in the OM – L-IRBIT usually more so than IRBIT – in parallel with the upregulation of NBCn1 and NBCn2. Single-cell RNAseq data (https://cello.shinyapps.io/kidneycellexplorer/) show that transcripts of IRBIT and L-IRBIT are present throughout the kidney. In the OM, the transcripts are most abundant in the PT, mTAL and collecting duct. Further studies will be required to test the hypothesis that our three challenges specifically cause IRBIT and L-IRBIT protein levels to rise in the mTAL. If so, the up regulation of IRBITs would increase their interaction with NBCn1 and NBCn2, multiplying the Na/HCO3 cotransport activity in the mTAL beyond what one would expect based simply on the increase in protein levels of NBCn1 and NBCn2.
Thus, we make our fifth proposal; that, in mTAL, the generally increased expression of the IRBITs – by virtue of their stimulation of NBCn1 and NBCn2, which play the roles proposed above – is the appropriate adaptive response to the three challenges.
We believe it is unlikely that the increased expression levels of the two NBCs (i.e. NBCn1 and NBCn2) and their two most powerful activators (i.e. the IRBITs) – produced by each of three challenges – is random. The increases are generally several-fold in magnitude and in each case, if the enhanced expression of IRBITs occurs in the mTAL, would synergize to produce massive increases in transport activity. Nevertheless, each of the three challenges produces a different quantitative pattern of changes in the four proteins (Figs 4,7,8 and 10), which is what one might expect if each challenge triggered a distinct regulatory pathway. Moreover, the oocyte data show that the IRBITs each stimulate NBCn1 and NBCn2 to markedly different degrees. Differential regulation of NBCn1 vs. NBCn2, or IRBIT vs. L-IRBIT, could exploit the unique properties of each protein. For example, NBCn1 is unique among NBCs in having an intrinsic Na+ conductance (Choi et al. 2000). A major difference between the IRBIT and L-IRBIT molecules is in the Nt extension of L-IRBIT. Different Nt variants of L-IRBIT differentially affect target specificity (Kawaai et al. 2017) and have markedly different levels of stimulation on NBCe1-B (Kawaai et al. 2017; Wang et al. 2020). Similarly, different Nt variants of both IRBITs differentially affect the effect of the phosphorylation of the PEST domain (Wang et al. 2020).
Potential modes of signalling
A question that arises is which signal-transduction systems could underlie the upregulation of the NBCs and IRBITs by three diverse challenges. In the case of MAc and MAlk, long-term decreases or increases, respectively, in pHi could increase the expression of all four proteins. RPTPγ, which appears to be activated in response to increased [CO2]o or decreased [HCO3−]o (Zhou et al. 2016) and which is present in the mTAL by single-cell RNAseq (https://cello.shinyapps.io/kidneycellexplorer/), could likewise contribute. In the case of HSD, increased Ang II levels, which lead to reduced Na+ reabsorption, could in part act by upregulating the NBCs and IRBITs.
In each of the above cases, the challenges could affect not only total levels of the NBC proteins, but expression at the basolateral membrane. Moreover, the signal-transduction processes could produce different phosphorylation patterns on different IRBIT vs. L-IRBIT variants, and therefore different degrees of activation of different targets.
Consideration of apparent inconsistencies
Thus far in the Discussion, we have argued that the observed results (i.e. upregulation of NBCn1, NBCn2, and the IRBITs) are appropriate responses to at least some of the consequences of the three disparate challenges (i.e. MAc, MAlk, and HSD). However, the reader may imagine that, for example, an adaptation that is appropriate for NH4+ reabsorption by the mTAL is simultaneously inappropriate for HCO3− reabsorption. Here, we will address apparent inconsistencies for each challenge in turn.
Under MAc caused by NH4Cl intake, would increased NBCn1 and NBCn2 activities suppress HCO3− reabsorption in mTAL, resulting in inappropriate excretion of HCO3− into the urine? Because MAc reduces plasma [HCO3−] and thus [HCO3−] in the glomerular filtrate, the requirement for HCO3− reabsorption (assuming GFR is constant) along the nephron is actually lower than normal. The proper adaptation of the kidney is the creation of new HCO3−, which it achieves in large part by increased ammoniagenesis (mainly in the PT) and H+ secretion. At the level of the mTAL, the critical response is increased NH4+ uptake across the apical membrane, ultimately for shunting into the collecting duct. Any diminution of mTAL HCO3− reabsorption would be largely inconsequential due to the decreased HCO3− delivery to the mTAL. Moreover, we speculate that the increases in NBCn1 and NBCn2 activities in the mTAL are just enough to titrate the increased amounts of entering NH4+, with little effect on JHCO3. This hypothesis requires testing in functional experiments.
One might worry that the increased NBCn1 and NBCn2 activities would decrease JNaCl. However, because NH4Cl treatment does not change the expression of AE2 in the medulla of rat kidney (Frische et al. 2004; Quentin et al. 2004), there is no reason to suspect that Cl− uptake across the basolateral membrane would rise. Although increased NBCn1 and NBCn2 activities would raise the Na+ backflux, we speculate that increased activity of basolateral Na+-K+ pump could balance this effect. Again, this hypothesis requires testing in functional experiments.
Under MAlk caused by NaHCO3 intake, would an increase NBCn1 and NBCn2 activities promote NH4+ reabsorption by the mTAL, causing an inappropriate increase in urinary acid excretion? The proper adaptation of the kidney to MAlk is to reduce HCO3− reclamation along the nephron (thereby increasing urinary HCO3− excretion) and to reduce the creation of new HCO3−, which it achieves by decreasing ammoniagenesis and H+ secretion. Thus, less NH4+ reaches the mTAL during MAlk, so that any increased propensity for NH4+ reabsorption would be of less consequence. During an NaHCO3 load (i.e. MAlk), increased NBCn1, NBCn2 and AE2 activities would decrease JNaCl. A reduction in JHCO3 would require that the activities of NBCn1 and NBCn2 increase more than that of AE2 (↑↑JNBCs > ↑JAE2 → ↓JHCO3, Fig. 13B). Again, this hypothesis requires testing in functional experiments.
Under HSD, would the increased NBCn1 and NBCn2 activities suppress HCO3− reabsorption by the mTAL? The proper – and observed – adaptation of the kidney to HSD is to reduce JNaCl while leaving acid-base homeostasis unaffected. The mTAL could achieve these effects by increasing the activities of NBCn1 plus NBCn2 to just match that of AE2 (↑↑JNBCs =↑↑JAE2 >↑JNa-K →↓JNa+, Fig. 13C). Such a perfectly matched upregulation would leave intracellular HCO3− homeostasis unaffected, and thus produce no change in either JHCO3 or JNH4+. Testing this hypothesis would require data on the effects of HSD on the activities of all three basolateral HCO3− transporters.
An earlier tubule-perfusion study (Good, 1990) has shown that chronic MAc, MAlk, and HSD – under the conditions of the experiments – all increase HCO3− reabsorbtion by the mTAL (paradoxically in the case of MAlk and HSD). These experiments also showed that MAc and MAlk both increase NH4+ reabsorption (paradoxically in the case of MAlk). Although the three paradoxical observations appear to conflict with our proposals, in fact they are not germane to our hypotheses for two major reasons. First, although the mTAL environment is hypertonic and the luminal composition of the mTAL depends heavily on events upstream in the nephron, investigators traditionally expose isolated perfused mTALs to luminal and basolateral solutions identical to those of an early PT. Second, to mimic a challenge, the luminal and basolateral solution compositions must appropriately shift to match those experienced by the mTAL in situ. For example, to mimic MAc, one would have to decrease luminal [HCO3−], greatly increase luminal [NH4+], and decrease basolateral pH and [HCO3−] – and do the opposite in the case of MAlk.
Potential sex effect
In the present study, we focus on female rats. We collected rat data at 1 and 2 weeks. Because rats come into oestrus about every 4–5 days, they would have gone through ~1.5 cycles for our 1-week experiments, and ~3 cycles for our 2-week experiments. Moreover, each group, at least for the whole-kidney data, had a minimum of eight rats. Thus, we believe that the effects of natural hormonal fluctuations should have averaged out over the course of our studies and number of animals.
The roles of transporters in renal electrolyte handling might be sex-specific (Veiras et al. 2017; Harris et al. 2019). The potential sexual differences remain to be addressed in the future.
Concluding remarks
In the present study, we make the surprising findings that MAc, MAlk and HSD each produce large increases in the expression of NBCn1, NBCn2 and the IRBITs. We propose that NBCn1 and NBCn2: (1) promote transepithelial NH4+ reabsorption during MAc by neutralizing intracellular NH; (2) inhibit NaCl reabsorption during NaHCO3-induced MAlk and also inhibit HCO3− reabsorption by opposing basolateral AE2; and (3) inhibit NaCl reabsorption (in concert with AE2) during Na+ loads by mediating net NaCl backflux across the basolateral membrane. (4) The IRBITs further amplify these roles of NBCn1 and NBCn2. Thus, NBCn1 and NBCn2 plus their potent activators, the IRBITs, appear to be at the nexus of adaptations to a broad array of physiological and pathophysiological stimuli.
Supplementary Material
Keypoints.
The roles of the Na+/HCO3− cotransporters NBCn1 and NBCn2 as well as their activators IRBIT and L-IRBIT in the regulation of the mTAL transport of NH4+, HCO3−, and NaCl are investigated.
Dietary challenges of NH4Cl, NaHCO3 or NaCl all increase the abundance of NBCn1 and NBCn2 in the outer medulla.
The three challenges generally produce parallel increases in the abundance of IRBIT and L-IRBIT in the outer medulla.
Both IRBIT and L-IRBIT powerfully stimulate the activities of the mTAL isoforms of NBCn1 and NBCn2 as expressed in Xenopus oocytes.
Our findings support the hypothesis that NBCn1, NBCn2, IRBIT and L-IRBIT appropriately promote NH4+ shunting but oppose HCO3− and NaCl reabsorption in the mTAL, and thus are at the nexus of the regulation pathways for multiple renal transport processes.
Funding
This work was supported by NSFC grants #81571388 (LMC), #31771294 (LMC), and #31571201 (YL), NIH grants U01-GM111251-05 (WFB) and R01-DK113197 (WFB), ONR grant N00014-16-1-2535 (WFB). RMA was supported by FAPESP grant (2018/22855-1). WFB gratefully acknowledges the support of the Myers/Scarpa endowed chair.
Biography
Jin-Lin Wang received her BSc degree in Biological Sciences from Xinyang Normal University, Henan, China, in 2013. She joined the School of Life Science and Technology at Huazhong University of Science and Technology, Wuhan, China, in 2013, and is now a PhD student. She is interested in the regulation of systemic acid-base and electrolyte balance, focusing on the physiological roles of the Na+/HCO3− cotransporters in the gastrointestinal tract and the kidneys.

Xiao-Yu Wang received her BSc degree in Biological Sciences from Huaibei Normal University, Anhui, China, in 2014. She joined the School of Life Science and Technology at Huazhong University of Science and Technology in 2014, and is now a PhD student. She is interested in the regulation of acid-base and electrolyte balance in the kidney, focusing on the expression of the IRBITs, and their roles in regulating the Na+/HCO3− cotransporters.

Footnotes
Additional information
Competing interests
The authors declared that no competing interest existed.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Statistical Summary Document
References
- Alper SL, Stuart-Tilley AK, Biemesderfer D, Shmukler B & Brown D (1997). Immunolocalization of AE2 anion exchanger in rat kidney. Am J Physiol 273, F601–F614. [DOI] [PubMed] [Google Scholar]
- Amlal H, Chen Q, Greeley T, Pavelic L & Soleimani M (2001). Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int 60, 1824–1836. [DOI] [PubMed] [Google Scholar]
- Amlal H, Paillard M & Bichara M (1994). NH4+ transport pathways in cells of medullary thick ascending limb of rat kidney. NH4+ conductance and K+/NH4+ (H+) antiport. J Biol Chem 269, 21962–21971. [PubMed] [Google Scholar]
- Ando H, Mizutani A, Matsu-ura T & Mikoshiba K (2003). IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem 278, 10602–10612. [DOI] [PubMed] [Google Scholar]
- Boedtkjer E, Praetorius J, Fuchtbauer EM & Aalkjaer C (2008). Antibody-independent localization of the electroneutral Na+,HCO3− cotransporter NBCn1 (Slc4a7) in mice. Am J Physiol Cell Physiol 294, C591–603. [DOI] [PubMed] [Google Scholar]
- Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Fuchtbauer AC, Simonsen U, Fuchtbauer EM & Aalkjaer C (2011). Disruption of Na+,HCO3− cotransporter NBCn1 (Slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in mice. Circulation 124, 1819–1829. [DOI] [PubMed] [Google Scholar]
- Boger CA, Gorski M, McMahon GM, Xu H, Chang YC, van der Most PJ, Navis G, Nolte IM, de Borst MH, Zhang W, Lehne B, Loh M, Tan ST, Boerwinkle E, Grams ME, Sekula P, Li M, Wilmot B, Moon JG, Scheet P, Cucca F, Xiao X, Lyytikainen LP, Delgado G, Grammer TB, Kleber ME, Sedaghat S, Rivadeneira F, Corre T, Kutalik Z, Bergmann S, Nielson CM, Srikanth P, Teumer A, Muller-Nurasyid M, Brockhaus AC, Pfeufer A, Rathmann W, Peters A, Matsumoto M, de Andrade M, Atkinson EJ, Robinson-Cohen C, de Boer IH, wang SJ, Heid IM, Gogele M, Concas MP, Tanaka T, Bandinelli S, Nalls MA, Singleton A, Tajuddin SM, Adeyemo A, Zhou J, Doumatey A, McWeeney S, Murabito J, Franceschini N, Flessner M, Shlipak M, Wilson JG, Chen G, Rotimi CN, Zonderman AB, Evans MK, Ferrucci L, Devuyst O, Pirastu M, Shuldiner A, Hicks AA, Pramstaller PP, Kestenbaum B, Kardia SLR, Turner ST, Study LC, Briske TE, Gieger C, Strauch K, Meisinger C, Meitinger T, Volker U, Nauck M, Volzke H, Vollenweider P, Bochud M, Waeber G, Kahonen M, Lehtimaki T, Marz W, Dehghan A, Franco OH, Uitterlinden AG, Hofman A, Taylor HA, Chambers JC, Kooner JS, Fox CS, Hitzemann R, Orwoll ES, Pattaro C, Schlessinger D, Kottgen A, Snieder H, Parsa A & Cohen DM (2017). NFAT5 and SLC4A10 loci associate with plasma osmolality. J Am Soc Nephrol 28, 2311–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois S, Meer LV, Wootla B, Bloch-Faure M, Chambrey R, Shull GE, Gawenis LR & Houillier P (2010). NHE4 is critical for the renal handling of ammonia in rodents. J Clin Invest 120, 1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MB & Green N (1973). Function of the thick ascending limb of Henle’s loop. Am J Physiol 224, 659–668. [DOI] [PubMed] [Google Scholar]
- Cabral PD & Herrera M (2012). Membrane-associated aquaporin-1 facilitates osmotically driven water flux across the basolateral membrane of the thick ascending limb. Am J Physiol Renal Physiol 303, F621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso G, Unwin R, Agulian S & Giebisch G (1991). Bicarbonate transport along the loop of Henle. I. Microperfusion studies of load and inhibitor sensitivity. J Clin Invest 88, 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso G, Unwin R, Ciani F, De Santo NG, De Tommaso G, Russo F & Giebisch G (1994). Bicarbonate transport along the loop of Henle. II. Effects of acid-base, dietary, and neurohumoral determinants. J Clin Invest 94, 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambrey R, St John PL, Eladari D, Quentin F, Warnock DG, Abrahamson DR, Podevin RA & Paillard M (2001). Localization and functional characterization of Na+/H+ exchanger isoform NHE4 in rat thick ascending limbs. Am J Physiol Renal Physiol 281, F707–717. [DOI] [PubMed] [Google Scholar]
- Choi I, Aalkjær C, Boulpaep EL & Boron WF (2000). An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405, 571–575. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Nielsen S & Praetorius J (2006). An anti-NH2-terminal antibody localizes NBCn1 to heart endothelia and skeletal and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 290, H172–H180. [DOI] [PubMed] [Google Scholar]
- Delpire E & Gagnon KB (2018). Na+-K+−2Cl− cotransporter (NKCC) physiological function in nonpolarized cells and transporting epithelia. Compr Physiol 8, 871–901. [DOI] [PubMed] [Google Scholar]
- Devogelaere B, Beullens M, Sammels E, Derua R, Waelkens E, van Lint J, Parys JB, Missiaen L, Bollen M & De Smedt H (2007). Protein phosphatase-1 is a novel regulator of the interaction between IRBIT and the inositol 1,4,5-trisphosphate receptor. Biochem J 407, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sober S, Parsa A, Luan J, Arora P,Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden F, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der HP, Kao WH, Sjogren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimaki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O’Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kahonen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Kottgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grassler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Artigas MS, Dong Y, Snieder H, Wang X, Zhu H, Lohman K, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S,Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A,Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stancakova A, Raffel LJ, Yao J, Kathiresan S, O’Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT Jr., Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, la-Korpela M, Kangas AJ, Lyytikainen LP, Soininen P, Tukiainen T, Wurtz P, Ong RT, Dorr M, Kroemer HK, Volker U, Volzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD & Zhai G (2011). Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton RA & Knepper MA (2007). Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87, 1083–1112. [DOI] [PubMed] [Google Scholar]
- Frische S, Zolotarev AS, Kim YH, Praetorius J, Alper S, Nielsen S & Wall SM (2004). AE2 isoforms in rat kidney: immunohistochemical localization and regulation in response to chronic NH4Cl loading. Am J Physiol Renal Physiol 286, F1163–1170. [DOI] [PubMed] [Google Scholar]
- Froissart M, Borensztein P, Houillier P, Leviel F, Poggioli J, Marty E, Bichara M & Paillard M (1992). Plasma membrane Na+-H+ antiporter and H+-ATPase in the medullary thick ascending limb of rat kidney. Am J Physiol 262, C963–970. [DOI] [PubMed] [Google Scholar]
- Garvin JL, Burg MB & Knepper MA (1988). Active NH4+ absorption by the thick ascending limb. Am J Physiol 255, F57–F65. [DOI] [PubMed] [Google Scholar]
- Good DW (1990). Adaptation of HCO3− and NH4+ transport in rat mTAL: effects of chronic metabolic acidosis and Na+ intake. Am J Physiol 258, F1345–1353. [DOI] [PubMed] [Google Scholar]
- Good DW, George T & Watts BA 3rd, (2006). Nongenomic regulation by aldosterone of the epithelial NHE3 Na+/H+ exchanger. Am J Physiol Cell Physiol 290, C757–763. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YM, Liu Y, Liu M, Wang JL, Xie ZD, Chen KJ, Wang DK, Occhipinti R, Boron WF & Chen LM (2017). Na+/HCO3− cotransporter NBCn2 mediates HCO3− reclamation in the apical membrane of renal proximal tubules. J Am Soc Nephrol 28, 2409–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AN, Lee HW, Fang L, Verlander JW & Weiner ID (2019). Differences in acidosis-stimulated renal ammonia metabolism in the male and female kidney. Am J Physiol Renal Physiol 317, F890–F905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SC & Andreoli TE (1984). Control of NaCl transport in the thick ascending limb. Am J Physiol 246, F745–756. [DOI] [PubMed] [Google Scholar]
- Hebert SC, Culpepper RM & Andreoli TE (1981). NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol 241, F412–F431. [DOI] [PubMed] [Google Scholar]
- Hennings JC, Andrini O, Picard N, Paulais M, Huebner AK, Cayuqueo IK, Bignon Y, Keck M, Corniere N, Bohm D, Jentsch TJ, Chambrey R, Teulon J, Hubner CA & Eladari D (2017). The ClC-K2 chloride channel is critical for salt handling in the distal nephron. J Am Soc Nephrol 28, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Yang D, Shcheynikov N, Ohana E, Shin DM & Muallem S (2013). Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3− cotransporters family. Proc Natl Acad Sci USA 110, 4105–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaai K, Ando H, Satoh N, Yamada H, Ogawa N, Hirose M, Mizutani A, Bonneau B, Seki G & Mikoshiba K (2017). Splicing variation of Long-IRBIT determines the target selectivity of IRBIT family proteins. Proc Natl Acad Sci USA 114, 3921–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikeri D, Azar S, Sun A, Zeidel ML & Hebert SC (1990). Na+-H+ antiporter and Na+-(HCO3−)n symporter regulate intracellular pH in mouse medullary thick limbs of Henle. Am J Physiol 258, F445–F456. [DOI] [PubMed] [Google Scholar]
- Kwon TH, Fulton C, Wang W, Kurtz I, Frokiær J, Aalkjær C & Nielsen S (2002). Chronic metabolic acidosis upregulates rat kidney Na-HCO3− cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol-Renal Physiol 282, F341–F351. [DOI] [PubMed] [Google Scholar]
- Laghmani K, Chambrey R, Froissart M, Bichara M, Paillard M & Borensztein P (1999). Adaptation of NHE-3 in the rat thick ascending limb: effects of high sodium intake and metabolic alkalosis. Am J Physiol 276, F18–F26. [DOI] [PubMed] [Google Scholar]
- Lee SK & Boron WF (2018). Exploring the autoinhibitory domain of the electrogenic Na+/HCO3− transporter NBCe1-B, from residues 28 to 62. J Physiol 596, 3637–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Boron WF & Parker MD (2012). Relief of autoinhibition of the electrogenic Na-HCO3 cotransporter NBCe1-B: role of IRBIT vs.amino-terminal truncation. Am J Physiol Cell Physiol 302, C518–C526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux G, Junco E, Perez R, Lemieux C & Allignet E (1985). The metabolic response of the kidney to acute sodium lactate alkalosis. Can J Physiol Pharmacol 63, 687–692. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin X, Wang DK, Guo YM, Gill HS, Morris N, Parker MD, Chen LM & Boron WF (2013a). Effects of optional structural elements, including two alternative amino termini and a new splicing cassette IV, on the function of NBCn1 (SLC4A7). J Physiol 591, 4983–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang DK, Jiang DZ, Qin X, Xie ZD, Wang QK, Liu M & Chen LM (2013b). Cloning and functional characterization of novel variants and tissue-specific expression of alternative amino and carboxyl termini of products of Slc4a10. PLoS One 8, e55974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa-Aziz R, Boron WF & Parker MD (2010). Using fluorometry and ion-sensitive microelectrodes to study the functional expression of heterologously-expressed ion channels and transporters in Xenopus oocytes. Methods 51, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa-Aziz R, Chen LM, Pelletier MF & Boron WF (2009). Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106, 5406–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM & Hamm LL (2001). Transport of NH3/NH4+ in oocytes expressing aquaporin-1. Am J Physiol-Renal Physiol 281, F255–F263. [DOI] [PubMed] [Google Scholar]
- Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjær C & Leipziger J (2004). Basolateral Na+-dependent HCO3− transporter NBCn1-mediated HCO3− influx in rat medullary thick ascending limb. J Physiol 555, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MD, Daly CM, Skelton LA & Boron WF (2007a). IRBIT functionally enhances the electroneutral Na+-coupled bicarbonate transporter NCBE by sequestering an N-terminal autoinhibitory domain. FASEB J 21, A1285. [Google Scholar]
- Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM & Boron WF (2008). Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl− self-exchange activity. J Biol Chem 283, 12777–12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MD, Skelton LA, Daly CM & Boron WF (2007b). IRBIT binds to and functionally enhances the electroneutral Na+-coupled bicarbonate transporters NBCn1, NDCBE and NCBE. FASEB J 21, A1285. [Google Scholar]
- Praetorius J, Kim YH, Bouzinova EV, Frische S, Rojek A, Aalkjær C & Nielsen S (2004). NBCn1 is a basolateral Na+-HCO3− cotransporter in rat kidney inner medullary collecting ducts. Am J Physiol-Renal Physiol 286, F903–F912. [DOI] [PubMed] [Google Scholar]
- Quentin F, Eladari D, Frische S, Cambillau M, Nielsen S, Alper SL, Paillard M & Chambrey R (2004). Regulation of the Cl−/HCO3− exchanger AE2 in rat thick ascending limb of Henle’s loop in response to changes in acid-base and sodium balance. J Am Soc Nephrol 15, 2988–2997. [DOI] [PubMed] [Google Scholar]
- Rocha AS & Kokko JP (1973). Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest 52, 612–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL & Boron WF (1997). Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature 387, 409–413. [DOI] [PubMed] [Google Scholar]
- Russell JM (2000). Sodium-potassium-chloride cotransport. Physiol Rev 80, 211–276. [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G & Mikoshiba K (2006). IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1). Proc Natl Acad Sci USA 103, 9542–9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober S, Org E, Kepp K, Juhanson P, Eyheramendy S, Gieger C, Lichtner P, Klopp N, Veldre G, Viigimaa M, Doring A, Kooperative Gesundheitsforschung in der Region Augsburg S, Putku M, Kelgo P, Study HYiE, Shaw-Hawkins S, Howard P, Onipinla A, Dobson RJ, Newhouse SJ, Brown M, Dominiczak A, Connell J, Samani N, Farrall M, Study MRCBGOH, Caulfield MJ, Munroe PB, Illig T, Wichmann HE, Meitinger T & Laan M (2009). Targeting 160 candidate genes for blood pressure regulation with a genome-wide genotyping array. PLoS One 4, e6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AM (1998). Expression of Cl−/HCO3− exchanger in the basolateral membrane of mouse medullary thick ascending limb. Am J Physiol 274, F358–364. [DOI] [PubMed] [Google Scholar]
- Udwan K, Abed A, Roth I, Dizin E, Maillard M, Bettoni C, Loffing J, Wagner CA, Edwards A & Feraille E (2017). Dietary sodium induces a redistribution of the tubular metabolic workload. J Physiol 595, 6905–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachel L, Shcheynikov N, Yamazaki O, Fremder M, Ohana E, Son A, Shin DM, Yamazaki-Nakazawa A, Yang CR, Knepper MA & Muallem S (2018). Modulation of Cl− signaling and ion transport by recruitment of kinases and phosphatases mediated by the regulatory protein IRBIT. Sci Signal 11, eaat5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL & McDonough AA (2017). Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28, 3504–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorum H, Kwon TH, Fulton C, Simonsen B, Choi I, Boron W, Maunsbach AB, Nielsen S & Aalkjær C (2000). Immunolocalization of electroneutral Na-HCO3− cotransporter in rat kidney. Am J Physiol-Renal Physiol 279, F901–F909. [DOI] [PubMed] [Google Scholar]
- Wang DK, Liu Y, Myers EJ, Guo YM, Xie ZD, Jiang DZ, Li JM, Yang J, Liu M, Parker MD & Chen LM (2015). Effects of Nt-truncation and coexpression of isolated Nt domains on the membrane trafficking of electroneutral Na+/HCO3− cotransporters. Sci Rep 5, 12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li H, Yang B, Guo L, Han X, Li L, Li M, Huang J & Gu D (2017). The hypertension risk variant RS820430 functions as an enhancer of SLC4A7. Am J Hypertens 30, 202–208. [DOI] [PubMed] [Google Scholar]
- Wang M, Wu H, Liu Y & Chen L-M (2020). Activation of mouse NBCe1-B by Xenopus laevis and mouse IRBITs: role of the variable Nt appendage of IRBITs. BBA-Biomembranes 1862, 183240. [DOI] [PubMed] [Google Scholar]
- Watts BA 3rd, & Good DW (1994). Effects of ammonium on intracellular pH in rat medullary thick ascending limb: mechanisms of apical membrane NH4+ transport. J Gen Physiol 103, 917–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ID & Verlander JW (2017). Ammonia transporters and their role in acid-base balance. Physiol Rev 97, 465–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Shcheynikov N & Muallem S (2011). IRBIT: it is everywhere. Neurochem Res 36, 1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchia M, Capolongo G, Rinaldi L & Capasso G (2018). The importance of the thick ascending limb of Henle’s loop in renal physiology and pathophysiology. Int J Nephrol Renovasc Dis 11, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Tomilin V, Mamenko M, Bhalla V & Pochynyuk O (2016). New perspective of ClC-Kb/2 Cl− channel physiology in the distal renal tubule. Am J Physiol Renal Physiol 310, F923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Skelton LA, Xu L, Chandler MP, Berthiaume JM & Boron WF (2016). Role of receptor protein tyrosine phosphatase gamma in sensing extracellular CO2 and HCO3. J Am Soc Nephrol 29, 2616–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.