Significance
Asymptomatic infections have been widely reported for COVID-19. However, many studies do not distinguish between the presymptomatic stage and truly asymptomatic infections. We conducted a systematic review and meta-analysis of COVID-19 literature reporting laboratory-confirmed infections to determine the burden of asymptomatic infections and removed index cases from our calculations to avoid conflation. By analyzing over 350 papers, we estimated that more than one-third of infections are truly asymptomatic. We found evidence of greater asymptomaticity in children compared with the elderly, and lower asymptomaticity among cases with comorbidities compared to cases with no underlying medical conditions. Greater asymptomaticity at younger ages suggests that heightened vigilance is needed among these individuals, to prevent spillover into the broader community.
Keywords: asymptomatic fraction, presymptomatic, silent transmission, novel coronavirus, comorbidity
Abstract
Quantification of asymptomatic infections is fundamental for effective public health responses to the COVID-19 pandemic. Discrepancies regarding the extent of asymptomaticity have arisen from inconsistent terminology as well as conflation of index and secondary cases which biases toward lower asymptomaticity. We searched PubMed, Embase, Web of Science, and World Health Organization Global Research Database on COVID-19 between January 1, 2020 and April 2, 2021 to identify studies that reported silent infections at the time of testing, whether presymptomatic or asymptomatic. Index cases were removed to minimize representational bias that would result in overestimation of symptomaticity. By analyzing over 350 studies, we estimate that the percentage of infections that never developed clinical symptoms, and thus were truly asymptomatic, was 35.1% (95% CI: 30.7 to 39.9%). At the time of testing, 42.8% (95% prediction interval: 5.2 to 91.1%) of cases exhibited no symptoms, a group comprising both asymptomatic and presymptomatic infections. Asymptomaticity was significantly lower among the elderly, at 19.7% (95% CI: 12.7 to 29.4%) compared with children at 46.7% (95% CI: 32.0 to 62.0%). We also found that cases with comorbidities had significantly lower asymptomaticity compared to cases with no underlying medical conditions. Without proactive policies to detect asymptomatic infections, such as rapid contact tracing, prolonged efforts for pandemic control may be needed even in the presence of vaccination.
COVID-19 surveillance provides real-time information about the epidemiological trajectory of the pandemic, informing risk assessments and mitigation policies around the world. Given that COVID-19 surveillance systems predominantly rely on symptom-based screening, the prevalence of asymptomatic infection is often not fully captured. Cross-sectional surveys, such as mass testing once an outbreak is identified, do not distinguish the truly asymptomatic from the presymptomatic. Often, the follow-up period after testing is too brief to ascertain whether patients subsequently develop symptoms. The percentage of silent infections identified by such studies is thus context specific, as it depends on the setting, phase of the epidemic, and efficiency of contact tracing. By contrast, the prevalence of truly asymptomatic infections should be stable across similar demographic settings, regardless of epidemiological trajectory and contact tracing.
Compounded by ambiguities about the different clinical manifestations of the disease, which can lead to misinterpretation of clinical and epidemiological studies (1), there have been substantial aberrations in reports and media coverage claiming the asymptomatic percentage to be as low as 4% (2, 3) or as high as 80 to 90% (4, 5). Similarly, the US Centers for Disease Control and Prevention guidelines for COVID-19 pandemic forecasting offer wide bounds for the asymptomatic percentage, ranging from 10 to 70% (6).
Previous meta-analyses of 41 studies (7), 13 studies (8), and 79 studies (9) estimate pooled asymptomaticity ranging from 16 to 20%. Two methodological issues limit the accuracy of these studies. First, pooled asymptomaticity reported in these studies is likely biased downward because they did not account for study designs which have a higher representation of cases experiencing symptoms (10). Second, one of the meta-analyses (7) did not consider biases in reported asymptomaticity that can arise from inadequate longitudinal follow-up. Studies that assess the symptom profile only at the time of testing or do not follow up symptoms for a sufficiently long time period cannot distinguish presymptomatic from asymptomatic infection, overestimating those that are truly asymptomatic.
Accurate estimates of true disease prevalence, including asymptomatic infections, are essential to calculate key clinical parameters, project epidemiological trajectories, and optimize mitigation measures. Clinical evidence indicates that viral loads among asymptomatic and symptomatic infections may be comparable (11–15). Unaware of their risk to others, individuals with silent infections are likely to continue usual behavior patterns. Accounting for silent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in the assessment of disease control measures is necessary to interrupt community transmission (16). Although the discrepancy between reported incidence and seroprevalence gives a sense of the extent of asymptomaticity, not all symptomatic cases are reported, and not all asymptomatic cases (for instance, those identified on the basis of exposure) are missed. Consequently, it is not sufficient to simply compare the reported cases to results from seroprevalence studies. We therefore conducted a systematic review and meta-analysis of COVID-19 literature reporting laboratory-confirmed infections to estimate the percentage of SARS-CoV-2 infections that are truly asymptomatic. We also investigated differences in asymptomaticity with respect to age, sex, comorbidity, study design, publication date, duration of symptom follow-up, geographic location, and setting.
Results
We identified a total of 114,124 abstracts based on our search criteria. After excluding duplicate and irrelevant studies, we used 390 in our meta-analyses (Fig. 1 and SI Appendix, Table S2). Most studies were conducted in China (n = 104, 27%), followed by the United States (n = 74, 19%), Italy (n = 21, 5%), and South Korea (n = 13, 3%). These studies included a total of 104,058 laboratory-confirmed COVID-19 cases, of which 25,050 exhibited no symptoms at the time of testing and 7,220 remained asymptomatic. We identified 170 studies that reported asymptomatic infections (11–13, 17–183), 332 studies that reported silent infections at the time of testing (10–12, 14, 17–20, 23–27, 31, 32, 35–40, 42–44, 46, 47, 49, 50, 52, 53, 56–58, 60–66, 68, 69, 73–75, 77–79, 81, 84, 87, 90–94, 97, 99, 101, 103, 104, 106, 111, 113–116, 118, 119, 121–123, 125, 127, 128, 131, 133, 135, 137, 138, 140, 143, 145, 146, 148–152, 154, 156, 158, 160–163, 166–170, 172–174, 176, 177, 179, 180, 182–405), and 143 that delineated presymptomatic and asymptomatic infections by following-up with those silently infected (11–13, 17–20, 22–29, 31–33, 35–40, 42–44, 46–54, 56–70, 72–75, 77–81, 83, 84, 87, 89–94, 96, 97, 99, 101, 103, 104, 106–109, 111–119, 121–125, 127–129, 131, 133, 135–138, 140, 141, 143, 145–156, 158–164, 166–170, 172–174, 176–183). Among the studies that reported follow-up of clinical symptoms after testing, 11.0% reported at time points at 1 wk to 2 wk, 33.8% reported at 2 wk to 3 wk, and 55.2% reported longer than 3 wk. Among the studies that reported asymptomatic infections, 58.8% reported zero index cases, either because cases were identified through a screening design or because the study only reported the cases that were identified through contact tracing. Of the 41.2% studies that reported data on index cases, these included household members, long-term care residents, members of the community, or travelers returning from COVID-19 hotspots (SI Appendix, Table S1). A summary of the risk of bias assessment is presented in SI Appendix, Table S2. Out of the 170 studies included in the calculation of asymptomaticity, 75 had low risk of bias, 10 had moderate risk of bias, and 85 had serious risk of bias.
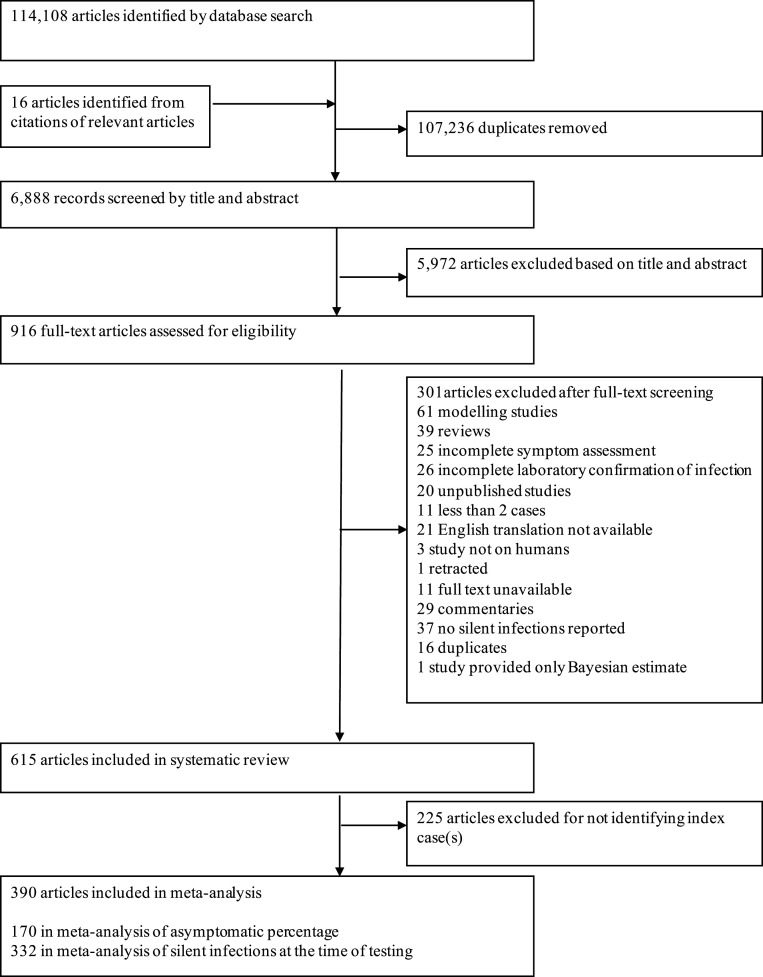
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the numbers of studies screened and included in the meta-analysis.
The percentage of cases that were truly asymptomatic among laboratory-confirmed cases was 35.1% (95% CI: 30.7 to 39.9%; Fig. 2). By contrast, a larger percentage of cases exhibited no symptoms at the time of testing (42.8%, 95% prediction interval: 5.2 to 91.1%) due to mischaracterization of presymptomatic cases as asymptomatic. To investigate the degree of mischaracterization, we considered a subset of studies that reported symptoms both at the time of testing and a minimum of 7 d after. Within this subset of studies, 31.8% (95% prediction interval: 5.6 to 78.7%) of cases exhibiting no symptoms at the time of testing progressed to develop symptoms. The percentage of truly asymptomatic cases among these studies was therefore 36.9% (95% CI: 31.8 to 42.4%), similar to that estimated for all studies reporting asymptomatic infections.
Fig. 2.
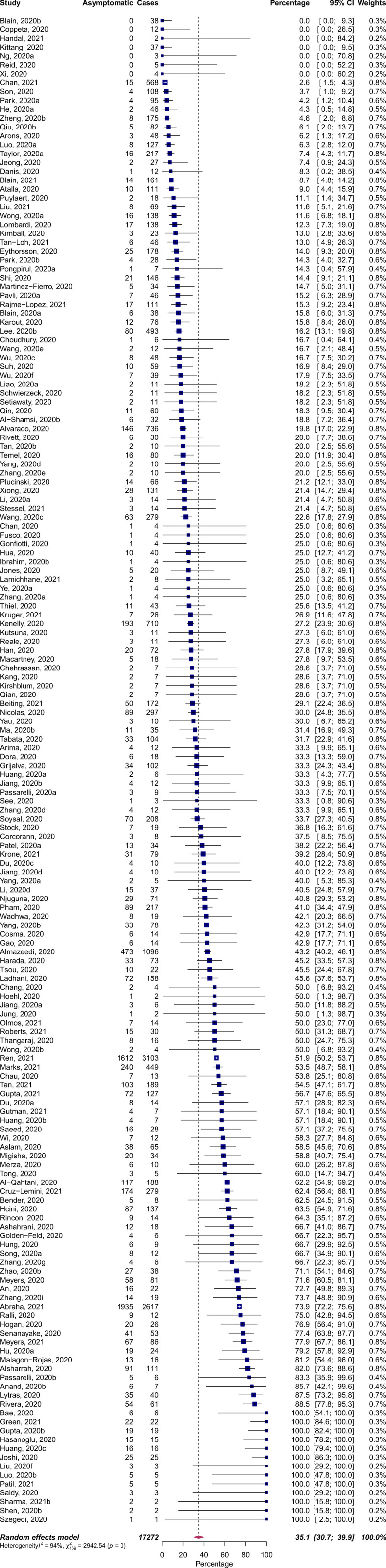
Pooled percentage of laboratory-confirmed COVID-19 cases which remained asymptomatic. Studies that did not report follow-up of silent infections or failed to identify index cases were excluded from the analysis.
These estimates were obtained after removing index cases from our calculations, correcting bias toward overrepresentation of symptomatic cases that would lead to underestimation of asymptomaticity. Without excluding index cases, estimates of asymptomatic infections using our two complementary approaches would be 27.8% (95% CI: 24.3 to 31.7%) and 29.4 (95% CI: 25.2 to 33.9%). To evaluate the impact of sample selection bias arising from higher participation among those experiencing symptoms, we next restricted our analysis to 25 studies in which complete screening of every individual present at the setting was performed. The pooled asymptomaticity among this smaller subset of studies was 47.3% (95% CI: 34.0 to 61.0%).
We found a statistically significant trend toward a lower asymptomatic percentage with increasing age (P < 0.01; Table 1). In pairwise comparisons, the asymptomatic percentage was significantly lower for the elderly, at 19.7% (95% CI: 12.7 to 29.4%) compared with 46.7% (95% CI: 32.0 to 62.0%) for children (P < 0.01). Asymptomaticity also varied across study settings (P = 0.03; Table 1). In particular, studies on long-term care facilities reported lower asymptomaticity compared with studies on healthcare facilities (P = 0.04) and household transmission (P = 0.04). We found no association between asymptomatic percentage and geographic location, study design, follow-up duration, or publication date (Table 1). We found that asymptomaticity in males was similar to that in females (log incidence rate ratio [IRR] 0.09, 95% CI −0.07 to 0.25, P = 0.27; SI Appendix, Fig. S1). Cases with comorbidities had lower asymptomaticity compared to cases with no underlying medical conditions (log IRR −0.43, 95% CI −0.82 to −0.04, P = 0.03; SI Appendix, Fig. S2).
Table 1.
Pooled estimates for percentages of all positive cases which remain asymptomatic stratified by age, gender, publication date, symptom follow-up duration, study design, and study setting
| n | Estimate (%) | CI (95%) | P value (test of overall effect) | |
| Age class | <0.01 | |||
| Children (0 y to 18 y) | 18 | 46.7 | 32.0 to 62.0 | |
| Adults (19 y to 59 y) | 17 | 32.1 | 22.2 to 43.9 | |
| Elderly (≥60 y) | 17 | 19.7 | 12.7 to 29.4 | |
| Study design | 0.10 | |||
| Population screening | 102 | 38.2 | 32.0 to 44.8 | |
| Others | 68 | 30.7 | 24.8 to 37.4 | |
| Publication date | 0.18 | |||
| January–April 2020 | 27 | 34.8 | 23.6 to 47.9 | |
| May–August 2020 | 69 | 29.5 | 24.2 to 35.4 | |
| September–December 2020 | 50 | 41.1 | 31.4 to 51.4 | |
| January–April 2021 | 24 | 38.4 | 25.6 to 53.1 | |
| Symptom follow-up duration | 0.07 | |||
| 7 d to 21 d | 73 | 40.6 | 32.9 to 48.6 | |
| 21+ d | 90 | 32.1 | 27.0 to 37.7 | |
| Setting | 0.03 | |||
| Community | 39 | 34.0 | 25.3 to 43.8 | |
| Healthcare facility | 81 | 38.5 | 31.6 to 45.9 | |
| Household | 18 | 42.5 | 30.9 to 54.9 | |
| Long-term care facility | 15 | 17.8 | 9.7 to 30.3 | |
| Others | 17 | 38.4 | 23.5 to 55.9 | |
| Geographic location | 0.78 | |||
| China | 50 | 33.6 | 26.1 to 42.0 | |
| United States | 28 | 33.3 | 22.6 to 46.1 | |
| Others | 92 | 36.8 | 30.4 to 43.6 |
Stratifications with statistically significant subgroup differences (P < 0.05) are in bold.
Egger’s test for asymptomatic percentage was significant (P = 0.04; SI Appendix, Fig. S3), providing evidence of potential small-study effects. We therefore conducted a sensitivity analysis by excluding studies with relatively small sample sizes (less than 10 infections). The pooled estimate in the restricted meta-analysis (33.1%; 95% CI: 28.0 to 38.5%) was similar to our original estimate, suggesting that our estimates are robust to publication bias.
Discussion
The SARS-CoV-2 pandemic infected more than 80 million people within a year and is still spreading rapidly despite widespread control efforts. The elements of the global response are similar to those deployed during the SARS-CoV-1 outbreak: detecting new cases through symptom-based surveillance, subsequent testing, and isolation of confirmed cases. In 2002, these measures achieved containment within 8 mo and fewer than 8,500 cases worldwide. Given that the aerosol and surface stability of the two viruses are similar (406), a crucial difference between the two outbreaks could be the role of silent infections in propagating transmission chains. Multiple clinical studies have indicated that viral loads in asymptomatic and symptomatic infections of COVID-19 may be similar (11–14, 354). Furthermore, the presymptomatic phase of SARS-CoV-2 is highly infectious (53), and transmission from those in this phase may be responsible for more than 50% of incidence (16). This is a striking difference from SARS-CoV-1 in which the infectiousness peaked at 12 d to 14 d after symptom onset (407). Although silent infections of SARS-CoV-1 were reported, no known transmission occurred from silently infected or even mildly symptomatic SARS cases.
Since the emergence of COVID-19, there has been much speculation about the silent transmission of the disease. Cross-sectional studies testing exposed individuals who do not exhibit symptoms often conflate asymptomatic infections with those in the presymptomatic phase, leading to substantial overestimation of asymptomatic infection. Longitudinal studies without sufficient follow-up similarly lead to overestimation of asymptomaticity (408). Additionally, inconsistent use of terminology has led to confusion, particularly when distinguishing infections which are silent at the time of testing from those which are truly asymptomatic (4, 5). A previous meta-analysis, for example, incorrectly includes infections in the presymptomatic phase to calculate pooled estimate of asymptomatic percentage (409). By contrast, several studies conducted early in the pandemic reported few asymptomatic infections, primarily due to restrictive testing criteria which focused on testing of severe cases that required hospitalization (410, 411). Inaccuracy in either direction is detrimental for public health. Overestimation of asymptomaticity engenders a perception that SARS-CoV-2 is less virulent, whereas underestimation skews key epidemiological parameters such as infection fatality rate and hospitalization rate upward, leading to suboptimal policy decisions.
To robustly estimate the asymptomatic percentage from studies with varying degrees of methodological vigor, we conducted two separate meta-analyses. In the first analysis, we estimated the asymptomatic percentage as 35.1% (95% CI: 30.7 to 39.9%), by including all studies with a duration of follow-up sufficient to identify asymptomatic infections. In the second analysis, we only included studies that both delineated silent infections at the time of testing and conducted follow-up to distinguish the presymptomatic stage from asymptomatic infections. With this analysis, we estimated the asymptomatic percentage as 36.9% (95% CI: 31.8 to 42.4%). Our estimates have overlapping CIs, which suggests that our pooled analysis is robust to methodological differences in symptom assessment. Our estimates are higher than the 15.6% (95% CI: 10.1 to 23.0%), 17% (95% CI: 14 to 20%), and 20% (95% CI: 17 to 25%) reported by three previous meta-analyses using 41 studies (7), 13 studies (8), and 79 studies (9). In large part, this difference arises because we excluded index cases from our calculation, correcting a bias that leads to underestimation of asymptomaticity. Our estimates of asymptomatic percentage without excluding index cases were 27.8% and 29.4%, for our two approaches. The lower bounds of 24% and 25%, for the two analyses overlaps with the range of the previous largest meta-analysis. Compared with other respiratory infections, the lower bound of our analyses is higher than the 13 to 19% estimated for influenza (412, 413), and the 13% for SARS-CoV-1 (414).
We found that 42.8% (95% prediction interval: 5.2 to 91.1%) of infections were silent at the time of testing. These cases have been incorrectly referred to as asymptomatic in previous studies (4, 5, 189, 239). This rate is context specific, as it is likely influenced by the association between symptomaticity and the time window when an infection is detectable or tested by RT-PCR. Additionally, the proportion of silent infections at the time of testing is highly sensitive to the efficiency of contact tracing. If most contacts are identified and tested swiftly, then nearly all infections will be silent at the time of testing. By contrast, if contact tracing is slow and incomplete, then a larger fraction of individuals will have developed symptoms by the time they are approached for testing, and a smaller proportion of those tested will be symptom-free. Reports of silent infections at the time of testing are also likely impacted by epidemic trajectory largely due to the predominance of recent infections in samples taken during the growth phase, in contrast with a higher proportion of older infections in samples taken during the declining phase. Unbiased measures of asymptomaticity, on the other hand, should be consistent across similar demographic settings, regardless of contact tracing and epidemic trajectory.
Several gaps remain in our understanding of asymptomatic carriage of COVID-19. Particularly, it is unclear why certain infections remain asymptomatic while the majority develop clinical symptoms. Our results indicate that children have greater asymptomaticity compared to the elderly. We also found that cases with comorbidities have lower asymptomaticity compared with cases with no underlying medical conditions. Additionally, studies on long-term care facilities reported lower asymptomaticity compared to other study settings. Given that the risk of severe illness is high among the elderly, the age association identified by our study implies that absence of symptoms may correlate with the tendency of developing milder symptoms. Case severity in SARS-CoV-2 patients has been linked to a cytokine storm which occurs more frequently in elderly patients (415, 416). Genetic (417), environmental risk factors, sex-linked differences (418), and cross-reactive immunity (419) might also contribute, although no studies have unequivocally demonstrated their association with either symptom status or severity.
Higher representation of asymptomatic SARS-CoV-2 infections among younger people has grave implications for control policies in daycares, schools, and universities. Settings with close, extensive contact among large groups of younger individuals are particularly susceptible to superspreader events of COVID-19 which may go undetected if surveillance focuses on symptomatic cases. This close congregation of relatively large groups similarly explains why influenza, mumps, and measles often spread more rapidly in schools and college campuses than in the broader community (420–422). As schools and universities convene in the midst of the COVID-19 pandemic, campus outbreaks are increasingly reported (423). Although COVID-19 severity is lower among young people, campus transmission with a large undetected component could more easily bridge to the rest of the population, fueling local and regional resurgence.
Our meta-analyses are subject to limitations, many related to the unprecedented pace of clinical research since the emergence of COVID-19. First, we found considerable heterogeneity in the percentage of asymptomatic infections. Subgroup analysis revealed that studies with longer follow-up reported lower asymptomaticity. Second, all reports of asymptomatic cases are confounded by the subjective and shifting definition of symptoms. For instance, the list of clinical manifestations associated with COVID-19 has expanded since the initial definitions (424). These changing definitions impact the classification of infections as asymptomatic or silent, and the more limited suite of symptoms initially considered indications of COVID-19 could bias early studies toward higher percentages in these categories. Nonetheless, we found no statistically significant differences in asymptomatic percentage when we stratified studies based on publication date. Third, in the studies included in our meta-analysis, it is possible that early mild symptoms occurring before a positive PCR test might go unrecorded, biasing the studies toward higher asymptomaticity. Fourth, although we corrected for the bias introduced by inclusion of predominantly symptomatic index cases, our estimates are still likely affected by sample selection bias, as participation is expected to be highest among those experiencing symptoms (10). Additionally, factors such as socioeconomic position, occupation, ethnicity, place of residence, internet and technological access, and scientific and medical interest could have contributed to nonrandom enrollment (425). To evaluate the effect of these biases, we calculated the pooled asymptomatic percentage using 25 studies that reported screening of all individuals in the study setting. Asymptomaticity among this smaller subset of studies was 47.3% (95% CI: 34.0 to 61.0%), with CIs that overlap with our primary analysis but the point estimate is higher than the base case CI. We therefore cannot rule out nonrandom sampling as a source of bias for estimation of the asymptomatic percentage.
In our meta-analysis, we excluded 225 studies that did not identify index cases. Additionally, 223 studies reported silent infections at the time of testing but were excluded from analysis of asymptomaticity for not reporting symptom assessment during follow-up for at least 7 d or for not specifying the duration of follow-up. Large-scale longitudinal surveys should prioritize the inclusion of these data to facilitate accurate estimation of the asymptomatic percentage. At minimum, such studies should report the number of index cases among their study participants, the clinical symptom status of individuals at the time of testing, the duration of symptom follow-up, and symptom status during the follow-up. Ideally, studies would additionally provide a full symptom profile both at time of testing and by the end of follow-up, to facilitate reclassification as case definitions are updated.
Estimating the extent of COVID-19 asymptomaticity is critical for calculating key epidemiological characteristics, quantifying the true prevalence of infection, and developing appropriate mitigation efforts. This meta-analysis also establishes a baseline for asymptomaticity, prior to widespread vaccination coverage. Amid concerns that vaccines may be less protective against infection than disease, widespread vaccination coverage may soon lead to a rise in the percentage of infections that present asymptomatically. The high prevalence of silent infections even at baseline, coupled with their transmission potential, necessitates accelerated contact tracing, testing, and isolation of infectious individuals, as symptom-based surveillance alone is inadequate for control.
Methods
Definition of Silent, Asymptomatic, and Presymptomatic Infection.
We defined silent infections as laboratory-confirmed COVID-19 cases that did not exhibit any clinical symptoms, including fever, upper respiratory symptoms, pneumonia, fatigue, headache, myalgia, dehydration, or gastrointestinal dysfunction, at the time of testing. Asymptomatic infections include those that continued to exhibit no clinical symptoms during at least 7 d of follow-up after testing. Presymptomatic cases were those that developed clinical symptoms subsequent to initial testing. The presymptomatic stage begins with the start of infectiousness and ends with the onset of symptoms (426).
Search Strategy and Selection Criteria.
We conducted a systematic review to identify studies reporting laboratory-confirmed COVID-19 cases without symptoms at the time of testing. Our search was inclusive of all studies that provided data regarding cases that were asymptomatic, presymptomatic, or both. We finalized systematic search criteria on May 1, 2020, and study collection was initiated by searching PubMed, EMBASE, Web of Science, and the World Health Organization Global Research Database on COVID-19 (427) weekly from inception through April 2, 2021, with no language restrictions. Our search terms included “SARS-CoV-2,” “novel coronavirus,” “coronavirus 2019,” “COVID-19,” “COVID 2019” AND “asymptomatic,” “no symptoms,” “presymptomatic,” “paucisymptomatic,” “sub-clinical,” “silent transmission,” “silent infection,” “without any symptoms,” and “without symptoms” (SI Appendix, Table S1). All studies of any design that included these terms, were published after January 1, 2020, and described the symptom status of COVID-19 cases were considered in the screening step. No changes were made to the search criteria after the study initiation on May 1, 2020. The study protocol is available in the Open Science Framework online public database, registration DOI: 10.17605/OSF.IO/ZCJ62.
All articles were double-screened (by P.S. and C.F.Z.) based on the title and abstract. Studies were excluded if they were 1) duplicate publications, 2) editorials, reviews, discussions, or opinion pieces, 3) ambiguous about the presence of silent infection, 4) modeling studies without primary data, 5) based on fewer than two cases, 6) not conducted in humans, or 7) retracted. All identified full-text articles were reviewed by P.S. and C.F.Z. For each full-text article, we manually searched references for additional relevant studies. Studies included in our meta-analysis either reported laboratory confirmations of COVID-19 at a single time point, providing a snapshot of disease prevalence in the study subjects, or reported longitudinal data over a period of follow-up.
Risk of bias was assessed independently by two authors, and consensus was achieved through discussion. We adapted the ROBINS-I checklist (428) to include seven items: 1) enrollment of all patients satisfying the criteria for inclusion, 2) enrollment of cases regardless of symptom status, 3) confirmation of cases using RT-PCR, 4) symptoms monitored by clinicians rather than self-reporting, 5) symptom assessment at the end of the follow-up period, 6) symptom follow-up duration of at least 7 d, and 7) loss to follow-up less than 5%.
Data Analysis.
We conducted a meta-analysis using the studies identified through our systematic review to determine the prevalence of those truly asymptomatic among infected individuals. To delineate true asymptomaticity from the combination of asymptomatic and presymptomatic infections, we pursued two complementary analyses: 1) a single-step analysis based on reports of those who were asymptomatic at the end of a follow-up period and 2) a two-step analysis first evaluating the percentage of infections without symptoms at the time of testing and then assessing asymptomaticity by subtracting those that progressed to develop symptoms. In the single-step analysis, we calculated asymptomaticity as the percentage of confirmed COVID-19 cases that continued to exhibit no clinical symptoms for at least 7 d after testing, whether or not symptom status was reported specifically at the time of testing. In the two-step analysis, we focused on a subset of studies that distinguished asymptomatic cases from those that were presymptomatic by reporting symptoms at time of testing as well as conducting follow-up of symptoms for at least 7 d after testing. In both analyses, we removed index case(s) from the denominator of our calculations to minimize representational bias that would result in overestimation of symptomaticity. As a sensitivity analysis, we repeated our calculations including index cases. For studies that did not follow a population screening design, we assumed that single infections without an epidemiological link were necessarily detected due to their symptoms. Therefore, we subset the calculations to include only those infections which were part of a cluster.
To calculate pooled estimates, study outcomes were logit transformed, each study was assigned a weight using the inverse variance method (429), the DerSimonian−Laird estimator was applied to evaluate between-study variance (430), and the Clopper–Pearson method was used to determine CIs (431). Given heterogeneity in asymptomatic percentages estimated across studies, we used a random-effects meta-analysis model, applying the Hartung and Knapp (432) method to adjust test statistics and CIs for the random effect. We evaluated small-study effects visually with a contour-enhanced funnel plot and statistically with Egger’s test (433). As a sensitivity analysis, we excluded studies with a small sample size (<10 infections), and we considered whether their removal impacted the pooling of results.
We conducted subgroup analysis stratified by age class, study design (population screening or not), publication date, duration of symptom follow-up, geographic location, and setting (community, healthcare facility, household, long-term care facilities, and other which encompassed schools, ships, conference, call centers, labor and delivery units, homeless shelters, and detention facilities). For subgroup analysis involving age class, we selected studies where all confirmed cases were either children (0 y to 18 y), adults (19 y to 59 y) or the elderly (≥60 y). We evaluated sex-based differences in asymptomaticity by selecting only those studies that stratified asymptomatic cases with respect to sex. For each of these studies we calculated the IRR, which was the ratio of the asymptomatic percentage in males relative to that in females. A similar analysis was performed to evaluate the asymptomaticity in cases with comorbidity relative to those without.
We next evaluated the impact of sample selection bias arising from higher participation among those experiencing symptoms in studies with voluntary participation. In this analysis, we calculated the pooled asymptomaticity after restricting to a smaller subset of studies that performed screening of every individual at the study setting. To avoid age-dependent bias in asymptomaticity, we removed studies where all participants belonged to a single age class (children, adults, or the elderly). Out of the 25 studies selected, 7 studies performed screening of all close household contacts (64, 80, 83, 103, 117, 131), 3 screened all flight passengers (28, 84, 91), and 2 screened all members of a tourist/pilgrim group (94, 129). Others were based on screening of healthcare workers (25, 110), inpatients admitted for non−COVID-19 reasons (19, 50, 59, 72, 108, 113), rigorously community screening (82, 166), travelers (18, 180), and those associated with a detention facility (92).
The meta-analysis and subgroup analyses were conducted using the metaprop function from the R package meta. Meta-analyses of sex-based and comorbidity-based differences in asymptomaticity were performed using the rma function from the R package metafor.
Supplementary Material
Acknowledgments
A.P.G. acknowledges funding from NSF Expeditions Grant 1918784, NIH Grant 1R01AI151176-01, NSF Grant RAPID-2027755, and the Notsew Orm Sands Foundation. S.M.M. was supported by the Canadian Institutes of Health Research [OV4 – 170643, COVID-19 Rapid Research] and Natural Sciences and Engineering Research Council of Canada Emerging Infectious Diseases Modelling Initiative (NSERC EIDM), Mathematics for Public Health (MfPH) grant.
Footnotes
The authors declare no competing interest.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2109229118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
Change History
August 27, 2021: Table 1 has been updated.
References
- 1.Hao X., et al., Reconstruction of the full transmission dynamics of COVID-19 in Wuhan. Nature 584, 420–424 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Dong Y., et al., Epidemiology of COVID-19 Among Children in China. Pediatrics 145, e20200702 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Heneghan C., Brassey J., Jefferson T., COVID-19: What proportion are asymptomatic? https://www.cebm.net/covid-19/covid-19-what-proportion-are-asymptomatic/. Accessed 29 May 2020.
- 4.Day M., Covid-19: Four fifths of cases are asymptomatic, China figures indicate. BMJ 369, m1375 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Mayor S., Covid-19: Nine in 10 pregnant women with infection when admitted for delivery are asymptomatic, small study finds. BMJ 369, m1485 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention , COVID-19 Pandemic Planning Scenarios. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios-archive/planning-scenarios-2020-05-20.pdf. Accessed 18 August 2020.
- 7.He J., Guo Y., Mao R., Zhang J., Proportion of asymptomatic coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Med. Virol. 93, 820–830 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byambasuren O., et al., Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: Systematic review and meta-analysis. J. Assoc. Med. Microbiol. Infect. Dis. Can., 5, 223–234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buitrago-Garcia D., et al., Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 17, e1003346 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gudbjartsson D. F., et al., Spread of SARS-CoV-2 in the Icelandic population. N. Engl. J. Med. 382, 2302–2315 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimball A., et al., Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility - King County, Washington, March 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 377–381 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arons M. M., et al., Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 382, 2081–2090 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoehl S., et al., Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N. Engl. J. Med. 382, 1278–1280 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thi Q. M. L., et al., Severe acute respiratory syndrome coronavirus 2 shedding by travelers, Vietnam, 2020. Emerging. Infect. Dis. J. 26, 1624–1626 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou R., et al., Viral dynamics in asymptomatic patients with COVID-19. Int. J. Infect. Dis. 96, 288–290 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moghadas S. M., et al., The implications of silent transmission for the control of COVID-19 outbreaks. Proc. Natl. Acad. Sci. U.S.A. 117, 17513–17515 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almazeedi S., et al., Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine 24, 100448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Qahtani M., et al., The prevalence of asymptomatic and symptomatic COVID-19 in a cohort of quarantined subjects. Int. J. Infect. Dis. 102, 285–288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Shamsi H. O., Coomes E. A., Aldhaheri K., Alrawi S., Serial screening for COVID-19 in asymptomatic patients receiving anticancer therapy in the United Arab Emirates. JAMA Oncol. 7, 129–131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsharrah D., et al., Clinical characteristics of pediatric SARS-CoV-2 infection and coronavirus disease 2019 (COVID-19) in Kuwait. J. Med. Virol. 93, 3246–3250 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarado G. R., et al., Symptom characterization and outcomes of sailors in isolation after a COVID-19 outbreak on a US aircraft carrier. JAMA Netw. Open 3, e2020981 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An P., Song P., Wang Y., Liu B., Asymptomatic patients with novel coronavirus disease (COVID-19). Balkan Med. J. 37, 229–230 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anand P., et al., Clinical profile, viral load, management and outcome of neonates born to COVID 19 positive mothers: A tertiary care centre experience from India. Eur. J. Pediatr. 180, 547–559 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arima Y., et al., Severe acute respiratory syndrome coronavirus 2 infection among returnees to Japan from Wuhan, China, 2020. Emerg. Infect. Dis. 26, 1596–1600 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alshahrani M. S., et al., Prevalence of the SARS-CoV-2 infection among post-quarantine healthcare workers. J. Multidiscip. Healthc. 13, 1927–1936 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aslam A., et al., SARS CoV-2 surveillance and exposure in the perioperative setting with universal testing and personal protective equipment (PPE) policies. Clin. Infect. Dis., 10.1093/cid/ciaa1607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atalla E., et al., Clinical presentation, course, and risk factors associated with mortality in a severe outbreak of COVID-19 in Rhode Island, USA, April-June 2020. Pathogens 10, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae S. H., et al., Asymptomatic transmission of SARS-CoV-2 on evacuation flight. Emerg. Infect. Dis. 26, 2705–2708 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bender W. R., Hirshberg A., Coutifaris P., Acker A. L., Srinivas S. K., Universal testing for severe acute respiratory syndrome coronavirus 2 in 2 Philadelphia hospitals: Carrier prevalence and symptom development over 2 weeks. Am. J. Obstet. Gynecol. MFM 2, 100226 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blain H., et al., Efficacy of a test-retest strategy in residents and health care personnel of a nursing home facing a COVID-19 outbreak. J. Am. Med. Dir. Assoc. 21, 933–936 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blain H., et al., Atypical clinical presentation of COVID-19 infection in residents of a long-term care facility. Eur. Geriatr. Med. 11, 1085–1088 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan J. F.-W., et al., A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 395, 514–523 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang L., Zhao L., Gong H., Wang L., Wang L., Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg. Infect. Dis. 26, 1631–1633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Vinh Chau N., et al., The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. bioRxiv [Preprint] (2020). 10.1101/2020.04.27.20082347. [DOI]
- 35.Chehrassan M., et al., Management of spine trauma in COVID-19 pandemic: A preliminary report. Arch. Bone Jt. Surg. 8, 270–276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppeta L., et al., Contact screening for healthcare workers exposed to patients with COVID-19. Int. J. Environ. Res. Public Health 17, 9082 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corcorran M. A., et al., Prolonged persistence of PCR-detectable virus during an outbreak of SARS-CoV-2 in an inpatient geriatric psychiatry unit in King County, Washington. Am. J. Infect. Control 49, 293–298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosma S., et al., The “scar” of a pandemic: Cumulative incidence of COVID-19 during the first trimester of pregnancy. J. Med. Virol. 93, 537–540 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danis K., et al., Cluster of coronavirus disease 2019 (COVID-19) in the French Alps, February 2020. Clin. Infect. Dis. 71, 825–832 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dora A. V., et al., Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for Veterans - Los Angeles, California, 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 651–655 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du W., et al., Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection 48, 445–452 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du W., et al., Persistence of SARS-CoV-2 virus RNA in feces: A case series of children. J. Infect. Public Health 13, 926–931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eythorsson E., et al., Clinical spectrum of coronavirus disease 2019 in Iceland: Population based cohort study. BMJ 371, m4529 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fusco F. M., et al., COVID-19 among healthcare workers in a specialist infectious diseases setting in Naples, Southern Italy: Results of a cross-sectional surveillance study. J. Hosp. Infect. 105, 596–600 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao Y., et al., A cluster of the corona virus disease 2019 caused by incubation period transmission in Wuxi, China. J. Infect. 80, 666–670 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonfiotti A., et al., Clinical courses and outcomes of five patients with primary lung cancer surgically treated while affected by severe acute respiratory syndrome coronavirus 2. Eur. J. Cardiothorac. Surg. 58, 598–604 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grijalva C. G., et al., Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1631–1634 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta N., et al., Transmission of SARS-CoV-2 infection by children: A study of contacts of index paediatric cases in India. J. Trop. Pediatr. 67, fmaa081 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han M. S., et al., Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr. 175, 73–80 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada S., et al., Control of a nosocomial outbreak of COVID-19 in a university hospital. Open Forum Infect. Dis. 7, ofaa512 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasanoglu I., et al., Higher viral loads in asymptomatic COVID-19 patients might be the invisible part of the iceberg. Infection 49, 117–126 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hcini N., et al., Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: A single-center prospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 257, 11–18 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He X., et al., Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26, 672–675 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Hogan C. A., et al., Large-scale testing of asymptomatic healthcare personnel for severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 27, 250–254 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu Z., et al., Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 63, 706–711 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hua C.-Z., et al., Epidemiological features and viral shedding in children with SARS-CoV-2 infection. J. Med. Virol. 92, 2804–2812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang L., et al., Initial CT imaging characters of an imported family cluster of COVID-19. Clin. Imaging 65, 78–81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang K., et al., A retrospective analysis of the epidemiology, clinical manifestations, and imaging characteristics of familial cluster-onset COVID-19. Ann. Transl. Med. 8, 747 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang Q., et al., Asymptomatic COVID-19 infection in patients with cancer at a cancer-specialized hospital in Wuhan, China - Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 24, 9760–9764 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Hung I. F.-N., et al., SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: A case series. Lancet Infect. Dis. 20, 1051–1060 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibrahim O. R., et al., COVID-19 in children: A case series from Nigeria. Pan Afr. Med. J. 35, 53 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeong T. H., et al., Real asymptomatic SARS-CoV-2 infection might be rare: Importance of careful interviews and follow-up. J. Korean Med. Sci. 35, e333 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang X.-L., et al., Transmission potential of asymptomatic and paucisymptomatic severe acute respiratory syndrome coronavirus 2 infections: A 3-family cluster study in China. J. Infect. Dis. 221, 1948–1952 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang Y., et al., Characteristics of a family cluster of Severe Acute Respiratory Syndrome Coronavirus 2 in Henan, China. J. Infect. 81, e46–e48 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang H., et al., Clinical features, laboratory findings and persistence of virus in 10 children with coronavirus disease 2019 (COVID-19). Biomed. J. 44, 94–100 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones N. K., et al., Effective control of SARS-CoV-2 transmission between healthcare workers during a period of diminished community prevalence of COVID-19. eLife 9, e59391 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joshi R. K., Ray R. K., Adhya S., Chauhan V. P. S., Pani S., Spread of COVID-19 by asymptomatic cases: Evidence from military quarantine facilities. BMJ Mil. Health 167, 217–218 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Jung J., et al., Investigation of a nosocomial outbreak of coronavirus disease 2019 in a paediatric ward in South Korea: Successful control by early detection and extensive contact tracing with testing. Clin. Microbiol. Infect. 26, 1574–1575 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang Y., et al., A retrospective view of pediatric cases infected with SARS-CoV-2 of a middle-sized city in mainland China. Medicine (Baltimore) 99, e23797 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karout L., et al., COVID-19 prevalence, risk perceptions, and preventive behavior in asymptomatic Latino population: A cross-sectional study. Cureus 12, e10707 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kennelly S. P., et al., Asymptomatic carriage rates and case fatality of SARS-CoV-2 infection in residents and staff in Irish nursing homes. Age Ageing 50, 49–54 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirshblum S. C., et al., Screening testing for SARS-CoV-2 upon admission to rehabilitation hospitals in a high COVID-19 prevalence community. PM R. 12, 1009–1014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kittang B. R., et al., Outbreak of COVID-19 at three nursing homes in Bergen. Tidsskr. Nor. Laegeforen., 10.4045/tidsskr.20.0405 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Kutsuna S., et al., SARS-CoV-2 screening test for Japanese returnees from Wuhan, China, January 2020. Open Forum Infect. Dis. 7, ofaa243(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ladhani S. N., et al., Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine 26, 100533 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J. Y., et al., Epidemiological and clinical characteristics of coronavirus disease 2019 in Daegu, South Korea. Int. J. Infect. Dis. 98, 462–466 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li W., et al., Virus shedding dynamics in asymptomatic and mildly symptomatic patients infected with SARS-CoV-2. Clin. Microbiol. Infect. 26, 1556.e1–1556.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J., et al., Comparative analysis of symptomatic and asymptomatic SARS-CoV-2 infection in children. Ann. Acad. Med. Singap. 49, 530–537 (2020). [PubMed] [Google Scholar]
- 79.Liao J., et al., Epidemiological and clinical characteristics of COVID-19 in adolescents and young adults. Innovation (N Y) 1, 100001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Z., et al., Investigation of a family cluster outbreak of COVID-19 indicates the necessity of CT screening for asymptomatic family members in close contact with confirmed patients. J. Thorac. Dis. 12, 3673–3681 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lombardi A., et al., Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin. Microbiol. Infect. 26, 1413.e9–1413.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo L., et al., Contact settings and risk for transmission in 3410 close contacts of patients with COVID-19 in Guangzhou, China: A prospective cohort study. Ann. Intern. Med. 173, 879–887 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo Y., et al., Asymptomatic SARS-CoV-2 infection in household contacts of a healthcare provider, Wuhan, China. Emerg. Infect. Dis. 26, 1930–1933 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lytras T., et al., High prevalence of SARS-CoV-2 infection in repatriation flights to Greece from three European countries. J. Travel Med. 27, taaa054 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma Y., et al., Characteristics of asymptomatic patients with SARS-CoV-2 infection in Jinan, China. Microbes Infect. 22, 212–217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Macartney K., et al., Transmission of SARS-CoV-2 in Australian educational settings: A prospective cohort study. Lancet Child Adolesc. Health 4, 807–816 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martinez-Fierro M. L., et al., The role of close contacts of COVID-19 patients in the SARS-CoV-2 transmission: An emphasis on the percentage of nonevaluated positivity in Mexico. Am. J. Infect. Control 49, 15–20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merza M. A., Haleem Al Mezori A. A., Mohammed H. M., Abdulah D. M., COVID-19 outbreak in Iraqi Kurdistan: The first report characterizing epidemiological, clinical, laboratory, and radiological findings of the disease. Diabetes Metab. Syndr. 14, 547–554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyers K. J., et al., A cross-sectional community-based observational study of asymptomatic SARS-CoV-2 prevalence in the greater Indianapolis area. J. Med. Virol. 92, 2874–2879 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Migisha R., et al., Early cases of SARS-CoV-2 infection in Uganda: Epidemiology and lessons learned from risk-based testing approaches - March-April 2020. Global. Health 16, 114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ng O.-T., et al., SARS-CoV-2 infection among travelers returning from Wuhan, China. N. Engl. J. Med. 382, 1476–1478 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Njuguna H., et al., Serial laboratory testing for SARS-CoV-2 infection among incarcerated and detained persons in a correctional and detention facility - Louisiana, April-May 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 836–840 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park S. Y., et al., Coronavirus disease outbreak in call center, South Korea. Emerg. Infect. Dis. 26, 1666–1670 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park J. H., Jang J. H., Lee K., Yoo S. J., Shin H., COVID-19 outbreak and presymptomatic transmission in pilgrim travelers who returned to Korea from Israel. J. Korean Med. Sci. 35, e424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Passarelli V. C., et al., Asymptomatic SARS-CoV-2 infections in hospitalized patients. Infect. Control Hosp. Epidemiol., 10.1017/ice.2020.441 (2020). [DOI] [Google Scholar]
- 96.Passarelli V. C., et al., Asymptomatic COVID-19 in hospital visitors: The underestimated potential of viral shedding. Int. J. Infect. Dis. 102, 412–414 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel M. C., et al., Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin. Infect. Dis. 71, 2920–2926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pavli A., et al., A cluster of COVID-19 in pilgrims to Israel. J. Travel Med. 27, taaa102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thai P. Q., et al., The first 100 days of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) control in Vietnam. Clin. Infect. Dis. 72, e334–e342 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plucinski M. M., et al., COVID-19 in Americans aboard the Diamond Princess cruise ship. Clin. Infect. Dis. 72, e448–e457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pongpirul W. A., et al., Clinical characteristics of patients hospitalized with coronavirus disease, Thailand. Emerg. Infect. Dis. 26, 1580–1585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Puylaert C. A. J., et al., Yield of screening for COVID-19 in asymptomatic patients prior to elective or emergency surgery using chest CT and RT-PCR (SCOUT): Multicenter study. Ann. Surg. 272, 919–924 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qian G., et al., COVID-19 transmission within a family cluster by presymptomatic carriers in China. Clin. Infect. Dis. 71, 861–862 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qin W., et al., The descriptive epidemiology of coronavirus disease 2019 during the epidemic period in Lu’an, China: Achieving limited community transmission using proactive response strategies. Epidemiol. Infect. 148, e132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qiu C., et al., Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China. J. Med. Virol. 92, 2027–2035 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ralli M., Morrone A., Arcangeli A., Ercoli L., Asymptomatic patients as a source of transmission of COVID-19 in homeless shelters. Int. J. Infect. Dis. 103, 243–245 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reale M. L., et al., SARS-CoV-2 infection in cancer patients: A picture of an Italian Onco-Covid unit. Front. Oncol. 10, 1722 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rincón A., et al., The keys to control a COVID-19 outbreak in a haemodialysis unit. Clin. Kidney J. 13, 542–549 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rivera F., et al., Prevalence of SARS-CoV-2 asymptomatic infections in two large academic health systems in Wisconsin. Clin. Infect. Dis., 10.1093/cid/ciaa1225 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rivett L., et al., Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. eLife 9, e58728 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saeed K., et al., Investigations, actions and learning from an outbreak of SARS-CoV-2 infection among healthcare workers in the United Kingdom. J. Infect. Prev. 22, 156–161 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ossami Saidy R. R., Globke B., Pratschke J., Schoening W., Eurich D., Successful implementation of preventive measures leads to low relevance of SARS-CoV-2 in liver transplant patients: Observations from a German outpatient department. Transpl. Infect. Dis. 22, e13363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwierzeck V., et al., First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clin. Infect. Dis. 72, 265–270 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.See K. C., et al., COVID-19: Four paediatric cases in Malaysia. Int. J. Infect. Dis. 94, 125–127 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Senanayake A. P., et al., Features of Covid-19 patients detected during community screening: A study from a rural hospital in Sri Lanka. Ceylon Med. J. 65, 67 (2020). [DOI] [PubMed] [Google Scholar]
- 116.Setiawaty V., et al., The identification of first COVID-19 cluster in Indonesia. Am. J. Trop. Med. Hyg. 103, 2339–2342 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shen J., et al., Characteristics of nosocomial infections in children screened for SARS-CoV-2 infection in China. Med. Sci. Monit. 26, e928835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi S. M., et al., Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J. Am. Med. Dir. Assoc. 21, 1378–1383.e1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Son H., et al., Epidemiological characteristics of and containment measures for COVID-19 in Busan, Korea. Epidemiol. Health 42, e2020035 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song W., et al., Clinical features of pediatric patients with coronavirus disease (COVID-19). J. Clin. Virol. 127, 104377 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soysal A., et al., Comparison of clinical and laboratory features and treatment options of 237 Comparison of clinical and laboratory features and treatment options of 237 symptomatic and asymptomatic children infected with SARS-CoV-2 in the early phase of the COVID-19 pandemic in Turkey. Jpn. J. Infect. Dis. 74, 273−279 (2021). [DOI] [PubMed] [Google Scholar]
- 122.Stock A. D., et al., COVID-19 infection among healthcare workers: Serological findings supporting routine testing. Front. Med. (Lausanne) 7, 471 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Suh H. J., et al., Clinical characteristics of COVID-19: Clinical dynamics of mild severe acute respiratory syndrome coronavirus 2 infection detected by early active surveillance. J. Korean Med. Sci. 35, e297 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Szegedi S., Huf W., Miháltz K., Vécsei-Marlovits P. V., Prevalence of SARS-CoV-2 infection in patients presenting for intravitreal injection. Spektrum Der Augenheilkunde 35 (70), 74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tabata S., et al., Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: A retrospective analysis. Lancet Infect. Dis. 20, 1043–1050 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tan Y.-P., et al., Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J. Clin. Virol. 127, 104353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taylor J., et al., Serial testing for SARS-CoV-2 and virus whole genome sequencing inform infection risk at two skilled nursing facilities with COVID-19 outbreaks - Minnesota, April-June 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1288–1295 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Temel H., et al., Evaluation of the clinical features of 81 patients with COVID-19: An unpredictable disease in children. J. Pediatr. Infect. Dis. 16, 47−52 (2021). [Google Scholar]
- 129.Vivian Thangaraj J. W., et al., A cluster of SARS-CoV-2 infection among Italian tourists visiting India, March 2020. Indian J. Med. Res. 151, 438–443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thiel S. L., et al., Flattening the curve in 52 days: Characterisation of the COVID-19 pandemic in the Principality of Liechtenstein - An observational study. Swiss Med. Wkly. 150, w20361 (2020). [DOI] [PubMed] [Google Scholar]
- 131.Tong Z.-D., et al., Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg. Infect. Dis. 26, 1052–1054 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsou T.-P., et al., Epidemiology of the first 100 cases of COVID-19 in Taiwan and its implications on outbreak control. J. Formos. Med. Assoc. 119, 1601–1607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wadhwa A., et al., Identification of presymptomatic and asymptomatic cases using cohort-based testing approaches at a large correctional facility - Chicago, Illinois, USA, May 2020. Clin. Infect. Dis. 72, e128–e135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y., et al., Characterization of an asymptomatic cohort of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infected individuals outside of Wuhan, China. Clin. Infect. Dis. 71, 2132–2138 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang G., et al., Infection, screening, and psychological stress of health care workers with COVID-19 in a non-frontline clinical department. Disaster Med. Public Health Prep., 10.1017/dmp.2020.428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wi Y. M., et al., Response system for and epidemiological features of COVID-19 in Gyeongsangnam-do Province in South Korea. Clin. Infect. Dis. 72, 661–667 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wong J., et al., High proportion of asymptomatic and presymptomatic COVID-19 infections in air passengers to Brunei. J. Travel Med. 27, taaa066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wong J., Jamaludin S. A., Alikhan M. F., Chaw L., Asymptomatic transmission of SARS-CoV-2 and implications for mass gatherings. Influenza Other Respir. Viruses 14, 596–598 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu J., et al., Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin. Infect. Dis. 71, 2099–2108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu S., et al., Understanding factors influencing the length of hospital stay among non-severe COVID-19 patients: A retrospective cohort study in a Fangcang shelter hospital. PLoS One 15, e0240959 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xi A., et al., Epidemiological and clinical characteristics of discharged patients infected with SARS-CoV-2 on the Qinghai Plateau. J. Med. Virol. 92, 2528–2535 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiong F., et al., Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J. Am. Soc. Nephrol. 31, 1387–1397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang M.-C., et al., A three-generation family cluster with COVID-19 infection: Should quarantine be prolonged? Public Health 185, 31–33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang R., Gui X., Xiong Y., Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw. Open 3, e2010182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang N., et al., In-flight transmission cluster of COVID-19: A retrospective case series. Infect. Dis. (Lond.) 52, 891–901 (2020). [DOI] [PubMed] [Google Scholar]
- 146.Yau K., et al., COVID-19 outbreak in an urban hemodialysis unit. Am. J. Kidney Dis. 76, 690–695.e1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ye F., et al., Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int. J. Infect. Dis. 94, 133–138 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang J., Tian S., Lou J., Chen Y., Familial cluster of COVID-19 infection from an asymptomatic. Crit. Care 24, 119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang W., et al., Secondary transmission of coronavirus disease from presymptomatic persons, China. Emerg. Infect. Dis. 26, 1924–1926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang H.-J., et al., Asymptomatic and symptomatic SARS-CoV-2 infections in close contacts of COVID-19 patients: A seroepidemiological study. Clin. Infect. Dis., 10.1093/cid/ciaa771 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang H., Chen R., Chen J., Chen B., COVID-19 transmission within a family cluster in Yancheng, China. Front. Med. (Lausanne) 7, 387 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang H., et al., A multi-family cluster of COVID-19 associated with asymptomatic and pre-symptomatic transmission in Jixi City, Heilongjiang, China, 2020. Emerg. Microbes Infect. 9, 2509–2514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao D., et al., Asymptomatic infection by SARS-CoV-2 in healthcare workers: A study in a large teaching hospital in Wuhan, China. Int. J. Infect. Dis. 99, 219–225 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng X., et al., Asymptomatic patients and asymptomatic phases of Coronavirus Disease 2019 (COVID-19): A population-based surveillance study. Natl. Sci. Rev. 7, 1527–1539 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Olmos C., et al., SARS-CoV-2 infection in asymptomatic healthcare workers at a clinic in Chile. PLoS One 16, e0245913 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roberts S. C., et al., Mass severe acute respiratory coronavirus 2 (SARS-CoV-2) testing of asymptomatic healthcare personnel - ERRATUM. Infect. Control Hosp. Epidemiol. 42, 625–626 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tan-Loh J., Cheong B. M. K., A descriptive analysis of clinical characteristics of COVID-19 among healthcare workers in a district specialist hospital. Med. J. Malaysia 76, 24–28 (2021). [PubMed] [Google Scholar]
- 158.Goldenfeld M., et al., Characteristics of clinically asymptomatic patients with SARS-CoV-2 infections, case series. Prehosp. Disaster Med. 36, 125–128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Green R., et al., COVID-19 testing in outbreak-free care homes: What are the public health benefits? J. Hosp. Infect. 111, 89–95 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Krone M., Noffz A., Richter E., Vogel U., Schwab M., Control of a COVID-19 outbreak in a nursing home by general screening and cohort isolation in Germany, March to May 2020. Euro Surveill. 26, 2001365 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Krüger S., et al., Performance and feasibility of universal PCR admission screening for SARS-CoV-2 in a German tertiary care hospital. J. Med. Virol. 93, 2890–2898 (2021). [DOI] [PubMed] [Google Scholar]
- 162.Reid R. J., Rosella L., Milijasevic N., Small L. N., Mass testing for asymptomatic COVID-19 infection among health care workers at a large Canadian hospital. J. Assoc. Med. Microbiol. Infect. Dis. Can. 5, 245–250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choudhury A., et al., COVID-19 in liver transplant recipients—A series with successful recovery. J. Clin. Transl. Hepatol. 8, 467–473 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Meyers K. J., et al., Follow-up of SARS-CoV-2 positive subgroup from the asymptomatic novel CORonavirus iNFection study. J. Med. Virol. 93, 2925–2931 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cruz-Lemini M., et al. Obstetric outcomes of SARS-CoV-2 infection in asymptomatic pregnant women. Viruses 13, 112 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abraha H. E., et al., Clinical features and risk factors associated with morbidity and mortality among patients with COVID-19 in northern Ethiopia. Int. J. Infect. Dis. 105, 776–783 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Beiting K. J., et al., Management and outcomes of a COVID-19 outbreak in a nursing home with predominantly Black residents. J. Am. Geriatr. Soc. 69, 1155–1165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Blain H., et al., Atypical symptoms, SARS-CoV-2 test results and immunisation rates in 456 residents from eight nursing homes facing a COVID-19 outbreak. Age Ageing 50, 641–648 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chan J., et al., COVID-19 in the New York City jail system: Epidemiology and health care response, March-April 2020. Public Health Rep. 136, 375–383 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gupta N., et al., Clinical profile and outcomes of asymptomatic vs. symptomatic travellers diagnosed with COVID-19: An observational study from a coastal town in South India. Drug Discov. Ther. 15, 1–8 (2021). [DOI] [PubMed] [Google Scholar]
- 171.Gutman M. J., et al., What was the prevalence of COVID-19 in asymptomatic patients undergoing orthopaedic surgery in one large United States City Mid-pandemic? Clin. Orthop. Relat. Res. 479, 1691–1699 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Handal N., et al., Comparison of SARS-CoV-2 infections in healthcare workers with high and low exposures to Covid-19 patients in a Norwegian University Hospital. Infect. Dis. (Lond.) 53, 420–429 (2021). [DOI] [PubMed] [Google Scholar]
- 173.Lamichhane S., Gupta S., Akinjobi G., Ndubuka N., Familial cluster of asymptomatic COVID-19 cases in a First Nation community in Northern Saskatchewan, Canada. Can. Commun. Dis. Rep. 47, 94–96 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu P., et al., Epidemiological and clinical features in patients with coronavirus disease 2019 outside of Wuhan, China: Special focus in asymptomatic patients. PLoS Negl. Trop. Dis. 15, e0009248 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Malagón-Rojas J. N., Mercado M., Gómez-Rendón C. P., SARS-CoV-2 and work-related transmission: Results of a prospective cohort of airport workers, 2020. Rev. Bras. Med. Trab. 18, 371–380 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marks M., et al., Transmission of COVID-19 in 282 clusters in Catalonia, Spain: A cohort study. Lancet Infect. Dis. 21, 629–636 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nicolás D., et al., A prospective cohort of SARS-CoV-2-infected health care workers: Clinical characteristics, outcomes, and follow-up strategy. Open Forum Infect. Dis. 8, ofaa592 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Patil U. P., Krishnan P., Abudinen-Vasquez S., Maru S., Noble L., Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) positive newborns of COVID-19 mothers after dyad-care: A case series. Cureus 13, e12528 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rajme-López S., et al., Large-scale screening for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) among healthcare workers: Prevalence and risk factors for asymptomatic and pauci-symptomatic carriers, with emphasis on the use of personal protective equipment (PPE). Infect. Control Hosp. Epidemiol., 10.1017/ice.2021.68 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ren R., et al., Asymptomatic SARS-CoV-2 infections among persons entering China from April 16 to October 12, 2020. JAMA 325, 489–492 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sharma R., et al., Perinatal outcome and possible vertical transmission of coronavirus disease 2019: Experience from North India. Clin. Exp. Pediatr. 64, 239–246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stessel B., et al., Evaluation of a comprehensive pre-procedural screening protocol for COVID-19 in times of a high SARS CoV-2 prevalence: A prospective cross-sectional study. Ann. Med. 53, 337–344 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tan J. K., et al., The prevalence and clinical significance of Presymptomatic COVID-19 patients: How we can be one step ahead in mitigating a deadly pandemic. BMC Infect. Dis. 21, 249 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abeysuriya S., et al., Universal screening for SARS-CoV-2 in pregnant women at term admitted to an East London maternity unit. Eur. J. Obstet. Gynecol. Reprod. Biol. 252, 444–446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Adorni F., et al., Self-reported symptoms of SARS-CoV-2 infection in a non-hospitalized population: Results from the large Italian web-based EPICOVID19 cross-sectional survey. JMIR Public Health Surveill. 6, e21866 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Aherfi S., Gautret P., Chaudet H., Raoult D., La Scola B., Clusters of COVID-19 associated with Purim celebration in the Jewish community in Marseille, France, March 2020. Int. J. Infect. Dis. 100, 88–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Albalate M., et al., High prevalence of asymptomatic COVID-19 in hemodialysis. Daily learning during first month of COVID-19 pandemic. Nefrologia (Engl. Ed.) 40, 279–286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Andrikopoulou M., et al., Symptoms and critical illness among obstetric patients with Coronavirus Disease 2019 (COVID-19) infection. Obstet. Gynecol. 136, 291–299 (2020). [DOI] [PubMed] [Google Scholar]
- 189.Baggett T. P., Keyes H., Sporn N., Gaeta J. M., Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA 323, 2191–2192 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bai Y., et al., Presumed asymptomatic carrier transmission of COVID-19. JAMA 323, 1406–1407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bai S. L., et al., Analysis of the first cluster of cases in a family of novel coronavirus pneumonia in Gansu Province. Zhonghua Yu Fang Yi Xue Za Zhi 54, E005 (2020). [DOI] [PubMed] [Google Scholar]
- 192.Berghoff A. S., et al., SARS-CoV-2 testing in patients with cancer treated at a tertiary care hospital during the COVID-19 pandemic. J. Clin. Oncol. 38, 3547–3554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bi Q., et al., Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study. Lancet Infect. Dis. 20, 911–919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Blitz M. J., et al., Universal testing for coronavirus disease 2019 in pregnant women admitted for delivery: Prevalence of peripartum infection and rate of asymptomatic carriers at four New York hospitals within an integrated healthcare system. Am. J. Obstet. Gynecol. MFM 2, 100169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Blumberg T. J., et al., Universal screening for COVID-19 in children undergoing orthopaedic surgery: A multicenter report. J. Pediatr. Orthop. 40, e990–e993 (2020). [DOI] [PubMed] [Google Scholar]
- 196.Brandstetter S., et al., Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatr. Allergy Immunol. 31, 841–847 (2020). [DOI] [PubMed] [Google Scholar]
- 197.Campbell K. H., et al., Prevalence of SARS-CoV-2 among patients admitted for childbirth in Southern Connecticut. JAMA 323, 2520–2522 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cardona-Hernandez R., et al., Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr. Diabetes 22, 202–206 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Carroll C., et al., Routine testing of close contacts of confirmed COVID-19 cases—National COVID-19 contact management programme, Ireland, May to August 2020. Public Health 190, 147–151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cattelan A. M., et al., An integrated strategy for the prevention of SARS-CoV-2 infection in healthcare workers: A prospective observational study. Int. J. Environ. Res. Public Health 17, 5785 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chang M. C., Seo W.-S., Park D., Hur J., Analysis of SARS-CoV-2 screening clinic (including drive-through system) data at a single university Hospital in South Korea from 27 January 2020 to 31 March 2020 during the COVID-19 outbreak. Healthcare (Basel) 8, 145 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chekhlabi N., et al., The epidemiological and clinical profile of COVID-19 in children: Moroccan experience of the Cheikh Khalifa University Center. Pan Afr. Med. J. 35, 57 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen P., et al., Clinical and demographic characteristics of cluster cases and sporadic cases of Coronavirus Disease 2019 (COVID-19) in 141 patients in the main district of Chongqing, China, between January and February 2020. Med. Sci. Monit. 26, e923985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen P., et al., Epidemiological and clinical characteristics of 136 cases of COVID-19 in main district of Chongqing. J. Formos. Med. Assoc. 119, 1180–1184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen Y., et al., Epidemiological characteristics of infection in COVID-19 close contacts in Ningbo city. Zhonghua Liu Xing Bing Xue Za Zhi 41, 667–671 (2020). [DOI] [PubMed] [Google Scholar]
- 206.Chen J., et al., Potential transmission of SARS-CoV-2 on a flight from Singapore to Hangzhou, China: An epidemiological investigation. Travel Med. Infect. Dis. 36, 101816 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen G., et al., Epidemiological analysis of 18 patients with COVID-19. Eur. Rev. Med. Pharmacol. Sci. 24, 12522–12526 (2020). [DOI] [PubMed] [Google Scholar]
- 208.Cheng H.-Y., et al., Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern. Med. 180, 1156–1163 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chiu C.-H., et al., Familial cluster of pneumonia and asymptomatic cases of COVID-19 in Taiwan. J. Formos. Med. Assoc. 119, 1560–1561 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Clarke C., et al., High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J. Am. Soc. Nephrol. 31, 1969–1975 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Crameri G. A. G., et al., Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill. 25, 2001542 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.D’Ambrosi F., et al., Management of gestational diabetes in women with a concurrent severe acute respiratory syndrome coronavirus 2 infection, experience of a single center in Northern Italy. Int. J. Gynaecol. Obstet. 152, 335–338 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stock da Cunha T., et al., The spectrum of clinical and serological features of COVID-19 in urban hemodialysis patients. J. Clin. Med. 9, 2264 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Silva J. H. D., Oliveira E. C. D., Hattori T. Y., Lemos E. R. S. D., Terças-Trettel A. C. P., Description of a COVID-19 cluster: isolation and testing in asymptomatic people as prevention strategies for local spread in Mato Grosso. Epidemiol. Serv. Saude 29, e2020264 (2020). [DOI] [PubMed] [Google Scholar]
- 215.Jesus M. C. S., et al., Family COVID-19 cluster analysis of an infant without respiratory symptoms. Rev. Soc. Bras. Med. Trop. 53, e20200494 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dhuyvetter A., Cejtin H. E., Adam M., Patel A., Coronavirus disease 2019 in pregnancy: The experience at an urban safety net hospital. J. Community Health 46, 267–269 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Díaz-Corvillón P., et al., Routine screening for SARS CoV-2 in unselected pregnant women at delivery. PLoS One 15, e0239887 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Donahue M., et al., Notes from the field: Characteristics of meat processing facility workers with confirmed SARS-CoV-2 infection - Nebraska, April-May 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1020–1022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Du H., et al., Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy 76, 510–532 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang D., et al., Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi 58, 269–274 (2020). [DOI] [PubMed] [Google Scholar]
- 221.Edlow A. G., et al., Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open 3, e2030455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Felice C., Di Tanna G. L., Zanus G., Grossi U., Impact of COVID-19 outbreak on healthcare workers in Italy: Results from a national E-survey. J. Community Health 45, 675–683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gaur A., et al., Clinico-radiological presentation of COVID-19 patients at a tertiary care center at Bhilwara Rajasthan, India. J. Assoc. Physicians India 68, 29–33 (2020). [PubMed] [Google Scholar]
- 224.Goldfarb I. T., et al., Universal SARS-CoV-2 testing on admission to the labor and delivery unit: Low prevalence among asymptomatic obstetric patients. Infect. Control Hosp. Epidemiol. 41, 1095–1096 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gong X., et al., Three infection clusters related with potential pre-symptomatic transmission of coronavirus disease (COVID-19), Shanghai, China, January to February 2020. Euro Surveill. 25, 2000228 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Graham N. S. N., et al., SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J. Infect. 81, 411–419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Grechukhina O., et al., Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. Am. J. Obstet. Gynecol. MFM 2, 100246 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Greiner J., et al., Characteristics and mechanisms to control a COVID-19 outbreak on a leukemia and stem cell transplantation unit. Cancer Med. 10, 237–246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gruskay J. A., et al., Universal testing for COVID-19 in essential orthopaedic surgery reveals a high percentage of asymptomatic infections. J. Bone Joint Surg. Am. 102, 1379–1388 (2020). [DOI] [PubMed] [Google Scholar]
- 230.Guo C.-X., et al., Epidemiological and clinical features of pediatric COVID-19. BMC Med. 18, 250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Han T., Outbreak investigation: Transmission of COVID-19 started from a spa facility in a local community in Korea. Epidemiol. Health 42, e2020056 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Han T., et al., The epidemiological characteristics of cluster transmission of coronavirus disease 2019 (COVID-19): A multi-center study in Jiangsu Province. Am. J. Transl. Res. 12, 6434–6444 (2020). [PMC free article] [PubMed] [Google Scholar]
- 233.He M., et al., Epidemiological and clinical characteristics of 35 children with COVID-19 in Beijing, China. Pediatr. Investig. 4, 230–235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hempel L., et al., SARS-CoV-2 infections in cancer outpatients-most infected patients are asymptomatic carriers without impact on chemotherapy. Cancer Med. 9, 8020–8028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Herraiz I., et al., Universal screening for SARS-CoV-2 before labor admission during Covid-19 pandemic in Madrid. J. Perinat. Med. 48, 981–984 (2020). [DOI] [PubMed] [Google Scholar]
- 236.Hijnen D., et al., SARS-CoV-2 transmission from presymptomatic meeting attendee, Germany. Emerg. Infect. Dis. 26, 1935–1937 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hong J.-M., et al., Epidemiological characteristics and clinical features of patients infected with the COVID-19 virus in Nanchang, Jiangxi, China. Front. Med. (Lausanne) 7, 571069 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hu X., et al., Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with Coronavirus Disease 2019 (COVID-19) pneumonia. Obstet. Gynecol. 136, 65–67 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ing A. J., Cocks C., Green J. P., COVID-19: In the footsteps of Ernest Shackleton. Thorax 75, 693–694 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Inui S., et al., Chest CT findings in cases from the cruise ship Diamond Princess with Coronavirus Disease (COVID-19). Radiol. Cardiothorac. Imaging 2, e200110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jatt L. P., et al., Widespread severe acute respiratory coronavirus virus 2 (SARS-CoV-2) laboratory surveillance program to minimize asymptomatic transmission in high-risk inpatient and congregate living settings. Infect. Control Hosp. Epidemiol. 41, 1331–1334 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu J.-Y., Chen T.-J., Hwang S.-J., Analysis of community-acquired COVID-19 cases in Taiwan. J. Chin. Med. Assoc. 83, 1087–1092 (2020). [DOI] [PubMed] [Google Scholar]
- 243.Jung C.-Y., et al., Clinical characteristics of asymptomatic patients with COVID-19: A nationwide cohort study in South Korea. Int. J. Infect. Dis. 99, 266–268 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Just J., Puth M.-T., Regenold F., Weckbecker K., Bleckwenn M., Risk factors for a positive SARS-CoV-2 PCR in patients with common cold symptoms in a primary care setting - A retrospective analysis based on a joint documentation standard. BMC Fam. Pract. 21, 251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Expert Taskforce for the COVID-19 Cruise Ship Outbreak , Epidemiology of COVID-19 outbreak on cruise ship quarantined at Yokohama, Japan, February 2020. Emerg. Infect. Dis. 26, 2591–2597 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kassem A. M., et al., SARS-CoV-2 infection among healthcare workers of a gastroenterological service in a tertiary care facility. Arab J. Gastroenterol. 21, 151–155 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bai K., et al., Clinical analysis of 25 COVID-19 infections in children. Pediatr. Infect. Dis. J. 39, e100–e103 (2020). [DOI] [PubMed] [Google Scholar]
- 248.Khalil A., Hill R., Ladhani S., Pattisson K., O’Brien P., Severe acute respiratory syndrome coronavirus 2 in pregnancy: Symptomatic pregnant women are only the tip of the iceberg. Am. J. Obstet. Gynecol. 223, 296–297 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ki M., Task Force for 2019-nCoV , Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol. Health 42, e2020007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Koizumi N., Siddique A. B., Andalibi A., Assessment of SARS-CoV-2 transmission among attendees of live concert events in Japan using contact-tracing data. J. Travel Med. 27, taaa096 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention , Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res. Perspect. 11, 8–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kute V. B., et al., Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: A multicenter cohort study from India. Transplantation 105, 851–860 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.LaCourse S. M., et al., Low prevalence of SARS-CoV-2 among pregnant and postpartum patients with universal screening in Seattle, Washington. Clin. Infect. Dis. 72, 869–872 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lai X., et al., Coronavirus Disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a Tertiary Hospital in Wuhan, China. JAMA Netw. Open 3, e209666 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lavezzo E., et al., Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 584, 425–429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Letizia A. G., et al., SARS-CoV-2 transmission among marine recruits during quarantine. N. Engl. J. Med. 383, 2407–2416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lewis S. S., et al., Early experience with universal pre-procedural testing for SARS-CoV-2 in a relatively low-prevalence area. Infect. Control Hosp. Epidemiol. 42, 341–343 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li Y., et al., Asymptomatic and symptomatic patients with non-severe Coronavirus Disease (COVID-19) have similar clinical features and virological courses: A retrospective single center study. Front. Microbiol. 11, 1570 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li J., et al., Aggressive quarantine measures reduce the high morbidity of COVID-19 in patients on maintenance hemodialysis and medical staff of hemodialysis facilities in Wuhan, China. Kidney Dis. 6, 271–283 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Li X., et al., Differences in clinical features and laboratory results between adults and children with SARS-CoV-2 infection. BioMed Res. Int. 2020, 6342598 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lin S., et al., Epidemiological and clinical characteristics of 161 discharged cases with coronavirus disease 2019 in Shanghai, China. BMC Infect. Dis. 20, 780 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liu J.-Y., Chen T.-J., Hwang S.-J., Analysis of imported cases of COVID-19 in Taiwan: A nationwide study. Int. J. Environ. Res. Public Health 17, 3311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention , The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 41, 145–151 (2020). [DOI] [PubMed] [Google Scholar]
- 264.Liu L., et al., Optimizing screening strategies for coronavirus disease 2019: A study from Middle China. J. Infect. Public Health 13, 868–872 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Liu S., et al., Clinical characteristics and risk factors of patients with severe COVID-19 in Jiangsu province, China: A retrospective multicentre cohort study. BMC Infect. Dis. 20, 584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Liu J., Huang J., Xiang D., Large SARS-CoV-2 outbreak caused by asymptomatic traveler, China. Emerg. Infect. Dis. 26, 2260–2263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Liu P., et al., Characteristics and effectiveness of the Coronavirus disease 2019 (COVID-19) prevention and control in a representative city in China. Med. Sci. Monit. 26, e927472 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lokken E. M., et al., Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am. J. Obstet. Gynecol. 223, 911.e1–911.e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.London V., et al., The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am. J. Perinatol. 37, 991–994 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lopez A. S., et al., Transmission dynamics of COVID-19 outbreaks associated with child care facilities—Salt Lake City, Utah, April-July 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1319–1323 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lu X., et al., SARS-CoV-2 infection in children. N. Engl. J. Med. 382, 1663–1665 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Field briefing: Diamond Princess COVID-19 cases, 20 Feb update. https://www.niid.go.jp/niid/en/2019-ncov-e/9417-covid-dp-fe-o2.html. Accessed 29 January 2021.
- 273.Ly T. D. A., et al., Pattern of SARS-CoV-2 infection among dependant elderly residents living in long-term care facilities in Marseille, France, March-June 2020. Int. J. Antimicrob. Agents 56, 106219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Maechler F., et al., Epidemiological and clinical characteristics of SARS-CoV-2 infections at a testing site in Berlin, Germany, March and April 2020-a cross-sectional study. Clin. Microbiol. Infect. 26, 1685.e7–1685.e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Maggiolo F., et al., SARS-CoV-2 infection in persons living with HIV: A single center prospective cohort. J. Med. Virol. 93, 1145–1149 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Malheiro R., et al., Effectiveness of contact tracing and quarantine on reducing COVID-19 transmission: A retrospective cohort study. Public Health 189, 54–59 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Maltezou H. C., et al., Transmission dynamics of SARS-CoV-2 within families with children in Greece: A study of 23 clusters. J. Med. Virol. 93, 1414–1420 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Maltezou H. C., et al., Children and adolescents with SARS-CoV-2 infection: Epidemiology, clinical course and viral loads. Pediatr. Infect. Dis. J. 39, e388–e392 (2020). [DOI] [PubMed] [Google Scholar]
- 279.Mao S., et al., Epidemiological analysis of 67 local COVID-19 clusters in Sichuan Province, China. BMC Public Health 20, 1525 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Marossy A., et al., A study of universal SARS-CoV-2 RNA testing of residents and staff in a large group of care homes in South London. J. Infect. Dis. 223, 381–388 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Martin C., et al., Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk healthcare workers and hospital staff. J. Hosp. Infect. 106, 102–106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Martini F., et al., On cancer, COVID-19, and CT scans: A monocentric retrospective study. J. Clin. Med. 9, 3935 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Massarotti C., et al., Asymptomatic SARS-CoV-2 infections in pregnant patients in an Italian city during the complete lockdown. J. Med. Virol. 93, 1758–1760 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Meena M. K., Singh M., Panda P. K., Bairwa M. K., Non-COVID area of a tertiary care hospital: A major source of nosocomial COVID-19 transmission. J. Family Community Med. 27, 212–215 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Melgosa M., et al., SARS-CoV-2 infection in Spanish children with chronic kidney pathologies. Pediatr. Nephrol. 35, 1521–1524 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Menachemi N., et al., Population point prevalence of SARS-CoV-2 infection based on a statewide random sample—Indiana, April 25-29, 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 960–964 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Menting T., et al., Low-threshold SARS-CoV-2 testing facility for hospital staff: Prevention of COVID-19 outbreaks? Int. J. Hyg. Environ. Health 231, 113653 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Miller E. S., Grobman W. A., Sakowicz A., Rosati J., Peaceman A. M., Clinical implications of universal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) testing in pregnancy. Obstet. Gynecol. 136, 232–234 (2020). [DOI] [PubMed] [Google Scholar]
- 289.Mostafa A., et al., Universal COVID-19 screening of 4040 health care workers in a resource-limited setting: An Egyptian pilot model in a university with 12 public hospitals and medical centers. Int. J. Epidemiol. 50, 50–61 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Namal E., Single center experience on screening oncology patients for covid-19 before anti-cancer treatment. Int. J. Hematol. Oncol. 30, 207–212 (2020). [Google Scholar]
- 291.Nishiura H., et al., The rate of underascertainment of novel coronavirus (2019-nCoV) infection: Estimation using Japanese passengers data on evacuation flights. J. Clin. Med. 9, 419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Norsa L., et al., Asymptomatic severe acute respiratory syndrome coronavirus 2 infection in patients with inflammatory bowel disease under biologic treatment. Gastroenterology 159, 2229–2231.e2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ornaghi S., et al., Performance of an extended triage questionnaire to detect suspected cases of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in obstetric patients: Experience from two large teaching hospitals in Lombardy, Northern Italy. PLoS One 15, e0239173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ossareh S., Bagheri M., Abbasi M., Abolfathi S., Bohlooli A., Role of screening for COVID-19 in hemodialysis wards, results of a single center study. Iran. J. Kidney Dis. 14, 389–398 (2020). [PubMed] [Google Scholar]
- 295.Oster Y., et al., Proactive screening approach for SARS-CoV-2 among healthcare workers. Clin. Microbiol. Infect. 27, 155–156 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Panagiotakopoulos L., et al., SARS-CoV-2 infection among hospitalized pregnant women: Reasons for admission and pregnancy characteristics—Eight U.S. Health Care Centers, March 1-May 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1355–1359 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Parri N., et al., Characteristic of COVID-19 infection in pediatric patients: Early findings from two Italian pediatric research networks. Eur. J. Pediatr. 179, 1315–1323 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Parri N., et al., COVID-19 in 17 Italian pediatric emergency departments. Pediatrics 146, e20201235 (2020). [DOI] [PubMed] [Google Scholar]
- 299.Patberg E. T., et al., Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 224, 382.e1–382.e18 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Patel A. B., et al., SARS-CoV-2 point prevalence among asymptomatic hospitalized children and subsequent healthcare worker evaluation. J. Pediatric Infect. Dis. Soc. 9, 617–619 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pavli A., et al., In-flight transmission of COVID-19 on flights to Greece: An epidemiological analysis. Travel Med. Infect. Dis. 38, 101882 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Payne D. C., et al., SARS-CoV-2 infections and serologic responses from a sample of U.S. Navy Service Members—USS Theodore Roosevelt, April 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 714–721 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Petersen I., Phillips A., Three quarters of people with SARS-CoV-2 infection are asymptomatic: Analysis of English household survey data. Clin. Epidemiol. 12, 1039–1043 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Pirnay J.-P., et al., Study of a SARS-CoV-2 outbreak in a Belgian military education and training center in Maradi, Niger. Viruses 12, 949 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Pollán M., et al., Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 396, 535–544 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Prabhu M., et al., Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: A prospective cohort study. BJOG 127, 1548–1556 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Pray I. W., et al., COVID-19 outbreak at an overnight summer school retreat—Wisconsin, July-August 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1600–1604 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Xingqiang P., et al., Study on transmission dynamic of 15 clusters of coronavirus disease 2019 cases in Ningbo. Zhonghua Liu Xing Bing Xue Za Zhi 41, E066 (2020). [DOI] [PubMed] [Google Scholar]
- 309.Qiu H., et al., Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infect. Dis. 20, 689–696 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rogers J. H., et al., Characteristics of COVID-19 in homeless shelters: A community-based surveillance study. Ann. Intern. Med. 174, 42–49 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Roxby A. C., et al., Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern. Med. 180, 1101–1105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sacco G., Foucault G., Briere O., Annweiler C., COVID-19 in seniors: Findings and lessons from mass screening in a nursing home. Maturitas 141, 46–52 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Huerta Saenz I. H., Elías Estrada J. C., Campos Del Castillo K., Muñoz Taya R., Coronado J. C., Características materno perinatales de gestantes COVID-19 en un hospital nacional de Lima, Perú [in Spanish]. Rev. Peru. Ginecol. Obstet., 10.31403/rpgo.v66i2245 (2020). [DOI] [Google Scholar]
- 314.Sakowicz A., et al., Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnant women. Am. J. Obstet. Gynecol. MFM 2, 100198 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Saluja M., Pillai D., Jeliya S., Bauddh N., Chandel R., COVID 19-clinical profile, radiological presentation, prognostic predictors, complications and outcome: A perspective from the Indian subcontinent. J. Assoc. Physicians India 68, 13–18 (2020). [PubMed] [Google Scholar]
- 316.Samrah S. M., et al., COVID-19 outbreak in Jordan: Epidemiological features, clinical characteristics, and laboratory findings. Ann. Med. Surg. (Lond.) 57, 103–108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sanchez G. V., et al., Initial and repeated point prevalence surveys to inform SARS-CoV-2 infection prevention in 26 skilled nursing facilities—Detroit, Michigan, March-May 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 882–886 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sastry S. R., et al., Universal screening for the SARS-CoV-2 virus on hospital admission in an area with low COVID-19 prevalence. Infect. Control Hosp. Epidemiol. 41, 1231–1233 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Saurabh S., et al., Dynamics of SARS-CoV-2 transmission among Indian nationals evacuated from Iran. Disaster Med. Public Health Prep., 10.1017/dmp.2020.393 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Savirón-Cornudella R., et al., Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) universal screening in gravids during labor and delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 256, 400–404 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Shen Q., et al., Novel coronavirus infection in children outside of Wuhan, China. Pediatr. Pulmonol. 55, 1424–1429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Sharma A. K., et al., Epidemiological and clinical profile of COVID-19 in Nepali children: An initial experience. J. Nepal Paediatr. Soc. 40, 202–209 (2020). [Google Scholar]
- 323.Shi L., et al., Quarantine at home may not enough!-from the epidemiological data in Shaanxi Province of China. BMC Res. Notes 13, 506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Shmakov R. G., et al., Clinical course of novel COVID-19 infection in pregnant women. J. Matern. Fetal Neonatal Med., 10.1080/14767058.2020.1850683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Singer J. S., et al., Low prevalence (0.13%) of COVID-19 infection in asymptomatic pre-operative/pre-procedure patients at a large, academic medical center informs approaches to perioperative care. Surgery 168, 980–986 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sugano N., Ando W., Fukushima W., Cluster of severe acute respiratory syndrome coronavirus 2 infections linked to music clubs in Osaka, Japan. J. Infect. Dis. 222, 1635–1640 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sun D., et al., Children infected with SARS-CoV-2 from family clusters. Front. Pediatr. 8, 386 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Sutton D., Fuchs K., D’Alton M., Goffman D., Universal screening for SARS-CoV-2 in women admitted for delivery. N. Engl. J. Med. 382, 2163–2164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tambe M. P., et al., An epidemiological study of laboratory confirmed COVID-19 cases admitted in a tertiary care hospital of Pune, Maharashtra. Indian J. Public Health 64, S183–S187 (2020). [DOI] [PubMed] [Google Scholar]
- 330.Xin T., et al., Clinical characteristics analysis of 13 cases of novel coronavirus infection in children in Changsha. Chinese Journal of Contemporary Pediatrics 22, 294–298 (2020).32312364 [Google Scholar]
- 331.Tang O., et al., Outcomes of nursing home COVID-19 patients by initial symptoms and comorbidity: Results of universal testing of 1970 residents. J. Am. Med. Dir. Assoc. 21, 1767–1773.e1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ali T., et al., Coronavirus disease-19: Disease severity and outcomes of solid organ transplant recipients: Different spectrum of disease in different populations? Transplantation 105, 121–127 (2021). [DOI] [PubMed] [Google Scholar]
- 333.Thompson J. W. Jr. et al., An epidemiologic study of COVID-19 patients in a state psychiatric hospital: High penetrance with early CDC guidelines. Psychiatr. Serv. 71, 1285–1287 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Tian S., et al., Characteristics of COVID-19 infection in Beijing. J. Infect. 80, 401–406 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Trahan M.-J., Mitric C., Malhamé I., Abenhaim H. A., Screening and testing pregnant patients for SARS-CoV-2: First-wave experience of a designated COVID-19 hospitalization centre in Montreal. J. Obstet. Gynaecol. Can. 43, 571–575 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Venkataram T., et al., Deployment of neurosurgeons at the warfront against coronavirus disease of 2019 (COVID-19). World Neurosurg. 144, e561–e567 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wang M., et al., Epidemiological characteristics and transmission dynamics of paediatric cases with coronavirus disease 2019 in Hubei province, China. J. Paediatr. Child Health 57, 637–645 (2021). [DOI] [PubMed] [Google Scholar]
- 338.Wanwan S., et al., Epidemiological characteristics of 2019 novel coronavirus family clustering in Zhejiang Province. Chin. J. Prev. Med 54, E027 (2020). [Google Scholar]
- 339.Xu X., et al., The cumulative rate of SARS-CoV-2 infection in Chinese hemodialysis patients. Kidney Int. Rep. 5, 1416–1421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yan J., et al., Coronavirus disease 2019 in pregnant women: A report based on 116 cases. Am. J. Obstet. Gynecol. 223, 111.e1–111.e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Yang J., et al., Clinical characteristics and eosinophils in young SARS-CoV-2-positive Chinese travelers returning to Shanghai. Front. Public Health 8, 368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yassa M., et al., Outcomes of universal SARS-CoV-2 testing program in pregnant women admitted to hospital and the adjuvant role of lung ultrasound in screening: A prospective cohort study. J. Matern. Fetal Neonatal Med. 33, 3820–3826 (2020). [DOI] [PubMed] [Google Scholar]
- 343.Cura Yayla B. C., et al., Characteristics and management of children with COVID-19 in Turkey. Balkan Med. J. 37, 341–347 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yayla B. C. C., Aykac K., Ozsurekci Y., Ceyhan M., Characteristics and management of children with COVID-19 in a tertiary care hospital in Turkey. Clin. Pediatr. 60, 170–177 (2021). [DOI] [PubMed] [Google Scholar]
- 345.Yılmaz K., et al., Evaluation of the novel coronavirus disease in Turkish children: Preliminary outcomes. Pediatr. Pulmonol. 55, 3587–3594 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ye L. X., et al., Investigation of a cluster epidemic of COVID-19 in Ningbo. Zhonghua Liu Xing Bing Xue Za Zhi 41, 2029–2033 (2020). [DOI] [PubMed] [Google Scholar]
- 347.Yombi J. C., De Greef J., Bernard P., Belkhir L., Testing of patients and coronavirus disease 2019 (COVID-19) infection before scheduled deliveries. J. Perinat. Med. 48, 995–996 (2020). [DOI] [PubMed] [Google Scholar]
- 348.Yue H., et al., Clinical characteristics of coronavirus disease 2019 in Gansu province, China. Ann. Palliat. Med. 9, 1404–1412 (2020). [DOI] [PubMed] [Google Scholar]
- 349.Zhan T., et al., Retrospective analysis of clinical characteristics of 405 patients with COVID-19. J. Int. Med. Res. 48, 300060520949039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zamzuri M. ‘A. I. A., et al., Epidemiological characteristics of COVID-19 in Seremban, Negeri Sembilan, Malaysia. Open Access Maced. J. Med. Sci. 8, 471–475 (2020). [Google Scholar]
- 351.Zhang L., Huang S., Clinical features of 33 cases in children infected with SARS-CoV-2 in Anhui Province, China—A multi-center retrospective cohort study. Front. Public Health 8, 255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Zhang S., et al., Factors associated with asymptomatic infection in health-care workers with severe acute respiratory syndrome coronavirus 2 infection in Wuhan, China: A multicentre retrospective cohort study. Clin. Microbiol. Infect. 26, 1670–1675 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Deng Z. Q., et al., Analysis on transmission chain of a cluster epidemic of COVID-19, Nanchang. Zhonghua Liu Xing Bing Xue Za Zhi 41, 1420–1423 (2020). [DOI] [PubMed] [Google Scholar]
- 354.Zou L., et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 382, 1177–1179 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.El-Sokkary R. H., et al., Characteristics and predicting factors of Corona Virus Disease-2019 (COVID-19) among healthcare providers in a developing country. PLoS One 16, e0245672 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Kristiansen M. F., et al., Epidemiology and clinical course of first wave coronavirus disease cases, Faroe Islands. Emerg. Infect. Dis. 27, 749–758 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Reale S. C., et al., Patient characteristics associated with SARS-CoV-2 infection in parturients admitted for labour and delivery in Massachusetts during the spring 2020 surge: A prospective cohort study. Paediatr. Perinat. Epidemiol. 35, 24–33 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Min Y., et al., Clinical characteristics of deceased hemodialysis patients affected by COVID-19. Int. Urol. Nephrol. 53, 797–802 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Huete-Pérez J. A., et al., First report on prevalence of SARS-CoV-2 infection among health-care workers in Nicaragua. PLoS One 16, e0246084 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Arnold F. W., Bishop S., Oppy L., Scott L., Stevenson G., Surveillance testing reveals a significant proportion of hospitalized patients with SARS-CoV-2 are asymptomatic. Am. J. Infect. Control 49, 281–285 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Bigelow B. F., et al., Outcomes of universal COVID-19 testing following detection of incident cases in 11 long-term care facilities. JAMA Intern. Med. 181, 127–129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Bayle C., et al., Asymptomatic SARS COV-2 carriers among nursing home staff: A source of contamination for residents? Infect. Dis. Now 51, 197–200 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Wang L., et al., Source investigation on a familiar cluster of coronavirus disease 2019 in Dandong city of Liaoning Province. Zhonghua Yu Fang Yi Xue Za Zhi 55, 120–122 (2021). [DOI] [PubMed] [Google Scholar]
- 364.Scheier T., et al., Universal admission screening for SARS-CoV-2 infections among hospitalized patients, Switzerland, 2020. Emerg. Infect. Dis. 27, 404–410 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Adetola H. H., et al., Clinical presentations and management of COVID-19 infected children seen in a district health facility in Kambia, northern Sierra Leone. Pan Afr. Med. J. 37, 28 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Camara E., et al., Epidemiological and clinical profile of children with Coronavirus disease (COVID-19) at the Center for the Treatment of Epidemics and Infection Prevention (CTEIP) of the University Hospital of Donka in Conakry. Pan Afr. Med. J. 37, 363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Jones A., et al., Assessment of day-7 postexposure testing of asymptomatic contacts of COVID-19 patients to evaluate early release from quarantine—Vermont, May-November 2020. MMWR Morb. Mortal. Wkly. Rep. 70, 12–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Khondaker T., et al., Clinical profile and outcome of COVID -19 in children with pre-existing renal disease. Int. J. Pediatr. Nephrol. 9, 1–6 (2021). [Google Scholar]
- 369.Marcus N., et al., Minor clinical impact of COVID-19 pandemic on patients with primary immunodeficiency in Israel. Front. Immunol. 11, 614086 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Martin-Villares C., Bernal-Sprekelsen M., Molina-Ramirez C. P., Bartolome-Benito M., COVID ORL ESP Collaborative Group , Risk of contagion of SARS-CoV-2 among otorhinolaryngologists in Spain during the “Two waves.” Eur. Arch. Otorhinolaryngol., 10.1007/s00405-020-06582-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Almadhi M. A., et al., The high prevalence of asymptomatic SARS-CoV-2 infection reveals the silent spread of COVID-19. Int. J. Infect. Dis. 105, 656–661 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Atakla H. G., et al., COVID-19 infection in pediatric subjects: Study of 36 cases in Conakry. Pan Afr. Med. J. 37, 42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Bender J. K., Brandl M., Höhle M., Buchholz U., Zeitlmann N., Analysis of asymptomatic and presymptomatic transmission in SARS-CoV-2 outbreak, Germany, 2020. Emerg. Infect. Dis. 27, 1159–1163 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Berry M., et al., Clinical stratification of pregnant COVID-19 patients based on severity: A single academic center experience. Am. J. Perinatol. 38, 515–522 (2021). [DOI] [PubMed] [Google Scholar]
- 375.Cabraal M. N. S., Samarawickrama R. I. U., Kodithuwakku K. A. R. R., Viswakula S. D., Lantra S. R., Nationwide descriptive study of COVID-19 in children below the age of 14 years in Sri Lanka. Sri Lanka J. Child Health 50, 103 (2021). [Google Scholar]
- 376.Cesilia C., Sudarmaji S., Setiabudi D., Nataprawira H. M., Case report of a COVID-19 family cluster originating from a boarding school. Paediatr. Indones. 61, 53–60 (2021). [Google Scholar]
- 377.Dbeibo L., et al., Assessment of a universal preprocedural screening program for COVID-19. Infect. Control Hosp. Epidemiol., 10.1017/ice.2021.40 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Dixon B. E., et al., Symptoms and symptom clusters associated with SARS-CoV-2 infection in community-based populations: Results from a statewide epidemiological study. PLoS One 16, e0241875 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Fakhim H., et al., Asymptomatic carriers of coronavirus disease 2019 among healthcare workers in Isfahan, Iran. Future Virol. 16, 93–98 (2021). [Google Scholar]
- 380.Hu S., et al., Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat. Commun. 12, 1533 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Isoldi S., et al., The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID-19: A 6-months prospective study. J. Med. Virol. 93, 3122–3132 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Jani S., et al., Clinical characteristics of mother-infant dyad and placental pathology in COVID-19 cases in predominantly African American population. AJP Rep. 11, e15–e20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Kute V. B., et al., A multicenter cohort study from India of 75 kidney transplants in recipients recovered after COVID-19. Transplantation 105, 1423–1432 (2021). [DOI] [PubMed] [Google Scholar]
- 384.Marcus J. E., et al., Risk factors associated with COVID-19 transmission among US air force trainees in a congregant setting. JAMA Netw. Open 4, e210202 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Miyahara R., et al., Familial clusters of coronavirus disease in 10 prefectures, Japan, February-May 2020. Emerg. Infect. Dis. 27, 915–918 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Myles P. S., et al., COVID-19 risk in elective surgery during a second wave: A prospective cohort study. ANZ J. Surg. 91, 22–26 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Nakajo K., Nishiura H., Transmissibility of asymptomatic COVID-19: Data from Japanese clusters. Int. J. Infect. Dis. 105, 236–238 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.de Miguel Negro M., et al., Pre-operative prevalence of asymptomatic carriers of COVID-19 in hospitals in Catalonia during the first wave after the resumption of surgical activity. Cir. Esp. (Engl. Ed.), 10.1016/j.ciresp.2021.01.014 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Oduro-Mensah E., et al., Clinical features of COVID-19 in Ghana: Symptomatology, illness severity and comorbid non-communicable diseases. Ghana Med. J. 5, 23–32 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Randremanana R. V., et al., The COVID-19 epidemic in Madagascar: Clinical description and laboratory results of the first wave, March-September 2020. Influenza Other Respi. Viruses 15, 457–468 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Sabetian G., et al., COVID-19 infection among healthcare workers: A cross-sectional study in southwest Iran. Virol. J. 18, 58 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Savirón-Cornudella R., et al., Screening of severe acute respiratory syndrome coronavirus-2 infection during labor and delivery using polymerase chain reaction and immunoglobulin testing. Life Sci. 271, 119200 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Singh V., Choudhary A., Datta M. R., Ray A., Maternal and neonatal outcomes of COVID-19 in pregnancy: A single-centre observational study. Cureus 13, e13184 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Sharma N., Seehra N., Kabra S., Pregnancy with covid-19 infection and fetomaternal outcomes. J. Evol. Med. Dent. Sci. 10, 23–27 (2021). [Google Scholar]
- 395.Soriano-Arandes A., et al., Household SARS-CoV-2 transmission and children: A network prospective study. Clin. Infect. Dis., 10.1093/cid/ciab228 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Stadler R. N., et al., Systematic screening on admission for SARS-CoV-2 to detect asymptomatic infections. Antimicrob. Resist. Infect. Control 10, 44 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Tawar S., Diva Reddy G., Ray S., Chawla N., Garg S., Rapid response and mitigation measures in control of COVID-19 cases in an industrial warehouse of Western Maharashtra, India. J. Mar. Med. Soc. 22, 220 (2020). [Google Scholar]
- 398.Tompkins L. K., et al., Mass SARS-CoV-2 testing in a dormitory-style correctional facility in Arkansas. Am. J. Public Health 111, 907–916 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Villa J., et al., Results of preoperative screening for COVID-19 correlate with the incidence of infection in the general population—A tertiary care experience. Hosp. Pract. 49, 216–220 (2021). [DOI] [PubMed] [Google Scholar]
- 400.Xie W., et al., Infection and disease spectrum in individuals with household exposure to SARS-CoV-2: A family cluster cohort study. J. Med. Virol. 93, 3033–3046 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Van Vinh Chau N., et al., The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. Clin. Infect. Dis. 71, 2679–2687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Xu H., et al., A follow-up study of children infected with SARS-CoV-2 from western China. Ann. Transl. Med. 8, 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Wu H. P., Clinical features of coronavirus disease 2019 in children aged <18 years in Jiangxi, China: An analysis of 23 cases. Chinese Journal of Contemporary Pediatrics 22, 419–424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Yaoling M., Analysis of the clinical characteristics of 115 children with novel coronavirus infection. Chinese Journal of Contemporary Pediatrics 22, 290 (2020).32312363 [Google Scholar]
- 405.Yun Z., Clinical features and chest CT findings of 2019 coronavirus disease in infants and young children. Chinese Journal of Contemporary Pediatrics 22, 215 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.van Doremalen N., et al., Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 382, 1564–1567 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Cheng P. K. C., et al., Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet 363, 1699–1700 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Oran D. P., Topol E. J., Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann. Intern. Med. 173, 362–367 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Kronbichler A., et al., Asymptomatic patients as a source of COVID-19 infections: A systematic review and meta-analysis. Int. J. Infect. Dis. 98, 180–186 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team , The novel coronavirus pneumonia emergency response epidemiology team, the epidemiological characteristics of an outbreak of 2019 novel Coronavirus Diseases (COVID-19)—China, 2020. China CDC Weekly 2, 113–122 (2020). [PMC free article] [PubMed] [Google Scholar]
- 411.Zhou X., Li Y., Li T., Zhang W., Follow-up of asymptomatic patients with SARS-CoV-2 infection. Clin. Microbiol. Infect. 26, 957–959 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Leung N. H. L., Xu C., Ip D. K. M., Cowling B. J., Review article: The fraction of influenza virus infections that are asymptomatic: A systematic review and meta-analysis. Epidemiology 26, 862–872 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Furuya-Kanamori L., et al., Heterogeneous and dynamic prevalence of asymptomatic influenza virus infections. Emerg. Infect. Dis. 22, 1052–1056 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wilder-Smith A., et al., Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg. Infect. Dis. 11, 1142–1145 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Perrotta F., et al., COVID-19 and the elderly: Insights into pathogenesis and clinical decision-making. Aging Clin. Exp. Res. 32, 1599–1608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Mehta P., et al., COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Carter-Timofte M. E., et al., Deciphering the role of host genetics in susceptibility to severe COVID-19. Front. Immunol. 11, 1606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Conti P., Younes A., Coronavirus COV-19/SARS-CoV-2 affects women less than men: Clinical response to viral infection. J. Biol. Regul. Homeost. Agents 34, 339–343 (2020). [DOI] [PubMed] [Google Scholar]
- 419.Le Bert N., et al., SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584, 457–462 (2020). [DOI] [PubMed] [Google Scholar]
- 420.Whyte D., et al., Mumps epidemiology in the mid-west of Ireland 2004-2008: Increasing disease burden in the university/college setting. Euro Surveill. 14, 19182 (2009). [PubMed] [Google Scholar]
- 421.Centers for Disease Control and Prevention , Mumps outbreak on a university campus—California, 2011. MMWR Morb. Mortal. Wkly. Rep. 61, 986–989 (2012). [PubMed] [Google Scholar]
- 422.Lessler J., et al., Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N. Engl. J. Med. 361, 2628–2636 (2009). [DOI] [PubMed] [Google Scholar]
- 423.Stein-Zamir C., et al., A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Euro Surveill. 25, 2001352 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.World Health Organization , Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 14 August 2020.
- 425.Griffith G. J., et al., Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 11, 5749 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Centers for Disease Control and Prevention , Coronavirus Disease 2019. (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. Accessed 27 May 2020.
- 427.World Health Organization , Global research on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov. Accessed 4 May 2020.
- 428.Sterne J. A., et al., ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Borenstein M., Hedges L. V., Higgins J. P. T., Rothstein H. R., A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1, 97–111 (2010). [DOI] [PubMed] [Google Scholar]
- 430.DerSimonian R., Laird N., Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- 431.Agresti A., Coull B. A., Approximate is better than “exact” for interval estimation of binomial proportions. null 52, 119–126 (1998). [Google Scholar]
- 432.Hartung J., Knapp G., On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat. Med. 20, 1771–1782 (2001). [DOI] [PubMed] [Google Scholar]
- 433.Egger M., Davey Smith G., Schneider M., Minder C., Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.