Abstract
Buruli ulcer (BU), the second most common mycobacterial disease in West Africa, is a necrotizing skin disease that can lead to high morbidity in affected patients. The disease is caused by Mycobacterium ulcerans (MU), whose major virulence factor is mycolactone. Although early infection can be treated with antibiotics, an effective preventative strategy is challenging due to unknown reservoir(s) and unresolved mode(s) of transmission. Further, disease occurrence in remote locations with limited access to health facilities further complicates disease burden and associated costs. We discuss here MU transmission hypotheses and investigations into environmental reservoirs and discuss successes and challenges of studying MU and Buruli ulcer across human, animal, and environmental interfaces. We argue that a One Health approach is needed to advance the understanding of MU transmission and designing management scenarios that prevent and respond to epidemics. Although previous work has provided significant insights into risk factors, epidemiology and clinical perspectives of disease, understanding the bacterial ecology, environmental niches and role of mycolactone in natural environments and during infection of the human host remains equally important to better understanding and preventing this mysterious disease.
Keywords: Mycobacterium ulcerans, Buruli ulcer, Transmission, One-health
1. Introduction
Human encroachment into natural habitats has contributed to expanding the range of many environmental pathogens, influencing the emergence of infectious diseases [1]. Well known examples include dengue, malaria, schistosomiasis, and leishmaniasis [2]. Although attracting less public awareness, environmentally transmitted non-tuberculosis mycobacterial (NTM) diseases cause substantive, yet often unappreciated illness burden [[3], [4], [5]]. Nearly every pathogenic NTM can cause soft tissue infections, though Mycobacterium ulcerans, the causative agent of Buruli ulcer (BU), M. fortuitum, M. abscessus, M. marinum, and M. leprae, are most commonly involved [6].
Though a neglected tropical disease, data have shown BU is the third most common mycobacteriosis following tuberculosis and leprosy [7]. Buruli ulcer has been reported in at least 33 countries, with highest prevalence in West Africa and Australia, though many countries do not report, and active case surveillance is not conducted in every country [8]. Further complicating, BU may have similar disease presentation to other cutaneous infections, and high recurrence and reinfection rates [9]. The disease can lead to high morbidity, particularly to affected rural populations, who, apart from the direct pathological impact of often large ulcers, are also socio-economically impacted [[10], [11], [12]]. Treatment includes a combination of clarithromycin and rifampicin, but patients are sometimes lost-to-follow-up because of the two-month treatment regime or need for hospitalization.
M. ulcerans produces a macrolide lipid, mycolactone, that is cytotoxic and the primary virulence determinant [13]. Genes for mycolactone are encoded on a 174 Kb plasmid (pMUM001), and variations in pMUM001 yield different mycolactone congeners among MU strains and ecovars, geographically distributed with varying virulence [14]. M. ulcerans and other mycolactone producing mycobacterium ecovars evolved from a common M. marinum ancestor through genome reduction and pMUM001 acquisition, progressing into environmental pathogens with documented genomic and toxin geographic diversity [15]. Plasmid pMUM001 is suggested to be acquired by MU at an expense of extensive gene loss during evolution from its progenitor M. marinum thereby suggesting its significant role in adaptation to a new environmental niche [15].
Buruli ulcer has a complex epidemiology where spatial and temporal heterogeneity in the MU environment leads to fluctuating heterogeneity in transmission, also depending on interactions between host(s), reservoir, and the pathogen [16] . Despite this basic understanding, transmission mechanisms have not yet been resolved. M. ulcerans has a doubling time between 24 and 84 h, depending on laboratory conditions, and growth can take months, making culture difficult [17,18]. This limitation contributes to narrow understanding of MU genetic variability in natural environmental populations. Likely there are numbers of sub-species yet to be discovered in environmental habitats, and we speculate this under studied area of species diversity leaves clinically relevant members underappreciated. And while the role of mycolactone in BU pathology is well documented, its function in natural environments is lacking. The coincidental evolution of virulence hypothesis states that many microbes acquire traits to outcompete or overcome biotic and abiotic forces during their normal life cycle in the outside-host environment [19]. However, such traits, such as toxin production, could confer virulence during host infection. Thus, the primary function of these “virulence factors” is to provide a fitness advantage for its producer in the environment and this is likely the case with mycolactone (Fig. 1). Mycolactone's macrolide structure suggests that it may be an antagonist to bacteria with quorum sensing machinery or may serve as a regulator of secondary metabolism, and recently published data demonstrate this antagonism occurs in vitro [20,21]. To understand mechanisms leading to transmission and pathogenesis, it is important to determine the reservoir(s) of MU, stress responses and community interactions in its natural niche and during host infection, and the role mycolactone plays in these interactions.
Fig. 1.
Proposed functions of mycolactone production in the M. ulcerans natural, aquatic environment and human host (mycolactone is shown as a white box inside M. ulcerans). Mycolactone could have identical functioning in both environments, such as polymicrobial interactions, or oxygen conditions, however, mycolactone production also confers virulence to a susceptible host.
M. ulcerans normally resides in the environment where oxygen, pH, and availability of nutrients likely modulate proliferation and mycolactone production [[22], [23], [24], [25], [26]]. Furthermore, human landscape disturbances have been associated with MU in the environment, leading to increased transmission and infection risk, and over seasons related to weather events and climate patterns [[27], [28], [29], [30], [31], [32]]. Exposure to water during daily activities such as farming, swimming, fishing and conducting household chores enhance BU risk [11]. Higher disease burden is observed in areas of heterogenous topography and in close proximity to rivers, wetlands and cultivations suggesting a significant BU association with aquatic environments and local irrigation [30,33]. Given these epidemiological associations, it is necessary to better understand how MU populations vary among habitats, geographic areas and through seasons, and what role mycolactone plays in transmission from these environments. Below we highlight data of MU detection in the environment and how this provides insight into potential reservoirs, vectors, and transmission. We finally argue that it is important to move beyond only clinical studies of pathogenesis and treatment to investigate the biology and ecology of MU and the role of mycolactone in the environment to implement preventive and control strategies associated with natural resources management and within the One Health context.
2. MU environmental detection
The most reliable method for MU detection in the environment is qPCR targeting IS2404 with detection of pMUM001 [34] genes such as the keto-reductase (KR) or enoyl-reductase (ER) gene [35]. Several groups have used a multiplexed PCR technique developed by Fyfe et al. that targets KR and Ct difference between IS2404 or IS2606 to differentiate MU from other mycolactone producing mycobacteria [35]. Furthermore, other groups have successfully targeted variable number tandem repeats (VNTR) to differentiate environmental and clinical samples [34,36,37]. Methods such as loop mediated isothermal amplification, and chromatographic and aptamer techniques to detect mycolactone are in various stages of development but are still being optimized for field detection [[38], [39], [40], [41]]. To our knowledge, there are only two reported instances of MU environmental cultivation as environmental contamination and longer MU incubation time causes culture difficulty [42,43].
2.1. MU in aquatic environments
M. ulcerans PCR detection in the environment was first reported by Ross et al., from samples of a swamp and a golf course irrigation system within a BU outbreak on Philip Island in Victoria, Australia [44]. M. ulcerans DNA has since been detected in various environmental samples such as aquatic invertebrates, water filtrand, aquatic plant biofilms, plant rhizospheres and soils in Ghana, Benin, Cameroon, French Guiana and Australia among others, by several research groups (Table 1 and Supplemental Table 1) [[34], [35], [36],[44], [45], [46], [47], [48], [49]] . M. ulcerans presence was also positively correlated with BU prevalence in Benin [34]. These collective findings suggest that MU is persistent in aquatic environments and is likely a resident member of associated microbial communities. Understanding the ecology of MU and role of mycolactone in multiple habitat types and ecosystems is becoming increasingly recognized as an important step in resolving transmission and developing prevention strategies.
Table 1.
Postulated modes of transmission, research supporting them, challenges to confirm them as mode of transmission and future research directions. *While mosquitoes are technically aquatic invertebrates, we separate them because of their postulated independent role in transmission as biting adults.
| Postulated hypothesis | Supporting research evidence | Challenges | Future directions |
|---|---|---|---|
| Amoebae | Survival of MU inside amoeba demonstrated by several studies [77,78] Earlier inflammation in mouse footpad observed for topical inoculation of MU-A. polyphaga culture compared to MU alone [79] |
Replication of MU inside amoebae not confirmed Study on field research showed no presence of MU in amoebae cultured from environmental samples [78] | More field investigations Investigations of replication of MU inside amoebae needs to be confirmed |
| *Aquatic invertebrates |
MU detected in several aquatic invertebrate taxa [32,38,45,[47], [48], [49], [50],52,53] Successful culture of MU from Hemiptera (Gerris spp) and salivary tissues of insects [38] Higher abundance of piercing predators in ER+ sites compared to ER- sites in lotic habitats) [52] Inoculation of MU found in saliva of water bugs developed BU lesion in mice [47] Experimental infection showing colonization of MU to salivary gland and other organs such as head, raptorial arms, thorax and guts [47,80] Development of BU infection in mice upon biting by experimentally infected Naucoridae [50] A case report of BU lesions following Belostomatidae bite reported [81] |
Role of the aquatic invertebrates in BUD transmission still controversial as these aquatic invertebrates do not normally bite humans. There have been no published confirmations of laboratory experimental infections of aquatic invertebrates. Bivalves, snails and other invertebrates that do not bite or otherwise physically injure humans or other animals have been show to harbor MU in numbers similar to mosquitoes, piercing insects and vertebrate feces. | Field studies investigating presence of biting insects in BU affected sites and presence of MU in them Investigations into ability of MU replication in these insects Investigations into role of these insects in mechanical transmission of BU |
| *Mosquitoes | Presence of MU in mosquitoes in Australia [55,56] Insect repellant, bed nets decreased BU risk [[56], [57], [58]] BU reported in areas with other mosquito transmitted diseases [82] Higher attraction and oviposition of mosquitoes in mycolactone containing sites [60] Mosquito larvae can feed on possum excreta contaminated with MU, which can colonize and sustain inside larva's gut and mouthparts [83] MU sustained inside mosquito larvae as well as in salivary gland and gut of Belostomatidae that fed on the larvae [84] | No MU detected in mosquitoes in Africa [48,59] Although MU sustained inside mosquito larvae as well as in salivary gland and gut of Belostomatidae that fed on the larvae, MU was not maintained at pupae and adult stages and adult mosquitoes were not able to mechanically transmit the bacteria [84] Studies have shown decreased MU survival during development of larvae from L1-L4 stage, and no MU was detected in adult stages [59] | Investigations into role of mosquitoes as a mechanical vector for BU. Field investigations on presence of MU in mosquitoes in Africa Investigation into discrepancy regarding the presence of MU DNA in Australia and Africa. |
| Marsupials and mammals | Presence of MU in feces of mammals and marsupials in Australia [65] MU in feces of possums and marsupials that were identical to clinical strains [[61], [62], [63]] BU lesions in body parts of several mammals such as ringtail, brushtail and mountain brushtail possums, mouse, grass cutter, goats, dogs and other mammals in Australia and Africa [64,66,67,70] | No studies have isolated viable MU from animal guts. MU was not detected from Benin in rodents and shrews and MU was not present in feces of domestic animals in Ghana [42,69] | The asymptomatic colonization of MU in animal guts is intriguing and requires further investigation regarding effects of anaerobic conditions and temperature, among other conditions that might impact MU survival, growth and mycolactone production. Further, the interaction of MU with other intestinal bacteria needs to be conducted. |
| Human-human (unlikely mode of transmission) | No genetic relatedness occurred in MU infecting different members in a family [75] Absence of MU in feces of BU patients [74] | BU induced due to human bite, suggesting more of a mechanical role of human has been reported [76] | – |
2.2. MU in non-mosquito aquatic invertebrates
Portaels et al. detected MU in the hemipteran insect family Naucoridae, aggressive aquatic predators that can fly and bite humans, thereby becoming the first to propose the role of aquatic invertebrates as reservoirs and potential vectors [49]. Subsequently, Marsollier et al. cultured MU from salivary tissues of these insects [50], and Portaels et al cultured MU from another hemipteran group of insects (Gerridae: Gerris spp) indicating the presence of viable MU [42]. M. ulcerans was detected in water bugs in several other studies conducted by Williamson et al. and Zogo et al. suggesting their potential role in BU transmission [36,51]. However, a study by Benbow et al showed no significant differences in Hemiptera abundance or their MU positivity between Ghanaian BU endemic and nonendemic sites [52]. Marion et al. detected MU in water bug saliva only in endemic regions of Cameroon, which upon inoculation into mice developed BU lesions suggesting presence of live bacteria in insects' saliva [53].
Mathematical modelling by Roche et al. showed that removing Oligochaeta in community network models decreased MU prevalence in an aquatic community [54], suggesting the aquatic food web structure may be important to mediating MU in the environment. From 90 sites in Ghana, Benbow et al found that Pleidae (Arthropoda: Hemiptera), Gerridae (Arthropoda: Hemiptera), Hydracarina (Arthropoda: Arachnida) and Libellulidae (Arthopoda: Odonata) were significant indicator taxa in lotic habitats positive for IS2404 and ER, and that macroinvertebrate communities significantly differed and had higher abundance of piercing predators in IS2404 and ER-positive sites compared to ER-negative sites in lotic habitats [55]. Similarly, Garchitorena et al observed that Lepidoptera and Hemiptera had high MU positivity, with MU being detected in Hemiptera throughout 11 months in a year [56]. Furthermore, in Japan, M. ulcerans subsp shinshuense was present in crayfish within a house backyard whose multiple household members had ulcers [57]. Bivalves, snails and other invertebrates that do not bite or otherwise physically injure humans or other animals can harbor MU in numbers similar to mosquitoes, piercing insects and vertebrate feces [27,52,55]. Taken together, these studies demonstrate that MU is consistently associated with aquatic macroinvertebrate communities, represented by both biting and non-biting species, and that relative composition of some species compared to others (i.e., food web structure) may play an important role in controlling its natural abundance.
2.3. MU in mosquitoes
In Australia, MU DNA has been detected in adult mosquitoes [58,59]. Lavender et al. observed that BU occurrence correlated with MU positivity in wild-caught mosquitoes [59], and documented that patients reported BU lesions at mosquito bite sites. Risk analysis also showed insect repellant use reduced BU risk, suggesting a role of mosquitoes in pathogen transmission [59,60]. In Africa, Landier et al. reported bed net use decreased BU risk in Cameroon [61]; however, Zogo et al. [51] found no MU positivity in mosquitoes collected in Benin. Similarly, Djouaka et al. detected presence of other IS2404 positive mycobacterial species, but not MU in mosquitoes collected in Benin [62]. The discrepancy in MU detection in mosquitoes in Africa and Australia is unclear and requires further investigation. Since mosquito larvae are part of some aquatic food webs, particularly in slow moving water, it is plausible that adult mosquitoes are simply indicators of a highly MU contaminated water source and play a minor or sporadic role in transmission. Given that there have been no vector competency studies, and that there is not one scientifically documented case of a bacterial pathogen being biologically vectored by mosquitoes, the role of these in transmitting MU will require additional scrutiny and evidence as discussed previously by Merritt et al. [27]. But a laboratory study in 2017 by Sanders et al demonstrated higher mosquito attraction and oviposition to sites containing higher mycolactone, thereby suggesting mycolactone as an interkingdom signal or cue [63], suggesting the mycolactone and MU may be an indicator for a suitable mosquito habitat. The need for new investigations into the role of mycolactone in the ecology of MU is becoming increasingly evident and is potentially the unknown information that could help explain such broad disparities among studies.
2.4. MU in mammals
BU lesions on mammals and MU in their feces have been reported, suggesting potential zoonotic transmission. In Australia, MU DNA in possum feces from endemic regions was identical to clinical strains of BU patients [64,65], and identical to a Victorian outbreak strain [66]. Also, BU infection ranging from asymptomatic gut colonization to complicated forms such as systemic BU or BU lesions of the face and sensitive organs has been reported in ringtail (Pseudocheiridae pseudodheirus), brushtail (Trichosurus vulpecula) and mountain brushtail possums (Trichosurus cunninghami) [67], and Röltgen et al. observed MU in bandicoot (Isoodon macrourus) feces [68].
Similarly, BU lesions were observed in the tail of a mouse in a Ghanaian community [69] and detected in feces of Thryonomys swinderianus, a mammal that lives nearby water resources and rice fields in Cote d'Ivoire and other African countries [47,70]. BU characteristics and MU positivity have been reported in domestic animals such as goats and dogs in Benin [71], but not in rodents and shrews [72], and MU was not present in feces of domestic animals in Ghana [73]. However, characteristic BU lesions or MU presence has been found in other mammals such as koalas, horses, alpacas, and cats [74]. MU DNA sequence patterns were similar in T. swinderianus spleen and rectal content in Côte d'Ivoire [48].
The asymptomatic MU colonization in animal guts is intriguing and requires further confirmation on bacterial viability inside the gut. While MU DNA has been detected, to our knowledge, no studies have isolated viable MU from animal guts. Additionally, investigations of effects of higher temperature and anaerobic environments that might impact MU survival, growth and mycolactone production and function would provide further insights on MU pathology inside the gut, and the interaction of MU with other intestinal bacteria also merits investigation.
2.5. MU in other organisms
Wilson et al. used targeted PCR and VNTR confirmation to detect MU in Leptopelis frogs [75]. MU infection was also reported in the Indian flap-shelled turtle (Lissemys punctata punctata) in Japan that was confirmed by IS2404 PCR and sequencing [76]. Additionally, BU occurred following a snake bite; however, the authors suggest that this could have been MU mechanical inoculation [77].
Despite speculation of an animal reservoir or zoonotic transmission, human-human transmission has been shown to be unlikely. O'Brien et al. observed that no genetic relatedness occurred in MU infecting different members in a family, and Sarfo et al. did not find MU in BU patient feces, suggesting that humans may not play any role as a reservoir or in transmission of BU [78,79]. However, BU induced by human bite, suggesting a potential mechanical role, has been reported [80].
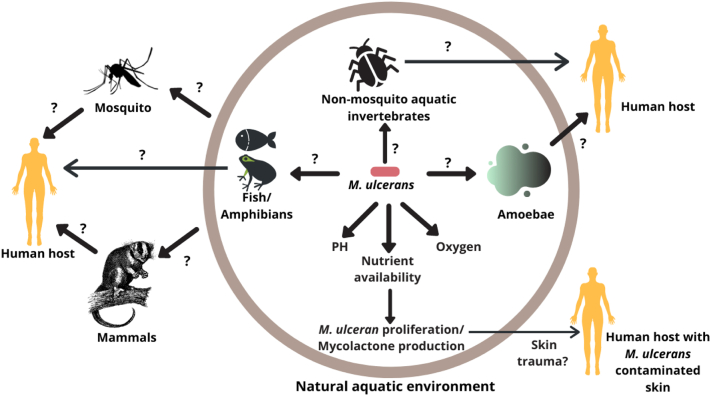
Given the high diversity of potential hosts among animals and habitats, it is clear that MU is distributed widely in various environments and in a range of abundances. However, how MU is transmitted, and how mycolactone functions outside of the host or may facilitate human transmission, is much less understood, with several groups proposing different modes of transmission, and a few suggesting complex, multiple modes of transmission that may vary by geography and environment (Fig. 2).
Fig. 2.
Proposed transmission mechanisms and potential reservoirs including amoebae, aquatic invertebrates, fish and frogs, mosquito vectors, marsupials and mammals, and puncture of M. ulcerans contaminated human skin that could lead to a conducive environment for M. ulcerans proliferation and mycolactone production. This is a simplistic rendition, as some of these hypotheses suggest complex, multiple modes of transmission that may vary by geography and environment.
3. Hypothesized modes of transmission
3.1. Skin trauma
One of the earliest hypothesized modes of transmission was directly through sharp plant cuts or through exposure to contaminated soil or water into the existing wound [[81], [82], [83], [84]]. Historical anecdotal evidence suggested that some environmental mycobacteria from plants, including MU, could be associated with patient isolates; however, MU was never confirmed [81]. In the 1990s, a reported association of a BU outbreak with a spray irrigation system in Australia led to the hypothesis that MU transmission occurred via aerosols introduced to the skin through injuries or infection to pre-existing cuts or wounds [44,85]. However, this hypothesis was not supported later, based on genomic analysis suggesting that MU reductive evolution and loss of various gene functions make MU unlikely to be free-living in the environment [58,86]. In 1999, Walsh et al. showed MU intradermal inoculation led to BU in armadillos [87]. Using a hairless guinea pig model, Williamson et al. demonstrated the inability of MU to cause infection of a passively inoculated abrasion site; however, infection was established upon subdermal injection of MU, establishing that puncture is necessary for inoculation and BU pathology under controlled laboratory conditions [37]. Wallace et al. further showed that minor puncture (<2 mm deep) or mosquito bite of MU contaminated skin can cause mechanical BU transmission [88]. Azumah et al. reported that BU was established when MU was topically applied to puncture at a 1.5 mm depth suggesting the possibility MU transmission in a deep wound or puncture [89]. Collectively, these experiments demonstrate that although MU may not be inoculated via passive infection through superficial cuts and wounds, its inoculation underneath the skin either through mechanical injury (anthropogenic, environmental puncture or invertebrate bites) or post-contamination of MU on established deep puncture may lead to transmission and BU pathogenesis.
3.2. Amoebae
M. ulcerans has been postulated to persist in amoebic cysts where Acanthamoeba and other species can harbor other pathogens such as M. marinum, M. avium and Legionella pneumophila facilitating transmission [90,91]. The co-cultivation of MU with A. polyphaga demonstrated intracellular survival for 2 weeks; however, there was a log CFU MU decrease [92]. There was low MU survival within A. castellanii at 28 days and at 42 days in another study, suggesting a possible role of amoeba as a host but less likely in transmission [91]. In the former, IS2404 positive DNA was found in 4.64% of amoebae cultivated from environmental sources; however, they were all negative for additional targets of IS2606 and KR genes [91]. In the latter, it was unclear whether MU replication was measured, but topical inoculation of MU-A. polyphaga culture to mouse footpads led to earlier inflammation compared to MU injections alone [89]. While these laboratory studies suggest a role for amoebae as reservoirs, more research is needed regarding intracellular survival and replication of MU in amoebae in natural environments, and their role in transmission.
3.3. Aquatic invertebrates
Several laboratory experiments have been conducted to study MU transmission by biting Hemiptera, including experimental infection of MU (but not mycolactone-mutant MU or M. marinum) to Naucoridae with MU in salivary glands, and bites that led to BU infection in a mouse model [50]. Further, it was demonstrated that an African MU isolate could colonize the Belostomatidae head, raptorial arms, thorax, salivary glands and gut; and then could be transferred to blow fly larvae (Diptera: Calliphoridae) (Phormia regina) during feeding [93]. There has been no observed clinical effects of MU to invertebrates, suggesting potential adaptation of MU as a commensal [50,93]. Similarly, inoculation of MU positive saliva derived from Belostomatidae caused BU lesions in mice [53]. A case report of BU lesions following a reported belostomatid bite was described by Marion et al. [94]. However, the potential of aquatic invertebrate vectors remains controversial as Naucoridae and Belostomatidae are not hematophagous and do not actively seek and attack humans. The chances of accidental biting and mechanical transmission either through a contaminated proboscis or piercing contaminated human skin in response to disturbance in their habitat still remains a possibility worth additional investigation [50,93].
3.4. Mosquitoes
As previously discussed, MU DNA has been detected in adult mosquitoes in Australia where mosquitoes are considered MU vectors. Tobias et al. showed that Aedes camptorhynhcus larvae can feed on MU contaminated possum excreta, which can colonize and sustain inside the mosquito larva gut and mouthparts [95]. Similarly, Wallace et al. showed that MU was sustained inside mosquito larvae but was not maintained at pupae and adult stages and adult mosquitoes were not able to mechanically transmit [96]. However, in that study, MU was maintained in guts and salivary glands of Belostomatidae that fed on the MU infected mosquito larvae.
Djouaka et al. observed decreased MU survival during development of larvae from the first to fourth instar stages, and no MU was detected in adults [62]. Although Wallace et al. showed no developmental defects in MU contaminated mosquito larvae, Hoxmeier et al did report decreased survival and developmental defects in Anopheles gambiae [96,97]. The developmental defect occurred due to disruption of lipid metabolism, a common feature observed in other pathogens such as M. tuberculosis and M. leprae [97]. The discrepancy in the results could be attributed to different species of mosquitoes used in the study as well as study design and analyses. Finally, as previously mentioned, Wallace et al. showed that minor puncture (<2 mm deep) or mosquito bite of MU contaminated skin can cause mechanical BU transmission [88].
4. Value of quality assurance
As discussed, MU detection from the environment relies heavily on PCR based methods. PCR is prone to yield false positive or negative results due to contamination, PCR inhibitors and low DNA concentration. [98]. A review of 22 papers investigating MU presence in more than one type of environmental matrix showed MU DNA in soil, animal excreta, plant, water, biofilm, detritus, fungi, moss, sediment and invertebrates (Supplemental Table 1). Overall, few studies confirmed MU in the environment by culture, instead using molecular methods for detection [43,99].
In most MU environmental studies, MU has been detected in a variety of analyzed samples and positivity was higher in BU endemic regions compared to non-endemic regions [34,47,70,100,101]. Although in one study BU endemicity did not predict MU positivity, and was suggested to be attributed to passive surveillance [36]. However, other studies detected little or no MU DNA in samples such as water and biofilm [46,102]. The reasons for these discrepancies are unclear but may be attributed to sample type, numbers, locations, seasons, and methodological or technical challenges. Additionally, there could be presence of other mycolactone producing mycobacteria in the environment that can give false positivity for MU that causes human disease.
Alternatively, these variations could be attributed to poor laboratory quality assurance. An External Quality Assessment (EQA) by Eddyani et al. showed extensive variation in laboratory performance of MU detection among clinical and environmental samples [98]. In that study, few samples were correctly identified by all laboratories, and only two laboratories correctly identified all samples, raising concerns over reliability of PCR data for clinical interpretation and environmental studies [98]. A follow up report of subsequent rounds of EQA was issued by the WHO in 2020 and indicated a high proportion of laboratories reporting both false–positive and false–negative results [103]. Authors suggested that poor quality control could impact WHO prevalence data as well as environmental and human MU and BU research conducted by these laboratories. Thus, strict laboratory internal and external quality control and assessment is crucial to correctly interpret PCR data that can aid to understand the MU reservoir and for accurate disease diagnosis. As a result of these EQA rounds, the WHO is supporting a BU laboratory network and new EQA program for PCR-based diagnosis in the WHO African Region [103]. Finally, PCR results cannot confirm bacterial viability. Employing newer methodology, such as molecular viability assays that take advantage of photoactivation of intercalating agents to differentiate between live and dead organisms would allow for detection of viable MU organisms in aquatic habitats, and better determination of MU replication and niche partitioning in these environments.
5. Toward a one health approach
M. ulcerans infection and progression to BU involves interaction between human hosts and the pathogen, which depends on the ecological niche of the pathogen, changing environmental conditions that affect the interactions and natural variability in abundance and communities that may mediate exposure. The link between ecological disturbance to aquatic water bodies and BU, invertebrate communities, and the known MU associations among most taxa, may provide management avenues of disease prevention. A glaring omission in the collective understanding of MU is the ecological and evolutionarily role of mycolactone in MU functioning and survival, and how this functioning may lead to risk of BU infection. Knowledge of the potential of a water source to be at high risk for MU abundance could be used to mediate the ecological disturbance or warn communities of risk. Prevalence and risk for transmission is at the nexus of several drivers that include landscape and aquatic ecosystem disturbances, pathogen ecological dynamics, food web interactions, human activity and behavior, and individual genetic and host factors. A major challenge in preventing BU is not having a foundational knowledge of transmission mechanisms, in order to implement an effective preventative strategy. Measuring drivers of MU and mycolactone ecological and evolutionary interactions in natural environments, with humans and with animals in concert that influence environmental persistence, colonization, virulence, and heterogeneity in transmission will allow capture of biological processes that generate BU disease patterns of occurrence in space and time.
To this end, a One-Health approach leading to holistic understanding of the complex interdependences of human, animal, microbial and ecosystem health influencing MU transmission is a key strategy for breakthroughs in BU control. Within this framework, a few recommended objectives within this strategy would include: 1. Integrated pathogen and disease surveillance in human, animal, and environmental sources; 2. Improved education and communication among community stakeholders, and human, animal, and environmental health experts; 3. Development of policy around upstream drivers of disease emergence (i.e. land use, water and soil exposure, water quality, etc); and 4. Development of strategies to encourage and increase political and financial commitment toward improving health capacities. Together, these objectives allow for rapid response and for strengthening prediction and prevention of BU and other environmentally transmitted infectious diseases. Challenges with institutional capacity, funding, and differences in multidisciplinary perspectives must be overcome to establish a synergistic global network of qualified individuals working locally, regionally, nationally, and internationally. Gaining a better picture of the global problem through enhanced human and environmental surveillance should be an urgent priority, with the goal to prevent exposure and infection, and reduce morbidity.
A Review of 22 papers investigating presence of MU in more than one environmental sample matrix. Abbreviations: MPM (Mycolactone Producing Mycobacteria); VNTR (Variable Number Tandem Repeat).
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
Acknowledgements
Funding: This work was supported by the National Science Foundation Directorate for Biological Sciences Ecology and Evolution of Infectious Diseases Program [Grant number: 1911457] and by Mississippi State University Department of Biological Sciences and Office of Research and Economic Development (Jordan start-up funds).
References
- 1.Guégan J.-F., Ayouba A., Cappelle J., de Thoisy B. Forests and emerging infectious diseases: unleashing the beast within. Environ. Res. Lett. 2020;15 doi: 10.1088/1748-9326/AB8DD7. [DOI] [Google Scholar]
- 2.Sutherst R.W. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 2004;17:136. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falkinham J.O. The changing pattern of nontuberculous mycobacterial disease. Can. J. Infect. Dis. 2003 doi: 10.1155/2003/323058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkinham J.O. Environmental sources of nontuberculous mycobacteria. Clin. Chest Med. 2015 doi: 10.1016/j.ccm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Prevots D.R., Marras T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria a review. Clin. Chest Med. 2015 doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honda J.R., Virdi R., Chan E.D. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front. Microbiol. 2018 doi: 10.3389/fmicb.2018.02029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manry J., Vincent Q.B., Johnson C., Chrabieh M., Lorenzo L., Theodorou I., Ardant M.-F., Marion E., Chauty A., Marsollier L., Abel L., Alcaïs A. Genome-wide association study of Buruli ulcer in rural Benin highlights role of two LncRNAs and the autophagy pathway. Commun. Biol. 2020;31(3):1–10. doi: 10.1038/s42003-020-0920-6. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization The Global Health Observatory: number of new reported cases of Buruli ulcer. Glob. Heal. Obs. 2021 https://www.who.int/data/gho/data/indicators/indicator-details/GHO/number-of-new-reported-cases-of-buruli-ulcer [Google Scholar]
- 9.Swenson C., Zerbe C.S., Fennelly K. Host variability in NTM disease: implications for research needs. Front. Microbiol. 2018 doi: 10.3389/fmicb.2018.02901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackumey M.M., Gyapong M., Pappoe M., Maclean C.K., Weiss M.G. Socio-cultural determinants of timely and delayed treatment of buruli ulcer: implications for disease control. Infect. Dis. Poverty. 2012 doi: 10.1186/2049-9957-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.N’krumah R.T.A.S., Koné B., Tiembre I., Cissé G., Pluschke G., Tanner M., Utzinger J. Socio-environmental factors associated with the risk of contracting Buruli ulcer in Tiassalé, South Côte d’Ivoire: a case-control study. PLoS Negl. Trop. Dis. 2016 doi: 10.1371/journal.pntd.0004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asiedu K., Etuaful S. Socioeconmoic implications of Buruli ulcer in Ghana: a three-year review. Trans. R. Soc. Trop. Med. Hyg. 1998;59:1015–1022. doi: 10.4269/ajtmh.1998.59.1015. [DOI] [PubMed] [Google Scholar]
- 13.Adusumilli S., Mve-Obiang A., Sparer T., Meyers W., Hayman J., Small P.L.C. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell. Microbiol. 2005 doi: 10.1111/j.1462-5822.2005.00557.x. [DOI] [PubMed] [Google Scholar]
- 14.Pidot S.J., Hong H., Seemann T., Porter J.L., Yip M.J., Men A., Johnson M., Wilson P., Davies J.K., Leadlay P.F., Stinear T.P. Deciphering the genetic basis for polyketide variation among mycobacteria producing mycolactones. BMC Genomics. 2008 doi: 10.1186/1471-2164-9-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yip M.J., Porter J.L., Fyfe J.A.M., Lavender C.J., Portaels F., Rhodes M., Kator H., Colorni A., Jenkin G.A., Stinear T. Evolution of mycobacterium ulcerans and other mycolactone-producing mycobacteria from a common Mycobacterium marinum progenitor. J. Bacteriol. 2007 doi: 10.1128/JB.01442-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreesen J.R., Bahl H., Gottschalk G. Clostridia. 1989. Introduction to the physiology and biochemistry of the genus Clostridium. [DOI] [Google Scholar]
- 17.Benbow M.E., Belinda H., Mosi L., Roberts S., Simmonds R., Jordan H.W. Hum. Emerg. Re-Emerging Infect. Bact. Mycotic Infect. 2015. Mycobacterium ulcerans and Buruli Ulcer; p. 841. [Google Scholar]
- 18.Portaels F., Johnson P., Meyers W. WHO/CDS/GBUI; Geneva, Switz: 2001. Buruli Ulcer: Diagnosis of Mycobacterium ulcerans Disease. [Google Scholar]
- 19.Adiba S., Nizak C., van Baalen M., Denamur E., Depaulis F. From grazing resistance to pathogenesis: the coincidental evolution of virulence factors. PLoS One. 2010 doi: 10.1371/journal.pone.0011882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y.S., Cao J.S., Yu H.Q. Impacts of environmental factors on AHL-producing and AHL-quenching activities of aerobic granules. Appl. Microbiol. Biotechnol. 2019 doi: 10.1007/s00253-019-10080-1. [DOI] [PubMed] [Google Scholar]
- 21.Dhungel L., Burcham L., Park J.Y., Sampathkumar H.D., Cudjoe A., Seo K.S., Jordan H. Responses to chemical cross-talk between the Mycobacterium ulcerans toxin, mycolactone, and Staphylococcus aureus. Sci. Rep. 2021;111(11):1–12. doi: 10.1038/s41598-021-89177-5. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanhueza D., Chevillon C., Colwell R., Babonneau J., Marion E., Marsollier L., Guégan J.F. Chitin promotes mycobacterium ulcerans growth. FEMS Microbiol. Ecol. 2016 doi: 10.1093/femsec/fiw067. [DOI] [PubMed] [Google Scholar]
- 23.Deshayes C., Angala S.K., Marion E., Brandli I., Babonneau J., Preisser L., Eyangoh S., Delneste Y., Legras P., De Chastellier C., Stinear T.P., Jackson M., Marsollier L. Regulation of mycolactone, the mycobacterium ulcerans toxin, depends on nutrient source. PLoS Negl. Trop. Dis. 2013 doi: 10.1371/journal.pntd.0002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanhueza D., Guégan J.F., Jordan H., Chevillon C. 2019. Environmental Variations in mycobacterium ulcerans Transcriptome: Absence of Mycolactone Expression in Suboptimal Environments, Toxins (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanhueza D., Chevillon C., Bouzinbi N., Godreuil S., Guégan J.F. Chitin increases mycobacterium ulcerans growth in acidic environments. Microbes Environ. 2018 doi: 10.1264/jsme2.ME17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palomino J.C., Obiang A.M., Realini L., Meyers W.M., Portaels F. Effect of oxygen on growth of mycobacterium ulcerans in the BACTEC system. J. Clin. Microbiol. 1998;36:3420–3422. doi: 10.1128/jcm.36.11.3420-3422.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merritt R.W., Walker E.D., Small P.L.C., Wallace J.R., Johnson P.D.R., Benbow M.E., Boakye D.A. Ecology and transmission of buruli ulcer disease: a systematic review. PLoS Negl. Trop. Dis. 2010 doi: 10.1371/journal.pntd.0000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Ravensway J., Benbow M.E., Tsonis A.A., Pierce S.J., Campbell L.P., Fyfe J.A.M., Hayman J.A., Johnson P.D.R., Wallace J.R., Qi J. Climate and landscape factors associated with buruli ulcer incidence in Victoria, Australia. PLoS One. 2012 doi: 10.1371/journal.pone.0051074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landier J., de Magny G.C., Garchitorena A., Guégan J.F., Gaudart J., Marsollier L., Le Gall P., Giles-Vernick T., Eyangoh S., Fontanet A., Texier G. Seasonal patterns of buruli ulcer incidence, Central Africa, 2002–2012. Emerg. Infect. Dis. 2015 doi: 10.3201/eid2108.141336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landier J., Gaudart J., Carolan K., Lo Seen D., Guégan J.F., Eyangoh S., Fontanet A., Texier G. Spatio-temporal patterns and landscape-associated risk of Buruli Ulcer in Akonolinga, Cameroon. PLoS Negl. Trop. Dis. 2014 doi: 10.1371/journal.pntd.0003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garchitorena A., Guegan J.F., Leger L., Eyangoh S., Marsollier L., Roche B. Mycobacterium ulcerans dynamics in aquatic ecosystems are driven by a complex interplay of abiotic and biotic factors. Elife. 2015;4 doi: 10.7554/eLife.07616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garchitorena A., Sokolow S.H., Roche B., Ngonghala C.N., Jocque M., Lund A., Barry M., Mordecai E.A., Daily G.C., Jones J.H., Andrews J.R., Bendavid E., Luby S.P., Labeaud A.D., Seetah K., Guégan J.F., Bonds M.H., De Leo G.A. Disease ecology, health and the environment: a framework to account for ecological and socio-economic drivers in the control of neglected tropical diseases. Philos. Trans. R. Soc. B Biol. Sci. 2017 doi: 10.1098/rstb.2016.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner T., Benbow M.E., Brenden T.O., Qi J., Johnson R.C. Buruli ulcer disease prevalence in Benin, West Africa: associations with land use/cover and the identification of disease clusters. Int. J. Health Geogr. 2008 doi: 10.1186/1476-072X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williamson H.R., Benbow M.E., Campbell L.P., Johnson C.R., Sopoh G., Barogui Y., Merritt R.W., Small P.L.C. Detection of mycobacterium ulcerans in the environment predicts prevalence of Buruli ulcer in Benin. PLoS Negl. Trop. Dis. 2012 doi: 10.1371/journal.pntd.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fyfe J.A.M., Lavender C.J., Johnson P.D.R., Globan M., Sievers A., Azuolas J., Stinear T.P. Development and application of two multiplex real-time PCR assays for the detection of mycobacterium ulcerans in clinical and environmental samples. Appl. Environ. Microbiol. 2007 doi: 10.1128/AEM.02971-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson H.R., Benbow M.E., Nguyen K.D., Beachboard D.C., Kimbirauskas R.K., McIntosh M.D., Quaye C., Ampadu E.O., Boakye D., Merritt R.W., Small P.L.C. Distribution of mycobacterium ulcerans in Buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLoS Negl. Trop. Dis. 2008 doi: 10.1371/journal.pntd.0000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson H.R., Mosi L., Donnell R., Aqqad M., Merritt R.W., Small P.L.C. Mycobacterium ulcerans fails to infect through skin abrasions in a Guinea pig infection model: implications for transmission. PLoS Negl. Trop. Dis. 2014 doi: 10.1371/journal.pntd.0002770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakyi S.A., Aboagye S.Y., Otchere I.D., Liao A.M., Caltagirone T.G., Yeboah-Manu D. RNA Aptamer that specifically binds to Mycolactone and serves as a diagnostic tool for diagnosis of Buruli ulcer. PLoS Negl. Trop. Dis. 2016 doi: 10.1371/journal.pntd.0004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Röltgen K., Cruz I., Ndung’u J.M., Pluschke G. Buruli Ulcer Mycobacterium Ulcerans Dis. 2019. Laboratory diagnosis of buruli ulcer: challenges and future perspectives. [DOI] [PubMed] [Google Scholar]
- 40.Sakyi S.A., Aboagye S.Y., Darko Otchere I., Yeboah-Manu D. Clinical and laboratory diagnosis of buruli ulcer disease: a systematic review. Can. J. Infect. Dis. Med. Microbiol. 2016 doi: 10.1155/2016/5310718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beissner M., Phillips R.O., Battke F., Bauer M., Badziklou K., Sarfo F.S., Maman I., Rhomberg A., Piten E., Frimpong M., Huber K.L., Symank D., Jansson M., Wiedemann F.X., Banla Kere A., Herbinger K.H., Löscher T., Bretzel G. Loop-mediated isothermal amplification for laboratory confirmation of Buruli ulcer disease—towards a point-of-care test. PLoS Negl. Trop. Dis. 2015 doi: 10.1371/journal.pntd.0004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portaels F., Meyers W.M., Ablordey A., Castro A.G., Chemlal K., de Rijk P., Elsen P., Fissette K., Fraga A.G., Lee R., Mahrous E., Small P.L.C., Stragier P., Torrado E., Van Aerde A., Silva M.T., Pedrosa J. First cultivation and characterization of mycobacterium ulcerans from the environment. PLoS Negl. Trop. Dis. 2008 doi: 10.1371/journal.pntd.0000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aboagye S.Y., Danso E., Ampah K.A., Nakobu Z., Asare P., Otchere I.D., Röltgen K., Yirenya-Tawiah D., Yeboah-Manu D. Isolation of nontuberculous mycobacteria from the environment of ghanian communities where buruli ulcer is endemic. Appl. Environ. Microbiol. 2016 doi: 10.1128/AEM.01002-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross B.C., Johnson P.D.R., Oppedisano F., Marino L., Sievers A., Stinear T., Hayman J.A., Veitch M.G.K., Robins-Browne R.M. Detection of mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl. Environ. Microbiol. 1997;10:4135–4138. doi: 10.1128/aem.63.10.4135-4138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotlowski R., Martin A., Ablordey A., Chemlal K., Fonteyne P.A., Portaels F. One-tube cell lysis and DNA extraction procedure for PCR-based detection of mycobacterium ulcerans in aquatic insects, molluscs and fish. J. Med. Microbiol. 2004 doi: 10.1099/jmm.0.45593-0. [DOI] [PubMed] [Google Scholar]
- 46.Vandelannoote K., Durnez L., Amissah D., Gryseels S., Dodoo A., Yeboah S., Addo P., Eddyani M., Leirs H., Ablordey A., Portaels F. Application of real-time PCR in Ghana, a Buruli ulcer-endemic country, confirms the presence of mycobacterium ulcerans in the environment. FEMS Microbiol. Lett. 2010 doi: 10.1111/j.1574-6968.2010.01902.x. [DOI] [PubMed] [Google Scholar]
- 47.Tian R.B.D., Niamké S., Tissot-Dupont H., Drancourt M. Detection of Mycobacterium ulcerans DNA in the environment, ivory coast. PLoS One. 2016 doi: 10.1371/journal.pone.0151567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammoudi N., Dizoe A.S., Regoui S., Davoust B., Drancourt M., Bouam A. Disseminated mycobacterium ulcerans infection in wild Grasscutters (Thryonomys swinderianus), Côte d’Ivoire. Am. J. Trop. Med. Hyg. 2019 doi: 10.4269/ajtmh.19-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portaels F., Elsen P., Guimaraes-Peres A., Fonteyne P.A., Meyers W.M. Insects in the transmission of Mycobacterium ulcerans infection. Lancet. 1999 doi: 10.1016/S0140-6736(98)05177-0. [DOI] [PubMed] [Google Scholar]
- 50.Marsollier L., Robert R., Aubry J., Saint André J.P., Kouakou H., Legras P., Manceau A.L., Mahaza C., Carbonnelle B. Aquatic insects as a vector for mycobacterium ulcerans. Appl. Environ. Microbiol. 2002 doi: 10.1128/AEM.68.9.4623-4628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zogo B., Djenontin A., Carolan K., Babonneau J., Jean-François Guegan S.E., Marion E. A field study in Benin to investigate the role of mosquitoes and other flying insects in the ecology of Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benbow M.E., Williamson H., Kimbirauskas R., McIntosh M.D., Kolar R., Quaye C., Akpabey F., Boakye D., Small P., Merritt R.W. Aquatic invertebrates as unlikely vectors of Buruli ulcer disease. Emerg. Infect. Dis. 2008 doi: 10.3201/eid1408.071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marion E., Eyangoh S., Yeramian E., Doannio J., Landier J., Aubry J., Fontanet A., Rogier C., Cassisa V., Cottin J., Marot A., Eveillard M., Kamdem Y., Legras P., Deshayes C., Saint-André J.P., Marsollier L. Seasonal and regional dynamics of M. ulcerans transmission in environmental context: deciphering the role of water bugs as hosts and vectors. PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roche B., Eric Benbow M., Merritt R., Kimbirauskas R., McIntosh M., Small P.L.C., Williamson H., Guégan J.F. Identifying the Achilles heel of multi-host pathogens: the concept of keystone “host” species illustrated by mycobacterium ulcerans transmission. Environ. Res. Lett. 2013 doi: 10.1088/1748-9326/8/4/045009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eric Benbow M., Kimbirauskas R., McIntosh M.D., Williamson H., Quaye C., Boakye D., Small P.L.C., Merritt R.W. 2014. Aquatic Macroinvertebrate Assemblages of Ghana, West Africa: Understanding the Ecology of a Neglected Tropical Disease, Ecohealth. [DOI] [PubMed] [Google Scholar]
- 56.Garchitorena A., Roche B., Kamgang R., Ossomba J., Babonneau J., Landier J., Fontanet A., Flahault A., Eyangoh S., Guégan J.F., Marsollier L. Mycobacterium ulcerans ecological dynamics and its association with freshwater ecosystems and aquatic communities: results from a 12-month environmental survey in Cameroon. PLoS Negl. Trop. Dis. 2014 doi: 10.1371/journal.pntd.0002879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohtsuka M., Kikuchi N., Yamamoto T., Suzutani T., Nakanaga K., Suzuki K., Ishii N. 2014. Buruli Ulcer Caused by Mycobacterium ulcerans Subsp Shinshuense: A Rare Case of Familial Concurrent Occurrence and Detection of Insertion Sequence 2404 in Japan, JAMA Dermatology. [DOI] [PubMed] [Google Scholar]
- 58.Johnson P.D.R., Azuolas J., Lavender C.J., Wishart E., Stinear T.P., Hayman J.A., Brown L., Jenkin G.A., Fyfe J.A.M. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg. Infect. Dis. 2007 doi: 10.3201/eid1311.061369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavender C.J., Fyfe J.A.M., Azuolas J., Brown K., Evans R.N., Ray L.R., Johnson P.D.R. Risk of Buruli ulcer and detection of mycobacterium ulcerans in mosquitoes in Southeastern Australia. PLoS Negl. Trop. Dis. 2011 doi: 10.1371/journal.pntd.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quek T.Y.J., Athan E., Henry M.J., Pasco J.A., Redden-Hoare J., Hughes A., Johnson P.D.R. Risk factors for mycobacterium ulcerans infection, southeastern Australia. Emerg. Infect. Dis. 2007 doi: 10.3201/eid1311.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Landier J., Boisier P., Piam F., Noumen-Djeunga B., Simé J., Wantong F.G., Marsollier L., Fontanet A., Eyangoh S. Adequate wound care and use of bed nets as protective factors against buruli ulcer: results from a case control study in Cameroon. PLoS Negl. Trop. Dis. 2011 doi: 10.1371/journal.pntd.0001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Djouaka R., Zeukeng F., Daiga Bigoga J., N’Golo Coulibaly D., Tchigossou G., Akoton R., Aboubacar S., Tchebe S.J.E., Nantcho Nguepdjo C., Adeoti R., Djegbe I., Tamo M., Mbacham W.F., Kakou-Ngazoa S.E., Ablordey A. Evidences of the low implication of mosquitoes in the transmission of mycobacterium ulcerans, the causative agent of Buruli ulcer. Can. J. Infect. Dis. Med. Microbiol. 2017 doi: 10.1155/2017/1324310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanders M.L., Jordan H.R., Serewis-Pond C., Zheng L., Benbow M.E., Small P.L., Tomberlin J.K. Mycobacterium ulcerans toxin, mycolactone may enhance host-seeking and oviposition behaviour by Aedes aegypti (L.) (Diptera: Culicidae) Environ. Microbiol. 2017 doi: 10.1111/1462-2920.13629. [DOI] [PubMed] [Google Scholar]
- 64.Carson C., Lavender C.J., Handasyde K.A., O’Brien C.R., Hewitt N., Johnson P.D.R., Fyfe J.A.M. Potential wildlife sentinels for monitoring the endemic spread of human Buruli ulcer in South-East Australia. PLoS Negl. Trop. Dis. 2014 doi: 10.1371/journal.pntd.0002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fyfe J.A.M., Lavender C.J., Handasyde K.A., Legione A.R., O’Brien C.R., Stinear T.P., Pidot S.J., Seemann T., Benbow M.E., Wallace J.R., McCowan C., Johnson P.D.R. A major role for mammals in the ecology of mycobacterium ulcerans. PLoS Negl. Trop. Dis. 2010 doi: 10.1371/journal.pntd.0000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lavender C.J., Stinear T.P., Johnson P.D.R., Azuolas J., Benbow M.E., Wallace J.R., Fyfe J.A.M. Evaluation of VNTR typing for the identification of Mycobacterium ulcerans in environmental samples from Victoria, Australia. FEMS Microbiol. Lett. 2008 doi: 10.1111/j.1574-6968.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 67.O’Brien C.R., Handasyde K.A., Hibble J., Lavender C.J., Legione A.R., McCowan C., Globan M., Mitchell A.T., McCracken H.E., Johnson P.D.R., Fyfe J.A.M. Clinical, microbiological and pathological findings of mycobacterium ulcerans infection in three Australian possum species. PLoS Negl. Trop. Dis. 2014 doi: 10.1371/journal.pntd.0002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Röltgen K., Pluschke G., Johnson P.D.R., Fyfe J. Mycobacterium ulcerans DNA in bandicoot excreta in Buruli ulcer–endemic area, Northern Queensland, Australia. Emerg. Infect. Dis. 2017 doi: 10.3201/eid2312.170780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narh C.A., Mosi L., Quaye C., Dassi C., Konan D.O., Tay S.C.K., de Souza D.K., Boakye D.A., Bonfoh B. Source tracking Mycobacterium ulcerans infections in the Ashanti Region, Ghana. PLoS Negl. Trop. Dis. 2015 doi: 10.1371/journal.pntd.0003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hammoudi N., Dizoe S., Saad J., Ehouman E., Davoust B., Drancourt M., Bouam A. Tracing Mycobacterium ulcerans along an alimentary chain in Côte d’Ivoire: a one health perspective. PLOS Neglected Tropical Diseases. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Djouaka F., Zeukeng Rousseau, Bigoga J.D., Kakou-Ngazoa R., Akoton Solange E., Tchigossou G., Coulibaly D.N., Jean-EudesmTchebe S., Aboubacar S., Nguepdjo C.N., Tossou E., Adeoti R., Nsonga T.M.N., Akpo Y., Djegbe I., Tamo M., Mbacham W.F., Ablordey A. Domestic animals infected with Mycobacterium ulcerans-Implications for transmission to humans. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durnez L., Suykerbuyk P., Nicolas V., Barrière P., Verheyen E., Johnson C.R., Leirs H., Portaels F. Terrestrial small mammals as reservoirs of mycobacterium ulcerans in Benin. Appl. Environ. Microbiol. 2010 doi: 10.1128/AEM.00199-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tobias N.J., Ammisah N.A., Ahortor E.K., Wallace J.R., Ablordey A., Stinear T.P. Snapshot fecal survey of domestic animals in rural Ghana for Mycobacterium ulcerans. PeerJ. 2016 doi: 10.7717/peerj.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh A., McBride W., Govan B., Pearson M. Potential animal reservoir of Mycobacterium ulcerans: a systematic review. Trop. Med. Infect. Dis. 2018 doi: 10.3390/tropicalmed3020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willson S.J., Kaufman M.G., Merritt R.W., Williamson H.R., Malakauskas D.M., Benbow M.E. Fish and amphibians as potential reservoirs of Mycobacterium ulcerans , the causative agent of Buruli ulcer disease. Infect. Ecol. Epidemiol. 2013 doi: 10.3402/iee.v3i0.19946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakaguchi K., Iima H., Hirayama K., Okamoto M., Matsuda K., Miyasho T., Kasamatsu M., Hasegawa K., Taniyama H. Mycobacterium ulcerans infection in an Indian flap-shelled turtle (Lissemys punctata punctata) J. Vet. Med. Sci. 2011;9:1217–1220. doi: 10.1292/jvms.10-0386. [DOI] [PubMed] [Google Scholar]
- 77.Hofer M., Hirschel B., Kirschner P., Beghetti M., Kaelin A., Siegrist C.-A., Suter S., Teske A., Bottger E.C. Disseminated osteomyelitis from mycobacterium ulcerans after a snakebite. N. Engl. J. Med. 1993 doi: 10.1056/NEJM199304083281405. [DOI] [PubMed] [Google Scholar]
- 78.Sarfo F.S., Lavender C.J., Fyfe J.A.M., Johnson P.D.R., Stinear T.P., Phillips R.O. Mycobacterium ulcerans DNA not detected in Faecal samples from Buruli ulcer patients: results of a pilot study. PLoS One. 2011 doi: 10.1371/journal.pone.0019611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Brien D.P., Wynne J.W., Buultjens A.H., Michalski W.P., Stinear T.P., Friedman N.D., Hughes A., Athan E. Exposure risk for infection and lack of human-to-human transmission of Mycobacterium ulcerans disease, Australia. Emerg. Infect. Dis. 2017 doi: 10.3201/eid2305.160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Debacker M., Zinsou C., Aguiar J., Meyers W.M., Portaels F. First case of mycobacterium ulcerans disease (Buruli ulcer) following a human bite. Clin. Infect. Dis. 2003 doi: 10.1086/367660. [DOI] [PubMed] [Google Scholar]
- 81.Barker D., Clancey J., Morrow R., Rao S. Transmission of Buruli disease. Br. Med. J. 1970;4:558. doi: 10.1136/bmj.4.5734.558-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barker D., Clancey J., Rao S. Mycobacteria of vegetation in Uganda. East Afr. Med. J. 1972;9:667–671. [PubMed] [Google Scholar]
- 83.Revill W., Barker D. Seasonal distribution of mycobacterial skin ulcers. Br. J. Prev. Soc. Med. 1972 doi: 10.1136/jech.26.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stanford J.L., Paul R.C. A preliminary report on some studies of environmental mycobacteria from Uganda. Ann. Soc. Belg. Med. Trop. 1920;1973 [PubMed] [Google Scholar]
- 85.Veitch M.G.K., Johnson P.D.R., Flood P.E., Leslie D.E., Street A.C., Hayman J.A. A large localized outbreak of mycobacterium ulcerans infection on a temperate southern Australian island. Epidemiol. Infect. 1997 doi: 10.1017/S0950268897008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stinear T.P., Seemann T., Pidot S., Frigui W., Reysset G., Garnier T., Meurice G., Simon D., Bouchier C., Ma L., Tichit M., Porter J.L., Ryan J., Johnson P.D.R., Davies J.K., Jenkin G.A., Small P.L.C., Jones L.M., Tekaia F., Laval F., Daffé M., Parkhill J., Cole S.T. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 2007 doi: 10.1101/gr.5942807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walsh D.S., Meyers W.M., Krieg R.E., Walsh G.P. Transmission of mycobacterium ulcerans to the nine-banded armadillo. Am. J. Trop. Med. Hyg. 1999 doi: 10.4269/ajtmh.1999.61.694. [DOI] [PubMed] [Google Scholar]
- 88.Wallace J.R., Mangas K.M., Porter J.L., Marcsisin R., Pidot S.J., Howden B., Omansen T.F., Zeng W., Axford J.K., Johnson P.D.R., Stinear T.P. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl. Trop. Dis. 2017 doi: 10.1371/journal.pntd.0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Azumah B.K., Addo P.G., Dodoo A., Awandare G., Mosi L., Boakye D.A., Wilson M.D. Experimental demonstration of the possible role of Acanthamoeba polyphaga in the infection and disease progression in Buruli Ulcer (BU) using ICR mice. PLoS One. 2017 doi: 10.1371/journal.pone.0172843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilson M.D., Boakye D.A., Mosi L., Asiedu K. In the case of transmission of mycobacterium ulcerans in buruli ulcer disease Acanthamoeba species stand accused. Ghana Med. J. 2011;45:31–34. doi: 10.4314/gmj.v45i1.68920. https://www.ncbi.nlm.nih.gov/pubmed/21572823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amissah N.A., Gryseels S., Tobias N.J., Ravadgar B., Suzuki M., Vandelannoote K., Durnez L., Leirs H., Stinear T.P., Portaels F., Ablordey A., Eddyani M. Investigating the role of free-living amoebae as a reservoir for mycobacterium ulcerans. PLoS Negl. Trop. Dis. 2014 doi: 10.1371/journal.pntd.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gryseels S., Amissah D., Durnez L., Vandelannoote K., Leirs H., de Jonckheere J., Silva M.T., Portaels F., Ablordey A., Eddyani M. Amoebae as potential environmental hosts for mycobacterium ulcerans and other mycobacteria, but doubtful actors in Buruli ulcer epidemiology. PLoS Negl. Trop. Dis. 2012 doi: 10.1371/journal.pntd.0001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mosi L., Williamson H., Wallace J.R., Merritt R.W., Small P.L.C. Persistent association of mycobacterium ulcerans with west African predaceous insects of the family belostomatidae. Appl. Environ. Microbiol. 2008 doi: 10.1128/AEM.01234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marion E., Chauty A., Yeramian E., Babonneau J., Kempf M., Marsollier L. A case of guilt by association: Water bug bite incriminated in M. ulcerans infection. Int. J. Mycobacteriol. 2014 doi: 10.1016/j.ijmyco.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 95.Tobias N.J., Seemann T., Pidot S.J., Porter J.L., Marsollier L., Marion E., Letournel F., Zakir T., Azuolas J., Wallace J.R., Hong H., Davies J.K., Howden B.P., Johnson P.D.R., Jenkin G.A., Stinear T.P. Mycolactone gene expression is controlled by strong SigA-like promoters with utility in studies of mycobacterium ulcerans and buruli ulcer. PLoS Negl. Trop. Dis. 2009 doi: 10.1371/journal.pntd.0000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wallace J.R., Gordon M.C., Hartsell L., Mosi L., Benbow M.E., Merritt R.W., Small P.L.C. Interaction of mycobacterium ulcerans with mosquito species: implications for transmission and trophic relationships. Appl. Environ. Microbiol. 2010 doi: 10.1128/AEM.00340-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoxmeier J.C., Thompson B.D., Broeckling C.D., Small P., Foy B.D., Prenni J., Dobos K.M. Analysis of the metabolome of Anopheles gambiae mosquito after exposure to mycobacterium ulcerans. Sci. Rep. 2015 doi: 10.1038/srep09242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eddyani M., Lavender C., De Rijk W.B., Bomans P., Fyfe J., De Jong B., Portaels F. Multicenter external quality assessment program for PCR detection of Mycobacterium ulcerans in clinical and environmental specimens. PLoS One. 2014 doi: 10.1371/journal.pone.0089407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marsollier L., Stinear T., Aubry J., Saint André J.P., Robert R., Legras P., Manceau A.L., Audrain C., Bourdon S., Kouakou H., Carbonnelle B. Aquatic plants stimulate the growth of and biofilm formation by mycobacterium ulcerans in axenic culture and harbor these Bacteria in the environment. Appl. Environ. Microbiol. 2004 doi: 10.1128/AEM.70.2.1097-1103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McIntosh M., Williamson H., Benbow M.E., Kimbirauskas R., Quaye C., Boakye D., Small P., Merritt R. Associations between mycobacterium ulcerans and aquatic plant communities of West Africa: implications for Buruli ulcer disease. Ecohealth. 2014 doi: 10.1007/s10393-013-0898-3. [DOI] [PubMed] [Google Scholar]
- 101.Pileggi S.M., Jordan H., Clennon J.A., Whitney E., Benbow M.E., Merritt R., McIntosh M., Kimbirauskas R., Small P., Boakye D., Quaye C., Qi J., Campbell L., Gronseth J., Ampadu E., Opare W., Waller L.A. Landscape and environmental influences on Mycobacterium ulcerans distribution among aquatic sites in Ghana. PLoS One. 2017 doi: 10.1371/journal.pone.0176375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eddyani M., De Jonckheere J.F., Durnez L., Suykerbuyk P., Leirs H., Portaels F. Occurrence of free-living amoebae in communities of low and high endemicity for Buruli ulcer in Southern Benin. Appl. Environ. Microbiol. 2008;74(21):6547–6551. doi: 10.1128/AEM.01066-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eyangoh S., Marion E., Chaitanya V S. Buruli Ulcer Laboratory Network and New External Quality Assessment Programme for PCR-based Diagnosis in the WHO African Region. 2021. https://www.who.int/publications/i/item/9789240007222
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Review of 22 papers investigating presence of MU in more than one environmental sample matrix. Abbreviations: MPM (Mycolactone Producing Mycobacteria); VNTR (Variable Number Tandem Repeat).