Highlights
-
•
Parkinson’s disease (PD) affects motor system and cognitive skills such as language.
-
•
We used machine learning to study language network connectivity in early-stage PD.
-
•
Connectivity was probed by classifying MEG responses to different spoken words.
-
•
PD vs controls were successfully classified using verbs and incorrectly inflected nouns.
-
•
Early-stage PD exhibits altered language network connectivity despite normal cognition.
Keywords: Parkinson’s disease (PD), Magnetoencephalography (MEG), Functional connectivity, Classification, Action verb, Morphosyntax
Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder, well-known for its motor symptoms; however, it also adversely affects cognitive functions, including language, a highly important human ability. PD pathology is associated, even in the early stage of the disease, with alterations in the functional connectivity within cortico-subcortical circuitry of the basal ganglia as well as within cortical networks. Here, we investigated functional cortical connectivity related to spoken language processing in early-stage PD patients. We employed a patient-friendly passive attention-free paradigm to probe neurophysiological correlates of language processing in PD patients without confounds related to active attention and overt motor responses. MEG data were recorded from a group of newly diagnosed PD patients and age-matched healthy controls who were passively presented with spoken word stimuli (action and abstract verbs, as well as grammatically correct and incorrect inflectional forms) while focussing on watching a silent movie. For each of the examined linguistic aspects, a logistic regression classifier was used to classify participants as either PD patients or healthy controls based on functional connectivity within the temporo-fronto-parietal cortical language networks. Classification was successful for action verbs (accuracy = 0.781, p-value = 0.003) and, with lower accuracy, for abstract verbs (accuracy = 0.688, p-value = 0.041) and incorrectly inflected forms (accuracy = 0.648, p-value = 0.021), but not for correctly inflected forms (accuracy = 0.523, p-value = 0.384). Our findings point to quantifiable differences in functional connectivity within the cortical systems underpinning language processing in newly diagnosed PD patients compared to healthy controls, which arise early, in the absence of clinical evidence of deficits in cognitive or general language functions. The techniques presented here may aid future work on establishing neurolinguistic markers to objectively and noninvasively identify functional changes in the brain’s language networks even before clinical symptoms emerge.
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterised by progressive loss of the midbrain dopaminergic neurons, primarily in the substantia nigra pars compacta, leading to a severe loss of nigrostriatal dopaminergic terminals, and subsequently, functional impairment of the basal ganglia (Dauer and Przedborski, 2003, Mullin and Schapira, 2015). PD pathology and neuronal degeneration are associated with intraneuronal accumulation of misfolded alfa-synuclein protein and the formation of Lewy bodies (Volpicelli-Daley et al., 2014, Volpicelli-Daley et al., 2011). These pathological processes start many years before patients present with cardinal clinical motor symptoms such as rest tremor, bradykinesia and rigidity (Braak and Del Tredici, 2009, Braak et al., 2002, Braak et al., 2003, Gaig and Tolosa, 2009).
Over the last decade, a large body of neuroimaging studies have shown that PD pathology is associated with alterations of functional connectivity within the cortico-subcortical circuitry of the basal ganglia (Göttlich et al., 2013, Hacker et al., 2012, Helmich et al., 2010, Kim et al., 2017, Putcha et al., 2015, Wu et al., 2011), even in the early stages of the disease (Fang et al., 2017, Luo et al., 2014, Olde Dubbelink et al., 2014, Olde Dubbelink et al., 2013). Furthermore, PD-related effects extend beyond the basal ganglia circuitry disrupting the functional connectivity at the cortical level (Amboni et al., 2015, Putcha et al., 2015, Rowe et al., 2002, Teramoto et al., 2016). Longitudinal resting-state MEG studies have found that PD patients in the earliest clinical stages of the disease exhibit alterations in delta and alpha band functional connectivity in the temporal cortical regions (Olde Dubbelink et al., 2013) as well as disruptions of whole-brain functional networks (Olde Dubbelink et al., 2014). These functional alterations evolved over time in a more widespread cortical patterns in association with the cognitive decline in these patients. Studies using functional magnetic resonance imaging (fMRI) reported that, relative to healthy controls, PD patients showed aberrant functional connectivity of the motor cortical areas during resting state (Wu et al., 2011) as well as during motor sequence tasks (Rowe et al., 2002). Furthermore, fMRI and EEG studies in PD demonstrated that reduced functional connectivity within frontoparietal networks is associated with cognitive decline (Amboni et al., 2015) as well as early executive dysfunction (Teramoto et al., 2016) in PD patients. Reduced functional connectivity of bilateral inferior parietal cortex as well as the right medial temporal lobe was found in cognitively unimpaired PD patients (Tessitore et al., 2012). Additionally, a recent meta-analysis found convergent evidence for abnormal resting-state functional connectivity of bilateral inferior parietal lobes in PD patients compared to healthy subjects and further suggested intrinsic connectivity of these regions as an early imaging biomarker of PD even before the motor symptoms. Importantly, some studies found altered cortico-subcortical connectivity of the right inferior parietal cortex as well as aberrant sensorimotor integration in high-risk groups for PD (Bäumer et al., 2007, Helmich et al., 2015).
The basal ganglia are connected to many cortical areas, including the motor and premotor cortices via the motor circuit, as well as to the prefrontal cortex, namely the dorsolateral prefrontal cortex and the lateral orbitofrontal cortex, via the prefrontal associative circuits (Alexander and Crutcher, 1990, Rodriguez-Oroz et al., 2009, Tekin and Cummings, 2002). It has been suggested that the dysfunction of this frontostriatal circuitry, caused by dopamine deficiency in PD, may compromise some aspects of language processing in PD patients (Birba et al., 2017), including action-related language (linguistic expressions related to motor or action contents; Bak, 2013, Cardona et al., 2013, Cardona et al., 2014), motor-language coupling (integration of action verb comprehension with ongoing manual actions; Cardona et al., 2013, García and Ibáñez, 2014, Ibáñez et al., 2013) as well as syntactic/grammatical processing (Dominey and Inui, 2009, García et al., 2017, Ullman, 2004, Ullman, 2008).
The processing of action-related language, in particular action verbs (such as kick or write), has been found to be deficient in PD patients, both in comprehension and production, as revealed by longer reaction times and lower performance accuracy for PD patients relative to healthy controls in behavioural tasks (e.g., action naming, action verb generation and comprehension; Bocanegra et al., 2015, Bocanegra et al., 2017, Boulenger et al., 2008, Fernandino et al., 2013, García et al., 2017, Herrera and Cuetos, 2012, Rodríguez-Ferreiro et al., 2009). PD patients have been shown to have impairments in processing action verbs compared to nouns (Bocanegra et al., 2015, Bocanegra et al., 2017, Boulenger et al., 2008, Rodríguez-Ferreiro et al., 2009) as well as compared to other verb categories, e.g. abstract verbs (Fernandino et al., 2013). Some studies have reported selective impairment in processing specific types of action verbs depending on the level of motion content (Bocanegra et al., 2017, Herrera et al., 2012b). These deficits have been found in early-stage PD irrespective of executive dysfunction (Bocanegra et al., 2015, Fernandino et al., 2013, García et al., 2017, Ibáñez et al., 2013, Rodríguez-Ferreiro et al., 2009).
It has been suggested that the processing of action-related language engages several parallel networks connecting the prefrontal, motor and temporal cortices with the basal ganglia (Cardona et al., 2013, García and Ibáñez, 2014). Neuroimaging studies have shown that, relative to healthy controls, PD patients exhibit alterations in functional connectivity between motor cortex and inferior frontal gyrus (Abrevaya et al., 2017) as well as between frontal and temporal cortical regions (Melloni et al., 2015) during the processing of action verbs. In both studies, aberrant functional connectivity was associated with disruptions of the basal ganglia circuitry (Abrevaya et al., 2017, Melloni et al., 2015).
Importantly, some studies have indicated that action-related language processing and motor-language coupling may be impaired in early PD before the onset of executive dysfunction (Ibáñez et al., 2013), and that these aspects (assessed with action-sentence compatibility effects) can be particularly affected in PD but not in other motor diseases (Cardona et al., 2014). Hence, the deficit in action verb processing may potentially serve as a neurocognitive marker of early PD (Cardona et al., 2013, García and Ibáñez, 2014, Ibáñez et al., 2013, Melloni et al., 2015).
PD patients have also been shown to have impairments in processing different levels of grammatical information including syntax (e.g., structure of phrases, clauses or sentences) and morphosyntax (e.g., subject-verb agreement), both in comprehension and production (Arnott et al., 2005, Grossman et al., 1992, Grossman et al., 2003, Hochstadt et al., 2006, Lee et al., 2003). Grammatical processing requires sequential and hierarchical processing of linguistic information in brain structures including the prefrontal cortex and the basal ganglia (Dominey and Inui, 2009, Ullman, 2001, Ullman, 2004), often with left-hemispheric lateralisation (Friederici et al., 2003b, Wilson et al., 2011). However, evidence on the neural correlates of syntactic processing in PD is scarce (Friederici et al., 2003a, Grossman et al., 2003). It has been shown that, compared to healthy controls, PD patients exhibit reduced activity in the prefrontal and right temporal cortices but also increased recruitment of the right inferior frontal gyrus during a sentence comprehension task (Grossman et al., 2003). Furthermore, an electrophysiological study by Friederici et al. (2003a) found aberrant neural responses reflecting late processes of syntactic integration of sentences in PD patients compared to controls, while the neural responses reflecting early automatic syntactic processes were relatively similar between the groups. The authors suggested that the damage in the cortico-subcortical network of basal ganglia in PD does not affect early automatic syntactic parsing during comprehension, but rather disrupts later syntactic integration (Friederici et al., 2003a). Interestingly, a recent study found that while asymptomatic individuals with a genetic risk for developing PD showed intact executive functions, they exhibited deficient processing of certain syntactic aspects that depend on grammatical mechanisms with minimal working memory reliance (identifying functional roles within predicates; García et al., 2017).
Taken together, there is converging evidence indicating that both action verb processing (Cardona et al., 2013, Cardona et al., 2014, García and Ibáñez, 2014, Ibáñez et al., 2013, Melloni et al., 2015) and syntactic processing (Dominey and Inui, 2009, García et al., 2017) are associated with cortico-subcortical circuits of the basal ganglia which may be partially disrupted in early PD prior to executive dysfunction and clinical manifestations (García et al., 2017, Ibáñez et al., 2013). Furthermore, PD literature points to abnormal cortico-cortical connectivity during processing of action verbs (Abrevaya et al., 2017, Melloni et al., 2015). More evidence comes from resting state studies reporting diverse alterations in the functional connectivity of cortico-subcortical networks and within cortical networks in the earliest clinical stages of PD (Olde Dubbelink et al., 2014, Olde Dubbelink et al., 2013), before cognitive impairments (Tessitore et al., 2012) and even preceding the emergence of clinical symptoms (Bäumer et al., 2007, Helmich et al., 2015).
Building on the aforementioned literature, it is plausible to hypothesise that cortico-cortical connectivity associated with the processing of action verbs and syntax may be altered early in PD prior to clinical evidence of cognitive or language impairments. Starting from this premise, we set out in the present study to investigate functional cortical connectivity of spoken language processing in early-stage PD patients using magnetoencephalography (MEG). The use of MEG allows disentangling of the rapidly evolving neural processes associated with the access of lexico-semantic and morphosyntactic information during speech processing with a temporal resolution on the millisecond scale, which is particularly important for such a highly dynamic function as language. The central question was whether early-stage PD patients, compared to healthy controls, would exhibit different functional connectivity within cortical language networks during processing of action verbs and morphosyntax. Crucially, these early-stage PD patients did not show significant deterioration in their general language or cognitive abilities in the clinical assessment. To answer this central question, we constructed classifiers to partition our subject population into PD patients and healthy controls based on the functional connectivity between brain regions previously identified as the core nodes of the cortical language system (Catani and Mesulam, 2008, Friederici, 2011, Friederici, 2012, Hickok and Poeppel, 2007) and action-related semantic networks (Tomasello et al., 2017).
2. Methods
2.1. Participants
Seventeen non-demented PD patients (nine males, mean age 66.2 ± 5.7, 14.5±2.8 years of education) and 15 healthy controls (five males, mean age 63.2 ± 4.4, 13.7 ± 2.4 years of education) participated in this study. All participants were right-handed native Danish speakers (handedness was assessed using Edinburgh Inventory; Oldfield, 1971). Participants’ overall cognitive functioning was assessed using the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005), and their general language abilities were assessed using the Boston Naming Test (Kaplan et al., 1983), the Similarities test from the Wechsler Adult Intelligence Scale-IV (WAIS-IV; Wechsler, 2008) and the Semantic Verbal Fluency test (produced items per one minute; Benton, 1968). No significant differences in gender, age or years of education were detected between the groups, neither were any group differences in the MoCA, Boston Naming Test, Similarities or Semantic Verbal Fluency scores (13 controls vs. 13 PD patients2). None of the controls reported any history of neurological or psychiatric disorders or drug abuse. PD patients fulfilled the United Kingdom PD Society Brain Bank criteria (Hughes et al., 1992). Disease severity was evaluated using the motor section of the Unified Parkinson's Disease Rating Scale UPDRS-III (Fahn et al., 1987) and the calculated Hoehn and Yahr staging (Hoehn and Yahr, 1967). All patients were at early stages of the disease (mean of disease duration 2.8 ± 1.7 years), and none of them had any psychiatric conditions or other neurological disorders. Patients were undergoing antiparkinsonian therapy at the time of testing. Recent conversion factors were used to calculate Levodopa equivalent dosage (LEDD; Tomlinson et al., 2010). Demographic and clinical characteristics are listed in Table 1. All participants gave written consent. The study was approved by the Central Denmark Region Committees on Biomedical Research Ethics (Nr. 1-10-72-323-16) and was carried out in accordance with the principles of the Declaration of Helsinki.
Table 1.
Demographic and clinical characteristics of participants. Values are given as mean (±SD). For the four neuropsychological tests denoted by asterisks (*) data were available only for 13 out of 17 PD patients and 13 out of 15 controls.
| PD patients | Healthy controls | t-test | p-value | |
|---|---|---|---|---|
| Gender | 9 males, 8 females | 5 males, 10 females | t(29.83) = 1.11 | 0.28 |
| Age | 66.24 (5.68) | 63.20 (4.43) | t(29.58) = 1.69 | 0.10 |
| Education | 14.50 (2.82) | 13.73 (2.44) | t(29.95) = 0.77 | 0.44 |
| MoCA* | 27.08 (1.98) | 26.92 (2.53) | t(22.67) = 0.17 | 0.86 |
| Boston Naming Test* | 28.54 (1.20) | 28.69 (1.25) | t(22.94) = 2.97 | 0.10 |
| WAIS-IV Similarities* | 23.92 (4.03) | 27.00 (5.02) | t(22.21) = 0.92 | 0.35 |
| Semantic Verbal Fluency (animals)* | 25.62 (6.95) | 27.92 (5.19) | t(23.96) = 0.10 | 0.75 |
| Disease duration (by 2018) | 2.82 (1.67) | |||
| Calculated H&Y | 2.63 (0.61) | |||
| UPDRS total | 34.13 (10.80) | |||
| Levodopa equivalent daily dose LEDD (mg) | 376.96 (209.60) |
2.2. Language stimuli
The stimuli consisted of a set of spoken Danish words (see Table 2) including two action verbs (‘slikke’ [slegə] (to lick) and ‘spytte’ [sbødə] (to spit)) and two abstract verbs (‘slutte’ [sludə] (to end) and ‘svække’ [svɛgə] (to weaken)). Abstract verbs were mainly used as a reference/control condition for the action verbs to allow better interpretation of the results. Additionally, an acoustically counterbalanced set of pseudowords was created by re-combining the same first and second syllables of the real verbs (i.e. ‘*slitte’ [sledə], ‘*spykke’ [sbøgə], ‘*slukke3’ [slugə] and ‘*svætte’ [svɛdə]). The stimuli were chosen to ensure a counterbalanced design where the same first syllables (‘sli-’ [sle],‘spy-’ [sbø], ‘slu-’ [slu], ‘svæ-’ [svɛ]) were equally present in words and pseudowords, and the same second syllables (here, ‘-kke’ [-gə] and ‘-tte’ [-də]) were used in action verbs, abstract verbs and pseudowords alike. This was implemented in order to balance the acoustic properties of stimulus groups and to ensure that the full recognition of the word forms was only possible after the second syllable onset, to which time point the brain responses were time-locked in the subsequent MEG analyses. The neural responses for pseudowords were not analysed as such.
Table 2.
Stimuli. Asterisks (*) denote pseudowords.
| Lexico-semantic condition | Words | Pseudowords |
|---|---|---|
| Action verbs | slikke [slegə] (to lick) spytte [sbødə] (to spit) |
*slitte [sledə] *spykke [sbøgə] |
| Abstract verbs | slutte [sludə] (to end) svække [svɛgə] (to weaken) |
*slukke [slugə] *svætte [svɛdə] |
| Morphosyntactic condition | Correct morphosyntax | Incorrect morphosyntax |
| Concrete nouns | stykket [sdøgəd] (the piece) slottet [slʌdəd] (the castle) |
*stykken [sdøgən] *slotten [slʌdən] |
| stokken [sdʌgən] (the stick) skytten [sgødən] (the shooter) |
*stokket [sdʌgəd] *skyttet [sgødəd] |
Morphosyntactic stimuli were created exploiting the Danish morphology where the definiteness of nouns is expressed, depending on the noun gender (neuter/common), by adding the morphemes -(e)t [(ə)d] (neuter gender) or -(e)n [(ə)n] (common gender) as suffixes. Thus, four nouns were chosen (‘stykke’ [sdøgə] (piece), ‘slot’ [slʌt] (castle), ‘stok’ [sdʌg] (stick) and ‘skytte’ [sgødə] (shooter)). Each of these nouns were cross-spliced with the correct inflectional morpheme (either -(e)t or ‐(e)n) to construct the correct morphosyntactic inflection expressing the definiteness of that particular noun. This resulted in four correct inflections (‘stykket’, ‘slottet’, ‘stokken’, ‘skytten’). The grammatically incorrect inflectional counterparts were constructed by cross-splicing each of the nouns with the incorrect inflectional morpheme (either -(e)t or ‐(e)n). The resulting incorrect inflections were ‘*stykken’, ‘*slotten’, ‘*stokket’, ‘*skyttet’. Note that identical base forms and suffixes were used in both sets of stimuli assuring that any correlates of morphosyntactic processing corresponding to correct or incorrect affixation were not differentially affected by acoustic stimulus properties.
The stimuli were made based on a digital recording (44.1 kHz, 32 bit) of a male native speaker of Danish. The first syllables were uttered with a following [-ə], separated by a silent gap (e.g., ‘sty-e’ [sdøʔə]). The second syllables (‘-kke’, ‘-tte’) were pronounced with a preceding schwa-sound [ə-] in order to avoid co-articulation biases in the final word stimuli. The morphosyntactic suffixes were produced with the preceding second syllables (e.g. ‘[ə]tten’ or ‘[ǝ]kket’). The stimuli were selected from several utterances and the original syllables were normalised by maximal peak amplitude. The redundant vowels following the first syllables were cut off, after which the first syllables were matched to have a duration of 315 ms (including 15-ms fade-in and fade-out envelopes), F0 of 100 Hz and equal loudness (estimated as total RMS). The two second syllable items were extracted from the preceding [ǝ-], matched for duration of 140 ms (with a 15-ms fade-out) and F0 of 135 Hz. The suffixed second syllables were created by splicing the extracted ‘-t’ and ‘-n’ suffixes of 110 ms duration (with 15-ms fade-out) onto the prepared ‘-kke’ and ‘-tte’ items at 60 ms, resulting in four syllables of 170 ms duration. Loudness of all second syllables was matched, after which a 3 dB decrease was applied to ensure a natural Danish stress pattern between the first and second syllables. Finally, the verb, noun, and pseudoword stimuli were constructed by cross-splicing each first syllable with the appropriate second syllable, separated by a 35-ms silent gap. Stimuli were edited using Praat 6.0.43 (Boersma and Weenink, 2018) and Adobe Audition CC (Adobe Inc., San Jose, CA).
2.3. Stimulus presentation
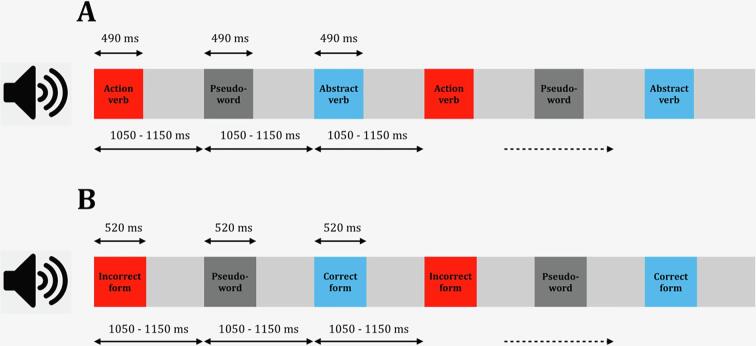
For stimulus presentation we adapted the so-called equiprobable paradigm, optimised for patient-friendliness, presentation time, and the number of linguistic contrasts tested simultaneously, as shown in previous MEG research using healthy participants (Hyder et al., 2020). Stimuli were presented in two sequences. In one sequence (lexical semantics condition), action and abstract verbs and matching pseudowords were presented equiprobably with 100 repetitions for each stimulus item (800 trials in total). Similarly in the other sequence (morphosyntactic condition), the correct and incorrect morphosyntactic forms were presented equiprobably with 100 repetitions for each stimulus item (800 trials in total). The stimulus onset asynchrony (SOA) was jittered between 1050 and 1150 ms with a mean of 1100 ms. Thus, the total duration of each sequence was ∼15 minutes, ensuring maximal patient comfort. All stimuli were pseudo-randomly presented (randomisation was done on subsequent trains of 40 stimuli to ensure a relatively even stimulus distribution across each sequence) such that two identical tokens were never presented immediately after each other (Fig. 1).
Fig. 1.
An illustration of the experimental paradigm. A: Action and abstract verbs and matching pseudowords were presented equiprobably with 100 repetitions for each stimulus item in a pseudo-randomised fashion (randomisation was done on subsequent trains of 40 stimuli such that two identical tokens were never presented immediately after each other). B: Correct and incorrect morphosyntactic forms were presented in the same fashion (equiprobably and pseudo-randomly with 100 repetitions for each stimulus item). The stimulus-onset-asynchrony (SOA) was jittered between 1050 and 1150 ms with a mean of 1100 ms. The total duration of each sequence was ∼15 min.
2.4. Procedure
MEG data were recorded in an electromagnetically shielded and acoustically isolated room (Vacuum Schmelzer Gmbh, Hanau, Germany). For each participant, the individual hearing threshold was estimated before starting the recording to ensure that the sound level among all participants was subjectively similar. Throughout the MEG session, auditory stimuli were presented at 40–50 dB (adjusted for patient’s individual comfort) above the individual hearing threshold using in-ear tubes (Etymotic ER-30). Stimuli were presented using Neurobehavioral Systems Presentation v16 (www.neurobs.com). During the MEG recording, participants were comfortably lying down on a non-magnetic patient bed without any task or any effort required from them. Participants were instructed to pay no attention to the presented auditory stimuli and to focus on watching a silent movie. The movie was displayed through a mirror projection screen placed at a suitable distance. Both stimulus sequences were divided into two sub-blocks of ∼7.5 minutes each with a short break (1–2 min) in-between to prevent fatigue. The order of the presentation of the four sub-blocks was randomised within each group.
2.5. Magnetoencephalographic recordings
MEG measurements were carried out using a CE-approved Elekta Neuromag Triux MEG system (Elekta Neuromag Oy, Helsinki, Finland). MEG data were recorded from 306 channels (102 magnetometers and 204 planar gradiometers) at 1000 Hz sampling rate with online high-pass and low-pass filtering at 0.01 Hz and 330 Hz, respectively. In addition, bipolar electrocardiogram (ECG) and two bipolar electrooculograms (horizontal and vertical EOG) were recorded to detect artifacts caused by heartbeats and eye movements. A continuous tracking of the participants’ head position and movement was done using four head position identification (HPI) coils. Prior to the MEG measurement, the positions of these HPI coils on the participant’s head were digitised along with the three cardinal landmarks (nasion and pre-auricular points) and additional scalp and facial points using a Polhemus FASTRAK setup (Polhemus, Vermont, USA).
2.6. MEG data preprocessing
For the first offline preprocessing step, MaxFilter v.2.2.15 Elekta Neuromag software was used (with subspace correlation limit of 0.96 and length of data buffering of 32 s) to reduce the noise from magnetic sources outside the head by applying the spatiotemporal signal-space separation technique (tSSS; Taulu and Simola, 2006). Compensation for head movements was made using the mean of head positions measured by HPI coils at the beginning of each recording block. Continuous MEG data were then preprocessed using MNE-Python version 0.19 (Gramfort et al., 2013). Data were high-pass filtered at 1 Hz and low-pass filtered at 40 Hz, which also removed power line noise (50 Hz) and its higher harmonics.
Artifact correction was done by applying independent component analysis (ICA) on the filtered MEG data using the fastica algorithm (Hyvärinen, 1999) as implemented in MNE-Python. For each subject, the smallest number of components required to explain more than 95% of the cumulative variance of the data was used for ICA fitting. The control group had a mean of 35 ± 2.5 components and the PD group had a mean of 30 ± 3.7 components. Identification of ICA components related to heartbeats was done using the find_bads_ecg algorithm in MNE-Python with the ctps method by which the detection of the ECG components is done using cross-trial phase statistics. Identification of ICA components related to eye movements was done using the find_bads_eog algorithm in MNE-Python which uses Pearson correlation between filtered EOG signal and filtered MEG data to detect EOG components. The automatic selection of ECG and EOG components was then checked visually to ensure the correct removal of artifactual components by plotting the interpolated magnetometers’ topographies of all ICA components and verifying that the topographies of the selected components corresponded to eye blinks and heart beats. Further verification was done by plotting the time courses of the components and comparing them with the recorded ECG and EOG signals, after which the artifact components were removed from the data. The control group had a mean of 2.6 ± 0.6 rejected artifactual components and the PD group had a mean of 2.4 ± 0.8 rejected artifactual components.
After ICA, the PD group had, on average, 0.9 ± 1.2 bad magnetometers in the lexico-semantic sequence, 1.1 ± 1.3 bad magnetometers in the morphosyntactic sequence and no bad gradiometers in either of the two sequences. For the control group, there were on average 0.4 ± 0.9 bad magnetometers in the semantics sequence, 0.6 ± 1.1 bad magnetometers in the morphosyntactic sequence, and no bad gradiometers in either of the two sequences. Data were then epoched from −50 ms to 1050 ms relative to stimulus onset with baseline correction at 0–350 ms (i.e., prior to word disambiguation point). Epochs with gradiometer amplitudes larger than 4000 (fT/cm) or magnetometer amplitudes larger than 4000 (fT) were rejected. For the PD group, a mean of 1.2 ± 1.4 and 2.0 ± 2.5 epochs were discarded in the lexico-semantic and morphosyntactic sequences, respectively. For the control group, a mean of 0.8 ± 0.8 and 0.8 ± 1.1) epochs were removed from further analysis in the lexico-semantic and the morphosyntactic sequences, respectively. For each participant, event-related fields (ERFs) were created by averaging the epochs of each stimulus type separately.
2.7. MRI data acquisition and processing
For each participant, a T1-weighted structural image of the brain was acquired using a 3 T Siemens Prisma MR scanner (MP2RAGE sequence with parameters: TR = 5 s; TE = 2.98 ms; in-plane resolution = 1x1 mm2; 176 sagittal slices, thickness = 1 mm; TI = 700|2500 ms (INV1|INV2); flip angle = 4|5 deg). The MP2RAGE UNI-images contain high-intensity background noise in areas outside and inside the skull. Tissue masks for air, bone, eyes and skin were first calculated for the INV2-image using the headreco utility in SimNIBS 2.1 (Nielsen et al., 2018). These were then applied to the UNI-image for inner skull surface extraction and tessellation. A single-layer Boundary Element Model (BEM) was thus created and used to compute the forward model for each participant. A surface-based source space was created on the estimated boundary surface between the white and grey matter using Freesurfer v6.0 (Dale et al., 1999). The decimated dipole grid had 4098 vertices in each hemisphere.
2.8. Source reconstruction
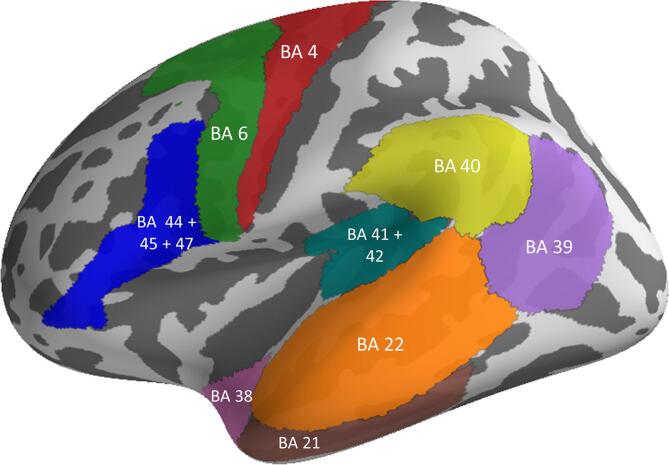
Source reconstruction was done using MNE-Python version 0.19. For each subject’s event-related responses, minimum norm source estimation (MNE; Hämäläinen and Ilmoniemi, 1994) was computed from all 306 MEG sensors with loose orientation (0.2) and depth weighting (0.8) constraints. Further analysis was done by defining a priori regions-of-interest (ROIs; Fig. 2) within individual participants’ source space on the basis of the PALS-B12 Brodmann Atlas parcellation of the cortical surface as implemented in Freesurfer (Van Essen, 2005). ROIs were defined on the basis of existing models of structural and functional connectivity of language processing (Catani and Mesulam, 2008, Friederici, 2011, Friederici, 2012, Hickok and Poeppel, 2007) as well as models of action semantic mechanisms (Tomasello et al., 2017), identifying fronto-temporo-parietal and precentral cortices related to language processing. Thus, our model included the following ROIs: primary auditory cortex and auditory belt (BA 41 + BA 42), superior temporal (BA 22), anterior temporal (BA 38), middle temporal (BA 21) and inferior frontal (pars opercularis BA 44, pars triangularis BA 45 and pars orbitalis BA 47) areas, angular gyrus (BA 39), supramarginal gyrus (BA 40), motor cortex (lateral segment of BA 4), and premotor cortex (lateral segment of BA 6). In total, we defined 18 bilateral ROIs (i.e., 9 ROIs in each hemisphere). For each participant’s source estimation, we extracted time series within each ROI using the pca_flip method as implemented in MNE-Python. This method applies singular vector decomposition (SVD) to the time series of the estimated sources within each ROI and uses the sign-adjusted and scaled first right-singular vector as the ROI’s time course. Scaling is done such that the power of the extracted time series equals the average power of all the time series at all vertices within the ROI. Sign adjustment is done based on the normal vectors at all vertices and the first left-singular vector. This method was mainly used to avoid random phase flipping in the extracted time series, and thus minimise signal cancellation.
Fig. 2.
A priori defined ROIs: primary auditory cortex and auditory belt (BA 41 + BA 42), superior temporal (BA 22), anterior temporal (BA 38), middle temporal (BA 21) and, inferior frontal (pars opercularis BA 44, pars triangularis BA 45 and pars orbitalis BA 47) areas, angular gyrus (BA 39), supramarginal gyrus (BA 40), motor cortex (lateral segment of BA 4) and premotor cortex (lateral segment of BA 6). ROIs were defined bilaterally in both hemispheres on the basis of the PALS-B12 Brodmann Atlas parcellation of the cortical surface, as implemented in Freesurfer.
2.9. Classification of PD patients and controls
Classification of PD patients and healthy controls was done based on the four word types independently. We tested if we could classify PD patients and healthy controls based on the correlation between the extracted time series from the ROIs during the processing of: (1) action verbs, (2) abstract verbs, (3) grammatically correct inflectional forms, and (4) grammatically incorrect inflectional forms. For each subject, a Spearman correlation coefficient was computed (while keeping the sign of the coefficient) for each pair of the extracted time series in source space, i.e., the extracted time series of source activity in each ROI was correlated with the time series of each of the other ROIs. Thus, for the 18 ROIs in our model, there were 153 different combinations of ROIs resulting in 153 correlation coefficients per subject. The correlation coefficients were computed starting at the word disambiguation point, i.e., at the onset of the second syllable of each stimulus. The calculation of correlation coefficients was done for each word type independently.
2.9.1. Classification pipeline
The classifier pipeline was constructed in MNE-Python using scikit-learn 0.22 (Abraham et al., 2014, Pedregosa et al., 2011). Classification features (153 correlation coefficients) were standardised (z-scored); the standardisation was done across all subjects (PD patients and controls). To avoid overfitting, the classification was cross-validated across all subjects. This was done by taking one subject (PD patient or control) as the test set and training the classifier on the rest of the subjects. The process was repeated until all participants had been used as test sets.
To reduce the dimensionality of the feature space comprising 153 features, we used a feature selection algorithm which selects features (according to a specific percentile) with the highest classification accuracy. Therefore, classification was done in two steps. The first step was to optimise the hyperparameter of the feature selection algorithm (percentile of features). This was done by performing a grid search to find the best percentile of features that yielded the highest classification accuracy across all subjects. The grid search spanned from 2% to 100% of the original feature space (153 features) with a step of 1% and was cross-validated across subjects. The second step consisted of two sub-steps and was cross-validated across subjects: First, feature selection was done by selecting the percentage of features according to the percentile found in the first step; second, a logistic regression classifier (C = 1, penalty = ‘L2’, solver = ‘L-BFGS’) was fitted using the selected features. We opted for logistic regression as it is a widely used linear classifier, and linear classifiers have been shown to perform better than non-linear classifiers on neural data (Misaki et al., 2010).
The statistical significance of the classification accuracy for each word type was tested using permutation tests (number of permutations: 5000, scoring: the area under the receiver operating characteristic curve (ROC-AUC; Hand and Till, 2001) and cross-validation with 10 stratified folds (Varoquaux et al., 2017)).
For the visualisation of the classifier performance for each word type, a normalised confusion matrix was computed and the precision and recall were calculated for each condition (see Table 3).
Table 3.
Results of classification and evaluation of the classifier performance for each word type for PD and healthy controls (HC) using normalised confusion matrices and precession-recall metrics (the results of all variants for each word type are concatenated in each of the 4 confusion matrices).
| Predicted PD | Predicted HC | Precision | Recall | Accuracy score | |
|---|---|---|---|---|---|
| Action verbs (p-value = 0.003) | |||||
| True PD | 82% | 18% | 0.785 | 0.733 | 0.781 |
| True control | 27% | 73% | |||
| Abstract verbs (p-value = 0.041) | |||||
| True PD | 76% | 24% | 0.692 | 0.600 | 0.688 |
| True control | 40% | 60% | |||
| Correct morphosyntactic forms (p-value = 0.384) | |||||
| True PD | 56% | 44% | 0.491 | 0.483 | 0.523 |
| True control | 52% | 48% | |||
| Incorrect morphosyntactic forms (p-value = 0.021) | |||||
| True PD | 68% | 32% | 0.627 | 0.616 | 0.648 |
| True control | 38% | 62% | |||
3. Results
In the following, we report the group classification results for each word type separately. Classification results for all word types are provided in Table 3. Full list of correlation coefficients from PD patients and controls are reported in supplemental data (Table S1).
3.1. Action verbs
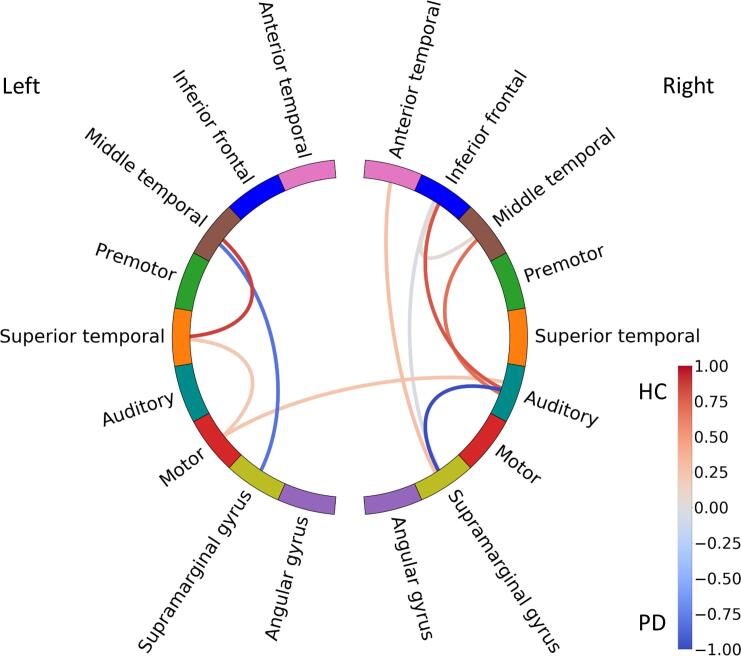
Classification using action verb responses was significant (p = 0.003) with an accuracy of 0.781. The calculated confusion matrix revealed that 73% of the data from controls and 82% of the data from PD patients were predicted correctly, hence, a total of 78% of the data from both groups was predicted correctly (recall = 0.733 and precision = 0.785) (see Table 3). The selected features (see Fig. 3) comprised 7% of the original feature space (i.e., 10 out of 153 connections).
Fig. 3.
Illustration of coefficients from the fitted logistic regression model for the action verb condition, depicted as colour-coded lines connecting respective areas. The values (coded as colour intensity) indicate the contribution to the overall class prediction: higher coefficient values in the red connections contribute more to classifying a participant as a healthy control (HC) and higher coefficient values in the blue connections contribute more to classifying a participant as a PD patient (PD). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Abstract verbs
Classification using abstract verb responses was also significant (p = 0.041) with an accuracy of 0.688. The calculated confusion matrix revealed that 60% of the data from controls and 76% of the data from PD patients were predicted correctly, hence, a total of 69% of the data from both groups was predicted correctly (recall = 0.6 and precision = 0.692) (see Table 3). The selected features (see Fig. 4) comprised 12% of the original feature space (i.e., 19 out of 153 connections).
Fig. 4.
Illustration of coefficients from the fitted logistic regression model for the abstract verb condition depicted as colour-coded lines connecting respective areas. The values (coded as colour intensity) indicate the contribution to the overall class prediction: higher coefficient values in the red connections contribute more to classifying a participant as a healthy control (HC) and higher coefficient values in the blue connections contribute more to classifying a participant as a PD patient (PD). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Correct morphosyntactic forms
No significant classification results were found for the correct morphosyntactic forms condition (p = 0.384). The calculated confusion matrix revealed that 48% of the data from controls and 56% of the data from PD patients were predicted correctly (recall = 0.483 and precision = 0.491) (see Table 3).
3.4. Incorrect morphosyntactic forms
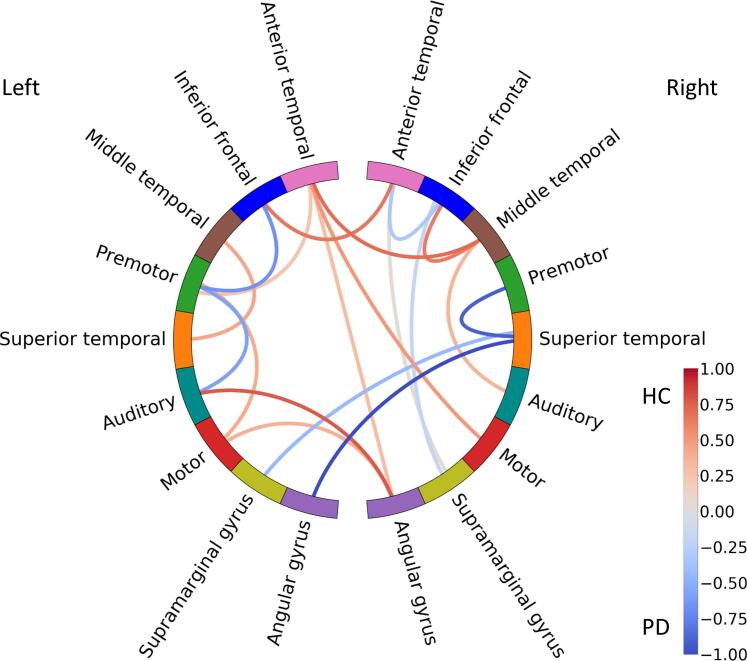
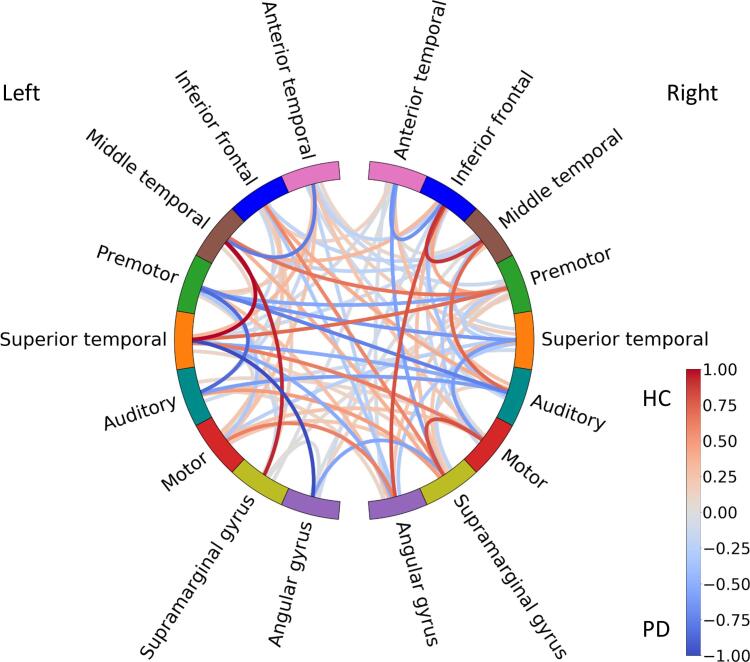
Classification using responses to incorrect morphosyntactic forms was significant (p = 0.021) with an accuracy of 0.648. The calculated confusion matrix revealed that 62% of the data from controls and 68% of the data from PD patients were predicted correctly, hence, a total of 65% of the data from both groups was predicted correctly (recall = 0.616 and precision = 0.627) (see Table 3). The selected features see (Fig. 5) comprised 58% of the original feature space (i.e., 89 out of 153 connections).
Fig. 5.
Illustration of coefficients from the fitted logistic regression model for the incorrect morphosyntactic form condition depicted as colour-coded lines connecting respective areas. The values (coded as colour intensity) indicate the contribution to the overall class prediction: higher coefficient values in the red connections contribute more to classifying a participant as a healthy control (HC) and higher coefficient values in the blue connections contribute more to classifying a participant as a PD patient (PD). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Using machine learning, we have demonstrated that newly diagnosed PD patients differ from healthy age-matched controls in the functional connectivity within the cortical networks active during automatic processing of spoken language. Differences in connectivity were found in unilateral and cross-callosal connections between the tested frontal, temporal and inferior-parietal cortices. Our results indicate that alterations in language network connectivity are present already in the early stages of PD, when overall cognitive functionality and verbal skills, such as naming, verbal conceptual reasoning and semantic verbal fluency are still relatively intact, as indicated by standard neuropsychological tests. Below we discuss the results and their implications in more detail (see also Table 3 and Table S1).
4.1. Classification based on action and abstract verbs
We obtained good classification performance based on the functional cortical connectivity during action verb processing with an accuracy of 0.781. With correct prediction of 78% of the full data across groups, action verbs showed numerically higher accuracy than the abstract verbs where PD patients and controls were classified with an accuracy of 0.688 and correct prediction of 69% of the full data across groups. Additionally, connections for the abstract verbs were more distributed relative to the more focal connectivity exhibited by the action verbs. These findings suggest more prominent differences in functional cortical connectivity for action verbs relative to abstract verbs processing and may help account for previous evidence from a behavioural study demonstrating that, compared to controls, PD patients were more impaired in processing action verbs than abstract verbs, which may be related to the use of the motor system (impaired in PD) in processing action-related referential semantics (Fernandino et al., 2013).
4.2. Classification based on morphosyntax
Our results showed that it was also possible to classify participants as PD patients and controls based on the functional cortical connectivity for automatic processing of incorrect morphosyntactic forms. The processing of incorrect morphosyntax yielded an accuracy of 0.648 with correct prediction of 65% of the full data across groups, while no significant classification results were obtained for the processing of correct morphosyntax.
It has been suggested that early, automatic stages of syntactic processing in PD is preserved (Friederici et al., 2003a, Longworth et al., 2005) and that PD patients are able to access morphosyntactic information in an automatic manner, but only for a short time which results in poor integrational processing due to the brief activation of this information (Arnott et al., 2005). In the context of automatic processing of grammar (when subjects’ attention is diverted away from language stimuli), healthy adults exhibit larger neural responses to grammatical violations relative to canonical contexts, which has been explained by morphosyntactic priming/pre-activation through existing connections between related morphemes or by an activation of an error-detection mechanism (Herrmann et al., 2009, Pulvermüller and Assadollahi, 2007, Pulvermüller and Shtyrov, 2003, Shtyrov et al., 2003). Thus, it may be that automatic parsing of non-canonical contexts is associated with complex patterns of functional connectivity and that PD patients potentially process grammatical violations in a different (either deficient or more resource-demanding) way than controls do, whereas these effects do not arise for well-formed combinations whose processing may still be preserved, at least in the early stages of the disease investigated in the present study.
4.3. Common functional connections across word types
Some common functional connections were observed across the different significant word-type conditions (see Fig. 6). In the left hemisphere, PD patients had lower correlation values relative to healthy controls in the connection between middle and superior temporal cortices during processing of action and abstract verbs as well as incorrect morphosyntax (see Table S1). In the right hemisphere, two common connections were observed between middle temporal cortex and inferior frontal gyrus as well as between middle temporal cortex and auditory cortex. For both connections, PD patients had lower correlation values relative to controls for action and abstract verbs as well as for processing incorrect morphosyntax (see Fig. 6 and Table S1).
Fig. 6.
Common features found across the three significant word-type conditions. PDs < HCs: lower correlation values for PD patients relative to controls. PDs > HCs: higher correlation values for PD patients relative to controls.
Functional interaction between the middle temporal cortex and the inferior frontal gyrus has previously been identified in both left and right hemispheres using combined anatomical and functional connectivity measures during spoken language comprehension in healthy adults (Saur et al., 2010). The functional connection between the middle temporal cortex and the ventrolateral prefrontal cortex is mediated by the ventral pathways (Saur et al., 2008) and presumably plays a role in linking linguistic input to stored knowledge (Hagoort et al., 2004). On the other hand, the connection between the middle temporal cortex and the auditory cortex may reflect early auditory stages of speech perception (e.g., phonetic/phonological processing) as well as the mapping of phonological forms onto lexico-semantic representations. Hence, the observed differences between the two groups in theses connections across the three significant word-type conditions may suggest potential alterations in the functional connectivity of the temporal cortical regions associated with auditory language processing in newly diagnosed PD patients. At this stage this interpretation is still speculative and needs further investigations to be validated.
The observed differences found in our study between PD patients and healthy controls emerging from the functional connectivity of the temporal cortical regions do not fully align with findings from a previous fMRI study reporting no significant differences between controls and PD patients in the functional connectivity of the temporal seed during listening to action verbs and nouns (Abrevaya et al., 2017). However, in that study the temporal seed was defined in the posterior portion of the superior temporal gyrus and only in the left hemisphere, whereas differences in the functional connectivity between PD patients and controls in our study were mainly found for the middle temporal cortex in both left and right hemispheres. Furthermore, the divergence between our findings and those of Abrevaya and colleagues may also be attributed to different experimental settings and language tasks, to variable patient populations, and to the fundamental differences between hemodynamic (fMRI) and electrophysiological (MEG) correlates of neural activity.
Another common connection across the tested word types was found in the right hemisphere between the supramarginal gyrus and the anterior temporal cortex with PD patients exhibiting lower correlation values compared to those of controls for both verbs, but vice versa for the incorrect morphosyntactic forms. The right supramarginal gyrus showed a common connection also with the right inferior frontal gyrus. This fronto-parietal connection was weaker in PD patients compared to controls during processing of action verbs and incorrect morphosyntactic forms but was stronger for abstract verbs (see Fig. 6 and Table S1). The presence of differences in functional connections for the right supramarginal gyrus corroborates previous findings from a recent meta-analysis demonstrating that, relative to healthy subjects, PD patients show consistent alterations in resting-state functional connectivity of the right supramarginal gyrus across studies, highlighting the important role of this region in PD pathology (Tahmasian et al., 2017).
4.4. Possible effects of dopaminergic medication on functional connectivity
PD patients in this study were tested while they were on their normal doses of dopaminergic medication. Thus, the observed functional connectivity in PD patients in our study may have been influenced not only by the disease as such, but also by their medication intake. If so, this may have affected the processing of action verbs the most since it is known to involve the motor circuits which, in turn, are directly affected by dopaminergic medication (Cardona et al., 2013, Herrera et al., 2012a, Herrera and Cuetos, 2012).
Neuroimaging studies that have systematically investigated the effects of dopaminergic medication on action semantic processing in PD patients have demonstrated that levodopa (the most common PD medication) has a selective neuro-modulatory effect on action perception with increased activity in cortical areas including the premotor and motor cortical regions in relation to levodopa administration (De Letter et al., 2012, Péran et al., 2013). Furthermore, in our recent study we found that deep brain stimulation of the subthalamic nucleus (STN-DBS) has selective influence on the neuromagnetic responses to action verbs relative to abstract verbs (Hyder et al., 2021). Together these findings suggest that processing of action verbs may benefit from the relative normalisation of the motor circuit activity induced by PD treatments. Such interpretation may account for the absence of discriminative functional connections between the motor, premotor and inferior frontal cortices in association with action verb processing in the present study. However, with the current experimental design this remains a speculation and requires further investigation controlling for the effect of PD medication. To the best of our knowledge, no neuroimaging study has systematically investigated the effects of dopaminergic medication on grammatical processing, hence, it is difficult to speculate about the potential effects of dopaminergic medication on the functional connectivity for morphosyntactic processing in PD patients, although, notably grammatical processing also involves frontostriatal circuitry (most importantly, inferior frontal gyrus; Dominey and Inui, 2009, Ford et al., 2013).
Neuroimaging studies have demonstrated that antiparkinsonian medication induces functional reorganisation at the cortical and subcortical levels in PD patients (Tahmasian et al., 2015). Furthermore, several studies have reported that antiparkinsonian medication enhances the functional connectivity within the sensorimotor network, particularly in the supplementary motor area (Esposito et al., 2013), as well as in the attention system (between the frontoparietal and the default mode networks; Dang et al., 2012). Such enhanced functional integration in PD patients during the intake of antiparkinsonian medication seems to be a consistent finding (Prodoehl et al., 2014). This evidence may partly account for the increased functional connectivity observed in PD patients compared to controls in some of the connections found in this study.
4.5. Functional connectivity in early-stage PD patients
Taken together, our findings show differences between early-stage PD patients and controls in functional cortical connectivity during automatic processing of spoken words. The functional connections observed across the different word types examined in this study indicate that, compared to controls, PD patients exhibit both reduced and increased functional connectivity between the tested temporal, inferior-parietal and frontal cortical regions during automatic verb processing as well as during the parsing of morphosyntactic violations.
It has been suggested that the initial disruption of the basal ganglia function due to dopamine deficiency is countered by compensatory processes and increased connectivity in other brain regions (Tahmasian et al., 2017). Increased resting state functional connectivity has been found in cognitively unimpaired PD patients suggesting that it may be an initial manifestation of altered brain function prior to cognitive decline (Gorges et al., 2015). The hyper-connectivity state in PD patients with normal cognition may thus reflect early compensatory or adaptive mechanisms to recruit other, less affected brain regions to maintain normal cognitive functions as long as possible (Gorges et al., 2015). Such findings may partly explain the increased connectivity observed in some functional connections in the early-stage PD patients compared to the controls in our study (however, see also the previous section 4.4 for a partly complementary explanation).
Cognitive decline in PD patients may be associated with transient processes from the hyper- toward the hypo-connectivity state (Gorges et al., 2015). Reduced functional connectivity within the frontoparietal network has been previously implicated in cognitive impairments (Amboni et al., 2015) as well as in early executive dysfunction (Teramoto et al., 2016) in PD patients. Evidence from a longitudinal MEG study demonstrated that, relative to healthy subjects, PD patients in the earliest clinical stages of the disease show reduced delta-band connectivity but increased alpha-band connectivity in the temporal cortical regions (Olde Dubbelink et al., 2013). With the progression of the disease, reduction in alpha-band connectivity was observed with a more widespread cortical pattern in association with the cognitive decline in these patients (Olde Dubbelink et al., 2013). Since our PD patients did not differ significantly from the healthy controls on cognitive and general language measures, a plausible interpretation of the observed hyper- and hypo-connectivity in PD patients relative to controls in this study is that they reflect PD-related functional reorganisation which can be detected at an early stage of the disease prior to any clinical evidence of cognitive or language dysfunctions. This suggestion requires further investigations which should involve more elaborate testing of different cognitive skills.
As already mentioned, dopaminergic medication may have exerted different effects on different cortical regions in our study. Whether testing PD patients in their “off” state (i.e., without influence of their dopaminergic medication) would have made the differences in functional connectivity between the two groups even more apparent or altered them somehow, is an open question. Thus, our findings do not directly generalise to unmedicated PD patients, which can be addressed in future studies.
Finally, inter-hemispheric connections were also observed. Despite the unilateral nature of the motor symptoms at the onset of PD indicating a potential imbalance in the neural activity between the two hemispheres (Cronin-Golomb, 2010), little is known about the inter-hemispheric functional connectivity in PD. Some fMRI studies have shown that PD patients exhibit reduced homotopic resting state functional connectivity in some cortical areas including supramarginal gyrus, motor cortex and primary somatosensory cortex (Luo et al., 2015). Furthermore, reduced functional connectivity between non-homotopic cortical regions including the right inferior frontal gyrus and left motor cortex has been found in PD patients with levodopa-induced dyskinesia in the ‘on’ state of antiparkinsonian medication (Cerasa et al., 2015), suggesting that alterations in inter-hemispheric coordination may play an important role in the pathophysiology of PD. Whether inter-hemispheric connections observed in our study reflect PD-related functional reorganisation detected at an early stage of the disease is an open question and such an interpretation must be treated with caution.
4.6. Limitations
Whereas the current data present significant new findings regarding the functional state of neurocognitive systems (language in particular) in early-stage PD patients, who overall exhibit no cognitive or language deficits, the present study is not without some limitations. A potential limitation of this study is a relatively small cohort of the participants caused by the restrictions involved in recruiting clinical populations; that said, other studies have used similar or even smaller samples of PD patients and controls and still obtained robust findings (Abrevaya et al., 2017, Ibáñez et al., 2013, Melloni et al., 2015, Péran et al., 2009). This limitation is partly also due to our strict quality control over the inclusion of early-stage PD patients and age-matched controls, which is also a strength of this study.
One other important aspect to mention is the relatively small number of stimulus items included. Balancing between ecological validity and strict control over stimulus features is an inherent challenge, which strongly influences decisions regarding experimental designs. Since we focused on pre-attentive electrophysiological responses to language stimuli (which are strongly affected by basic stimulus features, and any physical or psycholinguistic variance can confound or even invalidate findings), we employed a carefully selected set of stimulus items in each condition to ensure a counterbalanced stimulus design in which both acoustic and psycholinguistic properties of the stimuli were strictly controlled and balanced (Pulvermüller and Shtyrov, 2006).
Our analyses are based on a priori selected regions-of-interest (ROIs). It has been demonstrated that the choice of ROIs and connectivity estimators influence the functional interactions across brain regions (Dimitriadis et al., 2018). Similar to the current study, previous studies of functional brain connectivity during language-related tasks in PD patients and healthy adults have focused their analysis on regions typically engaged during language processing (Abrevaya et al., 2017, Saur et al., 2010). The purpose of our restricted analysis was mainly to allow for the classification of the two groups based on the functional connectivity between cortical regions that are known to be involved in language processing in general, and that are particularly implicated in the functional and structural connectivity models of language processing. Thus, this approach ensured that the results can be more plausibly attributed to language processing per se than to any general differences that are likely to emerge with a whole-brain approach. Nevertheless, as restricting the analysis to pre-defined ROIs inherently limits the space for establishing connections, we emphasise the necessity of further investigations using less restricted (e.g., whole-brain) analysis, which may potentially also be expanded to include oscillatory measures in different frequency bands (Jensen et al., 2019).
The lack of a behavioural task in the MEG was mainly due to our requirement of registering the neural responses to the linguistic input with minimal confounds related to attention and overt behavioural responses (e.g., button presses) that may be problematic for PD patients. A thorough language assessments of the participants could not be carried out for logistical reasons. Especially valuable would be to test for verb and morphosyntactic comprehension and production with standardised neuropsychological tests to assess these functions relative to normative performance, and then correlate the individual performance in the tasks with various neural measures.
A final limitation, as discussed before, is that PD patients in this study were tested while being under medication. Further replications of this study comparing patients in medicated and un-medicated states could provide important insights into PD-related functional cortical alterations and the possible effects of pharmaceutical treatments.
5. Conclusion
Overall, our findings provide evidence of differences in functional connectivity within the cortical systems underpinning language processing in newly diagnosed PD patients compared to healthy controls. They involve distributed networks including temporal, frontal and inferior-parietal cortices of both hemispheres and show successful classification results for the processing of action verbs, abstract verbs and morphosyntactic violations. Crucially, the results could be obtained in the absence of attention and stimulus-related tasks in a relatively simple, short and patient-friendly MEG recording session. The techniques presented here lay the ground for future work on establishing neurolinguistic markers to objectively and noninvasively identify functional alterations in language brain networks even before any clinical manifestations. Future replications and extensions of the present study with larger cohorts of participants and complementary methodological approaches are needed in order to fully understand the extents of its generalisability and potential applicability in the clinical practice.
CRediT authorship contribution statement
Rasha Hyder: Conceptualization, Methodology, Investigation, Formal analysis, Software, Resources, Data curation, Validation, Writing - original draft, Writing - review & editing. Mads Jensen: Conceptualization, Methodology, Formal analysis, Software, Supervision. Andreas Højlund: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision. Lilli Kimppa: Methodology, Resources, Writing - review & editing. Christopher J. Bailey: Software, Investigation, Writing - review & editing. Jeppe L. Schaldemose: Resources, Investigation. Martin B. Kinnerup: Resources, Investigation, Writing - review & editing. Karen Østergaard: Writing - review & editing, Supervision. Yury Shtyrov: Conceptualization, Methodology, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Acknowledgements
This work was supported by the Center of Functionally Integrative Neuroscience (Department of Clinical Medicine, Aarhus University), Danish Council for Independent Research (DFF 6110-00486, project 23776), Danish Association of Parkinson’s Disease (Parkinsonforeningen, project 30121), Basic Research Program of the NRU Higher School of Economics (HSE University) and Lundbeck Foundation (grants R140-2013-12951, R164-2013-15801). We declare no conflict of interest. We wish to thank neurologist Dr Adjmal Nahimi for his help in the clinical assessments of the PD patients included in this study.
Footnotes
Due to logistic difficulties in testing, neuropsychological data could not be obtained for a small subset of participants (4 PD patients and 2 healthy controls).
Note that the spelling ‘slukke’ actually refers to a real Danish verb (to turn off), but that verb is pronounced [slɔgə] and hence, the word form that is pronounced [slugə] (here indicated by an asterisk ‘*slukke’) and used in this experiment is a pseudoword in Danish.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102718.
Contributor Information
Rasha Hyder, Email: rasha.hyder@cfin.au.dk.
Yury Shtyrov, Email: yury@cfin.au.dk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abraham A., Pedregosa F., Eickenberg M., Gervais P., Mueller A., Kossaifi J., Gramfort A., Thirion B., Varoquaux G. Machine learning for neuroimaging with scikit-learn. Front. Neuroinf. 2014;8 doi: 10.3389/fninf.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrevaya S., Sedeño L., Fitipaldi S., Pineda D., Lopera F., Buritica O., Villegas A., Bustamante C., Gomez D., Trujillo N., Pautassi R., Ibáñez A., García A.M., Kumfor F. The Road less traveled: alternative pathways for action-verb processing in parkinson's disease. J. Alzheimers Dis. 2017;55(4):1429–1435. doi: 10.3233/JAD-160737. [DOI] [PubMed] [Google Scholar]
- Alexander G.E., Crutcher M.D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-L. [DOI] [PubMed] [Google Scholar]
- Amboni M., Tessitore A., Esposito F., Santangelo G., Picillo M., Vitale C., Giordano A., Erro R., de Micco R., Corbo D., Tedeschi G., Barone P. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. J. Neurol. 2015;262(2):425–434. doi: 10.1007/s00415-014-7591-5. [DOI] [PubMed] [Google Scholar]
- Arnott W.L., Chenery H.J., Murdoch B.E., Silburn P.A. Morphosyntactic and syntactic priming: an investigation of underlying processing mechanisms and the effects of Parkinson's disease. J. Neurolinguistics. 2005;18(1):1–28. doi: 10.1016/j.jneuroling.2004.02.002. [DOI] [Google Scholar]
- Bak T.H. The neuroscience of action semantics in neurodegenerative brain diseases. Curr. Opin. Neurol. 2013;26(6):671–677. doi: 10.1097/wco.0000000000000039. [DOI] [PubMed] [Google Scholar]
- Benton A.L. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6(1):53–60. doi: 10.1016/0028-3932(68)90038-9. [DOI] [Google Scholar]
- Birba A., García-Cordero I., Kozono G., Legaz A., Ibáñez A., Sedeño L., García A.M. Losing ground: Frontostriatal atrophy disrupts language embodiment in Parkinson's and Huntington's disease. Neurosci. Biobehav. Rev. 2017;80:673–687. doi: 10.1016/j.neubiorev.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Bocanegra Y., García A.M., Lopera F., Pineda D., Baena A., Ospina P., Alzate D., Buriticá O., Moreno L., Ibáñez A., Cuetos F. Unspeakable motion: Selective action-verb impairments in Parkinson's disease patients without mild cognitive impairment. Brain Lang. 2017;168:37–46. doi: 10.1016/j.bandl.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Bocanegra Y., García A.M., Pineda D., Buriticá O., Villegas A., Lopera F., Gómez D., Gómez-Arias C., Cardona J.F., Trujillo N., Ibáñez A. Syntax, action verbs, action semantics, and object semantics in Parkinson's disease: Dissociability, progression, and executive influences. Cortex. 2015;69:237–254. doi: 10.1016/j.cortex.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Boersma, P., & Weenink, D. (2018). Praat: doing phonetics by computer [Computer program]. Version 6.0.43, retrieved 1 September 2018 from http://www.praat.org/.
- Boulenger V., Mechtouff L., Thobois S., Broussolle E., Jeannerod M., Nazir T.A. Word processing in Parkinson's disease is impaired for action verbs but not for concrete nouns. Neuropsychologia. 2008;46(2):743–756. doi: 10.1016/j.neuropsychologia.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Braak H., Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv. Anat. Embryol. Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- Braak H., Del Tredici K., Bratzke H., Hamm-Clement J., Sandmann-Keil D., Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages) J. Neurol. 2002;249(Suppl 3):1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Braak H., Tredici K.D., Rüb U., de Vos R.A.I., Jansen Steur E.N.H., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24(2):197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Bäumer T., Pramstaller P.P., Siebner H.R., Schippling S., Hagenah J., Peller M., Gerloff C., Klein C., Münchau A. Sensorimotor integration is abnormal in asymptomatic Parkin mutation carriers: a TMS study. Neurology. 2007;69(21):1976–1981. doi: 10.1212/01.wnl.0000278109.76607.0a. [DOI] [PubMed] [Google Scholar]
- Cardona J.F., Gershanik O., Gelormini-Lezama C., Houck A.L., Cardona S., Kargieman L., Trujillo N., Arévalo A., Amoruso L., Manes F., Ibáñez A. Action-verb processing in Parkinson's disease: new pathways for motor-language coupling. Brain Struct. Funct. 2013;218(6):1355–1373. doi: 10.1007/s00429-013-0510-1. [DOI] [PubMed] [Google Scholar]
- Cardona J.F., Kargieman L., Sinay V., Gershanik O., Gelormini C., Amoruso L., Roca M., Pineda D., Trujillo N., Michon M., García A.M., Szenkman D., Bekinschtein T., Manes F., Ibáñez A. How embodied is action language? Neurological evidence from motor diseases. Cognition. 2014;131(2):311–322. doi: 10.1016/j.cognition.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Catani M., Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A., Koch G., Donzuso G., Mangone G., Morelli M., Brusa L., Quattrone A. A network centred on the inferior frontal cortex is critically involved in levodopa-induced dyskinesias. Brain. 2015;138(Pt 2):414–427. doi: 10.1093/brain/awu329. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A. Parkinson's disease as a disconnection syndrome. Neuropsychol. Rev. 2010;20(2):191–208. doi: 10.1007/s11065-010-9128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dang L.C., O'Neil J.P., Jagust W.J. Dopamine supports coupling of attention-related networks. J. Neurosci. 2012;32(28):9582–9587. doi: 10.1523/JNEUROSCI.0909-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. Parkinson's Disease: Mechanisms and Models. Neuron. 2003;39(6):889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- De Letter M., Van Borsel J., Santens P. An electrophysiological investigation of the effects of levodopa on semantic comprehension of action words in Parkinson’s Disease. J. Neurolinguistics. 2012;25(2):95–103. doi: 10.1016/j.jneuroling.2011.09.001. [DOI] [Google Scholar]
- Dimitriadis S.I., López M.E., Bruña R., Cuesta P., Marcos A., Maestú F., Pereda E. How to build a functional connectomic biomarker for mild cognitive impairment from source reconstructed MEG resting-state activity: the combination of ROI representation and connectivity estimator matters. Front. Neurosci. 2018;12:306. doi: 10.3389/fnins.2018.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominey P.F., Inui T. Cortico-striatal function in sentence comprehension: insights from neurophysiology and modeling. Cortex. 2009;45(8):1012–1018. doi: 10.1016/j.cortex.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Esposito F., Tessitore A., Giordano A., De Micco R., Paccone A., Conforti R., Tedeschi G. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson's disease by levodopa. Brain. 2013;136(Pt 3):710–725. doi: 10.1093/brain/awt007. [DOI] [PubMed] [Google Scholar]
- Fahn, S., Elton, R. L., & Members, U.P. (1987). Unified Parkinsons Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinsons Disease, 2, 153–163.
- Fang J., Chen H., Cao Z., Jiang Y., Ma L., Ma H., Feng T. Impaired brain network architecture in newly diagnosed Parkinson’s disease based on graph theoretical analysis. Neurosci. Lett. 2017;657:151–158. doi: 10.1016/j.neulet.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Fernandino L., Conant L.L., Binder J.R., Blindauer K., Hiner B., Spangler K., Desai R.H. Parkinson's disease disrupts both automatic and controlled processing of action verbs. Brain Lang. 2013;127(1):65–74. doi: 10.1016/j.bandl.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A.A., Triplett W., Sudhyadhom A., Gullett J., McGregor K., FitzGerald D.B., Mareci T., White K., Crosson B. Broca's area and its striatal and thalamic connections: a diffusion-MRI tractography study. Front. Neuroanat. 2013;7 doi: 10.3389/fnana.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The cortical language circuit: from auditory perception to sentence comprehension. Trends Cogn. Sci. 2012;16(5):262–268. doi: 10.1016/j.tics.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Kotz S.A., Werheid K., Hein G., von Cramon D.Y. Syntactic comprehension in Parkinson's disease: investigating early automatic and late integrational processes using event-related brain potentials. Neuropsychology. 2003;17(1):133–142. [PubMed] [Google Scholar]
- Friederici A.D., Rüschemeyer S.A., Hahne A., Fiebach C.J. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb. Cortex. 2003;13(2):170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Gaig C., Tolosa E. When does Parkinson's disease begin? Mov. Disord. 2009;24(S2):S656–S664. doi: 10.1002/mds.v24.2s10.1002/mds.22672. [DOI] [PubMed] [Google Scholar]
- García A.M., Ibáñez A. Words in motion: Motor-language coupling in Parkinson’s disease. Translational Neuroscience. 2014;5(2):152–159. doi: 10.2478/s13380-014-0218-6. [DOI] [Google Scholar]
- García A.M., Sedeño L., Trujillo N., Bocanegra Y., Gomez D., Pineda D., Villegas A., Muñoz E., Arias W., Ibáñez A. Language deficits as a preclinical window into parkinson's disease: evidence from asymptomatic parkin and dardarin mutation carriers. J. Int. Neuropsychol. Soc. 2017;23(2):150–158. doi: 10.1017/S1355617716000710. [DOI] [PubMed] [Google Scholar]
- Gorges M., Müller H.P., Lulé D., Pinkhardt E.H., Ludolph A.C., Kassubek J. To rise and to fall: functional connectivity in cognitively normal and cognitively impaired patients with Parkinson's disease. Neurobiol. Aging. 2015;36(4):1727–1735. doi: 10.1016/j.neurobiolaging.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Gramfort A., Luessi M., Larson E., Engemann D.A., Strohmeier D., Brodbeck C., Hämäläinen M. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013;7:267. doi: 10.3389/fnins.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M., Carvell S., Stern M.B., Gollomp S., Hurtig H.I. Sentence comprehension in Parkinson's disease: the role of attention and memory. Brain Lang. 1992;42(4):347–384. doi: 10.1016/0093-934x(92)90074-o. [DOI] [PubMed] [Google Scholar]
- Grossman M., Cooke A., DeVita C., Lee C., Alsop D., Detre J., Gee J., Chen W., Stern M.B., Hurtig H.I. Grammatical and resource components of sentence processing in Parkinson's disease: an fMRI study. Neurology. 2003;60(5):775–781. doi: 10.1212/01.WNL.0000044398.73241.13. [DOI] [PubMed] [Google Scholar]
- Göttlich M., Münte T.F., Heldmann M., Kasten M., Hagenah J., Krämer U.M. Altered resting state brain networks in Parkinson’s disease. PLoS ONE. 2013;8(10):e77336. doi: 10.1371/journal.pone.0077336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker C.D., Perlmutter J.S., Criswell S.R., Ances B.M., Snyder A.Z. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain. 2012;135(12):3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P., Hald L., Bastiaansen M., Petersson K.M. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304(5669):438–441. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- Hand D.J., Till R.J. A Simple generalisation of the area under the ROC curve for multiple class classification problems. Mach. Learn. 2001;45(2):171–186. doi: 10.1023/A:1010920819831. [DOI] [Google Scholar]
- Helmich R.C., Derikx L.C., Bakker M., Scheeringa R., Bloem B.R., Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb. Cortex. 2010;20(5):1175–1186. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- Helmich R.C., Thaler A., van Nuenen B.F.L., Gurevich T., Mirelman A., Marder K.S., Bressman S., Orr-Urtreger A., Giladi N., Bloem B.R., Toni I. Reorganization of corticostriatal circuits in healthy G2019S LRRK2 carriers. Neurology. 2015;84(4):399–406. doi: 10.1212/WNL.0000000000001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E., Cuetos F. Action naming in Parkinson's disease patients on/off dopamine. Neurosci. Lett. 2012;513(2):219–222. doi: 10.1016/j.neulet.2012.02.045. [DOI] [PubMed] [Google Scholar]
- Herrera E., Cuetos F., Ribacoba R. Verbal fluency in Parkinson's disease patients on/off dopamine medication. Neuropsychologia. 2012;50(14):3636–3640. doi: 10.1016/j.neuropsychologia.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Herrera E., Rodríguez-Ferreiro J., Cuetos F. The effect of motion content in action naming by Parkinson's disease patients. Cortex. 2012;48(7):900–904. doi: 10.1016/j.cortex.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Herrmann B., Maess B., Hasting A.S., Friederici A.D. Localization of the syntactic mismatch negativity in the temporal cortex: an MEG study. Neuroimage. 2009;48(3):590–600. doi: 10.1016/j.neuroimage.2009.06.082. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hochstadt J., Nakano H., Lieberman P., Friedman J. The roles of sequencing and verbal working memory in sentence comprehension deficits in Parkinson's disease. Brain Lang. 2006;97(3):243–257. doi: 10.1016/j.bandl.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder R., Højlund A., Jensen M., Johnsen E.L., Østergaard K., Shtyrov Y. STN-DBS affects language processing differentially in Parkinson’s disease: multiple-case MEG study. Acta Neurol. Scand. 2021:1–10. doi: 10.1111/ane.13423. [DOI] [PubMed] [Google Scholar]
- Hyder R., Højlund A., Jensen M., Østergaard K., Shtyrov Y. Objective assessment of automatic language comprehension mechanisms in the brain: Novel E/MEG paradigm. Psychophysiology. 2020;57(5) doi: 10.1111/psyp.13543. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Networks. 1999;10(3):626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M., Ilmoniemi R.J. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Compu. 1994;32(1):35–42. doi: 10.1007/bf02512476. [DOI] [PubMed] [Google Scholar]
- Ibáñez A., Cardona J.F., Dos Santos Y.V., Blenkmann A., Aravena P., Roca M., Hurtado E., Nerguizian M., Amoruso L., Gómez-Arévalo G., Chade A., Dubrovsky A., Gershanik O., Kochen S., Glenberg A., Manes F., Bekinschtein T. Motor-language coupling: direct evidence from early Parkinson's disease and intracranial cortical recordings. Cortex. 2013;49(4):968–984. doi: 10.1016/j.cortex.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Jensen M., Hyder R., Shtyrov Y. MVPA analysis of intertrial phase coherence of neuromagnetic responses to words reliably classifies multiple levels of language processing in the brain. eNeuro. 2019;6(4) doi: 10.1523/eneuro.0444-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Weintraub S., Goodglass H. Lea & Febiger; Philadelphia: 1983. Boston naming test. [Google Scholar]
- Kim J.Y., Criaud M., Cho S.S., Díez-Cirarda M., Mihaescu A., Coakeley S., Houle S. Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain. 2017;140(11):2955–2967. doi: 10.1093/brain/awx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Grossman M., Morris J., Stern M.B., Hurtig H.I. Attentional resource and processing speed limitations during sentence processing in Parkinson's disease. Brain Lang. 2003;85(3):347–356. doi: 10.1016/S0093-934X(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Longworth C.E., Keenan S.E., Barker R.A., Marslen-Wilson W.D., Tyler L.K. The basal ganglia and rule-governed language use: evidence from vascular and degenerative conditions. Brain. 2005;128(Pt 3):584–596. doi: 10.1093/brain/awh387. [DOI] [PubMed] [Google Scholar]
- Luo C.Y., Guo X.Y., Song W., Zhao B., Cao B., Yang J., Shang H.F. Decreased resting-state interhemispheric functional connectivity in Parkinson's disease. Biomed Res. Int. 2015;2015:1–8. doi: 10.1155/2015/692684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C.Y., Song W., Chen Q., Zheng Z.Z., Chen K., Cao B., Shang H.F. Reduced functional connectivity in early-stage drug-naive Parkinson's disease: a resting-state fMRI study. Neurobiol. Aging. 2014;35(2):431–441. doi: 10.1016/j.neurobiolaging.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Melloni M., Sedeño L., Hesse E., García-Cordero I., Mikulan E., Plastino A., Marcotti A., López J.D., Bustamante C., Lopera F., Pineda D., García A.M., Manes F., Trujillo N., Ibáñez A. Cortical dynamics and subcortical signatures of motor-language coupling in Parkinson’s disease. Sci. Rep. 2015;5(1) doi: 10.1038/srep11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M., Kim Y., Bandettini P.A., Kriegeskorte N. Comparison of multivariate classifiers and response normalizations for pattern-information fMRI. Neuroimage. 2010;53(1):103–118. doi: 10.1016/j.neuroimage.2010.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin S., Schapira A.H.V. Pathogenic Mechanisms of Neurodegeneration in Parkinson Disease. Neurol. Clin. 2015;33(1):1–17. doi: 10.1016/j.ncl.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J.D., Madsen K.H., Puonti O., Siebner H.R., Bauer C., Madsen C.G., Saturnino G.B., Thielscher A. Automatic skull segmentation from MR images for realistic volume conductor models of the head: Assessment of the state-of-the-art. Neuroimage. 2018;174:587–598. doi: 10.1016/j.neuroimage.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink K.T.E., Hillebrand A., Stoffers D., Deijen J.B., Twisk J.W.R., Stam C.J., Berendse H.W. Disrupted brain network topology in Parkinson’s disease: a longitudinal magnetoencephalography study. Brain. 2014;137(1):197–207. doi: 10.1093/brain/awt316. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink K.T.E., Stoffers D., Deijen J.B., Twisk J.W.R., Stam C.J., Hillebrand A., Berendse H.W. Resting-state functional connectivity as a marker of disease progression in Parkinson's disease: A longitudinal MEG study. NeuroImage: Clinical. 2013;2:612–619. doi: 10.1016/j.nicl.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., Dubourg V. Scikit-learn: machine learning in python. J. Mach. Learn. Res. 2011;12(Oct):2825–2830. [Google Scholar]
- Péran P., Cardebat D., Cherubini A., Piras F., Luccichenti G., Peppe A., Caltagirone C., Rascol O., Démonet J.-F., Sabatini U. Object naming and action-verb generation in Parkinson's disease: a fMRI study. Cortex. 2009;45(8):960–971. doi: 10.1016/j.cortex.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Péran P., Nemmi F., Méligne D., Cardebat D., Peppe A., Rascol O., Caltagirone C., Demonet J.F., Sabatini U. Effect of levodopa on both verbal and motor representations of action in Parkinson's disease: a fMRI study. Brain Lang. 2013;125(3):324–329. doi: 10.1016/j.bandl.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Prodoehl J., Burciu R.G., Vaillancourt D.E. Resting state functional magnetic resonance imaging in Parkinson's disease. Curr. Neurol. Neurosci. Rep. 2014;14(6):448. doi: 10.1007/s11910-014-0448-6. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F., Assadollahi R. Grammar or serial order?: discrete combinatorial brain mechanisms reflected by the syntactic mismatch negativity. J. Cogn. Neurosci. 2007;19(6):971–980. doi: 10.1162/jocn.2007.19.6.971. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F., Shtyrov Y. Automatic processing of grammar in the human brain as revealed by the mismatch negativity. Neuroimage. 2003;20(1):159–172. doi: 10.1016/s1053-8119(03)00261-1. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F., Shtyrov Y. Language outside the focus of attention: the mismatch negativity as a tool for studying higher cognitive processes. Prog. Neurobiol. 2006;79(1):49–71. doi: 10.1016/j.pneurobio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Putcha D., Ross R.S., Cronin-Golomb A., Janes A.C., Stern C.E. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson's disease. Neuroimage Clin. 2015;7:449–455. doi: 10.1016/j.nicl.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Ferreiro J., Menéndez M., Ribacoba R., Cuetos F. Action naming is impaired in Parkinson disease patients. Neuropsychologia. 2009;47(14):3271–3274. doi: 10.1016/j.neuropsychologia.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz M.C., Jahanshahi M., Krack P., Litvan I., Macias R., Bezard E., Obeso J.A. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8(12):1128–1139. doi: 10.1016/s1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Rowe J., Stephan K.E., Friston K., Frackowiak R., Lees A., Passingham R. Attention to action in Parkinson’s disease: impaired effective connectivity among frontal cortical regions. Brain. 2002;125(2):276–289. doi: 10.1093/brain/awf036. [DOI] [PubMed] [Google Scholar]
- Saur D., Kreher B.W., Schnell S., Kümmerer D., Kellmeyer P., Vry M.-S., Umarova R., Musso M., Glauche V., Abel S., Huber W., Rijntjes M., Hennig J., Weiller C. Ventral and dorsal pathways for language. PNAS. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D., Schelter B., Schnell S., Kratochvil D., Küpper H., Kellmeyer P., Kümmerer D., Klöppel S., Glauche V., Lange R., Mader W., Feess D., Timmer J., Weiller C. Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. Neuroimage. 2010;49(4):3187–3197. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Shtyrov Y., Pulvermüller F., Näätänen R., Ilmoniemi R.J. Grammar processing outside the focus of attention: an MEG study. J. Cogn. Neurosci. 2003;15(8):1195–1206. doi: 10.1162/089892903322598148. [DOI] [PubMed] [Google Scholar]
- Tahmasian M., Bettray L.M., van Eimeren T., Drzezga A., Timmermann L., Eickhoff C.R., Eickhoff S.B., Eggers C. A systematic review on the applications of resting-state fMRI in Parkinson's disease: Does dopamine replacement therapy play a role? Cortex. 2015;73:80–105. doi: 10.1016/j.cortex.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Tahmasian M., Eickhoff S.B., Giehl K., Schwartz F., Herz D.M., Drzezga A., van Eimeren T., Laird A.R., Fox P.T., Khazaie H., Zarei M., Eggers C., Eickhoff C.R. Resting-state functional reorganization in Parkinson's disease: An activation likelihood estimation meta-analysis. Cortex. 2017;92:119–138. doi: 10.1016/j.cortex.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S., Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006;51(7):1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Tekin S., Cummings J.L. Frontal–subcortical neuronal circuits and clinical neuropsychiatry: An update. J. Psychosom. Res. 2002;53(2):647–654. doi: 10.1016/S0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Teramoto H., Morita A., Ninomiya S., Akimoto T., Shiota H., Kamei S. Relation between resting state front-parietal EEG coherence and executive function in Parkinson's disease. Biomed Res. Int. 2016;2016:1–6. doi: 10.1155/2016/2845754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A., Esposito F., Vitale C., Santangelo G., Amboni M., Russo A., Corbo D., Cirillo G., Barone P., Tedeschi G. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79(23):2226–2232. doi: 10.1212/WNL.0b013e31827689d6. [DOI] [PubMed] [Google Scholar]
- Tomasello R., Garagnani M., Wennekers T., Pulvermüller F. Brain connections of words, perceptions and actions: a neurobiological model of spatio-temporal semantic activation in the human cortex. Neuropsychologia. 2017;98:111–129. doi: 10.1016/j.neuropsychologia.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Ullman M.T. A neurocognitive perspective on language: the declarative/procedural model. Nat. Rev. Neurosci. 2001;2(10):717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Ullman M.T. Contributions of memory circuits to language: the declarative/procedural model. Cognition. 2004;92(1):231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman M.T. In: Handbook of the Neuroscience of Language. Stemmer B., Whitaker H.A., editors. Elsevier; San Diego: 2008. CHAPTER 18 – the role of memory systems in disorders of language; pp. 189–198. [Google Scholar]
- Van Essen D.C. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Varoquaux G., Raamana P.R., Engemann D.A., Hoyos-Idrobo A., Schwartz Y., Thirion B. Assessing and tuning brain decoders: cross-validation, caveats, and guidelines. Neuroimage. 2017;145:166–179. doi: 10.1016/j.neuroimage.2016.10.038. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley L.A., Luk K.C., Lee V.M.Y. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite–like aggregates. Nat. Protoc. 2014;9(9):2135–2146. doi: 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley L., Luk K., Patel T., Tanik S., Riddle D., Stieber A., Meaney D., Trojanowski J., Lee V.-Y. Exogenous α-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. fourth ed. Pearson; San Antonio, TX: 2008. Wechsler Adult Intelligence Scale. [Google Scholar]
- Wilson S., Galantucci S., Tartaglia M., Rising K., Patterson D., Henry M., Ogar J., DeLeon J., Miller B., Gorno-Tempini M. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72(2):397–403. doi: 10.1016/j.neuron.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Long X., Wang L., Hallett M., Zang Y., Li K., Chan P. Functional connectivity of cortical motor areas in the resting state in Parkinson's disease. Hum. Brain Mapp. 2011;32(9):1443–1457. doi: 10.1002/hbm.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.