Abstract
Objectives
Due to the high interindividual variability in vancomycin pharmacokinetics, optimisation of its dosing is still challenging. This study aimed to explore vancomycin pharmacokinetics in adult patients and to propose an easy applicable dosing nomogram for initial treatment.
Methods
Vancomycin pharmacokinetics was calculated in a two-compartmental model based on therapeutic drug monitoring data. A linear regression model was used to explore the relationship between vancomycin elimination half-life and glomerular filtration rate estimated according the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Results
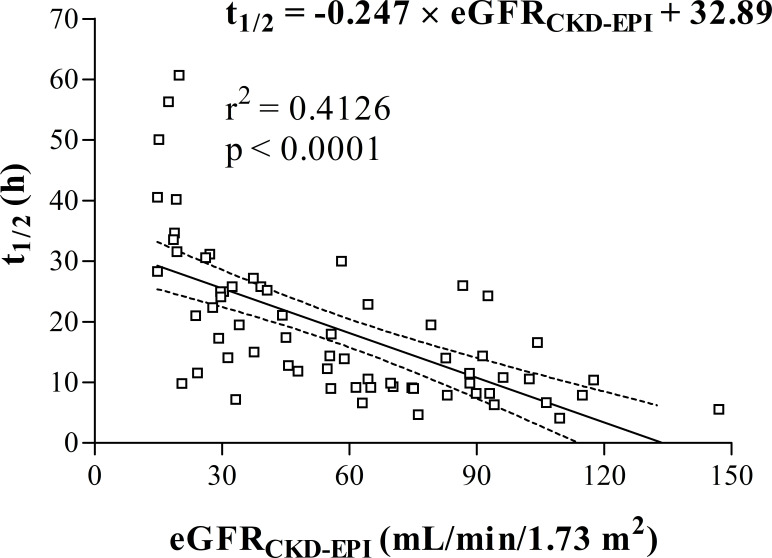
In the whole study population (n=66), vancomycin volume of distribution, clearance and half-life median (IQR) values were 0.69 (0.58–0.87) L/kg, 0.031 (0.022–0.050) L/h/kg and 14.4 (9.5–25.2) hours, respectively. Vancomycin half-life was associated with glomerular filtration rate (r2=0.4126, p<0.0001) according to the formula: t1/2 (h) = −0.247×eGFRCKD-EPI (mL/min/1.73 m2)+32.89. This relationship was used to construct a dosing nomogram.
Conclusions
We propose an easy-to-use dosing nomogram for vancomycin therapy initiation that allows individualisation of the dosing interval with respect to the administered dose size and functional renal status.
Keywords: vancomycin, pharmacokinetics, dosing nomogram, therapeutic drug monitoring, glomerular filtration, CKD-EPI
Introduction
Vancomycin, a glycopeptide antibiotic, is widely used for treating serious, nosocomial infections caused by gram-positive bacteria involving methicillin-resistant Staphylococcus aureus.1 Vancomycin exhibits time-dependent bactericidal activity against sensitive bacteria, while the ratio of the 24-hour area under the concentration–time curve to the minimal inhibitory concentration is considered the most adequate measure to predict the clinical and bacteriological outcomes of vancomycin treatment.2 In order to guard the clinical efficacy of vancomycin and prevent its toxicity, therapeutic drug monitoring (TDM) is recommended and routinely conducted in many centres around the world.1 3 It is recommended that trough serum vancomycin concentrations always be maintained above 10 mg/L to avoid development of resistance, while trough concentrations of 15–20 mg/L are recommended to improve penetration, increase the probability of obtaining optimal target serum concentrations and improve clinical outcomes in case of complicated infections (bacteraemia, endocarditis, osteomyelitis, meningitis, and hospital acquired pneumonia caused by S. aureus).1 However, since it is difficult to obtain multiple vancomycin plasma concentrations in routine clinical situations, TDM is typically carried out only at the time when estimated steady-state of plasma concentrations is achieved, while the initial doses must be estimated. The Summary of Product Characteristics recommends an initial vancomycin dose of 15–20 mg per kg of total body weight every 8–12 hours, which represents a relatively wide ranging daily dose of 30–60 mg/kg/day. This range may in fact be even greater due to rounding of doses according to the size of the vancomycin ampoules (multiples of 500 mg) in clinical routines. Whereas the majority of vancomycin dose is excreted unchanged by glomerular filtration,4 it is evident that the maintenance doses should be individualised according to the renal functional status. For example, it is unlikely that vancomycin 15 mg/kg every 12 hours for patients with a normal body weight and normal renal function will produce trough concentrations of 15–20 mg/L. The commonly used initial dosage regimen of 1 g every 12 hours thus often results in subtherapeutic trough concentrations.
The aim of this study is to propose an easy applicable dosing nomogram that would allow individualisation of the dosing interval with respect to administered dose size and functional renal status in the course of initial vancomycin therapy before TDM introduction.
Methods
Study design
An observational study was performed in adult patients treated with vancomycin intermittent infusion admitted to mixed wards of the General University Hospital, Charles University First Faculty of Medicine in Prague from January 2016 to December 2018. Patients meeting the following criteria were included: age ≥18 years, not receiving dialysis, receiving vancomycin for at least 3 days, and having at least two measured vancomycin serum concentrations in the course of therapy.
Since the study involved only analysis of routine clinical data, and at admission to the hospital all patients sign an approved general informed consent wherein they state, inter alia, that anonymous data can be used for research, study-specific ethics approval was waived.
Data retrieval
Clinical records of all evaluated patients were reviewed to collect information concerning gender, age, body weight, height, serial creatinine and vancomycin serum concentrations (sampling times included), and vancomycin dosing and administration times.
Creatinine values were measured using the Jaffe photometric method without deproteinisation on a Modular analyser (Roche Diagnostics, Basel, Switzerland), while vancomycin serum concentrations were measured by a turbidimetric inhibition immunoassay (Beckman Coulter, Brea, California, USA).
The glomerular filtration rate (GFR) was estimated for each patient according to the Chronic Kidney Disease Epidemiology Collaboration formula (eGFRCKD-EPI).5
Pharmacokinetic analysis
Individual vancomycin pharmacokinetic parameters—volume of distribution (Vd), clearance (CL) and elimination half-life (t1/2)—were calculated in a two-compartmental pharmacokinetic model with first-order elimination kinetics based on individual demographic and clinical data and observed vancomycin serum concentrations using MWPharm++ software (MediWare, Prague, Czech Republic). The vancomycin population pharmacokinetic model was individualised to maximise fitting of the simulated pharmacokinetic profile curve with observed concentration points in each patient. The fitting was performed using the Bayesian method.
Statistical analysis
Descriptive parameters—mean, SD, median, and IQR—were calculated using MS Excel 2010 (Microsoft Corporation, Redmond, USA). The χ2 test was used to compare categorical variables, and the linear regression model was used to evaluate the relationship between vancomycin elimination half-life and eGFRCKD-EPI using GraphPad Prism 3.02 (GraphPad Software, Inc, La Jolla, USA).
Initial dosing calculation
Dosing intervals appropriate to weight-normalised doses were derived from the following formula: t1/2 = ln2×(t2–t1)÷(lnC1–lnC2), where (t2–t1) represents the dosing interval of 8 and 12 hours, respectively, C2 represents vancomycin trough concentration of 10 and 20 mg/L, respectively (recommended range for vancomycin trough concentrations), C1 represents vancomycin peak concentration, which was calculated as dose (mg/kg)×median vancomycin Vd (0.69 L/kg)+15 mg/L (as a midpoint of recommended vancomycin steady-state trough levels), and t1/2 was replaced with eGFRCKD-EPI based on the observed relationship t1/2 (h) = –0.247×eGFRCKD-EPI (mL/min/1.73 m2)+32.89 (see statistical analysis and results).
Results
Sixty-six patients were enrolled into the study (44 males, 22 females). Their demographic and clinical characteristics are summarised in table 1. Vancomycin was administered as an intermittent intravenous infusion. Initial daily doses ranged from 8.5 to 42.9 mg per kg of body weight. The dosing regimen was 500, 1000 or 1500 mg every 8, 12 or 24 hours.
Table 1.
Study population (n=66)
| Median | IQR | Min–max | |
| Age (years) | 72 | 63–75 | 24–90 |
| Body weight (kg) | 79 | 70–94 | 55–133 |
| Height (cm) | 174 | 166–177 | 151–207 |
| Serum creatinine (µmol/L) | 113 | 78–178 | 38–369 |
| eGFRCKD-EPI (mL/min/1.73 m2) | 55.5 | 29.6–82.9 | 14.8–147.0 |
eGFRCKD-EPI is the glomerular filtration rate estimated according to CKD-EPI formula.
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate.
In total, 218 vancomycin serum concentrations for pharmacokinetic analysis were obtained (2–12 concentration points per patient; median=3). There were 163 concentration points taken as trough values, while 55 samples were taken as peak values. Subtherapeutic vancomycin serum trough concentrations were observed in 23 (35%) patients, while in 11 (17%) cases trough values exceeded the recommended range (10–20 mg/L). Vancomycin pharmacokinetic parameters are summarised in table 2.
Table 2.
Vancomycin pharmacokinetic data
| Vd (L) | Vd (L/kg) | CL (L/h) | CL (L/h/kg) | t1/2 (hours) | |
| Mean | 58.6 | 0.73 | 3.03 | 0.037 | 18.8 |
| SD | 15.0 | 0.19 | 1.78 | 0.022 | 12.2 |
| CV (%) | 26 | 26 | 59 | 58 | 65 |
| Median | 58.2 | 0.69 | 2.72 | 0.031 | 14.4 |
| IQR | 48.4–66.6 | 0.58–0.87 | 1.64–4.19 | 0.022–0.050 | 9.5–25.2 |
| Min–max | 35.0–104.4 | 0.37–1.19 | 0.40–9.83 | 0.007–0.110 | 4.1–60.7 |
CL, vancomycin clearance; Vd, vancomycin volume of distribution; t1/2, vancomycin elimination half-life.
The linear regression model showed that vancomycin elimination half-life was significantly related to estimated GFR (r2=0.4126, p<0.0001; figure 1). This relationship was described by the following equation: t1/2 (h) = −0.247×eGFRCKD-EPI (mL/min/1.73 m2)+32.89.
Figure 1.
Regression analysis of vancomycin elimination half-life (t1/2) and glomerular filtration rate estimated according to CKD-EPI formula (eGFRCKD-EPI). Dashed lines represent 95% CI. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate.
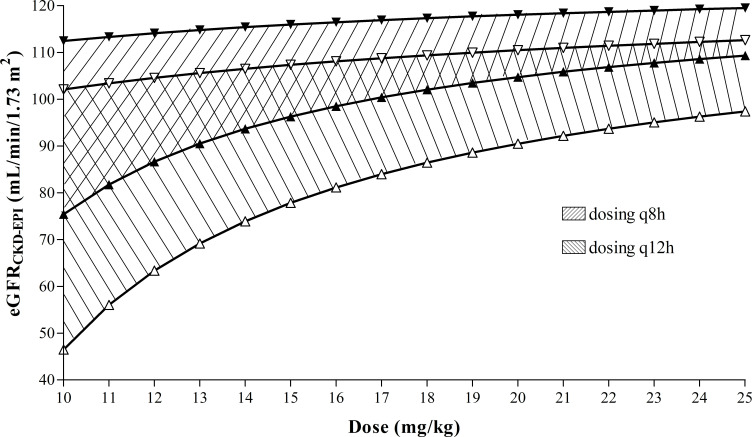
This relation was used for construction of an easy-to-use nomogram, which makes it possible to choose the optimal dosing interval for maintaining vancomycin trough concentrations in the therapeutic range (10–20 mg/L) in relation to the administered vancomycin dose size and functional renal status (figure 2).
Figure 2.
Nomogram for choosing the optimal dosing interval with respect to administered vancomycin dose size and patient functional renal status to achieve a target vancomycin steady-state trough concentration of 10–20 mg/L. Filled triangle symbols delimit zone for 12-hour dosing interval (q12h)l. Empty triangle symbols delimit zone for 8-hour dosing interval (q8h). Triangle symbols with upward vertices represent upper limit for vancomycin trough concentration (20 mg/L). Triangle symbols with downward vertices represent lower limit for vancomycin trough concentration (10 mg/L).
Simulation showed improved target vancomycin concentration attainment rate (p=0.05) when the truly administered doses were applied at dosing intervals recommended by the nomogram using each individual item of pharmacokinetic data; in 45 (68%) patients vancomycin concentrations would reach the trough target range (10–20 mg/L), while in nine (14%) or 12 (18%) patients the concentrations would remain above or under the target, respectively.
Discussion
Despite vancomycin becoming available for clinical use >50 years ago,6 optimisation of its dosing is still challenging; this is due to its narrow therapeutic range combined with high inter-individual pharmacokinetic variability, which has also been observed in our study with a coefficient of variation for t1/2 of 65%. Therefore, we tried to develop a simple tool to optimise initial vancomycin dosing in order to increase the attainment rate of vancomycin trough concentrations in the therapeutic range even before TDM introduction.
The nomogram shown in figure 2 makes it possible to estimate the optimal dosing interval with respect to the applied vancomycin dose size and renal function status. There are two hatched zones in the nomogram representing the 8-hour (area between empty triangle symbols) and 12-hour (area between filled triangle symbols) dosing interval. The triangle symbols with upward vertices represent the upper limit for the vancomycin trough concentration (20 mg/L), while the triangle symbols with downward vertices represent the lower limit for the vancomycin trough concentration (10 mg/L). Therefore, in the treatment of serious infections, it is advisable to follow the lower areas of the hatched zones. A 6-hour dosing interval should be considered in patients with an estimated GFR >110 mL/min/1.73 m2 (eg, patients with augmented renal clearance), while in patients with renal insufficiency, the dosing interval can be extended to 24 hours.
The use of the nomogram in the simulation resulted in a reduction of underdosed patients (to almost half), which we considered a satisfactory outcome for initial vancomycin therapy.
The main limitation of this study is that the accuracy of our nomogram was not validated prospectively. However, our study was based on objective pharmacokinetic data derived from demographic and clinical data recorded in the Hospital Information System. Therefore, the retrospective nature should not affect the validity of the results. However, the results of this exploratory study need to be confirmed in a prospectively conducted trial.
Measured creatinine clearance is unavailable in most patients at the time of initiation of vancomycin treatment, therefore calculated creatinine clearance was used to estimate the vancomycin maintenance doses.7 A nomogram based on serum creatinine concentrations has already been published previously, although it was based on the Cockcroft–Gault estimation for GFR estimation.8 The proposed nomogram in our study is based on the CKD-EPI equation, which is an up to date method for estimating GFR, whose superiority over the previous estimations for GFR has been described previously.9 10
Bayesian pharmacokinetic simulation is currently considered the gold standard for TDM. In this study we used a two-compartmental vancomycin population pharmacokinetic model with good predictive performance, as previously evaluated.11
We focused only on maintenance dosing in this study; however, in order to achieve a rapid attainment of the target concentration for seriously ill patients, a loading dose administration can be considered.12 13
Conclusion
We propose an easy-to-use dosing nomogram for vancomycin therapy initiation that allows individualisation of the dosing interval with respect to the administered dose size and functional renal status. However, due to the wide interindividual variability of vancomycin pharmacokinetics, this algorithm can be used only for the initiation of therapy, while further dosing still needs to be guided using the TDM.
What this paper adds.
What is already known on this subject
Summary of Product Characteristics recommends an initial vancomycin dose of 15–20 mg per kg of total body weight every 8–12 hours, which represents a relatively wide range of daily dose.
Whereas vancomycin is excreted unchanged by glomerular filtration, it is evident that the maintenance doses should be individualised according to the renal functional status.
What this study adds
We propose a dosing nomogram for vancomycin therapy initiation that allows individualisation of the dosing interval with respect to the administered dose size and functional renal status.
The nomogram is based on the CKD-EPI equation, which is an up to date method for estimating the glomerular filtration rate.
Footnotes
Contributors: All co-authors have contributed significantly to the publication and have agreed to the submission of the final manuscript.
Funding: The work was supported by the Charles University Project Progres Q25 and a grant No. SVV 260373.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults: summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2009;29:1275–9. [DOI] [PubMed] [Google Scholar]
- 2.Moise-Broder PA, Forrest A, Birmingham MC, et al. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004;43:925–42. 10.2165/00003088-200443130-00005 [DOI] [PubMed] [Google Scholar]
- 3.ZK Y, Tang HL, Zhai SD. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PLoS One 2013;8:e77169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matzke GR, Zhanel GG, Guay DRP. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet 1986;11:257–82. 10.2165/00003088-198611040-00001 [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine DP. Vancomycin: a history. Clin Infect Dis 2006;42(Suppl 1):S5–S12. 10.1086/491709 [DOI] [PubMed] [Google Scholar]
- 7.Pletz MW, Lipman J. Clinical measures for increased creatinine clearances and suboptimal antibiotic dosing. Intensive Care Med 2013;39:1322–4. 10.1007/s00134-013-2918-8 [DOI] [PubMed] [Google Scholar]
- 8.Thalakada R, Legal M, Lau TTY, et al. Development and validation of a novel vancomycin dosing nomogram for achieving high-target trough levels at 2 Canadian teaching hospitals. Can J Hosp Pharm 2012;65:180–7. 10.4212/cjhp.v65i3.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conil JM, Georges B, Breden A, et al. Estimation of glomerular filtration rate to adjust vancomycin dosage in critically ill patients: superiority of the chronic kidney disease epidemiology collaboration equation? Anaesth Intensive Care 2014;42:178–84. 10.1177/0310057X1404200203 [DOI] [PubMed] [Google Scholar]
- 10.Šíma M, Hartinger J, Štenglová Netíková I, et al. Creatinine clearance estimations for vancomycin maintenance dose adjustments. Am J Ther 2018;25:e602–4. 10.1097/MJT.0000000000000616 [DOI] [PubMed] [Google Scholar]
- 11.Sima M, Bakhouche H, Hartinger J, et al. Therapeutic drug monitoring of antibiotic agents: evaluation of predictive performance. Eur J Hosp Pharm 2019;26:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Šíma M, Hartinger J, Cikánková T, et al. Importance of vancomycin loading doses in intermittent infusion regimens. J Infect Chemother 2018;24:247–50. 10.1016/j.jiac.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 13.Šíma M, Hronová K, Hartinger J, et al. A simulation of loading doses for vancomycin continuous infusion regimens in intensive care. Infect Dis 2017;49:674–9. 10.1080/23744235.2017.1328741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.