Abstract
Background/purpose
Previous studies have shown that miR-874 is considered to be an important regulatory factor that participated in osteoclast differentiation. The role of miR-874-3p on osteoclast differentiation of human periodontal ligament fibroblast(hPDLF), however, is still unclear. This study was aimed to delve into the related molecular mechanism of miR-874-3p on hPDLF osteoclast differentiation.
Materials and methods
The qRT-PCR assays were applied to check miR-874-3p and WNT3A expression levels during the osteoclast differentiation of hPDLF. Alkaline phosphatase (ALP) activity assays and alizarin red staining assays were applied to appraise the degree of hPDLF osteoclast differentiation. Bioinformatics method and dual-luciferase reporter assay were employed together to anticipate and certify the interaction between miR-874-3p and WNT3A. Western blot assay was applied to examine the β-catenin and WNT3A expression in transfected hPDLF.
Results
In this study, the results indicated that the expression level of miR-874-3p was gradually down-regulated while WNT3A was concomitantly increased during osteogenic differentiation of hPDLF. Overexpression or knockdown of miR-874-3p would inhibit or promote WNT3A and β-catenin protein expression as well as osteogenic differentiation of hPDLF, respectively. Further research indicated that miR-874-3p directly regulated WNT3A expression via coupling with the 3′-UTR of WNT3A. Finally, upregulation of WNT3A expression levels rescues β-catenin expression levels and osteogenic differentiation of hPDLF inhibited by miR-874-3p was explored.
Conclusion
MiR-874-3p inhibits osteogenic differentiation of hPDLF through regulating Wnt/β-catenin pathway.
Keywords: MiR-874-3p, WNT3A, β-Catenin, hPDLF, Osteoclast differentiation
Introduction
The human periodontal ligament (PDL) is a dense connective tissue between the tooth root and alveolar bone. It has the function of fixing the tooth root and relieving the pressure generated during chewing.1 PDL is composed of different cell types, including cementoblasts, osteoblasts, endothelial cells, and fibroblasts.2 Fibroblasts are one of the most indispensable cell types in periodontal tissues and they play a crucial role in physiological function and pathological changes.3 Fibroblasts in PDL are reported to be the source of osteoblasts needed for sustaining alveolar bone reconstruction. There have developed multiple methods for periodontal regeneration by using cells extracted from PDL, and their potential in regulating osteoblasts and osteoclasts differentiation in periodontal tissue has been demonstrated.4 However, the molecular mechanism of osteoblastic differentiation of PDL fibroblasts has not been deciphered.
MicroRNAs (miRNAs) are small non-coding RNAs that may regulate thousands of genes.5 MicroRNAs mainly bind to the 3′ untranslated region of the target mRNA, and translation inhibition or degradation of the mRNA is caused.6 Studies have shown that miRNAs play a crucial role in the differentiation of periodontal cells. For example, miR-195-5p was reported to regulate osteogenic differentiation of periodontal ligament cells under mechanical loading.7 MiR-146a is capable of inducing the differentiation of periodontal ligament cells.8 MiR-214 promotes the differentiation of periodontal ligament stem cells into osteoblasts by Wnt/β-catenin pathway.9 The expression levels of miR-874-3p were reported to be decreased during osteogenic differentiation.6 MiR-874 can promote the proliferation and differentiation of osteoblasts in osteoporotic rats by inhibition of SUFU and activation of Hedgehog pathway.10 Nevertheless, other studies have shown that inhibition of miR-874-3P promotes the osteogenic differentiation of human periodontal ligament stromal cells.11 However, the role of miR-874-3p in the osteogenic differentiation of PDL fibroblasts remains to be further studied.
This study showed that the expression levels of miR-874-3p were decreased in differentiated human periodontal fibroblasts, and the upregulated miR-874-3p inhibited the differentiation of periodontal fibroblasts, while the results of miR-874-3p knockdown showed opposite results. Meanwhile, miR-884-3p was found to be able to regulate WNT3A expression in a targeted manner, thereby inhibiting the activity of Wnt/β-catenin pathway and reducing the differentiation of periodontal membrane fibroblasts.
Materials and methods
Cell culture and osteogenic differentiation
The human periodontal ligament fibroblast (hPDLF) was purchased from Lonza (Lonza, Basel, Switzerland). The cells were resuspended in culture medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco), 1% penicillin/streptomycin (Gibco), and 0.025 μg/mL amphotericin B (Sigma–Aldrich, St. Louis, MO, USA). The cells were cultured in a humidified 5% CO2 incubator at 37 °C. When the hPDLF reached 85% confluence, 50 μg/mL ascorbic acid (Sigma–Aldrich), 10 mM β-glycerophosphate (Sigma–Aldrich), and 100 nM dexamethasone (Sigma–Aldrich) were added in the culture medium to induce osteogenic differentiation.
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was extracted from collected cells by using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). The quantity and integrity of extracted total RNA were evaluated on a Nano Drop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Whole RNA was reversely transcribed into cDNA by using miRNA-specific TaqMan miRNA assay kit (Applied Biosystems, Foster City, CA, USA). qRT-PCR was applied by the SYBR Premix EX Taq (Takara, Beijing, China) in the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) to detect the expression levels of miR-874-3p. The PCR primers were designed and synthesized by Sangon Biological Engineering Technology (Sangon, Shanghai, China). U6 small nuclear RNA and β-actin were used as the endogenous reference genes to normalize miRNA and mRNA expression levels, respectively. The relative expression of miR-874-3p and WNT3A in each experimental group was analyzed using the 2-△△Ct method. All reactions were performed in triplicates. Primer sequences used in this study are shown in Table 1.
Table 1.
Primers for miR-874-3p, WNT3A, and reference genes.
| Gene | Primer | Sequence(5'→3') |
|---|---|---|
| miR-874-3p | Forward | GAACTCCACTGTAGCAGAGATGGT |
| Reverse | CATTTTTTCCACTCCTCTTCTCTC | |
| U6 | Forward | TCCTCCACGACAACCAAAACC |
| Reverse | TCTTTTCCCAAAATCCCAGACTC | |
| WNT3A | Forward | ATCGAGTTTGGTGGGATGGT |
| Reverse | CGCTGTCGTACTTGTCCTTG | |
| wt-WNT3A 3′UTR | Forward | TAGGCGATCGCTCGAGGCACCGGCCGCGGCTCCCC |
| Reverse | AATTCCCGGGCTCGAGTTTCGTCTAACTCCGTTGGACAGT | |
| mut-WNT3A 3′UTR | Forward | AATGGTCCGCTTTCCTGGAGCCAATGGCCCG |
| Reverse | AGGAAAGCGGACCATTTCCCGCCATGAGGGGCCAGGAAGG | |
| β-actin | Forward | GTGACGTTGACATCCGTAAAGA |
| Reverse | GCCGGACTCATCGTACTCC |
Western blot
Briefly, cells were washed in pre-cooling PBS buffer three times, and the total protein was separated by RIPA buffer (Beyotime, Shanghai, China). Protein concentration was detected by using BCA protein assay kits (CoWin Biotechnology, Cambridge, MA, USA). An equal amount of total proteins was electrophoresed to SDS-PAGE. Then, they were transferred to the polyvinylidene difluoride membranes (PVDF; Millipore, Boston, MA, USA) with blocked by 5% non-fat milk at room temperature for 1 h. The protein was identified by incubated with specific primary antibodies WNT3A (Rabbit Anti- WNT3A antibody, ab231178, 1:3000; Abcam, Cambridge, MA, USA), β-catenin (Rabbit Anti-beta Catenin antibody, ab6302, 1:3000; Abcam) and β-actin (Rabbit Anti-beta Actin antibody, ab8227, 1:3000; Abcam) overnight at 4 °C. Then, the membranes were further incubated with HRP-conjugated goat anti-rabbit immunoglobulin G secondary antibody (ab205718, 1:1500; Abcam) and the bands on the membranes were visualized by the ECL chemiluminescence reagent (Beyotime). The β-actin was used to normalize the amount of the analyzed samples and protein bands were quantified by gray value analysis by ImageJ software.
Cell transfection
The synthetic miR-874-3p mimics, miR-874-3p inhibitor, miR-control (NC), and miR-control (NC) inhibitor were acquired from GenePharma (GenePharma, Shanghai, China). After cells were cultured in 6-well plates for 24 h, miR-874-3p mimics, miR-874-3p inhibitor, miR-control (NC), and miR-control (NC) inhibitor were transfected into cells by Lipofectamine 2000 (Invitrogen).
Alkaline phosphatase (ALP) activity assays
Alkaline phosphatase enzyme activity was tested with the use of an ALP assay kit (Jiancheng Biotech, Nanjing, China). Briefly, the treated cells were washed twice with pre-cooling PBS and lysed with RIPA buffer (Beyotime). The lysate was incubated at 37 °C for 20 min, and the ALP enzymatic activity was assayed at 405 nm with the use of a spectrophotometer. The relative ALP activity was normalized to the protein content which was determined by using a BCA assay kit (Beyotime).
Alizarin red staining
The matrix mineralization deposition was detected by alizarin red staining (Leagene, Beijing, China). In brief, the cells were fixed with 4% paraformaldehyde for 30 min and rinsed with deionized water. Then, the cells were stained with 0.1% (w/v) Alizarin red for 15 min at pH 4.2. The matrix mineralization deposition appeared red and the stained cells were photographed after washing with deionized water for 15 min. The Alizarin red was desorbed with 10% cetylpyridinium chloride 15 min and measured by spectrophotometry at 540 nm with the use of a spectrophotometer. The staining results were normalized to the protein content which was determined by using a BCA assay kit (Beyotime).
Dual-luciferase reporter assay
The fragments of 3′-UTRs of WNT3A mRNA containing the wild-type (WT) and the mutant-type (MUT) binding sites of miR-874-3p anticipated by using TargetScan were cloned into pmirGLO luciferase report vector (Promega, Madison, WI, USA). The constructed luciferase reporters were called as WNT3A-WT and WNT3A-MUT, respectively. For luciferase assay, miR-874-3p inhibitor or miR-control (NC) were co-transfected with reporter plasmids into cells by Lipofectamine 2000 (Invitrogen). The luciferase activities were tested by the dual-luciferase kit (Promega) after 48 h transfection. Data are presented as the ratio of experimental (Renilla) luciferase to control (Firefly) luciferase.
Statistical analysis
All data are shown as the mean ± standard error of the mean from three independent experiments. GraphPad Prism 5 was used for analysis. Comparisons between two groups were performed using Student's t-test. P values of <0.01 (two-tailed) were considered to indicate a statistically significant difference.
Results
Expression level of miR-874-3p and WNT3A during osteogenic differentiation of hPDLF
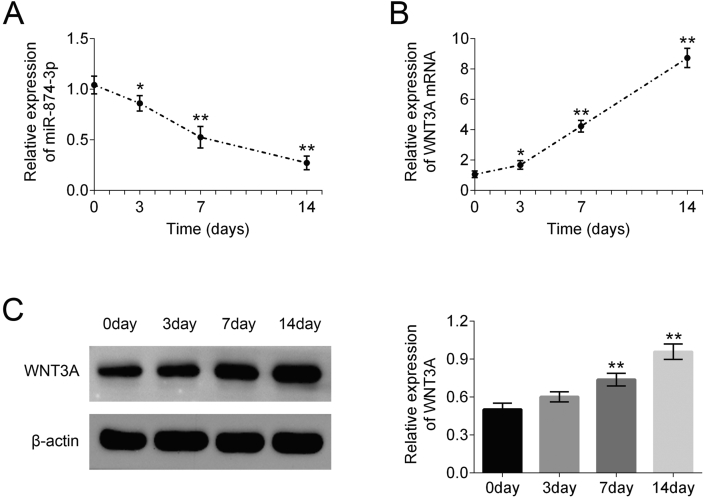
To better explore the role of miR-874-3p and WNT3A during in regulating the osteogenic differentiation potential of hPDLF, mRNA expression levels of miR-874-3p and WNT3A were first tested during osteogenic differentiation of hPDLF on the day 0, 3, 7, 14, respectively. The relative expression profile of miR-874-3p and WNT3A was monitored with the use of qRT PCR by the standard osteogenic differentiation method previously described. From this study, the expression level of miR-874-3p was gradually down-regulated after the induction of osteogenic differentiation of hPDLF (Fig. 1A). Meanwhile, the expression level of WNT3A was concomitantly increased (Fig. 1B). Furthermore, the mRNA protein expression levels of WNT3A were explored during osteogenic differentiation of hPDLF on the day 0, 3, 7, 14, respectively. The result demonstrated that the expression level of WNT3A protein decreased gradually with the osteogenic differentiation of hPDLF (Fig. 1C and D). These results indicate that the expression level of miR-874-3p was down-regulated and WNT3A was up-regulated during osteogenic differentiation of hPDLF.
Figure 1.
Expression level of miR-874-3p and WNT3A during osteogenic differentiation of hPDLF. (A) qRT-PCR analysis of miR-874-3p normalized to U6 at different days during osteogenic differentiation of hPDLF. (B) qRT-PCR analysis of WNT3A normalized to β-actin at different days during osteogenic differentiation of hPDLF. (C) Western blot analysis of protein levels of WNT3A normalized to β-actin at different days during osteogenic differentiation of hPDLF. ∗∗p < 0.01 versus control group and ##p < 0.01 versus LPS alone group. Data are presented as mean ± SD.
MiR-874-3p inhibits osteogenic differentiation of hPDLF
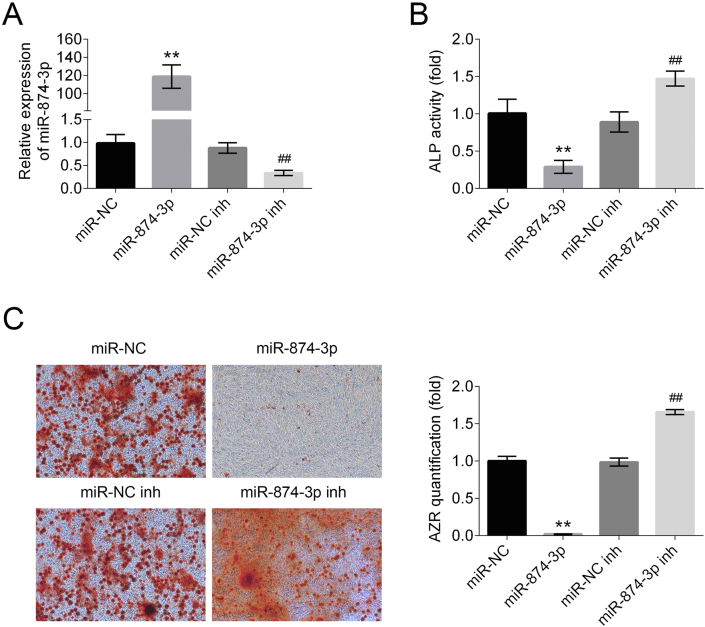
MiR-874-3p mimics, miR-874-3p inhibitor, miR-NC, and miR-NC inhibitor were synthesized and transfected into hPDLF as previously described to promote or inhibit miR-874-3p expression. The hPDLF were induced to undergo osteogenic differentiation for seven days after transfection. The expression levels of miR-874-3p in four kinds of groups were identified through qRT PCR (Fig. 2A). The miR-874-3p group showed obviously up-regulated miR-874-3p expression level, while that in the miR-874-3p inhibitor group was substantially down-regulated. Meanwhile, there were no significant changes in miR-874-3p expression levels in the miR-NC, and miR-NC inhibitor group. These results indicated that the transfection causes the overexpression of miR-874-3p in the miR-874-3p group, and suppresses miR-874-3p expression in the miR-874-3p inhibitor group. To clarify the effect of miR-874-3p on hPDLF osteogenic differentiation, ALP activity assays and Alizarin red staining were applied to four kinds of groups to characterize the degree of osteogenic differentiation of hPDLF. The results showed that overexpression of miR-874-3p dramatically inhibits osteoblastic differentiation, which was indicated by decreased ALP activity and matrix mineralization level in miR-874-3p overexpressing group compared to negative control group and blank control group (Fig. 2B and C). Taken together, these results indicated that miR-874-3p plays a negative role in the regulation of the osteogenic differentiation of hPDLF.
Figure 2.
MiR-874-3p inhibits osteogenic differentiation of hPDLF. (A) Expression levels of miR-874-3p in four groups of hPDLF after transfection. (B) ALP activity was measured after osteogenic differentiation for seven days. (C) Alizarin red staining was measured after osteogenic differentiation for seven days. ∗∗p < 0.01 versus control group and ##p < 0.01 versus LPS alone group. Data are presented as mean ± SD.
MiR-874-3p directly regulated WNT3A expression by binding to the 3′-UTR of WNT3A
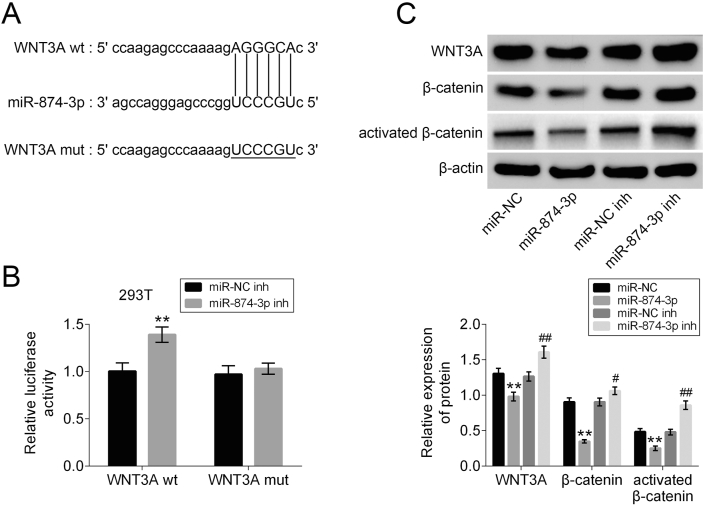
To explore the biomechanism of miR-874-3p in osteogenic differentiation of hPDLF, the mRNA binding sites were further anticipated in TargetScan (http://www.targetscan.org/vert_71/). The results indicated that WNT3A mRNA was a binding target of miR-874-3p. The anticipated 3′-UTRs of WNT3A mRNA binding to miR-874-3p were presented (Fig. 3A). In order to investigate whether WNT3A was a potential target of miR-874-3p, WNT3A WT and MUT fragments were cloned downstream of the firefly luciferase coding region. The results of this study indicated that the overexpression of miR-874-3p strikingly brought down the luciferase activity of the WNT3A-WT reporter gene, but had no effect on the WNT3A-MUT control (Fig. 3B). To further prove whether miR-874-3p regulated WNT3A expression, miR-874-3p mimics, miR-874-3p inhibitor, miR-NC, or miR-NC inhibitor were transfected into hPDLF to detect the expression levels of WNT3A, β-catenin and activated β-catenin (ABC) proteins. The Western blot assay results showed that high expression of miR-874-3p dramatically suppressed WNT3A, β-catenin and ABC protein expression compared with that in NC group. While miR-874-3p inhibitor significantly promoted WNT3A, β-catenin and ABC protein expression compared with that in NC-inh group (Fig. 3C). The data showed that WNT3A is a target of miR-874-3p and miR-874-3p represses Wnt3a and β-catenin protein expression level.
Figure 3.
MiR-874-3p directly regulated Wnt3a expression by binding to the 3′-UTR of WNT3A. (A) Forecast of miR-874-3p binding sites on target gene WNT3A by TargetScan. (B) Dual-luciferase assays were carried out after cells were co-transfected WNT3A-WT or WNT3A-MUT with miR-874-3p mimics or miR-control for 48 h. (C) The expression levels of WNT3A, β-catenin and ABC protein in cells transfected with miR-874-3p mimics, miR-874-3p inhibitor, miR-NC, or miR-NC inhibitor were determined by Western blot. β-actin was applied as the endogenous reference genes. ∗∗p < 0.01 versus control group and ##p < 0.01 versus LPS alone group. Data are presented as mean ± SD.
Upregulation of WNT3A alleviates repressed osteogenic differentiation of hPDLF promoted by miR-874-3p
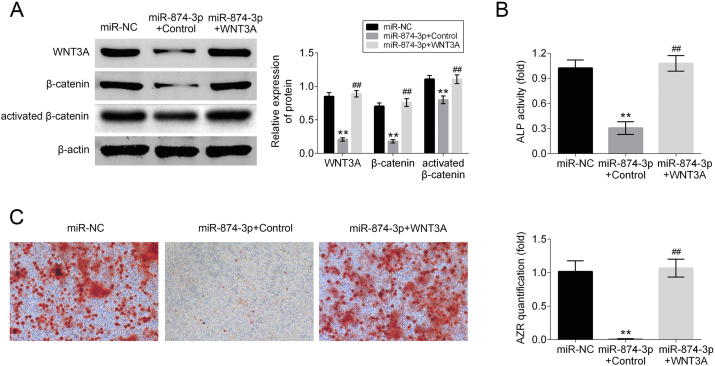
To determine whether miR-874-3p inhibits osteogenic differentiation of hPDLF by regulating WNT3A expression, a rescue experiment was performed. The hPDLF were transfected with either miR-NC or miR-874-3p mimics with either WNT3A overexpressing vector or vector control. Western blot analysis confirmed that miR-874-3p mimics can obviously suppress the expression of WNT3A, β-catenin and ABC in hPDLF while WNT3A overexpression vector can restore the expression levels of WNT3A, β-catenin and ABC proteins by strikingly promote the expression of WNT3A, β-catenin and ABC in hPDLF (Fig. 4A). To further verify whether WNT3A alleviates repressed osteogenic differentiation of hPDLF promoted by miR-874-3p, ALP activity assays and alizarin red staining were assessed in each group. The results indicated that miR-874-3p repressed osteogenic differentiation of hPDLF could be abrogated by WNT3A overexpression (Fig. 4B and C). Taken together, these results indicated that the effect of inhibition osteogenic differentiation of hPDLF is mediated by the miR-874-3p/WNT/β-catenin axis.
Figure 4.
Upregulation of WNT3A alleviates repressed osteogenic differentiation of hPDLF promoted by miR-874-3p. (A) The expression levels of WNT3A, β-catenin and ABC protein in cells transfected with either miR-NC or miR-874-3p mimics with either WNT3A overexpressing vector or vector control were determined by Western blot. β-actin was applied as the endogenous reference genes. (B) ALP activity and (C) Alizarin red staining was applied to detect the degree of osteogenic differentiation of hPDLF for each group. ∗∗p < 0.01 versus control group and ##p < 0.01 versus LPS alone group. Data are presented as mean ± SD.
Discussion
Recently, some studies have showed that hPDLF has the potential to differentiate into osteoblasts and osteoclasts in vivo.4 The osteogenic differentiation of hPDLF is accurately mediated by extracellular mechanical and intracellular molecular signals and it is a promising cell source for osteoblasts and osteoclasts in the periodontal ligament.12 Understanding the molecular regulatory mechanism of osteogenic differentiation of hPDLF may provide the novel therapeutic targets for periodontal ligament. However, the molecular regulatory mechanism of hPDLF fate determination remains poorly understood.
MiRNAs have been shown to play a important role in modulating of cell differentiation and growth processes.13,14 Many miRNAs are highly expressed in the osteogenesis system, contributing to the maintenance of normal tissue functions and homeostasis, and affecting cell differentiation.15 Moreover, many miRNAs are implicated in periodontal cell differentiation and disorders of miRNAs play a vital role in periodontal disease.16 Specifically, miR-320a was found to restrain osteogenic differentiation in osteoporosis. However, the specific molecular regulatory mechanism of osteogenic differentiation of hPDLF by miRNAs requires further investigation.17 This study disclosed that miR-874-3p was downregulated during the osteogenic differentiation of hPDLF, and the degree of osteogenic differentiation of hPDLF could be regulated by upregulating or downregulating the expression level of miR-874-3p. These results hint that miR-874-3p may act as an inhibiting factor in osteogenic differentiation of hPDLF.
A growing number of researches confirmed that miRNAs execute their functions by regulating the expression level of their target mRNAs.18 A previous study proved that overexpressed miR-143 accelerates osteogenic differentiation via TNF-α-dependent NF-κB signaling pathway.19 Moreover, miR-196a was reported to participate in osteogenic differentiation in osteoporotic mice via activation of GNAS-dependent Hedgehog pathway.20 In addition, adipose-derived stem cells osteogenic differentiation was reported to mediate through miR-665/IL6 axis via PI3K/Akt pathway.21 Another novel discovery of this study was that WNT3A is a target of miR-874-3p. WNT3A belongs to a gene family which encodes secreted signaling proteins. These proteins have been involved in several developmental processes. Here, the fact that miR-874-3p could target WNT3A was demonstrated by predicting the mRNA binding sites in TargetScan and performing the dual-luciferase assays. Moreover, high expression levels of miR-874-3p dramatically suppressed WNT3A and β-catenin protein expression. Furthermore, the results showed that miR-874-3p repressed osteogenic differentiation of hPDLF while this effect could be abrogated by WNT3A overexpression. Similarly, overexpression of WNT3A could also abolish the effect of miR-874-3p-induced low expression of β-catenin protein. These data indicate that WNT3A could alleviate repressed osteogenic differentiation of hPDLF promoted by miR-874-3p. However, to better define the role of miR-874-3p in osteogenic differentiation of hPDLF by targeting WNT3A and β-catenin, expression levels of WNT3A proteins should be detected after co-transfected with miRNA NC, miRNA mimics with control and miRNA mimics with β-catenin overexpressed plasmid in the not too distant future.
In conclusion, in this study, the fact that miR-874-3p and WNT3A would be downregulated and upregulated during osteogenic differentiation of hPDLF, respectively was discovered. Moreover, knockdown or overexpression of miR-874-3p would promote and inhibit osteogenic differentiation of hPDLF, respectively. Meanwhile, knockdown or overexpression of miR-874-3p could also promote and inhibit WNT3A and β-catenin protein expression, respectively. Finally, overexpression of WNT3A could rescue osteogenic differentiation of hPDLF. Figure out the role of miR-874-3p/Wnt3a/β-catenin in osteogenic differentiation of hPDLF could conceivably pave the path for advanced therapeutic targets in human periodontal ligament.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
Not applicable.
References
- 1.Fuchigami S., Nakamura T., Furue K., Sena K., Shinohara Y., Noguchi K. Recombinant human bone morphogenetic protein-9 potently induces osteogenic differentiation of human periodontal ligament fibroblasts. Eur J Oral Sci. 2016;124:151–157. doi: 10.1111/eos.12249. [DOI] [PubMed] [Google Scholar]
- 2.Kim T.I., Han J.E., Jung H.M., Oh J.H., Woo K.M. Analysis of histone deacetylase inhibitor-induced responses in human periodontal ligament fibroblasts. Biotechnol Lett. 2013;35:129–133. doi: 10.1007/s10529-012-0992-6. [DOI] [PubMed] [Google Scholar]
- 3.Inanç B., Elçin A.E., Elçin Y.M. Effect of osteogenic induction on the in vitro differentiation of human embryonic stem cells cocultured with periodontal ligament fibroblasts. Artif Organs. 2007;31:792–800. doi: 10.1111/j.1525-1594.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs C., Grimm S., Ziebart T., Walter C., Wehrbein H. Osteogenic differentiation of periodontal fibroblasts is dependent on the strength of mechanical strain. Arch Oral Biol. 2013;58:896–904. doi: 10.1016/j.archoralbio.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Wei F.L., Wang J.H., Ding G. Mechanical force-induced specific MicroRNA expression in human periodontal ligament stem cells. Cells Tissues Organs. 2014;199:353–363. doi: 10.1159/000369613. [DOI] [PubMed] [Google Scholar]
- 6.Hao Y., Ge Y., Li J., Hu Y., Wu B., Fang F. Identification of microRNAs by microarray analysis and prediction of target genes involved in osteogenic differentiation of human periodontal ligament stem cells. J Periodontol. 2017;88:1105–1113. doi: 10.1902/jop.2017.170079. [DOI] [PubMed] [Google Scholar]
- 7.Chang M., Lin H., Fu H., Wang B., Han G., Fan M. MicroRNA-195-5p regulates osteogenic differentiation of periodontal ligament cells under mechanical loading. J Cell Physiol. 2017;232:3762–3774. doi: 10.1002/jcp.25856. [DOI] [PubMed] [Google Scholar]
- 8.Hung P.S., Chen F.C., Kuang S.H., Kao S.Y., Lin S.C., Chang K.W. miR-146a induces differentiation of periodontal ligament cells. J Dent Res. 2010;89:252–257. doi: 10.1177/0022034509357411. [DOI] [PubMed] [Google Scholar]
- 9.Cao F., Zhan J., Chen X., Zhang K., Lai R., Feng Z. miR-214 promotes periodontal ligament stem cell osteoblastic differentiation by modulating Wnt/β-catenin signaling. Mol Med Rep. 2017;16:9301–9308. doi: 10.3892/mmr.2017.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J.C., Liu Z.G., Yu B., Zhang X.R. MicroRNA-874 targeting SUFU involves in osteoblast proliferation and differentiation in osteoporosis rats through the Hedgehog signaling pathway. Biochem Biophys Res Commun. 2018;506:194–203. doi: 10.1016/j.bbrc.2018.09.187. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y., Han Y., Guo R. Long non-coding RNA FER1L4 promotes osteogenic differentiation of human periodontal ligament stromal cells via miR-874-3p and vascular endothelial growth factor A. Stem Cell Res Ther. 2020;11:5. doi: 10.1186/s13287-019-1519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan M.Q., Tye C.E., Stein G.S., Lian J.B. Non-coding RNAs: epigenetic regulators of bone development and homeostasis. Bone. 2015;81:746–756. doi: 10.1016/j.bone.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z., Wang Z., Wang T., Yuan J., Wang X., Zhang Z. Inhibition of miR-34a-5p protected myocardial ischemia reperfusion injury-induced apoptosis and reactive oxygen species accumulation through regulation of Notch Receptor 1 signaling. Rev Cardiovasc Med. 2019;20:187–197. doi: 10.31083/j.rcm.2019.03.545. [DOI] [PubMed] [Google Scholar]
- 14.Altinbilek E., Ozturk D., Kavalci C. Neutrophil/lymphocyte ratio and Red blood cell distribution width are independent risk factors for 30-day mortality in gastrointestinal system bleeding patients. Signa Vitae. 2019;15:59–64. [Google Scholar]
- 15.Vienberg S., Geiger J., Madsen S., Dalgaard L.T. MicroRNAs in metabolism. Acta Physiol. 2017;219:346–361. doi: 10.1111/apha.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang N., Li Y., Wang G., Ding Y., Jin Y., Xu Y. Tumor necrosis factor-α suppresses adipogenic and osteogenic differentiation of human periodontal ligament stem cell by inhibiting miR-21/Spry 1 functional axis. Differentiation. 2017;97:33–43. doi: 10.1016/j.diff.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Wang C.G., Hu Y.H., Su S.L., Zhong D. LncRNA DANCR and miR-320a suppressed osteogenic differentiation in osteoporosis by directly inhibiting the Wnt/β-catenin signaling pathway. Exp Mol Med. 2020;52(8):1310–1325. doi: 10.1038/s12276-020-0475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P., Yang W., Wang G., Li Y. miR-143 suppresses the osteogenic differentiation of dental pulp stem cells by inactivation of NF-κB signaling pathway via targeting TNF-α. Arch Oral Biol. 2018;87:172–179. doi: 10.1016/j.archoralbio.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Zhong L.N., Zhang Y.Z., Li H., Fu H.L., Lv C.X., Jia X.J. Overexpressed miR-196a accelerates osteogenic differentiation in osteoporotic mice via GNAS-dependent Hedgehog signaling pathway. J Cell Biochem. 2019;120:19422–19431. doi: 10.1002/jcb.29166. [DOI] [PubMed] [Google Scholar]
- 21.Wu R., Ruan J., Sun Y. Long non-coding RNA HIF1A-AS2 facilitates adipose-derived stem cells (ASCs) osteogenic differentiation through miR-665/IL6 axis via PI3K/Akt signaling pathway. Stem Cell Res Ther. 2018;9:348. doi: 10.1186/s13287-018-1082-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]