Abstract
Background/purpose
Radiotherapy for head and neck cancer often causes severe oral mucositis. The purpose of this retrospective study was to further examine the risk factors for developing severe oral mucositis in patients with oral cancer undergoing radiotherapy as a compliment to a previous study performed by our group.
Materials and methods
A total of 181 patients with oral cancer undergoing radiotherapy were enrolled in the study. The association between a number of potential risk factors and grade 3 oral mucositis were analyzed using the cox proportional hazard model and a logistic regression analysis.
Results
Grade 3 oral mucositis occurred in 56 patients. The cox proportional hazard model analysis revealed that those with lower hemoglobin levels, concurrent cisplatin and cetuximab administration, and a larger number of teeth showed a significantly higher incidence of severe oral mucositis. Logistic regression analysis revealed that patients who had lower hemoglobin levels, received concurrent cisplatin or cetuximab treatment, and were not administered pilocarpine showed a significantly higher incidence of severe oral mucositis. The presence of teeth may stimulate the oral mucosa and become a risk factor for mucositis, and the administration of pilocarpine might reduce the risk.
Conclusion
This study describes the risk factors of severe radiation-induced oral mucositis in oral cancer patients and shows the possibility of risk reduction by pilocarpine. This information could help patients avoid painful mucositis.
Keywords: Oral mucositis, Radiotherapy, Oral cancer, Pilocarpine, Risk factors
Introduction
Radiotherapy (RT) is often used in the treatment of oral cancer. It can either be used alone, in combination with anticancer drugs (CRT), or in combination with molecular targeted drugs (BRT).1 RT causes a number of adverse effects including oral mucositis, xerostomia, taste disturbances, oral candidiasis, radiation-related dental caries, and osteoradionecrosis of the jaw. Oral mucositis in particular generates difficulties in eating due to the severe pain it causes, which decreases the patients' quality of life (QOL) and sometimes hinders the continuation of RT. Furthermore, oral mucositis may influence patient prognosis. Despite these issues, preventive strategies and therapeutic measures have not been established.2, 3, 4
We recently conducted a multicenter retrospective study to determine risk factors for oral mucositis and oral candidiasis in oral and oropharyngeal cancer patients undergoing RT, CRT, or BRT.5 Male oropharyngeal cancer patients with low hemoglobin, low leukocyte and lymphocyte counts, concurrent use of cisplatin and cetuximab, and peroral feeding developed grade 3 severe oral mucositis more frequently and more rapidly than patients did not have these risk factors. Conversely, other variables related to oral status, such as number of teeth, number of metal teeth, use of a spacer, and the administration of pilocarpine, were not correlated with the incidence of severe mucositis during RT. Therefore, this is a sub-analysis of the previous study.5 The primary site was limited to the oral cavity, and factors related to the development of severe oral mucositis during RT were examined.
Materials and methods
Patients
A total of 181 patients with squamous cell carcinoma of the oral cavity who underwent RT, CRT, or BRT at Nagasaki University Hospital, Kobe University Hospital, or Kansai Medical University Hospital were enrolled in the study. All patients underwent dental evaluation and panoramic radiograph examination prior to the start of RT. Teeth with severe periodontal disease, periapical lesion, carious stump, or root fracture were extracted at least 1 week prior to the start of RT. In addition, all patients received standard oral care by dentists and dental hygienists, which consisted of oral health instruction, removal of dental calculus (scaling), professional mechanical tooth cleaning (PMTC), removal of tongue coating with a toothbrush, and cleaning denture during RT. Furthermore, use of spacer to minimize radiation backscatter when patients had metal restorations, administration of pilocarpine to treat dry mouth, use of mouthwash containing local anesthetic, and administration of topical steroid ointment was done at the discretion of the dentist.
Evaluated data
The following data were investigated from the medical records and the panoramic radiographs: Sex, body mass index (BMI), presence of diabetes, levels of serum albumin and creatinine, hemoglobin, total leukocyte and lymphocyte counts, concurrent therapy (RT alone, CRT or BRT), type of RT (three-dimensional conformal radiation therapy [3D-CRT] or intensity modulated radiation therapy [IMRT]), total radiation dose, feeding route (oral feeding or tube feeding through gastric fistula), use of spacers, administration of pilocarpine hydrochloride (Salagen®, Kissei Pharmaceutical, Co., Ltd, Nagano, Japan), corticosteroid ointment (Dexaltin Oral Ointment®, Nihon Kayaku, Co., Ltd, Tokyo, Japan), the number of teeth, the number of metal restored teeth, alveolar bone loss (<1/2 or ≥1/2), and incidence of oral mucositis were noted. Oral stomatitis was categorized based on criteria from the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. According to these criteria, grade 3 oral stomatitis is defined as the inability to feed orally, or the presence of severe pain due to severe stomatitis.6 In this study, pain necessitating systemic administration of opioids was considered to be severe. The outcome of the study was the development of grade 3 oral mucositis, as this condition significantly reduces the patients' quality of life. Oral mucositis was observed for up to 90 days from the initiation of RT. Pilocarpine hydrochloride was administrated from the start to the end of RT.
Statistical analysis
All statistical analyses were performed using SPSS software (version 24.0; Japan IBM Co., Tokyo, Japan). The correlation between each variable and grade 3 oral mucositis was analyzed by the cox proportional hazard model and logistic regression analysis. The relationships between some of these factors and the occurrence of grade 3 oral mucositis were illustrated using the Kaplan–Meier method, and univariate analysis was performed using a log rank test. In all analyses, two-tailed p values < 0.05 were considered statistically significant.
Ethics
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research involving Human Subjects by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labor and Welfare of Japan. Ethical approval was obtained from the Institutional Review Boards (IRB) of Nagasaki University Hospital (No. 18091008), Kobe University Hospital (No. 180250), and Kansai University Hospital (No. 2018164). Japanese law does not require individual informed consent from participants in non-invasive observational trials such as the present study. Therefore, the need for informed consent was waived. As this was a retrospective study, patient identifiable information was removed and the research plan was published on the homepages of the participating hospitals websites, along with an opt-out option in accordance with IRB instructions.
Results
Table 1 contains summary statistics for the study cohort. This cohort was comprised of 181 total patients (121 males and 60 females), with a median age of 65.3 years. With respect to therapeutic treatments, 66 patients received RT alone, 99 patients received CRT, and 16 patients received BRT. A total of 161 patients underwent 3D-CRT and 20 patients underwent IMRT. Radiation therapy was performed with a standard fractionation of 2 Gy/day. The mean dose of RT was 63.7 Gy, within the range of 60–70 Gy. Pilocarpine was orally administered to 35 patients at 15 mg daily, with an average of 33.1 days (3–54 days).
Table 1.
Summary statistics of the patients.
| Factor | Number of patients or average value | |
|---|---|---|
| Age | 65.3 years | |
| Sex | Male | 121 |
| Female | 60 | |
| Body mass index (BMI) | 20.3 | |
| Diabetes | (−) | 152 |
| (+) | 29 | |
| Hemoglobin | 11.2 mg/dL | |
| Leukocyte | 3105/μL | |
| Lymphocyte | 449/μL | |
| Albumin | 3.59 g/dL | |
| Creatinine | 0.848 mg/dL | |
| Concurrent therapy | RT alone | 66 |
| RT + cisplatin | 99 | |
| RT + cetuximab | 16 | |
| Radiation method | 3D-CRT | 161 |
| IMRT | 20 | |
| Total dose | 63.7 Gy | |
| Use of spacer | (−) | 138 |
| (+) | 43 | |
| Pilocarpine hydrochloride | (−) | 146 |
| (+) | 35 | |
| Corticosteroid ointment | (−) | 111 |
| (+) | 70 | |
| Number of teeth | 15.6 | |
| Number of metal teeth | 5.56 | |
| Alveolar bone loss | <1/2 | 153 |
| ≥1/2 | 28 | |
| Oral mucositis | Grade 0–2 | 125 |
| Grade 3 | 56 | |
| Total | 181 |
RT: radiotherapy, 3D-CRT: three-dimensional conformal radiation therapy, IMRT: intensity modulated radiation therapy.
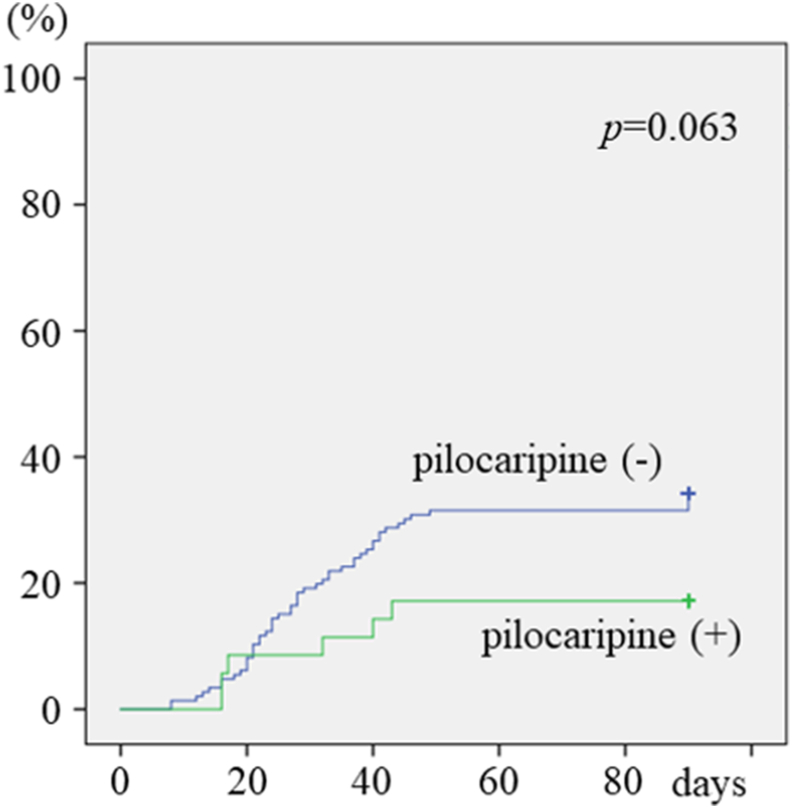
Grade 3 severe oral mucositis occurred in 56 (30.9%) patients. When analyzed using the cox proportional hazard model analysis, lower hemoglobin levels (p = 0.012), receiving concurrent cisplatin or cetuximab treatment (p = 0.010), and a larger number of teeth (p = 0.042) were each associated with a significantly higher incidence of severe oral mucositis (Table 2). Logistic regression analysis revealed that lower hemoglobin levels (p = 0.013), receiving concurrent cisplatin or cetuximab treatment (p = 0.006), and not receiving pilocarpine (p = 0.047) each correlated with a significantly higher incidence of severe oral mucositis (Table 2). A Kaplan–Meier curve showed that the administration of pilocarpine reduced grade 3 mucositis, although this effect was not significant (p = 0.063) (Fig. 1).
Table 2.
Relationship between each variable and the development of grade 3 oral mucositis.
| Factor | Cox proportional hazard model |
Logistic regression |
|||||
|---|---|---|---|---|---|---|---|
| p-value | HR | 95% CI | p-value | OR | 95% CI | ||
| Age | 0.333 | 0.136 | |||||
| Sex | Male vs. female | 0.164 | 0.093 | ||||
| Body mass index (BMI) | 0.377 | 0.106 | |||||
| Diabetes | (−) vs. (+) | 0.193 | 0.246 | ||||
| Hemoglobin | ∗0.012 | 0.833 | 0.723–0.961 | ∗0.013 | 0.755 | 0.606–0.942 | |
| Leukocyte | 0.634 | 0.670 | |||||
| Lymphocyte | 0.408 | 0.519 | |||||
| Albumin | 0.425 | 0.105 | |||||
| Creatinine | 0.334 | 0.255 | |||||
| Concurrent therapy | RT alone vs. RT + cisplatin vs. RT + cetuximab | ∗0.010 | 1.802 | 1.152–2.819 | ∗0.006 | 2.237 | 1.266–3.954 |
| Radiation method | 3D-CRT vs. IMRT | 0.945 | 0.572 | ||||
| Total dose | 0.449 | 0.432 | |||||
| Use of spacer | (−) vs. (+) | 0.963 | 0.309 | ||||
| Pilocarpine hydrochloride | (−) vs. (+) | 0.115 | ∗0.047 | 0.369 | 0.138–0.986 | ||
| Corticosteroid ointment | (−) vs. (+) | 0.875 | 0.560 | ||||
| Number of teeth | ∗0.042 | 1.03 | 1.001–1.060 | 0.097 | |||
| Number of metal teeth | 0.957 | 0.271 | |||||
| Alveolar bone loss | <1/2 vs. ≥1/2 | 0.809 | 0.597 | ||||
RT: radiotherapy, 3D-CRT: three-dimensional conformal radiation therapy, IMRT: intensity modulated radiation therapy, HR: hazard ratio, CI: confidence interval.
∗p < 0.05.
Figure 1.
Development of grade 3 oral mucositis in patients with and without pilocarpine administration.
Discussion
RT is part of a standard treatment regime for head and neck cancer. It is performed as either an initial treatment or as a postoperative therapy, with or without cisplatin or cetuximab.1,7 RT causes a number of adverse events, including oral mucositis, xerostomia, taste disturbance, trismus, dental caries, and osteoradionecrosis of the jaw; unfortunately effective prophylaxis treatments have not been determined.2, 3, 4
Kawashita et al.8 advocated for the use of a prophylactic bundle for radiation-induced adverse events in patients with head and neck cancers. This consisted of 1) extraction of infected teeth prior to the initiation of RT, 2) use of spacers to minimize radiation backscatter, 3) oral care, 4) administration of pilocarpine hydrochloride, 5) topical administration of corticosteroid ointments after the occurrence of mucositis, 6) care of affected skin, and 7) topical application of fluoride. Furthermore, a multicenter, randomized clinical trial was conducted to investigate the impact of topical steroid administration, spacers, and pilocarpine hydrochloride on the prevention of severe oral mucositis in oral cancer patients. The conclusion was that these treatments were effective in those undergoing RT alone, although the efficacy was not demonstrated in those undergoing CRT, because the chemotherapy regimens varied.9
Recently, we carried out a multicenter, retrospective analysis of 361 patients with oral or oropharyngeal cancer undergoing RT, CRT, or BRT. We reported that males, presence of oropharyngeal cancer, low hemoglobin levels, low leukocyte or lymphocyte counts, concurrent cisplatin or cetuximab treatment, and oral feeding were found to be significantly associated with a higher incidence of grade 3 oral mucositis.5 In that study, there was no relationship between the development of grade 3 oral mucositis and the administration of pilocarpine or the number of teeth present.5 In the present study, we focused on patients with oral cancer and performed a sub-analysis of the previous study. Low hemoglobin levels and concurrent use of cisplatin or cetuximab were risk factors for severe oral mucositis, similar to the results above in oral or oropharyngeal cancer patients.5 Interestingly, the current study showed that the administration of pilocarpine and the number of teeth might also influence the development and the time of development of grade 3 oral mucositis.
Pilocarpine is a cholinergic drug that mimics the effects of the chemical acetylcholine, which is produced by nerve cells. Pilocarpine stimulates the secretion of large amounts of saliva and sweat. It is used to treat dry mouth, also known as xerostomia, in Sjögren's syndrome. Xerostomia can also occur as a side effect of RT within head and neck cancer. The National Comprehensive Cancer network (NCCN) guidelines for head and neck cancer also recommended the use of pilocarpine to reduce xerostomia during RT.1 Scarantino et al. examined the efficacy of pilocarpine on xerostomia and mucositis during RT in 245 head and neck cancer patients in a randomized clinical trial.10 They concluded that the average unstimulated salivary flow was statistically greater in the pilocarpine group than in the placebo group, whereas the frequency of the development of grade 2–3 mucositis did not differ between the two groups. However, their study included only 63 patients with oral cancer, and differed from this study in that the outcome was mucositis at a grade of 2 or more, rather than just grade 3 mucositis. In our previous observational study of 326 patients with oral and oropharyngeal cancer, there was no significant difference in the incidence of grade 3 oral mucositis between patients who received pilocarpine and those who did not,5 as in their study.10 On the other hand, when focusing on patients with oral cancer as described in this study, the incidence rate of grade 3 oral mucositis was significantly higher in patients who had more teeth and significantly lower in those receiving pilocarpine. The reason for the observed difference between oral cancer and pharyngeal cancer is not clear. This sub-analysis of 181 patients with oral cancer revealed significantly less developed mucositis with the administration of pilocarpine. Furthermore, this study showed that the incidence of oral mucositis increased as the number of remaining teeth increased. We think these findings suggest that the site of mucositis was mostly localized to the tongue and buccal mucosa, where the mechanical stimulation of the teeth would be an aggravating factor. With this reasoning, the presence of teeth could increase the risk, while an increase in saliva may be a risk-mitigating factor (Fig. 2). Teeth are also a prime location for the growth of bacteria, especially when oral hygiene is poor. Bacteria can aggravate the site, inducing local inflammation and leading to the generation of pathological lesions on the mucosa. On the other hand, in those with oropharyngeal cancer, mucositis is often located in areas unrelated to tooth stimulation such as the soft palate, and so the number of teeth and the administration of pilocarpine did not correlate with the incidence of oral mucositis during RT in patients with this type of cancer.
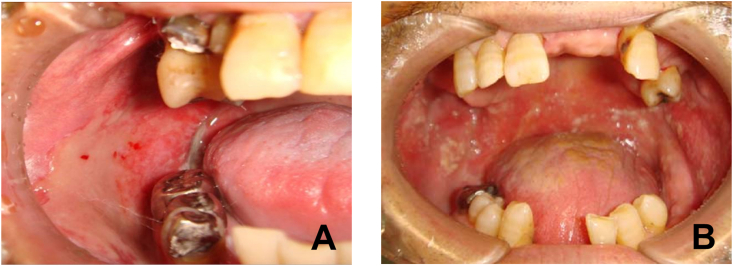
Figure 2.
Grade 3 oral mucositis during radiotherapy. A: Patients with oral cancer. Mucositis occurred in the buccal mucosa stimulated by teeth. B: Patients with oropharyngeal cancer. Mucositis occurred mainly within the soft palate and buccal mucosa, areas in which teeth could not play a causal role in the development of mucositis.
It is interesting that lower levels of hemoglobin and the lack of pilocarpine use (i.e., low unstimulated salivary flow) were associated with the incidence of grade 3 oral mucositis. Pels10 reported that low levels of salivary IgA in children with leukemia might result in the development and potentiation of oral lesions typical of mucositis during anti-tumor treatment. Furthermore, a significant decrease in salivary IgG and IgM concentrations during chemotherapy might also cause the potentiation of pathological lesions here as well. Bachmeier et al.11 demonstrated that there are increased levels of superoxide dismutase in the saliva of patients who developed mucositis and this could be a cellular defense mechanism of the oral mucosa. Thus, saliva not only protects the mucous membrane from mechanical stimulation of the teeth, but also some factors present in the saliva may protect the mucosa from radiation damage.
The present study has some limitations. First, it is a retrospective investigation with a small number of patients, and so generalization of the results may require a larger sample size. Second, there was no information on the saliva volume between patients administrated pilocarpine and those that were not. Although the study did not measure saliva volume, Scarantino et al. have shown that pilocarpine significantly increased unstimulated salivary flow in a randomized clinical trial.12 And third, the state of the teeth (contact with mucositis, presence of periodontal disease, caries and restorations) and the level of oral hygiene could not be investigated because of the retrospective nature of the study. Although these detailed dental findings were not investigated, the number of metal crowns and alveolar bone resorption of more than 1/2 were included in the independent variables, so it is thought that the state of the teeth and the periodontal tissues were examined to some extent.
To the best of our knowledge, we may be the first to report this potential relationship between pilocarpine and oral mucositis. In the future, intervention studies with more cases are needed in order to clarify these findings. Our findings indicate a possible relationship between teeth and mucositis, as well as the potentially preventive effect of pilocarpine on the development of mucositis in patients with oral cancer undergoing RT.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
We greatly appreciate all patients who participated in this trial. We thank Editage (www.editage.jp) for their English language editing services.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines) Head and Neck Cancers. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed 4 August 2019.
- 2.Rodriguez-Caballero A., Torres-Lagares D., Robles-Garcia M., Pachon-Ibanez J., Gonzalez-Padilla D., Gutierrez-Perez J.L. Cancer treatment-induced oral mucositis: a critical review. Int J Oral Maxillofac Surg. 2012;41:225–238. doi: 10.1016/j.ijom.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Lalla R.V., Bowen J., Barasch A. Mucositis guidelines leadership group of the multinational association of supportive care in cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120:1453–1461. [Google Scholar]
- 4.Moslemi D., Nokhandani A.M., Otaghsaraei M.T., Moghadamnia Y., Kazemi S., Moghadamnia A.A. Management of chemo/radiation-induced oral mucositis in patients with head and neck cancer: a review of the current literature. Radiother Oncol. 2016;120:13–20. doi: 10.1016/j.radonc.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Nishii M., Soutome S., Kawakita A. Factors associated with severe oral mucositis and candidiasis in patients undergoing radiotherapy for oral and oropharyngeal carcinomas: a retrospective multicenter study of 326 patients. Support Care Cancer. 2020;28:1069–1075. doi: 10.1007/s00520-019-04885-z. [DOI] [PubMed] [Google Scholar]
- 6.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed 4 August 2019.
- 7.Yanamoto S., Umeda M., Kioi M. Multicenter retrospective study of cetuximab plus platinum-based chemotherapy for recurrent or metastatic oral squamous cell carcinoma. Cancer Chemother Pharmacol. 2018;81:549–554. doi: 10.1007/s00280-018-3531-x. [DOI] [PubMed] [Google Scholar]
- 8.Kawashita Y., Hayashida S., Funahara M., Umeda M., Saito T. Prophylactic bundle for radiation-induced oral mucositis in oral or oropharyneal cancer patients. J Cancer Res Ther. 2014;2:9–13. [Google Scholar]
- 9.Kawashita Y., Koyama Y., Kurita H. Effectiveness of a comprehensive oral management protocol for the prevention of severe oral mucositis in patients receiving radiotherapy with or without chemotherapy for oral cancer: a multicenter, phase II, randomized controlled trial. Int J Oral Maxillofac Surg. 2019;48:857–864. doi: 10.1016/j.ijom.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Pels E.J. Oral mucositis and saliva IgA, IgG and IgM concentration during anti-tumor treatment in children suffering from acute lymphoblastic leukemia. Adv Clin Exp Med. 2017;26:1351–1358. doi: 10.17219/acem/64940. [DOI] [PubMed] [Google Scholar]
- 11.Bachmeier E., Mazzeo M.A., Lopez M.M. Mucositis and salivary antioxidants in patients undergoing bone marrow transplantation (BMT) Med Oral Patol Oral Cir Bucal. 2014;19:e444–e450. doi: 10.4317/medoral.19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarantino C., LeVegue F., Swann R.S. Effect of pilocarpine during radiation therapy: results of RTOG 97-09, a phase III randomized study in head and neck cancer patients. J Support Oncol. 2006;4:252–258. [PubMed] [Google Scholar]