Abstract
Sjögren's syndrome (SS) is a chronic autoimmune rheumatic disease characterized by a progressive lymphocytic infiltration of salivary glands, resulting in xerostomia and other oral diseases. The pathogenesis and mechanisms of SS on periodontal tissues are not well understood. Furthermore, results of two systemic reviews and meta-analyses in which compared periodontal parameters of patients with SS to healthy subjects were different. To determine whether periodontal conditions in SS were different from healthy controls, we re-examined the issue with a random-effect model, avoiding recruiting active controls and inadequate data conversion. Outcome measures included probing pocket depth (PPD), clinical attachment loss (CAL), plaque index (PI), and gingival index (GI). Recruited individuals comprised 198 patients with SS and 180 subjects for healthy controls. Quantitative analysis revealed higher PI (WMD = 0.76, 95% CI: 0.30, 1.23) and GI (WMD of total = 0.50, 95% CI: 0.01, 0.98) in SS patients who were not categorized into primary or secondary types of SS. PPD and CAL in SS patients was comparable with control subjects. However, heterogeneity was observed among included studies. Thus, results from this and previous analyses should be interpretated carefully, and a well-designed observational study regarding this issue should be conducted.
Keywords: Periodontal conditions, Sjögren's syndrome, Meta-analysis
Introduction
Ranking next to rheumatoid arthritis (RA), Sjögren's syndrome (SS) is the second most common autoimmune diseases.1 The estimated prevalence of primary SS (PSS) ranges from 0.9 to 6.0 per 1000 individual.1 Secondary SS (SSS) occurs secondary to other autoimmune rheumatic diseases. SS is characterized by lymphocytic infiltration of salivary and lacrimal glands, leading to xerostomia and keratoconjunctivitis sicca. Moreover, one third of these patients with this disease develop systemic complications, such as renal, pulmonary or neuro-logical manifestations. Around 5% of PSS patients will develop lymphoma, the most severe complication of the disease.1
Periodontal conditions and dental caries are two of the most important oral health issues, regarding their prevalence. In the periodontal aspect, inflammatory and immune reactions extend deep into the periodontal tissues, causing loss of tooth-supporting connective tissues and alveolar bone, and thus, periodontal disease (PD). PD is known to be associated with adverse pregnancy outcomes, cardiovascular diseases, stroke, pulmonary diseases and diabetes.2 A nationwide population-based study showed that patients with periodontal disease had an approximately 50% increased risk of subsequent SS.3 On the other hand, PD is also one of the complications of diabetes, RA and systemic lupus erythematosus (SLE). Clinical attachment loss (CAL) in patients with RA is 0.6 mm deeper than in control subjects.4 Patients with SLE had higher prevalence of bleeding on probing (BOP) and CAL, when compared to healthy subjects.5 Thus, the hyperactive immune responses of autoimmune diseases may worsen the periodontal condition.
The tango between periodontal and systemic inflammation suggests a link-up between the progress of PD and SS. The normal capillary network in healthy gingiva is reported to be replaced by a “cobweb” structure in subjects with SS.6 The dilated and twisting vasculization suggests the more inflammatory gingiva in SS subjects, compared to healthy individuals.7 However, whether periodontal parameters reflect an inflamed and destructed periodontal tissue in SS subjects is still controversial. Although a previous meta-analysis conducted by de Goés Soares et al.8 showed that plaque index (PI) and gingival index (GI) were larger in patients with SS than in controls, another one performed by Maarse et al.9 concluded that PPD, CAL, PI and GI in SS patients are comparable to controls. The contradicting results might be caused by the neglect of converting standard errors (SE) or standard errors of means (SEM) reported in 3 studies10, 11, 12 into standard deviation (SD) in the study of de Goés Soares et al. and including active controls in the study of Maarse et al. The potentially biased data reported in previous studies led to a demand for re-examining the issue. Thus, we re-examined differences between the periodontal status of SS and controls with the meta-analysis, avoiding using unconverted raw data and including active controls. Causes of heterogeneity were also explored by subgroup analyses and sensitivity analyses.
Materials and methods
Study selection criteria
This review was conducted according to the guide of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for reporting in systematic reviews and meta-analyses13 (checklist as supplement 1). Only quantitative studies that focused on the comparison of periodontal status between human subjects with or without PSS or SSS were included. To identify the link between periodontal parameters and SS, outcome indices included PPD, CAL, PI and GI. The meta-analysis of BOP was not performed because measurement tools among different studies varied.
Search methods
Published articles about human subjects were obtained from electronic databases of MEDLINE/PubMed, Web of Science, and the Cochrane database of systematic review and Embase until September 23rd, 2020. The search was conducted using the combination of the keywords ((((“oral condition” OR “dental condition” OR “oral complication”) OR (caries)) OR (periodontal disease)) OR (candidiasis)) AND (sjogren's syndrome). A manual search on the references of the articles in the review was also performed to identify relevant studies not identified by the search strategy created.
Quality assessment of methodological validity
Screening titles and abstracts of potentially relevant articles was performed before retrieving full articles, which were reviewed to verify if they fit all the inclusion criteria stated above. The literature selection and data extraction were done by two investigators (Y.H.C. and S.Y.W.). The quality of each study was assessed by Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) tool.14 Any disagreement on study inclusions, risk of bias assessment, or data extraction were resolved by discussions between the two investigators (Y.H.C., S.Y.W.).
Data extraction and synthesis of results
The following data items were extracted: authors, journal, publication year, countries and study institutions, diagnostic criteria, including and excluding criteria, number of subjects, study groups, ages, type and duration of SS, PPD, CAL, GI, and PI. Since distribution of PI or GI and medians of PPD were reported in the original articles of 2 studies,15,16 data of mean and SD were retrieved from the meta-analysis performed by Maarse et al.9 Data of SE and SEM reported in 3 studies10, 11, 12 were converted to SD for meta-analysis before combination for meta-analysis using the statistical software RevMan version 5.4. The effect size was estimated and reported as the standard mean difference (SMD). The 95% confidence interval (CI) was also calculated. A random-effects model was used because between-study heterogeneity was expected. The pooled effect was considered significant if p was <0.05. Between-study heterogeneity was assessed using Cochrane's Q and I2 statistics.17 The heterogeneity was substantial if the Q test showed a significant result (p < 0.05) and I2 > 70%. Publication bias for each outcome of interest was also investigated, by visual assessment in the funnel plot18 and the quantitative regression asymmetry test.19 Heterogeneity was investigated by the subgroup analyses,20 in which subjects were categorized into PSS, SSS and mix-SS. mix-SS, based on types of SS reported in the included studies. Mix-SS was defined as subjects whose type of SS was not defined in the included studies. Influences of each study were analyzed with sensitivity tests.21
Results
Study selection and characteristics of eligible studies
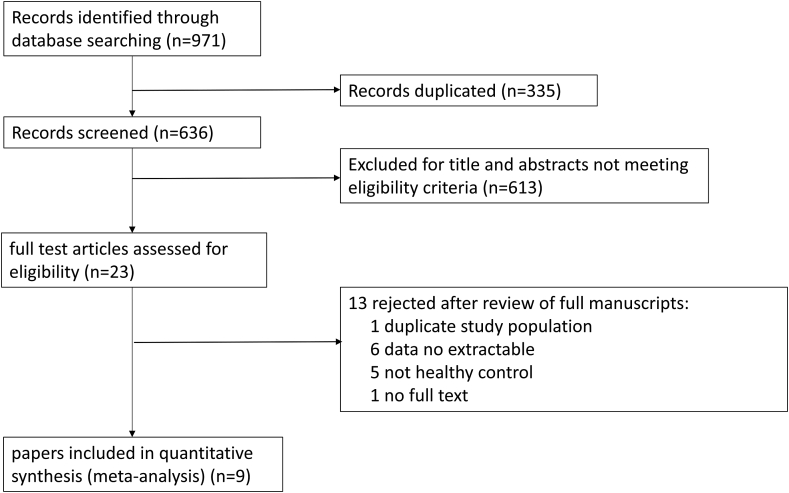
The search identified 971 studies potentially relevant to the review. Based on the assessment of the titles and abstracts (Fig. 1), full texts of 9 papers10, 11, 12,15,16,22, 23, 24, 25 (summarized in Table 1 and Table 2) were assessed for detailed examination and were enrolled into meta-analysis. A total of 378 individuals were recruited to these studies, including 198 patients with SS and 180 subjects for the control group. The included studies published between 1991 and 2010 were conducted across an international spectrum, i.e., the U.S.A, Brazil, Hungary, the UK, Denmark, Norway, and Turkey, representing participants from different cultural backgrounds.
Figure 1.
The PRISMA flow diagram of this study.
Table 1.
Summary of the included studies.
| Authors | Country | Diagnostic criteria of SS | Type of SS | Total number | N (N of females) | Age [years old] mean ± SE (range) | Duration of SS [years] mean ± SE (range) | Disease related to SSS (N) |
|---|---|---|---|---|---|---|---|---|
| Antoniazzi et al.10 | Brazil | 1993 European | PSS | 38 | 11 (11) | 48.1 ± 13.4 (NR) | PSS & SSS: S: 9.0 ± 5.0 (NR); D: 5.9 ± 3.7 (NR) |
RA (8) |
| SSS | 8 (8) | 53.8 ± 11.6 (NR) | ||||||
| control | 19 (19) | 49.8 ± 12.8 (NR) | ||||||
| Ergun et al.11 | Turkey | NR | mix-SS | 52 | 27 (NR) | 53.27 (26–78) | NR | RA (21), SLE (2) |
| control | 25 (NR) | 54.27 (25–94) | ||||||
| Kuru et al.22 | the UK | 1993 European | PSS | 29 | 8 (8) | 61.2 ± 14.4 (35–77) | D: 6.1 ± 5.7 (3–20) | |
| SSS | 10 (10) | 60.6 ± 11.8 (43–77) | D: 8.9 ± 7.6 (3–25) | |||||
| control | 11 (11) | 61.8 ± 13.9 (40–77) | ||||||
| Marton et al.23 | Hungary | 2002 AECG | PSS | 92 | 49 (46) | 55 ± 11 (32–76) | ||
| control | 43 (39) | 49 ± 15 (NR) | ||||||
| Najera et al.24 | the USA | 1993 European | mix-SS | 49 | 25 (23) | 60.92 ± 13.52 (28–80) | S: 7.76 ± NR (NR); D: 3.16 ± NR (NR) |
RA (2) |
| control | 24 (22) | 58.29 ± 12.09 (30–77) | ||||||
| Pedersen et al.16 | Norway | 1993 European | PSS | 30 | 16 (14) | median: 61.5 (40–80) | median: 3.5 (1–20) | |
| age- and sex-matched control | 14 (13) | median: 50 (39–70) | ||||||
| Pedersen et al.15 | Denmark | 2002 AECG, 1986 the Copenhagen criteria | PSS | 40 | 20 (20) | 60 ± 15 (NR) | S: 10 ± 7 (NR) D: 6 ± 7 (NR) |
|
| age- matched control | 20 (20) | 56 ± 13 (NR) | ||||||
| Rhodus and Michalowicz12 | the USA | 1993 European | PSS | 20 | 10 (10) | 56.7 ± NR (43–74) | D: 8.8 ± 4.8 (3–19) | |
| control | 10 (10) | 52.6 ± NR (32–65) | ||||||
| Tseng25 | the USA | NR | mix-SS | 28 | 14(14) | 52.9 ± 11.6 (NR) | D: NR ± NR (≥5) | NR |
| control | 14(14) | 53.7 ± 8.9 (NR) |
SS, Sjogren's syndrome; PSS, primary Sjogren's syndrome; SSS, secondary Sjogren's syndrome; mix-SS, primary Sjogren's syndrome and secondary Sjogren's syndrome; NR, not reported; SE, standard error of the mean; S: duration of symptoms; D, duration of the disease; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Table 2.
Overall parameters for primary, secondary and non-Sjögren's syndrome patients.
| Studies | study groups | n | PPD (mm) | statistical association (compared to control) | statistical association (SSS compared to PSS) |
|---|---|---|---|---|---|
| Antoniazzi et al.10 | PSS | 11 | 2.23 ± 0.09 (SE) | p > 0.05 | – |
| SSS | 8 | 2.62 ± 0.16 (SE) | p < 0.05 | p < 0.05 | |
| control | 19 | 2.10 ± 0.10 (SE) | – | – | |
| Ergun et al.11 | mix-SS | 37 | 1.88 ± 0.41 (SEM) | NS | – |
| control | 37 | 1.95 ± 0.63 (SEM) | – | – | |
| Kuru et al.22 | PSS | 8 | 1.78 ± 0.39 (SD) | NS | – |
| SSS | 10 | 2.04 ± 0.53 (SD) | NS | NS | |
| control | 11 | 2.04 ± 0.33 (SD) | – | – | |
| Marton et al.23 | PSS | 38 | 2.28 ± 1.09 (SD) | p < 0.05 | – |
| control | 34 | 1.82 ± 0.73 (SD) | – | – | |
| Najera et al.24 | mix-SS | 25 | 1.92 ± 0.38 (SD) | NS | – |
| control | 24 | 1.80 ± 0.27 (SD) | – | – | |
| Pedersen et al.16 | PSS | 16 | 2.32 ± 0.71 (SD) | NS | – |
| control | 14 | 2.71 ± 0.99 (SD) | – | – | |
| Pedersen et al.15 | PSS | 20 | 2.36 ± 1.01 (SD) | NS | – |
| control | 20 | 2.37 ± 1.01 (SD) | – | – | |
| Rhodus & Michalowicz12 | PSS | 10 | 3.49 ± 0.51 (SE) | NS | – |
| control | 10 | 3.02 ± 0.31 (SE) | – | – | |
| Tseng25 | mix-SS | 14 | 3.02 ± 0.31 (SD) | NS | – |
| control | 14 | 2.82 ± 0.36 (SD) | – | – |
| Studies | study groups | n | CAL (mm) | statistical association (compared to control) | statistical association (SSS compared to PSS) |
|---|---|---|---|---|---|
| Antoniazzi et al.10 | PSS | 11 | 2.57 ± 0.20 (SE) | NS | – |
| SSS | 8 | 3.67 ± 0.50 (SE) | p < 0.05 | p < 0.05 | |
| control | 19 | 2.40 ± 0.14 (SE) | – | – | |
| Kuru et al.22 | PSS | 8 | 2.14 ± 0.65 (SD) | NS | – |
| SSS | 10 | 2.76 ± 1.79 (SD) | NS | NS | |
| control | 11 | 2.60 ± 0.77 (SD) | – | – | |
| Najera et al.24 | mix-SS | 25 | 2.20 ± 0.48 (SD) | p < 0.05 | – |
| control | 24 | 1.96 ± 0.31 (SD) | – | – | |
| Rhodus & Michalowicz12 | PSS | 10 | 5.44 ± 0.39 (SE) | p < 0.10 | – |
| control | 10 | 2.70 ± 0.80 (SE) | – | – | |
| Tseng25 | mix-SS | 14 | 2.70 ± 0.80 (SD) | NS | – |
| control | 14 | 2.79 ± 0.46 (SD) | – | – |
| Studies | study groups | n | PI | statistical association (compared to control) | statistical association (SSS compared to PSS) |
|---|---|---|---|---|---|
| Antoniazzi et al.10 | Antoniazzi et al.14 | Antoniazzi et al.14 | Antoniazzi et al.14 | Antoniazzi et al.14 | Antoniazzi et al.14 |
| SSS | 8 | 1.28 ± 0.10 (SE) | p < 0.05 | p < 0.05 | |
| control | 19 | 0.73 ± 0.06 (SE) | – | – | |
| Kuru et al.22 | PSS | 8 | 1.18 ± 0.33 (SD) | NS | – |
| SSS | 10 | 1.44 ± 0.33 (SD) | NS | NS | |
| control | 11 | 1.44 ± 0.56 (SD) | – | – | |
| Najera et al.24 | mix-SS | 25 | 0.96 ± 0.42 (SD) | p < 0.05 | – |
| control | 24 | 0.65 ± 0.24 (SD) | – | – | |
| Pedersen et al.16 | PSS | 16 | 0.54 ± 0.31 (SD) | NS | – |
| control | 14 | 0.52 ± 0.18 (SD) | – | – | |
| Pedersen et al.15 | PSS | 20 | 0.61 ± 0.70 (SD) | NS | – |
| control | 20 | 0.60 ± 0.71 (SD) | – | – | |
| Rhodus & Michalowicz12 | PSS | 10 | 0.97 ± 0.46 (SE) | p < 0.10 | – |
| control | 10 | 0.65 ± 0.35 (SE) | – | – | |
| Tseng25 | mix-SS | 14 | 0.5 ± 0.35 (SD) | NS | – |
| control | 14 | 0.32 ± 0.28 (SD) | – | – |
| Studies | study groups | n | GI | statistical association (compared to control) | statistical association (SSS compared to PSS) |
|---|---|---|---|---|---|
| Antoniazzi et al.10 | PSS | 11 | 1.15 ± 0.07 (SE) | p < 0.05 | – |
| SSS | 8 | 1.19 ± 0.07 (SE) | p < 0.05 | p > 0.05 | |
| control | 19 | 0.71 ± 0.05 (SE) | – | – | |
| Kuru et al.22 | PSS | 8 | 1.47 ± 0.32 (SD) | NS | – |
| SSS | 10 | 1.47 ± 0.32 (SD) | NS | NS | |
| control | 11 | 1.52 ± 0.65 (SD) | – | – | |
| Najera et al.24 | mix-SS | 25 | 1.11 ± 0.49 (SD) | NS | – |
| control | 24 | 1.01 ± 0.33 (SD) | – | – | |
| Pedersen et al.16 | PSS | 16 | 0.49 ± 0.31 (SD) | NS | – |
| control | 14 | 0.48 ± 0.30 (SD) | – | – | |
| Pedersen et al.15 | PSS | 20 | 0.32 ± 0.56 (SD) | NS | – |
| control | 20 | 0.34 ± 0.60 (SD) | – | – | |
| Rhodus & Michalowicz12 | PSS | 10 | 1.37 ± 0.66 (SE) | NS | – |
| control | 10 | 0.98 ± 0.36 (SE) | – | – | |
| Tseng25 | mix-SS | 14 | 0.98 ± 0.36 (SD) | NS | – |
| control | 14 | 0.79 ± 0.28 (SD) | – | – |
PSS, primary Sjogren's syndrome SS; SSS, secondary Sjogren's syndrome; mix-SS, primary Sjogren's syndrome and secondary Sjogren's syndrome; PPD, periodontal probing depth; CAL, clinical attachment loss; PI, plaque index; GI, gingival index; SE, standard error of the mean; SD, standard deviation; NS, not significant.
Risk of bias
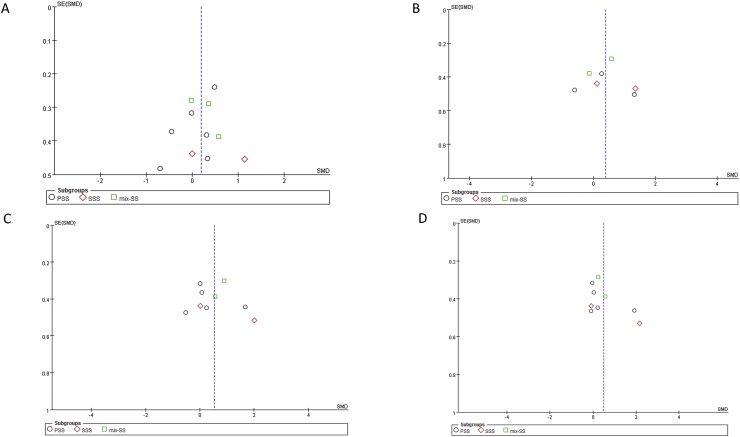
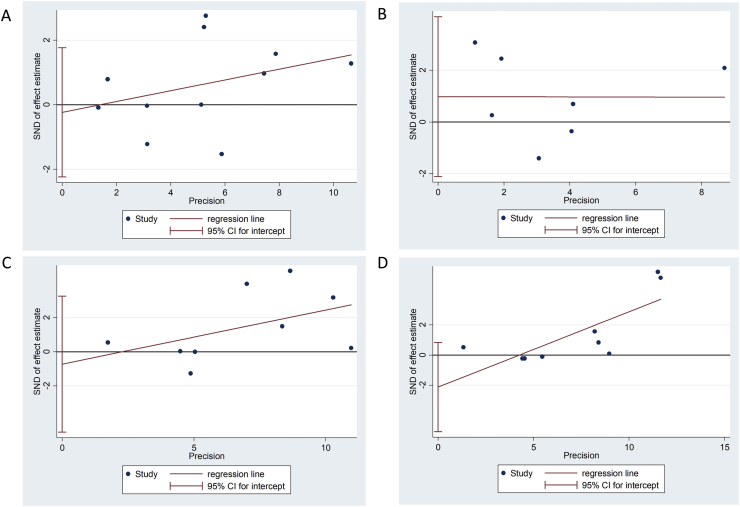
Quality assessment of the included studies is summarized in Table 3. Funnel plots (Fig. 2) and Egger's tests (Fig. 3) revealed no publication bias among the included studies. In the domain of “Bias due to confounding”, several confounding factors or variations were uncontrolled among studies, so the overall risk of bias assessment of all included studies were “moderate risk of bias”. The factors related to “Bias due to confounding” included SS-related factors (Table 1) and periodontal examination related factors (Table 4).
Table 3.
Risk of bias evaluation of the included studies.
| Domain | Antoniazzi et al.10 | Ergun et al.11 | Kuru et al.22 | Marton et al.23 | Najera et al.24 | Pedersen et al.16 | Pedersen et al.15 | Rhodus & Michalowicz12 | Tseng25 |
|---|---|---|---|---|---|---|---|---|---|
| Bias due to confounding | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk |
| Bias in selection of participants into the study | Low risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Bias in classification of interventions | X | X | X | X | X | X | X | X | X |
| Bias due to deviations from intended interventions | X | X | X | X | X | X | X | X | X |
| Bias due to missing data | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bias in measurement of outcomes | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bias in selection of the reported result | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Overall | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk |
Figure 2.
Funnel plots (A) probing pocket depth (PPD) (B) Clinical attachment loss (CAL) (C) Plaque index (PI) (D) gingival index (GI).
Figure 3.
Egger's test (A) probing pocket depth (PPD) (B) Clinical attachment loss (CAL) (C) Plaque index (PI) (D) gingival index (GI).
Table 4.
Periodontal status that is related heterogeneity.
| Author | selected teeth | probing sites/surface | Type of probes | Plaque index | Gingival index | Prior periodontal treatment | DM and smoking |
|---|---|---|---|---|---|---|---|
| Antoniazzi et al.10 | all erupted teeth, excluding 3rd molar | PPD and CAL of six sites (mesio-buccal, disto-buccal, mesio-lingual, mid-lingual and distal lingual) of each tooth; PI and GI of four sites (mesial, buccal, distal and lingual) | indicating what kind of probe they used | Silness & Loe31 | Loe32 | excluding patients receiving periodontal treatment. | recruiting three, two and five heavy smokers in PSS, SSS and control groups, respectively; excluding DM patients |
| Ergun et al.11 | not mentioned | four sites (buccal, mesial, lingual and distal) | Hu-Friedy periodontal probe with Williams's markings | approximal plaque index | not mentioned | ||
| Kuru et al.22 | all erupted teeth, excluding 3rd molar | four sites (disto-, mid-, mesio-buccal, mid-lingual) | the William's periodontal probe | Silness & Loe31 | Loe & Silness33 | ||
| Marton et al.23 | not mentioned | four sites of the selected teeth | the calibrated periodontal probe | not mentioned | not mentioned | ||
| Najera et al.24 | all erupted teeth, excluding 3rd molar | four sites (disto-, mid-, mesio-buccal, mid-lingual) | the Florida probe | Silness & Loe,31 Loe32 | five SS subjects and three control subjects who had received periodontal surgery | excluding DM patients in the control group | |
| Pedersen et al.16 | Ramfjord teeth | four sites (buccal, mesial, lingual and distal) | manual periodontal probe proposed by Loe | Loe32 | Loe32 | recruiting three smokers but no smokers in the control group | |
| Pedersen et al.15 | Ramfjord teeth | four sites (buccal, mesial, lingual and distal) | manual periodontal probe proposed by Loe | Loe32 | Loe32 | recruiting five smokers but no smoker in the control group | |
| Rhodus and Michalowicz12 | Ramfjord teeth | six sites of the selected teeth. | Michigan “O” probe with Willian's marking | not mentioned | excluding patients receiving periodontal treatment | excluding smokers | |
| Tseng25 | Ramfjord teeth +26 | mesio-facial surfaces of upper right first molar, upper left first premolar and first molar and lower right central incisor, and mesio-lingual surfaces of upper left central incisor, left lower first molar and lower right first premolar | Michigan “O”probe with Willian's marking | Loe32 | Silness & Loe31 |
Data analysis
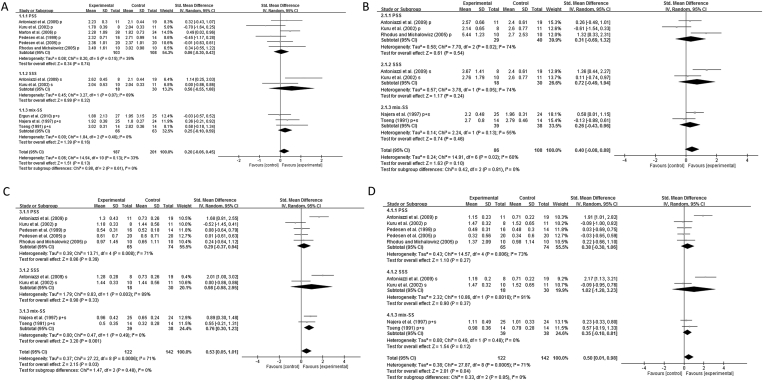
PPD and CAL of total SS subjects were provided in nine and five studies, respectively. The quantitative analysis revealed no deeper PPD (Fig. 4A, total) or CAL (Fig. 4B, total) in total SS patients (PPD: Weighted mean difference (WMD) = 0.20, 95% CI: −0.06, 0.45; CAL: WMD = 0.40, 95% CI: −0.08, 0.88), compared to the healthy controls. The subgroup analyses also showed that PPD or CAL in PSS, SSS or mix-SS subjects was not deeper than in controls (Fig. 4A and B: PSS, SSS and mix-SS).
Figure 4.
Forest plots showing the weighted mean difference (WMD) between Sjögren's Syndrome (SS) patients and non-SS patients (A) probing pocket depth (PPD) (B) Clinical attachment loss (CAL) (C) Plaque index (PI) (D) gingival index (GI).
PI and GI of total SS subjects were both provided in seven studies. PI (Fig. 4C, total) and GI (Fig. 4D, total) in total SS subjects were higher than in controls (PI: WMD of total = 0.53, 95% CI: 0.05, 1.01; GI: WMD of total = 0.50, 95% CI: 0.01, 0.98). However, the subgroup analyses revealed different profiles of both parameters among different groups. Quantitative analyses showed higher PI in patients with mix-SS (WMD = 0.76, 95% CI: 0.30, 1.23), but not in patients with PSS (WMD = 0.29, 95% CI: −0.37, 0.94) or SSS (WMD = 0.98, 95% CI: −0.98, 2.95; Fig. 4C). Meanwhile, GI was not higher in patients with PSS (WMD = 0.38, 95% CI: −0.30, 1.06), SSS (WMD = 1.02, 95% CI: −1.20, 3.23) or mix-SS (WMD = 0.35, 95% CI: −0.10, 0.81) than healthy controls.
Presence of significant heterogeneity for data of PI and GI in total SS subjects is suggested (Fig. 4C and D). The subgroup analyses showed I2 for PI of PSS, SSS and mix-SS were 71%, 89% and 0%. I2 for GI of PSS, SSS and mix-SS were 73%, 91% and 0%, indicating the presence of the heterogeneity. Based on the visual inspection of the forest plots, the study of Antoniazzi et al.10 was likely the outlier and contributed to the heterogeneity. The sensitivity test showed that removing the study of Antoniazzi et al.10 led to the conclusion of no difference in PI and GI of SS patients and controls (Table 5). Additionally, opposite results of PI or GI were also obtained by removing studies of Najera et al.24 or Tseng,25 two studies that compared the mix-SS group with controls. Removing the study of Kuru et al.22 also led to a conclusion that PPD and CAL were deeper in patients of SS than controls. Removing the study of Pedesen et al.16 resulted in deeper PPD in patients of SS than controls.
Table 5.
Sensitivity analyses of included studies.
| Indices | Excluded study | Standard mean difference (95% CI) | Heterogeneity (I2), % |
|---|---|---|---|
| PPD | Antoniazzi et al.10 | 0.12 (−0.13, 0.38) | 22 |
| Kuru et al.22 | 0.27 (0.02, 0.53)∗ | 26 | |
| Marton et al.23 | 0.15 (−0.13, 0.43) | 32 | |
| Pedesen et al.16 | 0.26 (0.01, 0.51)∗ | 22 | |
| Pedesen et al.15 | 0.22 (−0.06, 0.50) | 38 | |
| Rhodus and Michalowicz4 | 0.19 (−0.09,0.46) | 39 | |
| Ergun et al.11 | 0.23 (−0.06, 0.51) | 36 | |
| Najera et al.24 | 0.18 (−0.11, 0.46) | 39 | |
| Tseng25 | 0.16 (−0.11, 0.43) | 36 | |
| CAL | Antoniazzi et al.10 | 0.25 (−0.32, 0.82) | 60 |
| Kuru et al.22 | 0.62 (0.09, 1.14)∗ | 56 | |
| Rhodus and Michalowicz4 | 0.27 (−0.20, 0.75) | 55 | |
| Najera et al.24 | 0.36 (−0.23, 0.95) | 65 | |
| Tseng25 | 0.49 (−0.04, 1.02) | 60 | |
| PI | Antoniazzi et al.10 | 0.23 (−0.11, 0.58)∗ | 32 |
| Kuru et al.22 | 0.73 (0.20, 1.25) | 70 | |
| Pedesen et al.16 | 0.59 (0.05, 1.12) | 73 | |
| Pedesen et al.15 | 0.60 (0.07, 1.14) | 71 | |
| Rhodus and Michalowicz4 | 0.56 (0.03, 1.10) | 74 | |
| Najera et al.24 | 0.48(-0.07, 1.02)∗ | 72 | |
| Tseng25 | 0.53 (−0.02, 1.07)∗ | 74 | |
| GI | Antoniazzi et al.10 | 0.13 (−0.14, 0.41)∗ | 0 |
| Kuru et al.22 | 0.66 (0.08, 1.25) | 76 | |
| Pedesen et al.16 | 0.56 (0.02, 1.11) | 74 | |
| Pedesen et al.15 | 0.58 (0.03, 1.12) | 73 | |
| Rhodus and Michalowicz4 | 0.53 (−0.1, 1.08)∗ | 75 | |
| Najera et al.24 | 0.55 (−0.03, 1.12)∗ | 75 | |
| Tseng25 | 0.49 (−0.06, 1.04)∗ | 75 |
PPD, periodontal probing depth; CAL, clinical attachment loss; PI, plaque index; GI, gingival index; SE, standard error of the mean; SD, standard deviation; CI: confident interval.
∗ indicating results that are different from the data present in the meta-analysis of Fig. 4.
Discussion
Although the comparison of periodontal parameters in SS patients with healthy controls has been reported in two meta-analyses, this study outcompeted the previous ones by (1) correctly transferring the data of SE and SEM reported in 3 studies10, 11, 12 to SD for meta-analysis, in contrast to the study conducted by Antoniazzi et al.,10 and (2) removing two studies26,27 that included patients with sicca syndrome, other systemic autoimmune disease or subjects with anti-SSA/SSB autoantibodies as controls, in contrast to the study conducted by Maarse et al.9
This systematic review and meta-analysis showed that PI and GI in total SS patients were greater than the healthy controls. This might be due to the decrease of salivary glands functions, which are critically important for the maintenance of oral health. Xerostomia was shown to be associated with plaque accumulation and bleeding on probing.28 The change of salivary viscosity, the decline of self-cleaning ability, the reduction of lubricating ability, the loss of antimicrobial molecules could all contribute to plaque accumulation.10 Our study echoes with the meta-analyses conducted by de Goés Soares et al.8 In contrast, given that “researchers should avoid categorizing active and inactive controls into a generic control group to achieve meaningful estimates”,29 estimated effects of SS in the study of Maarse et al.9 were likely to be seriously confounded by differences in comparator group intensity.
Although the issue of heterogeneity was also detected in previous meta-analyses,8,9 we further explored it with strategies in additions to I2 and chi2 tests. The subgroup analysis, which were not performed genuinely in previous studies, showed that PI in PSS or SSS subjects, or GI in PSS, SSS or mix-SS subjects was not severer than in healthy controls. The sensitivity test showed that inclusion of three studies,10,24,25 two of which recruited mix-SS subjects,24,25 led to the significantly higher PI or GI in SS subjects. These findings are threats to the validity of the results. While SS is well known for its diverse presentation of the disease, it was not surprised that characteristics of subjects were varied among different observational studies. Thus, to include studies that are de facto the same, the core philosophy of meta-analysis,30 was very difficult. However, considering that the heterogeneity of PI and GI in the mix-SS subjects was absent (I2 = 0, Fig. 3C and D), the higher PI and GI in the mix-SS subjects than healthy controls might be convincing.
On the other hand, the difference between the results of total SS and subgroup analyses could be explained by the insufficient sample size in each subgroup. The result could be considered as a study testing whether different periodontal parameters in SS patients were different from controls. Using data from this meta-analysis to estimate sample size, 208 subjects should be recruited for sufficient statistical power. The sample sizes for the PSS and SSS subgroups were 139 and 49, relatively. Thus, the probability of detecting an actual existing effect of PSS or SSS was relatively low. Indeed, using statistical method to increase sample size is the strength of meta-analysis.
Additionally, we were unable to exclude publication bias even though there was no funnel plot asymmetry. It is possible that negative studies were not published. The heterogeneity comes from factors that would affect periodontal parameters, which were reviewed for the first time in Table 4. The confounding factors of periodontal disease, including DM, smoking and previous periodontal history/treatment, etc., were neither controlled well in most studies. Removing the study conducted by Najera et al.,24 which included subjects who received periodontal surgery, led to different conclusion for PI and GI (Table 5). Subjects in the included studies had different SS-diagnosed duration and medications, potentially impacting the progress of PD differently. Finally, while a study10 reported the medication for the treatment of the SS, information about treatments of SS subjects in other studies was limited. It is unclear how treatment may modify the disease course. This systematic review included a comprehensive and systematic literature search, quantitatively and qualitatively studying most periodontal aspects in SS, including PPD, CAL, PI and GI. It also corrected biased data from previous reports.
In conclusion, outcomes of the present meta-analysis highlighted negative impacts on PI and GI in subjects with SS. However, regarding the threat from heterogeneity, reported estimates might not be solid enough to conclude the effect of SS on periodontal conditions. Thus, a well-designed research with large sample sizes, matching of co-founding factors and simultaneous observation of periodontal and glandular changes is required to identify effects of SS on periodontal tissues. Meanwhile, due to the potential impacts of low salivary flow rate on plaque accumulation, dentists should keep reinforce the teeth cleaning ability of the patients with SS.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgement
Authors appreciate the generous advice from Professor Chia-Shu Lin, Department of Dentistry, National Yang Ming Chiao Tung University and Center for Evidence-based Medicine, Taipei Veterans General Hospital. All authors claim no funding source for this study.
References
- 1.Nocturne G., Mariette X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. Nat Rev Rheumatol. 2013;9:544–556. doi: 10.1038/nrrheum.2013.110. [DOI] [PubMed] [Google Scholar]
- 2.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 3.Lin C.Y., Tseng C.F., Liu J.M. Association between periodontal disease and subsequent Sjögren's syndrome: a nationwide population-based Cohort study. Int J Environ Res Publ Health. 2019;16 doi: 10.3390/ijerph16050771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Lozano B., Gonzalez-Febles J., Garnier-Rodriguez J.L. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: a case-control study. Arthritis Res Ther. 2019;21:27. doi: 10.1186/s13075-019-1808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutter-Locher Z., Smith T.O., Giles I., Sofat N. Association between systemic lupus erythematosus and periodontitis: a systematic review and meta-analysis. Front Immunol. 2017;8:1295. doi: 10.3389/fimmu.2017.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scardina G.A., Ruggieri A., Messina P. Periodontal disease and sjogren syndrome: a possible correlation? Angiology. 2010;61:289–293. doi: 10.1177/0003319709344576. [DOI] [PubMed] [Google Scholar]
- 7.Zoellner H., Chapple C.C., Hunter N. Microvasculature in gingivitis and chronic periodontitis: disruption of vascular networks with protracted inflammation. Microsc Res Tech. 2002;56:15–31. doi: 10.1002/jemt.10009. [DOI] [PubMed] [Google Scholar]
- 8.de Goes Soares L., Rocha R.L., Bagordakis E., Galvao E.L., Douglas-de-Oliveira D.W., Falci S.G.M. Relationship between Sjögren syndrome and periodontal status: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:223–231. doi: 10.1016/j.oooo.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Maarse F., Jager D.H.J., Alterch S. Sjögren's syndrome is not a risk factor for periodontal disease: a systematic review. Clin Exp Rheumatol. 2019;37(Suppl 118):225–233. [PubMed] [Google Scholar]
- 10.Antoniazzi R.P., Miranda L.A., Zanatta F.B. Periodontal conditions of individuals with Sjögren's syndrome. J Periodontol. 2009;80:429–435. doi: 10.1902/jop.2009.080350. [DOI] [PubMed] [Google Scholar]
- 11.Ergun S., Cekici A., Topcuoglu N. Oral status and candida colonization in patients with Sjögren's syndrome. Med Oral Patol Oral Cir Bucal. 2010;15:e310–e315. doi: 10.4317/medoral.15.e310. [DOI] [PubMed] [Google Scholar]
- 12.Rhodus N.L., Michalowicz B.S. Periodontal status and sulcular Candida albicans colonization in patients with primary Sjögren's syndrome. Quintessence Int. 2005;36:228–233. [PubMed] [Google Scholar]
- 13.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne J.A., Hernan M.A., Reeves B.C. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen A.M., Bardow A., Nauntofte B. Salivary changes and dental caries as potential oral markers of autoimmune salivary gland dysfunction in primary Sjögren's syndrome. BMC Clin Pathol. 2005;5:4. doi: 10.1186/1472-6890-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen A.M., Reibel J., Nordgarden H., Bergem H.O., Jensen J.L., Nauntofte B. Primary Sjögren's syndrome: salivary gland function and clinical oral findings. Oral Dis. 1999;5:128–138. doi: 10.1111/j.1601-0825.1999.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 19.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oxman A.D., Guyatt G.H. A consumer's guide to subgroup Analyses. Ann Intern Med. 1992;116:78–84. doi: 10.7326/0003-4819-116-1-78. [DOI] [PubMed] [Google Scholar]
- 21.Aurelio T. Assessing the influence of a single study in the meta-anyalysis estimate. Stata Technical Bulletin. 1999;8 [Google Scholar]
- 22.Kuru B., McCullough M.J., Yilmaz S., Porter S.R. Clinical and microbiological studies of periodontal disease in Sjögren syndrome patients. J Clin Periodontol. 2002;29:92–102. doi: 10.1034/j.1600-051x.2002.290202.x. [DOI] [PubMed] [Google Scholar]
- 23.Marton K., Boros I., Varga G. Evaluation of palatal saliva flow rate and oral manifestations in patients with Sjögren's syndrome. Oral Dis. 2006;12:480–486. doi: 10.1111/j.1601-0825.2005.01224.x. [DOI] [PubMed] [Google Scholar]
- 24.Najera M.P., al-Hashimi I., Plemons J.M. Prevalence of periodontal disease in patients with Sjögren's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol. 1997;83:453–457. doi: 10.1016/s1079-2104(97)90144-x. [DOI] [PubMed] [Google Scholar]
- 25.Tseng C.C. Periodontal status of patients with Sjögren's syndrome: a cross-sectional study. J Formos Med Assoc. 1991;90:109–111. [PubMed] [Google Scholar]
- 26.Le Gall M., Cornec D., Pers J.O. A prospective evaluation of dental and periodontal status in patients with suspected Sjögren's syndrome. Joint Bone Spine. 2016;83:235–236. doi: 10.1016/j.jbspin.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen A.M., Andersen T.L., Reibel J., Holmstrup P., Nauntofte B. Oral findings in patients with primary Sjögren's syndrome and oral lichen planus: a preliminary study on the effects of bovine colostrum-containing oral hygiene products. Clin Oral Invest. 2002;6:11–20. doi: 10.1007/s00784-001-0148-x. [DOI] [PubMed] [Google Scholar]
- 28.Mizutani S., Ekuni D., Tomofuji T. Relationship between xerostomia and gingival condition in young adults. J Periodontal Res. 2015;50:74–79. doi: 10.1111/jre.12183. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson P., Bergmark A. Compared with what? An analysis of control-group types in Cochrane and Campbell reviews of psychosocial treatment efficacy with substance use disorders. Addiction. 2015;110:420–428. doi: 10.1111/add.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S-j, Jiang H., Yang H. The dilemma of heterogeneity tests in meta-analysis: a challenge from a simulation study. PloS One. 2015;10 doi: 10.1371/journal.pone.0127538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silness J., Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 32.Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 33.Loe H., Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]