Abstract
Background/purpose
Pyroptosis is a form of programmed cell death dependent on the activation of caspase-1. Porphyromonas gingivalis (P. gingivalis) is a major pathogenic bacterium in periodontitis and its lipopolysaccharide (LPS) can trigger inflammation. However, whether P. gingivalis-LPS affects epithelial connections or triggers pyroptosis in the gingival epithelium is unknown.
Materials and methods
Gingival samples from human donors were collected and the expression levels of E-cadherin, nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), caspase-1/4/5, interleukin (IL)-18, and IL-1β were examined. P. gingivalis-LPS was injected into rat gingival sulcus to establish gingivitis models, and the expression levels of E-cadherin, NLRP3, caspase-1/11, IL-18, and IL-1β were compared via immunohistochemistry. The mRNA levels of E-cadherin, caspase-1, IL-18, and IL-1β were evaluated in oral mucosa epithelial cells (OMECs) and rat gingival tissues.
Results
In the present study, NLRP3 (p < 0.01), caspase-1 (p < 0.01), caspase-4 (p = 0.044), and IL-18 (p = 0.036) expression was greater in the human inflammatory gingival samples, whereas E-cadherin (p = 0.045) had the opposite presentation. Gingivitis models were successfully established in rats with the injection of P. gingivalis-LPS. NLRP3 (p = 0.015), caspase-1 (p < 0.01), caspase-11 (p < 0.01), and IL-18 (p = 0.041) were upregulated, whereas E-cadherin (p = 0.038) expression was decreased. Furthermore, E-cadherin mRNA was decreased while caspase-1, IL-18, and IL-1β mRNA levels were increased. The addition of a caspase-1 inhibitor reversed the expression changes.
Conclusion
P. gingivalis-LPS may effectively destroy the epithelial connection by triggering pyroptosis.
Keywords: Epithelial connection, Inflammation, Lipopolysaccharide, Pyroptosis
Introduction
Periodontitis is an oral chronic inflammatory disease characterized by periodontal tissue inflammation and alveolar bone absorption as a result of an immune response to pathogen invasion.1,2 In terms of the pathogenesis of periodontitis, proinflammatory factors are secreted into the gingival sulcus, thereby increasing the leukocyte count and gingival crevicular fluid volume.3 The junctional epithelium is subsequently impaired by pathogens, inflammatory cells, and cytokines,4 followed by the activation of osteoclasts and alveolar bone resorption.5 Therefore, the gingival epithelial barrier is the first line of defense against pathogenic invasion and its integrity plays an important role in preventing infections and gingival inflammation. Microorganisms represent another major contributing factor in the development of periodontitis, particularly Porphyromonas gingivalis (P. gingivalis), which is one of the major pathogenic bacteria associated with periodontal diseases. P. gingivalis secretes LPS, which can effectively induce the production of proinflammatory cytokines.6 LPS causes further destruction and disruption of the epithelial barrier by affecting the tight junctions among gingival epithelial cells.7
Pyroptosis (also called inflammatory cell death) refers to programmed cellular death and was first discovered in macrophages infected by salmonella typhi.8 Pyroptosis is dependent on caspase-1 activation, manifesting as nuclear shrinkage, chromatin fragmentation, membrane swelling, and rupturin.9 When pathogen-associated molecular patterns (PAMPs) invade host cells, intracellular sensor pattern recognition receptors (PRRs), nucleotide-binding oligomerization domain-like receptors (NLRs), and cytoplasmic absent in melanoma 2 (AIM2) proteins can recognize the signal and bind to apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC). Further, ASC may attach to caspase-1 to form a protein-inflammasome complex that participates in immune killing.10,11
If the epithelial connection is affected, it is easy for PAMPs to destroy tissue and accelerate the damage process.12 In the canonical pathway, PAMPs from pathogens activate caspase-1 through ASC, following the cleaving and formation of IL-18 and IL-1β. The activated IL-18 and IL-1β play key roles in inflammation and the upregulation of other proinflammatory cytokines associated with the inflammatory response.13,14 Additionally, inflammatory factors may open the pores of the cell membrane, together with an increase in inflammation, cell swelling, and rupture.15 In the non-canonical pathway, PAMPs directly activate caspase-4/5 in humans or caspase-11 in rats,16 which hydrolyzes Gasdermin-D into Gasdermin-N and regulates caspase-1 to induce pyroptosis.17
A number of researchers have reported the association of periodontitis and NLR activation through nucleotide-binding oligomerization domain-containing protein 1 (NOD1), NOD2, and NLRP6,18, 19, 20 which may indicate the development of pyroptosis in periodontitis. Periodontitis is a continuously progressive disease with no alveolar bone resorption in the early stages (gingivitis), corresponding to a remarkably lower inflammatory response than that in established periodontitis.21 However, literature reporting the relationship between pyroptosis and gingivitis is sparse and whether pyroptosis in periodontitis occurs at the gingivitis stage remains unknown. In the present study, we investigated the role of P. gingivalis-LPS to determine whether it triggered pyroptosis or affected epithelial connections in the gingival epithelium.
Materials and methods
Experimental ethics
The study was approved by the institutional research ethics committee at the Hospital of Stomatology, Jilin University (Ref No. SSJLU-2015045). Clinical samples were collected following the Declaration of Helsinki, 2013. Informed consent was obtained from each donor prior to participation in the present study. Animal experiments were conducted according to the ethical guidelines for animals.
Sample collection
Fifteen healthy and 15 inflammatory gingival tissue specimens were obtained from 30 donors (18 men and 12 women; age: 20–56 y, mean 35.8 y) who visited the Department of Periodontology and Oral and Maxillofacial Surgery, Hospital of Stomatology, Jilin University, China. The participants in the healthy group had good oral hygiene with no signs of periodontal diseases (probing depth (PD) < 3 mm and no bleeding on probing (BOP)). Healthy gingival tissue specimens were obtained by gingivectomy either during crown lengthening or following tooth extraction. In the inflammatory group (presenting with chronic periodontitis with PD > 5 mm), gingival tissue specimens were obtained during gingivectomy or flap surgery for hyperplastic or chronic periodontitis-associated gingival tissues. Donors who were adolescents, pregnant, smokers, or receiving active drug treatment, and those who had a history of systemic diseases (such as cardiovascular disease, diabetes, autoimmune disorders) were excluded. The gingival tissues were sectioned (4-μm thickness) for further immunohistochemistry analysis following dehydration and paraffin embedding.
Experimental gingivitis model
Twenty male Sprague Dawley (SD) rats (6–7 weeks old, 220–280 g) (HFK BioScience, Beijing, China) were housed and fed with sterile water and food under specific pathogen-free conditions. The SD rats were randomly divided into gingivitis and control groups (n = 10). The experimental model was established as described previously.22 For the gingivitis group rats, an aliquot of 10 μL (1 mg/mL) P. gingivalis-LPS (InvivoGen, San Diego, CA, USA) was injected into the bottom of the gingival sulci at the labial site of the maxillary central incisor every 2 days for a total of five times and control group rats were injected with saline.
Ten days later, the rats were executed by dislocation, the gingivae were excised and fixed in 10% formalin, and sections were produced following dehydration and paraffin embedding.
Immunohistochemistry
Immunochemical staining analysis was conducted as described previously.23 For this purpose, antibodies against IL-18 and E-cadherin were obtained from Biosynthesis Biotechnology Co., Ltd, Beijing, China. E-cadherin, NLRP3, IL-1β, caspase-1, caspase-4, and caspase-5 were supplied by ABclonal Biotech Co., Ltd (Wuhan, China) and caspase-11 was supplied by Abcam, Cambridge, UK. The dilution of caspase-11 for the rats was 1:200, and the concentrations of all other reagents were 1:100.
The sections were viewed and scored under a light microscope (Olympus, Tokyo, Japan) by two observers blinded to the methodology. Five microscope fields at × 400 were selected randomly and yellow or brown areas at the cytomembrane or cytoplasm were considered indicative of positive immunoreaction. The scores of positivity (0, 0–10% positivity; 1, 10–25% positivity; 2, 25–50% positivity; 3, 50–75% positivity; 4, 75–100% positivity) and positive intensity (0, negative; 1, weak; 2, moderate; 3, strong) were determined as described previously.23 The staining index was calculated using the following formula:
| Index value = Score of positivity (%) ∗ the staining intensity score. |
Cell culture
Oral mucosa epithelial cells (OMECs) were obtained from a healthy donor (male, 23 years) at the Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Jilin University, China as reported in a previous study.23 The OMECs were cultured in Keratinocyte Serum-Free Medium (InvivoGen). Cells grown from the fourth to sixth passages in the logarithmic phase were used in the experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
OMECs were cultured with P. gingivalis-LPS (1 μg/mL) or 1 mM of Z-YVAD-FMK (YVAD, a caspase-1 inhibitor; Abcam) for 24 h. Total RNA from OMECs and from the epithelium of rat gingivae was isolated using TRIeasy™ reagent (YEASEN, Shanghai, China) and the total RNA was used to synthesize cDNA with a reverse transcription kit (YEASEN). The mRNAs were expanded using the SYBR-Green Real-time PCR master mix (YEASEN) in a CFX96 RT-PCR Detection System (Bio-Rad, Hercules, CA, USA). The primer sequences are shown in Table 1. The qPCR cycling protocol consisted of the initial activation cycle at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s, extension at 72 °C for 20 s, and a final extension at 72 °C for 5 min. The gene expression levels were quantified using the comparative 2−ΔΔCT method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Table 1.
Primer sequences.
| Gene | Species | Sequences (5′- 3′) |
|---|---|---|
| E-cadherin | Homo | F: CGAGATCTACAAGTTCACCG |
| R: AGTGGAGCTTCAGCGTGATG | ||
| Rattus | F: TGAAGCCCAGGAAATACACCC | |
| R: GGATTAAAGGCGTGCACCAAC | ||
| caspase-1 | Homo | F: ACACGTCTTGCCCTCATTATCT |
| R: ATAACCTTGGGCTTGTCTTTCA | ||
| Rattus | F: TCAGGCTCAGAAGGGAATG | |
| R: CGCTGTACCCCAGATTTTGT | ||
| IL-18 | Homo | F: ATGCCTGATATCGACCGAAC |
| R: TGGCACACGTTTCTGAAAGA | ||
| Rattus | F: TGGCTGCTGAACCAGTAGAG | |
| R: CTAGAGGCCGATTTCCTTGG | ||
| IL-1β | Homo | F: CAGCAGCATCTCGACAAGAG |
| R: CATCATCCCACGAGTCACAG | ||
| Rattus | F: TGAACTCAACTGTGAAATAGC | |
| R: CCCAAGTCAAGGGCTTGGAA | ||
| GAPDH | Homo | F: GCCATGTACGTAGCCATCCA |
| R: GAACCGCTCATTGCCGATAG | ||
| Rattus | F: GGCACAGTCAAGGCTGAGAATG | |
| R: ATGGTGGTGAAGACGCCAGTA |
Statistical analysis
Statistical analyses were conducted using SPSS v22.0 software (IBM, New York, New York, USA). Comparisons among groups were assessed using the analysis of variance (ANOVA) and Student's t-test. Comparisons between factors were analyzed using the Pearson correlation test. A P-value of <0.05 was considered statistically significant.
Results
Expression of associated proteins in inflammatory and healthy gingival tissues
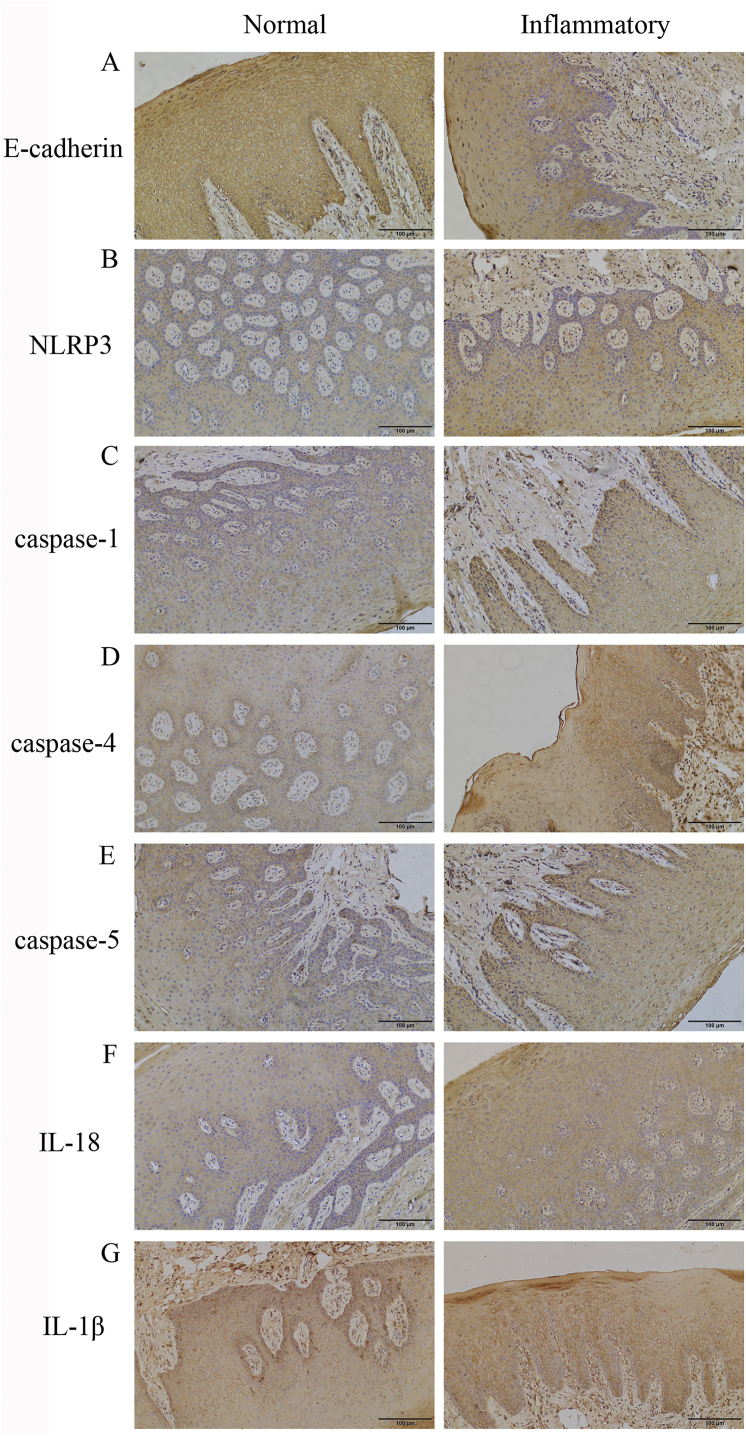
The expression levels of various proteins in inflammatory and healthy human and rat tissues are shown in Table 2. It is worth noting that the levels of NLRP3 (p < 0.01), caspase-1 (p < 0.01), caspase-4 (p = 0.044), and IL-18 (p = 0.036) expression in inflammatory tissues were significantly higher than those in normal tissues whereas the expression of E-cadherin had the opposite presentation (p = 0.045) (Fig. 1; Table 2). Although IL-1β (p = 0.533) and caspase-5 (p = 0.138) had relatively high expression in inflammatory tissues, a statistical index was not reached. Moreover, there was a positive association between caspase-1 and NLRP3 (r = 0.571, p < 0.01), IL-18 (r = 0.512, p = 0.021), and caspase-4 (r = 0.49, p = 0.028) and a negative correlation with E-cadherin (r = −0.527, p = 0.017). The results indicated the weakening of the epithelial connection and pyroptosis activation in the inflamed gingivae.
Table 2.
The expression of different proteins in human and rat gingivitis samples.
| Proteins | Index values (Human) |
p value | Proteins | Index values (Rats) |
p value | ||
|---|---|---|---|---|---|---|---|
| Inflammatory | Normal | Gingivitis | Control | ||||
| E-cadherin | 6.00 ± 1.47 | 4.50 ± 1.65 | 0.045 | E-cadherin | 4.47 ± 1.31 | 5.73 ± 1.22 | 0.038 |
| NLRP3 | 6.13 ± 1.72 | 3.83 ± 1.29 | <0.01 | NLRP3 | 5.33 ± 0.98 | 3.90 ± 1.36 | 0.015 |
| Caspase-1 | 6.07 ± 1.33 | 4.17 ± 1.15 | <0.01 | Caspase-1 | 5.93 ± 1.07 | 4.30 ± 1.36 | <0.01 |
| IL-18 | 6.10 ± 1.32 | 4.93 ± 0.97 | 0.036 | IL-18 | 6.20 ± 1.61 | 4.80 ± 1.20 | 0.041 |
| IL-1β | 5.53 ± 0.85 | 5.20 ± 1.42 | 0.533 | IL-1β | 5.00 ± 0.59 | 4.17 ± 1.43 | 0.105 |
| Caspase-4 | 5.93 ± 1.07 | 4.67 ± 1.50 | 0.044 | Caspase-11 | 4.67 ± 0.65 | 3.13 ± 1.16 | <0.01 |
| Caspase-5 | 5.40 ± 1.34 | 4.53 ± 1.15 | 0.138 | ||||
The data were statistically analyzed using the Student's t-test.
Figure 1.
Comparison of protein expression in healthy and inflammatory human gingival tissues ( × 200) (A) E-cadherin expression was reduced in inflammatory tissues (B, C, D, F) the expression levels of NLRP3, caspase-1, caspase-4, and IL-18 were upregulated in inflammatory tissues (E, G) no difference in the expression of caspase-5 and IL-1β was observed between the two groups.
P. gingivalis-LPS promoted gingivitis in the rat experimental model
In the experimental gingivitis model, all rats (n = 20) remained healthy without developing any systemic illnesses. Under macroscopic observation, the rats’ gingival papilla appeared red and swollen in the gingivitis group and the gingivae of nine out of 10 rats exhibited BOP (+).
In contrast, the control group rats showed no swelling of the gingival papillae and no BOP. Although the gingival papillae of a number of control group rats turned bright red, the intensity was weaker than that in the gingivitis group. In general, the gingival papillae reacted strongly in the gingivitis group rats and turned pink in the control group rats. Clinical observation of the labial papillae of the central incisors in the gingivitis group rats revealed inflammation, which demonstrated that P. gingivalis-LPS promoted the development of gingivitis.
Pyroptosis-associated protein activation in epithelium of gingivitis
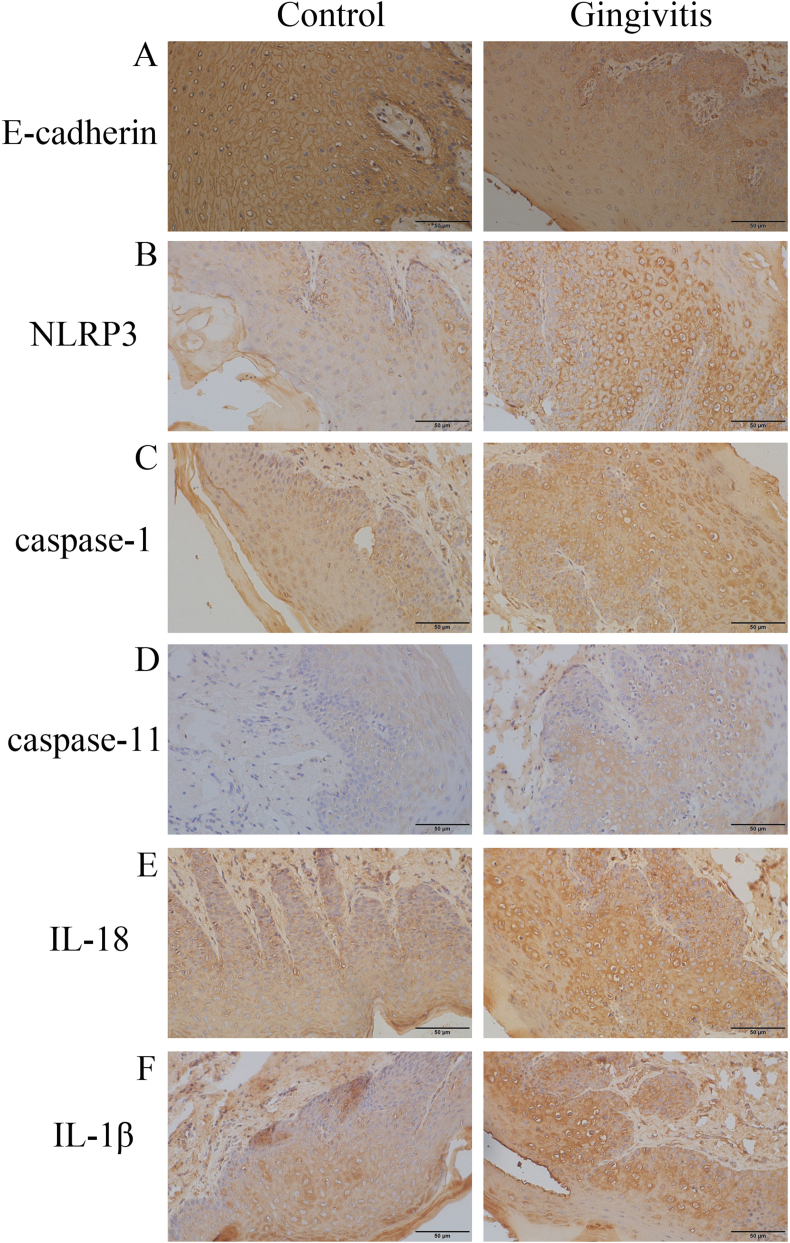
P. gingivalis-LPS effectively promoted the development of gingivitis by regulating the expression of the associated proteins (Fig. 2; Table 2). In the gingivitis group, the expression levels of NLRP3 (p = 0.015), caspase-1 (p < 0.01), caspase-11 (p < 0.01), and IL-18 (p = 0.041) were obviously higher than the levels in the control group; however, no significant change was observed in the expression level of IL-1β (p = 0.105). Furthermore, decreased E-cadherin expression was observed in the epithelium under the inflamed condition (p = 0.038). Similarly, a positive association was also observed between caspase-1 and NLRP3 (r = 0.631, p < 0.01), caspase-11 (r = 0.477, p = 0.033), and IL-18 (r = 0.728, p < 0.01), and an inverse association was observed between caspase-1 and E-cadherin (r = −0.529, p = 0.016) (Table 3). The results showed that P. gingivalis-LPS interfered with the epithelial connection and promoted the development of pyroptosis in the rats’ gingival epithelium.
Figure 2.
Expression of various proteins in rat gingivitis models ( × 400) (A) Levels of E-cadherin were lower in the gingivitis group (B, C, D, E) expression levels of NLRP3, caspase-1, caspase-11, and IL-18 were higher in gingivitis group compared to those in the control group (F) no difference existed in IL-1β expression.
Table 3.
Correlations of protein expression levels in human and rat gingival tissues.
| Proteins | Human |
Proteins | Rats |
||
|---|---|---|---|---|---|
| Correlation index | p value | Correlation index | p value | ||
| caspase-1 vs. E-cadherin | −0.527 | 0.017 | caspase-1 vs. E-cadherin | −0.529 | 0.016 |
| caspase-1 vs. NLRP3 | 0.571 | <0.01 | caspase-1 vs. NLRP3 | 0.631 | <0.01 |
| caspase-1 vs. IL-18 | 0.512 | 0.021 | caspase-1 vs. IL-18 | 0.728 | <0.01 |
| caspase-1 vs. IL-1β | 0.363 | 0.116 | caspase-1 vs. IL-1β | 0.389 | 0.09 |
| caspase-1 vs. caspase-4 | 0.49 | 0.028 | caspase-1 vs. caspase-11 | 0.477 | 0.033 |
| caspase-1 vs. caspase-5 | 0.372 | 0.106 | caspase-11 vs. E-cadherin | −0.425 | 0.062 |
| caspase-4 vs. caspase-5 | 0.326 | 0.161 | |||
The data were statistically analyzed using the Pearson correlation test.
P. gingivalis-LPS enhanced pyroptosis at the mRNA level
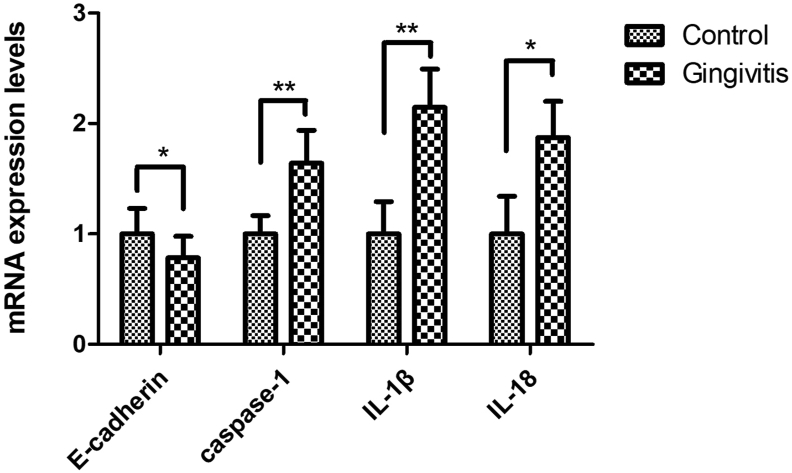
To confirm the reliability of P. gingivalis-LPS augmented pyroptosis in the gingivitis epithelium, we examined the mRNA expression utilizing rat gingivae (Fig. 3). The E-cadherin mRNA level was significantly decreased in the gingivitis group (p < 0.05), while the pyroptosis-related proteins caspase-1 (p < 0.01), IL-1β (p < 0.01), and IL-18 (p < 0.05) were significantly increased. These results indicated that gingivitis was associated with the destruction of epithelial connections and the process of pyroptosis.
Figure 3.
Expression of relative mRNA levels in rat gingivae. Epithelial connection protein E-cadherin was higher in the control group (p < 0.05); mRNA levels of the pyroptosis proteins caspase-1 (p < 0.01), IL-1β (p < 0.01), and IL-18 (p < 0.05) were higher in the gingivitis group than in the control group (n = 10 per group; ∗p < 0.05; ∗∗p < 0.01). The data were expressed as the mean ± SD and statistically analyzed using the Student's t-test.
P. gingivalis-LPS broke epithelial connection through caspase-1 activation
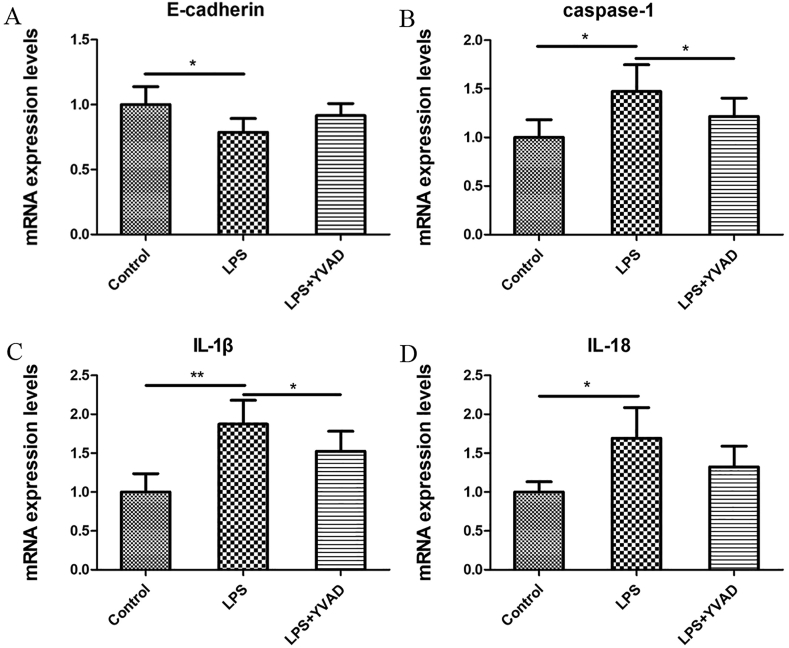
To determine the effect of pyroptosis on epithelial connections, OMECs were cultured with P. gingivalis-LPS and YVAD. The mRNA level of E-cadherin decreased (p < 0.05; Fig. 4A) and those of pyroptosis-associated proteins increased (Fig. 4B–D). Importantly, YVAD addition weakened the pyroptosis and reversed E-cadherin changes, which indicated that the pyroptosis process has a vital function in epithelial connections.
Figure 4.
Expression of relative mRNA levels in OMECs after stimulation with P. gingivalis-LPS for 24 h (A) the mRNA levels for the epithelial connection protein E-cadherin were decreased (p < 0.05) whereas (B) caspase-1 (p < 0.05) (C) IL-1β (p < 0.01), and (D) IL-18 (p < 0.05) mRNA expression levels were upregulated in OMECs. The addition of YVAD reversed these trends (∗p < 0.05; ∗∗p < 0.01). The data were expressed as the mean ± SD and statistically analyzed using the Student's t-test.
Discussion
In the present study, we used an animal model to demonstrate the association of P. gingivalis and its P. gingivalis-LPS with pyroptosis in the gingival epithelium. In addition, we investigated whether P. gingivalis-LPS triggers pyroptosis or affects the epithelial connection in the gingival epithelium at the early periodontitis stage. For this purpose, immunohistochemistry was performed to analyze the expression of NLRP3, caspase-1/4/5/11, IL-18, and IL-1β in human gingival tissues as well as in rat gingiva in which gingivitis was induced by injecting P. gingivalis-LPS into the gingival sulcus. We observed the promotion of pyroptosis and the destruction of epithelial connections corresponding to P. gingivalis-LPS-induced gingivitis. The expression of NLRP3, caspase-1, caspase-4, and IL-18 was greater in the inflammatory gingival samples. Periodontitis and gingivitis fall under two separate categories. The distinction lies in the degree of inflammation, but the effects of gingival inflammation are not different between the two diseases. Gingival inflammation (gingivitis) triggers the exudation and release of various proinflammatory factors (tumor necrosis factor-α, IL-6, IL-8, nitric oxide) into the gingival sulcus and increases the leukocyte count and gingival crevicular fluid.3 The composition (including enzymes, peptides, immunoglobulin, and local mediators) and amount of gingival crevicular fluid secretion vary according to the gingival health and can be used as a potential diagnostic tool.24 The junctional epithelium is also impaired by pathogens, inflammatory cells, and cytokines, leading to the activation of osteoclasts and alveolar bone resorption.5 Therefore, controlling the progression of inflammation and preventing its development from gingivitis to alveolar bone resorption are considered critical.
The expression of proteins associated with pyroptosis development in the gingival epithelium was evaluated in the present study. The data revealed a higher expression of caspase-1, NLRP3, and IL-18 in inflammatory gingivae than in healthy gingivae in both humans and rats (Figure 1, Figure 2; Table 2). Further, the expression of caspase-1 was positively correlated with that of NLRP3 and IL-18 and negatively correlated with that of E-cadherin, both in human and rat gingiva (Table 3). These results indicated that pyroptosis is activated by LPS during damage to the epithelial connection. Although the human samples were collected at the periodontitis stage while the samples from the rat model were collected at the gingivitis stage, there was no remarkable difference in the inflammation in the gingival tissues, suggesting that pyroptosis is a continuous process from gingivitis to periodontitis. In a recent study, Cheng et al. investigated pyroptosis in various stages of apical periodontitis and reported contrasting results and variations in caspase-1 expression associated with pyroptosis in various stages. In addition, it was confirmed that the inhibition of caspase-1 partially diminished the secretion of IL-1β, IL-6, and IL-8, as well as bone resorption.25 The differences in the results may be attributed to differences in the study designs. To ensure the integrity of pyroptosis activation signals and to detect non-canonical activation, the expression levels of caspase-4/5 in human samples and caspase-11 in rat samples were investigated in the present study. The expression of caspase-4 in inflammatory tissues was significantly higher than that in normal tissues, whereas caspase-5 showed no difference between the two groups in the human samples. Caspase-11 in the gingivitis group rats had a higher expression level than that in the control group rats, indicating the activation of non-canonical pyroptosis in the inflamed gingival tissues. Shi et al. reported that caspase-1, caspase-4, caspase-5, and caspase-11 are vital factors associated with the regulation of inflammation and pyroptosis. Although both caspase-4 and caspase-5 function in the non-canonical pathway, no correlation was found between the two factors, suggesting their general independence from each other to some extent.26,27
E-cadherin plays a substantial role in epithelial barrier connections as a function of adhesion among epithelial cells. The expression of E-cadherin in inflammatory tissues was lower than that in normal tissues in both human and rat gingivitis specimens. Similar results were observed for the E-cadherin mRNA level (Fig. 3). These findings suggested that decreased E-cadherin expression is associated with the destruction of epithelial connections and the activation of inflammatory responses.28 In addition, E-cadherin participates in the epithelial defense against pathogens through its vital function of connecting epithelial cells. Therefore, the downregulation of E-cadherin expression suggests the destruction of the protective barrier and apoptosis of epithelial cells.29 The resulting gingival epithelial barrier dysfunction facilitates further infection and inflammation expansion.30
P. gingivalis-LPS may have inhibited E-cadherin and pyroptosis activation in OMECs. However, the inhibition of caspase-1 and the associated by YVAD restored the E-cadherin expression level. These results demonstrated that pyroptosis development can disrupt epithelial connections.
Most studies have focused on pyroptosis in relation to periodontal diseases; however, reports on the relationship between non-canonical pyroptosis and gingivitis are sparse. Using immunohistochemistry, we observed that the expression of caspase-4 was significantly higher in inflammatory tissues compared to the expression in healthy tissues whereas caspase-5 showed no difference. In rat gingivitis models, caspase-11 expression was significantly higher in the gingivitis group. These results suggested that the canonical and non-canonical pathways of pyroptosis are activated simultaneously. In view of the fact that several factors function in both pathways, we performed correlation analyses to determine if consistencies exist in the two pathways. A moderately positive correlation was observed between caspase-1 and caspase-4 in human samples and between caspase-1 and caspase-11 in rat samples (Table 3), indicating a certain degree of cooperation between the two pyroptosis-activated pathways. Although the relationship between caspase-4 and caspase-5 is still unclear, no correlation was found between the two factors due to their involvement in non-canonical patterns in humans. Additionally, the IL-1β secreted in the non-canonical pathway may interfere with the results.27 Interestingly, the mRNA expression level of IL-1β in the gingivitis group was significantly higher than those in the control group (Fig. 3), whereas the protein levels were similar in the animal samples (Fig. 2F; Table 2). We speculated that continuous injection may have caused mechanical injury, which might increase the IL-1β expression. Overall, non-canonical pyroptosis can be activated by LPS and caspase-4/5/11 represents a new mode of pattern recognition in innate immunity.26,31 However, the underlying mechanism and balance between the canonical and non-canonical roles remain unknown. In addition, there is a need for further in vivo studies to ensure the reliability of the findings in this study and to confirm whether pyroptosis exists in the epithelium in other tissues.
The present study demonstrated that P. gingivalis-LPS might destroy epithelial connections by triggering pyroptosis. These findings present a new mechanism in the development of periodontitis and new insights for treatment. However, the regulation of pyroptosis and methods to control the progression require further investigation.
Declaration of competing interest
All authors declare no conflict of interests.
Acknowledgements
The study was supported by grants from the Department of Science and Technology of Jilin Province (20180101121JC; 20200404108YY) and the Finance Department of Jilin Province (JCSZ2019378-3; JCSZ2020304-24).
References
- 1.Listgarten M.A. Pathogenesis of periodontitis. J Clin Periodontol. 1986;13:418–430. doi: 10.1111/j.1600-051x.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 2.Najeeb S., Zafar M.S., Khurshid Z., Zohaib S., Almas K. The role of nutrition in periodontal health: an update. Nutrients. 2016;8:530. doi: 10.3390/nu8090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths G.S., Sterne J.A., Wilton J.M., Eaton K.A., Johnson N.W. Associations between volume and flow rate of gingival crevicular fluid and clinical assessments of gingival inflammation in a population of british male adolescents. J Clin Periodontol. 2008;19:464–470. doi: 10.1111/j.1600-051x.1992.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 4.Listgarten M.A., Ellegaard B. Experimental gingivitis in the monkey: relationship of leukocyte counts in junctional epithelium, sulcus depth, and connective tissue inflammation scores. J Periodontal Res. 1973;8:199–214. doi: 10.1111/j.1600-0765.1973.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 5.Adriana D.B., Isabella G., Silvia C., Maria G. Periodontal disease: linking the primary inflammation to bone loss. Clin Dev Immunol. 2013:1–7. doi: 10.1155/2013/503754. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Zeng J., Wang X., Zheng M., Luan Q. P53 mediates lipopolysaccharide-induced inflammation in human gingival fibroblasts. J Periodontol. 2018;89:1142–1151. doi: 10.1002/JPER.18-0026. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y., Zhang L., Zhang Y. EphrinA1/EphA2 promotes epithelial hyperpermeability involving in lipopolysaccharide-induced intestinal barrier dysfunction. J Neurogastroenterol Motil. 2020;26:397–409. doi: 10.5056/jnm19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan M.A., Cookson B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 9.Galluzzi L., Vitale I., Abrams J.M. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miao E.A., Rajan J.V., Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogura Y., Sutterwala F.S., Flavell R.A. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Guo W., Wang P., Liu Z.H., Ye P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. Int J Oral Sci. 2018;10:e8. doi: 10.1038/ijos.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamkanfi M., Dixit V. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Genco R.J., Van Dyke T.E. Prevention: reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol. 2010;7:479–480. doi: 10.1038/nrcardio.2010.120. [DOI] [PubMed] [Google Scholar]
- 15.Liu X., Zhang Z., Ruan J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Man S.M., Kanneganti T.D. Gasdermin D: the long-awaited executioner of pyroptosis. Cell Res. 2015;25:1183–1184. doi: 10.1038/cr.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X. Formation of membrane pores by gasdermin-N causes pyroptosis. Sci China Life Sci. 2016;59:1071–1073. doi: 10.1007/s11427-016-5109-3. [DOI] [PubMed] [Google Scholar]
- 18.Chaves de Souza J.A., Frasnelli S.C., Curylofo-Zotti F.A., Ávila-Campos M.J., Spolidório L.C., Zamboni D.S. NOD1 in the modulation of host-microbe interactions and inflammatory bone resorption in the periodontal disease model. Immunology. 2016;149:374–385. doi: 10.1111/imm.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchesan J., Jiao Y., Schaff R.A. TLR4, NOD1 and NOD2 mediate immune recognition of putative newly identified periodontal pathogens. Mol Oral Microbiol. 2016;31:243–258. doi: 10.1111/omi.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W., Liu J., Wang W., Wang Y., Ouyang X. NLRP6 induces pyroptosis by activation of caspase-1 in gingival fibroblasts. J Dent Res. 2018;97:1391–1398. doi: 10.1177/0022034518775036. [DOI] [PubMed] [Google Scholar]
- 21.Özcan E., Saygun N.I., Serdar M.A., Kurt N. Evaluation of the salivary levels of visfatin, chemerin, and progranulin in periodontal inflammation. Clin Oral Invest. 2015;19:921–928. doi: 10.1007/s00784-014-1308-0. [DOI] [PubMed] [Google Scholar]
- 22.Wang R., Ma Z., Wang X., Wang Q., Chen Y., Chang X. Preparation of biodegradable chitosan thermosensitive hydrogel for minocycline-HCl gelatin microspheres and its effects on experimental rat gingivitis. J Pract Stomatol. 2009;23:503–506. [Google Scholar]
- 23.Li Y., Li J., Sun J. Expression of RAD51 and its clinical impact in oral squamous cell carcinoma. Anal Cell Pathol. 2020;2020:1827676. doi: 10.1155/2020/1827676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurshid Z., Mali M., Naseem M., Najeeb S., Zafar M. Human gingival crevicular fluids (GCF) proteomics: an overview. Dent J. 2017;5:12–19. doi: 10.3390/dj5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng R., Feng Y., Zhang R., Liu W., Lei L., Hu T. The extent of pyroptosis varies in different stages of apical periodontitis. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2018;1864:226–237. doi: 10.1016/j.bbadis.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Shi J., Zhao Y., Wang Y. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 27.Viganò E., Diamond C.E., Spreafico R., Balachander A., Sobota R.M., Mortellaro A. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun. 2015;6:8761–8773. doi: 10.1038/ncomms9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shie J.H., Kuo H.C. Higher levels of cell apoptosis and abnormal e-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome. BJU Int. 2011;108:E136–E141. doi: 10.1111/j.1464-410X.2010.09911.x. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Du J., Liu F., Wang X., Li Y. Role of caspase-3/e-cadherin in helicobacter pylori-induced apoptosis of gastric epithelial cells. Oncotarget. 2017;8:59204–59216. doi: 10.18632/oncotarget.19471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park K., Jin J., Hu Y., Zhou K., Ma J.X. Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am J Pathol. 2011;178:688–698. doi: 10.1016/j.ajpath.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahoo M., Lantier L., Re F. Role of canonical and non-canonical inflammasomes during burkholderia infection. Curr Top Microbiol Immunol. 2016;397:199–214. doi: 10.1007/978-3-319-41171-2_10. [DOI] [PubMed] [Google Scholar]