Abstract
AIM
To investigate the safety and efficacy of using a one-step viscoelastic agent technique for posterior chamber phakic implantable collamer lens with a central hole (ICL V4c) implantation for myopia correction.
METHODS
The one-step viscoelastic agent technique for ICL V4c implantation was used in 100 eyes of 52 patients. Refractive outcomes, intraocular pressure (IOP), corneal endothelial cell, and corneal densitometry values were evaluated at 1d, 1wk, 1 and 3mo postoperatively.
RESULTS
All the surgeries were uneventful. No corrected distance visual acuity was lost after 3mo. IOP was 16.12±3.18 mm Hg before surgery, and 14.74±3.08 mm Hg at 1d and 14.50±2.56 mm Hg at 3mo after surgery (P<0.05). Corneal endothelial cell density was 2580±242 cell/mm2, the coefficient of variation in cell size was 42.11%±7.92%, and the percentage of hexagonal cells was 40.98%±9.46% before surgery. No significant difference was found when these outcomes were compared between the studied time points (P>0.05). The corneal densitometry values of the central 2 mm and 2 to 6 mm areas showed similar regularities. After surgery, the values significantly increased at 1d, then decreased to the preoperative values at 1wk, and then continued to decrease at 3mo (P<0.05).
CONCLUSION
The one-step viscoelastic agent technique for ICL V4c implantation is found to be safe and effective for myopia correction and causes little disturbance to the cornea.
Keywords: phakic intraocular lens, V4c, viscoelastic agent, myopia, intraocular pressure, corneal endothelial cell, corneal densitometry
INTRODUCTION
The use of posterior chamber phakic implantable collamer lens with a central hole (ICL V4c) implantation has grown rapidly since it was approved in China in 2014. Its safety and efficacy have gained wide acceptance[1]–[5]. The central hole design of the ICL V4c helps maintain the intraocular pressure (IOP) without performing an additional peripheral iridotomy, which is needed with a conventional ICL (without a central hole). Thus, compared to implantation of conventional ICLs, ICL V4c implantation is less traumatic, and makes the perioperative preparation more efficient. The 0.36-mm central hole improves aqueous humor circulation, and potentially decreases the risk of pupillary block glaucoma and anterior subcapsular opacification[6]. Therefore, ICL V4c implantation has replaced conventional ICL implantation as a mainstream surgical procedure.
Improvements in surgical technique are also required in addition to ICL design innovation. We refer to the conventional ICL V4c implantation procedure as a two-step viscoelastic agent technique, where the viscoelastic agent is injected twice[1]–[5]. After a lot of clinical practice, the procedures were simplified, and a one-step viscoelastic agent technique for ICL V4c implantation was developed (details in the Methods section). The present study investigated the safety and efficacy of this one-step technique for ICL V4c implantation for myopia correction. We focused mainly on its impact on the cornea during the 3-month observational period.
SUBJECTS AND METHODS
Ethical Approval
The study complies with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Eye and ENT Hospital, Fudan University. Informed consent was obtained from all patients after a detailed explanation of the possible risks and benefits of the study.
This is a non-randomized prospective study (ChiCTR1900024713). All the patients underwent routine ophthalmic examinations at the Refractive Surgery Center of the Department of Ophthalmology, Eye and ENT Hospital of Fudan University (Shanghai, China) and met the surgical requirements for ICL V4c (STAAR Surgical Company, Monrovia, CA, USA) implantation. Exclusion criteria were: corneal endothelial cell density (ECD) ≤2000 cell/mm2, and a history of ocular surgery, cataract, glaucoma, amblyopia, retinal detachment, neuro-ophthalmic diseases, and ocular inflammatory diseases.
One hundred eyes of 52 patients who underwent the one-step viscoelastic agent technique for ICL V4c implantation were enrolled in the study. Mean preoperative spherical equivalent (SE) was -11.48±5.06 D (range: -2.50 to -28.75 D), and mean age was 28.88±7.12y (range: 18 to 47y).
Surgical Procedure
The ICL V4c (STAAR Surgical Company, Monrovia, CA, USA) is a posterior chamber intraocular lens designed with a 360 µm central hole. It corrects up to -18.00 D myopic spherical refraction and up to -5.00 D cylindrical refraction[1]–[2]. ICL V4c implantation procedures were performed by an experienced surgeon (Zhou XT). Pupils were dilated 1h before surgery. ICL V4c was then implanted via a 3-mm temporal corneal incision using an injector cartridge. Then, a moderate viscoelastic surgical agent, 1% sodium hyaluronate, was injected into the anterior chamber. Thereafter, the ICL V4c lens was placed in the posterior chamber. The viscoelastic surgical agent was washed away using a balanced salt solution. Postoperative medications included antibiotic eye drops, non-steroidal anti-inflammatory eye drops, steroidal eye drops, and artificial eye drops.
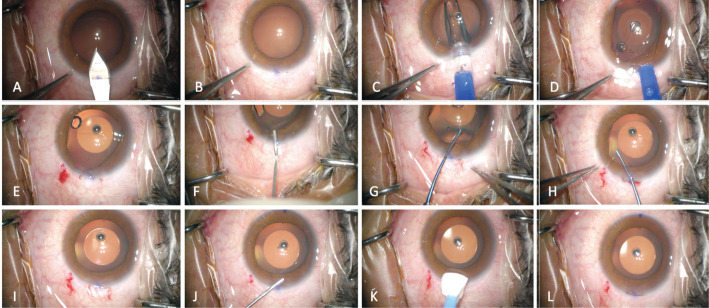
The differences between the two-step and one-step techniques are as follows. During the conventional two-step viscoelastic agent technique for ICL V4c implantation, the viscoelastic agent is injected twice. The first injection is administered after the corneal incision was made. At that point, a viscoelastic agent is injected to maintain the anterior chamber depth. The second injection is performed after the ICL lens is injected. The viscoelastic agent is then injected to protect the endothelial cells from mechanical injuries when adjusting the ICL haptics into the posterior chamber. After the ICL is placed in an appropriate position, the viscoelastic agent is washed away[7]. Figure 1 shows a detailed step-by-step diagram of the surgical procedures. The main difference between the two-step and one-step techniques is that the first injection of the viscoelastic agent is skipped in the latter, and the ICL V4c is immediately implanted after the corneal incision is made. The viscoelastic surgical agent is then injected, and then, the ICL V4c haptics is placed into the posterior chamber, and the viscoelastic surgical agent is washed away.
Figure 1. Detailed surgical procedure of the one-step viscoelastic agent technique for ICL V4c implantation.
A: A 3-mm temporal clear corneal incision is made; B: The anterior chamber depth is stable, indicating that a good self-closing incision has been made; C: A Toric ICL V4c lens is implanted by an injector cartridge; D: The ICL V4c lens unfolds smoothly, and three haptics are placed in the posterior chamber and one in the anterior chamber; E: The anterior chamber depth remains stable after the injector is removed; F: A little amount of viscoelastic surgical agent is injected above the haptic in the anterior chamber; G: The haptic in the anterior chamber is placed in the posterior chamber using a manipulator; H: Adjustments of the ICL V4c position are made; I: The anterior chamber depth remains stable after the ICL V4c is implanted; J: The viscoelastic surgical agent is then washed away using a balanced salt solution; K: Self-closing of the incision is finally checked; L: The ICL V4c lens implantation procedures is completed.
Measurements
Postoperative IOP measurement was conducted every hour for 3 to 5h after surgery. Paracentesis through corneal incision was performed to prevent sustained IOP elevation if it exceeded 30 mm Hg. The patients were followed up at 1d, 1wk, 1 and 3mo postoperatively. Corneal endothelial cells in the central cornea were assessed using a non-contact specular microscope (EM-3000; Tomey, Nagoya, Japan). In addition to corneal ECD, the coefficient of variation in cell size (CV) and the percentage of hexagonal cells (6A) are two important parameters used to evaluate corneal endothelial cells. The former represents polymegethism or morphological variation, and the latter represents the degree of polymorphism of the corneal endothelial cells[8].
Corneal densitometry and the ICL V4c vault measurement were performed using a rotating Scheimpflug camera (Pentacam, Oculus, Germany). Corneal densitometry values represent the degree of transparency of the cornea, which is expressed in grayscale units and ranges from 0 (maximum transparency) to 100 (completely opaque, minimum transparency). The system software automatically divides the cornea into four layers. The anterior layer (LA) and posterior layer (LP) represent the anterior 120 µm and posterior 60 µm of the cornea, respectively. The central layer (LC) refers to the volume between two boundary layers. The total layer (LT) refers to the entire cornea, from the epithelium to the endothelium. For each eye, the average corneal densitometry values of all four layers were analysed in two areas. The central zone was analysed from 0 to 2 mm, and the relatively central annuli zone was analysed from 2 to 6 mm, based on their distance from the corneal apex[9]–[10]. IOP was measured using a non-contact tonometer (NCT; Canon, Japan).
Statistical Analysis
Statistical analysis was performed using SAS V9.3 software (Cary, NC, USA). To determine the within-subject correlation, a linear mixed model was used to detect the differences among the variables, including the IOP, corneal endothelial cell, and corneal densitometry measurements between the study time points. The statistical significance level was set at a P value of less than 0.05.
RESULTS
All the surgeries were uneventful. No vision-threatening complications, including uncontrollable IOP increase, cataract formation, Toric lens rotation, or retinal detachment, were observed during the 3-month follow-up period.
Three hours after surgery, four eyes had IOP exceeding 30 mm Hg (maximum 35 mm Hg) and corneal paracentesis was conducted. After 3h postoperatively, two of the four eyes had persistently higher IOP (24-26 mm Hg) compared with the preoperative value (18-20 mm Hg). However, no tendency to increase was seen in the measurements conducted every 30min for another 3 times. Through routine postoperative medication, IOP of all the eyes decreased to baseline value at 1d postoperatively. IOP was 16.12±3.18 mm Hg (range: 10.4 to 22.3 mm Hg) before surgery and 14.74±3.08 mm Hg (range: 8.1 to 22.9 mm Hg), 15.48±2.72 mm Hg (range: 9.6 to 22.8 mm Hg), 14.30±2.36 mm Hg (range: 9.6 to 20.0 mm Hg), and 14.50±2.56 mm Hg (range: 8.7 to 20.9 mm Hg) at 1d, 1wk, 1 and 3mo after surgery (P<0.05), respectively.
At postoperative 3mo, SE decreased from preoperative -11.48±5.06 to -0.46±1.42 D (range: +1.38 to -6.60 D). The ratio between postoperative uncorrected distance visual acuity (UDVA) and preoperative corrected distance visual acuity (CDVA; efficacy index) was 1.07±0.26 (range: 0.50 to 2.00). The ratio between postoperative CDVA and preoperative CDVA (safety index) was 1.21±0.25 (range: 1.0 to 2.00). None of the eyes lost one or more lines of the CDVA. The mean vault was 562±265 µm (range: 180 to 1160 µm) at 3mo.
Before surgery, corneal ECD was 2580±242 cell/mm2 (range: 2049 to 3143 cell/mm2), CV was 42.11%±7.92% (range: 23% to 80%), and 6A was 40.98%±9.46% (range: 24% to 74%). No significant difference in these variables was found when comparing between the time points (P>0.05; Table 1).
Table 1. ECD, CV, and 6A in the central cornea in 3mo after one-step viscoelastic agent technique for ICL V4c implantation for myopia correction.
| Parameters | ECD (cell/mm2) | CV (%) | 6A (%) |
| Preoperative | 2580±242 | 42.11±7.92 | 40.98±9.46 |
| 1d | 2560±224 | 41.24±5.64 | 40.36±8.83 |
| 1wk | 2551±240 | 41.41±5.87 | 40.44±9.11 |
| 1mo | 2548±258 | 42.32±6.44 | 39.69±8.47 |
| 3mo | 2624±249 | 39.84±5.75 | 42.33±7.84 |
| P | 0.237 | 0.105 | 0.394 |
ECD: Endothelial cell density; CV: The coefficient of variation in cell size; 6A: The percentage of hexagonal cells; ICL V4c: Posterior chamber phakic implantable collamer lens with a central hole; SD: Standard deviation.
mean±SD
Corneal densitometry values of the central zone from 0 to 2 mm (Table 2) and relatively central annuli zone area from 2 to 6 mm (Table 3) of the four corneal layers showed similar regularities. These values significantly increased at 1d after surgery (P<0.05), then decreased to the preoperative values at 1wk postoperatively (P>0.05), and then continued to decrease at 3mo after surgery (P<0.05).
Table 2. Corneal densitometry values of the central 2 mm zone of 4 layers.
| Parameters | LA | LC | LP | LT |
| Preoperative | 18.94±1.30 | 11.55±0.64 | 9.79±0.57 | 13.42±0.75 |
| 1d | 20.26±3.97a | 12.24±3.37a | 10.52± 2.61a | 14.33±3.22a |
| 1wk | 18.96±1.13 | 11.52±0.52 | 9.85±0.55 | 13.45±0.60 |
| 1mo | 18.43±1.06 | 11.39±0.56 | 9.74±0.63 | 13.19±0.65 |
| 3mo | 17.15±1.36a | 10.72±0.76a | 9.26±0.75 | 12.39±0.88a |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
LA: Anterior layer; LC: Central layer; LP: Posterior layer; LT: Total layer. aP<0.05 vs preoperative values.
mean±SD
Table 3. Corneal densitometry values of the relatively central annuli zone from 2 to 6 mm of 4 layers.
| Parameters | LA | LC | LP | LT |
| Preoperative | 16.85±1.15 | 10.33±0.56 | 8.84±0.54 | 12.01±0.69 |
| 1d | 18.01±2.55a | 10.86±1.42a | 9.09±0.81a | 12.65±1.52a |
| 1wk | 16.87±1.11 | 10.32±0.48 | 8.91±0.49 | 12.03±0.60 |
| 1mo | 16.39±0.92 | 10.19±0.51 | 8.81±0.58 | 11.80±0.58 |
| 3mo | 15.31±1.26a | 9.59±0.68a | 8.38±0.70a | 11.10±0.82a |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
LA: Anterior layer; LC: Central layer; LP: Posterior layer; LT: Total layer. a P<0.05 vs preoperative values.
mean±SD
DISCUSSION
ICL V4c is the latest generation of ICLs; it eliminates the need for preoperative laser peripheral iridotomy. It was proven to be safe and effective for the correction of myopia[1]–[2]. Based on the conventional two-step viscoelastic agent technique for ICL V4c implantation, and years of clinical practice, a one-step viscoelastic agent technique for ICL V4c implantation (the one-step technique) was developed. This is an improvement over and simplified version of the conventional ICL implantation procedures. This study explored whether the one-step technique is suitable for clinical application by observing the anterior segment performance after surgery.
No vision threatening complications were observed, and no CDVA was lost. The efficacy and safety indices were 1.07 and 1.21, respectively, in this study. These refractive outcomes are comparable to those reported in previous studies, suggesting that the one-step technique is safe and effective for visual acuity improvement in myopia correction when compared with the conventional technique[1]–[2],[11]. The mean vault was 562 µm, which was within the acceptable range. The lowest value was 180 µm. In cases of low vault after ICL implantation, if there is no evidence of cataract development, clinical follow-up could be an appropriate approach instead of immediate lens exchange[12]–[13].
Viscoelastic agents are important for anterior chamber maintenance and corneal endothelium protection in ophthalmic surgeries. However, retained viscoelastic agents are considered the main cause of ocular hypertension after ICL implantation. The viscoelastic remnant may obstruct the trabecular meshwork and the canal of Schlemm and cause an increase of IOP[14]–[15]. During the conventional two-step procedures, the viscoelastic agent is injected twice into the anterior chamber, before and after the ICL V4c lens is injected[7]. It is easy to flush away the viscoelastic agent in the anterior chamber in front of the ICL lens. However, the ICL lens must be crossed to flush away the viscoelastic agent in the posterior chamber. This is difficult to achieve fully because of the limited operating space. The remaining viscoelastic agent could jam the central hole and lead to pupillary block ocular hypertension. Thus, the key step that prevents retained viscoelastic agent-related IOP increase is a thorough irrigation of the anterior and posterior chambers[16]. Skipping the first viscoelastic agent injection procedure greatly reduces the amount of viscoelastic residue, decreasing the risk of pupillary block ocular hypertension due to retention of the viscoelastic agent. The corneal paracentesis rate was 4% and the highest postoperative IOP was 35 mm Hg. This indicates that the rate of IOP increase caused by the viscoelastic remnant is low and can be treated in the early period through IOP monitoring after surgery. After one day, postoperative IOP was stable, and none of the patients experienced incontrollable ocular hypertension during the 3-month observational period.
In a recent report, anterior subcapsular cataract (ASC) was induced during implantation of ICL V4c by cannula irrigation as a stream was forced onto the capsule through the central hole during the viscoelastic agent removal process[17]. The one-step technique also makes it possible to avoid the posterior chamber viscoelastic agent removal procedure. Thus, it decreases the risk of ASC due to operative trauma.
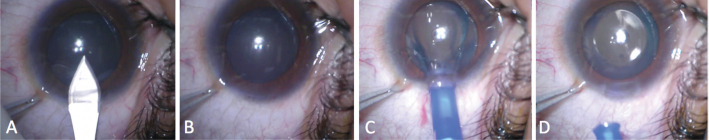
Moreover, the one-step technique reduces the operation time and improves the efficiency of the operation. Figure 2 shows an extreme example of the one-step viscoelastic agent technique for ICL V4c implantation, which could be referred to as a viscoelastic surgical agent-free technique for ICL V4c implantation. In this example, after the ICL V4c was injected, the four haptics of a non-toric ICL V4c were directly placed in the posterior chamber without adjustment; consequently, it was possible to skip the second step of viscoelastic surgical agent injection and removal procedures.
Figure 2. Viscoelastic surgical agent-free technique for ICL V4c implantation.
A: A 3-mm temporal clear corneal incision is made; B: The anterior chamber depth is stable, indicating that a good self-closing incision has been made; C: A non-toric ICL V4c lens is implanted by an injector cartridge; D: The ICL V4c lens unfolds smoothly, and four haptics are placed in the posterior chamber without adjustment. The ICL V4c lens implantation procedures is completed without viscoelastic surgical agent injection.
During the ICL-V4c implantation procedure, the intraocular space for surgical operation is limited, and corneal endothelial cell damage or corneal edema is a potential complication. Acute corneal endothelial cell loss usually occurs if the anterior chamber space is unstable and surgical instruments may touch the corneal endothelial layer. Therefore, endothelial cell monitoring is important for the safety assessment of ICL implantation[18]. Chen et al[7] and Yang et al[19] monitored corneal ECD for 12mo or more after the conventional two-step technique for ICL V4c implantation. They both reported that no significant reductions in ECD at the 3-month follow-up visit were noted. In the current study, all the corneal endothelial cell parameters, including ECD, CV, and 6A, were stable over time using the one-step technique. Goukon et al[8] reported that ICL V4c implantation may have advantages over conventional ICL implantation only in terms of maintaining the density in the superior regions, possibly because preoperative laser iridotomies are unnecessary. This suggests that reducing unnecessary operation procedures would lead to less disturbance to the corneal endothelial cells. In the current study, the simplified one-step technique did not induce a significant change in either the density or morphology of the central corneal endothelial cells. The corneal endothelium was effectively protected by viscoelastic injection only once. In terms of surgical skill, surgeons need to master the conventional method before using the one-step technique. The key point of the one-step technique is that its effectiveness is based on good self-close corneal incision making, which ensures a stable anterior chamber depth. When this occurs, the first viscoelastic injection can be skipped and the ICL injection can be directly performed. The ICL V4c injection is another important part of the procedure because the lens has to be kept wet. In addition, the lens incarceration in the corneal incision and rotation in the anterior chamber should be avoided. When using the one-step technique, if any situation emerges that impacts the anterior chamber stability, surgeons should switch to the conventional method to avoid mechanical disturbance to the corneal endothelial cell, crystalline lens, or iris.
In the current study, we also obtained densitometry values of the central 6-mm corneal area. This allowed an assessment of the impact of the one-step technique on the central optical zone. Physiological and pathological changes in the corneal composition can lead to changes in corneal transparency. A decrease in corneal transparency can be revealed by an increase in corneal densitometry value. This often occurs when an edema or inflammation occurs[20]–[21]. In the current study, it was found that corneal densitometry peaks and then declined to the baseline value in the early period (1d) after the one-step technique ICL V4c implantation procedure. Transient increase in corneal densitometry value usually occurs due to transient epithelial defects or mild corneal edema. Moreover, corneal transparency improved within 3mo postoperatively. This further proved that the one-step technique causes minimal disturbance to the cornea. Chen et al[7] investigated corneal densitometry changes in 12mo after the conventional two-step technique for ICL V4c implantation. They found that corneal densitometry increased slightly at postoperative one day and then decreased gradually. Hence, their results are consistent with those of the present study. Therefore, compared with the conventional surgical method, the simplified one-step method showed equal safety for the corneal transparency.
The surgical procedures in the present study were performed by an experienced surgeon. This avoided confounding effects derived from surgical technique variability. One limitation is that this study evaluated ECD of the central area of the cornea. Hence, peripheral endothelial damage was not measured. However, the corneal densitometry assessed not only the central area (0-2 mm) but also the relative peripheral area (2 to 6 mm) of the cornea. Thus, it provided information on a wider central optical zone of the cornea[22]. Tekin et al[23] reported that ECD, CV, and 6A were statistically significantly correlated with the corneal densitometry values in all layers, indicating that corneal densitometry values can potentially be used as indicators of the health of the corneal endothelium. Both corneal endothelial cells and corneal densitometry values were evaluated in this study. Therefore, a relatively comprehensive corneal condition evaluation was performed. Another limitation is that the participants in this study were recruited only from a single refractive centre in China. Thus, the current study was limited by its single-centre design and monoracial background. Further multi-centric studies and long-term observation periods are needed.
In conclusion, the one-step viscoelastic agent technique for ICL V4c implantation is safe and effective for myopia correction and causes little disturbance to the cornea.
Acknowledgments
Authors' contributions: Concept and design: Miao HM, Zhao F, Wang XY, Zhou XT. Data collection: Miao HM, Zhao F, Niu LL, Zhao J, Zhou XT. Analysis and interpretation: Miao HM, Zhao F, Niu LL. Writing the article: Miao HM, Zhao F, Zhao J. Critical revision of the article: Miao HM, Zhao J, Wang XY, Zhou XT.
Foundations: Supported in part by the National Natural Science Foundation of China for Young Scholars (No.81700872); the National Natural Science Foundation of China (No.81770955); the Project of Shanghai Science and Technology (No.17411950200; No.20410710100); the Joint Research Project of New Frontier Technology in Municipal Hospitals (No.SHDC12018103); the Clinical Research Plan of SHDC (No.SHDC2020CR1043B); the Project of Shanghai Xuhui District Science and Technology (No.2020-015).
Conflicts of Interest: Miao HM, None; Zhao F, None; Niu LL, None; Zhao J, None; Wang XY, None; Zhou XT, None.
REFERENCES
- 1.Miao HM, Chen X, Tian M, Chen YJ, Wang XY, Zhou XT. Refractive outcomes and optical quality after implantation of posterior chamber phakic implantable collamer lens with a central hole (ICL V4c) BMC Ophthalmol. 2018;18(1):1–7. doi: 10.1186/s12886-018-0805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan Z, Miao H, Zhao F, Wang X, Chen X, Li M, Zhou X. Two-year outcomes of visian implantable collamer lens with a central hole for correcting high myopia. J Ophthalmol. 2018;2018:8678352. doi: 10.1155/2018/8678352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamiya K, Shimizu K, Igarashi A, Kitazawa Y, Kojima T, Nakamura T, Oka Y, Matsumoto R. Posterior chamber phakic intraocular lens implantation: comparative, multicentre study in 351 eyes with low-to-moderate or high myopia. Br J Ophthalmol. 2018;102(2):177–181. doi: 10.1136/bjophthalmol-2017-310164. [DOI] [PubMed] [Google Scholar]
- 4.Lisa C, Naveiras M, Alfonso-Bartolozzi B, Belda-Salmerón L, Montés-Micó R, Alfonso JF. Posterior chamber collagen copolymer phakic intraocular lens with a central hole to correct myopia: one-year follow-up. J Cataract Refract Surg. 2015;41(6):1153–1159. doi: 10.1016/j.jcrs.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Huseynova T, Ozaki S, Ishizuka T, Mita M, Tomita M. Comparative study of 2 types of implantable collamer lenses, 1 with and 1 without a central artificial hole. Am J Ophthalmol. 2014;157(6):1136–1143. doi: 10.1016/j.ajo.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Fujisawa K, Shimizu K, Uga S, et al. Changes in the crystalline lens resulting from insertion of a phakic IOL (ICL) into the porcine eye. Graefes Arch Clin Exp Ophthalmol. 2007;245(1):114–122. doi: 10.1007/s00417-006-0338-y. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Shen Y, Xu HP, Wang XY, Zhou XT. One-year natural course of corneal densitometry in high myopic patients after implantation of an implantable collamer lens (model V4c) BMC Ophthalmol. 2020;20(1):50. doi: 10.1186/s12886-020-1320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goukon H, Kamiya K, Shimizu K, Igarashi A. Comparison of corneal endothelial cell density and morphology after posterior chamber phakic intraocular lens implantation with and without a central hole. Br J Ophthalmol. 2017;101(11):1461–1465. doi: 10.1136/bjophthalmol-2016-309363. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Jian W, Sun L, Li M, Han T, Son J, Zhou X. One-year follow-up of changes in corneal densitometry after accelerated (45 mW/cm2) transepithelial corneal collagen cross-linking for keratoconus: a retrospective study. Cornea. 2016;35(11):1434–1440. doi: 10.1097/ICO.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ní Dhubhghaill S, Rozema JJ, Jongenelen S, Ruiz Hidalgo I, Zakaria N, Tassignon MJ. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Invest Ophthalmol Vis Sci. 2014;55(1):162–168. doi: 10.1167/iovs.13-13236. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Miao HM, Naidu RK, Wang XY, Zhou XT. Comparison of early changes in and factors affecting vault following posterior chamber phakic implantable collamer lens implantation without and with a central hole (ICL V4 and ICL V4c) BMC Ophthalmol. 2016;16(1):161. doi: 10.1186/s12886-016-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Córdoba A, Graue-Hernández EO, Gómez-Bastar A, Navas A. Long-term follow-up of persistent low vault after implantable collamer lens exchange. J Cataract Refract Surg. 2019;45(4):519–522. doi: 10.1016/j.jcrs.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Lopez F, Bouza-Miguens C, Tejerina V, et al. Long-term assessment of crystalline lens transparency in eyes implanted with a central-hole phakic collamer lens developing low postoperative vault. J Cataract Refract Surg. 2021;47(2):204–210. doi: 10.1097/j.jcrs.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 14.Senthil S, Choudhari NS, Vaddavalli PK, Murthy S, Reddy JC, Garudadri CS. Etiology and management of raised intraocular pressure following posterior chamber phakic intraocular lens implantation in myopic eyes. PLoS One. 2016;11(11):e0165469. doi: 10.1371/journal.pone.0165469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzer MP, Tetz MR, Auffarth GU, Welt R, Völcker HE. Effect of Healon5 and 4 other viscoelastic substances on intraocular pressure and endothelium after cataract surgery. J Cataract Refract Surg. 2001;27(2):213–218. doi: 10.1016/s0886-3350(00)00568-x. [DOI] [PubMed] [Google Scholar]
- 16.Pan AP, Wen LJ, Shao X, Zhou KJ, Wang QM, Qu J, Yu A. A novel ophthalmic viscosurgical device-free phakic intraocular lens implantation makes myopic surgery safer. Eye Vis (Lond) 2020;7:18. doi: 10.1186/s40662-020-00185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinwender G, Varna-Tigka K, Shajari M, Kohnen T. Anterior subcapsular cataract caused by forceful irrigation during implantation of a posterior chamber phakic intraocular lens with a central hole. J Cataract Refract Surg. 2017;43(7):969–974. doi: 10.1016/j.jcrs.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu K, Kamiya K, Igarashi A, Shiratani T. Early clinical outcomes of implantation of posterior chamber phakic intraocular lens with a central hole (hole ICL) for moderate to high myopia. Br J Ophthalmol. 2012;96(3):409–412. doi: 10.1136/bjophthalmol-2011-300148. [DOI] [PubMed] [Google Scholar]
- 19.Yang W, Zhao J, Sun L, Zhao J, Niu LL, Wang XY, Zhou XT. Four-year observation of the changes in corneal endothelium cell density and correlated factors after implantable collamer lens V4c implantation. Br J Ophthalmol. 2021;105(5):625–630. doi: 10.1136/bjophthalmol-2020-316144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savini G, Huang JH, Lombardo M, Serrao S, Schiano-Lomoriello D, Venanzio S, Ducoli P. Objective monitoring of corneal backward light scattering after femtosecond laser-assisted LASIK. J Refract Surg. 2016;32(1):20–25. doi: 10.3928/1081597X-20151207-08. [DOI] [PubMed] [Google Scholar]
- 21.Han T, Zhao J, Shen Y, Chen YJ, Tian M, Zhou XT. A three-year observation of corneal backscatter after small incision lenticule extraction (SMILE) J Refract Surg. 2017;33(6):377–382. doi: 10.3928/1081597X-20170420-01. [DOI] [PubMed] [Google Scholar]
- 22.Otri AM, Fares U, Al-Aqaba MA, Dua HS. Corneal densitometry as an indicator of corneal health. Ophthalmology. 2012;119(3):501–508. doi: 10.1016/j.ophtha.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Tekin K, Sekeroglu MA, Kiziltoprak H, Yilmazbas P. Corneal densitometry in healthy corneas and its correlation with endothelial morphometry. Cornea. 2017;36(11):1336–1342. doi: 10.1097/ICO.0000000000001363. [DOI] [PubMed] [Google Scholar]