Abstract
Background/purpose
Burning mouth syndrome (BMS) is defined as a chronic burning pain of the oral mucosa with no associated clinical signs or apparent extraneous cause. Limited epidemiologic data have been published. In this study, we probed the nationwide registered database to assess the prevalence of BMS in Taiwan.
Materials and methods
A retrospective study was conducted to analyze the dental dataset compiled by the Taiwan's National Health Insurance Research Database from 2004 to 2013. The diagnosis of BMS was identified in accordance with the International Classification of Disease, Ninth revision. In addition, sex and age were analyzed by multivariate Poisson regression.
Results
The prevalence of BMS revealed from 2.24 (per 104) to 3.11 (per 104) over past 10 year period. Female has higher risk of BMS than male (RR: 1.39; 95% CI: 1.37–1.41). The 50–59 years old group, 60–69 years old group, and >70 years old group had 1.55-, 2.06-, and 2.58-fold risk than 40–49 years old group for BMS (p < 0.001), respectively.
Conclusion
Taken together, this is the first reported nationwide population based prevalence data for BMS in Taiwan. The risk for BMS is highly associated with female and advancing age.
Keywords: Burning mouth syndrome, Prevalence, Register-based, Nationwide population, Taiwan
Introduction
Burning mouth syndrome (BMS), an idiopathic chronic pain disorder, is characterized by a burning sensation of the oral cavity for which no medical or dental cause can be found.1 The International Headache Society defined BMS as an intraoral burning or dysesthetic sensation in the oral mucous membrane and tongue that recur for more than 2 h per day for more than 3 months, without clinically evident causative lesions.2 The etiology of BMS remains poorly understood that resulted in the treatment is quite challenging. Patients who suffer from BMS do not always display a consistence of clinical features. Previously, Lin et al.3 reported that BMS is highly associated with (1) the deficiency of hemoglobin, iron, and vitamin B12, (2) the abnormally high blood homocysteine level, and (3) the positivity of serum gastric parietal cell antibody. Recently, a series studies were further evaluated the above mentioned factors and relationship of BMS.4, 5, 6, 7, 8, 9 These highly scientific evidences from clinical findings and laboratory testing may provide the new insight of BMS.
The estimated prevalence of BMS in the general population varies widely in the review articles. Women are more likely to have BMS than men and the prevalence increases with advancing age.10,11 This syndrome seems more prevalent in menopausal women, comorbid psychosocial, psychiatric, and neurodegenerative disorders.1,10,11
Due to the lack of rigorous diagnostic criteria of BMS in previous publications, little is known about the accurate prevalence of BMS. In this study, the prevalence of BMS was identified in accordance with the International Classification of Disease, Ninth revision from Taiwan's National Health Insurance Research Database (NHIRD).
Materials and methods
Data source
After approved by the Ethics Review Board at the Chung Shan Medical University Hospital (CS2-15071). Dental dataset (DN),12 a part of NHIRD which contains dental original claim data for ambulatory care expenditures by dental visit, was used for this study.
Patient identification and assessment
The diagnosis of BMS was identified in accordance with the ICD-9 codes 781.1(disturbances of sensation of smell and taste), 529.0 (glossitis), and 529.6 (glossodynia). In this study, we identified dental visit patients with diagnosed BMS during the period between January 1, 2004 and December 31, 2013 for annual prevalence rate of BMS. To increase the validity of diagnoses in the administrative data set, we only captured dental visit patients who received ≥3 diagnoses of BMS. In addition, sex and age were analyzed by multivariate Poisson regression. Age stratification was divided by people aged within 0–19, 20–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years old.
Statistical analysis
The relative risk of BMS from 2004 to 2013 after adjusting for year, age, and sex was evaluated by multivariate Poisson regression. Relative risk (RR) and 95% confidence interval (CI) of BMS were calculated. All statistical analyses were performed with the SPSS version 19 (SPSS, Chicago, IL, USA). p < 0.05 was considered statistically significant.
Results
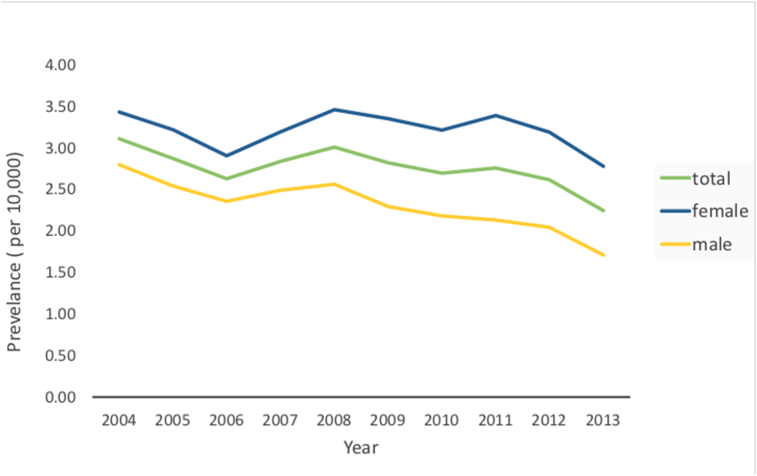
The time trends for the prevalence of BMS in Taiwan from 2004 to 2013 is illustrated in Fig. 1. The prevalence was higher among women than among men. The sex-specific annual prevalence of BMS from 2004 to 2013 is presented in Table 1. The prevalence of BMS was ranged from 2.24 (per 104) to 3.11 (per 104) over past 10 year period. The average annual prevalence was 2.76 (per 104). The average female-to-male ratio was 1.37.
Figure 1.
Time trends for the prevalence of BMS in Taiwan from 2004 to 2013. The prevalence was higher among female than among male.
Table 1.
Prevalence of BMS by sex in Taiwan.
|
Year |
Total |
Female |
Male |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | BMS | P | N | BMS | P | N | BMS | P | |
| 2004 | 22,689,122 | 7043 | 3.11 | 11,147,537 | 3820 | 3.43 | 11,541,585 | 3223 | 2.79 |
| 2005 | 22,770,383 | 6531 | 2.87 | 11,207,943 | 3600 | 3.21 | 11,562,440 | 2931 | 2.53 |
| 2006 | 22,876,527 | 6001 | 2.62 | 11,284,820 | 3273 | 2.90 | 11,591,707 | 2728 | 2.35 |
| 2007 | 22,958,360 | 6506 | 2.83 | 11,349,593 | 3618 | 3.19 | 11,608,767 | 2888 | 2.49 |
| 2008 | 23,037,031 | 6917 | 3.00 | 11,410,680 | 3942 | 3.45 | 11,626,351 | 2975 | 2.56 |
| 2009 | 23,119,772 | 6509 | 2.82 | 11,483,038 | 3843 | 3.35 | 11,636,734 | 2666 | 2.29 |
| 2010 | 23,162,123 | 6232 | 2.69 | 11,526,898 | 3697 | 3.21 | 11,635,225 | 2535 | 2.18 |
| 2011 | 23,224,912 | 6400 | 2.76 | 11,579,238 | 3920 | 3.39 | 11,645,674 | 2480 | 2.13 |
| 2012 | 23,315,822 | 6090 | 2.61 | 11,642,503 | 3708 | 3.18 | 11,673,319 | 2382 | 2.04 |
| 2013 | 23,373,517 | 5238 | 2.24 | 11,688,843 | 3243 | 2.77 | 11,684,674 | 1995 | 1.71 |
P: Prevalence rate per 10,000 population; N: number; BMS: burning mouth syndrome.
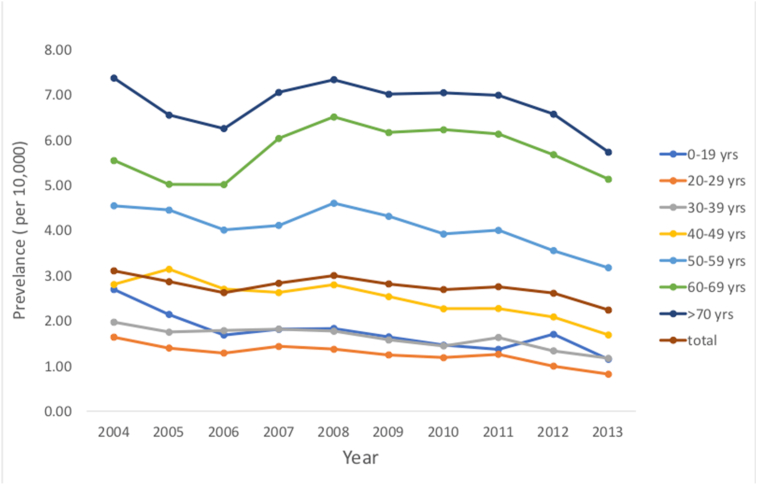
The average and median age of BMS by sex in Taiwan is shown in Table 2. The mean age of patients with BMS was 47.85 years old. The prevalence of BMS from 2004 to 2013 stratified by age is shown in Fig. 2.
Table 2.
Average and median age of BMS by sex in Taiwan.
|
year |
Total |
Male |
Female |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Mean | Median | SD | Mean | Median | SD | |
| 2004 | 42.3 | 45.0 | 23.9 | 42.1 | 44.0 | 24.7 | 42.6 | 46.0 | 23.1 |
| 2005 | 44.1 | 47.0 | 22.8 | 42.9 | 45.0 | 23.6 | 45.1 | 48.0 | 22.0 |
| 2006 | 46.0 | 49.0 | 22.1 | 45.3 | 48.0 | 22.7 | 46.6 | 49.0 | 21.6 |
| 2007 | 46.9 | 50.0 | 22.4 | 46.1 | 48.0 | 23.1 | 47.5 | 51.0 | 21.8 |
| 2008 | 48.0 | 51.0 | 22.2 | 46.4 | 49.0 | 23.5 | 49.2 | 53.0 | 21.0 |
| 2009 | 48.8 | 52.0 | 21.9 | 47.0 | 50.0 | 23.2 | 50.1 | 54.0 | 20.9 |
| 2010 | 50.1 | 54.0 | 21.6 | 48.5 | 51.0 | 22.8 | 51.3 | 55.0 | 20.7 |
| 2011 | 50.5 | 54.0 | 21.4 | 48.1 | 51.0 | 22.7 | 52.0 | 56.0 | 20.5 |
| 2012 | 49.8 | 55.0 | 22.8 | 46.8 | 50.0 | 24.5 | 51.7 | 56.0 | 21.5 |
| 2013 | 52.0 | 56.0 | 21.4 | 49.4 | 54.0 | 23.1 | 53.6 | 57.0 | 20.2 |
N: number; BMS: burning mouth syndrome.
Figure 2.
Age-specific group in the prevalence of BMS in Taiwan from 2004 to 2013.
The relative risk of BMS from 2004 to 2013 for sex is illustrated in Table 3. The risk decreased with the increment of the year (RR: 0.98; 95% CI: 0.98–0.98, p < 0.001). Females had higher risk of BMS than males (RR: 1.39; 95% CI: 1.37–1.41, p < 0.001).
Table 3.
Relative ratio of BMS from 2004 to 2013 by multivariate Poisson regression for sex.
| RR | 95% C.I. |
p value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Year (per 1 year) | 0.98 | 0.98 | 0.98 | <0.001 |
| Sex (ref: Male) | ||||
| Female | 1.39 | 1.37 | 1.41 | <0.001 |
RR: Relative ratio; BMS: burning mouth syndrome.
The relative risk of BMS from 2004 to 2013 for year stratification is shown in Table 4. The risk decreased with the increment of the year (RR: 0.97; 95% CI: 0.97–0.97, p < 0.001). When comparing with 40–49 years old group, 50–59 years old group, 60–69 years old group, and >70 years old group had 1.55-, 2.06-, and 2.58-fold risk for BMS (p < 0.001), respectively.
Table 4.
Relative ratio of BMS from 2004 to 2013 by multivariate Poisson regression for age.
| RR | 95% C.I. |
p value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Year (per 1 year) | 0.97 | 0.97 | 0.97 | <0.001 |
| age (ref: 40–49 years) | ||||
| 0–19 years | 0.73 | 0.71 | 0.74 | <0.001 |
| 20–29 years | 0.53 | 0.52 | 0.55 | <0.001 |
| 30–39 years | 0.69 | 0.67 | 0.70 | <0.001 |
| 50–59 years | 1.55 | 1.52 | 1.58 | <0.001 |
| 60–69 years | 2.06 | 2.02 | 2.11 | <0.001 |
| ≧70 years | 2.58 | 2.53 | 2.63 | <0.001 |
RR: Relative ratio; BMS: burning mouth syndrome.
Discussion
To the best of our knowledge, this is the first longitudinal study for BMS survey in Taiwan. The prevalence of BMS revealed from 2.24 (per 104) to 3.11 (per 104) from 2004 to 2013. As illustrated in Table 5, there are highly variable among the present study and previous published prevalence data.13, 14, 15 The reasons may be explained by the use of different study designs, diagnosis criteria, and geographic regions. This study based on Taiwan's NHIRD represented by a nationwide population may reflect more accurate prevalence of BMS.
Table 5.
The comparisons of limited epidemiological data of burning mouth syndrome (BMS).
| Sampling (period) |
Assessment of BMS | Sex predominant | Age distribution (years old) |
Prevalence | |
|---|---|---|---|---|---|
| Su. et al. present study (Taiwan) |
Nationwide population (2004–2013) |
Insurance dataset | Female | Mean age: 47.85 Mean age: male 46.26 Mean age: female 48.97 Significant: > 30–39 years old group |
Total: 2.76 (per 104) Male: 2.31 (per 104) Female: 3.21 (per 104) |
| Tammiala-Salonen et al.13 (Finnish) |
600 volunteers (289 men and 311 women) (10-year observation) |
Questionnaires Clinical exam | Female |
Mean age: male 51 Mean age: female 57 Significant: female > 30–39 years old group |
Total: 1–15% (dependent on the criteria for BMS) |
| Bergdahl & Bergdahl14 (Sweden) | 1427 volunteers (669 men and 758 women) (year no shown) |
Questionnaires Clinical exam | Female |
Mean age: male 59.1 Mean age: female 56.9 Significant: > 30–39 years old group |
Total: 3.7%; Male: 1.6% Female: 5.5% |
| Kohorst et al.15 (USA) |
Residents of Olmsted County (2000–2009) |
Clinical exam | Female | Mean age: 59.4 Significant: > 50 years old group |
Total: 10.33 (per 104) Male: 3.40 (per 104) Female: 16.95 (per 104) |
Our data reveal that BMS is an uncommon disease highly associated with female. The results were in agreement with previous studies.13, 14, 15 Menopausal women may suffer from xerostomia due to the decreased levels of estrogen and progesterone.10 The review articles suggest that psychogenic disturbances are associated with BMS more frequently in women than men.10,11 Consistently, recent Korean retrospective population-based cohort study reported that female BMS patients are associated with an increasing incidence of depression and anxiety.16
The nature of BMS diagnosis is quite challenging. It evokes the discussion that “Is burning mouth a syndrome or a disorder”. The variable BMS prevalence in previous might be due to the lack of consistent BMS diagnostic criteria. Therefore, the true prevalence of BMS is difficult to reach. Many studies did not distinguish between the symptom of oral burning rather than BMS itself.17,18 Taken together, the appropriately definition the dental/medical condition of BMS is very important.
The present survey has several strengths. This is the first nationwide retrospective study evaluating long term prevalence of BMS in Taiwan. With the same methodology to investigate the prevalence of BMS from NHIRD, the results from this cross-sectional analysis from 2004 to 2013 could be more reliable. The use of insurance register-based database can provide the nationwide population sample size, generalizability, and statistical power to assess the prevalence of BMS in Taiwan. In addition, numerous epidemiology surveys based on DN for oral diseases have been already published,19, 20, 21 which suggests that this study has good reliability.
However, there are still some limitations in this study. First, BMS is a chronic pain and dysesthesia disorder without specific ICD-9 code. The diagnosis of BMS was based on the disturbances of sensation of smell and taste (781.1), glossitis (529.0), and glossodynia (529.6) according to a previous publication.22 Due to this is a register-based study, the prevalence of BMS may be underestimated. Second, the diagnosis of BMS was solely based on ICD-9 codes from DN which may be less accurate than the diagnosis from the actual medical records. The use of Longitudinal Health Insurance Database 2010 which contains all of the original claim data of NHIRD may overcome this shortcoming in the further study. Third, the potential risk factors that could contribute to the sensation of oral burning, including pain intensity, medication intake, nutritional status, smoking habits, alcohol consumption, socioeconomic status, and family history of local and systemic diseases can not be obtained from DN. In addition, the relevant clinical variables, such as blood pressure, imaging results, pathology findings, and serum laboratory data were also unavailable in this study. Fourth, the actual locations of typical symptom characteristics were also not obtained in this databank. Finally, the retrospective cross-section design in this survey, a cohort design will be better for the assessment of BMS prevalence.
Taken together, we report the first nationwide population based prevalence data for BMS in Taiwan. The data reveal that BMS is highly associated with advancing age and females. Further studies should explore the potential effect of potential factors such as concurrent disease and medication use on the prevalence of BMS.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This work is supported by a grant from Chung Shan Medical University Hospital (CSH-2017-A-024).
References
- 1.Sun A., Wu K.M., Wang Y.P., Lin H.P., Chen H.M., Chiang C.P. Burning mouth syndrome: a review and update. J Oral Pathol Med. 2013;42:649–655. doi: 10.1111/jop.12101. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS) 3rd ed. vol. 38. 2018. The international classification of Headache disorders. Cephalalgia; pp. 1–211. [DOI] [PubMed] [Google Scholar]
- 3.Lin H.P., Wang Y.P., Chen H.M., Kuo Y.S., Lang M.J., Sun A. Significant association of hematinic deficiencies and high blood homocysteine levels with burning mouth syndrome. J Formos Med Assoc. 2013;112:319–325. doi: 10.1016/j.jfma.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Chiang M.L., Jin Y.T., Chiang C.P., Wu Y.H., Chang J.Y.F., Sun A. Anemia, hematinic deficiencies, hyperhomocysteinemia, and gastric parietal cell antibody positivity in burning mouth syndrome patients with vitamin B12 deficiency. J Dent Sci. 2020;15:34–41. doi: 10.1016/j.jds.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Y.T., Chiang M.L., Wu Y.H., Chang J.Y.F., Wang Y.P., Sun A. Anemia, hematinic deficiencies, hyperhomocysteinemia, and gastric parietal cell antibody positivity in burning mouth syndrome patients with iron deficiency. J Dent Sci. 2020;15:42–49. doi: 10.1016/j.jds.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang C.P., Wu Y.H., Wu Y.C., Chang J.Y., Wang Y.P., Sun A. Anemia, hematinic deficiencies, hyperhomocysteinemia, and serum gastric parietal cell antibody positivity in 884 patients with burning mouth syndrome. J Formos Med Assoc. 2020;119:813–820. doi: 10.1016/j.jfma.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Chiang M.L., Chiang C.P., Sun A. Anemia, hematinic deficiencies, and gastric parietal cell antibody positivity in burning mouth syndrome patients with or without hyperhomocysteinemia. J Dent Sci. 2020;15:214–221. doi: 10.1016/j.jds.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang M.L., Wu Y.H., Chang J.Y., Wang Y.P., Wu Y.C., Sun A. Anemia, hematinic deficiencies, and hyperhomocysteinemia in gastric parietal cell antibody-positive and -negative burning mouth syndrome patients. J Formos Med Assoc. 2021;120:819–826. doi: 10.1016/j.jfma.2020.08.032. [DOI] [PubMed] [Google Scholar]
- 9.Jin Y.T., Wu Y.C., Wu Y.H., Chang J.Y., Chiang C.P., Sun A. Anemia, hematinic deficiencies, hyperhomocysteinemia, and gastric parietal cell antibody positivity in burning mouth syndrome patients with or without microcytosis. J Dent Sci. 2021;16:608–613. doi: 10.1016/j.jds.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritchie A., Kramer J.M. Recent advances in the etiology and treatment of burning mouth syndrome. J Dent Res. 2018;97:1193–1199. doi: 10.1177/0022034518782462. [DOI] [PubMed] [Google Scholar]
- 11.Wu S., Zhang W., Yan J., Noma N., Young A., Yan Z. Worldwide prevalence estimates of burning mouth syndrome: a systematic review and meta-analysis. Oral Dis. 2021 doi: 10.1111/odi.13868. In press. [DOI] [PubMed] [Google Scholar]
- 12.National Health Insurance Administration . 2020. Ministry of Health and welfare, Taiwan, R.O.C. National Health insurance Research database. Data subsets. Assessed from nhird.nhri.org.tw/en (dated on 2020.12.30) [Google Scholar]
- 13.Tammiala-Salonen T., Hiidenkari T., Parvinen T. Burning mouth in a Finnish adult population. Community Dent Oral Epidemiol. 1993;21:67–71. doi: 10.1111/j.1600-0528.1993.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 14.Bergdahl M., Bergdahl J. Burning mouth syndrome: prevalence and associated factors. J Oral Pathol Med. 1999;28:350–354. doi: 10.1111/j.1600-0714.1999.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 15.Kohorst J.J., Bruce A.J., Torgerson R.R., Schenck L.A., Davis M.P.D. The prevalence of burning mouth syndrome: a population-based study. Br J Dermatol. 2015;172:1654–1656. doi: 10.1111/bjd.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J.Y., Kim Y.S., Ko I., Kim D.K. Association between burning mouth syndrome and the development of depression, anxiety, dementia, and Parkinson disease. JAMA Otolaryngol Head Neck Surg. 2020;146:561–569. doi: 10.1001/jamaoto.2020.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki N., Mashu S., Toyoda M., Nishibori M. Oral burning sensation: prevalence and gender differences in a Japanese population. Pain Pract. 2010;10:306–311. doi: 10.1111/j.1533-2500.2010.00361.x. [DOI] [PubMed] [Google Scholar]
- 18.Thoppay J.R., De Rossi S.S., Ciarrocca K.N. Burning mouth syndrome. Dent Clin. 2013;57:497–512. doi: 10.1016/j.cden.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Yang S.F., Wang Y.H., Su N.Y. Changes in prevalence of pre-cancerous oral submucous fibrosis from 1996-2013 in Taiwan: a nationwide population-based retrospective study. J Formos Med Assoc. 2018;117:147–152. doi: 10.1016/j.jfma.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y.T., Wang Y.H., Yu H.C., Yu C.H., Chang Y.C. Time trend in the prevalence of oral lichen planus based on Taiwanese National Health Insurance Research Database 1996-2013. J Dent Sci. 2018;13:274–280. doi: 10.1016/j.jds.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y.H., Chen Y.T., Chiu Y.W., Yu H.C., Chang Y.C. Time trends in the prevalence of diagnosed sialolithiasis from Taiwanese Nationwide Health Insurance Dental dataset. J Dent Sci. 2019;14:365–369. doi: 10.1016/j.jds.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee C.F., Lin K.Y., Lin M.C., Lin C.L., Chang S.N., Kao C.H. Sleep disorders increase the risk of burning mouth syndrome: a retrospective population-based cohort study. Sleep Med. 2014;15:1405–1410. doi: 10.1016/j.sleep.2014.06.009. [DOI] [PubMed] [Google Scholar]