Summary
Background
Although recent studies have suggested that naturally acquired Human papillomavirus (HPV) antibodies are partly protective against subsequent homotypic infection, the extent of protection remains indecisive. Here, we evaluate the protective effect of neutralizing and IgG antibodies simultaneously.
Methods
In a cohort of 3634 women aged 18-45 years from the control arm of a phase III trial of the HPV-16/18 bivalent vaccine, participants were tested for neutralizing antibodies by pseudovirion-based neutralization assay (PBNA) and IgG antibodies by enzyme-linked immunosorbent assay (ELISA) at baseline. HPV-16/18 incident and persistent infections were identified using cervical specimens periodically collected during the 5·5 years of follow-up. The protective effects of HPV-16/18 neutralizing and IgG antibodies against homotypic infection were assessed using a Cox proportional hazard model.
Findings
For the persistent infection (PI) endpoints of HPV-16/18 lasting for over 6/12 months, a prevalence of type-specific neutralizing antibodies was highly protective (6-month PI: hazard ratio (HR) = 0·16, 95% confidence interval (CI): 0·04, 0·65; 12-month PI: HR = 0·23, 95% CI: 0·06, 0·94), whereas a prevalence of IgG antibodies was associated with minor and non-significant protection (6-month PI: HR = 0·66, 95% CI: 0·40, 1·09; 12-month PI: HR = 0·66, 95% CI: 0·36, 1·20). After increasing the cut-off value to the median IgG level, the risk of 6-month PI was significantly lower in seropositive vs seronegative women (HR = 0·38, 95% CI: 0·18, 0·83).
Interpretation
Naturally acquired antibodies are associated with a substantially reduced risk of subsequent homotypic infection.
Funding
NSFC; The Fujian Province Health Education Joint Research Project; Xiamen Science and Technology Major Project; CIFMS; and Xiamen Innovax.
Research in context.
Evidence before this study
We searched PubMed for studies published between January 1, 2000, and July 30, 2020, using the search terms “human papillomavirus” or “HPV” and “serology” or “seropositivity” or “natural immunity” or “natural infection” and examined the reference lists of eligible publications. The search was not limited to English-language publications.
Infection with human papillomavirus (HPV) may elicit an antibody response, and measurement of the antibody response is highly dependent on the assay used. Several serological assays are used to measure type-specific antibodies, of which the pseudovirion-based neutralization assay (PBNA) measures total neutralizing antibodies that functionally block the entry of pseudovirions into cultured cells, which are expected to be the primary immune mechanism for protection against HPV infection. However, this assay is highly labour-intensive and is therefore seldom used in large epidemiological studies. The virus-like particle-based enzyme-linked immunosorbent assay (VLP-ELISA), which measures a broad spectrum of IgG antibodies binding with the L1 VLP coating, including neutralizing and non-neutralizing antibodies, is most commonly used as an HPV immunity indicator. Recent studies have consistently suggested that natural antibody responses confer partial protection, especially at higher antibody titres. The majority of previous studies investigating the protection of naturally acquired antibodies used IgG as the marker, and limited work has been carried out to determine the protective effect against HPV based on neutralizing assays. Wentzensen et al and Lin et al compared the protective effect of naturally acquired antibodies obtained with different assays and suggested that the point estimates of protection for neutralizing antibodies measured by the competitive Luminex immunoassay (cLIA) or by secreted alkaline phosphatase protein neutralization assay (SEAP-NA) were stronger than those for the IgG antibodies measured by VLP-ELISA. However, these two studies were case-control studies with small sample sizes that investigated the effect of antibodies on HPV transient infection. A better quantification of the protective effect against subsequent HPV infection based on neutralizing assays requires a much larger study.
Added value of this study
Our study is the first to evaluate the natural immunity conferred with HPV neutralizing antibodies measured by PBNA in a cohort with a large sample size and long-term follow-up and to compare the protective effect with that of total IgG antibodies measured by VLP-ELISA under different cut-off settings at the same time. We found that naturally acquired antibodies are associated with a substantially reduced risk of subsequent homotypic HPV infection. Neutralizing antibodies are a more specific indicator for protective natural immunity, and binding IgG can serve as a surrogate indicator after setting a proper cut-off value.
Implications of all the available evidence
Our study shows that natural antibodies provide considerable protection against future infection. These findings provide more objective parameters for vaccine cost-effectiveness analysis and for further optimizing HPV vaccination strategies, especially designing the target population of catch-up programmes, which is of great significance for achieving the global strategic goal of eliminating cervical cancer in 2030 put forth by the WHO with a currently insufficient supply of HPV vaccines.
Alt-text: Unlabelled box
1. Introduction
Human papillomavirus types 16 and 18 (HPV-16 and HPV-18) infections cause approximately 70% of cases of invasive cervical cancer worldwide.[1] More than 80% of sexually active individuals will be infected with HPV during their lifetime; although the majority can clear the infection in approximately 2 years, the remaining individuals develop persistent infection that might induce cancers.[2] Host immune responses are likely to be a critical mechanism for preventing, controlling and eliminating HPV infection. It is thought that clearance of HPV infection is mainly mediated by the cellular immune response, whereas antibody responses theoretically help prevent subsequent infections.[3]
Several prophylactic HPV vaccines have been available since 2006, including HPV-16/18 bivalent vaccine (Cervarix®, GSK, and Cecolin®, Xiamen Innovax), HPV-6/11/16/18 quadrivalent vaccine (Gardasil®, Merck), and HPV-6/11/16/18/31/33/45/52/58 9-valent vaccine (Gardasil ®9, Merck). However, the current insufficient supply of the vaccines, although expected to be temporary, has greatly slowed the pace of wide implementation, especially in resource-limited areas where the burden of cervical cancer is high. It is important to develop HPV vaccination strategies based on cost-effectiveness analysis, which is of great significance for achieving the global strategic goal of eliminating cervical cancer in 2030 put forth by the WHO.[4] Mathematical modelling has been used to estimate the cost-effectiveness of HPV vaccination, which requires realistic assumptions for the transmission and infection clearance of HPV, as well as the extent and duration of acquired immunity after infection clearance.[5] Laprise JF et al[6] suggested that the results of cost-effectiveness analysis were sensitive to assumptions about natural immunity; hence, a better understanding of the extent of preventive immunity raised by natural HPV infection is crucial to accurately model the effectiveness of different vaccination strategies.
Several HPV serological assays with different properties are currently available: the pseudovirion-based neutralization assay (PBNA), which measures total neutralizing antibodies that can block the entry of HPV type-specific pseudovirions into cultured cells and are thought to be a biologically relevant subset of the antibodies; the competitive Luminex immunoassay (cLIA), designed to measure antibodies competing with a dominant type-specific monoclonal antibody against a neutralizing epitope and thus has the potential to detect the majority of the neutralizing antibodies; and the virus-like particle-based enzyme-linked immunosorbent assay (VLP-ELISA), which measures a broad spectrum of neutralizing and non-neutralizing IgG antibodies binding with the coating L1 VLP.[7] These three assays are technically different and measure different aspects of the HPV humoral immune status.
Approximately 50%-70% of individuals infected with HPV-16/18 have detectable type-specific serum antibodies.[8], [9], [10], [11], [12] Whether these naturally acquired antibodies protect against future infections has been debated in earlier studies,[13], [14], [15] which might be due to the heterogeneity of antibody assays, relatively small sample sizes, analytic techniques and different end points. Nevertheless, recent studies have consistently suggested that natural HPV antibodies are partly protective, especially at higher antibody titres.[16], [17], [18] However, the immunity indicator used in these studies has mostly been binding IgG antibodies measured by VLP-ELISA. The sensitivity and specificity of ELISAs are largely determined by the characteristics of coating HPV L1 antigen, which lead to high heterogenicity among different ELISAs and might cause inconsistency among different studies. Neutralizing antibodies are expected to be the primary immune mechanism for protection against HPV infection, and for most virology studies, they are thought to be the gold standard for serology investigation. PBNA theoretically measures total HPV type-specific neutralizing antibodies; however, due to the complicated experimental platform and its labour-consuming characteristics, very limited work has been done to determine the protective effect of HPV natural immunity based on neutralizing antibody assays.
Wentzensen et al[19] and Lin et al[7] compared the protective effect of natural antibodies with different assays and suggested that the point estimates of protection for neutralizing antibodies measured by cLIA or by secreted alkaline phosphatase protein neutralization assay (SEAP-NA) were stronger than those for IgG antibodies measured by VLP-ELISA. However, these two studies were case-control studies with small sample sizes (N = 933 and N = 388, respectively) and investigated the effect of antibodies on HPV transient infection. A better quantification of the protection against subsequent HPV infection based on neutralizing assay requires a much larger study. Here, we evaluate the protection against HPV-16/18 infection of naturally acquired antibodies in a 66-month follow-up period based on the PBNA assay in a cohort of women from the control arm of a large multicentre phase III trial of the novel Escherichia coli-based recombinant HPV-16 and -18 bivalent vaccine (Cecolin®), expected to determine the protective extent of natural HPV antibodies. As VLP-ELISA is a much easier platform to set up than PBNA, we compared the protective effect between neutralizing antibodies and that of total IgG antibodies measured by ELISA and explored the potential for IgG antibodies to act as a surrogate for neutralizing antibodies to investigate the protective effect of naturally induced antibodies.
2. Methods
2.1. Study population and procedures
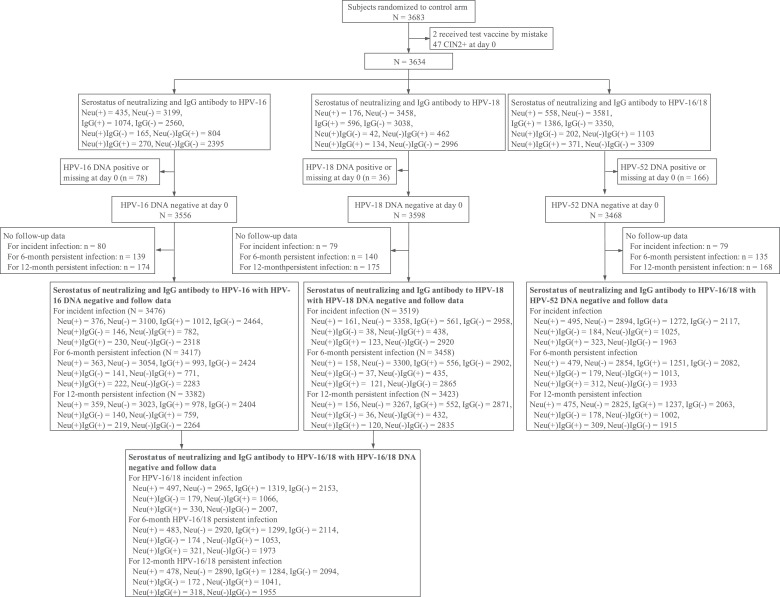
Study participants were women randomized to the control arm of the multicentre, randomized, double-blind, controlled phase III trial of the HPV-16/18 bivalent vaccine Cecolin® (ClinicalTrials.gov, NCT01735006). The enrolled participants were healthy women aged 18 to 45 years old who were not pregnant, were immunocompetent, and had 1-4 sexual partners. The methodologies, inclusion and exclusion criteria have been previously described in detail.[20] Our analysis included women DNA negative for HPV-16 and/or HPV-18, with no high-grade cervical intraepithelial lesion or cancer (CIN2+) at baseline, who had received at least one control vaccine dose and had at least one effective follow-up visit (Figure 1).
Figure 1.
Flow chart of the participants. CIN2+: High-grade cervical intraepithelial lesion or cancer; Neu (+): Seropositive for neutralizing antibodies; Neu (-): Seronegative for neutralizing antibodies; IgG (+): Seropositive for IgG antibodies; IgG (-): Seronegative for IgG antibodies; Neu (-) IgG (-): Seronegative for both neutralizing and IgG antibodies; Neu (-) IgG (+): Seronegative for neutralizing antibodies but seropositive for IgG antibodies; Neu (+) IgG (-): Seropositive for neutralizing antibodies but seronegative for IgG antibodies; Neu (+) IgG (+): Seropositive for both neutralizing and IgG antibodies;
Serum samples of all participants were collected at day 0 before vaccination. Gynaecological examinations were performed at day 0 and months 7, 12, 18, 24, 30, 42, 54, and 66. At these visits, endocervical swab samples were collected for Papanicolaou testing and HPV DNA typing. Cytology results were reported according to the Bethesda system-2001. Women with abnormal cytological test results, excluding atypical squamous cells of undetermined significance (ASC-US)/high-risk HPV-negative, were referred for colposcopy and biopsied if necessary according to the colposcopy management algorithm. Paraffin-embedded biopsied tissue specimens diagnosed as cervical intraepithelial neoplasia grade 1 or more severe (CIN1+) were also typed for HPV DNA.[20] Written informed consent was obtained from all participants, and the protocol was approved by independent ethics committees.
2.2. Antibody detection
Anti-HPV-16 and -18 of baseline serum samples were tested by both PBNA and ELISA. Detailed procedures have been described previously.[20], [21], [22] In brief, for PBNA, the serum samples were serially diluted starting at 1:20 in 2-fold increments and then mixed with HPV pseudovirions, after which the mixtures were transferred to 293FT cell monolayers and incubated at 37°C and 5% CO2. A positive sample was defined as one that caused a 50% reduction or more in green fluorescent protein expression (GFP) compared with the negative control, and the neutralization titres were defined as the highest dilution of positive samples. The cut-off titres of PBNA were set as 1:20. For ELISA, each well of a 96-well microtitre plate was coated with HPV L1 VLPs expressed by E. coli, and then serially diluted serum samples were added. The optical density was read at 450/620 nm. The positive samples were quantified using standard curves of references traceable to the World Health Organization international standards for antibodies against HPV-16 (NIBSC code 05/134) or HPV-18 (NIBSC code 10/140) expressed in international units (IUs), and the cut-off values were 3·0 IU/ml for HPV-16 and 2·1 IU/ml for HPV-18.
2.3. HPV DNA detection
HPV DNA testing was performed using the HPV DNA enzyme immunoassay (DEIA) (Labo Biomedical Products, the Netherlands). Samples with positive findings were further typed by broad spectrum PCR SPF10-LiPA 25 (Version 1) (Labo Biomedical Products) for 13 oncogenic HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and 12 non-oncogenic HPV types (6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 70, and 74) and by HPV-16/18-specific polymerase chain reactions (HPV TS16/18, Labo Biomedical Products). A positive result for HPV-16 or HPV-18 was defined as the presence of the relevant type of HPV DNA by either LiPA or HPV TS16/18.
2.4. Statistical analysis
2.4.1. Exposure variables
The main exposure variables were HPV-16 and -18 serostatus, which were expressed as binary variables (seropositive or seronegative) at enrolment. To increase the statistical power of the analysis, we also combined the protective effects of natural antibodies against future homotypic infections with HPV-16 and HPV-18. According to the serostatus of neutralizing and IgG antibodies to HPV-16 and HPV-18, women were classified as three analysis set groups: 1) seronegative for both HPV-16 and HPV-18 neutralizing antibodies (HPV-16/18 Neu (-)); seropositive for HPV-16 and/or HPV-18 neutralizing antibodies (HPV-16/18 Neu (+)); 2) seronegative for both HPV-16 and HPV-18 IgG antibodies (HPV-16/18 IgG (-)); seropositive for HPV-16 and/or HPV-18 IgG antibodies (HPV-16/18 IgG (+)); 3) seronegative for both HPV-16 neutralizing and IgG antibodies and seronegative for both HPV-18 neutralizing and IgG antibodies (HPV-16/18 Neu (-) IgG (-)); seronegative for HPV-16 neutralizing antibodies but seropositive for HPV-16 IgG antibodies and/or seronegative for HPV-18 neutralizing antibodies but seropositive for HPV-18 IgG antibodies (HPV-16/18 Neu (-) IgG (+)); seropositive for HPV-16 neutralizing antibodies but seronegative for HPV-16 IgG antibodies and/or seropositive for HPV-18 neutralizing antibodies but seronegative for HPV-18 IgG antibodies (HPV-16/18 Neu (+) IgG (-)); seropositive for both HPV-16 neutralizing and IgG antibodies and/or seropositive for both HPV-18 neutralizing and IgG antibodies (HPV-16/18 Neu (+) IgG (+)) (Table 1).
Table 1.
Categorization scheme of exposure.
| Exposure | HPV-16 serostatus | HPV-18 serostatus | The endpoint type of observation | ||
|---|---|---|---|---|---|
| Neu | IgG | Neu | IgG | ||
| HPV-16/18 Neu (-) | - | NA | - | NA | HPV-16 and HPV-18 |
| HPV-16/18 Neu (+) | + | NA | - | NA | HPV-16 |
| - | NA | + | NA | HPV-18 | |
| + | NA | + | NA | HPV-16 and HPV-18 | |
| HPV-16/18 IgG (-) | NA | - | NA | - | HPV-16 and HPV-18 |
| HPV-16/18 IgG (+) | NA | + | NA | - | HPV-16 |
| NA | - | NA | + | HPV-18 | |
| NA | + | NA | + | HPV-16 and HPV-18 | |
| HPV-16/18 Neu (-) IgG (-) | - | - | - | - | HPV-16 and HPV-18 |
| HPV-16/18 Neu (-) IgG (+) | - | + | - | - | HPV-16 |
| - | + | + | - | HPV-16 | |
| - | + | + | + | HPV-16 | |
| - | - | - | + | HPV-18 | |
| + | - | - | + | HPV-18 | |
| + | + | - | + | HPV-18 | |
| - | + | - | + | HPV-16 and HPV-18 | |
| HPV-16/18 Neu (+) IgG (-) | + | - | - | - | HPV-16 |
| + | - | - | + | HPV-16 | |
| + | - | + | + | HPV-16 | |
| + | - | + | - | HPV-16 and HPV-18 | |
| - | - | + | - | HPV-18 | |
| - | + | + | - | HPV-18 | |
| + | + | + | - | HPV-18 | |
| HPV-16/18 Neu (+) IgG (+) | + | + | - | - | HPV-16 |
| + | + | - | + | HPV-16 | |
| + | + | + | - | HPV-16 | |
| + | + | + | + | HPV-16 and HPV-18 | |
| - | - | + | + | HPV-18 | |
| - | + | + | + | HPV-18 | |
| + | - | + | + | HPV-18 | |
Neu: neutralizing antibodies, IgG: IgG antibodies; +: seropositive; -: seronegative. NA: not applicable, meaning regardless of the serostatus.
2.4.2. Outcome variables
Endpoints evaluated were newly detected infection, 6/12-month persistent infection (6-m PI and 12-m PI), separately defined as (1) incident infection: detection of the specific-type HPV DNA at least once during the follow-up period; (2) 6/12 m PI: detection of the same HPV type in at least 2 samples not interrupted by negative samples over a minimum of 150 days (6-m PI) and of 300 days (12-m PI).
2.4.3. Statistical methods
Incidence was calculated as the number of detected events divided by the total person-time. Person-time was calculated as the sum of the follow-up for each participant expressed in years. The follow-up period started on the day after the first vaccination and ended on the date of the first occurrence of the type-specific endpoint or the date of the last effective gynaecological visit. The analysis unit for grouped infection was based on the individual, and infection that occurred in the relative baseline serostatus cohort of the corresponding type was considered an endpoint event (Table 1). The relationship between exposure and risk of newly detected infections was assessed using a Cox proportional hazard model. The Kaplan-Meier method was used to construct the cumulative incidence of infections by serostatus group. Log-rank tests were used to compare the differences in the cumulative incidence across groups. All statistical analyses were conducted using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
2.4.4. Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all of the data, as well as the final responsibility to submit for publication.
3. Results
A total of 3634 participants were included in the study (Figure 1). The seroprevalences of HPV-16 neutralizing antibodies and IgG antibodies were 12·0% (435 of 3634) and 29·6% (1074 of 3634), respectively. For HPV-18, the seroprevalences of neutralizing antibodies and IgG antibodies were 4·8% (176 of 3634) and 16·4% (596 of 3634), respectively. Baseline characteristics among women with different serostatuses are shown in Table 2 and Tables S1-2 in the Supplementary Tables. The mean age of the participants was approximately 30 years and was similar in different serostatus groups. At baseline, compared with women who were negative for both neutralizing and IgG antibodies, women with neutralizing antibodies more frequently had abnormal cytological findings (ASC-US+) (11·5% vs 7·1%, P=0·0011), while the prevalence was the same in women with IgG antibodies (8·4% vs 7·1%, P=0·1515).
Table 2.
Baseline characteristics of the participants in different HPV-16/18 serostatus groups.
| Neutralizing antibodies |
Binding antibodies |
Combination of neutralizing and binding antibodies |
||||||
|---|---|---|---|---|---|---|---|---|
| Neu (-) | Neu (+) | IgG (-) | IgG (+) | Neu (-) IgG (-) | Neu (-) IgG (+) | Neu (+) IgG (-) | Neu (+) IgG (+) | |
| Number of participants | 3076 | 558 | 2248 | 1386 | 2082 | 1103 | 202 | 371 |
| Mean age ± SD (y) | 29·9 ± 7·4 | 29·9 ± 7·2 | 30·0 ± 7·4 | 29·7 ± 7·2 | 30·0± 7·4 | 29·8 ± 7·2 | 30·4 ± 7·5 | 29·8 ± 7·1 |
| Cytological findings at day 0, n (%) # | ||||||||
| Normal (NILM) | 2859 (93·0) | 493 (88·4) | 2085 (92·8) | 1267 (91·4) | 1933 (92·8) | 1020 (92·5) | 183 (90·6) | 323 (87·1) |
| Abnormal (ASC-US+) | 215 (7·0) | 64 (11·5) | 162 (7·2) | 117 (8·4) | 148 (7·1) | 82 (7·4) | 19 (9·4) | 47 (12·7) |
| ASC-US | 138 (4·5) | 45 (8·1) | 111 (4·9) | 72 (5·2) | 101 (4·9) | 47 (4·3) | 13 (6·4) | 33 (8·9) |
| HC2 (-) | 88 (2·9) | 21 (3·8) | 72 (3·2) | 37 (2·7) | 68 (3·3) | 24 (2·2) | 5 (2·5) | 16 (4·3) |
| HC2 (+) | 50 (1·6) | 24 (4·3) | 39 (1·7) | 35 (2·5) | 33 (1·6) | 23 (2·1) | 8 (4·0) | 17 (4·6) |
| LSIL | 69 (2·2) | 14 (2·5) | 45 (2·0) | 38 (2·7) | 42 (2·0) | 30 (2·7) | 4 (2·0) | 11 (3·0) |
| HSIL | 1 (0.0) | 4 (0·7) | 2 (0·1) | 3 (0·2) | 1 (0·1) | 1 (0·1) | 1 (0·5) | 3 (0·8) |
| ASC-H | 5 (0·2) | 1 (0·2) | 3 (0·1) | 3 (0·2) | 3 (0·1) | 3 (0·3) | 1 (0·5) | 0 (0) |
| AIS/AGC | 2 (0·1) | 0 (0) | 1 (0·0) | 1 (0·1) | 1 (0·1) | 1 (0·1) | 0 (0) | 0 (0) |
| Unsatisfactory | 2 (0·1) | 1 (0·2) | 1 (0·0) | 2 (0·1) | 1 (0·1) | 1 (0·1) | 0 (0) | 1 (0·3) |
Neu (-): Seronegative for both HPV-16 and HPV-18 neutralizing antibodies; Neu (+): Seropositive for HPV-16 and/or HPV-18 neutralizing antibodies; IgG (-): Seronegative for both HPV-16 and HPV-18 IgG antibodies; IgG (+): Seropositive for HPV-16 and/or HPV-18 IgG antibodies; Neu (-) IgG (-): Seronegative for both HPV-16 neutralizing and IgG antibodies and seronegative for both HPV-18 neutralizing and IgG antibodies; Neu (-) IgG (+): Seronegative for HPV-16 neutralizing antibodies but seropositive for HPV-16 IgG antibodies and/or seronegative for HPV-18 neutralizing antibodies but seropositive for HPV-18 IgG antibodies; Neu (+) IgG (-): Seropositive for HPV-16 neutralizing antibodies but seronegative for HPV-16 IgG antibodies and/or seropositive for HPV-18 neutralizing antibodies but seronegative for HPV-18 IgG antibodies; Neu (+) IgG (+): Seropositive for both HPV-16 neutralizing and IgG antibodies and/or seropositive for both HPV-18 neutralizing and IgG antibodies
NILM: negative for intraepithelial lesion or malignancy; ASC-US: Atypical squamous cells of undetermined significance; LSIL: low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; ASC-H: atypical squamous cells cannot exclude high-grade lesion; AIS: adenocarcinoma in situ; AGC: atypical glandular cells; HC2 (-): negative on the Hybrid Capture-2 test; HC2 (+): positive on the Hybrid Capture-2 test.
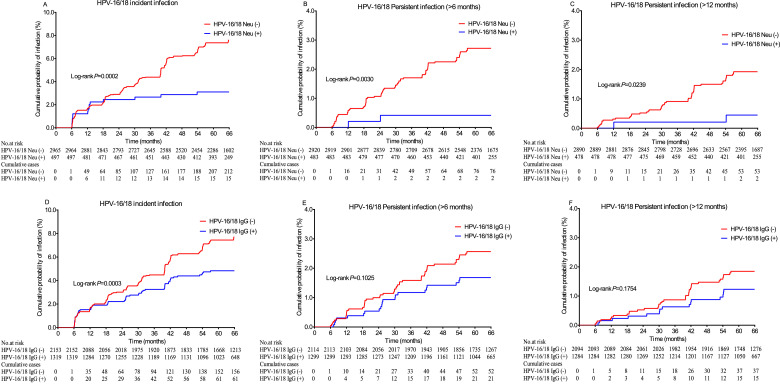
The observed protective effects of neutralizing and IgG antibodies against subsequent infection were very similar in univariate and age-adjusted analyses (Table 3). In age-adjusted analyses, both neutralizing antibodies and IgG antibodies were associated with protection against subsequent HPV-16/18 incident infections. Although the point estimate of the neutralizing antibodies showed stronger protection, the difference was not significant (Neu: HR=0·41, 95% CI, 0·25, 0·67; IgG: HR=0·60, 95% CI, 0·46, 0·79). We observed that neutralizing antibodies significantly lowered the risk of subsequent homotypic HPV-16/18 persistent infection (6-m PI: HR = 0·16, 95% CI: 0·04, 0·65; 12-m PI: HR = 0·23, 95% CI: 0·06, 0·94) but that the presence of IgG antibodies modestly and non-significantly lowered the risk of subsequent homotypic HPV-16/18 persistent infection (6-m PI: HR = 0·66, 95% CI: 0·40, 1·09; 12-m PI: HR = 0·66, 95% CI: 0·36, 1·20). Moreover, concomitant positivity for IgG antibodies did not significantly increase the preventive effects of neutralizing antibodies (Table 3). The Kaplan-Meier plots showed that the preventive effects of neutralizing antibodies did not decline with time for at least 5 years (Figure 2).
Table 3.
The risk of newly detected HPV-16/18 infection according to the HPV-16/18 serostatus.
| HPV-16/18 serostatus* | No. of participants | Person-years | No. of events | Incidence (95% CI), per 100 person-years | Hazard ratio (95% CI) | P Value | Adjusted Hazard ratio (95% CI) # | P Value # |
|---|---|---|---|---|---|---|---|---|
| Endpoint: HPV-16/18 incident infection | ||||||||
| Neu (-) | 2965 | 14,970 | 253 | 1·69 (1·49, 1·91) | 1·00 | •• | 1·00 | •• |
| Neu (+) | 497 | 2525 | 17 | 0·67 (0·42, 1·08) | 0·40 (0·25, 0·66) | 0·0003 | 0·41 (0·25, 0·67) | 0·0003 |
| IgG (-) | 2153 | 10,875 | 190 | 1·75 (1·52, 2·01) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 1319 | 6691 | 70 | 1·05 (0·83, 1·32) | 0·60 (0·46, 0·80) | 0·0003 | 0·60 (0·46, 0·79) | 0·0003 |
| Neu (-) IgG (-) | 2007 | 10,164 | 179 | 1·76 (1·52, 2·04) | 1·00 | •• | 1·00 | •• |
| Neu (-) IgG (+) | 1066 | 5409 | 58 | 1·07 (0·83, 1·39) | 0·62 (0·46, 0·83) | 0·0014 | 0·61 (0·46, 0·82) | 0·0011 |
| Neu (+) IgG (-) | 179 | 902 | 5 | 0·55 (0·23, 1·33) | 0·33 (0·14, 0·80) | 0·0146 | 0·34 (0·14, 0·81) | 0·0158 |
| Neu (+) IgG (+) | 330 | 1687 | 12 | 0·71 (0·40, 1·25) | 0·41 (0·23, 0·73) | 0·0024 | 0·41 (0·23, 0·73) | 0·0025 |
| Endpoint: 6-month persistent HPV-16/18 infection | ||||||||
| Neu (-) | 2920 | 15,283 | 76 | 0·50 (0·40, 0·62) | 1·00 | •• | 1·00 | •• |
| Neu (+) | 483 | 2559 | 2 | 0·08 (0·02, 0·31) | 0·16 (0·04, 0·64) | 0·0097 | 0·16 (0·04, 0·65) | 0·0102 |
| IgG (-) | 2114 | 11,112 | 52 | 0·47 (0·36, 0·61) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 1299 | 6784 | 21 | 0·31 (0·20, 0·47) | 0·66 (0·40, 1·09) | 0·1052 | 0·66 (0·40, 1·09) | 0·1022 |
| Neu (-) IgG (-) | 1973 | 10,378 | 50 | 0·48 (0·37, 0·64) | 1·00 | •• | 1·00 | •• |
| Neu (-) IgG (+) | 1053 | 5470 | 20 | 0·37 (0·24, 0·57) | 0·75 (0·45, 1·27) | 0·2852 | 0·75 (0·45, 1·26) | 0·2784 |
| Neu (+) IgG (-) | 174 | 913 | 1 | 0·11 (0·02, 0·78) | 0·23 (0·03, 1·64) | 0·1406 | 0·23 (0·03, 1·65) | 0·1435 |
| Neu (+) IgG (+) | 321 | 1711 | 1 | 0·06 (0·01, 0·41) | 0·12 (0·02, 0·88) | 0·0364 | 0·12 (0·02, 0·88) | 0·0367 |
| Endpoint: 12-month persistent HPV-16/18 infection | ||||||||
| Neu (-) | 2890 | 15,320 | 53 | 0·35 (0·26, 0·45) | 1·00 | •• | 1·00 | •• |
| Neu (+) | 478 | 2550 | 2 | 0·08 (0·02, 0·31) | 0·23 (0·06, 0·93) | 0·0390 | 0·23 (0·06, 0·94) | 0·0408 |
| IgG (-) | 2094 | 11,135 | 37 | 0·33 (0·24, 0·46) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 1284 | 6783 | 15 | 0·22 (0·13, 0·37) | 0·66 (0·36, 1·21) | 0·1786 | 0·66 (0·36, 1·20) | 0·1756 |
| Neu (-) IgG (-) | 1955 | 10,398 | 36 | 0·35 (0·25, 0·48) | 1·00 | •• | 1·00 | •• |
| Neu (-) IgG (+) | 1041 | 5475 | 14 | 0·26 (0·15, 0·43) | 0·74 (0·40, 1·36) | 0·3277 | 0·73 (0·40, 1·36) | 0·3222 |
| Neu (+) IgG (-) | 172 | 910 | 1 | 0·11 (0·02, 0·78) | 0·32 (0·04, 2·30) | 0·2552 | 0·32 (0·04, 2·32) | 0·2592 |
| Neu (+) IgG (+) | 318 | 1704 | 1 | 0·06 (0·01, 0·42) | 0·17 (0·02, 1·23) | 0·0793 | 0·17 (0·02, 1·24) | 0·0798 |
Neu (-): Seronegative for both HPV-16 and HPV-18 neutralizing antibodies; Neu (+): Seropositive for HPV-16 and/or HPV-18 neutralizing antibodies; IgG (-): Seronegative for both HPV-16 and HPV-18 IgG antibodies; IgG (+): Seropositive for HPV-16 and/or HPV-18 IgG antibodies; Neu (-) IgG (-): Seronegative for both HPV-16 neutralizing and IgG antibodies and seronegative for both HPV-18 neutralizing and IgG antibodies; Neu (-) IgG (+): Seronegative for HPV-16 neutralizing antibodies but seropositive for HPV-16 IgG antibodies and/or seronegative for HPV-18 neutralizing antibodies but seropositive for HPV-18 IgG antibodies; Neu (+) IgG (-): Seropositive for HPV-16 neutralizing antibodies but seronegative for HPV-16 IgG antibodies and/or seropositive for HPV-18 neutralizing antibodies but seronegative for HPV-18 IgG antibodies; Neu (+) IgG (+): Seropositive for both HPV-16 neutralizing and IgG antibodies and/or seropositive for both HPV-18 neutralizing and IgG antibodies.
Adjusted for continuous age at enrolment.
Figure 2.
Kaplan-Meier estimates of the cumulative incidence of HPV-16/18 incident infection (A, D), persistent infection (>6 months) (B, E) and persistent infection (>12 months) (C, F) among different HPV-16/18 serostatus groups. The log-rank test was used to analyse the differences among different serostatuses. HPV-16/18 Neu (-): seronegative for both HPV-16 and HPV-18 neutralizing antibodies; HPV-16/18 Neu (+): seropositive for HPV-16 and/or HPV-18 neutralizing antibodies; HPV-16/18 IgG (-): seronegative for both HPV-16 and HPV-18 IgG antibodies; HPV-16/18 IgG (+): seropositive for HPV-16 and/or HPV-18 IgG antibodies.
The protective effect of binding IgG antibodies based on increased cut-off values determined from quartile IgG antibody levels of seropositive participants was also analysed. After increasing the cut-off value, positive IgG status showed stronger protection efficacy against subsequent HPV infection. With setting the cut-off to the median level (6·6 IU/ml for HPV-16 and 4·0 IU/ml for HPV-18), the risk of HPV-16/18 incident infection and 6-month PI was statistically lower in seropositive vs seronegative women (incident infection: HR = 0·54, 95% CI: 0·38, 0·78; 6-m PI: HR = 0·38, 95% CI: 0·18, 0·83). The point estimate protective effect of IgG peaked at the cut-off setting of the median IgG level, which was still lower than that of PBNA neutralizing antibodies, and the calculated protection efficacy was not further increased by increasing the cut-off value to the 75% quantile (Q3) (Table 4). When analysing the different combined IgG and neutralizing antibody statuses, moderate protection against 6-month persistent infection was observed in HPV-16/18 Neu (-) IgG (+) compared with HPV-16/18 Neu (-) IgG (-); however, the point estimate was also weaker than HPV-16/18 Neu (+) IgG (-) (HR = 0·43, 95% CI: 0·19, 1·00 vs HR = 0·13, 95% CI: 0·02, 0·95) (Table S3 in the Supplementary Tables).
Table 4.
The risk of newly detected HPV-16/18 infection according to HPV-16/18 IgG serostatus depended on different cut-off values.
| HPV-16/18 serostatus* | No. of participants | Person-years | No. of events | Incidence (95% CI), per 100 person-years | Hazard ratio (95% CI) | P Value | Adjusted Hazard ratio (95% CI) # | P Value# |
|---|---|---|---|---|---|---|---|---|
| Endpoint: HPV-16/18 incident infection | ||||||||
| Cut-off (Q1): HPV-16: 4·4; HPV-18: 2·8 | ||||||||
| IgG (-) | 2394 | 12,090 | 205 | 1·70 (1·48, 1·94) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 1072 | 5441 | 56 | 1·03 (0·79, 1·34) | 0·61 (0·46, 0·82) | 0·0011 | 0·61 (0·45, 0·82) | 0·0010 |
| Cut-off (Q2): HPV-16: 6·6; HPV-18: 4·0 | ||||||||
| IgG (-) | 2751 | 13,855 | 233 | 1·68 (1·48, 1·91) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 711 | 3639 | 33 | 0·91 (0·64, 1·28) | 0·54 (0·38, 0·78) | 0·0009 | 0·54 (0·38, 0·78) | 0·0009 |
| Cut-off (Q3): HPV-16: 10·1; HPV-18: 7·6 | ||||||||
| IgG (-) | 3084 | 15,548 | 260 | 1·67 (1·48, 1·89) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 374 | 1886 | 19 | 1·01 (0·64, 1·58) | 0·60 (0·38, 0·95) | 0·0300 | 0·61 (0·38, 0·96) | 0·0344 |
| Endpoint: 6-month persistent infection | ||||||||
| Cut-off (Q1): HPV-16: 4·4; HPV-18: 2·8 | ||||||||
| IgG (-) | 2352 | 12,338 | 59 | 0·48 (0·37, 0·62) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 1055 | 5527 | 16 | 0·29 (0·18, 0·47) | 0·60 (0·35, 1·05) | 0·0725 | 0·60 (0·35, 1·04) | 0·0708 |
| Cut-off (Q2): HPV-16: 6·6; HPV-18: 4·0 | ||||||||
| IgG (-) | 2702 | 14,143 | 70 | 0·49 (0·39, 0·63) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 701 | 3705 | 7 | 0·19 (0·09, 0·40) | 0·38 (0·18, 0·83) | 0·0149 | 0·38 (0·18, 0·83) | 0·0154 |
| Cut-off (Q3): HPV-16: 10·1; HPV-18: 7·6 | ||||||||
| IgG (-) | 3032 | 15,876 | 79 | 0·50 (0·40, 0·62) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 367 | 1925 | 4 | 0·21 (0·08, 0·55) | 0·42 (0·15, 1·14) | 0·0875 | 0·42 (0·15, 1·15) | 0·0921 |
| Endpoint: 12-month persistent infection | ||||||||
| Cut-off (Q1): HPV-16: 4·4; HPV-18: 2·8 | ||||||||
| IgG (-) | 2328 | 12,365 | 41 | 0·33 (0·24, 0·45) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 1044 | 5523 | 13 | 0·24 (0·14, 0·41) | 0·71 (0·38, 1·32) | 0·2754 | 0·71 (0·38, 1·32) | 0·2714 |
| Cut-off (Q2): HPV-16: 6·6; HPV-18: 4·0 | ||||||||
| IgG (-) | 2675 | 14,184 | 47 | 0·33 (0·25, 0·44) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 693 | 3696 | 6 | 0·16 (0·07, 0·36) | 0·49 (0·21, 1·14) | 0·0977 | 0·49 (0·21, 1·15) | 0·1006 |
| Cut-off (Q3): HPV-16: 10·1; HPV-18: 7·6 | ||||||||
| IgG (-) | 3002 | 15,919 | 54 | 0·34 (0·26, 0·44) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 362 | 1921 | 4 | 0·21 (0·08, 0·55) | 0·61 (0·22, 1·69) | 0·3429 | 0·62 (0·23, 1·72) | 0·3580 |
IgG (-): Seronegative for both HPV-16 and HPV-18 IgG antibodies; IgG (+): Seropositive for HPV-16 and/or HPV-18 IgG antibodies;
Adjusted for continuous age at enrolment.
When natural immunity to HPV-16 and HPV-18 was analysed independently, limited to the sample size and low incidence, the data did not show a significantly reduced risk of infection by HPV-16 or HPV-18. However, similar trends were observed for the preventive effects of neutralizing and IgG antibodies (Table 5, Tables S4-6 in the Supplementary Tables, and Figures S1-2 in the Supplementary Figures).
Table 5.
The risk of newly detected HPV-16 infection according to HPV-16 serostatus.
| HPV-16 serostatus* | No. of subjects | Person-years | No. of events | HPV-16 incidence (95%CI) per 100 person-years | Hazard ratio (95%CI) | P Value | Adjusted Hazard ratio (95% CI) # | P Value # |
|---|---|---|---|---|---|---|---|---|
| Endpoint: incident infection | ||||||||
| Neu (-) | 3100 | 15,855 | 178 | 1·12 (0·97, 1·30) | 1·00 | •• | 1·00 | •• |
| Neu (+) | 376 | 1888 | 15 | 0·79 (0·48, 1·32) | 0·72 (0·43, 1·23) | 0·2276 | 0·73 (0·43, 1·24) | 0·2400 |
| IgG (-) | 2464 | 12,612 | 140 | 1·11 (0·94, 1·31) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 1012 | 5131 | 53 | 1·03 (0·79, 1·35) | 0·93 (0·68, 1·28) | 0·6734 | 0·93 (0·68, 1·27) | 0·6469 |
| Neu (-) IgG (-) | 2318 | 11,880 | 135 | 1·14 (0·96, 1·35) | 1·00 | •• | 1·00 | •• |
| Neu (-) IgG (+) | 782 | 3975 | 43 | 1·08 (0·80, 1·46) | 0·96 (0·68, 1·35) | 0·8056 | 0·95 (0·67, 1·34) | 0·7729 |
| Neu (+) IgG (-) | 146 | 732 | 5 | 0·68 (0·28, 1·64) | 0·63 (0·26, 1·54) | 0·3087 | 0·64 (0·26, 1·56) | 0·3222 |
| Neu (+) IgG (+) | 230 | 1155 | 10 | 0·87 (0·47, 1·61) | 0·77 (0·40, 1·46) | 0·4190 | 0·77 (0·41, 1·46) | 0·4242 |
| Endpoint: 6-month persistent infection | ||||||||
| Neu (-) | 3054 | 16,004 | 59 | 0·37 (0·29, 0·48) | 1·00 | •• | 1·00 | •• |
| Neu (+) | 363 | 1920 | 2 | 0·10 (0·03, 0·42) | 0·28 (0·07, 1·15) | 0·0781 | 0·28 (0·07, 1·16) | 0·0801 |
| IgG (-) | 2424 | 12,733 | 47 | 0·37 (0·28, 0·49) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 993 | 5190 | 14 | 0·27 (0·16, 0·46) | 0·73 (0·40, 1·32) | 0·2966 | 0·73 (0·40, 1·32) | 0·2908 |
| Neu (-) IgG (-) | 2283 | 11,991 | 46 | 0·38 (0·29, 0·51) | 1·00 | •• | 1·00 | •• |
| Neu (-) IgG (+) | 771 | 4013 | 13 | 0·32 (0·19, 0·56) | 0·84 (0·45, 1·56) | 0·5793 | 0·84 (0·45, 1·55) | 0·5681 |
| Neu (+) IgG (-) | 141 | 742 | 1 | 0·13 (0·02, 0·96) | 0·35 (0·05, 2·53) | 0·2976 | 0·35 (0·05, 2·56) | 0·3030 |
| Neu (+) IgG (+) | 222 | 1177 | 1 | 0·08 (0·01, 0·60) | 0·22 (0·03, 1·60) | 0·1350 | 0·22 (0·03, 1·61) | 0·1362 |
| Endpoint: 12-month persistent infection | ||||||||
| Neu (-) | 3023 | 16,021 | 40 | 0·25 (0·18, 0·34) | 1·00 | •• | 1·00 | •• |
| Neu (+) | 359 | 1912 | 2 | 0·10 (0·03, 0·42) | 0·42 (0·10, 1·73) | 0·2287 | 0·42 (0·10, 1·73) | 0·2301 |
| IgG (-) | 2404 | 12,753 | 32 | 0·25 (0·18, 0·35) | 1·00 | •• | 1·00 | •• |
| IgG (+) | 978 | 5180 | 10 | 0·19 (0·10, 0·36) | 0·77 (0·38, 1·56) | 0·4634 | 0·77 (0·38, 1·56) | 0·4615 |
| Neu (-) IgG (-) | 2264 | 12,012 | 31 | 0·26 (0·18, 0·37) | 1·00 | •• | 1·00 | •• |
| Neu (-) IgG (+) | 759 | 4009 | 9 | 0·22 (0·12, 0·43) | 0·87 (0·41, 1·82) | 0·7044 | 0·87 (0·41, 1·82) | 0·7017 |
| Neu (+) IgG (-) | 140 | 741 | 1 | 0·13 (0·02, 0·96) | 0·52 (0·07, 3·81) | 0·5201 | 0·52 (0·07, 3·83) | 0·5224 |
| Neu (+) IgG (+) | 219 | 1171 | 1 | 0·09 (0·01, 0·61) | 0·33 (0·05, 2·42) | 0·2756 | 0·33 (0·05, 2·42) | 0·2762 |
Neu (-): Seronegative for HPV-16 neutralizing antibodies; Neu (+): Seropositive for HPV-16 neutralizing antibodies; IgG (-): Seronegative for HPV-16 IgG antibodies; IgG (+): Seropositive for HPV-16 IgG antibodies; Neu (-) IgG (-): Seronegative for both HPV-16 neutralizing and IgG antibodies; Neu (-) IgG (+): Seronegative for HPV-16 neutralizing antibodies but seropositive for HPV-16 IgG antibodies; Neu (+) IgG (-): Seropositive for HPV-16 neutralizing antibodies but seronegative for HPV-16 IgG antibodies; Neu (+) IgG (+): Seropositive for both HPV-16 neutralizing and IgG antibodies;
Adjusted for continuous age at enrolment.
4. Discussion
In this study, we observed an association between HPV-16/18 seropositivity and protection from subsequent infection over 5·5 years of follow-up, though the extent of protection was dependent on the serological indicators used. Naturally acquired HPV-16/18 neutralizing antibodies significantly reduced the risk of subsequent 6-month infection by 84%, while the binding IgG antibodies modestly and non-significantly lowered the risk by 34% during the 66-month follow-up period. After increasing the cut-off value of IgG antibodies to the median IgG antibody level of seropositive participants, IgG antibodies also showed a significant protective effect against future infection, albeit with a relatively lower point estimate (62%) than those of neutralizing antibodies.
The strength of our study is its first assessment of natural immunity conferred with neutralizing antibodies measured by PBNA in a cohort with a large sample size and long-term follow-up, and the laboratory measurements were fully validated to support the licensure of a new vaccine.[20] At the same time, we compared the protective effect of total IgG antibodies measured by VLP-ELISA under different cut-off settings with that of PBNA neutralizing antibody.
Previous studies suggested that the risk of subsequent HPV infection showed a decreasing trend with increasing homotypic HPV-specific IgG titres.[16], [18] Our study also showed that the protective effect of IgG antibodies was mild at the original cut-off value but that it increased after enhancing the IgG cut-off value set. After increasing the cut-off to the median IgG level of seropositive participants, positive IgG status also showed higher protection efficacy against subsequent HPV infection, and the protective effect could not be further increased by increasing the cut-off value to the 75% quartile. Nonetheless, the point estimate of the protective effect of positive IgG (at its optimal cut-off value) remained slightly lower than that of neutralizing antibodies (HR: 0·38 vs 0·16 for the 6-m PI endpoint) (Table 3 and Table 4). The data imply that neutralizing antibodies are a more specific indicator for protective natural immunity assays and that binding IgG can serve as a surrogate indicator after setting a proper cut-off value.
Based on the original cut-off (3·0 IU/ml for HPV-16 and 2·1 IU/ml for HPV-18) of our ELISA, the reported seroprevalences of IgG antibodies were 30·2% (461 of 1528) for HPV-16 and 16·0% (244 of 1528) for HPV-18 in Chinses women aged 18-25 years old (internal data), which were comparable to those obtained with ELISA conducted by GSK in Jiangsu, China (30·5% for HPV-16 and 16·0% for HPV-18) in the same age group.[23] Zhao H et al[21] also showed that when detecting unvaccinated serum samples, the agreements between our ELISA and the ELISA test from GSK were 0·87 and 0·83 for HPV-16 and HPV-18, respectively, which suggest a high level of agreement between the two ELISA tests. These data suggest that the original cut-off setting of our ELISA assay is acceptable and comparable with other widely used ELISAs, which also implies that the cut-off of IgG should be carefully reset for protection effect analysis. It should be noted that although the available data implicated that the characteristics of the ELISA used for this work doses not differ obviously from the other widely used ELISA test (by GSK), the possibility of lack of specificity and/or other limitations of the ELISA used in this study could not be absolutely excluded.
The extent of preventive immunity raised by natural HPV infection is one of the key parameters of vaccine cost-effectiveness analysis. Functional neutralizing antibody measured by PBNA is a more specific indicator for these protection investigations. However, PBNA is highly labour intensive and therefore seldom used in large epidemiological studies. ELISAs, which are high-throughput and easier to set up, are more practical and thus widely used in epidemiological studies; there is also high heterogenicity among different ELISAs because the method is influenced by the characteristics of coating HPV L1 antigen, which may be a reason for the fluctuation of the protection extent in different HPV natural immunity studies. A meta-analysis showed an approximately 35% and 30% decreased risk of subsequent infection with HPV-16 and HPV-18 among women who were seropositive for corresponding type IgG antibodies, respectively.[24] Based on the PBNA method, we showed that women with seropositive HPV-16/18 neutralizing antibodies had a significant 84% reduced risk of subsequent 6-month persistent infection, indicating that natural antibodies provide considerable protection against future infection. The findings provide a more objective parameter for the evaluation of vaccine cost-effectiveness.
One of the limitations in our study and other similar studies is the potential imbalance between seropositive and seronegative women in the risk of exposure to HPV due to differences in sexual behaviour and many other confounding factors. All participants in our study had 1-4 sexual partners at entry. We did not collect information about covariates, such as sexual behaviours and condom use, which are known to be associated with the risk of acquiring HPV. Nonetheless, some studies have shown that these covariates do not play an important role, with very similar results obtained with univariate and multivariable adjusted analyses.[16], [17], [18] A previous study suggested that women seropositive had a higher mean number of sexual partners and a higher incidence of a history of chlamydia infection.[15] Our data also showed that women seropositive for neutralizing antibodies were more likely to be diagnosed with cytological abnormalities. Thus, women seropositive for HPV-16/18 antibodies at baseline might be individuals with a higher behaviour risk of HPV infection, and the true preventive effects of antibodies might be underestimated in the above analyses. To determine whether this is true, we analysed the incidence of HPV-52 infection in women who were negative for HPV-52 DNA at baseline and with different HPV-16/18 serostatuses. HPV-52 is the most prevalent HPV type in healthy Chinese women,[25] with no or very limited cross-protection effect with HPV-16/18.[26] A 1·44-fold (95% CI: 1·08-1·91) higher risk of HPV-52 incident infection was observed in the HPV-16/18 Neu (+) subgroup than in the Neu (-) subgroup (Table S7 in the Supplementary Tables). Although not significant, the risks of persistent HPV-52 infection in the HPV-16/18 Neu (+) group were also higher than those in the HPV-16/18 Neu (-) group. By comparison, the risks of HPV-52 incident infection and persistent infection in the HPV-16/18 IgG (+) group were approximately the same as those in the IgG (-) group. Hence, the protective effect of neutralizing antibodies observed in our study might not be overestimated, which consolidates the conclusion that natural antibodies provide considerable protection against future infection.
Another limitation of our analysis is our inability to evaluate the effect of HPV neutralizing antibody titres; thus, we were not able to determine an accurate antibody threshold value for a defined reduction rate in infection. As the mean neutralizing antibody levels acquired from natural infection are very low (with 8·6 IU/ml for HPV-16 and 5·0 IU/ml for HPV-18 in women aged 18-26 years),[27] our data indicated that the protective antibody level of HPV might be even lower. Although a correlate of vaccine protection cannot be inferred from natural history studies, it would be plausible to allocate more resources to explore the one-shot vaccination programme for a quick reach of the global goal of eliminating cervical cancer.
In addition, it was not possible to determine whether an infection was a new infection or reactivation of a previous infection. Evidence exists that women can experience reactivation or redetection of a type-specific infection after a period of non-detection.[28] Based on this assumption, some infections considered new may indeed be persistent, which would bias the assessment of the relationship between natural antibodies and the risk of new infection. Furthermore, the protective role of naturally acquired HPV-16/18 antibodies in related cytology or disease was not clearly established due to limited power (small number of events in the follow-up) in the study.
In conclusion, our analysis showed that HPV natural immunity is considerably protective against future infection. Neutralizing antibodies are a highly specific indicator for HPV protective natural immunity, and binding IgG can serve as a surrogate indicator after setting a proper cut-off value. More studies are needed to explore the long-term protectiveness persistence and dynamics of natural immunities.
Declaration of Competing Interest
Ting Wu reports grants from National Natural Science Foundation of China (Grant number: 82073562). Youlin Qiao reports grants from Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (Grant number: 2017-I2M-B&R-03 and 2016-I2M-1–019). Jun Zhang reports grants from The Fujian Province Health Education Joint Research Project (Grant number: 2019-WJ-05). Ningshao Xia reports grants from Xiamen Science and Technology Major Project (Grant number: 3502Z20193009), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (Grant number: No. 2019RU022), and Xiamen Innovax Biotech Company. Ting Wu, Youlin Qiao, Jun Zhang, Fanghui Zhao, Wen Chen, Chao Zhao, and Lihui Wei report honoraria and travel expenses of invited lectures for educational events from Xiamen Innovax Biotech Company, outside the submitted work. Zhijie Lin, Bizhen Lin and Huirong Pan are employees of Xiamen Innovax Biotech Company. All other authors declare no conflicts of interest.
Acknowledgments
Contributors
T Wu, Y Qiao, F Zhao, N Xia, J Zhang, X Yao and W Chen contributed to the study design. X Yao, W Chen, C Zhao, L Wei, Y Hu, M Li, Z Lin, B Lin, X Liu, Y Hong, Q Li, Q Pan, X Zhang, M Li, Y Zhao, L Zhang, H Xu, F Hu, J Zhao, Y Huang, W Sheng, Y Zheng, S Hu, S Huang and H Pan contributed to sample collection and experiment or data interpretation. X Yao, T Wu, Y Su, and J Zhang contributed to the data analysis and writing of the report. All authors reviewed or revised the manuscript and approved the final draft for submission.
Acknowledgements
We thank all the study participants and research staff of the HPV-003 Study Group for their contributions to the present study. In addition, we also thank Dr. Allan Hildesheim of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute, National Institutes of Health for his helpful comments on this study.
Data sharing statement
The data of the study are available for reasonable request for academic purpose. To gain access, please contact the corresponding author (Ting Wu, wuting@xmu.edu.cn) for further details.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100196.
Contributor Information
Fanghui Zhao, Email: zhaofangh@cicams.ac.cn.
Youlin Qiao, Email: qiaoy@cicams.ac.cn.
Ting Wu, Email: wuting@xmu.edu.cn.
Jun Zhang, Email: zhangj@xmu.edu.cn.
Ningshao Xia, Email: nsxia@xmu.edu.cn.
Appendix. Supplementary materials
References
- 1.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best practice & research Clinical obstetrics & gynaecology. 2018;47:2–13. doi: 10.1016/j.bpobgyn.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Stanley MA, Sterling JC. Host responses to infection with human papillomavirus. Current problems in dermatology. 2014;45:58–74. doi: 10.1159/000355964. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem. https://www.who.int/publications/i/item/9789240014107 (Nov 17, 2020), Accessed 13th Jan 2021.
- 5.Matthijsse SM, Hontelez JAC, Naber SK. The estimated impact of natural immunity on the effectiveness of human papillomavirus vaccination. Vaccine. 2015;33(41):5357–5364. doi: 10.1016/j.vaccine.2015.08.079. [DOI] [PubMed] [Google Scholar]
- 6.Laprise JF, Chesson HW, Markowitz LE. Effectiveness and Cost-Effectiveness of Human Papillomavirus Vaccination Through Age 45 Years in the United States. Annals of internal medicine. 2020;172(1):22–29. doi: 10.7326/M19-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin SW, Ghosh A, Porras C. HPV16 seropositivity and subsequent HPV16 infection risk in a naturally infected population: comparison of serological assays. PLoS One. 2013;8(1):e53067. doi: 10.1371/journal.pone.0053067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coseo S, Porras C, Hildesheim A. Seroprevalence and Correlates of Human Papillomavirus 16/18 Seropositivity Among Young Women in Costa Rica. Sexually Transmitted Diseases. 2010;37(11):706–714. doi: 10.1097/OLQ.0b013e3181e1a2c5. [DOI] [PubMed] [Google Scholar]
- 9.Porras C, Bennett C, Safaeian M. Determinants of seropositivity among HPV-16/18 DNA positive young women. Bmc Infectious Diseases. 2010:10. doi: 10.1186/1471-2334-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faust H, Jelen MM, Poljak M, Klavs I, Ucakar V, Dillner J. Serum antibodies to human papillomavirus (HPV) pseudovirions correlate with natural infection for 13 genital HPV types. J Clin Virol. 2013;56(4):336–341. doi: 10.1016/j.jcv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Tong Y, Ermel A, Tu WZ, Shew M, Brown DR. Association of HPV types 6, 11, 16, and 18 DNA detection and serological response in unvaccinated adolescent women. Journal of Medical Virology. 2013;85(10):1786–1793. doi: 10.1002/jmv.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jun Z, Xin W, Xuelian C. Levels of Human Papillomavirus 16/18 Neutralizing Antibody among 18~45 year-old Women in Liuzhou, Guangxi Province, China: Association with precancerous Lesions of Cervical Cancer. Chinese Journal of Virology. 2017;33(03):354–360. [Google Scholar]
- 13.Ho GY, Studentsov Y, Hall CB. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J Infect Dis. 2002;186(6):737–742. doi: 10.1086/342972. [DOI] [PubMed] [Google Scholar]
- 14.Viscidi RP, Schiffman M, Hildesheim A. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13(2):324–327. doi: 10.1158/1055-9965.epi-03-0166. [DOI] [PubMed] [Google Scholar]
- 15.Olsson SE, Kjaer SK, Sigurdsson K. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Human vaccines. 2009;5(10):696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 16.Safaeian M, Porras C, Schiffman M. Epidemiological Study of Anti-HPV16/18 Seropositivity and Subsequent Risk of HPV16 and -18 Infections. Jnci-J Natl Cancer I. 2010;102(21):1653–1662. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellsague X, Naud P, Chow SN. Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired human papillomavirus type 16/18 antibodies: analysis of the control arm of PATRICIA. J Infect Dis. 2014;210(4):517–534. doi: 10.1093/infdis/jiu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safaeian M, Castellsague X, Hildesheim A. Risk of HPV-16/18 Infections and Associated Cervical Abnormalities in Women Seropositive for Naturally Acquired Antibodies: Pooled Analysis Based on Control Arms of Two Large Clinical Trials. J Infect Dis. 2018;218(1):84–94. doi: 10.1093/infdis/jiy112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wentzensen N, Rodriguez AC, Viscidi R. A Competitive Serological Assay Shows Naturally Acquired Immunity to Human Papillomavirus Infections in the Guanacaste Natural History Study. Journal of Infectious Diseases. 2011;204(1):94–102. doi: 10.1093/infdis/jir209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao YL, Wu T, Li RC. Efficacy, Safety, and Immunogenicity of an Escherichia coli-Produced Bivalent Human Papillomavirus Vaccine: An Interim Analysis of a Randomized Clinical Trial. Journal of the National Cancer Institute. 2020;112(2):145–153. doi: 10.1093/jnci/djz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, Lin ZJ, Huang SJ. Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination. Human vaccines & immunotherapeutics. 2014;10(3):740–746. doi: 10.4161/hv.27619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Zhao H, Yao X. Comparing immunogenicity of the Escherichia coli-produced bivalent human papillomavirus vaccine in females of different ages. Vaccine. 2020;38(39):6096–6102. doi: 10.1016/j.vaccine.2020.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Zhao FH, Zhu FC, Chen W. Baseline prevalence and type distribution of human papillomavirus in healthy Chinese women aged 18-25 years enrolled in a clinical trial. Int J Cancer. 2014;135(11):2604–2611. doi: 10.1002/ijc.28896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N. Natural Acquired Immunity Against Subsequent Genital Human Papillomavirus Infection: A Systematic Review and Meta-analysis. J Infect Dis. 2016;213(9):1444–1454. doi: 10.1093/infdis/jiv753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei F, Yin K, Wu X. Human papillomavirus prevalence and associated factors in women and men in south China: a population-based study. Emerging microbes & infections. 2016;5(11):e119. doi: 10.1038/emi.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochi H, Kondo K, Matsumoto K. Neutralizing antibodies against human papillomavirus types 16, 18, 31, 52, and 58 in serum samples from women in Japan with low-grade cervical intraepithelial neoplasia. Clinical and vaccine immunology: CVI. 2008;15(10):1536–1540. doi: 10.1128/CVI.00197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu YM, Guo M, Li CG. Immunogenicity noninferiority study of 2 doses and 3 doses of an Escherichia coli-produced HPV bivalent vaccine in girls vs. 3 doses in young women. Science China Life sciences. 2020;63(4):582–591. doi: 10.1007/s11427-019-9547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Insinga RP, Perez G, Wheeler CM. Incidence, duration, and reappearance of type-specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1585–1594. doi: 10.1158/1055-9965.EPI-09-1235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.