Summary
Background
Spirometric lung function impairment is an independent predictor of respiratory and cardiovascular disease, and mortality across a broad range of socioeconomic backgrounds and environmental settings. No contemporary studies have explored these relationships in a predominantly regional/remote First Nations population, whose health outcomes are worse than for non-First Nations populations, and First Nations people living in urban centres.
Methods
This was a retrospective cohort study of 1,734 adults (1,113 First Nations) referred to specialist respiratory outreach clinics in the state of Queensland, Australia from February 2012 to March 2020. Regression modelling was used to test associations between lung function and mortality and cardiovascular disease.
Findings
At the time of analysis (August 2020), 189 patients had died: 88 (47%) from respiratory causes and 38 (20%) from cardiovascular causes. When compared to patients with forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) Z-scores of >0 to -1, patients with Z-scores <-1 were at elevated mortality risk (HR=3•2, 95%CI 1•4-7•4; HR=2•6, 95%CI 1•3-5•1), and elevated cardiovascular disease risk (OR=1•5, 95%CI 1•1-2•2; OR=1•6, 95%CI 1•2-2•3). FEV1/FVC% Z-scores <-1 were associated with increased overall mortality (HR=1•6, 95%CI 1•1-2•3), but not cardiovascular disease (OR=1•1, 95%CI 0•8-1•4). These associations were not affected by First Nations status.
Interpretation
Reduced lung function even within the clinically normal range is associated with increased mortality, and cardiovascular disease in First Nations Australians. These findings highlight the importance of lung function optimisation and inform the need for future investment to improve outcomes in First Nations populations.
Funding
None.
Keywords: Respiratory Medicine, First Nations, Cardiovascular Disease, lung function, spirometry, Outcomes
Research in context.
Evidence before this study: We searched PubMed for relevant publications exploring associations between lung function and cardiovascular disease, and mortality. We searched for articles published between database inception to February 2020, using search terms “spirometr*”, “lung function”, “cardiovascular”, “mortality”, and “survival”. We found analyses of data from large-scale cohort studies, literature reviews, and a meta-analysis that examined these associations. One large-scale cohort study included participants from a diverse range of socioeconomic backgrounds and environments. Across all studies, strong associations between FEV1 and FVC indices were found. Individually, however, there is disagreement as to whether only FEV1, FVC, or both, independently predict mortality and cardiovascular disease. Notably, we found only one study published in 1988 that included predominantly First Nations participants; this was the only study to have found any association between FEV1/FVC% and mortality, but not for other spirometry lung indices. Limitations of that study include that it is no longer contemporary (spirometry data collected in 1970/71), reference values applied were not reported, and other likely confounders (e.g., tobacco smoking, co-morbidities) were not accounted for.
Added value of this study: In this cohort of predominantly First Nations Australians, we found that any lung function impairment, even within the clinical normal range of 0 to -1•64 Z-scores, is associated with increased respiratory and cardiovascular disease risk, and mortality. We provide supportive evidence that both FEV1 and FVC independently predict these outcomes, and to our knowledge, this is only the second study to find a significant association between FEV1/FVC% and mortality. Furthermore, this is the only study to establish this association while correcting for factors such as smoking, BMI, and co-morbidities.
Implications of all the available evidence: Our findings highlight the importance of lung function optimisation, particularly in the First Nations Australian population, who like other global First Nations populations, are at heightened risk of chronic respiratory and cardiovascular diseases, and early mortality. In the context of recent research findings which show that lung function catch-up growth is possible in children, it is vital that preservation and optimisation of lung function occurs in First Nations children to alter lung function trajectories and prevent adverse outcomes in adulthood.
Alt-text: Unlabelled box
1. Introduction
It is increasingly accepted that lung function is an independent prognostic marker not only of respiratory conditions but also of cardiovascular disease and overall mortality [1,2] even in asymptomatic people without respiratory disease [3]. The relationship between forced expiratory volume in 1 second (FEV1) and cardiovascular disease mortality has been explored in several large population-based cohort studies [1,2,4,5]. The prospective urban rural epidemiology (PURE) study with lung function data from 126,359 people from 17 countries found that reduced FEV1 (even within the clinically normal range) was associated with increased mortality and cardiovascular disease, with an effect size greater than conventional risk factors such as smoking and hypertension [6]. Similar correlations have been observed with forced vital capacity (FVC) measurements, but only rarely with the FEV1/FVC ratio [1,3,5,6].
Several mechanisms have been put forward to explain the relationship between lung function and cardiovascular disease. Systemic inflammation, [2] when associated with chronic lung conditions such as chronic bronchitis, [7] has emerged as the most likely mechanism. Furthermore, airflow limitation is an independent predictor of vascular injury and atherosclerosis [4,8]. The strong associations of both FEV1 and FVC impairments with coronary artery disease are present even among lifetime non-smokers [9]. More recently, lung function impairment has also been associated with congestive heart failure after adjusting for left ventricular function and cardiac stress [1].
Several large population-based cohort studies have included participants from a range of socioeconomic backgrounds and environmental settings [2,6]. While the PURE study [6] included participants from high and low income countriesacross urban and rural areas, only one previously published study has specifically examined data from First Nations populations [10]. This is particularly important as First Nations people are among the most disadvantaged groups [11] and First Nations Australians are more affected by chronic disease than their non-First Nations counterparts [12]. Chronic respiratory disease among First Nations peoples is approximately 2•5 times higher than for non-First Nations, [13] while cardiovascular disease burden is 1•2 times higher and cardiovascular-related mortality 1•6 times higher [12]. The relative poorer health of First Nations Australians is further worsened with increasing geographical remoteness [14]. Thus, it is important to confirm the prognostic value of lung function in First Nations people, particularly those in regional and remote areas.
Since 2011, we have been part of the Indigenous Respiratory Outreach Care (IROC) program, a specialist respiratory outreach service in Queensland that delivers care directly to regional and remote communities with high First Nations populations. The visiting specialist teams consist of a First Nations project officer, respiratory physician, nurse, and respiratory scientist. Lung function testing is routinely performed by patients at IROC clinics before physician consultation. Using data of adults treated at IROC clinics, we evaluated spirometric measures of lung function, co-morbidities, and demographic factors as predictors of cardiovascular and respiratory morbidity and mortality of the cohort. Our study's aims were to, firstly, determine what demographic and disease factors are associated with reduced lung function, and secondly, to evaluate the prognostic value of spirometric lung function with future cardiovascular disease and mortality.
2. Methods
2.1. Study design, setting, and participants
This is an eight-year retrospective cohort study (February 2012-March 2020) of adults referred to the IROC service through their primary care physicians, First Nations health workers or self-referral. Clinics were held in regional and remote Queensland communities. Inclusion criteria were adults aged 18 years at IROC clinic visit, who were medically reviewed and had spirometry performed by a trained respiratory scientist. Ethical approval and a waiver of patient consent for this study was granted by The Prince Charles Hospital Human Research Ethics Committee (reference: HREC/2019/QPCH/58452).
2.2. Lung function testing
Spirometry was performed according to American Thoracic Society (ATS) and European Respiratory Society (ERS) criteria [15] using an EasyOne Pro or EasyOne Pro Lab (ndd Medizintechnik) portable machine. Patients were tested with a nose clip while seated. Global Lung Function Initiative (GLI) 2012 reference values [16] were applied to spirometry tests to derive Z-scores for each patient. For patients who self-identified as First Nations, the GLI 2012 “Other/mixed” classification was chosen, [17] while the “Caucasian” classification was used for non-First Nations patients.
2.3. Data verification
Patient demographics, lung function data, respiratory diagnosis, smoking and household smoking status, and comorbidities recorded by a respiratory physician for each patient were collected. Data for mortality and cause of death data was censored in August 2020 and collected from electronic medical records, using death certificates where available, or discharge summaries. Where cause of death was unclear or no information was available, cause of death was flagged as “unknown”. All information was transferred into a separate study database, and missing information was completed where possible using electronic and paper chart medical records.
2.4. Definitions and statistical analysis
Pre-bronchodilator spirometry at first visit to IROC was used as the baseline for this study. We analysed the data using categories described by the PURE study [6] where FEV1 Z-scores ≥0 are grouped as “no impairment”, <0 to -1 as “mild impairment”, <-1 to -2 as “moderate impairment”, and <-2 as “severe impairment”. We note that only Z-scores ≤-1•64 (lowest 5% of the reference population) are considered clinically abnormal [16].
Ever-smoker status was assigned to patients who indicated they had smoked in their lifetime, and together with household smoke exposure status were determined using clinic questionnaires completed during their clinic visit. Chronic respiratory disease includes respiratory physician diagnoses of chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, or other chronic pulmonary disease. Cardiovascular disease includes ischaemic heart disease, congestive heart failure, stroke or transient ischaemic attack (TIA), and cardiac valve disease.
Adjusted hazard ratios (HRs) for mortality were calculated for patient demographics, baseline lung function status, arterial hypertension, and type 2 diabetes using a multivariable Cox proportional hazards model after testing the proportional hazards assumption. Due to the small event numbers, patients within the no impairment and mild impairment groups were combined into a single category for the survival analysis. Multivariable logistic regression modelling was used to generate odds ratios (ORs) for respiratory and cardiovascular disease. Main effects were fit for age, sex, First Nations status, body mass index (BMI), smoking status, lung function, hypertension, and type 2 diabetes. Interaction terms (First Nations status and FEV1 or FVC impairment) were used in each model to explore effect modification of First Nations status on associations between lung function status and outcomes. Stata16•1 (StataCorp LLC) was used for statistical analyses; 2-tailed p-values <0•05 were considered significant.
Role of funding sources: The funding source had no role in the study design, secondary data analysis, data interpretation or manuscript writing.
3. Results
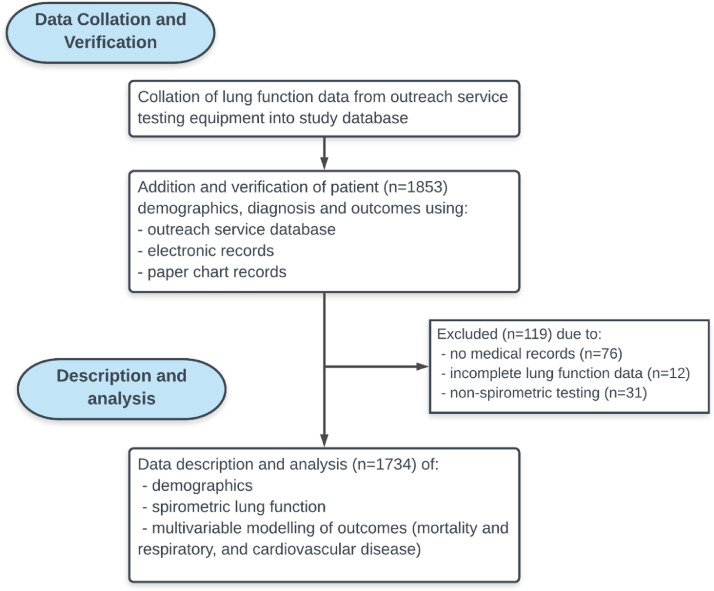
Of the 1,853 patients with lung function data, we were unable to obtain medical records for 76 patients while 43 patients had lung function data that was incomplete or did not include spirometry (Figure 1). Spirometry, demographic, chronic respiratory disease, and comorbidity data for the cohort included in this analysis are summarised in Table 1 and categorised by FEV1 Z-scores. The median age of the remaining patients included in this cohort (n=1,734) was 57 years (IQR 48-67) (First Nations=55 years, IQR 46-64; non-First Nations=63 years, IQR 53-72); 1,013 (58%) were females and 1,113 (64%) First Nations Australians. The median follow-up time was 4 years (IQR 2-5) from the time patients were first seen at IROC, to the time of collation and verification (August 2020) of the data. During this period, 189 (11%) patients died; 88 (47%) from respiratory causes, 38 (20%) from cardiovascular causes, 38 (20%) from other causes including renal disease, non-pulmonary cancers, and 25 (13%) from unknown causes.
Figure 1.
Flow diagram of study data collection, verification, and analysis.
Table 1.
Summary of patient demographics by zFEV1.
| Clinically normal range | Clinically abnormal range | ||||
|---|---|---|---|---|---|
| zFEV1 ≥0 (n=173) | zFEV1 <0 to -1 (n=326) | zFEV1 <-1 to -2 (n=469) | zFEV1 <-2 (n=766) | ||
| Median (IQR) | |||||
| FEV1 (% predicted) | 107•0 (102•8-112•3) | 91•4 (88•5-95•2) | 78•7 (74•8-82•2) | 54•0 (40•9-63•7) | |
| FVC (% predicted) | 107•4 (100•6-112•6) | 93•3 (88•1-98•9) | 82•2 (76•8-88•6) | 66•9 (56•7-75•9) | |
| FEV1/FVC | 0•82 (0•78-0•86) | 0•80 (0•75-0•83) | 0•77 (0•70-0•82) | 0•65 (0•52-0•75) | |
| n (%) < LLN (Z <-1.64) | 3 (<1%) | 20 (<1%) | 76 (16%) | 450 (59%) | |
| n (%) | |||||
| Sex | |||||
| Female | 108 (62%) | 192 (59%) | 281 (59•9%) | 432 (56%) | |
| Male | 65 (38%) | 134 (41%) | 188 (40•1%) | 334 (44%) | |
| First Nations | 97 (56%) | 200 (61%) | 319 (68•0%) | 497 (65%) | |
| Age (years) | 53 (40-63) | 55 (45-66) | 57 (46-68) | 59 (51-68) | |
| BMI (kg/m2) | |||||
| Underweight (<18•5) | 1 (<0•1%) | 10 (<1%) | 21 (5%) | 49 (6%) | |
| Healthy (18•5-24•9) | |||||
| 33 (19%) | 63 (19%) | 70 (15%) | 167 (22%) | ||
| Overweight (25-29•9) | |||||
| 49 (28%) | 75 (23%) | 111 (24%) | 182 (24%) | ||
| Obese (≥30) | |||||
| 90 (52%) | 178 (55%) | 267 (57%) | 368 (48%) | ||
| Smoking (n=1571) | |||||
| Current | 27 (16%) | 97 (30%) | 175 (37%) | 303 (40%) | |
| Former | |||||
| 43 (25%) | 98 (30%) | 151 (32%) | 270 (35%) | ||
| Never | 76 (44%) | 93 (29%) | 115 (25%) | 123 (16%) | |
| Household smoke (n=1396) | |||||
| Yes | |||||
| 43 (25%) | 96 (29%) | 150 (32%) | 280 (37%) | ||
| No | 88 (51%) | 158 (49%) | 247 (53%) | 330 (43%) | |
| Hypertension | 40 (23%) | 101 (31%) | 174 (37%) | 307 (40%) | |
| Chronic respiratory disease | 104 (60%) | 218 (67%) | 360 (77%) | 663 (87%) | |
| Cardiovascular disease | 29 (17%) | 48 (15%) | 96 (21%) | 218 (29%) | |
| Cancers | 13 (6%) | 14 (4%) | 14 (<1%) | 44 (6%) | |
| Deaths | 1 (<1%) | 15 (5%) | 32 (7%) | 141 (18%) | |
Clinically normal range encompasses Z-scores >=-1.64.
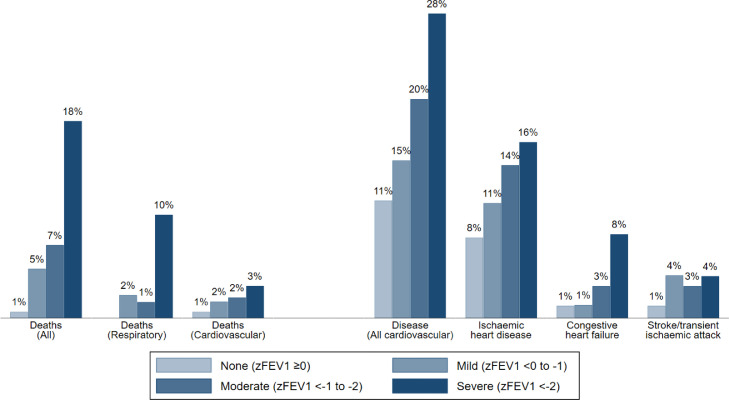
With worsening lung function (lower Z-score categories), the proportion of patients with chronic respiratory disease and cardiovascular disease increased, as well as the number of deaths (Table 2). The frequency of cardiovascular disease (ischaemic heart, heart failure) and mortality (all, respiratory, and cardiovascular) based on baseline FEV1 categories depict graded increases with decreasing FEV1 (Figure 2). Likewise, there were graded increases with decreasing FVC (Supplement Figure S1).
Table 2.
Summary of outcomes by First Nations status by FEV1 category.
| Clinically normal range | Clinically abnormal range | |||
|---|---|---|---|---|
| zFEV1 ≥0 | zFEV1 <0 to -1 | zFEV1 <-1 to -2 | zFEV1 <-2 | |
| n (%) | ||||
| Deceased | ||||
| First Nations | 0/97 (0%) | 9/200 (5%) | 20/319 (6%) | 77/497 (15%) |
| Non-First Nations | 1/76 (1%) | 5/125 (5%) | 12/150 (8%) | 64/269 (24%) |
| Respiratory disease | ||||
| First Nations | 33/97 (34%) | 134/200 (67%) | 242/319 (76%) | 427/497 (86%) |
| Non-First Nations | 40/76 (54%) | 84/126 (67%) | 118/150 (79%) | 236/269 (88%) |
| Cardiovascular disease | ||||
| First Nations | 10/97 (10%) | 26/200 (13%) | 69/319 (22%) | 133/497 (27%) |
| Non-First Nations | 9/76 (12%) | 22/126 (17%) | 27/150 (18%) | 85/269 (32%) |
Figure 2.
Percentages of patients within zFEV1 impairment categories assigned to each outcome.
Cox regression analyses of baseline spirometry data (FEV1, FVC, and FEV1/FVC%) relating to mortality are presented in Table 3. Like the data for frequency of disease, there were graded increases in mortality with decreasing FEV1, FVC, and FEV1/FVC% Z-scores. Associations with mortality were still evident in additional modelling for FEV1/FVC% that included adjustment for the presence of respiratory disease (p=0•04). Risk of death for First Nations patients was similar to their non-First Nations counterparts for FEV1 (p for interaction=0•70), FVC (p for interaction=0•89), and FEV1/FVC% (p for interaction=0•84).
Table 3.
Hazard ratios (HR) for mortality in three multivariable models.
| FEV1 | FVC | FEV1/FVC% | |||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | ||
| Age (per 10-year increase) | 1•85 (1•61, 2•11) | <0•01 | 1•89 (1•65, 2•17) | <0•01 | 1•85 (1•61, 2•11) | <0•01 | |
| Sex | Female | 0•75 (0•56, 1•01) | 0•06 | 0•75 (0•56, 1•01) | 0•06 | 0•81 (0•60, 1•09) | 0•17 |
| First Nations status | 1•38 (0•51, 3•75) | 0•53 | 1•00 (0•44, 2•26) | 1•00 | 1•10 (0•65, 1•86) | 0•73 | |
| BMI | Underweight | 1•82 (1•07, 3•09) | <0•01 | 1•80 (1•06, 3•07) | <0•01 | 1•85 (1•09, 3•14) | <0•01 |
| Normal | Reference | Reference | Reference | ||||
| Overweight | 0•77 (0•53, 1•12) | 0•80 (0•54, 1•16) | 0•72 (0•50, 1•05) | ||||
| Obese | 0•45 (0•31, 0•65) | 0•43 (0•29, 0•62) | 0•45 (0•31, 0•66) | ||||
| Smoking*(n=1571) | Current | 1•56 (0•98, 2•49) | 0•14 | 1•62 (1•02, 2•58) | 0•09 | 1•72 (1•07, 2•76) | 0•06 |
| Former | 1•49 (0•96, 2•33) | 1•58 (1•01, 2•46) | 1•61 (1•03, 2•53) | ||||
| Never | Reference | Reference | Reference | ||||
| Spirometry | Z-score | ||||||
| Clinically normal | ≥0 to -1 | Reference | <0•01 | Reference | <0•01 | Reference | 0•02 |
| <-1 to -2 | 2.11 (0•83, 5.38) | 2•57 (1•31, 5.05) | 1•71 (0.93, 3•14) | ||||
| Clinically abnormal | <-2 | 5.05 (2•30, 11.1) | 4.99 (2•97, 9.30) | 2•10 (1•23, 3•57) | |||
| Hypertension | 1•37 (1.01, 1•86) | 0•045 | 1•35 (1•00, 1•84) | 0•05 | 1•31 (0•96, 1•79) | 0•09 | |
| Type 2 diabetes | 1•67 (1•19, 2•36) | <0•01 | 1•51 (1•08, 2•13) | 0•02 | 1•80 (1•27, 2•55) | <0•01 | |
*Patients with missing smoking data (n=163) included in modelling but excluded from global Wald test.
Adjusted HRs (Table 3) also depict a significant reduction in risk for patients who were overweight or obese, while underweight patients and patients with type 2 diabetes were at significantly increased mortality risk. When compared to a combined normal-to-mild group (>0 to-1 Z-scores), patients in a combined moderate/severe group (<-1 Z-scores) for FEV1 and FVC were at >2•5 times higher risk of mortality (HR 3•2, 95%CI 1•4-7•4; HR=2.6, 95%CI 1•3-5•1), and 1•6 times higher risk with moderate/severe impairment in FEV1/FVC% (HR=1•6, 95%CI 1•1-2•3).
For respiratory disease, logistic regression (Tables 4 and 5) showed increased risk in patients with any FEV1, FVC, or FEV1/FVC% (Supplement Table S1) impairment. Adjusted risk of cardiovascular disease was higher for all patients with any FEV1 or FVC impairment, current and former smokers, and patients with hypertension and/or type 2 diabetes. First Nations patients were at similar risk for both cardiovascular disease (FEV1 p for interaction=0•38, FVC p for interaction=0•43, FEV1/FVC% p for interaction=0•34) and respiratory disease (FEV1 p for interaction=0•16, FVC p for interaction=0•27, FEV1/FVC% p for interaction=0•73) as their non-First Nations counterparts. We also found females were at significantly reduced risk of cardiovascular disease. When compared to a combined normal/mild group (>=-1 Z-score), patients in a combined moderate/severe group (<-1 Z-scores) were at greater risk of cardiovascular disease for both FEV1 and FVC (OR 1•5, 95%CI 1•1-2•2; OR=1•6, 95%CI 1•2-2•3). We found no relationship between impaired FEV1/FVC% and cardiovascular disease (OR=1•1, 95%CI 0•8-1•4).
Table 4.
Odds ratios (ORs) for respiratory and cardiovascular disease in two multivariable models with FEV1.
| Respiratory disease | Cardiovascular disease | ||||||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | ||||
| Age (per 10-year increase) | 1•21 (1•11, 1•33) | <0•01 | 1•57 (1•41, 1•76) | <0•01 | |||
| Sex | Female | 0•99 (0•77, 1•28) | 0•95 | 0•69 (0•54, 0•89) | <0•01 | ||
| First Nations status | 1•87 (0•96, 3•66) | 0•07 | 0•98 (0•35, 2•77) | 0•59 | |||
| BMI | Underweight | 0•99 (0•54, 1•82) | 0•42 | 1•01 (0•53, 1•91) | 0•15 | ||
| Normal | Reference | Reference | |||||
| Overweight | 1•10 (0•76, 1•59) | 1•45 (0•98, 2•15) | |||||
| Obese | 1•29 (0•93, 1•80) | 1•07 (0•74, 1•55) | |||||
| Smoking*(n=1571) | Current | 1•07 (0•76, 1•50) | 0•27 | 1•71 (1•17, 2•50) | 0•02 | ||
| Former | 1•31 (0•92, 1•86) | 1•52 (1•06, 2•19) | |||||
| Never | Reference | Reference | |||||
| FEV1 | Z-score | ||||||
| Clinically normal | ≥0 | Reference | <0•01 | Reference | 0•02 | ||
| <0 to -1 | 1•64 (0•87, 3•12) | 1•29 (0•53, 3•15) | |||||
| <-1 to -2 | 2•79 (1•46, 5•33) | 1•07 (0•46, 2•48) | |||||
| Clinically abnormal | <-2 | 6•02 (3•17, 11•42) | 2•15 (0•98, 4•71) | ||||
| Hypertension | 1•26 (0•93, 1•71) | 0•14 | 2•15 (1•63, 2•83) | <0•01 | |||
| Type 2 diabetes | 0•95 (0•69, 1•33) | 0•78 | 2•44 (1•82, 3•27) | <0•01 | |||
*Patients with missing smoking data (n=163) included in modelling but excluded from global Wald test.
Table 5.
Odds ratios (ORs) for respiratory and cardiovascular disease in two multivariable models with FVC.
| Respiratory disease | Cardiovascular disease | ||||||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | ||||
| Age (per 10-year increase) | 1•25 (1•14, 1•36) | <0•01 | 1•61 (1•44, 1•80) | <0•01 | |||
| Sex | Female | 1•06 (0•58, 1•92) | 0•85 | 0•68 (0•53, 0•88) | <0•01 | ||
| First Nations status | 1•52 (0•85, 2•70) | 0•16 | 0•71 (0•31, 1•59) | 0•40 | |||
| BMI | Underweight | 1•06 (0•58, 1•92) | 0•61 | 0•97 (0•49, 1•90) | 0•11 | ||
| Normal | Reference | Reference | |||||
| Overweight | 1•07 (0•74, 1•54) | 1•45 (0•98, 2•14) | |||||
| Obese | 1•23 (0•89, 1•70) | 1•03 (0•71, 1•49) | |||||
| Smoking*(n=1571) | Current | 1•21 (0•87, 1•68) | 0•11 | 1•77 (1•22, 2•58) | <0•01 | ||
| Former | 1•45 (1•02, 2•06) | 1•55 (1•08, 2•22) | |||||
| Never | Reference | Reference | |||||
| FVC | Z-score | ||||||
| Clinically normal | ≥0 | Reference | <0•01 | Reference | <0•01 | ||
| <0 to -1 | 1•64 (0•92, 2•90) | 0•81 (0•38, 1•74) | |||||
| <-1 to -2 | 2•07 (1•15, 3•73) | 1•09 (0•52, 2•28) | |||||
| Clinically abnormal | <-2 | 3•61 (1•89, 6•88) | 2•18 (1•07, 4•44) | ||||
| Hypertension | 1•29 (0•96, 1•75) | 0•09 | 2•19 (1•66, 2•88) | <0•01 | |||
| Type 2 diabetes | 0•92 (0•66, 1•28) | 0•62 | 2•26 (1•68, 3•03) | <0•01 | |||
*Patients with missing smoking data (n=163) included in modelling but excluded from global Wald test.
4. Discussion
In our study of 1,734 adults (approximately two-thirds First Nations Australians), we found that any FEV1, FVC, and FEV1/FVC% impairment, even within clinically normal ranges, was associated with significantly increased risk of all-cause mortality. Type 2 diabetes (T2DM) and being underweight were associated with increased risk, while obesity was associated with decreased risk of overall mortality. In multivariable regression of respiratory and cardiovascular disease, we found that FEV1, FVC, and FEV1/FVC% impairment were associated with increased risk of respiratory disease, whilst FEV1 and FVC impairment, male sex, ever-smoker status, and co-morbidities (T2DM and hypertension) were associated with increased cardiovascular disease risk. First Nations status did not alter risk of respiratory or cardiovascular disease, or mortality. Notably, in our unique cohort, our findings were similar to that of the recent PURE study, which suggested FEV1 and FVC, even within the clinically normal ranges, were associated with mortality and cardiovascular disease risk in their large prospective cohort of 126,359 adults [6].
Our study is novel for several reasons. Firstly, our data confirms the applicability of the PURE study [6] findings of the relationship of lung function to future cardiovascular and respiratory morbidity, and mortality in a predominantly First Nations population in a regional/remote setting. Secondly, we also found that in addition to the well-known importance of FEV1 and FVC, the FEV1/FVC ratio has an equally significant association with mortality in First Nations Australians. Finally, our study is one of the first, and the only contemporaneous study, to be largely based on a First Nations population. This is important as the burden of chronic illness among First Nations Australians is high and cardiovascular illness is the largest cause of mortality [6]. Confirming the applicability of international studies to First Nations Australians is evidence that may promote strategic resource allocation to improve outcomes for this population.
Our findings are concordant with data from the Whitehall II Study (n=4,817) in which mean baseline FEV1 and FVC were significantly lower (-0•4 litres, p<0•001; -0•5 litres, p<0•001) among those participants who were deceased at census [2]. While FEV1 often receives greater attention within the literature, FVC has also been strongly associated with survival outcomes [3,10,18,19]. An analysis of 7,489 participants in the Atherosclerosis Risk in Communities (ARIC) study by Burney et al. found higher FVC significantly reduced mortality risk (HR 0•90, 95%CI 0•81-1•00), while higher FEV1 (HR 0•98, 95%CI 0•80-1•07) did not [3]. The relationship between FEV1/FVC% and mortality is often unexplored except for a small number of studies. An analysis of 12,703 participants from the Gutenberg Health Study by Magnussen et al, found no association between FEV1/FVC% with survival (HR 0•94, 0•85-1•04) [1]. To our knowledge, the only study that has demonstrated a significant association between FEV1/FVC% and mortality was a study of two First Nations populations in Papua New Guinea, where the mean FEV1/FVC% was significantly greater at baseline in those participants who were still alive at census versus those who had died (difference=6•5%, p<0•001) [10]. One explanation for this finding is that our data is from a respiratory service, where airway obstruction is a common element to many diseases seen and treated. When we re-analysed the data, adding adjustment for the presence of respiratory disease to our modelling, our findings remained the same, thus, we are confident that respiratory disease is not the underlying reason.
We observed similar results when analysing cardiovascular outcomes, where any FEV1 or FVC impairment, even within the clinically normal range, significantly increased cardiovascular disease risk, independent of other factors such as age, sex, smoking status, and BMI. This relationship is now well supported by numerous studies, summarised in a recent review [5]. The Coronary Artery Risk Development in Young Adults (CARDIA) study (n=3,000) found that subclinical lung function decline was associated with two distinct cardiopulmonary disease states at follow-up [20]. FEV1 and FVC decline with preserved FEV1/FVC%, a restrictive spirometry pattern, was associated with left ventricular (LV) hypertrophy (LV mass in grams, β=3•84, p<0•0001) at year 25 of follow-up [20]. Decline in FEV1, with associated FEV1/FVC% decline, was associated with lowered cardiac output at year 5 (LV cardiac output in litres/minute, β=-0•07, p=0•03) and lowered left atrial internal dimension (LAID) at year 25 (LAID in cm, β=-0•034, p=0•0001) of follow-up [20]. Our data are consistent with the notion that graded increases in cardiovascular disease prevalence occur with increasing FEV1 and FVC impairment. Indeed, a meta-analysis of 12 datasets found that patients with COPD were more likely to have cardiovascular disease than controls or the general population (OR 2•46, 95%CI 2•02–3•00) [21].
Findings for First Nations patients within this cohort were similar to the whole group, confirming the applicability of lung function as a predictor of future outcomes to the First Nations population of Australia. First Nations status did not significantly influence mortality, nor cardiovascular or respiratory disease risk. This is likely due to First Nations individuals making up most of the whole group. For this study we used the “Other/mixed” GLI category to produce reference values and Z-scores, based on previous findings that this is the most suitable ethnic category for First Nations children [17]. Currently, this category has yet to be validated for use in the adult First Nations population and so lung function interpretation within this population is more difficult. We also note that First Nations representation increased with worsening FEV1 impairment and was highest in the moderate and severe FEV1 categories of impairment.
Our study supports existing evidence within the literature that spirometric lung function is an independent predictor of future mortality and cardiovascular disease risk and provides up-to-date evidence that these findings are applicable in First Nations populations. This highlights the importance of lung function optimisation, particularly in First Nations populations that continue to be at heightened risk of respiratory and cardiovascular diseases, and reduced life expectancies [12]. Recent research in lung function trajectories suggests that impaired (but still clinically normal) FEV1 in childhood or young adulthood are associated with lower lung function in adulthood, and associated increased cardiopulmonary and all-cause mortality [22], [23], [24], [25]. Importantly, there is now evidence that early identification and management of respiratory illness in early life may precipitate catch-up growth, and alter the lung function trajectories of children treated [25,26]. We have previously shown that specialist respiratory outreach services that are tailored to the needs of target communities can significantly improve the lung function of children in First Nations communities [27]. Continued optimisation of lung function in children and adults in First Nations populations through services such as these, while concurrently addressing other risk factors such as those identified in our study (e.g. smoking, hypertension, type 2 diabetes) are important to improve future outcomes for this at-risk population.
Our study has several limitations. Firstly, our analysis is limited somewhat by a smaller sample size when compared to other larger-scale epidemiological studies discussed above. Secondly, our analysis is of data from a clinical service, and so selection bias may limit the applicability of our findings to wider regional and remote First Nations and non-First Nations Australian populations. While the IROC service allows self-referral, the proportion of patients who were self-referred is unknown. Thirdly, data for amount of smoking were low quality and often incomplete, and so were not included in our model, thus limiting the ability of our analysis to determine the impact of smoking on outcomes. Fourthly, while all lung function testing was performed by experienced respiratory scientists, these results are clinically reported values and a minority may not meet full ATS/ERS criteria [15]. Inclusion of these tests may alter associations between lung function impairment and outcomes. Finally, death certificate data was not available for most patients via electronic medical records, and so discharge summaries (discharge due to patient death) were used for these patients instead. While each of these discharge summaries were considered by medical doctors involved in this study, it is possible the true cause of death may have been misassigned in a minority of cases, which may affect our results.
Our findings suggest that reduced FEV1, FVC, and FEV1/FVC%, even within the clinically normal range, are independently associated with increased mortality for both First Nations and non-First Nations Australians living in regional and remote areas. Reduced FEV1 and FVC were also independently associated with increased cardiovascular disease risk in both groups. We conclude that known associations between lung function and both increased mortality and cardiovascular disease risk are applicable to First Nations and non-First Nations Australians living in regional and remote areas. Further, we found a novel association between FEV1/FVC% and mortality risk in both First Nations and non-First Nations Australians that persisted after adjusting for the presence of respiratory disease. In the content of recent research into lung function trajectories, these findings highlight a need for significant emphasis to be placed on lung function optimisation in children and young adults, particularly in First Nations populations who have a substantially heightened risk of chronic lung and heart disease, and early mortality, in adulthood. ‘Closing the gap’ for First Nations Australians requires a broad socioeconomic approach, [28] as well as attention to factors that influence lung trajectories, much of which occurs in utero and early childhood [25].
5. Author contributions
Study concept and design: A.J.C., A.B.C., J.M., M.M.
Data collation: A.J.C., A.D., K.F., P.M.
Data verification: A.J.C., A.D., K.F., M.M.
Statistical analysis: A.J.C., M.C.
All authors contributed to the interpretation of the data, participated in the writing and critical revision of the manuscript, and have approved the final version for submission. A.J.C. and M.M. verified the underlying data.
6. Data sharing statement
In line with the wishes expressed by the Menzies-based First Nations Reference Group, data collected for this study will not be made publicly available in any form.
Declaration of Competing Interest
The authors received no specific funding for this work. AJC is supported by a National Health and Medical Research Council (NHMRC) Postgraduate Scholarship (APP2003334), had full access to all the data in the study and had final responsibility for the decision to submit for publication. JM reports grants from the Children's Hospital Foundation (RPC0772019) during the conduct of the study. ABC reports grants from the NHMRC (APP1154302) and Children's Health Foundation (top-up #50286); other fees to the institution from work relating to being a IDMC Member of an unlicensed vaccine (GSK), and an advisory member of study design for unlicensed molecule for chronic cough (Merck) outside the submitted work. TB reports grants from during the conduct of the study; AD is a paid employee of Metro North Hospital and Health Services which also administers the Indigenous Respiratory Outreach (IROC) Program for Queensland Health, and provides clinical services to IROC clinics. KF reports grants from Various competitive funding bodies, non-financial support from Industry, occasional honorariums from various Universities, various international funding bodies, and Cochrane Clinical Answers, occasional royalty from UpToDate, outside the submitted work; and Paid employee of Metro North Hospital and Health Services which also administers the IROC program for Queensland Health. MM reports other from Children's Hospital Foundation, and grants from the NHMRC during the conduct of the study.
Acknowledgements
We acknowledge and thank the First Nations Health Workers, First Nations project officers, doctors, nurses, and clinical measurements scientists on the IROC program for their tireless efforts.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100188.
Appendix. Supplementary materials
References
- 1.Magnussen C, Ojeda FM, Rzayeva N, Zeller T, Sinning CR, Pfeiffer N. FEV1 and FVC predict all-cause mortality independent of cardiac function - Results from the population-based Gutenberg Health Study. Int J Cardiol. 2017;234:64–68. doi: 10.1016/j.ijcard.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Sabia S, Shipley M, Elbaz A, Marmot M, Kivimaki M, Kauffmann F. Why does lung function predict mortality? Results from the Whitehall II Cohort Study. Am J Epidemiol. 2010;172(12):1415–1423. doi: 10.1093/aje/kwq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax. 2011;66(1):49–54. doi: 10.1136/thx.2010.147041. [DOI] [PubMed] [Google Scholar]
- 4.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 5.Ramalho SHR, Shah AM. Lung function and cardiovascular disease: A link. Trends Cardiovasc Med. 2020 doi: 10.1016/j.tcm.2019.12.009. Jan 3:S1050-1738(20)30002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duong M, Islam S, Rangarajan S, Leong D, Kurmi O, Teo K. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): an international, community-based cohort study. Lancet Glob Health. 2019;7(5):e613–ee23. doi: 10.1016/S2214-109X(19)30070-1. [DOI] [PubMed] [Google Scholar]
- 7.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Symptoms of chronic bronchitis and the risk of coronary disease. The Lancet. 1996;348(9027):567–572. doi: 10.1016/S0140-6736(96)02374-4. [DOI] [PubMed] [Google Scholar]
- 8.Chandra D, Gupta A, Strollo PJ, Jr., Fuhrman CR, Leader JK, Bon J. Airflow Limitation and Endothelial Dysfunction. Unrelated and Independent Predictors of Atherosclerosis. Am J Respir Crit Care Med. 2016;194(1):38–47. doi: 10.1164/rccm.201510-2093OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158(12):1171–1181. doi: 10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 10.Anderson HR, Vallance P, Bland JM, Nohl F, Ebrahim S. Prospective study of mortality associated with chronic lung disease and smoking in Papua New Guinea. Int J Epidemiol. 1988;17(1):56–61. doi: 10.1093/ije/17.1.56. [DOI] [PubMed] [Google Scholar]
- 11.Kirmayer LJ, Brass G. Addressing global health disparities among Indigenous peoples. The Lancet. 2016;388(10040):105–106. doi: 10.1016/S0140-6736(16)30194-5. [DOI] [PubMed] [Google Scholar]
- 12.Australian Health Ministers’ Advisory Council. Aboriginal and Torres Strait Islander Health Performance Framework 2017 Report. Canberra; 2017.
- 13.Australian Institute of Health and Welfare. The burden of chronic respiratory conditions in Australia: a detailed analysis of the Australian Burden of Disease Study 2011. Canberra; 2017.
- 14.Australian Institute of Health and Welfare. Australia's health 2014. Canberra; 2014.
- 15.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blake TL, Chang AB, Chatfield MD, Marchant JM, McElrea MS. Global Lung Function Initiative-2012 'other/mixed' spirometry reference equation provides the best overall fit for Australian Aboriginal and/or Torres Strait Islander children and young adults. Respirology. 2020;25(3):281–288. doi: 10.1111/resp.13649. [DOI] [PubMed] [Google Scholar]
- 18.Olofson J, Skoogh BE, Bake B, Svardsudd K. Mortality related to smoking habits, respiratory symptoms and lung function. Eur J Respir Dis. 1987;71(2):69–76. [PubMed] [Google Scholar]
- 19.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279(8):585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 20.Cuttica MJ, Colangelo LA, Shah SJ, Lima J, Kishi S, Arynchyn A. Loss of Lung Health from Young Adulthood and Cardiac Phenotypes in Middle Age. Am J Respir Crit Care Med. 2015;192(1):76–85. doi: 10.1164/rccm.201501-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 22.Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373(2):111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 23.Agusti A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5(12):935–945. doi: 10.1016/S2213-2600(17)30434-4. [DOI] [PubMed] [Google Scholar]
- 24.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6(7):535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 25.Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019;7(4):358–364. doi: 10.1016/S2213-2600(18)30529-0. [DOI] [PubMed] [Google Scholar]
- 26.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souëf PN. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6(7):526–534. doi: 10.1016/S2213-2600(18)30099-7. [DOI] [PubMed] [Google Scholar]
- 27.Collaro AJ, Chang AB, Marchant JM, Rodwell LT, Masters IB, Chatfield MD. Pediatric Patients of Outreach Specialist Queensland Clinics Have Lung Function Improvement Comparable to That of Tertiary Pediatric Patients. Chest. 2020;158(4):1566–1575. doi: 10.1016/j.chest.2020.03.084. [DOI] [PubMed] [Google Scholar]
- 28.Closing the gap for Aboriginal health. The Lancet. 2019;393(10173) doi: 10.1016/S0140-6736(19)30405-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.