CHEMISTRY Correction for “The pervasive threat of lead (Pb) in drinking water: Unmasking and pursuing scientific factors that govern lead release,” by Raymond J. Santucci Jr and John R. Scully, which was first published September 8, 2020; 10.1073/pnas.1913749117 (Proc. Natl. Acad. Sci. U.S.A. 117, 23211–23218).
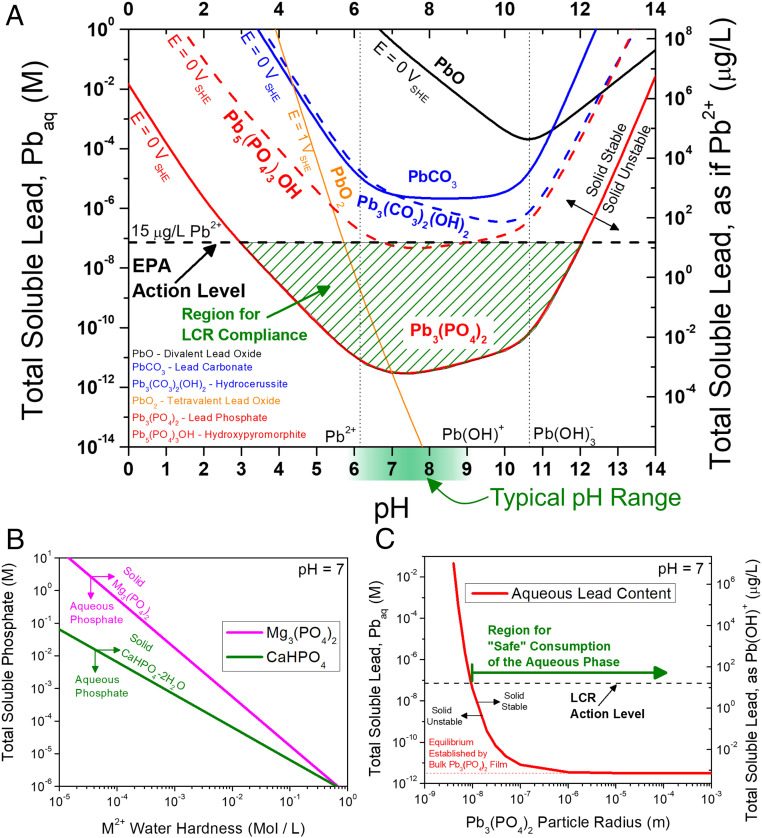
The authors note that Fig. 1 and its corresponding legend appeared incorrectly: “We would like to report a correction concerning a mathematical error in the calculation of the equilibrium expression for Pb5(PO4)3OH hydroxypyromorphite. This error was the result of a failure to distribute a (1/5) coefficient across the entire equilibrium expression for Pb5(PO4)3OH. This affects the red dashed line on the graph in Fig. 1A. The effect of this error is to shift the equilibria of this compound to higher (more soluble) levels of aqueous lead. A reinvestigation of the other expressions revealed no further mathematical errors. Only Fig. 1A was affected by the mathematical error. Fig. 1 B and C are unaffected. The correction of Fig. 1A concerns the equilibrium of Pb5(PO4)3OH, graphically depicted by the dashed red line.
Fig. 1.
Thermodynamic diagrams exhibiting the types of calculations that describe the Pb–drinking water system. (A) Chemical stability diagram highlighting the relative stabilities of various Pb-based compounds as a function of pH and total soluble Pb concentration. The EPA action limit of 15 μg/L Pb2+ is included for reference (horizontal dashed line). Diagrams were constructed for a representative drinking water chemistry where the concentrations of carbonate and chloride are 1 mM each, and an inhibitor concentration of [PO43−] = 0.1 mM utilizing thermodynamic data in Lange’s Handbook of Chemistry (37) except for the hydroxylated carbonate and phosphate compounds (dashed lines), which were taken from the American Water Works Association (8). (B) The effect of water hardness in limiting available phosphate (aqueous) in drinking water. (C) The effect of decreasing particle size on destabilizing the solid phase assuming a surface energy of 3 J/m2 for Pb3(PO4)2 particles in water.
The mathematical error can be seen in the open source data workbook included with the article on the worksheet labeled “Fig. 1a Chem. Stability Diagram”. The equilibrium expression is calculated in column U, where it can be seen that there is a value of “(1)” instead of a value of “(1/5)”. The error is expressed as follows:
| [Original] |
| [Correction] |
A minor typographical error was also found concerning a subscript in the formula for the hydroxypyromorphite phase in the legend of Fig. 1A. The subscript has been corrected in Fig. 1A. The error is expressed as follows:
| [Original] |
| [Correction] |
The authors would like to thank Dr. Murray McBride (Cornell) and Dr. Daniel Giammar (Washington University in St. Louis) for their helpful discussions leading to detection of this error.” The corrected figure and its corrected legend appear below.
In addition, the authors note that on page 23214, left column, first paragraph, line 4, “As shown, only the Pb-phosphate films (Fig. 1A, red lines) can fix the total concentration of soluble Pb below the EPA action level (black horizontal dashed line) over a range of pH from 3 to 14+ depending on the nature of the phosphate film (the green shaded region indicates range of “compliant” water achieved by phosphate-based films)” should instead appear as “As shown, only the Pb-phosphate films (Fig. 1A, red lines) can fix the total concentration of soluble Pb below the EPA action level (black horizontal dashed line) over a range of pH from 3 to 12 depending on the nature of the phosphate film (the green shaded region indicates range of “compliant” water achieved by phosphate-based films).”