Summary
The Wnt/β-catenin pathway is a highly conserved, frequently mutated developmental and cancer pathway. Its output is defined mainly by β-catenin’s phosphorylation- and ubiquitylation-dependent proteasomal degradation, initiated by the multi-protein β-catenin destruction complex. The precise mechanisms underlying destruction complex function have remained unknown, largely because of the lack of suitable in vitro systems. Here we describe the in vitro reconstitution of an active human β-catenin destruction complex from purified components, recapitulating complex assembly, β-catenin modification, and degradation. We reveal that AXIN1 polymerization and APC promote β-catenin capture, phosphorylation, and ubiquitylation. APC facilitates β-catenin’s flux through the complex by limiting ubiquitylation processivity and directly interacts with the SCFβ-TrCP E3 ligase complex in a β-TrCP-dependent manner. Oncogenic APC truncation variants, although part of the complex, are functionally impaired. Nonetheless, even the most severely truncated APC variant promotes β-catenin recruitment. These findings exemplify the power of biochemical reconstitution to interrogate the molecular mechanisms of Wnt/β-catenin signaling.
Keywords: Wnt/beta-catenin signalling, beta-catenin destruction complex, adenomatous polyposis coli (APC), axis inhibition protein (AXIN), glycogen synthase kinase 3 (GSK3), casein kinase 1 (CK1), SCFβ-TrCP, biochemistry, ubiquitin, colorectal cancer
Graphical abstract
Highlights
-
•
β-Catenin destruction complex function recapitulated with purified proteins
-
•
AXIN1 polymers and APC promote β-catenin capture, phosphorylation, and ubiquitylation
-
•
Oncogenic APC truncation mutants are hypomorphs promoting β-catenin recruitment
-
•
APC directly binds the ubiquitylation machinery
Ranes et al. reconstitute the β-catenin destruction complex from purified proteins, thereby recapitulating its assembly and biochemical activities and enabling the mechanistic interrogation of its scaffolding proteins AXIN1 and APC. AXIN1 polymerization and APC facilitate the recruitment, phosphorylation, and ubiquitylation of β-catenin. Oncogenic truncation of APC partially preserves APC function.
Introduction
The highly conserved Wnt/β-catenin signaling pathway coordinates key events in early embryogenesis, tissue homeostasis, and regeneration, governing stem cell maintenance, cell fate specification, and cell proliferation (Clevers et al., 2014; Steinhart and Angers, 2018). It is one of the most frequently mutated pathways in cancer (Sanchez-Vega et al., 2018). The degree of its activation is the outcome of balanced activities of two opposing multi-protein complexes that determine the fate of newly synthesized cytoplasmic β-catenin: the β-catenin destruction complex (DC) and the Wnt signalosome (Gammons and Bienz, 2018; Stamos and Weis, 2013; van Kappel and Maurice, 2017). The DC predominates at basal Wnt/β-catenin signaling and, via phosphorylation-dependent ubiquitylation, earmarks β-catenin for proteasomal degradation. Receptor engagement by Wnt growth factors converts the DC into a receptor-associated Wnt signalosome complex, where β-catenin phosphorylation and ubiquitylation are attenuated, resulting in increased levels and nuclear accumulation of β-catenin and the expression of β-catenin/T cell factor/lymphoid enhancer-binding factor (TCF/LEF) target genes (Gammons and Bienz, 2018).
In the DC, the scaffolding proteins and tumor suppressors axis inhibition protein 1 (AXIN1) and adenomatous polyposis coli (APC) collaborate to co-recruit the kinases casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β) jointly with their substrate β-catenin (Stamos and Weis, 2013; van Kappel and Maurice, 2017; Figure 1A). This enables the sequential phosphorylation of an N-terminal β-catenin phosphodegron and the subsequent ubiquitylation of newly synthesized β-catenin by a SKP1-CUL1-F box (SCF) E3 ubiquitin ligase complex containing the substrate recruitment component β-TrCP (SCFβ-TrCP) (Amit et al., 2002; Jiang and Struhl, 1998; Li et al., 2012; Liu et al., 2002; Orford et al., 1997; Stamos and Weis, 2013; van Kappel and Maurice, 2017; Winston et al., 1999; Wu et al., 2003). Beta-catenin poly-ubiquitylation triggers its proteasomal degradation (Aberle et al., 1997).
Figure 1.
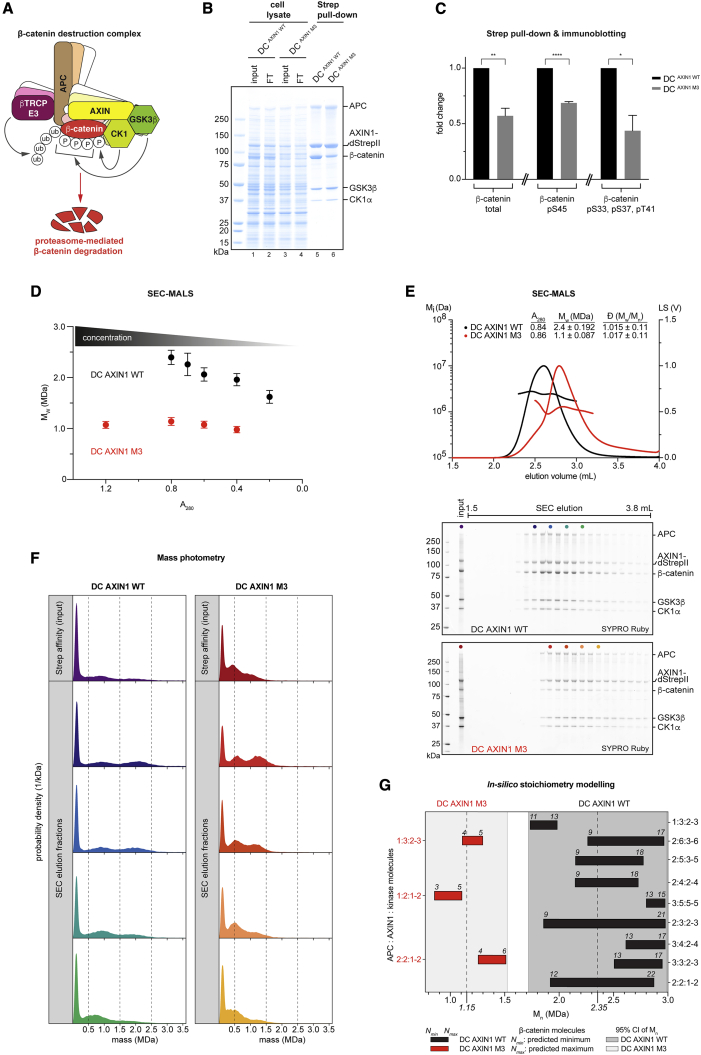
AXIN1 polymerization is a major contributor to the concentration-dependent DC stoichiometry
(A) Schematic representation of the DC. See Figure S1A for further details.
(B) DC variants containing either wild-type or polymerization-deficient (M3) AXIN1, affinity-purified via AXIN1-dStrepII, and analyzed using SDS-PAGE and Coomassie staining. FT, flowthrough.
(C) Quantification of total β-catenin recovered with AXIN1-dStrepII and its phosphorylation, normalized to recovered total β-catenin. Data are from immunoblot analyses of experiments as shown in (B). See Figure S1B for a representative set of immunoblots. Results are means from three independent experiments ± SEM. Statistical analyses were performed using an unpaired Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
(D) SEC-MALS of the DC containing either wild-type or M3 AXIN1 at different input concentrations. Data points are mean weight average molecular weights (MW) ± uncertainty (see STAR Methods). See Figure S1D for SDS-PAGE analysis of input samples and Figures S1E and S1F for SEC-MALS data and representative SDS-PAGE analyses of elution fractions.
(E) SEC-MALS of the DC containing either wild-type or M3 AXIN1 at a single input concentration. Light scattering intensities at 90° and calculated Mi values are plotted. Input A280, MW, and dispersities (Ð) over the peak areas with uncertainties are tabulated. Peak fractions were analyzed using SDS-PAGE and Sypro Ruby staining.
(F) Mass photometry of input and SEC-MALS elution fractions (indicated by colored dots in E).
(G) In silico modeling of DC stoichiometry. Predicted stoichiometries are shown on the right- or left-hand y axes for the DC with wild-type or M3 AXIN1, respectively. Dashed lines indicate mean number average molecular weight (Mn) values from SEC-MALS; shaded areas indicate corresponding 95% confidence intervals.
The central DC scaffold, AXIN1, directly binds and assembles all components of the core DC, strongly boosting β-catenin phosphorylation (Dajani et al., 2003; Hamada et al., 1999; Ikeda et al., 1998; Willert et al., 1999), in part by suppressing phosphorylation of substrates competing with β-catenin (Gavagan et al., 2020). Its low average cellular concentration (Lee et al., 2003; Tan et al., 2012) requires AXIN1 to form filamentous polymers for efficient β-catenin degradation (Fiedler et al., 2011). The second DC scaffold, APC, harbors up to ten β-catenin binding sites, a subset of which are phospho-regulated by the DC kinases (Eklof Spink et al., 2001; Ha et al., 2004; Liu et al., 2006; Rubinfeld et al., 1993; Su et al., 1993), as well as three AXIN1 docking motifs (Behrens et al., 1998; Kishida et al., 1998; Spink et al., 2000; Stamos and Weis, 2013; van Kappel and Maurice, 2017; Figure S1A). Loss of varying numbers of these motifs by APC truncating mutations within the intestinal stem cell compartment confers high basal β-catenin levels and initiates up to 80% of colorectal cancers (CRCs) (Barker and Clevers, 2006; Kohler et al., 2008, 2009; Morin et al., 1997; Munemitsu et al., 1995; Novellasdemunt et al., 2017; Roberts et al., 2011; Rubinfeld et al., 1997; Zhang and Shay, 2017), the third most common cancer type worldwide (Bray et al., 2018). Although the function of AXIN1 in the DC is relatively well understood, that of APC is less clear (van Kappel and Maurice, 2017). Several non-mutually exclusive functions have been proposed for APC, among them the capture of β-catenin in the cytoplasm for its modification (Ha et al., 2004) or cytoplasmic retention (Krieghoff et al., 2006; Roberts et al., 2011), the transfer of phosphorylated β-catenin from AXIN1 to the ubiquitin-proteasome system (Kimelman and Xu, 2006), or the protection of β-catenin from de-phosphorylation (Su et al., 2008) or de-ubiquitylation (Novellasdemunt et al., 2017).
Our understanding of the DC is shaped largely by genetic, cell biological, cellular biochemical, and structural studies (Stamos and Weis, 2013; van Kappel and Maurice, 2017; and references above). A minimal DC has been constructed in mammalian cells (Pronobis et al., 2017), and the reconstitution of DC function in mammalian cell (Su et al., 2008) or Xenopus egg (Salic et al., 2000) extracts has been the closest step toward achieving full DC reconstitution in vitro. Still, both these systems offer limited control over the factors present in the experimental setup. The biochemical reconstitution of the DC from purified components, thus far hampered by the lack of purified, recombinant, full-length human AXIN1 and APC, has long been sought after, as it would enable the controlled interrogation of DC function in a reductionist system without confounding cellular factors.
Here we describe the in vitro reconstitution of the core DC and recapitulate β-catenin capture, phosphorylation, ubiquitylation, and degradation, using purified components. We demonstrate that oncogenic APC mutations in CRC impart deficiencies to DC assembly and activities.
Results
Biochemical reconstitution of the DC
To interrogate the mechanism of the core DC, we expressed the recombinant human complex in insect cells and affinity-purified it through a C-terminal double-StrepII tag on AXIN1. We included AXIN1 either in its wild-type or non-polymerizable mutant form (M3) (Fiedler et al., 2011). All full-length DC components (AXIN1, APC, CK1α, GSK3β, and β-catenin) were present in the purified complex (Figure 1B). However, loss of AXIN1 polymerization significantly reduced β-catenin recruitment (Figures 1B, 1C, and S1B), in agreement with the essential role of AXIN1 self-assembly in destabilizing β-catenin in vivo (Fiedler et al., 2011). Phosphorylation of β-catenin, APC, and AXIN1 is critical to the DC function (Stamos and Weis, 2013; van Kappel and Maurice, 2017). Immunoblotting revealed that a subpopulation of β-catenin within the complex was phosphorylated on the authentic phosphodegron sites targeted by CK1α and GSK3β (S45 and S33/S37/T41, respectively; Figure S1B; Liu et al., 2002). Loss of AXIN1 polymerization also significantly reduced degron phosphorylation (Figures 1C and S1B). Treatment of the purified DC with λ-phosphatase increased APC and AXIN1 mobility in SDS-PAGE and abolished detectable β-catenin phosphorylation (Figures S1C). Conversely, ATP addition reduced AXIN1 mobility and further augmented β-catenin phosphorylation, showing that phosphorylation of these components was not saturated (Figure S1C).
We next analyzed the DC’s mass by size exclusion chromatography coupled to in-line multi-angle light scattering (SEC-MALS). AXIN1 (92 kDa) is expected to bind a single copy each of β-catenin (85 kDa), CK1α (39 kDa), and GSK3β (47 kDa). Disregarding AXIN1 and APC multimerization (Fiedler et al., 2011; Kunttas-Tatli et al., 2014), we reasoned that up to three AXIN1 molecules could be bound by a single APC molecule (312 kDa) through APC’s SAMP repeats. APC would bind up to ten β-catenin molecules through its 15Rs and 20Rs. The resulting overall theoretical molecular weight would amount to 1,951 kDa (Figure S1A). At its highest attainable concentration, the DC displayed an average molecular weight of 2,395 ± 141 kDa (Figures 1D and S1D–S1F), indicating additional higher order contributions to DC stoichiometry. Dilution of the complex decreased the measured molecular weights, in line with the anticipated concentration dependency of complex assembly (Figures 1D and S1D–S1F). The size of the DC was not saturated at its highest concentration, but we were unable to concentrate the complex by ultrafiltration. AXIN1 polymerization is a major contributor to the overall size of the DC: even at a higher concentration than that for the wild-type DC, the AXIN1 M3 mutant complex displayed an average molecular weight of only 1,072 ± 68 kDa, and its dilution had no impact on its overall mass (Figures 1D and S1D–S1F). As for affinity purification, the eluting complex showed an increased β-catenin occupancy with wild-type compared with M3 AXIN1 (Figures S1E and S1F).
We next used mass photometry to measure the DC’s mass distribution at the single-molecule level (Sonn-Segev et al., 2020; Young et al., 2018). We first performed SEC-MALS on DCs containing either wild-type or M3 AXIN1 (Figure 1E), with results comparable with those shown in Figure 1D. We next analyzed the SEC-MALS input material and fractions across the elution peaks (Figure 1E, indicated by colored dots on gels) using mass photometry (Figure 1F). Molecular weight distributions were broad. The dominance of events below 0.2 MDa indicated DC dissociation upon dilution for mass photometry (<100 nM) (Sonn-Segev et al., 2020), even within the short time frame (≈5 min). This generated multiple lower molecular weight events from the parental complex, in line with the dynamic, concentration-dependent assembly of the DC (Barua and Hlavacek, 2013; Nong et al., 2021; Pronobis et al., 2017; Schaefer and Peifer, 2019). Nonetheless, we still detected high-molecular weight species (Figure 1F). Although most events measured for DC AXIN1 M3 were less than 1.5 MDa, the wild-type DC displayed a broader mass distribution ranging up to ≈3 MDa (Figure 1F). In conclusion, mass photometry further illustrates the importance of AXIN1 polymerization in DC assembly.
We performed in silico modeling to predict possible stoichiometries within the DC that satisfy both the molecular weights observed in SEC-MALS and constraints on the basis of our own and published findings on DC assembly (Figure 1G; Table S1; see STAR Methods for details). Modeling suggests that the DC can attain different stoichiometries for a given molecular weight range. Although AXIN1 polymerization and the APC/AXIN1 ratio are important stoichiometry determinants, the degree of β-catenin incorporation contributes most to the stoichiometry variability (Figure 1G; Table S1).
Taken together, we show that AXIN1 polymerization governs the concentration-dependent assembly of the DC, recruitment of β-catenin, and complex composition.
Oncogenic APC variants are part of the DC
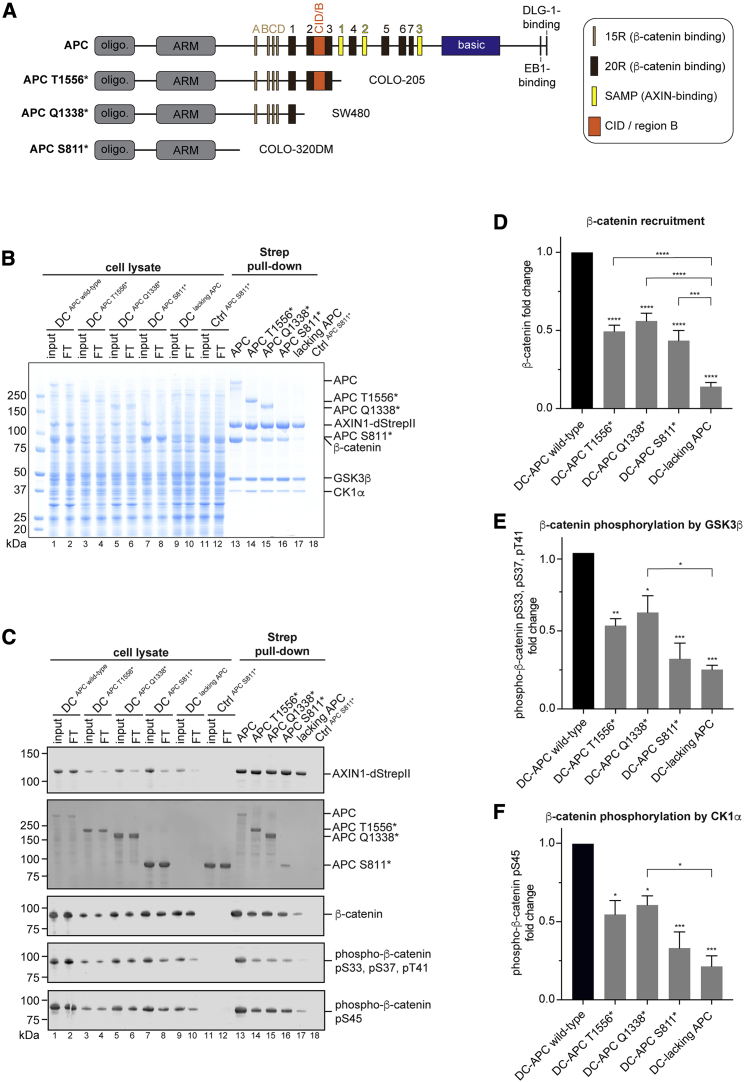
We next assessed the contribution of APC to DC assembly by purifying different DC variants. The kinases robustly co-purified with AXIN1, regardless of APC’s presence (Figure 2B, lanes 17 and 13). Although only sub-stoichiometric amounts of β-catenin were recovered in the absence of APC (Figures 2B and 2C, lane 17, and Figure 2D), APC co-expression robustly increased β-catenin co-purification (Figures 2B and 2C, lane 13, and Figure 2D). Analyzing the phosphorylation status of β-catenin using immunoblotting, we observed that APC omission not only limited β-catenin recruitment but also reduced its degron phosphorylation by ≈75% (Figure 2C, compare lanes 13 and 17; Figures 2E and 2F).
Figure 2.
Oncogenic APC variants, although part of the DC, impair β-catenin recruitment and phosphorylation
(A) Schematic representation of APC and its oncogenic truncation variants from the indicated CRC cell lines. See Table S10 for details of APC truncations.
(B) DCs purified via AXIN1-dStrepII with the indicated co-expressed APC variants. Samples were analyzed using SDS-PAGE and Coomassie staining.
(C) Immunoblot analyses of the lysates and affinity-purified material shown in B. Immunoblotting likely underestimates APC (312 kDa) abundance because of lower transfer efficiency compared with truncation variants.
(D) Quantification of total β-catenin recovered with AXIN1, assessed using immunoblotting. Signals were not normalized for β-catenin levels in the lysates, as not all β-catenin may be available for DC binding.
(E and F) Quantification of phosphorylation status (pS33, pS38, pS41; pS45) of recovered β-catenin, normalized for the amounts of recovered total β-catenin.
Results shown in (D)–(F) are means from three independent experiments ± SEM. Statistical analyses were performed using one-way ANOVA with the Bonferroni test for multiple comparisons. Asterisks above bars (without brackets) refer to comparisons to the no-APC condition (black bar). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant. See Tables S2–S4 for details of statistical analyses.
Germline and sporadic nonsense mutations give rise to truncated APC variants lacking large C-terminal stretches, with variable losses of AXIN1-binding SAMP repeats and β-catenin-binding 15R and 20R repeats, depending on the truncation site (van Kappel and Maurice, 2017; Zhang and Shay, 2017; Figure 2A). To explore whether these truncated variants can be part of the recombinant DC and facilitate β-catenin recruitment and phosphorylation, we expressed and purified the DC in the context of APC mutant variants found in commonly used CRC cell lines (APC T1556∗ from COLO-205, APC Q1338∗ from SW480, and APC S811∗ from COLO-320DM; Figure 2A; Table S10). All APC variants were expressed (Figures 2B and 2C, lanes 1–12), and APC T1556∗ and APC Q1338∗ robustly co-purified with AXIN1 (Figures 2B and 2C, lanes 14 and 15). This was surprising, given the absence of AXIN1-binding SAMP repeats in both these APC variants. APC S811∗, although co-purifying at much reduced levels, as anticipated given the loss of all 15R, 20R, and SAMP repeats, nonetheless was detectable within the complexes (Figures 2B and 2C, lane 16). A parallel control pull-down from cells expressing only APC S811∗ (without any other DC components) indicated that APC S811∗ inclusion into the DC was specific (Figures 2B and 2C, lane 18). The three truncation variants, including APC S811∗, still stimulated β-catenin recruitment (Figures 2B–2D). Both GSK3β- and CK1α-dependent β-catenin phosphorylation were reduced upon APC truncation. This was especially apparent for APC S811∗, for which β-catenin phosphorylation was comparable with that observed upon APC omission (Figures 2E and 2F). Expression of APC T1556∗, but not APC omission or expression of any other APC variant, reproducibly increased the electrophoretic mobility of AXIN1 in SDS-PAGE, suggesting altered post-translational modification, and lowered levels of AXIN1 and β-catenin (Figures 2B and 2C).
In conclusion, APC truncation variants are hypomorphs still able to incorporate into the DC, in line with previous cell-based biochemical studies (Li et al., 2012; Novellasdemunt et al., 2017; Voloshanenko et al., 2013). APC promotes both the recruitment of β-catenin to the DC and its phosphorylation. Surprisingly, even an extreme CRC mutant variant of APC lacking all β-catenin-binding motifs still supports β-catenin recruitment. Although APC 15R and 20R motifs are therefore not strictly required for facilitating β-catenin recruitment, they play a role in promoting β-catenin phosphorylation.
APC facilitates β-catenin phosphorylation and ubiquitylation
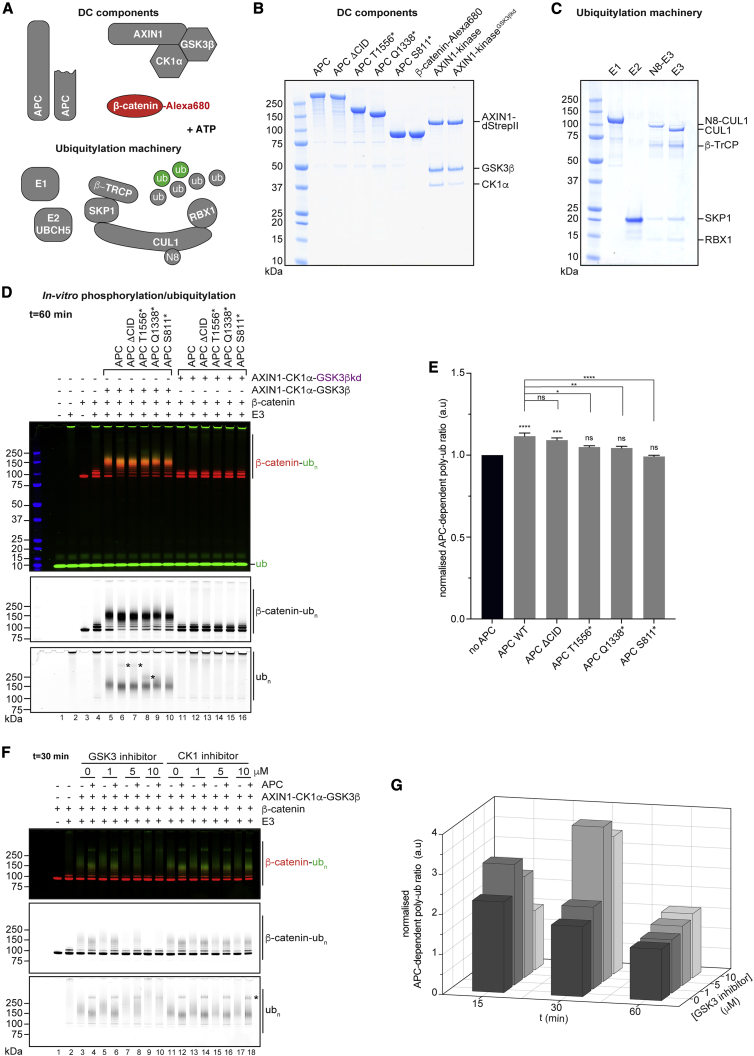
We next aimed to reconstitute the biochemical activities of the DC to investigate the consequences of lost AXIN1 polymerization and oncogenic APC truncation. We individually expressed full-length APC, the aforementioned CRC truncation variants, and an APC variant lacking the so-called β-catenin-inhibitory domain (CID), which is essential for the suppression of β-catenin levels in cells (Choi et al., 2013; Kohler et al., 2009; Liu et al., 2006; Novellasdemunt et al., 2017; Roberts et al., 2011). We separately produced in vitro-dephosphorylated β-catenin (Figures 3A and 3B). We further generated an AXIN1-CK1α-GSK3β (AXIN1-kinase) complex containing either the wild-type proteins, non-polymerizable AXIN1 M3, and/or a kinase-dead (kd) GSK3β variant (KD85/181AN) (Figures 3A, 3B, and S5E). To reconstitute the ubiquitylation reaction, we supplied the E1 ubiquitin-activating enzyme UBE1, the E2 ubiquitin-conjugating enzyme UBCH5a and the E3 ubiquitin ligase complex composed of β-TrCP, SKP1, CUL1, and RBX1, with the latter being in vitro neddylated on CUL1 (Rabut and Peter, 2008; Figure 3C). To track β-catenin modification by SDS-PAGE and multi-channel fluorimetry, we labeled β-catenin with an Alexa Fluor 680 fluorophore and used fluorescein-ubiquitin (Figures 3A and 3B). Upon combining the relevant components, we initiated β-catenin phosphorylation and ubiquitylation by ATP addition, terminating the reactions at different time points (see Figures 3D and 3E for 60 min time point and Figures S2 and S3 for additional time points).
Figure 3.
Reconstitution of the biochemical activity of the DC
(A) Schematic representation of the DC and accessory components for biochemical assays.
(B) Purified DC components analyzed using SDS-PAGE and Coomassie staining. kd, kinase-dead.
(C) Purified ubiquitylation machinery components.
(D) In vitro DC activity assay (reaction time 60 min). Top panel: dual-channel fluorimetry of SDS-PAGE gel with β-catenin in red and ubiquitin in green. Middle and bottom panels: separated channels. Asterisks in the bottom panel mark ubiquitylated APC variants. See Figure S2 for additional time points and Figure S3 for analyses of β-catenin phosphorylation.
(E) Quantification of APC-dependent β-catenin poly-ubiquitylation. See Figures S2B and S2C for quantification details. Data are means from three independent experiments ± SEM. Statistical significance was tested using one-way ANOVA with the Bonferroni test for multiple comparisons. Asterisks above bars (without brackets) refer to comparisons to the no-APC condition (black bar). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant. See Table S5 for details of statistical analyses.
(F) In vitro DC activity assay (as in D, but at 30 min time point) ± GSK3 or CK1 inhibitors at the indicated concentrations. The asterisk in the bottom panel marks ubiquitylated APC. See Figures S4A and S4B for additional time points and analyses of β-catenin phosphorylation.
(G) Quantification of APC-dependent β-catenin poly-ubiquitylation ± GSK3 inhibitor, on the basis of data shown in (F) (n = 1 representative experiment). See Figure S4C for CK1 inhibitor quantification.
The ubiquitylation machinery (E1, E2, E3 complex and ubiquitin) produced free poly-ubiquitin (Figure 3D, lane 2). Un-modified β-catenin appeared as a single band, and addition of the ubiquitylation machinery without the AXIN1-kinase complex resulted in the moderate (mostly mono- and di-) ubiquitylation of β-catenin (Figure 3D, lanes 3 and 4). Further addition of the AXIN1-kinase complex gave rise to β-catenin poly-ubiquitylation (Figure 3D, lane 5), which was further augmented by APC, leading to the near complete depletion of un-modified β-catenin (Figure 3D, lane 6). Given that β-catenin was present in excess (1 μM β-catenin, 150 nM AXIN1-kinase complex, 200 nM APC, and 100 nM SCFβ-TrCP), the near stoichiometric poly-ubiquitylation of β-catenin indicates that the reconstituted DC releases β-catenin upon modification and subsequently captures new β-catenin molecules. Although the proportion of modified β-catenin increased, the average ubiquitin chain length decreased in the presence of APC, indicating that APC reduces the processivity of β-catenin ubiquitylation, increasing throughput of the DC (Figure 3D). By dividing the poly-ubiquitylated β-catenin Alexa Fluor 680 signal by the total β-catenin signal in each lane, we compared the extent of β-catenin poly-ubiquitylation between reactions (Figures S2B and S2C). We subsequently quantified the β-catenin poly-ubiquitylation attributed to APC versus AXIN1-kinase complex only (Figure 3D, lanes 6–10 compared with lane 5; Figures 3E and S2D). Although wild-type APC and its ΔCID variant moderately but significantly increased β-catenin poly-ubiquitylation, the APC truncation mutants did not (Figure 3E). We observed a small but statistically significant decrease in β-catenin poly-ubiquitylation with the APC truncations compared with wild-type APC (Figure 3E). Catalytic inactivation of GSK3β resulted in background levels of β-catenin poly-ubiquitylation comparable with those without AXIN1-kinase complex (Figure 3D, compare lanes 11–16 with lane 4). We observed similar trends at the 15 and 30 min reaction time points (Figure S2D).
Interestingly, we noted discrete ubiquitylation at molecular weights corresponding to wild-type, ΔCID, and T1556∗ APC variants (Figure 3D, lower panel, lanes 6–8), suggesting that APC is a substrate of SCFβ-TrCP. The β-catenin poly-ubiquitylation signal may mask any potential signal from APC Q1338∗ and S811∗; we were therefore unable to ascertain the ubiquitylation status of these two variants. APC ubiquitylation was dependent on active GSK3β (Figure 3D, lanes 12–14).
We confirmed phosphodegron phosphorylation in poly-ubiquitylated β-catenin using immunoblotting (Figure S3A, lanes 5–10). The much reduced β-catenin phosphorylation kinetics with kinase-dead GSK3β revealed that wild-type and ΔCID APC promote residual β-catenin phosphorylation (Figure S3A, lanes 12 and 13). This led us to optimize our experimental setup, reasoning that the rapid progression of β-catenin phosphorylation with wild-type GSK3β may mask potential effects of APC on β-catenin modification. To attenuate the reaction kinetics while using wild-type GSK3β, we titrated GSK3 or CK1 inhibitors and monitored β-catenin modification across different time points. Indeed, with the GSK3 inhibitor, we observed a dose-dependent suppression in β-catenin phosphorylation and poly-ubiquitylation, which was more extensive in the absence than presence of APC (Figure 3F for 30 min time point; Figures S4A and S4B for 15 and 60 min time points). This sensitization of the assay toward APC addition displayed a maximum at 30 min with 5 μM GSK3 inhibitor (Figure 3G). In contrast, we did not observe this sensitization using a CK1 inhibitor (Figures 3F and S4A–S4C), possibly because of its limited potency toward CK1α (Rena et al., 2004), especially at the high ATP concentration (5 mM) in the reactions.
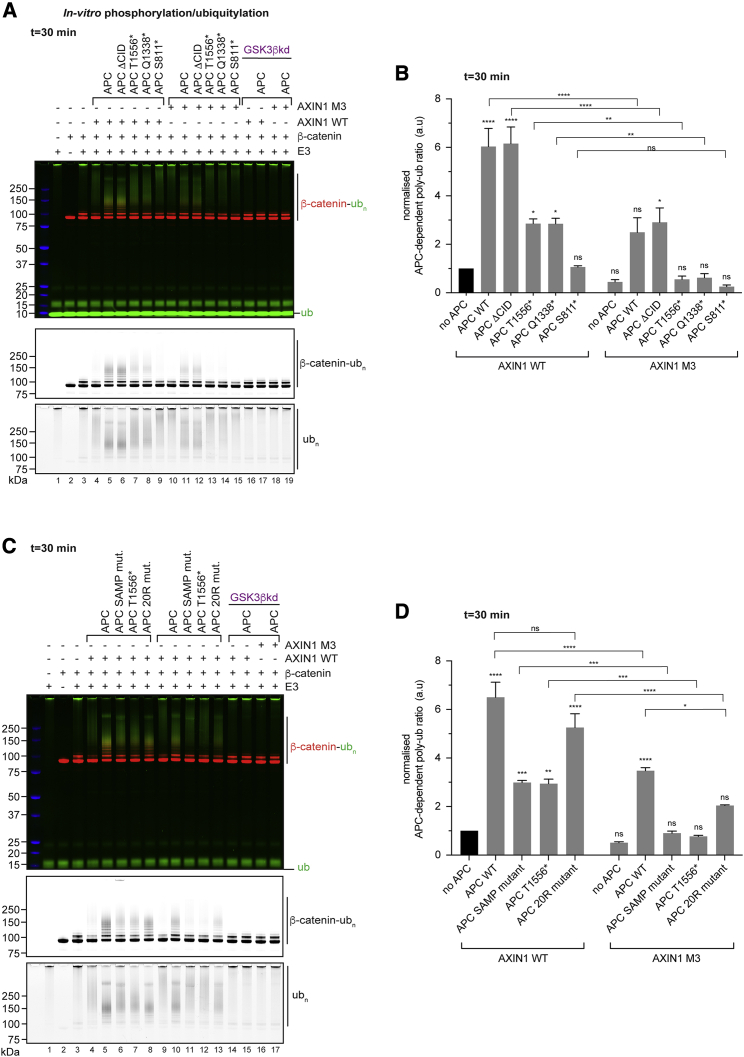
We next revisited the impact of APC truncations under the sensitized reaction conditions (5 μM GSK3 inhibitor, t = 30 min [Figures 4A and 4B] or t = 60 min [Figures S5A and S5B]). Wild-type and ΔCID APC stimulated β-catenin modification ≈6-fold (Figures 4A and 4B). This stimulation was reduced by ≈50% for the T1556∗ and Q1338∗ truncation variants and completely abolished for the S811∗ variant, with a similar trend after 60 min (Figures 4A, 4B, S5A, and S5B). Under the sensitized conditions, APC retained its ability to limit poly-ubiquitylation processivity (Figure S6A). Although CRC-associated APC truncations were less able to stimulate β-catenin poly-ubiquitylation, APC ΔCID behaved identically to wild-type APC (Figures 4A, 4B, S5A, and S5B), suggesting that the essential role of the CID region in cells (Choi et al., 2013; Kohler et al., 2009; Liu et al., 2006; Novellasdemunt et al., 2017; Roberts et al., 2011) is mediated by other factors not present in our in vitro system.
Figure 4.
Oncogenic APC truncation and impaired AXIN1 polymerization limit DC activity
(A and C) In vitro DC activity assays in the presence of 5 μM GSK3 inhibitor. Data are displayed as in Figure 3D. APC and AXIN1 mutant variants were used as indicated.
(B and D) Quantification of the APC-dependent poly-ubiquitylation of β-catenin. Data are means from three independent experiments ± SEM.
Statistical analyses were performed using one-way ANOVA with the Bonferroni test for multiple comparisons. Asterisks above bars (without brackets) refer to comparisons to the no-APC condition (black bar). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant. See Tables S6 and S8 for details of statistical analyses, Figure S5 for data from the 60 min time point and protein quality control, and Figures S6B and S6C for analysis of poly-ubiquitin linkage type.
We also probed the contribution of AXIN1 polymerization to the activity of the DC containing either wild-type or truncated APC. Loss of AXIN1 polymerization (M3) significantly reduced the APC-dependent activity of the DC (Figures 4A, 4B, S5A, and S5B). With AXIN1 M3, wild-type and ΔCID APC were only able to stimulate β-catenin ubiquitylation to levels similar to those attained with APC T1556∗ and APC Q1338∗ in the context of wild-type AXIN1 (Figures 4A, 4B, S5A, and S5B). Therefore, AXIN1 polymerization is critical to DC activity, in line with the reduced ability of AXIN1 M3 to recruit β-catenin (see above).
Both AXIN1 and β-catenin binding define APC function in the DC
We next explored the mechanism underlying the deficiency of APC truncation variants to promote β-catenin ubiquitylation. Any known APC truncation in CRC removes both a number of AXIN-binding SAMP repeats and 20R β-catenin binding sites (van Kappel and Maurice, 2017; Zhang and Shay, 2017). To dissect the contributions of either AXIN1 or β-catenin binding to APC function, we generated point mutant variants of APC, inactivating either all AXIN1-binding SAMP repeats (Spink et al., 2000) or abolishing the 20R phosphorylation sites responsible for turning 20Rs into high-affinity β-catenin binding sites (Ha et al., 2004; Liu et al., 2006; Table S10). The impairment of APC-dependent β-catenin poly-ubiquitylation upon loss of all SAMP repeats was comparable with that seen for APC T1556∗ (≈50%), which additionally lacks four of the six β-catenin-binding 20R repeats (4–7) (Figures 4C, 4D, and 2A). Both mutants also behaved similarly to each other in the context of non-polymerizable AXIN1 M3, where they were unable to significantly stimulate β-catenin poly-ubiquitylation (Figures 4C and 4D). This suggests that loss of AXIN1 binding is the primary cause for the deficiency of APC T1556∗ and that additional loss of 20R 4–7 has a limited effect. Concordantly, mutation of all 20R phosphorylation sites accounted only for a small, non-significant reduction of β-catenin poly-ubiquitylation relative to wild-type APC in the context of wild-type AXIN1 and a weaker (≈40%) but significant reduction of β-catenin poly-ubiquitylation in the context of AXIN1 M3 (Figures 4C and 4D). We note that the 20R mutations can, however, be considered a less severe mutation than those of the SAMP repeats. We made similar observations at the 60 min reaction time point (Figures S5C and S5D). The stronger impact of the APC S811∗ truncation, which removes all direct β-catenin binding sites (Figures 4A, 4B, S5A, and S5B) illustrates that 15Rs and 20Rs nonetheless contribute to APC function in our in vitro system, implying possible redundancy. In summary, we show that both AXIN1 and β-catenin binding define the function of APC in the DC and that the loss of APC-AXIN1 interaction largely accounts for the impact of APC truncations that preserve at least some β-catenin binding sites.
AXIN1 polymerization and APC control β-catenin phosphorylation
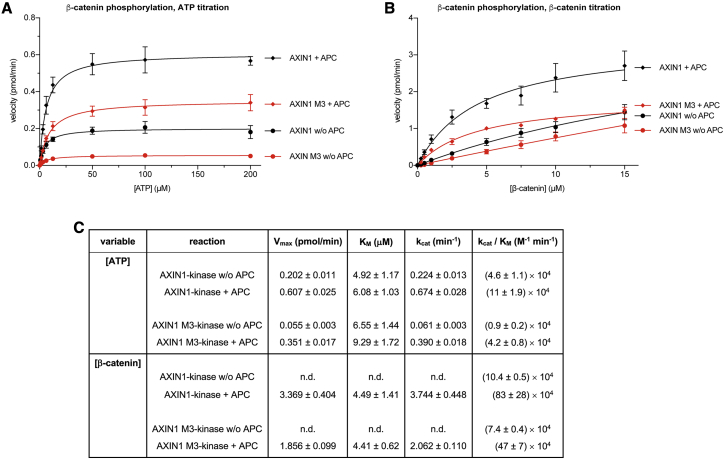
The contribution of APC toward β-catenin phosphorylation (Figures 2E, 2F, and S3A) prompted us to characterize the β-catenin phosphorylation kinetics using a radiometric assay (Figures 5 and S7; see STAR Methods for details). Our experimental setup did not distinguish between β-catenin degron-specific phosphorylation and potential other phosphorylation events on the protein. We therefore generated a β-catenin phosphodegron mutant variant (4A) as a control (Table S10). These mutations abolished the pS45 and pS33, pS37, pT41 signals corresponding to CK1α- and GSK3β-dependent phosphorylation, respectively (Figure S7A), which is unsurprising, but also very much reduced overall β-catenin phosphorylation, as detected by autoradiography (Figure S7B). This demonstrated that the bulk of β-catenin phosphorylation in vitro occurs on the phosphodegron. The auto-inhibitory pS9 phosphorylation of GSK3β (Dajani et al., 2001; Frame et al., 2001) was barely detected and will therefore have a limited impact on the in vitro kinase reactions (Figure S7A).
Figure 5.
AXIN1 polymerization and APC regulate kinase activity in the DC
Kinetic kinase assays to assess β-catenin phosphorylation.
(A) β-Catenin-directed activity of an AXIN1-kinase complex containing either wild-type or polymerization-deficient (M3) AXIN1 ± APC, at different ATP concentrations. See Figure S7 for control reactions and representative autoradiographs. Data points are means from three independent experiments ± SEM.
(B) β-Catenin-directed kinase activity as in (A), but at different β-catenin concentrations.
(C) Summary of kinase activity parameters. Data are means from three independent experiments ± SEM. n.d., not determined because of lack of reaction velocity saturation.
Titrating ATP while maintaining a fixed concentration of β-catenin and wild-type or M3 AXIN1-kinase complex, we measured the initial rates of β-catenin phosphorylation (Figures 5A, S7C, and S7D). Addition of APC had no (within error) or only a minor effect on the apparent kinase KM (ATP) in either the wild-type or M3 AXIN1-kinase containing reactions (Figure 5C). Furthermore, KM (ATP) was comparable for the wild-type and M3 AXIN1-kinase complexes. Therefore, neither inclusion of APC nor AXIN1 polymerization affect ATP binding. In contrast, APC increased the apparent kcat for wild-type and M3 AXIN1-kinase reactions by ≈3- and ≈6-fold and thereby the apparent reaction efficiencies (kcat/KM) by ≈2- and ≈5-fold, respectively (Figure 5C). In the absence or presence of APC, AXIN1 polymerization increased the apparent reaction efficiency by ≈5- or ≈3-fold, respectively (Figure 5C). The apparent reaction efficiencies of the wild-type AXIN1-kinase complex without APC and the AXIN1 M3-kinase complex with APC were comparable, suggesting that AXIN1 polymerization and APC have overlapping functions (Figure 5C).
We next performed β-catenin titrations at a saturating ATP concentration of 200 μM to explore whether the increased phosphorylation efficiencies are due to an effect of β-catenin binding or another mechanism (Figures 5B, S7E, and S7F). In the absence of APC, the initial velocities of neither the wild-type nor the M3 AXIN1-kinase complex reactions were saturated with up to 15 μM β-catenin (Figure 5B). We could therefore only determine the apparent kcat/KM reaction efficiencies for these conditions (Figure 5C; see STAR Methods for details). Both the wild-type and M3 AXIN1-kinase complexes experienced a strong (≈8- and ≈6-fold, respectively) increase in reaction efficiencies upon APC addition (Figures 5B and 5C). In the presence of APC, where we could reliably measure all kinetic parameters, AXIN1 polymerization had no effect on the apparent kinase KM (β-catenin); however, we observed a modest (≈2-fold) increase in kcat and the corresponding reaction efficiency (Figures 5B and 5C). These observations suggest that under the reaction conditions, AXIN1 polymerization promotes β-catenin phosphorylation not through enhancing β-catenin binding but through directly affecting the maximum phosphorylation rates the kinases can attain. This is surprising in the light of our findings described above (Figures 1B, 1C, 1E, and 1G), which would predict a change in KM.
Taken together, AXIN1 polymerization and APC binding both increase the DC’s phosphorylation efficiency. We note that AXIN1 and APC are both CK1α and GSK3β substrates (Ha et al., 2004; Ikeda et al., 1998; Kim et al., 2013; Liu et al., 2006; Rubinfeld et al., 1996; Figures S7C–S7F) and may act as β-catenin competitors in our assays, complicating the interpretation of the derived parameters.
APC interfaces the DC with the ubiquitylation machinery
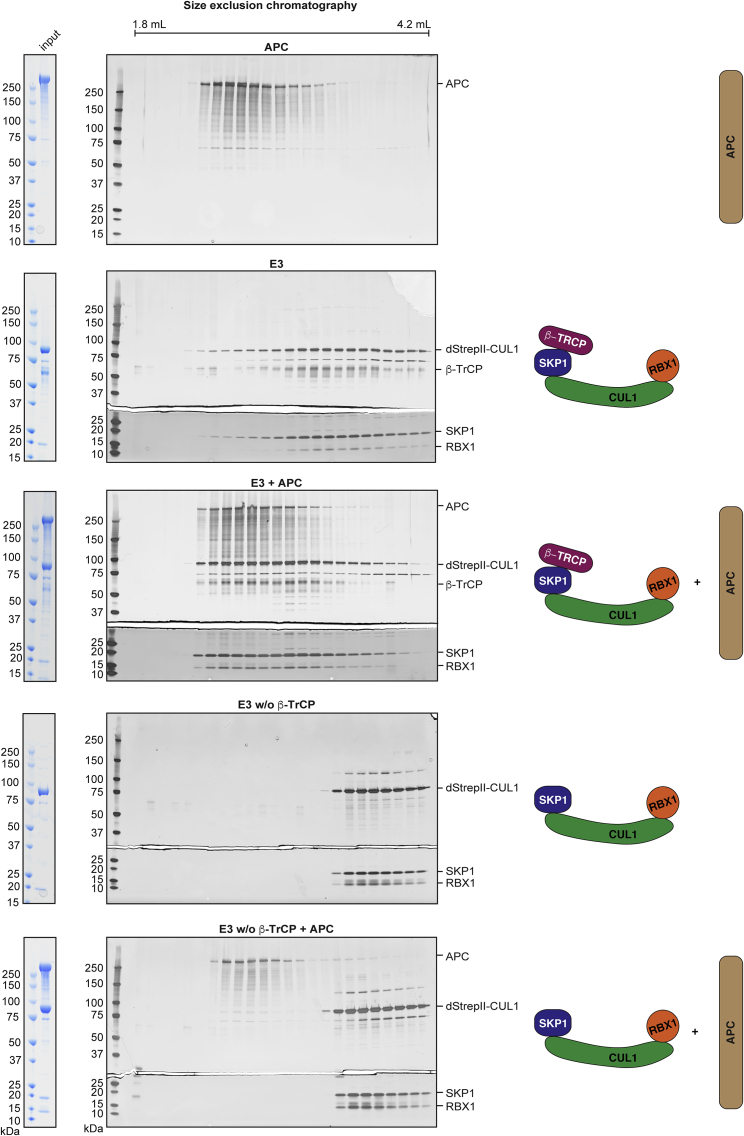
Among the many mutually compatible roles proposed for APC in the DC, one is to communicate with SCFβ-TrCP (Kimelman and Xu, 2006; Pronobis et al., 2015; Su et al., 2008; Xu and Kimelman, 2007). Reasoning this role may involve a physical interaction of APC with the E3 ubiquitin ligase complex, we performed analytical gel filtration experiments. To benchmark our experimental setup, we confirmed that the elution of phosphorylated but not non-phosphorylated β-catenin is affected by addition of SCFβ-TrCP (Figures S8B and S8C), in agreement with the well-understood phosphorylation-dependent interaction of β-catenin with β-TrCP (Wu et al., 2003). The APC elution peak was centered around 2.2 mL and that of SCFβ-TrCP around 2.8 mL. Mixing of both components resulted in a substantially earlier elution of SCFβ-TrCP, in a range similar to that for APC alone (Figure 6), in line with the largely intrinsically disordered APC dominating the elution behavior of an APC-SCFβ-TrCP complex. Purifying SCFβ-TrCP, we obtained two populations, the full E3 complex and one lacking β-TrCP (Figure S8A). This enabled us to test the role of β-TrCP in the E3-APC interaction. Omission of β-TrCP abolished the elution shift by APC (Figure 6), suggesting that the interaction either occurs directly through β-TrCP or through another β-TrCP-dependent mechanism. A recently proposed interaction of Drosophila Axin with the β-TrCP ortholog Slimb (Schaefer et al., 2020) prompted us to perform the same experiment with AXIN1. However, we observed only a small population of SCFβ-TrCP shifting compared with APC (Figure S8D), indicating that APC is the predominant DC component binding to the E3 complex in our system. Our in vitro system will be a valuable tool to decipher the precise molecular mechanisms by which APC communicates the activities of the DC to the ubiquitylation machinery to control Wnt/β-catenin signaling.
Figure 6.
Interaction of APC with the SCFβ-TrCP E3 ubiquitin ligase complex
The indicated proteins and complexes were resolved by size exclusion chromatography and fractions analyzed using SDS-PAGE and Coomassie (input) or silver staining (SEC fractions). The top and bottom parts of the gels were separated for silver staining of SKP1 and RBX1 to avoid overstaining of the other components. See Figure S8 for purification of the E3 complexes and analyses of β-catenin and AXIN1 interactions with SCFβ-TrCP.
Reconstitution of β-catenin proteasomal degradation
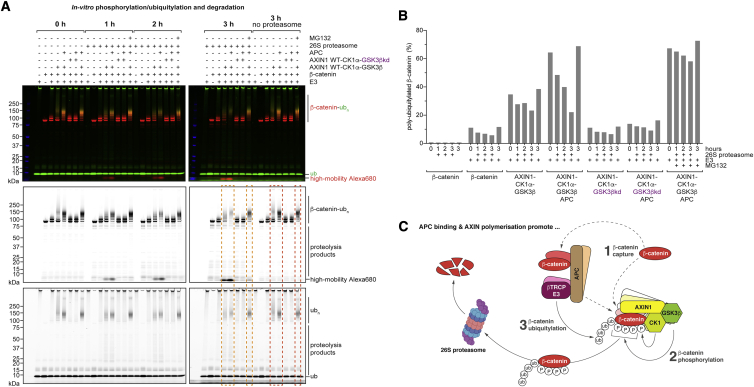
To interrogate whether poly-ubiquitylation of β-catenin is sufficient for its proteasomal degradation in vitro, we next sought to recapitulate the final step of β-catenin processing, its degradation. We confirmed that β-catenin is indeed modified with proteasome-targeting K48-linked ubiquitin chains in vitro (Figures S6B and S6C). We next performed β-catenin phosphorylation/ubiquitylation assays, adding purified human 26S-proteasome 60 min after initiating the reactions. As observed previously, the AXIN1-kinase complex and APC stimulated β-catenin poly-ubiquitylation in a GSK3β-dependent manner (Figures 7A and 7B). We observed a time-dependent degradation of poly-ubiquitylated β-catenin by the 26S-proteasome, which was severely inhibited by the proteasome inhibitor MG132 (Figures 7A and 7B). We further noted the proteasome-dependent accumulation of a high-mobility Alexa Fluor 680 degradation product, which correlated with the extent of β-catenin poly-ubiquitylation (Figure 7A). We conclude that our in vitro system fully recapitulates the biochemical functions of the core DC from component assembly to phosphorylation-dependent ubiquitylation of β-catenin and its proteasomal degradation.
Figure 7.
In vitro reconstitution of ubiquitylation-dependent β-catenin degradation by the proteasome
(A) In vitro DC activity assay in the presence of 5 μM GSK3 inhibitor. After 60 min, 26S-proteasome was added for the indicated time periods. Data are displayed as in Figure 3D. The high-mobility Alexa 680 signal (bottom of the gel) accumulates in a proteasome- and ubiquitylation-dependent manner. Areas boxed in orange and red-orange highlight the differences in β-catenin levels with or without incubation with proteasome for 3 h, respectively.
(B) Quantification (as in Figure S2B) of the percentage of poly-ubiquitylated β-catenin from the data shown in (A).
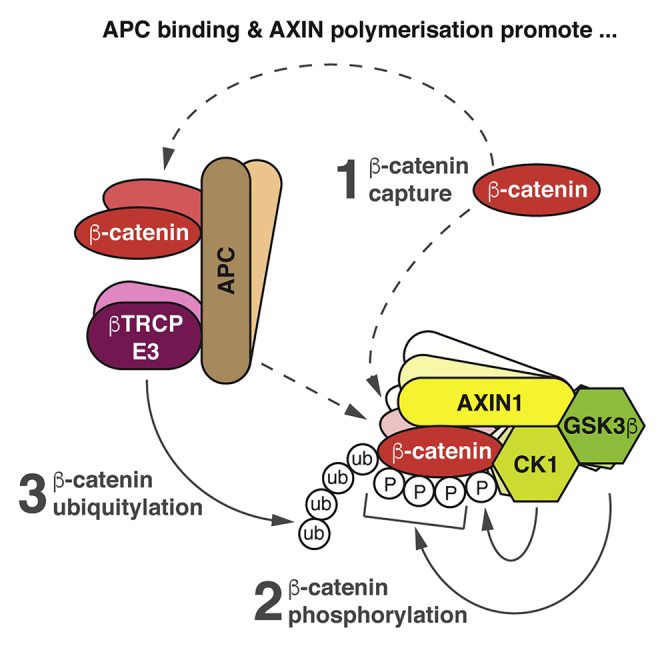
(C) Model summarizing functions of AXIN1 polymerization and APC in the DC. APC promotes β-catenin recruitment, both by direct binding and by facilitating β-catenin binding by AXIN1. Likewise, AXIN1 polymerization enhances β-catenin recruitment. Both APC binding and AXIN1 polymerization boost the efficiency of β-catenin phosphorylation. Increased β-catenin phosphorylation translates into increased ubiquitylation of β-catenin. APC may further promote β-catenin ubiquitylation through a direct interaction with SCFβ-TrCP. Dashed arrows represent interactions, solid arrows enzymatic modifications. The 26S-proteasome was created using BioRender.com.
Discussion
A recent interrogation of the cBioPortal for Cancer Genomics database (Cerami et al., 2012) highlights APC, AXIN2, and β-catenin mutations in ≈67%, ≈5%, and ≈6% of colorectal carcinomas, respectively (Bugter et al., 2021). Other cancer types with DC mutations include hepatocellular, endometrial, adrenocortical, gastric, and bladder urothelial carcinoma as well as melanoma (Bugter et al., 2021). This underpins the significance of understanding the molecular mechanisms of the DC. The biochemical reconstitution of the core DC from purified, recombinant proteins enables detailed mechanistic studies of the DC and its regulators. The latter include protein phosphatases (van Kappel and Maurice, 2017), the poly-ADP-ribosyltransferase tankyrase (Mariotti et al., 2017), ubiquitin ligases (Tauriello and Maurice, 2010), and components of the Wnt signalosome (Gammons and Bienz, 2018).
We show that AXIN1 polymerization is a major determinant of DC assembly and that both AXIN1 polymerization and APC promote β-catenin recruitment, phosphorylation, and ubiquitylation. Despite the loss of all AXIN1-binding SAMP repeats and numerous or all known β-catenin binding sites, APC truncation variants found in CRC are still part of the DC and act as functional hypomorphs. Stimulation of β-catenin ubiquitylation by AXIN1 polymerization and APC correlates with β-catenin phosphorylation. Beyond a simple phosphorylation-dependent effect, APC may promote β-catenin ubiquitylation by recruiting the SCFβ-TrCP complex to the DC. These insights led us to propose a model describing roles of polymeric AXIN1 and APC in the DC (Figure 7C). We further reconstituted the ubiquitylation- and proteasome-dependent degradation of β-catenin, enabling future investigations of DC-dependent β-catenin processing at every step, namely recruitment, phosphorylation, ubiquitylation, and proteolysis.
DC assembly and stoichiometry
We observe a concentration-dependent contribution of AXIN1 polymerization to the overall size of the DC. Rather than describing the DC as a stoichiometrically well-defined multi-protein machine, it is more appropriately portrayed as a dynamic assembly whose composition responds to the concentration of its components, in line with previous proposals (Schaefer et al., 2018; Xue et al., 2013). The molecular weights of the complexes attainable indicate that the extent of AXIN1 polymerization is limited in our system, which agrees with the relatively high (low-micromolar) Kd of AXIN1 DIX domains (Kan et al., 2020). Using parameters derived from SEC-MALS, we calculated the DCs extinction coefficients and concentrations across the SEC-MALS elution peaks (see STAR Methods). For the DC with wild-type AXIN1, the estimated concentration in the input sample was 415 nM; its maximum concentration at the detector was 56 nM. In SEC-MALS experiments performed on the isolated AXIN1 DIX domain at >10-fold higher protein concentration (6.6 μM in the input and 0.71 μM at the detector), molecular weights compatible with only 1 or 2 protomers were observed (Kan et al., 2020). Despite the comparably low DC concentrations in our study, we still observe a contribution of AXIN1 polymerization to the size of the DC, both in SEC-MALS and even in mass photometry, likely because of avidity provided by APC. The main consequence of AXIN1 polymerization is an increased recruitment of β-catenin to the DC. Although our current setup does not lend itself to the precise determination of DC stoichiometries, we used in silico modeling to determine a subset of possible DC stoichiometries that satisfy the observed molecular weights. Our analysis indicates that AXIN1 polymerization provides the DC with a broader range of possible stoichiometries and increased β-catenin recruitment. Our model also reveals potential stoichiometries at which the wild-type DC is nearly or fully saturated with β-catenin.
These stoichiometries obtained in vitro may differ from those in cells, where DC-accessory factors, additional regulatory events, potential effects of molecular crowding (Rivas and Minton, 2016), and the subcellular context add to the complexity. Indeed, our work and that of Kan et al. (2020) points toward stoichiometry differences between DCs assembled in vitro and those observed in cells by light microscopy (Schaefer et al., 2018). Cytoplasmic puncta in the Wingless interstripes within the epidermis of Drosophila embryos, equivalents of the DC known as β-catenin degradasomes, were measured to contain tens to hundreds of Axin molecules (Schaefer et al., 2018). DC-associated factors influencing the size of the DC may include the poly-ADP-ribosyltransferase tankyrase (Martino-Echarri et al., 2016; Mariotti et al., 2017) or the Hippo signaling protein YAP/TAZ, which has been proposed to complex with the DC (Azzolin et al., 2014). These and other mechanisms may lead to still higher local concentrations of AXIN1 in cells than those we achieved in this study.
Overlapping functions of AXIN1 polymerization and APC
The functional overlap of AXIN1 polymerization and APC in DC activities illustrates the redundancy and robustness inherent to the DC (Pronobis et al., 2017). Although loss of AXIN1 polymerization reduces DC activity, APC can still stimulate the complex. This partial mechanistic redundancy likely ensures that the DC efficiently maintains low levels of newly synthesized β-catenin, given the large impact leaky β-catenin target gene expression would have on cellular decision making.
Functions of AXIN1 polymers
Polymerization of AXIN1 is required for its function in the Wnt/β-catenin pathway (Fiedler et al., 2011; Schwarz-Romond et al., 2007) and, as we show here, for normal DC assembly. AXIN1 polymerization likely increases its avidity for other DC components. This was apparent in our pull-down experiments for β-catenin, but lower experimental concentrations of components may be needed to reveal a potential avidity effect in APC recruitment, particularly if the AXIN1-APC interaction is of higher affinity. In turn, clustering of AXIN1 by APC may support AXIN1 polymerization. A contribution of Apc to the formation cytoplasmic Axin puncta has been revealed in Apc null Drosophila tissues, in line with roles for both Axin and Apc in the formation of polymeric assemblies (Mendoza-Topaz et al., 2011). AXIN1 has been proposed to adopt different conformations (Kim et al., 2013; Wang et al., 2013), and we speculate that polymerization may, in addition to increasing local AXIN1 concentration, allosterically expose the AXIN1 β-catenin binding site. However, in the light of enhanced β-catenin binding conferred by AXIN1 polymerization (Figures 1B, 1C, 1E, and 1G), it is surprising that AXIN1 polymerization promotes β-catenin phosphorylation through increasing kcat rather than decreasing KM. Future studies will address the underlying mechanism.
Functions of APC
Many non-mutually exclusive functions have been postulated for APC (Gammons and Bienz, 2018; Ha et al., 2004; Kimelman and Xu, 2006; Krieghoff et al., 2006; Kunttas-Tatli et al., 2014; Mendoza-Topaz et al., 2011; Novellasdemunt et al., 2017; Pronobis et al., 2017; Roberts et al., 2011; Stamos and Weis, 2013; Su et al., 2008; Tacchelly-Benites et al., 2018; van Kappel and Maurice, 2017). Our in vitro system offers novel opportunities for studying the precise effects of APC on DC assembly and its biochemical activities.
DC assembly
As does AXIN1 polymerization, APC promotes β-catenin recruitment to the DC, in agreement with previous proposals and the notion that β-catenin-binding 15R and 20R motifs in the extensive intrinsically disordered regions of APC act like a “fishing net” to retrieve cytoplasmic β-catenin (Ha et al., 2004; Kimelman and Xu, 2006; van Kappel and Maurice, 2017). APC truncation variants observed in CRC are still incorporated into the DC, as observed in cellular biochemistry studies (Li et al., 2012; Novellasdemunt et al., 2017; Voloshanenko et al., 2013). With loss of SAMP repeats in APC T1556∗ and APC Q1338∗ (Figure 2A), we hypothesize that the interaction of these APC variants with AXIN1 occurs largely indirectly through β-catenin. Indeed, β-catenin has been shown to simultaneously bind AXIN1 and a non-phosphorylated 20R3 motif from APC but not its phosphorylated variant, in agreement with structural findings (Ha et al., 2004). APC 15R motifs are expected to bind β-catenin simultaneously with AXIN1 (Ha et al., 2004).
Surprisingly, even the most extensive truncation mutant tested (APC S811∗, found in COLO-320DM cells) can be part of the DC and promote β-catenin recruitment. How APC S811∗ can achieve this in the absence of known β-catenin binding sites remains unknown. APC S811∗, and also the other APC variants tested, may bind AXIN1 through its armadillo repeat domain (Pronobis et al., 2015), and this APC-AXIN1 interaction may promote a conformational change in AXIN1 that enables β-catenin binding to AXIN1 (Kim et al., 2013; Wang et al., 2013). The partially retained function of APC S811∗ offers a potential explanation for why a complete loss of APC is selected against during colorectal carcinogenesis (Albuquerque et al., 2002).
APC and enzymatic functions of the DC
We find that APC promotes both β-catenin phosphorylation and ubiquitylation. Given the absence of DC accessory components in our setup, this cannot be explained by APC-dependent protection of β-catenin from de-phosphorylation (Su et al., 2008) or de-ubiquitylation (Novellasdemunt et al., 2017), or the partition of the DC from the Wnt signalosome (Mendoza-Topaz et al., 2011), although each of these scenarios is likely relevant in the cellular context. Our mutagenesis studies illustrate the importance of APC’s AXIN1 and β-catenin binding sites for DC-dependent modification of β-catenin.
Phosphorylation
We unequivocally show that APC promotes β-catenin phosphorylation, clarifying previous uncertainties around this question (e.g., Novellasdemunt et al., 2017). We find that neither APC incorporation nor AXIN1 polymerization affect KM (ATP) and thereby ATP binding by the kinases. Instead, both APC binding and AXIN1 polymerization stimulate kcat, leading to an enhanced catalytic efficiency kcat/KM. As we did not achieve saturation of the initial β-catenin phosphorylation velocities when titrating β-catenin in vitro, we could not deduce whether APC renders β-catenin phosphorylation more efficient by lowering the KM (β-catenin), as our pull-down experiments would suggest. Our inability to achieve saturation of β-catenin phosphorylation rates by titrating β-catenin may reflect distinct reaction conditions from those used by Gavagan et al. (2020); this includes the presence of full-length AXIN1 and APC, which are also substrates of CK1α and GSK3β (Ha et al., 2004; Ikeda et al., 1998; Kim et al., 2013; Liu et al., 2006; Rubinfeld et al., 1996).
Ubiquitylation
We observe that APC limits the ubiquitin chain length on β-catenin, and thereby processivity, while increasing the stoichiometry of β-catenin ubiquitylation, indicating that APC promotes the flux of β-catenin through the DC and SCFβ-TrCP. Further supporting a role of APC in communicating with the ubiquitylation machinery, we show that APC interacts with the SCFβ-TrCP complex independently of β-catenin and that this interaction is mediated by β-TrCP. AXIN1, however, appears to interact with SCFβ-TrCP much more weakly than APC, at least under our experimental conditions. These observations differ from those of Schaefer et al. (2020), in which the Drosophila β-TrCP ortholog Slimb was shown to co-localize and co-immunoprecipitate with Axin but not Apc2. Our data further suggest that APC is a substrate of SCFβ-TrCP, potentially being mono-ubiquitylated (Figure S6B), with hitherto unknown functional consequences.
Role of the 20R2-CID region of APC
Previous studies have revealed that the 20R2-CID region in APC is critical for β-catenin ubiquitylation and degradation (Choi et al., 2013; Kohler et al., 2009; Liu et al., 2006; Novellasdemunt et al., 2017; Roberts et al., 2011). As 20R2-CID does not bind β-catenin, the underlying mechanism has remained unclear. In contrast to these cell-based studies, our in vitro system does not ascribe a function to the CID, strongly suggesting that the core DC lacks additional factors or the cellular context required for the CID to fulfil its role. Indeed, several studies proposed additional regulators that may act through the 20R2-CID region, including the serine/threonine protein phosphatase PP1, α-catenin, and the de-ubiquitylating enzyme USP7 (Choi et al., 2013; Novellasdemunt et al., 2017; Su et al., 2008).
In summary, the reconstitution of the DC provides detailed insights into the functions of the DC scaffolds AXIN1 and APC and opens new avenues for the mechanistic dissection of Wnt/β-catenin signaling. The system further provides important tools for the identification and development of molecular probes and potential therapeutics to modulate this highly cancer-relevant pathway.
Limitations of the study
This study presents a prototypic application of our reductionist system to interrogate the DC at mechanistic detail. For technical reasons, the experiments were carried out at higher than cellular concentrations of the relevant proteins (Lee et al., 2003; Tan et al., 2012), which in part may limit or override the requirement for scaffolding to drive interactions or biochemical reactions. Where DC core components were co-expressed prior to their purification, the cellular context may still affect their interplay. Similarly, the phosphorylation status of AXIN1 and APC, known to regulate the DC (van Kappel and Maurice, 2017), was not controlled in our assays. AXIN and APC paralogs (AXIN2, APC2) will also need to be considered. Future developments of the system will address these and other limitations referred to above.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-AXIN1 | Cell Signaling Technology | Cat# 2087; RRID: AB_2274550 |
| anti-APC | Novus Biologicals | Cat# NB100-667; RRID: AB_521749 |
| anti-β-catenin | Cell Signaling Technology | Cat# 610153; RRID: 397554 |
| anti-phospho-β-catenin pS45 | Cell Signaling Technology | Cat# 9564; RRID: AB_331150 |
| anti-phospho-β-catenin pS33/pS37/pT41 | Cell Signaling Technology | Cat# 9561; RRID: AB_331729 |
| anti-non-phospho-β-catenin S33/S37/T41 | Cell Signaling Technology | Cat# 8814; RRID: AB_11127203 |
| anti-GSK3β | Cell Signaling Technology | Cat# 12456; RRID: AB_2636978 |
| anti-GSK3β pS9 | Cell Signaling Technology | Cat# 5558; RRID: AB_10013750 |
| anti-StrepMAB-Classic | IBA Life Sciences | Cat# 2-1507; RRID: AB_513133 |
| anti-ubiquitin P4D1 clone | Enzo Life Sciences | Cat# BML-PW0930; RRID: AB_10998070 |
| anti-ubiquitin K48 linkage-specific | Abcam | Cat# ab140601; RRID: AB_2783797 |
| anti-ubiquitin K63 linkage-specific | Abcam | Cat# ab179434; RRID: AB_2737584 |
| IRDye 680RD donkey anti-mouse | LI-COR | Cat# 926-68072; RRID: AB_10953628 |
| IRDye 800CW donkey anti-rabbit | LI-COR | Cat# 926-32213; RRID: AB_621848 |
| IRDye 800CW donkey anti-mouse | LI-COR | Cat# 926-32212; RRID: AB_621847 |
| Bacterial and virus strains | ||
| E. coli BL21-CodonPlus(DE3)-RIL | Agilent | Cat# 230245 |
| E. coli DH10 MultiBacTurbo | ATG:biosynthetics GmbH (Bieniossek et al., 2008) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Protease inhibitor cocktail tablets | Thermo Scientific | Cat# A32965 |
| AEBSF | Melford Laboratories Ltd | Cat# A20010 |
| β-Glycerophosphate | Sigma-Aldrich | Cat# G9422 |
| StrepTactin-Sepharose resin | IBA Life Sciences | Cat# 2-1201 |
| Cellfectin II | Thermo Scientific | Cat# 11496015 |
| Penicillin-Streptomycin | Thermo Scientific | Cat# 15070063 |
| Alexa Fluor® 680 C2 Maleimide | Thermo Scientific | Cat# A20344 |
| Coenzyme A | Sigma-Aldrich | Cat# C3019 |
| Sfp1 | Dr Alessandro Vannini lab | N/A |
| Lambda phosphatase | Dr Gideon Coster lab | N/A |
| APPB1-Uba3 | Enzo Life Sciences | Cat# PRT112 |
| UbcH12 | Enzo Life Sciences | Cat# UW9145 |
| His6-NEDD8 | Enzo Life Sciences | Cat# UW9225 |
| N-terminally fluorescein-labeled ubiquitin | Boston Biochem. | Cat# U-580 |
| ubiquitin | Boston Biochem. | Cat# U-100H |
| K48-linked ubiquitin chains | R&D Systems | Cat# UC-230 |
| K63-linked ubiquitin chains | R&D Systems | Cat# U-C-330 |
| UBA1 | Dr Edward Morris lab | N/A |
| GSK3 inhibitor CHIR-99021 | Sigma-Aldrich | Cat# 361571 |
| CK1 inhibitor D4476 | Sigma-Aldrich | Cat# 218696 |
| ATP regeneration solution | Enzo Lifesciences | Cat# BML-EW9810 |
| 26S proteasome | Boston Biochem. | Cat# e-365 |
| Proteasome inhibitor MG132 | Sigma-Aldrich | Cat# M7449 |
| γ-32P-ATP | Hartmann Analytics | Cat# SCP-301 |
| Pierce™ silver stain kit | Thermo Scientific | Cat# 24612 |
| SYPRO Ruby protein stain | Thermo Scientific | Cat# S12000 |
| InstantBlue protein stain | Abcam | Cat# 119211 |
| NativeMark™ unstained protein standard | Thermo Scientific | Cat# LC0725 |
| Precision Plus Protein™ standards | Bio-Rad | Cat:# 1610373 |
| Experimental models: Cell lines | ||
| S. frugiperda Sf9 insect cells | Thermo Scientific | Cat# 11496015 |
| T. ni High-Five insect cells | Thermo Scientific | Cat# B85502 |
| Recombinant DNA | ||
| pLIB vector | Jan-Michael Peters lab, IMP (Weissmann et al., 2016) | N/A |
| pBIG1a vector | Jan-Michael Peters lab, IMP (Weissmann et al., 2016) | N/A |
| pBIG1b vector | Jan-Michael Peters lab, IMP (Weissmann et al., 2016) | N/A |
| pBIG2ab vector | Jan-Michael Peters lab, IMP (Weissmann et al., 2016) | N/A |
| pLIB_ APC | GenScript (this paper) | N/A |
| pLIB_ APC T1556∗ | This paper, see Table S10 | N/A |
| pLIB_ APC Q1338∗ | This paper, see Table S10 | N/A |
| pLIB_ APC S811∗ | This paper, see Table S10 | N/A |
| pLIB_dStrepII-TEV-APC | GenScript (this paper) | N/A |
| pLIB_dStrepII-TEV-APC SAMP mutant | GenScript (this paper, see Table S10) | N/A |
| pLIB_dStrepII-TEV-APC 20R phospho mutant | GenScript (this paper, see Table S10) | N/A |
| pLIB_dStrepII-TEV-APC ΔCID | This paper, see Table S10 | N/A |
| pLIB_dStrepII-TEV-APC T1556∗ | This paper | N/A |
| pLIB_dStrepII-TEV-APC Q1338∗ | This paper | N/A |
| pLIB_dStrepII-TEV-APC S811∗ | This paper | N/A |
| pBIG1b_APC | This paper | N/A |
| pBIG1b_APC T1556∗ | This paper | N/A |
| pBIG1b_APC Q1338∗ | This paper | N/A |
| pBIG1b_APC S811∗ | This paper | N/A |
| pLIB_AXIN1-TEV-dStrepII | GenScript (this paper) | N/A |
| pLIB_AXIN1 M3-TEV-dStrepII | This paper, see Table S10 | N/A |
| pLIB_AXIN1 | This paper | N/A |
| pLIB_AXIN1 M3 | This paper, see Table S10 | N/A |
| pLIB_dStrepII-TEV-β-catenin | GenScript (this paper) | N/A |
| pLIB_dStrepII-TEV-β-catenin-ybbR | This paper, see Table S10 | N/A |
| pLIB_dStrepII-TEV-β-catenin S33A, S37A, T41A | This paper, see Table S10 | N/A |
| pLIB_β-catenin | This paper | N/A |
| pLIB_CK1α | GenScript (this paper) | N/A |
| pLIB_GSK3β | GenScript (this paper) | N/A |
| pLIB_GSK3β K85N, D181N | This paper, see Table S10 | N/A |
| pLIB_β-TrCP | GenScript (this paper) | N/A |
| pLIB_CUL1 | GenScript (this paper) | N/A |
| pLIB_SKP1 | GenScript (this paper) | N/A |
| pLIB_RBX1 | GenScript (this paper) | N/A |
| pBIG1a_AXIN1-kinase complex | This paper, see Table S11 | N/A |
| pBIG1a_AXIN1-kinase GSK3β-dead complex | This paper, see Table S11 | N/A |
| pBIG1a_AXIN1 M3-kinase complex | This paper, see Table S11 | N/A |
| pBIG1a_AXIN1-kinase-β-catenin complex | This paper, see Table S11 | N/A |
| pBIG2ab_wild-type DC (destruction complex) | This paper, see Table S11 | N/A |
| pBIG2ab_DC_APC T1556∗ complex | This paper, see Table S11 | N/A |
| pBIG2ab_DC_APC Q1338∗ complex | This paper, see Table S11 | N/A |
| pBIG2ab_DC_APC S811∗ complex | This paper, see Table S11 | N/A |
| pBIG2ab_DC_AXIN1 M3 complex | This paper, see Table S11 | N/A |
| pBIG2ab_SCFβ-TrCP E3 ligase | This paper, see Table S11 | N/A |
| pProEx HIS6-TEV-UBCH5c | Frank Sicheri lab (Chou et al., 2012) | N/A |
| Software, algorithms, databases | ||
| ImageJ (Fiji) | NIH | RRID: SCR_002285 |
| ImageJ Linearize GelData plugin | https://rsb.info.nih.gov/ij/plugins/linearize-gel-data.html | N/A |
| GraphPad Prism | GraphPad | RRID: SCR_002798 |
| Image Studio Lite | LI-COR | RRID: SCR_013715 |
| ImageQuant | GE Healthcare | RRID: SCR_04246 |
| ASTRA version 7.3.1 | Wyatt Technology | RRID: SCR_016255 |
| AcquireMP acquisition software | Refeyn Ltd | Cat# 600-1 |
| DiscoverMP analysis software | Refeyn Ltd | Cat# 600-1 |
| Scientific Python Development Environment | https://www.spyder-ide.org/ | RRID: SCR_008394 |
| DC stoichiometry algorithm | This paper | N/A |
| ExPASy ProtParam | https://web.expasy.org/protparam/ (Gasteiger et al., 2005) | RRID: SCR_018087 |
| UniProt | https://www.uniprot.org (UniProt Consortium, 2021) | RRID:SCR_004426 |
| Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) |
http://www.rcsb.org/pdb/ (Berman et al., 2002) PDB: 1EMU (Spink et al., 2000) PDB: 1V18 (Ha et al., 2004) PDB: 1H8F (Dajani et al., 2001) |
RRID:SCR_012820 |
| Deposited data | ||
| Original data and gel/Western blot images | This paper; Mendeley Data | https://doi.org/10.17632/wynmtr788p.1 |
| Other | ||
| Insect-Xpress cell medium | Lonza | Cat# BELN12-730Q |
| NuPAGE™ 4 – 12% Bis-Tris SDS-PAGE gels | Thermo Scientific | Cat# NP0321; Cat# WG1402; Cat# WG1403A |
| StrepTrap HP column | GE Healthcare | Cat# 28907547 |
| HiLoad 16/60 Superdex 200 column | GE Healthcare | Cat# 28989335 |
| HiLoad 16/600 Superose 6 column | GE Healthcare | Cat# 29323952 |
| WTC-50N5 column | Wyatt Technology | Cat# WTC-50N5 |
| WTC-100N5 column | Wyatt Technology | Cat# WTC-100N5 |
| DAWN® MALS detector | Wyatt Technology | Cat# N1018; RRID: SCR_020896 |
| Optilab refractometer | Wyatt Technology | Cat# N1001OP |
| Agilent 1260 Infinity II HPLC system | Agilent | RRID: SCR_019354 |
| Odyssey CLx fluorescence imager | LI-COR | RRID: SCR_014579 |
| Typhoon FLA 9500 | GE Healthcare | RRID: SCR_019957 |
| High Precision Coverslips | VWR | Cat# 630-2187 |
| CultureWell™ gaskets | Sigma-Aldrich | Cat# GBL103250 |
| Refeyn OneMP mass photometer | Refeyn Ltd | Cat# 100-01 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Sebastian Guettler (sebastian.guettler@icr.ac.uk).
Materials availability
Reagents generated in this study will be made available upon request, but we may require a completed Materials Transfer Agreement if there is potential for commercial application.
Experimental model and subject details
The E1 protein, λ-phosphatase and Sfp enzyme (all obtained as gifts; see below) were produced in Escherichia coli. The E2 protein was expressed in Escherichia coli BL21-CodonPlus(DE3)-RIL cells. All other proteins and protein complexes were expressed in either Sf9 (Spodoptera frugiperda) or High-Five (Trichoplusia ni) insect cells.
Method details
Cloning and expression of recombinant proteins and complexes
cDNA constructs of DC components and the SCFβ-TrCP E3 ligase complex were codon-optimized for E.coli expression by GenScript and were subcloned into the pLIB vector (Weissmann et al., 2016) under control of the polyhedron gene promoter of the Autographa californica nuclear polyhedrosis virus (AcNPV). The codon-optimized DC cDNAs were inserted into the pLIB vector as follows:
BamHI - Kozak sequence (AGCCGCCACC) - Start codon - insert - Stop codon - HindIII
Affinity-tagged versions were synthesized by extending the ORF with a double-StrepII (dStrepII) tag followed (for N-terminal tags) or preceded (for C-terminal tags) by a TEV protease site. Tag and TEV site sequences were as follows:
N-terminal: MSA-WSHPQFEK-(GGGS)2-GGSA-WSHPQFEK-GA-ENLYFQG
C-terminal: GA-ENLYFQG-SA-WSHPQFEK-(GGGS)2-GGSA-WSHPQFEK ∗stop codon.
See Table S10 for plasmid details. Multi-protein expression constructs were assembled using the biGBac system (Weissmann et al., 2016) to generate single baculoviruses with core destruction complex (DC) variants, sub-complexes and the SCFβ-TrCP E3 ubiquitin ligase complex (Table S11). Correct assembly was verified by restriction digest and sequencing of the open reading frames. Further construct details are available upon request.
Viral bacmids for the expression of individual subunits (from the pLIB vector) or complexes (from the pBIG vector) were generated using Tn7 transposition in DH10 MultiBacTurbo E. coli competent cells (Bieniossek et al., 2008). Insect cells were grown in Insect-Express media (# BELN12-730Q, Lonza) supplemented with 1% Penicillin-Streptomycin (# 15070063, Thermo Scientific). 2 mL of 5 × 105 cells/mL adherent Sf9 cells (# 11496015, Thermo Scientific) were transfected with purified bacmids using Cellfectin II reagent (# 10362100, Thermo Scientific) to generate recombinant baculoviruses. P1 virus was harvested after 72 h of incubation without shaking at 27°C. All of the ≈2 mL of harvested media was used to infect a fresh 30-mL culture of Sf9 cells (5 × 105 cells/mL) for a second round of viral amplification at 27°C and shaking at 130 rpm. Virus-containing media were harvested (P2 virus) once cell viability had dropped to ≈80%, and stored at 4°C.
Destruction (sub-) complexes were expressed in 0.25-L cultures of Sf9 cells, whereas the SCFβ-TrCP E3 ubiquitin ligase complex and individual DC components in 0.5-L cultures of High-Five cells (# B85502, Thermo Scientific). The amplified viruses were used to infect 1 × 106 cells/mL of Sf9 or High-Five cells and incubated at 27°C, 130 rpm. Cultures were harvested by centrifugation (250 x g for 10 min at 4°C) when cell viability had reached between 90 – 80%, which typically took approximately 72 h, and cell pellets were stored at −80°C.
APC purification
High-Five cell pellets were resuspended in a buffer containing 50 mM HEPES-NaOH pH 7.5, 750 mM NaCl, 10 mM β-mercaptoethanol, 5% glycerol, 1 mM AEBSF protease inhibitor and Pierce protease inhibitor tablets, EDTA-free (# A32965, Thermo Scientific). Lysates were briefly sonicated, centrifuged at 45,000 x g for 45 min to remove insoluble material and filtered through a 0.45-μm filter. The cleared lysate was loaded onto a 5-mL StrepTrap HP column (GE Healthcare) and washed with 20 column volumes (CV) of wash buffer (identical to lysis buffer but lacking protease inhibitors). dStrepII-fusion proteins were eluted with 5 mM d-desthiobiotin in wash buffer. The eluted peak fractions were pooled and dialysed overnight at 4°C against 50 mM HEPES-NaOH pH 7.5, 500 mM NaCl, 2 mM TCEP, 5% glycerol and His6-Strep-TEV protease to cleave the dStrepII affinity tag. Residual affinity tag, uncleaved protein and His6-Strep-TEV protease were removed through a subtractive Strep affinity chromatography step using a 5-mL StrepTrap HP column. The cleaved APC proteins were concentrated and flash-frozen in liquid nitrogen.
β-catenin purification
High-Five cell pellets were resuspended in a buffer containing 50 mM HEPES-NaOH pH 7.5, 500 mM NaCl, 10 mM β-mercaptoethanol and Pierce protease inhibitor tablets, EDTA-free. The subsequent steps until after loading of the cleared lysate onto the 5-mL StrepTrap column were performed as described for the purification of APC (see section above). Unbound material was removed with 20 CV of wash buffer, followed by equilibration with 2 CV of wash buffer containing 2 mM MnCl2. Purified λ-phosphatase (kind gift from Dr Jerome Gouge and Dr Gideon Coster) at 20 μg/mL in 3 CV wash buffer containing 2 mM MnCl2 was passed through the StrepTrap column. The column was capped and incubated at room temperature for 30 min to de-phosphorylate β-catenin. Lambda phosphatase was removed with 20 CV of wash buffer (without MnCl2) and the de-phosphorylated β-catenin eluted with 5 mM d-desthiobiotin. The eluted peak fractions were pooled and dialysed overnight at 4°C against 50 mM HEPES-NaOH pH 7.5, 150 mM NaCl, 2 mM TCEP and His6-StrepII-tagged TEV protease to cleave the dStrepII affinity tag. Residual affinity tag, uncleaved protein and His6-Strep-TEV protease were removed through a subtractive Strep affinity chromatography step using a 5-mL StrepTrap HP column.
β-catenin-ybbR Alexa Fluor® 680 labeling
A CoA-fluorophore conjugate can be covalently attached to a serine residue in a short ybbR peptide by the Bacillus subtilis Sfp phosphopantetheinyl transferase (Yin et al., 2005). Purified Sfp enzyme was kindly provided by Dr Erin Cutts, and the labeling of β-catenin-ybbR was performed as described (Yin et al., 2006) or with the following modifications. Alexa Fluor® 680 maleimide (# A20344, Thermo Scientific) at 2.5 mM was conjugated to 0.5 mM Coenzyme A (# C3019, Sigma-Aldrich) in 100 mM sodium phosphate pH 7.0 reaction buffer, with five-fold excess of fluorophore to ensure near-complete conjugation of CoA. The reaction was incubated in the dark for 2 h at room temperature, and unconjugated Alexa Fluor® 680 maleimide was quenched with 12.5 mM DTT and incubation for a further 15 min. The entire sample was used in the following β-catenin labeling reaction: CoA conjugation reaction with ≈0.5 mM Alexa Fluor® 680-CoA, 26 μM β-catenin-ybbR, 6 μM Sfp enzyme and 10 mM MgCl2. The reaction was performed in the dark for 1.5 h at room temperature, and reaction components were separated from labeled β-catenin-Alexa Fluor® 680 by size exclusion chromatography on a HiLoad 16/60 Superdex 200 column (prep grade, GE Healthcare) equilibrated in 50 mM HEPES-NaOH pH 7.5, 150 mM NaCl, 2 mM TCEP, 5% glycerol. Pure protein fractions were pooled, concentrated and flash-frozen in liquid nitrogen.
Purification of the destruction complex and subcomplexes
Sf9 cell pellets were resuspended in 50 mM HEPES-NaOH pH 7.5, 150 mM NaCl, 10 mM β-mercaptoethanol, 5% glycerol, 10 mM β-glycerophosphate, 1 mM AEBSF and Pierce protease inhibitor tablets, EDTA-free. Lysates were briefly sonicated and centrifuged at 25,000 x g for 45 min to remove insoluble material. Cleared lysates were incubated in batch with 1 – 2 mL of pre-equilibrated Strep-Tactin Sepharose resin (IBA Lifesciences) at 4°C rotating for 2 h. Resin was washed with > 50 CV of wash buffer (lysis buffer lacking protease and phosphatase inhibitors), and protein complexes were eluted with wash buffer supplemented with 50 mM d-biotin. Attempts to concentrate the purified destruction (sub-) complexes resulted in their precipitation. To obtain more concentrated samples, the protein complexes were eluted with biotin, which competes with the dStrepII tag more efficiently than desthiobiotin on the Strep-Tactin resin. The eluted peak fractions were pooled and dialysed overnight at 4°C against 50 mM HEPES-NaOH pH 7.5, 150 mM NaCl, 2 mM TCEP, 5% glycerol, then flash-frozen in liquid nitrogen.
SCFβ-TrCP E3 ligase complex purification and in-vitro neddylation
High-Five cell pellets were resuspended in a buffer containing 50 mM HEPES-NaOH pH 7.5, 200 mM NaCl, 10 mM β-mercaptoethanol, 0.1% NP-40, 5% glycerol and Pierce protease inhibitor tablets, EDTA-free. Lysates were briefly sonicated, centrifuged at 45,000 x g for 45 min to remove insoluble material and filtered through a 0.45-μm filter. The cleared lysate was loaded onto a 5-mL StrepTrap HP column (GE Healthcare), washed with 20 CV of wash buffer (lysis buffer lacking NP-40 and protease inhibitors), and the protein complex was eluted with 5 mM d-desthiobiotin. The peak protein fractions were pooled and subjected to size exclusion chromatography on a HiLoad 16/600 Superose 6 column (prep grade, GE Healthcare), equilibrated in 50 mM HEPES-NaOH pH 7.5, 200 mM NaCl, 2 mM TCEP, 5% glycerol. The elution fractions containing all the components of the SCFβ-TrCP E3 ligase complex were pooled, concentrated and flash-frozen in liquid nitrogen. Elution fractions from a second peak corresponding to a complex without β-TrCP were processed likewise.
The in-vitro neddylation was performed in the following reaction: 8 μM SCFβ-TrCP E3 ligase complex, 350 nM APPBP1-Uba3 (# PRT112, Enzo Lifesciences), 1.8 μM UbcH12 (# UW9145, Enzo Lifesciences), 10 μM His6-NEDD8 (# UW9225, Enzo Lifesciences), 10 mM MgCl2 and 1.25 mM ATP. The reaction was incubated for 10 min at 37°C and stopped by the addition of DTT at a final concentration of 15 mM. A Strep-affinity chromatography on a 5-mL StrepTrap HP column was performed to remove the reaction components from the neddylated E3 ligase complex. The bound material was washed with 20 CV of wash buffer (50 mM HEPES-NaOH pH 7.5, 200 mM NaCl, 2 mM TCEP, 5% glycerol) and eluted with 5 mM d-desthiobiotion. Elution fractions containing neddylated SCFβ-TrCP E3 ligase were pooled, concentrated and flash-frozen in liquid nitrogen.
E1 and E2 purifications
Purified recombinant human E1 enzyme (UBA1, ubiquitin-like modifier-activating enzyme 1), expressed in E. coli, was generously provided by Dr Aylin Morris-Davies and Dr Edward Morris. The bacterial expression plasmid for the human E2 ubiquitin-conjugating enzyme (pProEx His6-TEV- UBCH5c) was provided by Dr Frank Sicheri (Chou et al., 2012). Human UBCH5c was expressed as His6-TEV fusion protein in E. coli BL21-CodonPlus(DE3)-RIL (# 230245, Agilent) grown in 2 L of LB media. Expression was induced at an OD600 of 0.6 with 1 mM IPTG overnight at 18°C. Cells were harvested by centrifugation, resuspended in a buffer containing 50 mM HEPES-NaOH pH 7.5, 150 mM NaCl, 5 mM imidazole, 10 mM β-mercaptoethanol, EDTA-free Pierce protease inhibitor tablets, lysed by sonication and centrifuged at 45,000 x g for 45 min to remove insoluble material. Lysates were filtered through a 0.45-μm filter and loaded onto a 5-mL HisTrap HP affinity column (GE Healthcare). The bound material was washed with 20 CV of wash buffer (lysis buffer lacking protease inhibitors). His6-TEV-UBCH5c was eluted with a linear imidazole gradient (5 to 250 mM imidazole) in a buffer containing 50 mM HEPES-NaOH 7.5, 150 mM NaCl and 5 mM β-mercaptoethanol. The peak fractions containing relatively pure protein were pooled and dialysed overnight at 4°C against 50 mM HEPES-NaOH pH 7.5, 150 mM NaCl and 2 mM TCEP, concentrated and flash-frozen in liquid nitrogen.
SEC-MALS
Affinity-purified DCs (wild-type and a mutant variant containing polymerization-deficient AXIN1 M3 (Fiedler et al., 2011)) were quantified by UV spectroscopy at 280 nm (A280). Given the unknown stoichiometry of the complexes, an accurate determination of their concentrations was not possible as extinction coefficients could not be reliably estimated. Therefore, serial dilutions of the complexes were made on the basis of A280. 90 μL samples of different dilutions were resolved by size exclusion chromatography on an Agilent 1260 Infinity II HPLC system with a WTC-100N5 column (Wyatt Technology). The separation was performed in 50 mM HEPES-NaOH pH 7.5, 150 mM NaCl, 2 mM TCEP, 5% glycerol at a flow rate of 0.25 mL/min with collection of 100 μL elution fractions. 25 μL of elution fractions and 15 μL of the DC complexes, representing one sixth of input material, were analyzed by SDS-PAGE. Light scattering and differential refractive index (dRI) of the samples were analyzed in-line using the DAWN® and Optilab instruments from Wyatt Technologies, respectively. Data analysis, using the Zimm light scattering model and a dn/dc of 0.185 mL/g, was performed using Wyatt’s ASTRA software version 7.3.1. Weight average molecular weights (Mw), number average molecular weights (Mn) and dispersities (Ð) with their associated uncertainties for the elution peak areas were obtained through the ASTRA software data analysis (see ASTRA 7 User’s Guide for details). See the “In-silico stoichiometry modelling of the DC and estimation of DC molar concentrations” section below for details on estimating DC concentrations in input samples and eluates.
Assessment of SCFβ-TrCP E3 ligase interactions with DC components
Size exclusion chromatography of affinity-purified SCFβ-TrCP E3 ligase (as described above) resulted in the separation of fully assembled SCFβ-TrCP E3 ligase and a distinct subpopulation of SCF E3 ligase lacking β-TrCP (SCFw/o β-TrCP E3 ligase). All subsequent binding analyses were performed in buffer containing 50 mM HEPES-NaOH pH 7.5, 200 mM NaCl, 2 mM TCEP. Purified DC components APC, AXIN1 WT or β-catenin at 1.5 μM were incubated with 1.5 μM of either SCFβ-TrCP E3 ligase or SCFw/o β-TrCP E3 ligase for 30 min on ice. For each individual set of samples, i.e., APC - SCFβ-TrCP E3 ligase and APC - SCFw/o β-TrCP E3 ligase, the individual components were also incubated with equal volumes of the respective protein buffer instead of the component being omitted. As a positive control, 1.5 μM β-catenin was phosphorylated using 75 nM AXIN1-CK1α-GSK3β complex in the presence of 5 mM MgCl2 and 1 mM ATP, by incubating at 25°C for 60 min and terminating the reaction by addition of 10 mM EDTA. A similar reaction lacking ATP was conducted in parallel as a negative (non-phosphorylated β-catenin) control.
90 μL of the samples were resolved by size exclusion chromatography on an Agilent 1260 Infinity II HPLC system with a WTC-50N5 column (Wyatt Technology). The setup, including detectors and fraction collector, was the same as for the SEC-MALS experiments described above. The separation was performed in 50 mM HEPES-NaOH pH 7.5, 200 mM NaCl, 2 mM TCEP at a flow rate of 0.25 mL/min with collection of 100 μL elution fractions. 10 μL of elution fractions were resolved by SDS-PAGE (4 – 12% gradient Bis-Tris polyacrylamide gels (Life Technologies)), and proteins were detected by silver staining using the Pierce™ Silver stain kit. Separately, one sixth of input materials (15 μL) was analyzed by SDS-PAGE and Coomassie staining. Phosphorylation of β-catenin was detected through immunoblot analysis of the input samples and elution fractions using anti-β-catenin and anti-phospho-β-catenin pS33, pS37, pT41 antibodies.
β-catenin phosphorylation and ubiquitylation reactions
The concentrations of proteins added to the reactions were determined by UV spectroscopy (A280) using theoretical extinction coefficients calculated using the ProtParam tool in ExPASy (Gasteiger et al., 2005) for the fully reduced forms of the proteins. For the AXIN1-kinase complex, we assumed a 1:1:1 AXIN1:CK1α:GSK3β stoichiometry to calculate the theoretical extinction coefficient. All reactions were performed in the following reaction buffer: 50 mM sodium phosphate pH 7.0, 150 mM NaCl, 1 mM TCEP. Salt contributions from the proteins added to the reactions were less than 50 mM NaCl. Master mixes were made of reaction components whenever possible to facilitate reaction assembly and to minimize variability between individual reactions. Complete reactions of 30 μL were prepared on ice from three components; the concentrations listed refer to the final concentrations in the 30 μL reactions: I. Ubiquitin mix (10 μL per reaction): reaction buffer containing 10 μM recombinant human ubiquitin (# U-100H, Boston Biochem.), 1 μM N-terminally fluorescein-labeled human ubiquitin (# U-580, Boston Biochem.), 50 nM E1 (human UBA1), 1 μM E2 (UBCH5c), 10 mM MgCl2, 5 mM ATP added just before starting the reactions. II. DC components (10 μL per reaction): reaction buffer lacking NaCl (due to the high NaCl contribution from the APC protein stocks) containing 1 μM β-catenin-Alexa Fluor® 680, 200 nM APC, 150 nM AXIN1-kinase complex. Equal volumes of different APC proteins and AXIN1-kinase complex variants were added to reactions, respectively, by diluting the more highly concentrated stocks such that the stocks had the same concentrations as that which was least concentrated among them. Dilution ensured equal salt carryover between different reactions. In addition, equal volumes of corresponding protein buffers were used when proteins were omitted from the reaction. GSK3 inhibitor CHIR-99021 (# 361571, Sigma-Aldrich) or CK1 inhibitor D4476 (# 218696, Sigma-Aldrich) were added at this step, at the final concentrations stated in the figure legends; vehicle solution was used in control reactions. III. E3 ligase complex (10 μL per reaction): 100 nM SCFβ-TrCP E3 ligase complex in reaction buffer. The reaction parts II and III were combined and incubated at room temperature for 10 min. Likewise, reaction part I was also prepared, but without ATP, and incubated at room temperature for 10 min. The reaction was started by adding ATP to reaction part I and combining it with II and III. Reactions were incubated under agitation (500 rpm) at 25°C in a Thermomixer (Eppendorf) and terminated at the specified time points by adding 9 μL of the progressing reaction mixture to 9 μL of 2x SDS-PAGE loading buffer and heating to 95°C for 5 min. Typically, 7 μL of boiled samples were resolved on 4 – 12% gradient Bis-Tris polyacrylamide gels (Life Technologies) and imaged for β-catenin-Alexa Fluor® 680, fluorescein-ubiquitin and the protein standards on a Typhoon FLA 9500 biomolecular imager (GE Healthcare). Signals were quantified using ImageQuant software (GE Healthcare). The protein standards (# 1610373, Bio-Rad) were imaged at 635 nm excitation wavelength, which to a lesser extent also triggers Alexa Fluor® 680 fluorescence. The raw image contained β-catenin-Alexa Fluor® 680 signals obtained at 635 nm and 685 nm excitation wavelengths. The β-catenin-Alexa Fluor® 680 signals obtained at 635 nm excitation wavelength were masked, leaving only the lane showing the protein standards. The β-catenin-Alexa Fluor® 680, fluorescein-ubiquitin and the protein standards images were merged back to obtain the final multicolor images presented. Where downstream immunoblot analyses were performed, the same gels were subsequently used to transfer the proteins onto nitrocellulose membranes. These were scanned for β-catenin-Alexa Fluor® 680 on the Typhoon imager before being processed for immunoblot detection and analyzed on an Odyssey infrared imaging system (LI-COR).
In-vitro phosphorylation and de-phosphorylation
The reactions were set up with 40 μL of affinity-purified DCs (A280 of 0.4) in a final reaction volume of 45 μL. For the phosphorylation reactions, affinity-purified DCs were incubated with 10 mM MgCl2, 5 mM ATP for 30 min at 25°C. Reactions were terminated by adding 15 μL of 4x SDS-PAGE loading buffer, followed by heating to 95°C for 5 min. Where indicated, affinity-purified DCs were de-phosphorylated in a reaction containing 2 mM MnCl2 and 2 μM λ-phosphatase for 30 min at 25°C and reactions terminated by adding 15 μL of 4x SDS-PAGE loading buffer, followed by heating to 95°C for 5 min.
β-catenin proteasomal degradation
In-vitro β-catenin ubiquitylation reactions of 30 μL were set up as described above (β-catenin phosphorylation and ubiquitylation reactions) in the presence of 5 μM GSK3 inhibitor and incubated for 60 min under agitation (500 rpm) at 25°C. Immediately afterward, reactions were placed on ice. 5 μL of each reaction were mixed with 5 μL of 2x SDS-PAGE loading buffer and heated to 95°C for 5 min; these samples served as input references for the subsequent proteasome reactions. The β-catenin proteasomal degradation was performed in the following buffer: 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 2 mM ATP, 5 mM MgCl2, 1 mM TCEP, 0.2 mg/mL BSA, 2% glycerol supplemented with 1x ATP regeneration solution (# BML-EW9810, Enzo Lifesciences) and 25 nM human 26S proteasome (# e-365, Boston Biochem). Control reactions were further supplemented with 10 μM MG132 (# M7449, Sigma-Aldrich) in the presence or absence of 26S proteasome. 5 μL of each ubiquitylation reaction were distributed over four microcentrifuge tubes and incubated in a total reaction volume of 15 μL under agitation (500 rpm) at 25°C. The reactions were stopped after 1, 2 and 3 h by adding 5 μL of 4x SDS-PAGE loading buffer and heating to 95°C for 5 min. The samples were resolved on 4 – 12% gradient Bis-Tris polyacrylamide gels (Life Technologies) and imaged for β-catenin-Alexa Fluor ® 680 and fluorescein-ubiquitin on a Typhoon FLA 9500 biomolecular imager (GE Healthcare). Signals were quantified using ImageQuant software (GE Healthcare).
Radiometric kinetic kinase assays
All reactions were performed in the following reaction buffer: 50 mM HEPES-NaOH pH 7.0, 150 mM NaCl, 10 mM MgCl2, 1 mM TCEP at room temperature. Reactions were initiated by adding 200 μM unlabelled ATP with 0.9 μCi γ-32P-ATP (# SCP-301, Hartmann Analytics) or serial dilutions of this ATP mix. Master mixes were made of reaction components whenever possible to facilitate reaction assembly. Assays were set up in a 96-well PCR plate using a multichannel pipette to ensure simultaneous initiation and termination of parallel reactions. Each 30 μL reaction in either an ATP titration or β-catenin titration experiment was set up from two components as specified below; the concentrations listed refer to the final concentrations in the 30 μL reactions:
ATP titration reactions
I. ATP mixture (6 μL per reaction): ATP buffer (50 mM HEPES-NaOH pH 7.0, 150 mM NaCl, 1 mM TCEP) containing 200 μM unlabelled ATP and 0.9 μCi γ-32P-ATP. A two-fold serial dilution of a 200-μM ATP master mix was made in ATP buffer with each dilution volume sufficient for at least 2.5 reactions; ATP concentrations were 200, 100, 50, 12.5, 6.26, 3.125, 1.56, 0.78 μM.
II. DC components mixture (24 μL per reaction): reaction buffer lacking NaCl (due to the high NaCl contribution from the APC protein) containing 1 μM β-catenin, 200 nM APC (or an equal volume of APC protein buffer added to reactions without APC), 150 nM AXIN1-kinase complex, 10 mM MgCl2 and incubated for 10 min at room temperature.
6 μL of ATP mixture were placed in individual wells on a 96-well PCR plate; a separate set of wells contained 29 μL of the DC components mixture.
β-catenin titration reactions
I. ATP mixture (6 μL per reaction): ATP buffer (50 mM HEPES-NaOH pH 7.0, 150 mM NaCl, 1 mM TCEP) containing 200 μM unlabelled ATP and 0.9 μCi γ-32P-ATP.
II. DC components mixture (24 μL per reaction): reaction buffer lacking NaCl (due to the high NaCl contribution from the APC protein) and containing 200 nM APC (or an equal volume of APC protein buffer added to reactions without APC), 150 nM AXIN1-kinase complex, 10 mM MgCl2 and serial dilutions of β-catenin (15, 10, 7.5, 5, 2.5, 1.0, 0.5, 0.25 μM). A master mix of the reaction components without β-catenin was placed in 96-well PCR plates, each well containing solution sufficient for 1.25 reactions. Subsequently, β-catenin from serial dilutions was mixed into the same wells, completing the DC component mixture.
6 μL of ATP mixture were placed in individual wells on a 96-well PCR plate.
To start the reactions, 24 μL of the DC components mixture was transferred into the wells containing the ATP mixture using a multichannel pipette and mixed by pipetting 3 times before starting the time course. Using a multichannel pipette, the reactions were terminated at specific time points (0.5, 1, 2 and 5 min) by pipetting 6 μL of the reaction volume into wells containing 6 μL of 2x SDS-PAGE loading buffer. At the end of the experiment, the samples were transferred to microcentrifuge tubes and heated to 95°C for 5 min. For the ATP titration reactions, the complete contents of the boiled samples were resolved on 4 – 12% gradient Bis-Tris polyacrylamide gels. For the β-catenin titrations, the boiled reaction samples containing more than 1 μM β-catenin were diluted with 1x SDS-PAGE loading buffer to prevent gel overloading. The samples were diluted to obtain a similar loading concentration of β-catenin as in the boiled sample for the 1.0 μM reaction. 12 μL of the diluted, boiled samples for the 15, 10, 7.5, 5, 2.5 μM reactions and the complete boiled samples from the other reactions (12 μL) were resolved on 4 – 12% gradient Bis-Tris polyacrylamide gels. All polyacrylamide gels were stained with Coomassie prior to drying on chromatography paper (Whatman). To enable quantification of the extent of protein phosphorylation, two-fold serial dilutions of the left-over ATP mixture containing unlabelled ATP and γ-P32-ATP were made for each experiment and specific amounts of the ATP mixture (12.5, 6.25, 3.125, 1.563, 0.781, 0.390, 0.195 pmol) were spotted onto nitrocellulose membrane strips and air-dried. For each experiment, the dried polyacrylamide gels and the corresponding nitrocellulose membrane strips were simultaneously exposed to a phosphorimaging screen (GE Healthcare) and scanned on a Typhoon FLA 9500 biomolecular imager (GE Healthcare).
Control reactions to validate the β-catenin-4A phospho mutant (S33A, S37A, T41A, S45A) and assess GSK3β serine 9 phosphorylation were performed as described above with the following changes: reactions contained 200 μM unlabelled ATP and 0.9 μCi γ-32P-ATP (for radiometric reactions only), 1 μM β-catenin and 200 nM APC. The reactions were terminated after 5 min by pipetting 6 μL of the reaction volume into wells containing 6 μL of 2x SDS-PAGE loading buffer.
The phosphorimaging files were analyzed using ImageJ (Fiji) (Schindelin et al., 2012). The raw files (.gel), which use a square root algorithm for signal compression, were normalized prior to gel densitometry analysis using the ImageJ “Linearize GelData” plugin (https://rsb.info.nih.gov/ij/plugins/linearize-gel-data.html). Background-corrected signal intensities of phosphorylated (32P) β-catenin were converted into picomoles using the standard curve generated from the background-corrected signal intensities of known ATP (γ-32P-ATP) quantities in serial dilutions.
The initial reaction rates (pmol/min) were obtained from the slopes of linear fits to data describing the amounts of generated product (pmol phosphorylated β-catenin) over time (min) at the different concentrations of either ATP or β-catenin. The initial rates were plotted as a function of [ATP] or [β-catenin] by nonlinear least-squares regression fit and kinetic parameters, Vmax (pmol/μL), KM (μM) and kcat (min-1) determined using the Michaelis-Menten equation:
To calculate kcat, the total enzyme concentration [ET] in the reactions needs to be known. The precise kinase concentrations in the AXIN1-CK1α-GSK3β complexes are not known in the experimental setup. Therefore, the concentration of the complete AXIN1-kinase complex was used as simplification, giving an [ET] of 0.9 pmol based on 6 μL of 150 nM AXIN1-kinase complex. (Note that as per convention, [ET] is not entered as a concentration but as an amount.) Standard errors for Vmax, KM and kcat reported in Figure 5C were derived from the non-linear least-squares fits to the Michaelis-Menten equation. For the β-catenin reactions in the absence of APC, which did not saturate, the catalytic efficiencies (kcat/KM) were obtained from the linear fit to the plots of the initial rates against [β-catenin], and the associated standard errors were derived from the linear regression fit. For reactions reaching saturation, standard errors for kcat/KM were propagated from individual kcat and KM values using the following equation:
= kcat
= KM
= kcat/KM
= standard error
Mass photometry
Instrument setup
Microscope coverslips (High Precision, No. 1.5, 24 50 mm, # 630-2187, VWR) were cleaned twice, sequentially with Milli-Q water, 100% isopropanol, Milli-Q water. Washed coverslips were dried with compressed air. Reusable silicone CultureWell™ gaskets (# GBL103250, Sigma-Aldrich) were cleaned sequentially with Milli-Q water, 100% Isopropanol, Milli-Q water and dried with compressed air. Cleaned gaskets were placed on the cleaned coverslips and mounted on the sample stage of a Refeyn OneMP mass photometer (Refeyn Ltd, Oxford, UK). All measurements were performed in acquisition buffer containing 50 mM HEPES-NaOH pH 7.5, 150 mM NaCl and filtered through a 0.2-μm filter. Each measurement was performed in a separate well. Data were acquired using the Refeyn AcquireMP 2.3.1 software. Mass photometry movies of 30,000 frames were recorded from a 10 10 μm instrument field of view at a frame rate of 362.4 Hz with a frame binning value of 4, resulting in an effective frame rate of 90.6 Hz. For each acquisition, 15 μL of acquisition buffer were pipetted into a well, and the focal position was identified and secured in place using the autofocus functionality in the software.
Sample preparation and acquisition
Same-day, affinity-purified AXIN1 wild-type and AXIN1 M3 DCs at A280 = 0.84 and 0.86, respectively, were subjected to SEC-MALS analysis under conditions similar to those reported above. For accurate detection of single events during data acquisition, the recommended final protein concentration in the acquisition wells should be below 100 nM (Sonn-Segev et al., 2020). As stated above, the precise concentration of the DCs could not be reliably estimated. By determining the A280 values of the SEC-MALS input samples and peak elution fractions and performing several test acquisitions, a sample concentration corresponding to an A280 of ≈0.01 in the acquisition wells enabled detection of individual single events below saturation levels during acquisition. For instance, 2 μL of a 1:10 dilution in acquisition buffer of the input samples and 2 to 3 μL of undiluted elution fractions were used for the acquisitions. Input samples were diluted just prior to each respective data acquisition. Upon addition of the samples to the wells, data acquisition was started after 10 s to allow for autofocus stabilization.
Data processing
Data were processed and analyzed using Refeyn DiscoverMP 2.3.0 software by performing three main steps: (1) background removal, (2) identification of particle landing events on the coverslip acquisition field, and (3) particle fitting to extract maximum contrast. The following default fitting parameters were used; threshold 1 (related to the particle contrast relative to the background noise) was set to 1.5, threshold 2 (related to the radial symmetry of the particle signature) was set to 0.25, and 5 ratiometric frames were binned. Individual particle contrasts from each individual movie were converted to mass using a contrast-to-mass (C2M) calibration. From these data, kernel density estimates (KDEs) were generated for each sample using a Gaussian kernel with a fixed bandwidth of 25 kDa.
Calibration procedure: Contrast-to-mass calibration was performed in the acquisition buffer. NativeMark™ unstained protein standard (# LC0725, Thermo Scientific), which contains proteins in the range of 20 to 1200 kDa, was diluted 1:100 in acquisition buffer, and 2.5 μL were added to an acquisition well for measurement. The following masses were used to generate a standard calibration curve in the DiscoverMP software: 66, 146, 480 and 1048 kDa.
In-silico stoichiometry modeling of the DC and estimation of DC molar concentrations
Inputs
The following inputs were used for modeling of DC stoichiometries:
-
i)
Predicted molecular weights and extinction coefficients (ε) of the individual proteins in the DC, from Expasy ProtParam (Gasteiger et al., 2005). As protein complexes can have similar molecular weights without sharing similar ε, by taking ε into account, we were able to narrow down the modeled stoichiometries.
-
ii)
Means of molecular weights obtained from individual SEC-MALS data slices (Mi) for the DC with AXIN1 WT or DC with AXIN1 M3 across the peak (DC AXIN1 WT: 2.3 – 3.0 mL; DC AXIN1 M3: 2.5 – 3.2 mL). Mean molecular weights are number average molecular weights (Mn).
-
iii)
Mean molar extinction coefficients (in M-1 cm-1) of the DC calculated from the parameters recorded by SEC-MALS for individual data slices as follows:
The differential refractive index (dRI) of a sample solution relative to the solvent is directly proportional to the sample concentration. Hence, the sample concentration (c) can be calculated from the dRI measurements and the protein-specific refractive index increment dn/dc of 0.185 mL g-1 as follows:
With the concentration determined and converted into g L-1, the extinction coefficient (ε) in L g-1 cm-1 is calculated using the Beer-Lambert law equation:
where is absorbance measured at 280 nm (A280) in absorbance units (AU), and is length of light path (1 cm). ε is then converted into the molar units (M-1 cm-1) by multiplying ε with the calculated molar masses (g mol-1) from MALS.
Performing these calculations across the elution peak, we obtained the concentrations of the DC at the detector in SEC-MALS for each data slice for the SEC-MALS experiment shown in Figure 1E. The maximum concentration measured for the DC with wild-type AXIN1 was 56 nM; that for the DC with M3 AXIN1 was 94 nM. We also calculated the average ε of the DC (2,024,161 ± 367,667 M-1 cm-1 for the DC with wild-type AXIN1 and 980,087 ± 275,179 M-1 cm-1 for that with M3 AXIN1; variance, SD). This enabled us to estimate the molar concentrations of the DC in the SEC-MALS input samples: 415 nM for the DC with wild-type AXIN1, and 877 nM for that with M3 AXIN1. Note, however, that due to the highly concentration-dependent composition of the DC, the molar extinction coefficients will also vary with concentration, especially for the DC containing wild-type AXIN1.
Conditions
The DC stoichiometries are modeled on the conditions that:
-
1
DC components are APC, AXIN1, CK1α, GSK3β and β-catenin.
-
2
The molecular weight of the DC is the sum of molecular weights of its components at a given stoichiometry.
-
3
The extinction coefficient of the DC is the sum of the individual values of its components at a given stoichiometry.
-
4
Molecular weight and of the DC at the modeled stoichiometry must satisfy the input values ± 1.96 standard deviations from the mean values calculated from SEC-MALS (95% confidence interval).
-
5
At least one of each component is present in the complex.
-
6
The number of molecules in the DC takes the form of a positive integer.
-
7
DC stoichiometries are modeled by incrementing the number of molecules of each component by 1 in the order of conditions 8 – 14.
-
8
At least two AXIN1 molecules are present in the DC with wild-type AXIN1, as we observe that AXIN1 polymerization is a major DC size determinant.
-
9
CK1α and GSK3β occupancy on AXIN1 is at least 50%, and at most both kinases are bound to AXIN1. This is to mitigate possible modeling errors in the case of incomplete occupancy of kinase binding sites on AXIN1.
-
10
Each APC binds at least one and a maximum of ten β-catenin molecules through four 15R and six 20R repeats, as 20R2 does not bind β-catenin (Liu et al., 2006).
-
11
Each APC binds one to three AXIN1 molecules via three available SAMP repeats.
-
12
At least one molecule of β-catenin is bound to each AXIN1 or APC molecule present in the complex.
-
13
For a given AXIN1:APC stoichiometry, at least one more molecule of β-catenin is present in the DC containing wild-type AXIN1 compared to the DC containing AXIN1 M3.
-
14
The number of β-catenin molecules in the DC with AXIN1 M3 does not exceed the number of β-catenin molecules in the DC with wild-type AXIN1.
Conditions 9 and 12-14 are based on the SDS-PAGE analysis of affinity-purified material and the elution fractions from SEC-MALS. As a consequence of condition 9, the number of β-catenin molecules can increase if AXIN1 is not saturated with kinases.
The algorithm first imports the inputs, sets the constraints on the stoichiometries that can be modeled and starts from the minimum number of AXIN1 molecules (AXIN1 M3 n = 1, AXIN1 WT n = 2). For each AXIN1 molecule, the algorithm then explores all possible combinations of the DC in the order of conditions specified above. Modeled stoichiometries which satisfy all of the set theoretical limits on the APC:AXIN1:kinases:β-catenin ratios and attain a molecular weight and ε falling within the range calculated from SEC-MALS are written in the final output file. The molecular weights plotted in Figure 1G correspond to number average molecular weights (Mn). The Python scripts for DC stoichiometry modeling are available for download as Data S1 in the online version of the article.
Antibodies and reagents
Primary antibodies used were anti-β-catenin (# 610153, BD Transduction Laboratories), anti-phospho-β-catenin pS33, pS37, pT41 (# 9561, Cell Signaling Technology), anti-non-phospho-β-catenin S33, S37, T41 (#8814, Cell Signaling Technology), anti-phospho-β-catenin pS45 (# 9564, Cell Signaling Technology), anti-StrepMAB-Classic (# 2-1507-001, IBA Life Sciences) to detect dStrepII-AXIN1, anti-AXIN1 (# 2087, Cell Signaling Technology), anti-APC (# NB100-667, Novus Biologicals), anti-GSK3β (# 12456, Cell Signaling Technology), anti-phospho-GSK3β pS9 (# 5558, Cell Signaling Technology), anti-ubiquitin K48 linkage-specific (# ab140601, Abcam), anti-ubiquitin K63 linkage-specific (# ab179434, Abcam), anti-ubiquitin P4D1 clone (# BML-PW0930, Enzo Lifesciences). Secondary antibodies for immunoblotting with detection using the Odyssey infrared imaging system (LI-COR) were IRDye 800CW donkey anti-mouse (# 926-32212, LI-COR), IRDye 800CW donkey anti-rabbit (# 926-32213, LI-COR), IRDye 680RD donkey anti-mouse (# 926-68072, LI-COR). Recombinant human poly-ubiquitin chains were obtained from commercial source: K63-linked chains (# UC-330, R&D Systems), K48-linked chains (# UC-230, R&D Systems).
Quantification and statistical analysis
Various β-catenin signals from in-vitro ubiquitylation reactions were analyzed using ImageQuant software (GE Healthcare). Phosphorylated β-catenin levels from in vitro γ-32P-ATP kinetic reactions were analyzed using ImageJ (Fiji) 1.53c software, and the kinetic parameters were determined as described above using GraphPad Prism 8.2.1 software. All statistical analyses were performed using GraphPad Prism 8.2.1 software. Statistical significance was calculated using either an unpaired Student’s t test or one-way ANOVA analysis with Bonferroni test for multiple comparisons. p < 0.05 was considered significant.
Acknowledgments
We thank Gideon Coster and Jerome Gouge for λ-phosphatase, Erin Cutts for Sfp1, Aylin Morris-Davies and Edward Morris for UBA1, and Frank Sicheri for the UBCH5C expression construct. We thank Jan-Michael Peters (IMP, Vienna, Austria) for providing the biGBac system. We thank Refeyn, particularly Tomás de Garay, for help with mass photometry. We thank Elton Zeqiraj, Sonja Lorenz, Gideon Coster, and members of the S.G. team for helpful comments on the manuscript. Work in S.G.’s laboratory has been funded by Cancer Research UK, first through a Career Establishment Award (C47521/A16217), followed by a Programme Foundation Award (C47521/A28286), by the Wellcome Trust through an Investigator Award (214311/Z/18/Z), by the Lister Institute of Preventive Medicine through a Lister Institute Research Prize Fellowship, and by the Institute of Cancer Research (ICR).
Author contributions
S.G. conceived the project with input from M.R. S.G. and M.R. planned and M.R. conducted all experiments. M.Z. expressed and purified the E3 complex. S.S. performed the in silico DC stoichiometry analysis. R.K. cloned the DC expression construct. S.G. and M.R. wrote the manuscript with contributions from all authors.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science.
Published: August 4, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.molcel.2021.07.013.
Supporting citations
The following references appear in the Supplemental information: Morrone et al. (2012).
Supplemental information
Data and code availability
-
•
Original data and gel/Western blot images have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is listed in the Key resources table.
-
•
All original code is available in this paper’s supplemental information (Data S1).
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
References
- Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque C., Breukel C., van der Luijt R., Fidalgo P., Lage P., Slors F.J., Leitão C.N., Fodde R., Smits R. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum. Mol. Genet. 2002;11:1549–1560. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- Amit S., Hatzubai A., Birman Y., Andersen J.S., Ben-Shushan E., Mann M., Ben-Neriah Y., Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Barker N., Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- Barua D., Hlavacek W.S. Modeling the effect of APC truncation on destruction complex function in colorectal cancer cells. PLoS Comput. Biol. 2013;9:e1003217. doi: 10.1371/journal.pcbi.1003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Jerchow B.A., Würtele M., Grimm J., Asbrand C., Wirtz R., Kühl M., Wedlich D., Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Berman H.M., Battistuz T., Bhat T.N., Bluhm W.F., Bourne P.E., Burkhardt K., Feng Z., Gilliland G.L., Iype L., Jain S. The Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- Bieniossek C., Richmond T.J., Berger I. MultiBac: multigene baculovirus-based eukaryotic protein complex production. Curr. Protoc. Protein Sci. 2008;Chapter 5 doi: 10.1002/0471140864.ps0520s51. Unit 5.20. [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Bugter J.M., Fenderico N., Maurice M.M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat. Rev. Cancer. 2021;21:5–21. doi: 10.1038/s41568-020-00307-z. [DOI] [PubMed] [Google Scholar]
- Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.H., Estarás C., Moresco J.J., Yates J.R., 3rd, Jones K.A. α-Catenin interacts with APC to regulate β-catenin proteolysis and transcriptional repression of Wnt target genes. Genes Dev. 2013;27:2473–2488. doi: 10.1101/gad.229062.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y.-C., Keszei A.F.A., Rohde J.R., Tyers M., Sicheri F. Conserved structural mechanisms for autoinhibition in IpaH ubiquitin ligases. J. Biol. Chem. 2012;287:268–275. doi: 10.1074/jbc.M111.316265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Loh K.M., Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- Dajani R., Fraser E., Roe S.M., Yeo M., Good V.M., Thompson V., Dale T.C., Pearl L.H. Structural basis for recruitment of glycogen synthase kinase 3beta to the axin-APC scaffold complex. EMBO J. 2003;22:494–501. doi: 10.1093/emboj/cdg068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani R., Fraser E., Roe S.M., Young N., Good V., Dale T.C., Pearl L.H. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–732. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Eklof Spink K., Fridman S.G., Weis W.I. Molecular mechanisms of beta-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC-beta-catenin complex. EMBO J. 2001;20:6203–6212. doi: 10.1093/emboj/20.22.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler M., Mendoza-Topaz C., Rutherford T.J., Mieszczanek J., Bienz M. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating β-catenin. Proc. Natl. Acad. Sci. U S A. 2011;108:1937–1942. doi: 10.1073/pnas.1017063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S., Cohen P., Biondi R.M. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- Gammons M., Bienz M. Multiprotein complexes governing Wnt signal transduction. Curr. Opin. Cell Biol. 2018;51:42–49. doi: 10.1016/j.ceb.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M.R., Appel R.D., Bairoch A. In: The Proteomics Protocols Handbook. Walker J.M., editor. Humana Press; 2005. Protein identification and analysis tools on the ExPASy server; pp. 571–607. [Google Scholar]
- Gavagan M., Fagnan E., Speltz E.B., Zalatan J.G. The scaffold protein Axin promotes signaling specificity within the Wnt pathway by suppressing competing kinase reactions. Cell Syst. 2020;10:515–525.e5. doi: 10.1016/j.cels.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha N.-C., Tonozuka T., Stamos J.L., Choi H.-J., Weis W.I. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol. Cell. 2004;15:511–521. doi: 10.1016/j.molcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Hamada F., Tomoyasu Y., Takatsu Y., Nakamura M., Nagai S., Suzuki A., Fujita F., Shibuya H., Toyoshima K., Ueno N., Akiyama T. Negative regulation of Wingless signaling by D-axin, a Drosophila homolog of axin. Science. 1999;283:1739–1742. doi: 10.1126/science.283.5408.1739. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Kishida S., Yamamoto H., Murai H., Koyama S., Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Kan W., Enos M.D., Korkmazhan E., Muennich S., Chen D.-H., Gammons M.V., Vasishtha M., Bienz M., Dunn A.R., Skiniotis G., Weis W.I. Limited dishevelled/Axin oligomerization determines efficiency of Wnt/β-catenin signal transduction. eLife. 2020;9:531. doi: 10.7554/eLife.55015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-E., Huang H., Zhao M., Zhang X., Zhang A., Semonov M.V., MacDonald B.T., Zhang X., Zhang X., Garcia Abreu J. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Xu W. Beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- Kishida S., Yamamoto H., Ikeda S., Kishida M., Sakamoto I., Koyama S., Kikuchi A. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J. Biol. Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- Kohler E.M., Derungs A., Daum G., Behrens J., Schneikert J. Functional definition of the mutation cluster region of adenomatous polyposis coli in colorectal tumours. Hum. Mol. Genet. 2008;17:1978–1987. doi: 10.1093/hmg/ddn095. [DOI] [PubMed] [Google Scholar]
- Kohler E.M., Chandra S.H.V., Behrens J., Schneikert J. Beta-catenin degradation mediated by the CID domain of APC provides a model for the selection of APC mutations in colorectal, desmoid and duodenal tumours. Hum. Mol. Genet. 2009;18:213–226. doi: 10.1093/hmg/ddn338. [DOI] [PubMed] [Google Scholar]
- Krieghoff E., Behrens J., Mayr B. Nucleo-cytoplasmic distribution of β-catenin is regulated by retention. J. Cell Sci. 2006;119:1453–1463. doi: 10.1242/jcs.02864. [DOI] [PubMed] [Google Scholar]
- Kunttas-Tatli E., Roberts D.M., McCartney B.M. Self-association of the APC tumor suppressor is required for the assembly, stability, and activity of the Wnt signaling destruction complex. Mol. Biol. Cell. 2014;25:3424–3436. doi: 10.1091/mbc.E14-04-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Salic A., Krüger R., Heinrich R., Kirschner M.W. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li V.S.W., Ng S.S., Boersema P.J., Low T.Y., Karthaus W.R., Gerlach J.P., Mohammed S., Heck A.J.R., Maurice M.M., Mahmoudi T., Clevers H. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Liu C., Li Y., Semenov M., Han C., Baeg G.-H., Tan Y., Zhang Z., Lin X., He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Liu J., Xing Y., Hinds T.R., Zheng J., Xu W. The third 20 amino acid repeat is the tightest binding site of APC for beta-catenin. J. Mol. Biol. 2006;360:133–144. doi: 10.1016/j.jmb.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Mariotti L., Pollock K., Guettler S. Regulation of Wnt/β-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding. Br. J. Pharmacol. 2017;174:4611–4636. doi: 10.1111/bph.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino-Echarri E., Brocardo M.G., Mills K.M., Henderson B.R. Tankyrase inhibitors stimulate the ability of tankyrases to bind Axin and drive assembly of β-catenin degradation-competent Axin puncta. PLoS ONE. 2016;11:e0150484. doi: 10.1371/journal.pone.0150484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Topaz C., Mieszczanek J., Bienz M. The adenomatous polyposis coli tumour suppressor is essential for Axin complex assembly and function and opposes Axin’s interaction with Dishevelled. Open Biol. 2011;1:110013. doi: 10.1098/rsob.110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin P.J., Sparks A.B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K.W. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Morrone S., Cheng Z., Moon R.T., Cong F., Xu W. Crystal structure of a Tankyrase-Axin complex and its implications for Axin turnover and Tankyrase substrate recruitment. Proc. Natl. Acad. Sci. U S A. 2012;109:1500–1505. doi: 10.1073/pnas.1116618109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. U S A. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong J., Kang K., Shi Q., Zhu X., Tao Q., Chen Y.-G. Phase separation of Axin organizes the β-catenin destruction complex. J. Cell Biol. 2021;220:e202012112. doi: 10.1083/jcb.202012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novellasdemunt L., Foglizzo V., Cuadrado L., Antas P., Kucharska A., Encheva V., Snijders A.P., Li V.S.W. USP7 is a tumor-specific WNT activator for APC-mutated colorectal cancer by mediating β-catenin deubiquitination. Cell Rep. 2017;21:612–627. doi: 10.1016/j.celrep.2017.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford K., Crockett C., Jensen J.P., Weissman A.M., Byers S.W. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J. Biol. Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- Pronobis M.I., Rusan N.M., Peifer M. A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient βcatenin destruction. eLife. 2015;4:e08022. doi: 10.7554/eLife.08022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronobis M.I., Deuitch N., Posham V., Mimori-Kiyosue Y., Peifer M. Reconstituting regulation of the canonical Wnt pathway by engineering a minimal β-catenin destruction machine. Mol. Biol. Cell. 2017;28:41–53. doi: 10.1091/mbc.E16-07-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabut G., Peter M. Function and regulation of protein neddylation. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 2008;9:969–976. doi: 10.1038/embor.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G., Bain J., Elliott M., Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5:60–65. doi: 10.1038/sj.embor.7400048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas G., Minton A.P. Macromolecular crowding in vitro, in vivo, and in between. Trends Biochem. Sci. 2016;41:970–981. doi: 10.1016/j.tibs.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D.M., Pronobis M.I., Poulton J.S., Waldmann J.D., Stephenson E.M., Hanna S., Peifer M. Deconstructing the ßcatenin destruction complex: mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol. Biol. Cell. 2011;22:1845–1863. doi: 10.1091/mbc.E10-11-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B., Souza B., Albert I., Müller O., Chamberlain S.H., Masiarz F.R., Munemitsu S., Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Albert I., Porfiri E., Fiol C., Munemitsu S., Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Albert I., Porfiri E., Munemitsu S., Polakis P. Loss of beta-catenin regulation by the APC tumor suppressor protein correlates with loss of structure due to common somatic mutations of the gene. Cancer Res. 1997;57:4624–4630. [PubMed] [Google Scholar]
- Salic A., Lee E., Mayer L., Kirschner M.W. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol. Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vega F., Mina M., Armenia J., Chatila W.K., Luna A., La K.C., Dimitriadoy S., Liu D.L., Kantheti H.S., Saghafinia S., Cancer Genome Atlas Research Network Oncogenic signaling pathways in The Cancer Genome Atlas. Cell. 2018;173:321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer K.N., Peifer M. Wnt/beta-catenin signaling regulation and a role for biomolecular condensates. Dev. Cell. 2019;48:429–444. doi: 10.1016/j.devcel.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer K.N., Bonello T.T., Zhang S., Williams C.E., Roberts D.M., McKay D.J., Peifer M. Supramolecular assembly of the beta-catenin destruction complex and the effect of Wnt signaling on its localization, molecular size, and activity in vivo. PLoS Genet. 2018;14:e1007339. doi: 10.1371/journal.pgen.1007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer K.N., Pronobis M.I., Williams C.E., Zhang S., Bauer L., Goldfarb D., Yan F., Major M.B., Peifer M. Wnt regulation: exploring Axin-Disheveled interactions and defining mechanisms by which the SCF E3 ubiquitin ligase is recruited to the destruction complex. Mol. Biol. Cell. 2020;31:992–1014. doi: 10.1091/mbc.E19-11-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T., Metcalfe C., Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J. Cell Sci. 2007;120:2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- Sonn-Segev A., Belacic K., Bodrug T., Young G., VanderLinden R.T., Schulman B.A., Schimpf J., Friedrich T., Dip P.V., Schwartz T.U. Quantifying the heterogeneity of macromolecular machines by mass photometry. Nat. Commun. 2020;11:1772. doi: 10.1038/s41467-020-15642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink K.E., Polakis P., Weis W.I. Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J. 2000;19:2270–2279. doi: 10.1093/emboj/19.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamos J.L., Weis W.I. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z., Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145:dev146589. doi: 10.1242/dev.146589. [DOI] [PubMed] [Google Scholar]
- Su L.K., Vogelstein B., Kinzler K.W. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Su Y., Fu C., Ishikawa S., Stella A., Kojima M., Shitoh K., Schreiber E.M., Day B.W., Liu B. APC is essential for targeting phosphorylated beta-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol. Cell. 2008;32:652–661. doi: 10.1016/j.molcel.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Tacchelly-Benites O., Wang Z., Yang E., Benchabane H., Tian A., Randall M.P., Ahmed Y. Axin phosphorylation in both Wnt-off and Wnt-on states requires the tumor suppressor APC. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.W., Gardiner B.S., Hirokawa Y., Layton M.J., Smith D.W., Burgess A.W. Wnt signalling pathway parameters for mammalian cells. PLoS ONE. 2012;7:e31882. doi: 10.1371/journal.pone.0031882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello D.V.F., Maurice M.M. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle. 2010;9:3700–3709. doi: 10.4161/cc.9.18.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49(D1):D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kappel E.C., Maurice M.M. Molecular regulation and pharmacological targeting of the β-catenin destruction complex. Br. J. Pharmacol. 2017;174:4575–4588. doi: 10.1111/bph.13922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshanenko O., Erdmann G., Dubash T.D., Augustin I., Metzig M., Moffa G., Hundsrucker C., Kerr G., Sandmann T., Anchang B. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat. Commun. 2013;4:2610. doi: 10.1038/ncomms3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yin J., Chen D., Nie F., Song X., Fei C., Miao H., Jing C., Ma W., Wang L. Small-molecule modulation of Wnt signaling via modulating the Axin-LRP5/6 interaction. Nat. Chem. Biol. 2013;9:579–585. doi: 10.1038/nchembio.1309. [DOI] [PubMed] [Google Scholar]
- Weissmann F., Petzold G., VanderLinden R., Huis In ’t Veld P.J., Brown N.G., Lampert F., Westermann S., Stark H., Schulman B.A., Peters J.-M. biGBac enables rapid gene assembly for the expression of large multisubunit protein complexes. Proc. Natl. Acad. Sci. U S A. 2016;113:E2564–E2569. doi: 10.1073/pnas.1604935113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K., Logan C.Y., Arora A., Fish M., Nusse R. A Drosophila Axin homolog, Daxin, inhibits Wnt signaling. Development. 1999;126:4165–4173. doi: 10.1242/dev.126.18.4165. [DOI] [PubMed] [Google Scholar]
- Winston J.T., Strack P., Beer-Romero P., Chu C.Y., Elledge S.J., Harper J.W. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Xu G., Schulman B.A., Jeffrey P.D., Harper J.W., Pavletich N.P. Structure of a β-TrCP1-Skp1-β-catenin complex: destruction motif binding and lysine specificity of the SCF(β-TrCP1) ubiquitin ligase. Mol. Cell. 2003;11:1445–1456. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
- Xu W., Kimelman D. Mechanistic insights from structural studies of beta-catenin and its binding partners. J. Cell Sci. 2007;120:3337–3344. doi: 10.1242/jcs.013771. [DOI] [PubMed] [Google Scholar]
- Xue B., Romero P.R., Noutsou M., Maurice M.M., Rüdiger S.G.D., William A.M., Jr., Mizianty M.J., Kurgan L., Uversky V.N., Dunker A.K. Stochastic machines as a colocalization mechanism for scaffold protein function. FEBS Lett. 2013;587:1587–1591. doi: 10.1016/j.febslet.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Straight P.D., McLoughlin S.M., Zhou Z., Lin A.J., Golan D.E., Kelleher N.L., Kolter R., Walsh C.T. Genetically encoded short peptide tag for versatile protein labeling by Sfp phosphopantetheinyl transferase. Proc. Natl. Acad. Sci. U S A. 2005;102:15815–15820. doi: 10.1073/pnas.0507705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Lin A.J., Golan D.E., Walsh C.T. Site-specific protein labeling by Sfp phosphopantetheinyl transferase. Nat. Protoc. 2006;1:280–285. doi: 10.1038/nprot.2006.43. [DOI] [PubMed] [Google Scholar]
- Young G., Hundt N., Cole D., Fineberg A., Andrecka J., Tyler A., Olerinyova A., Ansari A., Marklund E.G., Collier M.P. Quantitative mass imaging of single biological macromolecules. Science. 2018;360:423–427. doi: 10.1126/science.aar5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Shay J.W. Multiple roles of APC and its therapeutic implications in colorectal cancer. J. Natl. Cancer Inst. 2017;109:55. doi: 10.1093/jnci/djw332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Original data and gel/Western blot images have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is listed in the Key resources table.
-
•
All original code is available in this paper’s supplemental information (Data S1).
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.