Abstract
Background
Real-world clinical data to support the use of casirivimab–imdevimab for the treatment of outpatients with mild to moderate coronavirus disease-19 (COVID-19) is needed. This study aimed to assess the outcomes of casirivimab–imdevimab treatment of mild to moderate COVID-19.
Methods
A retrospective cohort of 696 patients who received casirivimab–imdevimab between December 4, 2020 and April 9, 2021 was compared to a propensity-matched control of 696 untreated patients with mild to moderate COVID-19 at Mayo Clinic sites in Arizona, Florida, Minnesota, and Wisconsin. Primary outcome was rate of hospitalization at days 14, 21 and 28 after infusion.
Findings
The median age of the antibody-treated cohort was 63 years (interquartile range, 52–71); 45·5% were ≥65 years old; 51.4% were female. High-risk characteristics were hypertension (52.4%), body mass index ≥35 (31.0%), diabetes mellitus (24.6%), chronic lung disease (22.1%), chronic renal disease (11.4%), congestive heart failure (6.6%), and compromised immune function (6.7%). Compared to the propensity-matched untreated control, patients who received casirivimab–imdevimab had significantly lower all-cause hospitalization rates at day 14 (1.3% vs 3.3%; Absolute Difference: 2.0%; 95% confidence interval (CI): 0.5–3.7%), day 21 (1.3% vs 4.2%; Absolute Difference: 2.9%; 95% CI: 1.2–4.7%), and day 28 (1.6% vs 4.8%; Absolute Difference: 3.2%; 95% CI: 1.4–5.1%). Rates of intensive care unit admission and mortality at days 14, 21 and 28 were similarly low for antibody-treated and untreated groups.
Interpretation
Among high-risk patients with mild to moderate COVID-19, casirivimab–imdevimab treatment was associated with a significantly lower rate of hospitalization.
Funding
Mayo Clinic.
Keywords: Monoclonal antibodies, Casirivimab, Imdevimab, Covid-19, Outcomes
Research in context.
Evidence before this study
Casirivimab–imdevimab are anti-spike monoclonal antibodies that have been authorized for use, under the emergency use authorization, for the treatment of high-risk patients with mild to moderate coronavirus disease-19. This authorization is based on the results of reduction in viral load among treated patients in the randomized placebo-controlled clinical trial that enrolled only 275 patients. Clinical outcomes of the use of casirivimab–imdevimab is needed.
Added value of this study
This retrospective cohort study enrolled 1392 treated and propensity-matched untreated patients and provides real-world data on the clinical outcomes of casirivimab–imdevimab treatment. In this cohort of exclusively high-risk patients with mild to moderate coronavirus disease-19, there was a significant reduction in all-cause hospitalization in patients who received treatment with casirivimab–imdevimab, compared to propensity-matched untreated control. The findings in this study provide the real-world clinical outcome needed to support the use of these specific anti-spike monoclonal antibodies in patients with mild to moderate coronavirus disease-19.
Implication of all the available evidence
The clinical outcomes data reported in this study, complements the virologic outcomes data in the randomized controlled trial, and collectively provide data to support the use of casirivimab–imdevimab as early treatment of high-risk patients with mild to moderate coronavirus disease-19.
Alt-text: Unlabelled box
1. Introduction
The United States (US) Food and Drug Administration (FDA) has authorized the emergency use of anti-spike monoclonal antibody therapies for the treatment of mild to moderate coronavirus disease-19 (COVID-19) [1], [2], [3]. These neutralizing monoclonal antibodies, which were originally identified and isolated from B cells and plasma of donors who have recovered from COVID-19, inhibit the interaction of the spike protein of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) with the human ACE-2 receptors, thereby preventing viral attachment, cell entry and infectivity [4].
Casirivimab and imdevimab are two recombinant human IgG1 monoclonal antibodies that bind non-competitively to non-overlapping epitopes of the spike protein receptor-binding domain of SARS-CoV-2, thereby blocking the viral entry into host cells. On November 21, 2020, the combination of casirivimab–imdevimab received FDA emergency use authorization (EUA) for the outpatient treatment of high-risk patients with mild to moderate COVID-19 [2]. This was based on an early phase randomized placebo controlled clinical trial of 275 patients that demonstrated significant reduction in viral load among patients who received the casirivimab–imdevimab combination [5]. There were also lower rates of hospitalization and medically attended visits among the antibody-treated patients, but the low number of clinical events did not allow for robust statistical analysis. Accordingly, national society guidelines did not initially endorse the use of these monoclonal antibodies for the routine treatment of mild to moderate COVID-19.
Recently, the yet to be published results of the ongoing clinical trials have demonstrated clinical benefits, prompting the Infectious Diseases Society of America and the US National Institutes of Health to endorse the use of casirivimab–imdevimab for treatment of high-risk patients with mild to moderate COVID-19 [5], [6], [7]. In this study, we report the real-world outcomes of casirivimab–imdevimab among an exclusively high-risk population with mild to moderate COVID-19. We specifically investigated the primary outcome of 28-day hospitalization among high-risk patients who received casirivimab–imdevimab and compared this to a cohort of untreated propensity-matched control. The rates of intensive care unit admission and mortality were also assessed as secondary outcomes.
2. Methods
2.1. Monoclonal antibody treatment program
The Mayo Clinic Monoclonal Antibody Treatment (MATRx) program was established on November 7, 2020 in order to prepare for the impending EUAs for monoclonal antibodies for the outpatient treatment of COVID-19. The details of this treatment program have been reported [8]. A multidisciplinary team reviewed patients for eligibility for monoclonal antibody therapy using both electronic health record (EHR) registry tools for internal patients, and a self- and clinician-referred process for patients external to the health system.
The criteria for casirivimab–imdevimab infusion was guided by the FDA EUA. In particular, patients who were 18 years and older were eligible for casirivimab–imdevimab treatment if they had symptoms of mild to moderate COVID-19 (e.g., cough, sore throat, headache, body aches, fever, and constitutional symptoms), were within 10 days of symptom onset, and had at least one of the following criteria: age ≥65 years, body mass index (BMI) ≥35, diabetes mellitus, chronic kidney disease, immunosuppressive medication use, or an immunocompromising condition. Patients 55 years and older qualified if they had hypertension, cardiovascular disease, or chronic lung disease. Patients with clinical manifestations of severe COVID-19 (e.g., new or worsening hypoxemia) and those requiring hospitalization for COVID-19 were excluded. There is separate eligibility criteria for patients between 12 and 17 years, but we did not include adolescent patients in our study.
All eligible patients were approached by the MATRx members for education and consenting. All consenting patients subsequently received one-hour infusion of casirivimab (1200-mg dose) and imdevimab (1200-mg dose) at our COVID-19 infusion facilities [8]. Patients were monitored for vital signs and oxygenation status prior to, during, and for one hour after infusion. Patients were asked to report adverse reactions and were followed by telemedicine through remote monitoring program.
Other COVID-19 directed antiviral drug or immunomodulator treatment, such as remdesivir or corticosteroid therapy, respectively, were not provided to patients with mild to moderate disease in the outpatient setting. These treatment regimens were available only to patients who developed severe COVID-19 that requires hospitalization.
2.2. Study design and participants
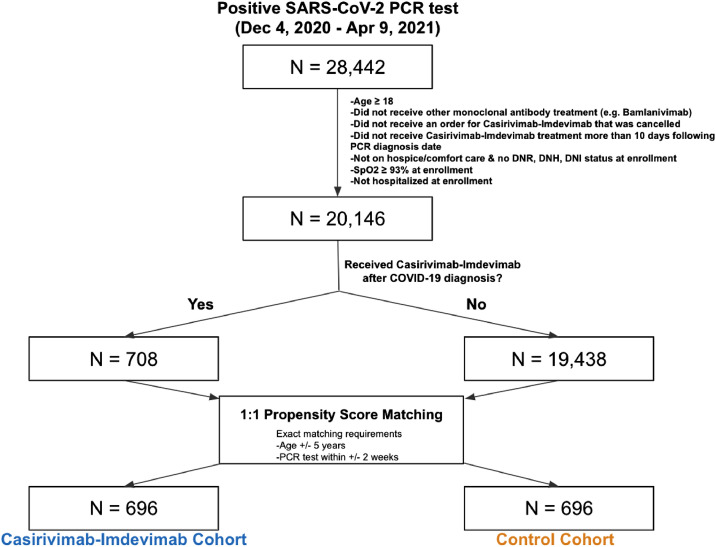
This retrospective study was conducted among adult (≥ 18 years old) patients identified from the Mayo Clinic EHR database with positive SARS-CoV-2 PCR tests between December 4, 2020 and April 9, 2021. The start date December 4, 2020 was selected as it was the earliest test date for a patient infused with casirivimab–imdevimab. The study end date was selected as the most recent date with available data. The participant selection algorithm (Fig. 1) resulted into two cohorts that were balanced for relevant demographic and clinical covariates: (1) treated patients who received casirivimab–imdevimab infusion, and (2) control patients who did not receive anti-spike monoclonal antibody after diagnosis of COVID-19. This retrospective study adhered to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Fig. 1.
Cohort selection criteria and patient counts. The diagram shows the inclusion criteria and propensity score matching procedure that was used to construct the Casirivimab–Imdevimab and control cohorts. In each box, the number of patients, starting with the full study population of 28,442 patients in the Mayo Clinic Electronic Health record database who had at least one positive SARS-CoV-2 PCR test between December 4, 2020 and April 9, 2021.
2.3. Participant selection and propensity score matching
The study population was selected from the pool of adult patients with COVID-19 who met the following criteria: (1) had not received bamlanivimab with or without etesevimab at any time during the study period, (2) did not have a canceled casirivimab–imdevimab order, (3) were not on hospice or comfort care, (4) did not have a do not intubate (DNI), do not resuscitate (DNR), or do not hospitalize (DNH) status, (5) had minimum SpO2 of >93%, and (6) were not currently hospitalized at the time of positive PCR test or casirivimab–imdevimab infusion. For each patient in the treated cohort, the enrollment date was defined as the date of casirivimab–imdevimab infusion.
Propensity score matching was performed to select matched controls balanced on covariates that may influence casirivimab–imdevimab administration (Table 1) [9]. Propensity scores were computed for each patient by fitting an L1-regularized logistic regression model to predict which of the two cohorts the patient was in, as a function of the covariates detailed in next section [10]. To identify a matched control for each antibody-treated patient, a set of control patients with the same age (+/− 5 years) and PCR diagnosis date (+/− 7 days) was considered, and the patient with the closest propensity score was selected, if the propensity score difference was less than the selected threshold. If the control patient (1) had a minimum SpO2 < 93%, (2) was hospitalized, (3) had an active DNR, DNI or DNH status, (4) was receiving only palliative or comfort care, or (5) was deceased on or before the date of study enrollment, then a new control patient (the next nearest neighbor by propensity score) was selected. This process was repeated until an eligible match was found. If an eligible match was not found, the search was expanded to the set of control patients with age +/− 5 years and PCR diagnosis dates +/− 14 days relative to the patient treated with casirivimab–imdevimab. If the expanded search did not find any control patients, the casirivimab–imdevimab-treated patient was dropped from the analysis which led to a decrease in size of our treated cohort to 696 patients. The caliper threshold was set to 0.1 * pooled standard deviation of the propensity scores in the logit space. For each control patient, the enrollment date was defined based on the number of days between the positive PCR test and casirivimab–imdevimab infusion for the matched treated patient (Supplementary Figure 1). Patients in the control cohort could not have received casirivimab–imdevimab at a later time because the EUA only allows use for the first 10 days since symptom onset. All of the code for propensity score matching procedure was written in Python. The software package “sklearn” (v0.20.3) was used to train the L1-regularized logistic regression models.
Table 1.
Clinical characteristics of matched and unmatched cohorts. Demographics, body mass index, comorbidities, and immunosuppressant use for the treatment and control cohorts before and after propensity score matching. Comorbidities were determined using International Classification of Diseases (ICD) codes recorded in the past 5 years relative to the first positive SARS-CoV-2 PCR testing date for each patient. In the last column, the Standardized Mean Difference (SMD) is defined as the mean of the covariate in the casirivimab–imdevimab cohort minus the mean of the covariate in the control cohort, divided by the pooled standard deviation. Covariates with SMD < 0.25 are considered moderately balanced and covariates with SMD < 0.1 are considered highly balanced.
| After matching | Before matching |
|||||
|---|---|---|---|---|---|---|
| Clinical covariate | Casirivimab – imdevimab cohort (n= 696) | Control cohort (n= 696) | Standardized Difference | Casirivimab – imdevimab cohort (n= 708) | Control cohort (n= 19,438) | Standardized Difference |
| Age (years) | ||||||
| Median | 63 | 63 | 63 | 43 | ||
| Interquartile Range | (52, 71) | (52, 71) | (52, 71) | (30, 56) | ||
| <65 years old | 378 (54·3%) | 386 (55·5%) | 0·02 | 384 (54·2%) | 17,169 (88·3%) | 1·04 |
| 65–75 years old | 208 (29·9%) | 195 (28·0%) | 0·04 | 211 (29·8%) | 1471 (7·6%) | 0·81 |
| >75 years old | 110 (15·8%) | 115 (16·5%) | 0·02 | 113 (16·0%) | 798 (4·1%) | 0·57 |
| Sex | ||||||
| Female | 356 (51·1%) | 375 (53·9%) | 0·05 | 363 (51·3%) | 9806 (50·4%) | 0·02 |
| Male | 340 (48·9%) | 321 (46·1%) | 0·05 | 345 (48·7%) | 9627 (49·5%) | 0·02 |
| Race | ||||||
| American Indian | 3 (0·4%) | 2 (0·3%) | 0·02 | 3 (0·4%) | 91 (0·5%) | 0·01 |
| Asian | 8 (1·1%) | 5 (0·7%) | 0·04 | 8 (1·1%) | 466 (2·4%) | 0·08 |
| Black/African American | 26 (3·7%) | 28 (4·0%) | 0·01 | 27 (3·8%) | 583 (3·0%) | 0·05 |
| White/Caucasian | 645 (92·7%) | 646 (92·8%) | 0·01 | 656 (92·7%) | 16,033 (82·5%) | 0·27 |
| Other | 8 (1·1%) | 10 (1·4%) | 0·03 | 8 (1·1%) | 747 (3·8%) | 0·14 |
| Unknown | 6 (0·9%) | 5 (0·7%) | 0·02 | 6 (0·8%) | 1518 (7·8%) | 0·26 |
| Ethnicity | ||||||
| Hispanic | 29 (4·2%) | 32 (4·6%) | 0·02 | 29 (4·1%) | 1384 (7·1%) | 0·12 |
| Non-Hispanic | 655 (94·1%) | 653 (93·8%) | 0·01 | 667 (94·2%) | 16,150 (83·1%) | 0·3 |
| Unknown | 12 (1·7%) | 11 (1·6%) | 0·01 | 12 (1·7%) | 1904 (9·8%) | 0·28 |
| Site | ||||||
| Scottsdale, Arizona | 139 (20·0%) | 156 (22·4%) | 0·06 | 139 (19·6%) | 3086 (15·9%) | 0·1 |
| Jacksonville, Florida | 132 (19·0%) | 125 (18·0%) | 0·03 | 137 (19·4%) | 2399 (12·3%) | 0·21 |
| Mayo Clinic Health Systems | 357 (51·3%) | 363 (52·2%) | 0·02 | 363 (51·3%) | 10,961 (56·4%) | 0·1 |
| Rochester, Minnesota | 69 (9·8%) | 52 (7·5%) | 0·08 | 69 (9·7%) | 2960 (15·2%) | 0·15 |
| Body Mass Index (kg/m2) | ||||||
| Underweight (< 18·5) | 1 (0·1%) | 2 (0·3%) | 0·03 | 1 (0·1%) | 102 (0·5%) | 0·05 |
| Normal weight (18·5 to 25) | 68 (9·8%) | 67 (9·6%) | 0 | 68 (9·6%) | 2234 (11·5%) | 0·06 |
| Overweight (25 to 30) | 145 (20·8%) | 147 (21·1%) | 0·01 | 148 (20·9%) | 2972 (15·3%) | 0·16 |
| Obese - class 1 (30 to 35) | 129 (18·5%) | 133 (19·1%) | 0·01 | 130 (18·4%) | 2329 (12·0%) | 0·2 |
| Obese - class 2 (35 to 40) | 116 (16·7%) | 119 (17·1%) | 0·01 | 120 (16·9%) | 933 (4·8%) | 0·55 |
| Obese - class 3 (≥ 40) | 95 (13·6%) | 95 (13·6%) | 0 | 98 (13·8%) | 720 (3·7%) | 0·52 |
| Unknown | 142 (20·4%) | 133 (19·1%) | 0·03 | 143 (20·2%) | 10,148 (52·2%) | 0·64 |
| Comorbidity | ||||||
| − Hypertension | 363 (52·2%) | 365 (52·4%) | 0·01 | 374 (52·8%) | 3091 (15·9%) | 0·99 |
| − Chronic Pulmonary disease | 151 (21·7%) | 135 (19·4%) | 0·06 | 158 (22·3%) | 1934 (9·9%) | 0·41 |
| − Diabetes Mellitus w/o Complications | 98 (14·1%) | 77 (11·1%) | 0·09 | 105 (14·8%) | 665 (3·4%) | 0·6 |
| − Cancer (Local) | 94 (13·5%) | 79 (11·4%) | 0·07 | 98 (13·8%) | 628 (3·2%) | 0·57 |
| − Peripheral Vascular Disease | 92 (13·2%) | 93 (13·4%) | 0 | 97 (13·7%) | 661 (3·4%) | 0·54 |
| − Renal Disease | 80 (11·5%) | 79 (11·4%) | 0 | 80 (11·3%) | 780 (4·0%) | 0·36 |
| − Diabetes Mellitus w/ | 66 (9·5%) | 53 (7·6%) | 0·07 | 69 (9·7%) | 400 (2·1%) | 0·51 |
| Complications | 65 (9·3%) | 58 (8·3%) | 0·04 | 69 (9·7%) | 584 (3·0%) | 0·38 |
| − Liver Disease - Mild | 64 (9·2%) | 48 (6·9%) | 0·08 | 67 (9·5%) | 446 (2·3%) | 0·46 |
| − Congestive Heart Failure | 53 (7·6%) | 37 (5·3%) | 0·09 | 57 (8·1%) | 455 (2·3%) | 0·36 |
| − Cerebrovascular Disease | 44 (6·3%) | 31 (4·5%) | 0·08 | 48 (6·8%) | 268 (1·4%) | 0·44 |
| − Myocardial Infarction | 35 (5·0%) | 28 (4·0%) | 0·05 | 36 (5·1%) | 242 (1·2%) | 0·33 |
| − Connective Tissue Disease | 20 (2·9%) | 12 (1·7%) | 0·08 | 21 (3·0%) | 184 (0·9%) | 0·2 |
| − Cancer (Metastatic) | 15 (2·2%) | 11 (1·6%) | 0·04 | 15 (2·1%) | 164 (0·8%) | 0·14 |
| − Peptic Ulcer Disease | 7 (1·0%) | 10 (1·4%) | 0·04 | 7 (1·0%) | 77 (0·4%) | 0·09 |
| − Paraplegia/Hemiplegia | 5 (0·7%) | 3 (0·4%) | 0·04 | 6 (0·8%) | 91 (0·5%) | 0·05 |
| − Dementia | 3 (0·4%) | 1 (0·1%) | 0·05 | 3 (0·4%) | 74 (0·4%) | 0·01 |
| − Liver Disease - Moderate/Severe | 1 (0·1%) | 0 (0·0%) | 0·05 | 1 (0·1%) | 14 (0·1%) | 0·03 |
| − HIV/AIDS | 44 (6·3%) | 44 (6·3%) | 0 | 49 (6·9%) | 263 (1·4%) | 0·45 |
| Immunosuppressant use | ||||||
| Time from PCR date to casirivimab–imdevimab infusion (days) | ||||||
| − Mean | 2·61 | 2·60 | ||||
| − Standard deviation | 1·25 | 1·25 | ||||
The effectiveness of covariate balancing between casirivimab–imdevimab-treated and control cohort was assessed using the standardized difference. All covariates showed standardized differences < 0.1 confirming that the cohorts were reasonably balanced for reliable downstream comparisons (Table 1). The success of balancing was also confirmed by comparing the age distribution (Supplementary Fig. 2) and the prevalence of each categorical covariate (Supplementary Fig. 3) in the two cohorts before and after propensity matching.
2.4. Demographic and clinical covariates
To perform the propensity matching described above, demographic and clinical covariates which could influence the likelihood of casirivimab–imdevimab administration were considered (Table 1). Demographic covariates considered included age, sex, race, and ethnicity. Race and ethnicity were determined based on multiple choice questions with fixed categories and were considered in this study in order to control for social determinants of health and other potential confounding factors. Clinical covariates were derived from the Charlson Comorbidity Index and identified for each patient on the basis of International Classification of Diseases (ICD)-9 and ICD-10 codes recorded in the 5 years prior to the SARS-CoV-2 PCR testing date.
Other covariates considered during the propensity score matching included hypertension, BMI, immunosuppressive medication usage, and location of infusion. Hypertension was determined using ICD-10 codes recorded in the 5 years prior to the PCR testing date. BMI was calculated using the most recently recorded weight (between one year before and one week after COVID-19 diagnosis) and height (between age 18 and one week after COVID-19 diagnosis). Immunosuppressive medication usage was determined using medication orders active or completed in the year prior to the PCR testing date up to the end of the study period. Location of infusion was incorporated into the covariate balancing analysis post-hoc. This study included participants from all four major sites in Mayo Clinic Health Systems: Scottsdale, Arizona; Jacksonville, Florida; Rochester, Minnesota; and other Mayo Clinic Health Systems locations in Minnesota and Wisconsin. Due to the small number of sites, this variable was modelled as a fixed effect.
2.5. Outcomes
The clinical outcomes that were assessed and compared between the casirivimab–imdevimab-treated and control cohorts at days 14, 21, and 28 after enrollment were rates of hospitalization, ICU admission, and death. Hospitalization rate was considered as the primary outcome, while ICU admission and death were secondary exploratory outcomes. The indications for hospital admission were not pre-defined but was dependent on provider assessment of the patient clinical status in real-time, and this is often due to progression of disease severity. Patients with enrollment dates within 14, 21, or 28 days of the study end date (April 9, 2021) were considered right-censored at that date.
2.6. Ethics approval and patient consent
The Mayo Clinic Institutional Review Board approved this study. Informed consent was waived and patients without research authorization were excluded.
2.7. Statistical analysis
The incidence of each outcome - hospitalization, ICU admission, and death - was assessed at daily intervals with a Kaplan-Meier analysis. The Kaplan-Meier estimate of incidence and corresponding confidence intervals at 14, 21, and 28 days was computed for the casirivimab–imdevimab and control cohorts. To compare the rates of incidence between the cohorts, the difference of incidence rates was computed along with a 95% confidence interval. This confidence interval around the difference was computed using a delta-method-based approach [11]. In particular, the standard error of the difference was where SEGreenwood,1 and SEGreenwood,2 are the Greenwood estimates of the standard error of the Kaplan-Meier estimate at a point. Survival analysis was performed using the “lifelines” package (v0.25.6) in Python [12]. The confidence intervals around the incidence rates for each individual cohort were computed using the exponential Greenwood approach used by the lifelines package [11,12].
In addition, the average number of days in hospital and ICU were compared for the two cohorts. In particular, the absolute difference in days along with 95% confidence intervals for these outcomes at the 14 day, 21 day, and 28 day time points were reported. At each time point, patients with insufficient follow-up data were excluded from the analysis.
2.8. Role of funding sources
Mayo Clinic provided intramural funds for the conduct of this study. Mayo Clinic did not have any direct role in data collection, analysis, interpretation, and the decision to publish the research.
3. Results
3.1. Patient population
Of the 28,442 adult patients with positive SARS-CoV-2 PCR tests during the study period, the participant selection algorithm (Fig. 1) resulted into two cohorts that were balanced for relevant demographic and clinical covariates: (1) casirivimab–imdevimab-treated patients (n = 696), and (2) control patients (n = 696) who did not receive monoclonal antibody infusions (Table 1). The mean time from PCR date to casirivimab–imdevimab infusion was 2.61 days (median, 2 days) (Supplementary Figure 1). The demographic and clinical characteristics are listed in Table 1. All patients have medical comorbidity or a characteristic that suggested high-risk for progression to severe COVID-19. The most common comorbidities were hypertension (52.4%), BMI ≥35 kg/m2 (31.0%), diabetes mellitus (24.6%), chronic lung disease (22.1%), chronic renal disease (11.4%), congestive heart failure (6.6%), and compromised immune function (6.7%).
3.2. All-cause hospitalization
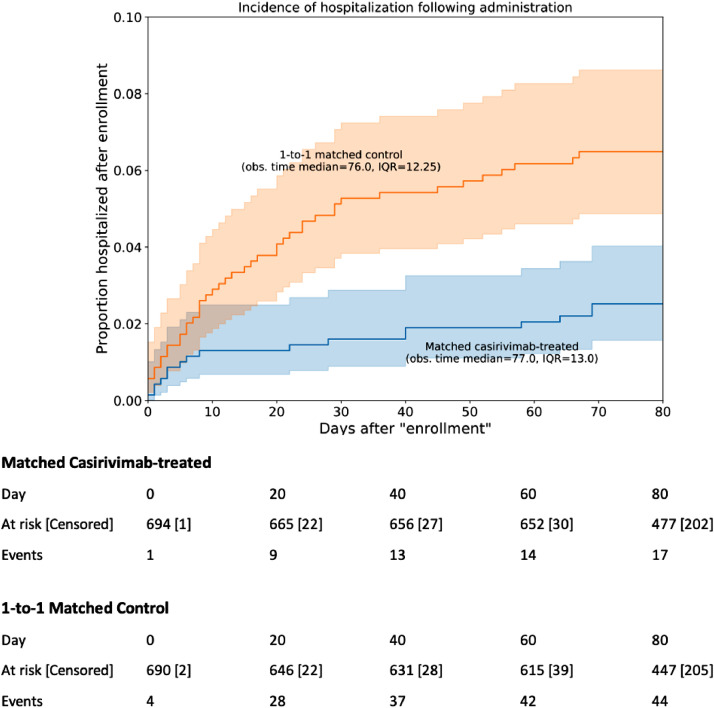
All-cause hospitalization rates were significantly lower in the casirivimab–imdevimab group than the propensity-matched cohort at day 14 (1.3% vs 3.3%; Absolute Difference: 2.0%; 95% CI: 0.50–3.7%), day 21 (1.3% vs 4.2%; Absolute Difference: 2.9%; 95% CI: 1.2%−4.7%), and day 28 (1.6% vs 4.8%; Absolute Difference: 3.2%; 95% CI: 1.4–5.1%) (Table 2). Patients who were treated with casirivimab–imdevimab had significantly more hospitalization-free days compared to the propensity-matched cohort (Table 3). Kaplan-Meier survival analysis showed significant difference in the rates of hospitalization-free survival between the casirivimab–imdevimab-treated and propensity-matched untreated control (log-rank test p-value 3 × 10−4; Fig. 2).
Table 2.
Hospitalization, intensive care unit admission, and mortality rate outcomes for the matched casirivimab–imdevimab and control cohorts• For each outcome measure, patient incidence rates are provided at 14 days, 21 days, and 28 days• Results of a logistic regression (with covariates consisting of all the covariates which were balanced, as well as the treatment) applied on the matched cohorts are shown• The columns include: (1) Casirivimab–imdevimab cohort: Percentage of patients with the outcome variable in the Casirivimab–imdevimab cohort among the patients with follow-up data available, along with 95% confidence intervals, (2) Control cohort: Percentage of patients with the outcome variable in the propensity-matched control cohort among the patients with follow-up data available, along with 95% confidence intervals, (3) Risk difference: Absolute difference in risk between the treatment and control cohorts, along with 95% confidence intervals.
| Outcome | Casirivimab–imdevimab cohort (696 patients) | Control cohort (696 patients) | Risk difference (95% CI) |
|---|---|---|---|
| Hospitalization | |||
| 14 day 21 day 28 day | 1·3% (0·7%, 2·5%) 1·3% (0·7%, 2·5%) 1·6% (0·9%, 2·9%) | 3·3% (2·2%, 5·0%) 4·2% (3·0%, 6·0%) 4·8% (3·5%, 6·7%) | 2·0% (0·5%, 3·7%) 2·9% (1·2%, 4·7%) 3·2% (1·4%, 5·1%) |
| Intensive Care Unit admission | |||
| 14 day 21 day 28 day | 0·73% (0·3%, 1·7%) 0·73% (0·3%, 1·7%) 0·73% (0·3%, 1·7%) | 0·87% (0·4%, 1·9%) 0·87% (0·4%, 1·9%) 1·0% (0·5%, 2·1%) | 0·15% (−0·8%, 1·1%) 0·15% (−0·8%, 1·1%) 0·30% (−0·7%, 1·3%) |
| Mortality | |||
| 14 day 21 day 28 day | 0·15% (0·0%, 1·0%) 0·15% (0·0%, 1·0%) 0·15% (0·0%, 1·0%) | 0·44% (0·1%, 1·4%) 0·44% (0·1%, 1·4%) 0·59% (0·2%, 1·6%) | 0·29% (−0·3%, 0·9%) 0·29% (−0·3%, 0·9%) 0·33% (−0·2%, 1·1%) |
Table 3.
Hospital and Intensive Care Unit (ICU) length of stay outcomes for the casirivimab–imdevimab and control cohorts. For each outcome measure, the mean value and standard deviation is provided for the subset of patients with follow-up data available at 14 days, 21 days, and 28 days. The columns include: (1) Casirivimab–imdevimab cohort: mean (standard deviation) for the outcome variable among the patients in the Casirivimab–imdevimab cohort with follow-up data available, (2) Control cohort: mean (standard deviation) for the outcome variable among the patients in the propensity matched control cohort with follow-up data available, (3) Absolute difference in days: mean number of hospital/ICU days in the Casirivimab–imdevimab cohort minus the mean number of hospital/ICU days in the control cohort, along with a 95% confidence interval for this difference.
| Outcome | Casirivimab – Imdevimab (n= 696) | Control (n= 696) | Absolute difference (95% CI) |
|---|---|---|---|
| Number of patients with follow-up | |||
| 14 day | 679 | 679 | |
| 21 day | 673 | 674 | |
| 28 day | 668 | 671 | |
| Number of days in hospital | |||
| 14 day | 0·06 (0·64) | 0·14 (0·92) | 0·08 (−0·01, 0·16) |
| 21 day | 0·07 (0·80) | 0·18 (1·20) | 0·12 (0·01, 0·29) |
| 28 day | 0·07 (0·81) | 0·23 (1·45) | 0·17 (0·04, 0·29) |
| Number of days in Intensive Care Unit | |||
| 14 day | 0·03 (0·46) | 0·03 (0·44) | 0·00 (−0·05, 0·05) |
| 21 day | 0·03 (0·46) | 0·03 (0·49) | 0·00 (−0·06, 0·05) |
| 28 day | 0·03 (0·47) | 0·03 (0·49) | 0·00 (−0·06, 0·05) |
Fig. 2.
Kaplan-Meier curves of hospitalization rates for casirivimab–imdevimab patients and propensity matched controls. For each patient in the casirivimab–imdevimab cohort (n = 696), the index date is set to be the date of infusion, and for each patient in the control cohort (n = 696), the index date is set using the date of infusion for the matched control. 95% confidence intervals are displayed for each curve, along with the median and interquartile range (IQR) of the follow-up time for the cohort. A table showing the number of events along with the number of patients at risk and censored over time is shown below. A log-rank test against the hypothesis of equal hazard rates gives p-value 3 × 10−4.
3.3. Intensive care unit admissions and all-cause mortality
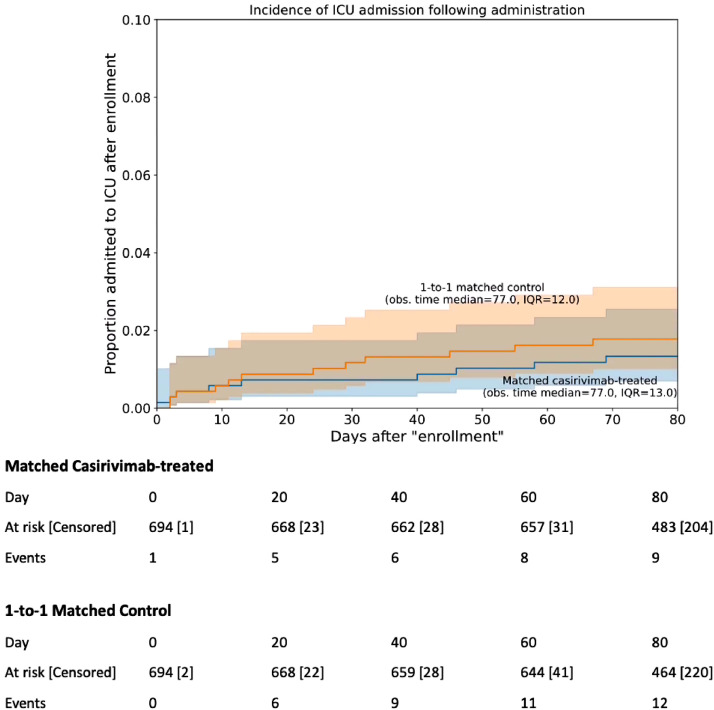
All-cause ICU admission rates were similarly low and were not significantly different between the casirivimab–imdevimab-treated and the untreated cohorts (Table 2; Fig. 3). At day 28, five of the 668 patients treated with casirivimab–imdevimab were admitted to the ICU, compared to seven of 671 untreated patients (Table 2). Five patients died from any cause (including only one patient who received casirivimab–imdevimab). Among four patients who did not receive monoclonal antibody, two died from respiratory progression of COVID-19, one had a cerebrovascular accident, and one had sudden cardiac death. The single patient who died in the casirivimab–imdevimab group succumbed secondary to metabolic derangements in the setting of dehydration, possibly related to COVID-19.
Fig. 3.
Kaplan-Meier curves of Intensive Care Unit (ICU) admission rates for casirivimab–imdevimab patients and propensity matched controls. For each patient in the casirivimab–imdevimab cohort (n = 696), the index date is set to be the date of infusion, and for each patient in the control cohort (n = 696), the index date is set using the date of infusion for the matched control. 95% confidence intervals are displayed for each curve, along with the median and interquartile range (IQR) of the follow-up time for the cohort. A table showing the number of events along with the number of patients at risk and censored over time is shown below. A log-rank test against the hypothesis of equal hazard rates gives p-value 0.51.
3.4. Adverse events
Adverse events were reported in seven patients. Four reported fever, two reported shortness of breath, two reported nausea, and one patient reported chest pain, headache, or flushing. No patient had anaphylaxis. All adverse events were mild (NCI Grade 1) and did not require hospitalization.
4. Discussion
This retrospective study shows that casirivimab–imdevimab treatment of mild to moderate COVID-19 was associated with a statistically significant decrease in the rate of all-cause hospitalization during the first 28 days after infusion. The 1.6% rate of hospitalization at day 28 in our study was comparable to the data from the initial clinical trial that compared casirivimab–imdevimab with placebo [5]. In that placebo-controlled trial, the reported rate of hospitalization and medically attended visits was 3% (6 of 182 patients) [5]. However, our study was limited only to hospitalization as our primary outcome, and we did not account for the other medically attended visits.
By virtue of the strict US FDA EUA criteria, 100% of the casirivimab–imdevimab-treated cohort had at least one risk factor for severe COVID-19 [2]. Thus, in contrast to the placebo-controlled trial that included all patients regardless of risk profile, our study was limited exclusively to patients at high-risk of progression to severe COVID-19, with hypertension, obesity and diabetes as the most common comorbidities. Almost half of the population was over 65 years of age. Previous studies have identified these characteristics and medical conditions as predisposing factors for severe and critical COVID-19 [13,14]. The low rate of hospitalization observed in our study is therefore highly clinically relevant. As previous studies have suggested that patients with previously noted medical comorbidities are at higher risk of severe and critical illness, treatment with casirivimab–imdevimab may have mitigated this progression in our exclusively high-risk patient population.
In contrast to the higher rates of hospitalization (up to 15% among the high-risk group) reported among untreated patients in the casirivimab–imdevimab clinical trial [5,15], our untreated control population consisting exclusively of high-risk patients only had approximately 5% hospitalization rate at day 28. This lower rate of hospitalization among our untreated cohort in this study could be due to multiple factors including earlier testing and improved monitoring. Mayo Clinic had implemented a COVID-19 Frontline Care Team which established an extensive Remote Monitoring Program that manages the high-risk patients in the outpatient setting [16,17]. During the early part of the pandemic in March to July 2020, Mayo Clinic's multidisciplinary approach to the care of COVID-19 patients reported a hospitalization rate of 11.4% and mortality of 1.2% among all patients [18]. This outcome has further improved over time, as providers have become familiar with management of patients [19,20]. This improvement in clinical outcomes is reflected in the low rates of ICU admission and mortality in the untreated high-risk control population. This is also consistent with studies from other centers which have shown that the outcomes of COVID-19 have improved over time [21,22].
Based on this study, it is estimated that, in the first 28 days of follow up of 668 patients treated with casirivimab–imdevimab, there were over 110 hospital days saved. However, these results should be interpreted in the context of the specific population and healthcare institution. As indicated, our program proactively identified eligible patients and sought them for medication education and consenting, resulting in rapid time to infusion of the medication. Our observations suggest that casirivimab–imdevimab treatment has allowed patients to remain in the comfort of their homes as they recover from COVID-19, thereby minimizing the burden to the hospital systems.
This study has several limitations. First, this was an observational cohort study, and does not provide the scientific rigor of a randomized clinical trial. Performing a randomized trial was not feasible due to the ethical implications of withholding a drug authorized for emergency use in the treatment of high-risk patients. To overcome this, propensity score matching was performed to identify a cohort of untreated patients that were matched based on demographic characteristics, medical comorbidities, and risk profiles. Both cohorts were evaluated during the same temporal window in order to minimize confounders due to staffing, clinical care and resource availability, and circulating variants. The data suggest this process tightly matched the treated and untreated groups across many clinical variables and demographics. Second, the retrospective study design is another limitation that may not have captured all the outcomes of patients who may have received subsequent care in other institutions. This is mitigated by the aforementioned extensive outpatient remote monitoring and follow up program, which continued to follow patients virtually during the isolation period [17]. Also, only patients with documented follow up were included in the analysis of outcomes at days 14, 21 and 28. Third, this study focused on the combination of casirivimab–imdevimab and did not include patients who received bamlanivimab monotherapy or its combination with etesevimab. The clinical outcomes reported here therefore only apply to one specific monoclonal antibody (casirivimab–imdevimab), and this must be considered in the face of variable susceptibility of emerging variants in the community. Fourth, the study population was predominantly Caucasian, and further studies will need to be performed to validate the findings in other populations. Fifth, the outcomes data was derived from a single multi-site healthcare system which proactively screened and consented all the identified eligible patients leading to the rapid infusion of monoclonal antibody, and thus, the results may not be generalizable to other systems with different practices and processes [8]. The cost of monoclonal antibodies and its infusion should also be considered in their adoption for clinical use. In the US, casirivimab–imdevimab was distributed free by the federal government. However, there are costs related to its infusion, which we waived for all uninsured and underinsured patients. Nonetheless, the cost-benefit ratio should be considered in implementing this clinical practice. Sixth, despite the large patient population of almost 1400 treated and untreated patients and the strong statistical significance, the magnitude of absolute reduction in all-cause hospitalization was small. Extrapolating our findings to a larger population level, with over 47,000 new cases a day in the United States and 836,000 new cases a day globally as of May 8, 2021, the magnitude of even a small change is significantly amplified [23]. Seventh, even if both study cohorts were matched based on social and demographic factors, our study did not account for socioeconomic factors that could have influenced a patient's decision to seek hospitalization. However, our remote monitoring program would have identified the patients who progressed to severe illness that would have warranted hospitalization. Finally, the overall low rates of ICU admission and mortality limited our ability to determine any significant difference between the treated and untreated cohorts, despite having the largest cohort of COVID-19 patients treated with casirivimab–imdevimab outside of clinical trials.
In conclusion, this real-world study on the outcomes of the emergency use of casirivimab–imdevimab suggests that it is associated with a reduction in all-cause hospitalization. Adverse events were uncommon and mild. There were low rates of all-cause mortality and ICU admission in both the treated and untreated populations. Larger patient populations may be needed to determine if there is an association with the exploratory secondary outcomes.
Funding
Mayo Clinic
Contributors
Concept and Design: Razonable, Pawlowski, Ganesh
Acquisition, Analysis, or Interpretation of Data: Razonable, Pawlowski, Lenehan, Puranik, Venkatakrishnan, O'Horo, Badley, Ganesh
Drafting of the Manuscript: Razonable, Pawlowski, Ganesh
Critical Revision of the Manuscript: all authors
Statistical Analysis: Pawlowski, Lenehan, Puranik, Venkatakrishnan, O'Horo, Ganesh, Razonable
Administrative, Technical, or Material Support: Destro Borgen, Hedin
Supervision: Razonable, Bierle, Speicher, Seville, Tulledge-Scheitel, Wilker, Hanson, Ganesh
Data sharing statement
The dataset generated and analyzed during the study can be made available by the corresponding author, RRR, on reasonable request. RRR, CP, and RG are responsible and accessed the raw data involved with the study.
Declaration of Competing Interest
Dr. Arndt has nothing to disclose.
Dr. Arndt has nothing to disclose.
Dr. Badley reports personal fees from Abbvie, personal fees from Gilead, personal fees from Freedom Tunnel, personal fees from Pinetree Therapeutics, personal fees from Primmune, personal fees from Immunome, personal fees from Flambeau Diagnostics, personal fees from Corvus, personal fees from Equilium, personal fees from Excision Biotherapeutics, personal fees from Zentilis, personal fees from Nference, outside the submitted work
Dr. Bierle has nothing to disclose.
Ms. Destro Borgen has nothing to disclose.
Dr. Ganesh has nothing to disclose.
Dr. Hanson has nothing to disclose.
Michelle Hedin has nothing to disclose.
Dr. Lenehan reports other from Janssen, outside the submitted work.
Dr. O'Horo reports personal fees from Bates College, personal fees from Elsevier, Inc, grants from Nference, Inc, outside the submitted work.
Dr. Pawlowski reports personal fees from nference, outside the submitted work.
Dr. Puranik reports personal fees from nference, outside the submitted work.
Dr. Razonable reports grants from Regeneron, grants from Roche, grants from Gilead, personal fees from Novartis, outside the submitted work.
Dr. Seville has nothing to disclose.
Dr. Speicher has nothing to disclose.
Dr. Tulledge-Scheitel has nothing to disclose.
Dr. Venkatakrishnan reports personal fees from nference, outside the submitted work.
Dr. Wilker has nothing to disclose.
Acknowledgments
The writing group thanks all the members of the MATRx team (Supplement). They are affiliated with the Mayo Clinic and did not receive compensation for their work in establishing the Mayo Clinic Monoclonal Antibody Infusion Program.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101102.
Appendix. Supplementary materials
References
- 1.xxx An EUA for bamlanivimab-a monoclonal antibody for COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.24415. [DOI] [PubMed] [Google Scholar]
- 2.xxx An EUA for casirivimab and imdevimab for COVID-19. Med Lett Drugs Ther. 2020;62(1614):201–202. [PubMed] [Google Scholar]
- 3.xxx An EUA for bamlanivimab and etesevimab for COVID-19. Med Lett Drugs Ther. 2021;63(1621):49–50. [PubMed] [Google Scholar]
- 4.Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreich D.M., Sivapalasingam S., Norton T. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhimraj A, Morgan R, Schumaker AH, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 05/10/ 2021. [DOI] [PMC free article] [PubMed]
- 7.NIH. Anti-SARS-CoV-2 monoclonal antibodies. Available at: https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/. Accessed May 10, 2021.
- 8.Razonable R.R., Aloia N.C.E., Anderson R.J. Mayo Clinic Proceedings; 2021. A framework for outpatient infusion of anti-spike monoclonal antibodies to high-risk patients with mild to moderate coronavirus disease-19: the mayo clinic model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caliendo M., Kopeinig S. Some practical guidance for the implementation of propensity score matching. J Econ Surv. 2008;22(1):31–72. [Google Scholar]
- 10.Tian Y., Schuemie M.J., Suchard M.A. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018;47(6):2005–2014. doi: 10.1093/ije/dyy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosmer D.W., Lemeshow S., May S. Wiley-Interscience; Hoboken, NJ: 2008. Applied survival analysis: regression modeling of time-to-event data. [Google Scholar]
- 12.Davidson-Pilon C, Kalderstam J, Zivich P, et al. CamDavidsonPilon/lifelines: v0. 21.3. Zenodo 2019.
- 13.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P., Nirula A., Heller B. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crane S.J., Ganesh R., Post J.A., Jacobson N.A. Telemedicine consultations and follow-up of patients with COVID-19. Mayo Clin Proc. 2020;95(9s) doi: 10.1016/j.mayocp.2020.06.051. S33-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganesh R., Salonen B.R., Bhuiyan M.N. Managing patients in the COVID-19 pandemic: a virtual multidisciplinary approach. Mayo Clinic Proc: Innovat, Qual Outcomes. 2021;5(1):118–126. doi: 10.1016/j.mayocpiqo.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Horo J.C., Cerhan J.R., Cahn E.J. Mayo Clinic Proceedings; 2020. Outcomes of COVID-19 with the mayo clinic model of care and research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Group R.C., Horby P., Lim W.S. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Vidal C., Cozar-Llisto A., Meira F. Trends in mortality of hospitalised COVID-19 patients: a single centre observational cohort study from Spain. Lancet Reg Health Eur. 2021;3 doi: 10.1016/j.lanepe.2021.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz L.I., Jones S.A., Cerfolio R.J. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16(2):90–92. doi: 10.12788/jhm.3552. [DOI] [PubMed] [Google Scholar]
- 23.Medicine JHU. Coronavirus resource center. Available at: https://coronavirus.jhu.edu/. Accessed 06/02/ 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.