Abstract
Introduction
Avoidance of inhaled bird antigens is essential to prevent hypersensitivity pneumonitis disease progression. The aim of the present study was to develop a sandwich enzyme link immunoassay (ELISA) and an immunochromatographic test (ICT) and compare their ability to detect pigeon antigens in environmental samples.
Methods
An amplified sandwich ELISA using pigeon serum as a calibration standard and a ICT using gold-labeled anti-pigeon serum antibodies for the rapid detection of pigeon antigens in environmental samples were developed. Twenty-two different airborne samples were collected and analysed using both methods. Strip density values obtained with ICT were calculated and compared with the concentrations determined by the ELISA method for pigeon antigens. Strips results were also visually analysed by five independent evaluators.
Results
The ELISA method to quantify pigeon antigen had a broader range (58.4 and 10,112.2 ng/ml), compared to the ICT assay (420 to 3360 ng/ml). A kappa index of 0.736 (p < 0.0001) was obtained between the observers evaluating the ICT strips. The results of the ELISA and the relative density of the ICT showed a highly significant correlation (rs:0.935; p < 0.0001). Bland-Altman plot also confirmed excellent agreement between the two methods (mean difference: −1.626; p < 0.0001).
Conclusions
Since there was a good correlation between both assays, we can conclude that the rapid and simple ICT assay is a good and valid alternative, which does not require expensive equipment, for the validated ELISA technique.
Keywords: Hypersensitivity pneumonitis, Immunochromatographic test, Pigeon antigen, Strip assay
Graphical abstract
Highlights
-
•
Demonstrating antigen exposure is critical in the diagnosis and management of hypersensitivity pneumonitis.
-
•
An immunochromatographic test is compared with an ELISA method for the measurement of pigeon antigens in the environment.
-
•
The immunochromatographic test described was highly consistent with the validated ELISA and does not require specific skills.
-
•
This method may prove to be a useful tool for predicting the progression of bird-related hypersensitivity pneumonitis.
1. Introduction
Hypersensitivity pneumonitis (HP) is an interstitial lung disease characterized by inflammation in bronchioles and alveoli (Selman, 2004). It occurs in some genetically predisposed individuals after recurrent inhalation of particular environmental antigens, usually avian and fungal proteins. HP is characterized by fever, dyspnea, chest tightness/discomfort and/or cough (Quirce et al., 2016), and it has recently been classified into two different forms: an acute form characterized by inflammation and a chronic form characterized by fibrosis, according to the intensity and frequency of antigen exposure, clinical evaluation, pulmonary function tests and radiologic findings (Lacasse et al., 2009; Vasakova et al., 2017).
Bird-related hypersensitivity pneumonitis (BRHP) occurs after inhalation exposure to avian antigens. It can be caused by either direct exposure, i.e., through breeding birds, or indirect exposure, i.e., due to the presence of nearby dovecotes, flocks of pigeons in parks, feather duvets, and so on (Shirai et al., 2017; Morell et al., 2008). It has been demonstrated that the most common antigenic sources are droppings, feathers and blooms (Vasakova et al., 2019; Raghu et al., 2020). Next to pigeon antigens, other types of avian antigen such as those associated with parrots, parakeets, chickens, ducks, etc., can cause BRHP (Kokkarinen et al., 1994). Currently, it seems that the prevalence of BRHP is underestimated. A Japanese epidemiological study conducted between 2000 and 2009 revealed that 60% of chronic cases of HP were caused by avian antigens (Okamoto et al., 2013), and it has been shown that, in some patients, this disease can be caused by exposure to feather duvets or pillows (Morell et al., 2013).
For patients with BRHP, complete avoidance of inhaled avian antigens is essential to improve the respiratory symptoms and prevent disease progression (Vourlekis et al., 2004). Some BRHP cases possibly become chronic because of the persistence of their exposure to low levels of antigen (Borderías et al., 2010). To control exposure to avian antigen levels in the environment, air measurements are essential for these patients (Agache and Rogozea, 2013). Previous studies have measured the concentration of avian antigens in collected dust and air samples by using direct, inhibition or sandwich enzyme-linked immunoassays (ELISA) (Craig et al., 1992; Tsutsui et al., 2015), while others used an antigen-capture ELISA with signal amplification in order to improve sensitivity and detect small amounts of antigen (Kuramochi et al., 2010). All these techniques are costly and time-consuming and require access to laboratories with sophisticated equipment and trained personnel, and so they are not readily adaptable to field use. Therefore, considerable efforts are being made to develop easy-to-use analytical methods that are able to guide legislation on the concentration of these proteins in the air, and may also allow consumers to monitor their levels.
The immunochromatographic test (ICT) is a rapid semiquantitative allergen estimation method, easy to perform and interpret, portable, and cheap. In this test, a sample with labeled specific antibodies is mixed and incubated with a strip. Then, the immunocomplexes formed migrate by capillarity through the nitrocellulose membrane and bind to the line of immobilized specific capture antibodies if the sample contains the specific antigen (Bogdanovic et al., 2006; Tsay et al., 2002; Álvarez-Simon et al., 2014).
The aim of the present study was to develop and validate a sensitive sandwich enzyme link immunoassay technique and a rapid ICT to detect pigeon antigens in environmental air samples and to determine whether the results obtained with the two methods were comparable.
2. Material and methods
2.1. Preparation of rabbit anti-pigeon polyclonal antibodies and colloidal gold labeling
Anti-pigeon polyclonal antibodies were produced by Kaneka Eurogentec S.A., Seraing, Belgium. In Brief, a commercial pigeon serum (Rockland Immunochemicals Inc., Limerick, Ireland), with a protein concentration of 15.5 mg/ml determined previously by the Bicinchoninic acid (BCA) method (Pierce Chemical Co., Rockford, USA), was subcutaneous injected into two New Zealand white rabbits. Three booster injections were administered at 4-week intervals. The use of the animals for this purpose was approved by the firm's Animal Ethics Committee.
The IgG fraction of the polyclonal antisera of both rabbits was isolated on immobilized Protein A–agarose columns (Pierce Chemical Co., Rockford, USA), and then eluted to Excellulose columns (Pierce Chemical Co., Rockford, USA) to desalt and exchange the buffer to phosphate buffer saline (PBS). Purified and desalt IgG fractions from each rabbit were named Pol J and Pol K. The protein concentration of the IgG fraction of the sera was 7.7 mg/ml (Pol J) and 6.4 mg/ml (Pol K), as determined by the BCA method.
A portion of rabbit anti-pigeon polyclonal antibody from one rabbit (Pol J) was biotinylated using the EZ links Sulfo-NHS-LC-Biotinylation Kit (Pierce Chemical Co.) according to the manufacturer's instructions. Another portion of Pol J was labeled with colloidal gold by BBI Solutions (British Biocell International, Cardiff, UK). Briefly, the antibody was conjugated to a 40 nm gold colloid using passive adsorption and was stable at least for 10 months. Then, the conjugate was blocked with bovine serum albumin (BSA) (Pierce Chemical Co., Rockford, USA) and the resulting conjugate was concentrated by ultrafiltration to give a final optical density (OD) (520 nm) of 10 and suspended in a 2 mM disodium tetraborate buffer pH 7.2, containing 0.095% sodium azide.
2.2. Sample collection and analysis
Twenty-two air samples were collected from two different dovecotes in the province of Barcelona with a medium-volume automated air sampler (Flite 3, Vertex technics S.L, Barcelona, Spain) containing nitrocellulose filters with a 0.65 μm pore size (Millipore S.A.S., Molsheim, France). The automated air sampler had a pole 115 cm high with a support at the top for the nitrocellulose filter. Dust was collected at a flow of 12 l/min during 1 h, 2 h, 4 h, 8 h, 12 h or 24 h. Air samples of environments without avian antigens were also collected at the same flow for 1 h, 2 h, 4 h or 8 h, for use as negative controls. Antigens were extracted from the filters in 1.5 ml PBS/0.2% BSA/0.5% Tween-20 (pH 7.4) buffer overnight at 4 °C. Filters were discarded and the eluates were stored at −20 °C until being analysed in parallel by the anti-pigeon sandwich ELISA and the ICT.
2.3. Characterization of pigeon serum and air samples by 1D and 2D electrophoresis
For 1D electrophoresis performance, Laemmli sample buffer 4× (Bio-Rad, Madrid, Spain) was diluted in 2-mercaptoethanol (Sigma-Aldrich Quimica S.L, Madrid, Spain) according to the manufacturer's instructions. Pigeon serum (850 μg protein) or air sample (60 ng protein) were mixed with the previous mixture at a 3:1 proportion and heated for 5 min at 90 °C in the water bath. Subsequently, samples were loaded onto preparative Criterion TGX Stain-Free gels 4–20% precast polyacrylamide gel (Bio-Rad, Madrid, Spain) according to the manufacturer's manual, and electrophoresis was performed at 200 V for 40 min.
For 2D electrophoresis, pigeon serum (100 μg protein) was mixed with rehydration solution (pure H2O/8.5 M Urea/2% Chaps/0.5% immobilized pH gradient (IPG) buffer/0.002% Bromophenol blue) and loaded onto IPG strips (IPG strip, pH 3–10, 7 cm, General Electric Healthcare, Boston, USA). Isoelectric focusing (IEF) was then carried out using the IPGphor IEF system (General Electric Healthcare, Boston, USA) following the manufacturer's instructions. IPG strips were then equilibrated and loaded onto Mini-PROTEAN TGX Stain-Free gels 4–20% (7 cm, IPG/prep, Bio-Rad, Madrid, Spain) and run at 200 V for 30 min.
In both cases, Precision Plus Protein™ Unstained Standards (Bio-Rad, Madrid, Spain) were used as molecular weight (MW) markers with a MW ranging from 10 to 250 kDa. After 1D and 2D electrophoresis, gels were imaged in a Gel Doc EZ Imager (Bio-Rad, Madrid, Spain) and proteins were stained with Coomassie brilliant blue (Bio-Rad, Madrid, Spain) or blotted to a membrane.
2.4. Immunoblotting of pigeon serum
1D and 2D gels of pigeon serum were blotted onto polyvinylidene difluoride (PVDF) membranes using the Trans-Blot Turbo transfer system (Bio-Rad, Madrid, Spain). Then, membranes were washed with PBS and blocked with 5% nonfat dry milk (Bio-Rad, Madrid, Spain) for 1 h at room temperature (RT) under gentle shaking. After three washing steps with PBS/0.05 T (PBS with 0.05% Tween-20), membranes were incubated with rabbit anti-pigeon polyclonal antibody Pol J or Pol K diluted 1/5000 in PBS for 2 h at RT with gentle shaking. After three washing steps with PBS/0.05 T, membranes were incubated with goat anti-rabbit IgG-HRP (H + L, SouthernBiotech, Birmingham, USA) diluted 1/20,000 in PBS for 1 h at RT. Membranes were then washed three times with PBS/0.05 T and incubated with the enzyme substrate for 5 min at RT (Kit Clarity Western ECL Substrate, Bio-Rad, Madrid, Spain), according to the supplier's recommendations. Image acquisition was carried out with the Odyssey Fc Imaging System and the Image Studio Lite software (LI-COR, Nebraska, USA).
2.5. Sandwich ELISA for pigeon antigens
Wells of high-binding microtiter plates (Costar, Cambridge, USA) were coated overnight at 4 °C with Pol K (0.1 μg protein/well) in PBS buffer (pH 7.4). The wells were blocked (200 μl/well) with PBS/1% BSA/0.05% Tween-20 for 1 h at 37 °C. After washing three times with PBS/0.1 T (PBS with 0.1% Tween 20), plates were incubated for 1 h at 37 °C with 100 μl/well of filter elutes, negative controls (eluate of a white filter) and pigeon antigen standards containing 6.7–27,500 ng protein/ml. After three washes with PBS/0.1 T, plates were incubated for 1 h at 37 °C with 100 μl/well of Pol J biotinylated at 1:15,000 dilution in PBS/1% BSA/0.05% Tween-20. Streptavidin (Sigma-Aldrich Quimica S.L, Madrid, Spain) at 1/10,000 dilution in PBS/1% BSA/0.05% Tween-20 was added (100 μl/well) after washing again three times with PBS/0.1 T. After streptavidin incubation for 1 h at 37 °C, wells were washed three times and the reaction was developed with 30% H2O2 and 3,3′,5,5′-tetramethylbenzidine in acetate buffer (pH 5.5) at 25 °C in the dark. After 20 min, the reaction was stopped with 2 M H2SO4 and read at 450 nm with a plate reader (Infinite M Nano, Tecan, Männedorf, Switzerland). The standard curve was constructed from seven data points using a four-parameter logistic curve fit. Results are expressed in ng/ml, referring to the protein content of the standard preparation. Magellan software (Tecan Austria GmbH, Grödig, Austria) was used to calculate the results.
The lower limit of detection (LOD) was defined as the concentration of the lowest point on the standard curve, with the optical density (OD) value at this point having to be at least 0.05 above the blank. The lower limit of quantification (LOQ) was defined, according to the curve fitting function, as the concentration that gave the OD of the lowest point on the standard curve plus 20% of the highest point on the standard curve. The upper LOQ was defined as the concentration that gave 80% of the OD at the highest point on the standard curve. Following these premises, the determinations were largely limited to the log-linear part of the curve.
The inter-assay coefficient of variation (CV) was determined by analysing two points of the standard curve in duplicate on 10 different days. Thus, the CV of each point was calculated as the standard deviation of the mean of 10 measurements in duplicate (SD/mean x 100).
2.6. Specificity of the ELISA
Cross-reactivity of the ELISA assay towards other types of bird protein was tested using serum of small parrot, parakeet and parrot. Sera from those birds were obtained by centrifugation of collected blood samples. Subsequently, protein concentration of sera was determined by the BCA method (Pierce Chemical Co., Rockford, USA). Characterization of the different avian sera was performed by 1D and 2D electrophoresis following the protocol described above.
2.7. Immunochromatographic strip test
The capture antibody Pol K (960 ng/strip) was applied to direct cast backed nitrocellulose nitrate membranes (Unisart 1UN95E CN95, Sartorius Stedim Biotech, Goettingen, Germany) in a line format at 3 mm from the application end of the strip (Test line). At 6 mm, a goat antirabbit IgG antibody (Southern Biotechnology Associates, Inc., Birmingham, USA) was bound at 600 ng/strip to provide a positive control (Control line). Membranes were dried for 1 h at 37 °C and assembled as follows: on an adhesive support, an absorbent pad was layered and the membrane was placed overlapping it by 2 mm and cut into individual strips of 4 mm in width.
After optimization, 20 μl of environmental samples or pigeon serum standards were mixed 1:1 in a microtiter plate well with gold-labeled anti-pigeon Pol J antibody solution with an OD of 4. After forward pipetting, the gold-labeled Pol J antibody was allowed to react during 10 min at RT with pigeon antigens of the samples to form a complex. Then, a test strip was dipped in each well for 30 min at RT to allow antigen-antibody complexes to diffuse across the nitrocellulose membrane. These complexes reacted with the specific anti-pigeon Pol K antibodies immobilized on the test line forming a pink-purple line. The excess of colloidal dye antibody conjugate migrated further and reacted with the goat anti-rabbit IgG antibody in the control line.
Strips were scanned with a scanner (HP Scanjet G4050, Hewlett-Packard Enterprise, California, USA) and test line intensities were analysed by densitometry with ImageJ 1.52a (National Institutes of Health, USA). To determine the limit of detection of the strip assay, pigeon serum (Rockland Immunochemicals Inc., Limerick, Ireland) with a protein concentration of 27.5 mg/ml, previously determined by the BCA method, was used in a series of concentrations from 52.5 to 6720 ng/ml and tested in triplicate. In parallel, test lines of environmental samples were also read with the naked eye by five independent observers who classified the strips as negative (52.5 ng/ml), positive (420 ng/ml) and double positive (3360 ng/ml) comparing their color intensity with a 3-point standard curve (Fig. 1).
Fig. 1.
Three-point standard curve of pigeon serum proteins to evaluate the test line of the environmental strips as negative (52.5 ng/ml), positive (420 ng/ml) or double positive (3360 ng/ml). The figure shows the scanning of each standard curve strip and their densitometry analyses. C, control line; T, test line.
2.8. Statistical analysis
The Shapiro-Wilk normality test was calculated for pigeon aeroallergen levels measured by the ELISA method and for relative density determined from immunochromatographic strips. Spearman's rank correlation coefficient (rs) was calculated between pigeon aeroallergen levels and strip density values since all data showed a non-normal distribution. Bland-Altman analysis was performed to determine the agreement between the two assays. After logit transformation of the data, the difference between the paired measurements was plotted against the mean of the measurements. The immunochromatographic strip results were visually analysed by five independent evaluators, and Fleiss' Kappa test was used to calculate the degree of agreement between the different raters.
Statistical analyses were performed using GraphPad Prism 6 for Windows (version 6.01, GraphPad Software Inc., San Diego, California, USA) and IBM SPSS Statistics (version 26, IBM Corporation, Armonk, New York, USA). Differences with a p-value <0.05 (two-tailed) were considered to be significant.
3. Results
3.1. Characterization of pigeon serum and immunoblotting
Fig. 2A shows the different protein bands separated by 1D electrophoresis from pigeon serum (Fig. 2A, lane 2) and one of the air sample (Fig. 2A, lane 3) collected from the dovecotes. Different bands were observed with molecular weights ranging from 10 to 250 KDa. Some bands were found in common between the serum and the air sample, mostly between 25 and 65 kDa.
Fig. 2.
Protein composition of pigeon serum and an air sample eluate collected from the dovecote by one and two dimensional electrophoresis and Western blots with antibodies Pol K and Pol J. 2A) Lane 1, a molecular weight size marker in KDa; lane 2, Coomassie-stained 1D gel of pigeon serum; lane 3, Coomassie-stained 1D gel of an 8 h air sample; lane 4 and 5, immunoblots with Pol K and Pol J against pigeon serum, respectively; lane 6 and 7, 8 h air sample immunoblot with Pol K and Pol J, respectively; lane 8 and 9, pigeon immunoblot and air sample immunoblot, respectively, with serum of a patient with BRHP due to pigeon exposure. 2B) Lane 1, molecular weight marker in KDa; lane 2, Coomassie-stained 2D gel of pigeon serum; lane 3 and 4, Pol K and Pol J immunoblots against pigeon serum, respectively.
Fig. 2B (lane 2, 3 and 4) shows different spots of proteins from pigeon serum separated by molecular weight and isoelectric point in two dimensions. The observed proteins were mostly in the range of 20 to 25 KDa and 50 to 75 KDa.
Immunoblotting in one and two dimensions showed that the polyclonal antibodies Pol K and Pol J were able to detect a wide range of proteins from pigeon serum (Fig. 2A, lane 4 and 5, and 2 B, lane 3 and 4) and from the air sample (Fig. 2A, line 6 and 7). Serum from a patient diagnosed with BRHP due to pigeon exposure showed a similar pattern of recognition to those of the polyclonal antibodies Pol K and Pol J (Fig. 2A, line 8 and 9).
3.2. Characterization of the ELISA method
The sandwich ELISA against pigeon proteins was able to detect a range of concentrations between 6.7 and 27,500 ng/ml in the standard curve. However, the lower and upper LOQ determined the working range of concentrations between 58.4 and 10,112.2 ng/ml. The standard curve was formed by seven different concentrations of pigeon serum proteins that followed a sigmoidal shape (Fig. 3A. The inter-CV using two different points of the standard curve was 2.76% and 7.35% respectively. In the negative controls the antigen concentration was undetectable. As regards the specificity of the method, the sandwich ELISA showed some cross-reactivity with small parrot, parakeet and parrot. Fifty percent of the OD of the highest point on the pigeon standard curve was reached at protein concentrations of 54,344, 74,806 and 172,175 ng/ml for small parrot, parakeet and parrot respectively (Fig. 3A). One and two-dimensional electrophoresis showed that these birds had some protein bands/spots of similar size and/or charge with pigeon that might explain this cross-reactivity (Fig. 3B).
Fig. 3.
Protein composition of bird sera (small parrot, parakeet and parrot serum) by one and two-dimensional electrophoresis (3A) and cross-reactivity of these sera with a sandwich ELISA for pigeon antigen detection (3B).
3.3. Characterization of the immunochromatographic strip assay
The rapid test was able to detect a range of concentrations from 420 to 3360 ng/ml (Fig. 4). The lower LOD (420 ng/ml) was considered as the minimum amount of pigeon serum proteins from the standard curve giving a well-defined pink-purple band at the test line (Fig. 4A). The upper LOD (3360 ng/ml) was considered as the maximum amount of pigeon serum proteins producing a more intense pink-purple band at the test line compared to the previous concentration (Fig. 4A). In the negative controls the antigen concentration was undetectable.
Fig. 4.
Immunochromatographic strip assay standard curve to determine the lower and upper limits of detection. 4A) Scanning of the test line of each standard curve strip. 4B) Graph of relative density results (mean ± SD) at each concentration of pigeon serum proteins using a four parameter logistic curve.
3.4. Inter-reader agreement of immunochromatographic strip assay results
According to the observers' interpretations of the strips as negative, positive or double positive, a kappa index of 0.574 (CI 0.479–0.668; p < 0.0001) was obtained. However, when considering positive and double positive results as a single category the kappa index increased (0.736, CI 0.605–0.867, p < 0.0001). The observers were able to detect visually pigeon allergens in a concentration range of 141 to 3360 ng/ml in air sample eluates.
3.5. Comparison between ELISA and immunochromatographic strip assay
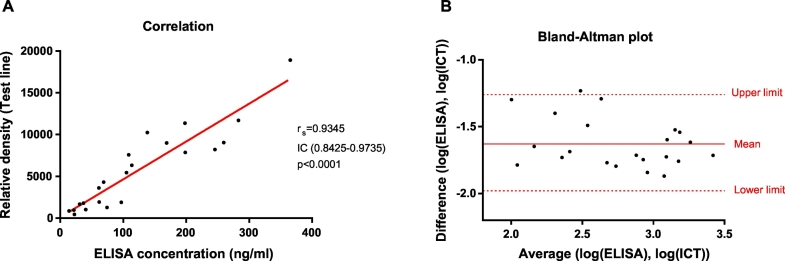
A strong correlation was observed between the concentration determined by the ELISA method and the relative density results of the strip assay with a rs coefficient of 0.935 (CI 0.843–0.974; p < 0.0001) (Fig. 5A). The Bland-Altman plot also confirmed a good agreement between the methods with a mean difference of −1.626 (CI -1.988 to −1.264; p < 0.0001) (Fig. 5B).
Fig. 5.
Comparison of pigeon aeroallergen concentration determined by ELISA method and density values calculated with the immunochromatographic strip assay. 5A) Spearman's rank correlation. 5B) Bland-Altman plot.
4. Discussion
We developed and validated two sensitive methods to detect pigeon antigens in environmental air samples: an ELISA and an immunochromatographic strip assay. Although other studies describing the quantification of avian antigens have been reported (Craig et al., 1992; Tsutsui et al., 2015; Kuramochi et al., 2010), to our knowledge, this is the first in which an ICT is presented and compared with an ELISA method for the measurement of pigeon antigens in the environment. The study demonstrates that the methods have enough sensitivity, specificity, reproducibility, and applicability for the detection of environmental exposure to pigeon antigens.
Chronic HP is difficult to identify and is often misdiagnosed as idiopathic pulmonary fibrosis (Raghu et al., 2020; Morell et al., 2013). Therefore, demonstrating antigen exposure and evaluating the effects of allergen avoidance measures are critical in the diagnosis and management of this disease. Tsutsui et al. reported that the concentration of avian antigen present in household dust correlated with the prognosis of chronic bird-related HP (Tsutsui et al., 2015). Chronic HP has been assumed to progress as a result of persistent exposure to very small amounts of antigen. Moreover, avian antigens were reported to persist in the patient's house six months after removal of all birds (Morell et al., 2008; Craig et al., 1992). Therefore, it is important to examine the environmental levels of antigen after diagnosis and during antigen avoidance in order to facilitate the control of this disease.
Environmental monitoring of airborne antigens is a time-consuming process that commonly comprises three steps: sampling aeroallergens on a filter, elution of the allergens, and determination of allergen levels by an allergen-specific ELISA (Gómez-Ollés et al., 2006). Here, we first obtained polyclonal antibodies with high specific reactivity to pigeon serum antigens and developed a validated ELISA method using those antibodies. Second, we developed and validated a novel ICT using the same antibodies used in the ELISA method. Previous studies have proposed that ICT may be a valid alternative to the ELISA, as it speeds up the last step of the process (Bogdanovic et al., 2006; Tsay et al., 2002; Álvarez-Simon et al., 2014).
The antigenic components associated with the development of BRHP was described using biochemical analyses and immunoassays. The main antigenic substances suspected were immunoglobulins, serum albumin, and intestinal mucin, from droppings, blooms and pigeon sera (Shirai et al., 2017; de Beer et al., 1990; Rouzet et al., 2017). Owing to the difficulty of obtaining antigenic extracts from intestinal mucin or bloom, pigeon serum is the antigen source most often used to measure specific antibodies in this type of HP (Rodrigo et al., 2000). The immunoblotting results of the present study demonstrated that the polyclonal antibodies used in the methods developed were able to detect the relevant pigeon antigens and that most of the antigenic proteins identified were also abundant in droppings and blooms (Rouzet et al., 2017). Rouzet et al. (2017) found proteins with a molecular weight range between 20 and 200 kD in bird dropping extracts, and that IGLL-1 (Immunoglobulin lambda-like polypeptide-1, 24.5 KDa) and ProE (Proproteinase E, 20.5 KDa) were the antigenic proteins with the most clinical interest as biomarkers for BRHP diagnosis. In this regard, Shirai et al. (2017) in an immunoblot analysis of sera from patients with BRHP, identified multiple immunoreactive bands in pigeon serum at various molecular weights, especially IGLL-1, similar to those obtained in the present study.
Moreover, the cross-reactivity study with other birds demonstrated elevated specificity (Fig. 3). Sera of small parrot, parakeet and parrot seem to cross-react slightly with pigeon serum, probably because they have common sera proteins (as observed in the 2D electrophoresis). Another possible reason was suggested by Rouzet et al. (2014). in a study designed to determine the specificity of immunogenic proteins of the droppings of three bird species; those authors reported that although no proteins were found common to all extracts, cross-reactions to avian antigens from the three species of bird were observed.
The sandwich ELISA using these polyclonal antibodies has a high sensitivity and specificity and has demonstrated its value for measuring pigeon antigens, even at relatively low concentrations. In this study the levels of pigeon antigens were assessed with respect to a reference preparation (pigeon antigen standard) containing 6.7–27,500 ng of protein/ml. This standard proved to be a suitable reference preparation for the measurement of pigeon exposure, as shown by the results of the SDS-PAGE gel (Fig. 2) and the comparison of the proteins of this standard with the air sample eluates collected from the dovecote. These assessments demonstrated that this standard is composed of the proteins contained in the air.
Although the ELISA is a very useful technique for detecting soluble antigens and meets most of the main requirements for analysing pigeon antigens (i.e., reproducibility, good specificity and acceptable sensitivity), it is a time-consuming process that requires skilled personnel and expensive laboratory equipment. Therefore, cost-efficient, less labor-intensive technological procedures that can be performed by non-specialist laboratory staff are needed in order to monitor pigeon antigen levels. ICT can provide fast qualitative or even semi-quantitative results within minutes with very good analytical sensitivity. Furthermore, the characteristics of their design and construction make them especially suitable for field testing and for use by non-laboratory-skilled personnel (Bogdanovic et al., 2006; Tsay et al., 2002). Fast antigen monitoring systems has been shown to be highly beneficial to diminishing exposure (Tsutsui et al., 2015; Kuramochi et al., 2010), therefore, rapid immunoassay for the assessment of pigeon antigens would be a very useful tool.
The visual interpretation of the ICT showed high levels of sensitivity and specificity, comparable to those recorded with other rapid assays measuring other aeroallergens (Bogdanovic et al., 2006; Tsay et al., 2002; Álvarez-Simon et al., 2014; Koets et al., 2011). Furthermore, the line intensity (analysed by densitometry, an objective measurement) of samples categorized as negative was significantly lower than that of positive and double positive samples. The LOD, determined as the minimum amount of allergen capable of producing a clearly visible signal in the test line of the standard in the rapid immunoassay, was 420 ng/ml. Unexpectedly, in the visual analysis of our test results, the independent observers detected pigeon antigen concentrations below the theoretical LOD determined for the assay; depending on the rater, the actual LOD of the assay with real field samples ranged from 141 to 416 ng/ml. This observation has also been reported in other studies: for example, Tsay et al. (2002) graded the line intensity of some samples analysed as medium or high even although the allergen levels analysed by ELISA were below the theoretical LOD of the rapid assay. Similarly, in the studies by Bogdanovic et al. (2006) and Álvarez-Simon et al. (2014), some samples with allergen levels below the sensitivity of the rapid test were classified as positive. Moreover, visual interpretation of rapid assays is inherently subjective, and depends on factors such as the rater's color perception. Therefore, it is important to assess inter-rater agreement. In this study, the agreement was graded as substantial, according to the classification recommended by Landis and Koch (1977).
The main advantage of this ICT is the speed of the analysis, and the fact that it can be performed in anon-laboratory environments. Although speed may not be decisive in environmental monitoring, it may constitute a real advantage for industrial hygiene monitoring, as some of the sampling procedures widely used in occupational hygiene take only a short time (for example, surface wipe sampling, dust sampling or bulk sampling). In this context, some studies have assessed the environmental measurement of avian antigen in dust (Craig et al., 1992; Kuramochi et al., 2010; Sema et al., 2018; Curtis et al., 2002). For example, Craig et al. (1992) reported that avian antigen could be detected in dust samples for prolonged periods of time after bird removal and environmental clean-up. Curtis et al. (2002) also reported that pigeon antigen was found in dust and air samples from a pigeon-infected school.
One limitation of these methods is the problem of discriminating between safe and unsafe environments. In this sense, data on environmental levels of pigeon antigen to which patients with bird-related hypersensitivity pneumonitis are exposed were lacking in the present study. It may be difficult to establish thresholds for allergen exposures, due to inter-individual variations in susceptibility to both sensitization and disease elicitation. These differences may be caused by genetic variations, age-dependent effects, or lifestyle factors (Maciag and Phipatanakul, 2020). Smoking, for example, may promote sensitization due to an adjuvant effect (Carlos et al., 2009). Moreover, different proteins have different sensitization potencies (Krutz et al., 2020). In any case, avoiding exposure to causal agents is crucial in HP management and is a decisive factor in prognosis, because progression can broadly be prevented with appropriate antigen avoidance. Moreover, identification of the offending antigen source at work is important for the determination of antigen-specific IgG antibodies.
In conclusion, we developed an ICT to monitor pigeon aeroallergen levels. The strip assay described was rapid, simple, sensitive, highly consistent with the validated ELISA and does not require expensive equipment or specific skills. This method for monitoring levels of pigeon antigen may prove to be a useful and versatile tool for predicting the progression of bird-related hypersensitivity pneumonitis.
Funding
SSD is researcher supported by CIBER (Instituto de Salud Carlos III, Spain), and MJC is a researcher supported by the Miguel Servet programme of the Instituto de Salud Carlos III (MSII17/00025). This project was supported by the Fundació Catalana de Pneumologia (FUCAP), Sociedad Española de Patología Respiratoria (SEPAR), FIS PI15/01954 (Instituto de Salud Carlos III), Fondo Europeo de Desarrollo Regional (FEDER) and European Union's Horizon 2020 research and innovation programme under grant agreement No 874707. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
CRediT authorship contribution statement
Silvia Sánchez-Díez: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. María-Jesús Cruz: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. Daniel Álvarez-Simón: Methodology, Writing – review & editing. Tomás Montalvo: Conceptualization, Methodology, Writing – review & editing. Xavier Muñoz: Writing – review & editing. Peter M. Hoet: Methodology, Writing – review & editing. Jeroen A. Vanoirbeek: Methodology, Writing – review & editing. Susana Gómez-Ollés: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Lotfi Aleya
References
- Agache I.O., Rogozea L. Management of hypersensivity pneumonitis. Clin. Transl. Allergy. 2013;3(1):5. doi: 10.1186/2045-7022-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Simon D., Cruz M.J., Untoria M.D. A rapid test for soy aeroallergens exposure assessment. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovic J., Koets M., Sander I. Rapid detection of fungal α-amylase in the work environment with a lateral flow immunoassay. J. Allergy Clin. Immunol. 2006;118(5):1157–1163. doi: 10.1016/j.jaci.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Borderías L., Morell F., Vera J., Briz H., Muñoz X., Cruz M.J. Neumonitis por hipersensibilidad crónica por estorninos. Exposición antigénica no masiva pero persistente. Arch. Bronconeumol. 2010;46(11):607–609. doi: 10.1016/j.arbres.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Carlos E.B.C., Gómezc R.M., Rodrigo B.C., Canonicab G.W. Impact of environmental tobacco smoke and active tobacco smoking on the development and outcomes of asthma and rhinitis. Curr. Opin. Allergy Clin. Immunol. 2009;9(2):136–140. doi: 10.1097/ACI.0b013e3283294038. [DOI] [PubMed] [Google Scholar]
- Craig TJ, Hershey J, Engler RJ, Davis W, Carpenter GB, Salata K. Bird antigen persistence in the home environment after removal of the bird. Ann. Allergy. 1992;69(6):510–512. http://www.ncbi.nlm.nih.gov/pubmed/1471783. Accessed May 2, 2019. [PubMed]
- Curtis L, Lee B-S, Cai D, et al. Pigeon allergens in indoor environments: a preliminary study. Allergy. 2002;57(7):627–631. http://www.ncbi.nlm.nih.gov/pubmed/12100304. Accessed May 2, 2019. [DOI] [PubMed]
- de Beer P.M., Bouic P.J., Joubert J.R. Identification of a “disease-associated” antigen in pigeon breeder’s disease by western blotting. Int. Arch. Allergy Immunol. 1990;91(4):343–347. doi: 10.1159/000235139. [DOI] [PubMed] [Google Scholar]
- Gómez-Ollés S., Cruz M.J., Renström A., Doekes G., Morell F., Rodrigo M.J. An amplified sandwich EIA for the measurement of soy aeroallergens. Clin. Exp. Allergy. 2006;36(9):1176–1183. doi: 10.1111/j.1365-2222.2006.02542.x. [DOI] [PubMed] [Google Scholar]
- Koets M., Renström A., Zahradnik E., Bogdanovic J., Wouters I.M., Van Amerongen A. Rapid one-step assays for on-site monitoring of mouse and rat urinary allergens. J. Environ. Monit. 2011;13(12):3475–3480. doi: 10.1039/c1em10658a. [DOI] [PubMed] [Google Scholar]
- Kokkarinen J., Tukiainen H., Seppä A., Terho E.O. Hypersensitivity pneumonitis due to native birds in a bird ringer. Chest. 1994;106(4):1269–1271. doi: 10.1378/CHEST.106.4.1269. [DOI] [PubMed] [Google Scholar]
- Krutz N.L., Kimber I., Maurer-Stroh S., Gerberick G.F. Determination of the relative allergenic potency of proteins: hurdles and opportunities. Crit. Rev. Toxicol. 2020;50(6):521–530. doi: 10.1080/10408444.2020.1793895. [DOI] [PubMed] [Google Scholar]
- Kuramochi J., Inase N., Takayama K., Miyazaki Y., Yoshizawa Y. Detection of indoor and outdoor avian antigen in management of bird-related hypersensitivity pneumonitis. Allergol. Int. 2010;59(2):223–228. doi: 10.2332/allergolint.09-OA-0161. [DOI] [PubMed] [Google Scholar]
- Lacasse Y., Selman M., Costabel U. Classification of hypersensitivity pneumonitis. Int. Arch. Allergy Immunol. 2009;149(2):161–166. doi: 10.1159/000189200. [DOI] [PubMed] [Google Scholar]
- Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- Maciag M.C., Phipatanakul W. Prevention of asthma: targets for intervention. Chest. 2020;158(3):913–922. doi: 10.1016/j.chest.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell F., Roger À., Reyes L., Cruz M.J., Murio C., Muñoz X. Bird fancier’s lung: a series of 86 patients. Medicine (Baltimore) 2008;87(2):110–130. doi: 10.1097/MD.0b013e31816d1dda. [DOI] [PubMed] [Google Scholar]
- Morell F., Villar A., Montero M.-Á. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir. Med. 2013;1(9):685–694. doi: 10.1016/S2213-2600(13)70191-7. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Miyazaki Y., Ogura T. A nationwide epidemiological survey of chronic hypersensitivity pneumonitis in Japan. Respir. Investig. 2013;51(3):191–199. doi: 10.1016/j.resinv.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Quirce S., Vandenplas O., Campo P. Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy. 2016;71(6):765–779. doi: 10.1111/all.12866. [DOI] [PubMed] [Google Scholar]
- Raghu G., Remy-Jardin M., Ryerson C.J. Diagnosis of hypersensitivity pneumonitis in adults. An official ATS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020;202(3):e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo M.J., Benavent M.I., Cruz M.J. Detection of specific antibodies to pigeon serum and bloom antigens by enzyme linked immunosorbent assay in pigeon breeder’s disease. Occup. Environ. Med. 2000;57(3):159–164. doi: 10.1136/OEM.57.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzet A., Reboux G., Rognon B. Immunogenic proteins specific to different bird species in bird fanciers lung. J. Toxicol. Environ. Heal - Part A Curr. Issues. 2014;77(12):724–730. doi: 10.1080/15287394.2014.889616. [DOI] [PubMed] [Google Scholar]
- Rouzet A., Reboux G., Dalphin J.-C. An immunoproteomic approach revealed antigenic proteins enhancing serodiagnosis performance of bird fancier’s lung. J. Immunol. Methods. 2017;450:58–65. doi: 10.1016/j.jim.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Selman M. Hypersensitivity pneumonitis: a multifaceted deceiving disorder. Clin. Chest Med. 2004;25(3):531–547. doi: 10.1016/j.ccm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Sema M., Miyazaki Y., Tsutsui T., Tomita M., Eishi Y., Inase N. Environmental levels of avian antigen are relevant to the progression of chronic hypersensitivity pneumonitis during antigen avoidance. Immunity, Inflamm. Dis. 2018;6(1):154–162. doi: 10.1002/iid3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Furusawa H., Furukawa A. Protein antigen of bird-related hypersensitivity pneumonitis in pigeon serum and dropping. Respir. Res. 2017;18(1):65. doi: 10.1186/s12931-017-0555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay A., Williams L., Mitchell E.B., Chapman M.D., Multi-Centre Study Group A rapid test for detection of mite allergens in homes. Clin. Exp. Allergy. 2002;32(11):1596–1601. doi: 10.1046/j.1365-2222.2002.01533.x. http://www.ncbi.nlm.nih.gov/pubmed/12569980 (Accessed May 2, 2019) [DOI] [PubMed] [Google Scholar]
- Tsutsui T., Miyazaki Y., Kuramochi J., Uchida K., Eishi Y., Inase N. The amount of avian antigen in household dust predicts the prognosis of chronic bird-related hypersensitivity pneumonitis. Ann. Am. Thorac. Soc. 2015;12(7):1013–1021. doi: 10.1513/AnnalsATS.201412-569OC. [DOI] [PubMed] [Google Scholar]
- Vasakova M., Morell F., Walsh S., Leslie K., Raghu G. Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am. J. Respir. Crit. Care Med. 2017;196(6):680–689. doi: 10.1164/rccm.201611-2201PP. [DOI] [PubMed] [Google Scholar]
- Vasakova M., Selman M., Morell F., Sterclova M., Molina-Molina M., Raghu G. Hypersensitivity pneumonitis: current concepts of pathogenesis and potential targets for treatment. Am. J. Respir. Crit. Care Med. May 2019 doi: 10.1164/rccm.201903-0541PP. rccm.201903-0541PP. [DOI] [PubMed] [Google Scholar]
- Vourlekis J.S., Schwarz M.I., Cherniack R.M. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am. J. Med. 2004;116(10):662–668. doi: 10.1016/j.amjmed.2003.12.030. [DOI] [PubMed] [Google Scholar]