STRUCTURED ABSTRACT
Objective:
This study aims to validate a previously reported recurrence clinical risk score (CRS).
Summary of Background Data:
Salvage transplantation after hepatocellular carcinoma (HCC) resection is limited to patients who recur within Milan criteria (MC). Predicting recurrence patterns may guide treatment recommendations.
Methods:
An international, multicenter cohort of R0 resected HCC patients were categorized by MC status at presentation. CRS was calculated by assigning 1 point each for initial disease beyond MC, multinodularity, and microvascular invasion. Recurrence incidences were estimated using competing risks methodology, and conditional recurrence probabilities were estimated using the Bayes theorem.
Results:
From 1992 to 2015, 1023 patients were identified, of whom 613 (60%) recurred at a median follow-up of 50 months. CRS was well validated in that all 3 factors remained independent predictors of recurrence beyond MC (HR 1.5–2.1, all p<0.001) and accurately stratified recurrence risk beyond MC, ranging from 19% (CRS 0) to 67% (CRS 3) at 5 years. Among patients with CRS 0, no other factors were significantly associated with recurrence beyond MC. The majority recurred within 2 years. After 2 years of recurrence-free survival, the cumulative risk of recurrence beyond MC within the next 5 years for all patients was 14%. This risk was 12% for patients with initial disease within MC and 17% for patients with initial disease beyond MC.
Conclusions:
CRS accurately predicted HCC recurrence beyond MC in this international validation. While the risk of recurrence beyond MC decreased over time, it never reached zero.
Keywords: Hepatocellular carcinoma, recurrence pattern, clinical risk score, Milan criteria, transplant, resection
Mini-abstract:
This study validated the clinical risk score to predict and stratify recurrence beyond Milan criteria following resection of hepatocellular carcinoma. Clinical risk score is composed of 3 risk factors, with 1 point given each for initial disease beyond MC, multinodular disease, and presence of microvascular invasion.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a complex and heterogeneous disease. It is endemic in many regions, increasing in incidence, and is the third leading cause of cancer-related mortality worldwide.1–3 Hepatocellular carcinoma remains a challenge to treat in part due to its high recurrence rates and the limitations of potentially curative therapies, such as liver transplantation and resection.4, 5
As a potentially curative procedure, liver transplantation has theoretical advantages over liver resection, as it not only removes the tumor but also the at-risk liver remnant.6, 7 However, in reality, it is plagued by many problems, such as the need for lifelong immunosuppression, the scarcity of donor organs, and the high dropout rates during the long waitlist.8–10 Liver resection also has its limitations and disadvantages. The adequacy of the future liver remnant often limits extensive resection especially in cirrhotic patients and the high 5-year recurrence rates of up to 70% post resection have been reported.5, 11, 12 The optimal treatment strategy should ideally be guided by a rigorous risk-versus-benefit assessment, but even such analysis does not always provide a clear answer. While transplantation is clearly the superior modality in patients with compromised liver function, when intention-to-treat analysis is used, liver transplantation and liver resection may have comparable long-term outcome.13–16
Due to the significant recurrence rates post liver resection, understanding the pattern of HCC recurrence becomes important in influencing treatment strategy, especially for the patients within both resection and transplantation (e.g. Milan) criteria.17 In another words, if we can accurately predict that a patient’s HCC will not recur beyond Milan criteria, then salvage transplantation may be a viable potential treatment, and an upfront resection perhaps may be the best option for this patient. Resection followed by salvage transplantation have shown to yield comparable long term outcome in well selected patients compared to primary transplantation.18 However, the challenge remains in the accurate prediction of the recurrence pattern after resection of HCC.
Several prognostic nomograms have been created and compared against conventional staging systems, but they resulted in concordance index of approximately 0.54 – 0.62 on external validation.19–22 The heterogeneous nature of HCC tumor, underlying liver disease, and patient populations make prediction of recurrence challenging. We have previously developed a clinical risk score (CRS) to predict recurrence patterns after resection of HCC from 1 institution.23 CRS is composed of 3 risk factors, with 1 point given each for initial disease beyond Milan criteria, multinodular disease, and presence of microvascular invasion.23 This international, multi-center study is aimed to validate the CRS for prediction of HCC recurrence beyond Milan criteria as it may help guide treatment recommendations and surveillance.
METHODS
Study patients
With the approval of the Institutional Review Board at each center, we retrospectively analyzed 1023 consecutive patients who underwent resection of HCC with curative intent during 1992 to 2015. This included 628 patients from Singapore General Hospital, 124 patients from Erasmus Medical Center, 115 patients from University of Montreal, 94 patients from Washington University School of Medicine, and 62 patients from Memorial Sloan Kettering Cancer Center (MSKCC). Patients from MSKCC used to derive the CRS in the prior study were not included in this validation study.23
All patients in this study underwent primary R0 liver resection for HCC. Patients who had prior ablation, embolization, resection, or liver transplantation for HCC were not evaluated. Patients with fibrolamellar HCC or combined HCC-cholangiocarcinoma were also not included. From consecutive patients who underwent HCC resection, we excluded those who had microscopic positive, grossly positive, or unknown margins (n=73). We also excluded patients with perioperative death or loss of follow up within 90 days of resection in order to focus on evaluation of recurrence outcome (n=99). Patients were considered for resection if they had resectable tumors, adequate liver remnant taking in account of their liver metabolic function, and absence of any distant metastasis.
Clinicopathological variables
Medical records were reviewed for clinical variables including age at resection, gender, underlying liver disease, Child-Pugh classification, model for end-stage liver disease (MELD) score, and preoperative laboratory results such as α-fetoprotein (AFP) and platelet. Operative details included the number of segments resected and operative blood loss. Major hepatectomy was defined as ≥ 3 Couinaud segments. Pathological data included presence of multiple tumor nodules or satellites, largest tumor diameter, tumor differentiation, microvascular invasion, cirrhosis, steatosis, and extracapsular extension.
All patients were given a CRS 0 to 3, with 1 point given each for initial disease beyond MC, multinodular disease, and presence of microvascular invasion.23 Initial disease beyond MC was defined as 1 tumor at >5cm in size or >3 tumors with each tumor >3cm in size noted on preoperative abdominal imaging, presence of macroscopic vascular invasion or extrahepatic spread.17 There were 18 patients (2%) with unknown initial disease MC and thus information obtained from operative pathology was used to derive their initial disease MC status. Patients with unknown initial disease and pathologic MC were excluded from the study since initial disease MC is necessary to construct the CRS. Multinodular disease was defined as > 1 tumor nodules and satellites noted on operative pathology. Status of microvascular invasion was also based on pathological examination.
Details at the time of first recurrence were also documented, including recurrence pattern within or beyond MC and primary treatment modality. The dates of recurrence, last follow up, and death were also recorded to obtain recurrence-free survival (RFS) and overall survival (OS).
Statistical analysis
Clinical and pathological characteristics stratified by initial disease MC status were summarized using frequency and percentage for categorical covariates, and the median and range for continuous covariates. Categorical and continuous covariates were compared using Fisher’s exact test and Wilcoxon rank-sum test, respectively. Recurrence-free and overall survival were estimated using Kaplan-Meier methods and were compared using the log-rank test. Cumulative incidence of any recurrence and recurrence beyond MC were estimated using competing risks methods and compared using Gray’s test.24 Multivariable competing risk regression model was used to examine the effect of CRS components on recurrence beyond MC.25 Cumulative incidence function was used to estimate conditional recurrence probabilities among patients who were alive and free of recurrence at 2 years. Statistical analyses were performed using SAS Version 9.4 (SAS Institute, INC., Cary, NC, USA) or R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). P-values were 2-sided and <0.05 were considered statistically significant.
RESULTS
Clinical and pathological characteristics
In this international validation study, 1023 patients were included, of whom 531 patients (52%) had initial disease within MC and 492 patients (48%) had initial disease beyond MC. Clinical and pathological characteristics of the entire cohort and comparisons of two groups are shown (Table 1). On univariate analysis, patients with initial disease beyond MC were significantly associated with female, non-viral hepatitis liver disease, normal bilirubin level, normal platelet level, higher AFP level, major hepatectomy, higher operative blood loss, multiple nodules or satellites, large tumor size, presence of microvascular invasion, absence of cirrhosis, and presence of extracapsular extension.
Table 1.
Clinical and pathological characteristics of patients with initial disease within versus beyond Milan criteria (MC).
| Total (n=1023) | Initial disease within MC (n=531) | Initial disease beyond MC (n=492) | p-value | |
|---|---|---|---|---|
| Demographic and history | ||||
| Age | 63 (14 – 88) | 63 (20 – 88) | 63 (14 – 88) | 0.98 |
| Male | 758 (74%) | 412 (78%) | 346 (70%) | <0.01 |
| Liver disease Hepatitis B Hepatitis C Hepatitis B+C Alcoholic NASH Hemochromatosis None |
373 (36%) 108 (11%) 8 (1%) 10 (1%) 27 (3%) 4 (0%) 361 (35%) |
227 (43%) 66 (12%) 6 (1%) 9 (2%) 18 (3%) 3 (1%) 120 (23%) |
146 (30%) 42 (9%) 2 (0%) 1 (0%) 9 (2%) 1 (0%) 241 (49%) |
<0.01 |
| Child Pugh class A B |
913 (89%) 83 (8%) |
471 (89%) 44 (8%) |
442 (90%) 39 (8%) |
0.82 |
| MELD score <10 ≥10 |
724 (71%) 242 (24%) |
366 (69%) 131 (25%) |
358 (73%) 111 (23%) |
0.37 |
| Preoperative tests | ||||
| Bilirubin >1, mg/dl | 304 (30%) | 182 (34%) | 122 (25%) | <0.01 |
| INR >1.12 | 297 (29%) | 153 (29%) | 144 (29%) | 1.00 |
| Creatinine >1.3, mg/dl | 118 (12%) | 64 (12%) | 54 (11%) | 0.56 |
| Albumin, g/dL <2.8 2.8–3.5 >3.5 |
58 (6%) 231 (23%) 712 (70%) |
36 (7%) 111 (21%) 372 (70%) |
22 (4%) 120 (24%) 340 (69%) |
0.12 |
| Platelet, k/mcL <160 160–400 >400 |
276 (27%) 697 (68%) 38 (4%) |
197 (37%) 321 (60%) 5 (1%) |
79 (16%) 376 (77%) 33 (7%) |
<0.01 |
| AFP, ng/ml <6.1 6.1–99.9 100–1000 >1000 |
315 (31%) 321 (31%) 135 (13%) 144 (14%) |
171 (32%) 201 (38%) 72 (14%) 41 (8%) |
144 (29%) 120 (24%) 63 (13%) 103 (21%) |
<0.01 |
| Operative data | ||||
| Major Hepatectomy | 397 (39%) | 112 (21%) | 285 (58%) | <0.01 |
| Operative blood loss, ml | 500 (3 – 8000) | 300 (5 – 4000) | 600 (3 – 8000) | <0.01 |
| Pathological data | ||||
| Multiple nodules or satellites 2 tumors 3 tumors ≥ 4 tumors |
256 (25%) 174 (68%) 54 (21%) 28 (11%) |
90 (17%) 70 (78%) 14 (16%) 6 (7%) |
166 (34%) 104 (63%) 40 (24%) 22 (13%) |
<0.01 |
| Largest tumor size, cm | 4.5 (0.3 – 25) | 3 (0.3 – 7.5) | 8 (1 – 25) | <0.01 |
| Differentiation Well Moderate Poor |
140 (14%) 684 (67%) 74 (7%) |
83 (16%) 357 (67%) 33 (6%) |
57 (12%) 327 (66%) 41 (8%) |
0.11 |
| Microvascular invasion | 358 (35%) | 133 (25%) | 225 (46%) | <0.01 |
| Cirrhosis | 384 (38%) | 268 (50%) | 116 (24%) | <0.01 |
| Steatosis | 173 (17%) | 79 (15%) | 94 (19%) | 0.11 |
| Extracapsular extension | 34 (3%) | 4 (1%) | 30 (6%) | <0.01 |
MC Milan criteria, NASH nonalcoholic steatohepatitis, MELD model for end-stage liver Disease, INR international normalized ratio, AFP α-fetoprotein.
Categorical variables are expressed as frequency (percentage). Sum of percentages less than 100% indicate missing values. Continuous variables are expressed as median (range).
Recurrence and survival
With a median follow-up of 50 months among survivors, 613 patients developed a recurrence by the end of the study, resulting a 5-year cumulative incidence of any recurrence of 62% [95% CI: 59%−64%], and recurrence beyond MC of 35% [95% CI: 32%−38%] (Fig. 1). In both groups of patients with initial disease within and beyond MC, more patients developed intrahepatic than extrahepatic recurrence (46% vs. 10%, and 38% vs. 25%, respectively). However, when examining recurrence pattern as being within or beyond MC in the multivariate analysis, having initial disease beyond MC was independently associated with recurrence beyond MC (HR 1.95, 95%CI: 1.55–2.44, p<0.001) after controlling for multinodular disease and microvascular invasion.
Fig. 1.
Recurrence patterns of patients with initial disease within versus beyond Milan criteria.
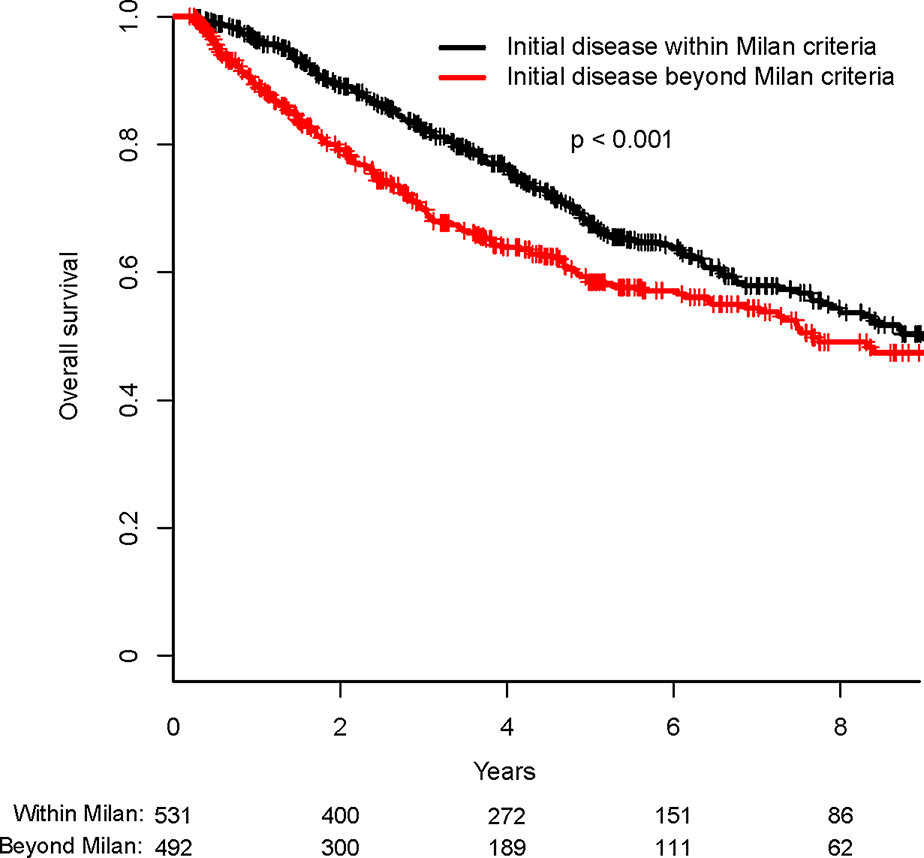
Among the entire cohort of 1023 patients, median RFS was 2.2 years (95% CI: 2.0–2.5 years) and median OS was 8.4 years (95% CI: 7.5–10.8 years). Patients with initial disease within MC had longer RFS than those with initial disease beyond MC (median RFS of 3.0 years vs. 1.5 year, p<0.001) (Fig. 2A). In addition, patients with initial disease within MC had OS significantly longer than those with initial disease beyond MC (median OS of 9.1 vs. 7.8 years, p=0.003) (Fig. 2B).
Fig. 2.
Recurrence-free survival (Fig. 2A) and overall survival (Fig. 2B) of patients with initial disease within versus beyond Milan criteria.
Recurrence treatment consisted of curative intent therapies, such as additional resection, salvage liver transplantation, or ablation, as well as noncurative treatments such as embolization, systemic chemotherapy, radiation or other best supportive care. Among patients with initial disease within MC who developed recurrence (n=298), 64 (21%) were amenable to subsequent resection, 7 (2%) received salvage liver transplantation, 41 (14%) underwent serial ablation or embolization, 45 (15%) underwent chemoradiation or other supportive care, and 141 (47%) patients did not have treatment documented. Among patients with initial disease beyond MC who developed recurrence (n=315), their treatments included second resection in 42 (13%) patients, salvage liver transplantation in 2 (1%) patients, serial ablation or embolization in 33 (10%) patients, chemoradiation or other supportive care in 112 (36%) patients, whereas treatment was not reported for the remaining 126 (40%) patients.
Validation of clinical risk score
Clinical risk score (CRS) was previously created to predict cumulative incidence of recurrence beyond MC.23 The 3 variables included were initial disease beyond MC, multinodular tumors, and microvascular invasion, which were all significantly associated with recurrence beyond MC (HR 1.95, 1.51, 2.12, all p<0.001, respectively) (Table 2). One point was assigned to each of the 3 variables to obtain the scores 0 to 3. Patients with CRS 0, 1, 2, and 3 had 5-year incidence of recurring beyond MC at 19%, 34%, 49%, and 67%, respectively (p<0.001) (Fig. 3).
Table 2.
Factors in the clinical risk score (CRS) were independently associated with recurrence beyond Milan criteria (MC).
| CRS factors | HR comparison | HR (95% CI) | p-value |
|---|---|---|---|
| Preoperative disease beyond MC | Beyond vs. within MC | 1.95 (1.55–2.44) | <0.001 |
| Multiple nodules or satellites | Yes vs. no | 1.51 (1.19–1.92) | <0.001 |
| Microvascular invasion | Yes vs. no | 2.12 (1.70–2.64) | <0.001 |
HR hazard ratio, CI confidence interval.
Fig. 3.
Clinical risk score stratified cumulative incidences of recurrence beyond Milan criteria.
Based on this CRS, patients with a score of 0 had 1-, 3- and 5-year RFS rates of 83%, 56% and 41%, respectively, which were significantly better than patients with a CRS of 3, who had 1-, 3- and 5-year RFS rates of 33%, 17% and 10%, respectively (p<0.001) (Fig. 4A). The median RFS for patient with CRS 0, 1, 2, and 3 were 48, 29, 16, and 6 months, respectively (p<0.001). Similarly, patients with CRS of 0 had 1-, 3- and 5-year OS of 96%, 86% and 71%, respectively, whereas patients with a CRS of 3 had 1-, 3- and 5-year OS of 82%, 54% and 45%, respectively (p<0.001) (Fig. 4B). The median OS for patient with CRS 0, 1, 2, and 3 were 128, 109, 88, and 43 months, respectively (p<0.001).
Fig. 4:
Clinical risk score stratified recurrence-free survival (Fig. 4A) and overall survival (Fig. 4B).
Among patients with CRS 0 (n=332), 59 (18%) developed recurrence beyond MC despite having none of the 3 major risk factors. On further analysis, none of the other clinical and pathological variables evaluated distinguished patients who recurred beyond MC (Supplemental Table 1). One notable finding was that 48 (81%) patients with CRS 0 with recurrence beyond MC were treated at Singapore, the majority of whom were male (88%) with hepatitis B (50%) and cirrhosis (54%) found on initial resection. Their median time to recurrence beyond MC was 1.4 years (range: 0.3–16 years).
Patients with CRS 0 treated at Western centers had 1-, 3- and 5-year cumulative incidences of recurrence beyond MC of 2%, 10% and 13%, respectively, which were significantly better than patients treated at Singapore, who had 1-, 3- and 5-year recurrence rates of 9%, 17% and 22%, respectively (p=0.029) (Supplemental Fig. 1A). Although the cumulative incidences of recurrence beyond MC in patients with CRS 0 were significantly different in those treated at Singapore compared to Western centers, the recurrence rates were not different in patients with CRS 1 to 3 (p=0.701, 0.827, 0.680, respectively) (Supplemental Fig. 1B to D), even though clinical and pathological characteristics were very different in these patient populations (Supplemental Table 2).
Conditional recurrence probabilities
Cumulative risk of recurrence beyond MC in all patients after resection was 35% at 5 years. Most of the risk of recurrence beyond MC occurred during the first 2 years after resection, which was 28% at 2 years. While the risk decreased over time, it never reached zero. If patients had 2 and 5 years of recurrence-free follow-up, the cumulative risk of recurrence beyond MC was 14% and 4%, respectively, for the subsequent 5 years. After patients had 2 years of recurrence-free follow-up, the risk of recurrence beyond MC was 17% in the next 5 years if the initial disease was beyond MC compared to 12% with initial disease within MC (Fig. 5). Although the risk never reached zero, the majority of patients were eligible for salvage transplantation following resection and especially after 2 or 5 years of recurrence-free follow-up.
Fig. 5.
Conditional probability of recurrence beyond Milan criteria after 2 years of recurrence-free survival.
DISCUSSION
In this international study of 1023 patients, we showed that a previously reported CRS validated well and accurately stratified the risks of recurrence beyond MC.23 Initial disease beyond MC, multinodular disease, and presence of microvascular invasion are known poor prognostic factors.19–22 The CRS has the advantage of being a simple scoring system and specifically derived in the context of evaluating the pattern of recurrence for consideration of salvage liver transplantation.
Although long-term outcome after liver transplantation is excellent and is associated with significantly lower recurrence rate compared to resection given their stringent selection, transplantation is limited by graft availability.26–28 Resection followed by salvage transplantation is a potential option in well-selected patients.18, 29 However, recurrence beyond MC generally eliminates the transplant option, except in selected patients with intrahepatic-only recurrence using the extended criteria and with potential downstaging procedures.30, 31 The CRS accurately stratified patients’ risk of recurrence beyond MC, ranging from 19% (CRS 0) to 67% (CRS 3) at 5 years. We found that the cumulative incidence of recurrence beyond MC by 5 years after resection was 35%. Thus, majority of patients submitted to resection were still eligible for salvage liver transplantation if they recurred, and especially those with low CRS.
Although multinodular tumors and microvascular invasion were based on pathological evaluation, patients with initial disease within MC were by definition having CRS 2 or less. In this study, if patients were known to have 1 tumor within MC on preoperative imaging (n=495), 98% remained either CRS 0 or 1. Although the tumors appeared solitary on imaging, 11% of these patients had additional satellite lesions and 23% had microvascular invasion, leading to a change in CRS to 1 if either was detected. By contrast, only 2% of these patients were upstaged to CRS 2 by having both additional satellites and microvascular invasion. Thus, in patients with solitary tumor and initial disease within MC, but with low MELD score and long expected waitlist period, a trial of upfront resection with close surveillance may allow patients to undergo early definitive treatment. With the upfront resection, the vast majority of these patients retained transplant eligibility, with the risk of recurrence beyond MC at 5 years ranging from 19% to 34% for CRS 0 to 1, respectively.
In patients with high CRS who have high risk of recurrence beyond MC, the optimal initial treatment is uncertain. On one hand, liver transplantation may be a better initial treatment strategy, given that many patients will become transplant ineligible with recurrence. In our cohort, patients with multiple tumors but within MC on preoperative imaging (n=36), 42% had microvascular invasion, hence upgrading their CRS to 2. Patients with CRS 2 had 49% 5-year cumulative incidence of recurrence beyond MC. While liver transplantation may be a better initial treatment for these patients, the disease extent and high CRS may also indicate an aggressive tumor biology and that may be better clarified by initial resection. However, this question requires further study. In patients with high CRS, upfront resection should be followed by close surveillance, which may allow detection of recurrence before progression to beyond MC.
Among patients with CRS 0, it is important to note that 18% of patients developed recurrence beyond MC, despite having none of the 3 major risk factors. These patients were initially eligible for liver transplantation but subsequently lost an opportunity for salvage transplantation. There were no additional clinical or pathological variables evaluated that distinguished those who recurred beyond MC. The only notable finding was that 81% of these patients were treated at Singapore and had a predominant history of hepatitis B and cirrhosis. While viral hepatitis is the leading cause of HCC in Singapore and other countries in Asia, non-alcoholic fatty liver disease is becoming a leading risk factor for HCC in the Western centers.32–34 The differences in underlying liver disease and associated genetic alterations may account for higher rate of recurrence beyond MC among patients with CRS 0 treated at Singapore.3, 35, 36 Although some studies found a lower rate of recurrence among non-hepatitis cohorts, others concluded a higher or similar rate of recurrence as the hepatitis cohorts.35, 37, 38 Further studies are needed to identify those high-risk patients, such as by evaluating their hepatitis viral load, infiltrative inflammatory markers, or genomic differences.
Despite a diverse patient population in this study, the CRS accurately stratified risk of recurrence beyond MC for patients treated at both Singaporean and Western centers. Although the risk of recurrence beyond MC in Singaporean patients with CRS 0 were significantly higher than those treated at Western centers, both of their recurrence rates were still significantly lower than those with CRS 1 or with higher scores. In addition, patients with CRS 1, 2, or 3 did not have significantly higher risk of recurrence beyond MC in those treated at Singapore compared to Western centers. In the entire cohort, compared to patients treated at Western centers, those treated at Singapore were predominantly male patients with viral hepatitis and worse liver reserve, underwent resection for smaller but more multinodular tumors, and found to have higher rate of cirrhosis and lower rate of steatosis. However, despite of these clinical and pathological differences in patients treated at Singapore versus Western centers, CRS was able to capture their risk of recurrence beyond MC similarly.
Most of the risk of recurrence beyond MC occurred within first 2 years postoperatively and it decreased with time but never reached zero. This may reflect different underlying etiology for recurrence. Prior studies have suggested that early recurrence within 2 years was due to cancer dissemination from the original tumor via microvascular invasion, whereas late recurrence was due to de novo cancer formation from underlying liver hepatitis and cirrhosis.12, 39–41 In our study, a total of 37 patients developed recurrence after 5 years, of whom only 2 (5%) patients had microvascular invasion, but 27 (73%) patients had history of viral hepatitis B or C and 13 (35%) patients had pathological cirrhosis on their initial resections. Thus, long-term follow-up is essential after resection, especially in patients with underlying hepatitis and cirrhosis, since effective therapies may allow long-term disease control.
The strength of this study is having the large number of patients treated at 5 different international centers to validate the CRS. In addition, having a diverse patient population with heterogeneous underlying liver diseases allows the finding of this study to be applicable to both Western and Asian centers. The major limitation of this study is its retrospective nature and the associated biases. Additionally, while other variables, such as AFP or serum inflammatory indices, may have some value for predicting recurrence beyond MC, these were not specifically evaluated in this study, given that the primary aim was to validate the CRS as initially described. Further studies may identify additional variables that will further refine the CRS and improve its predictive power.
In conclusion, we validated the ability of a previously reported CRS to predict risk of recurrence beyond MC in a multinational patient cohort. The results have implications for determining treatment recommendations and for identifying those in need of closer and long-term surveillance. It is important to recognize that majority of patients eligible for primary resection remained eligible for salvage transplantation after recurrence. While the risk of recurrence beyond MC decreased over time, it never reached zero.
DISCUSSANTS
PREDICTION OF RECURRENCE BEYOND MILAN CRITERIA AFTER RESECTION OF HEPATOCELLULAR CARCINOMA – AN INTERNATIONAL VALIDATION OF A CLINICAL RISK SCORE
DISCUSSANT
DR. CARLOS ESQUIVEL (Stanford, CA): Thank you. I enjoyed the presentation very much, and thanks for sending me the manuscript in advance.
The reported incidence of recurrence of HCC after liver transplantation is somewhere between 12 and 25%. After resection, the reported incidence of recurrence is quite high, as high as 70%.
In this particular study, the cumulative recurrence rate after resection was 60%. However, the strength of this study was being a multi-institutional, five cities, and four nations. The population sample was big enough to conduct the analysis.
The goal was to validate a clinical score system formula to predict which patients would have recurrence beyond the Milan criteria. And, I assume, that the significance of identifying such patients would be not to waste organs since these patients are not suitable candidates for transplantation. Formulas that are based on clinical factors are difficult to validate. There are many factors, maybe more than 10 that have been implicated in the recurrence of HCC, because HCC is a very heterogeneous and complex malignancy.
There were some interesting findings which may be due to institutional variability. For example, in the patients with a clinical score of zero, meaning they were low risk, 20% of them or 19% to be accurate, ended up with recurrence. And then, the other finding was that the group from Singapore had a recurrence significantly higher than that of Western centers.
So, my first question is, how did the authors dealt with the variability in interpretation from pathologists who look at the specimens, and from radiologists reading the films, particularly when they were looking for recurrence?
The second question, why did the authors not use the alpha-fetoprotein as a surrogate marker for tumor biology? This is the best biomarker we have so far.
The authors also mentioned that an optimal approach was to do resection, and if the patient had recurrence, then proceed with salvage liver transplantation. By reviewing the data, less than 2% of their patients went on to transplantation. So, it almost contradicts your hypothesis. Could you elaborate and explain why so few patients made it to transplantation?
And then my last question is, if you say to your patients: “If you have a liver transplant and the recurrence rate is going to be 15%, however, if you get a liver resection, your recurrence rate is 60% (using your figures), but if the tumor comes back, you only have a 2% chance of getting a liver transplant, what do you think your patients will do?
Thank you very much. I congratulate the authors because it is a challenge to come up with a formula that can help us manage these difficult patients, and also running a multi-institutional, international trial is a very difficult thing to do. Thanks again!
PREDICTION OF RECURRENCE BEYOND MILAN CRITERIA AFTER RESECTION OF HEPATOCELLULAR CARCINOMA – AN INTERNATIONAL VALIDATION OF A CLINICAL RISK SCORE
CLOSING DISCUSSANT
DR. JIAN ZHENG: Thank you, Dr. Esquivel, for the discussion. To answer your first question, we did evaluate tumor size and number of tumors by radiological and by pathological assessments. There were discrepancies between the two. For example, in patients who presented with initial disease within the Milan criteria, 11% of the tumors examined on pathology were greater than 1 centimeter larger or smaller compared to tumor size observed on radiology. In addition, in patients who presented with 1 tumor within the Milan criteria by radiology, 11% had additional satellites identified on pathology. Furthermore, 5% of tumors thought to be within the Milan criteria by radiology were actually beyond the criteria by pathology. In our clinical risk score, the initial disease Milan criteria status was based on preoperative imaging because that’s the information we would have when patient is being considered for liver transplantation versus resection. However, multinodular disease and microvascular invasion in our clinical risk score were based on pathological assessments, consistent with our prior study.
Regarding your second question, we did not evaluate alpha?fetoprotein given that our primary aim was to validate the CRS. Alpha-fetoprotein was evaluated in the original study but it was not found to be a significant predictor of recurrence beyond Milan criteria and thus it was not evaluated here. However, we have retrospectively obtained preoperative levels of alpha-fetoprotein from our patients, and thus it can be evaluated in our future study.
Regarding your third question, we had very few patients who underwent salvage transplantation. One of the reasons is that it is not a standard practice given scarcity of liver graft, whereas resection and locoregional therapies may be more accessible to some of our eligible patients. We also need more data on the outcome of salvage transplantation as we only have data from small series. Another reason for few patients who received salvage transplantation was because the median MELD score was only 8 at the time of the initial resection. Unless their liver function deteriorated significantly at the time of their recurrence, they may not meet the criteria for salvage transplantation. We also had missing treatment information on some patients, likely because all of our collaborators are tertiary referral centers, and thus patients may receive recurrence treatments in their local hospitals. In particular, in the Singapore center, salvage transplantation rates are also low due to several reasons- the donor rate is very low therefore the waiting time is very long, the MELD exception for HCC is only 15 in Singapore, thus often, HCC patients are not high on the waiting list. Most of the liver grafts goes to patients with much higher MELD score e.g. fulminant liver failures or advanced cirrhosis
Regarding your last question, liver transplantation does have the advantage over resection in that the recurrence rate is significantly lower. We hope that this simple CRS can help prioritize patients based on their recurrence risk. In patients with 1 tumor within the Milan criteria preoperatively, 98% remained CRS 0 or 1, which translated to 19% to 34% of risk of recurrence beyond the Milan criteria at 5 years. In patients with low CRS, it may help reduce the long waitlist for patients to undergo resection first and with transplantation option deferred for recurrence. In patients with high CRS and high risk of recurrence beyond Milan criteria after resection, upfront liver transplantation may be a better option for the patients, but it may not be the best allocation of scarce organs given these patients’ high risk of recurrence. Additional studies are needed to evaluate patients who are at high risk for recurrence after resection but may be acceptable risk for utilization of liver transplantation. Upfront liver transplantation for these high CRS patients may perhaps be considered in countries with a short waiting time or in the living donor setting. If these high CRS patients are not able to receive an upfront liver transplant, they should have closer surveillance after liver resection so as to ensure if and when a recurrence occurs, hopefully, it will still be within criteria for a potential salvage transplantation.
PREDICTION OF RECURRENCE BEYOND MILAN CRITERIA AFTER RESECTION OF HEPATOCELLULAR CARCINOMA – AN INTERNATIONAL VALIDATION OF A CLINICAL RISK SCORE
DISCUSSANT
DR. KARIM HALAZUN (New York, NY): This is a very good study and it’s great to see this big data and very interesting to see that you included some transplant centers yet still maintained a high volume of resections in patients who are within Milan.
I have a couple of questions.
Number one. There are markers that allow us to predict which tumors that are small and within criteria are actually bad tumors and bad biologically, one of which is alpha-fetoprotein; others are PIVKA-II and neutrophil-to-lymphocyte ratio. You already answered that you didn’t really look at that but it would be interesting to see which small tumors are actually aggressive that you could have predicted preoperatively rather than relied on microvascular invasion later on. It’s also interesting to see how many patients within Milan recurred.
One of the advantages we have in transplantation is, number one, we have the whole organ to look at afterwards so we can then reassess which patients are actually outside Milan, but we thought were in Milan, because our radiology is not exactly accurate, and there are multiple papers showing a big disconcordance between radiology and pathology. But the other advantage of transplantation is we have time, and you alluded to that. So even patients who are within Milan who we wait six to nine months to transplant, who then progress, are essentially re-assessed. I’m wondering how many patients in your study actually got some kind of bridging therapy before resection. How many got chemoembolized or received RFA before resection?
PREDICTION OF RECURRENCE BEYOND MILAN CRITERIA AFTER RESECTION OF HEPATOCELLULAR CARCINOMA – AN INTERNATIONAL VALIDATION OF A CLINICAL RISK SCORE
CLOSING DISCUSSANT
DR. JIAN ZHENG: Thank you for your question. As part of our exclusion criteria, we excluded patients who had any preoperative therapy. Yes, four out of five collaborating institutions have transplant programs, and many patients received downstaging therapy to bridge onto liver transplantation. However, we did not include patients who underwent locoregional therapies or liver transplantation. We only included patients who underwent first-time liver resection.
It would be interesting and important to evaluate other known prognostic factors, such as alpha?fetoproteins and serum inflammatory indices, to help identify small tumors that perhaps may be more aggressive than their appearance and would not do as well with the liver graft. We have retrospectively collected some of these preoperative variables for future study to improve our CRS.
Pathological information is helpful to guide treatment and surveillance of these patients. Yes, we would not know microvascular invasion status prior to surgery since biopsy is not routinely performed and there may be sampling error with biopsy. Our group at Memorial Sloan Kettering Cancer Center is currently working on using quantitative image analysis of preoperative abdominal scan to predict patients who may have microvascular invasion. In the near future, this may serve as an additional tool to determine microvascular invasion status and guide treatment of those small, aggressive tumors.
Supplementary Material
Acknowledgments
Grant: This work was supported in part by NIH/NCI P30 CA008748 Cancer Center Support Grant.
Footnotes
Conflicts of interest: William Chapman is the Founder of Pathfinder Therapeutics and a member of the Advisory Board of Novartis. Other co-authors have no disclosure.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. The Lancet 2012; 379(9822):1245–1255. [DOI] [PubMed] [Google Scholar]
- 3.Choo SP, Tan WL, Goh BK, et al. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53(3):1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg 2003; 238(5):703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle MB, Vachharajani N, Maynard E, et al. Liver transplantation for hepatocellular carcinoma: long-term results suggest excellent outcomes. J Am Coll Surg 2012; 215(1):19–28; discussion 28–30. [DOI] [PubMed] [Google Scholar]
- 7.Chapman WC, Klintmalm G, Hemming A, et al. Surgical treatment of hepatocellular carcinoma in North America: can hepatic resection still be justified? J Am Coll Surg 2015; 220(4):628–37. [DOI] [PubMed] [Google Scholar]
- 8.Cha CH, Ruo L, Fong Y, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg 2003; 238(3):315–21; discussion 321–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turcotte S, Dematteo RP. Resection versus transplantation for early hepatocellular carcinoma: more art than science. Ann Surg 2012; 256(6):892–3. [DOI] [PubMed] [Google Scholar]
- 10.Dhir M, Melin AA, Douaiher J, et al. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg 2016. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Li C, Wen T, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol 2015; 27(8):933–40. [DOI] [PubMed] [Google Scholar]
- 12.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. Journal of Hepatology 2003; 38(2):200–207. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30(6):1434–40. [DOI] [PubMed] [Google Scholar]
- 14.Pelletier SJ, Fu S, Thyagarajan V, et al. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl 2009; 15(8):859–68. [DOI] [PubMed] [Google Scholar]
- 15.Facciuto ME, Rochon C, Pandey M, et al. Surgical dilemma: liver resection or liver transplantation for hepatocellular carcinoma and cirrhosis. Intention-to-treat analysis in patients within and outwith Milan criteria. HPB (Oxford) 2009; 11(5):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012; 55(1):132–40. [DOI] [PubMed] [Google Scholar]
- 17.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334(11):693–9. [DOI] [PubMed] [Google Scholar]
- 18.Bhangui P, Allard MA, Vibert E, et al. Salvage Versus Primary Liver Transplantation for Early Hepatocellular Carcinoma: Do Both Strategies Yield Similar Outcomes? Ann Surg 2016; 264(1):155–63. [DOI] [PubMed] [Google Scholar]
- 19.Cho CS, Gonen M, Shia J, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg 2008; 206(2):281–91. [DOI] [PubMed] [Google Scholar]
- 20.Ang SF, Ng ES, Li H, et al. The Singapore Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PLoS One 2015; 10(4):e0118658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang P, Qiu J, Li J, et al. Nomograms for Pre- and Postoperative Prediction of Long-term Survival for Patients Who Underwent Hepatectomy for Multiple Hepatocellular Carcinomas. Ann Surg 2016; 263(4):778–86. [DOI] [PubMed] [Google Scholar]
- 22.Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg 2015; 261(5):939–46. [DOI] [PubMed] [Google Scholar]
- 23.Lee SY, Konstantinidis IT, Eaton AA, et al. Predicting recurrence patterns after resection of hepatocellular cancer. HPB (Oxford) 2014; 16(10):943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Annals of Statistics 1988; 16(3):1141–1154. [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 1999; 94(446):496–509. [Google Scholar]
- 26.Dhir M, Lyden ER, Smith LM, et al. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: a meta-analysis. HPB (Oxford) 2012; 14(9):635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapisochin G, Castells L, Dopazo C, et al. Single HCC in cirrhotic patients: liver resection or liver transplantation? Long-term outcome according to an intention-to-treat basis. Ann Surg Oncol 2013; 20(4):1194–202. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Z, Liang W, Milgrom DP, et al. Liver transplantation versus liver resection in the treatment of hepatocellular carcinoma: a meta-analysis of observational studies. Transplantation 2014; 97(2):227–34. [DOI] [PubMed] [Google Scholar]
- 29.Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology 2000; 31(4):899–906. [DOI] [PubMed] [Google Scholar]
- 30.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33(6):1394–403. [DOI] [PubMed] [Google Scholar]
- 31.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009; 10(1):35–43. [DOI] [PubMed] [Google Scholar]
- 32.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013; 47Suppl:S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 2011; 15(2):223–43, vii-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016; 122(11):1757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Utsunomiya T, Shimada M, Kudo M, et al. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg 2015; 261(3):513–20. [DOI] [PubMed] [Google Scholar]
- 36.Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 2015; 47(5):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omichi K, Shindoh J, Yamamoto S, et al. Postoperative Outcomes for Patients with Non-B Non-C Hepatocellular Carcinoma: A Subgroup Analysis of Patients with a History of Hepatitis B Infection. Ann Surg Oncol 2015; 22Suppl 3:1034–40. [DOI] [PubMed] [Google Scholar]
- 38.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma (hcc) in non alcoholic fatty liver disease (NAFLD): A multicenter prospective study. Hepatology 2015. [DOI] [PubMed] [Google Scholar]
- 39.Hirokawa F, Hayashi M, Asakuma M, et al. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol 2016; 25(1):24–9. [DOI] [PubMed] [Google Scholar]
- 40.Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol 2009; 51(5):890–7. [DOI] [PubMed] [Google Scholar]
- 41.Zheng J, Kuk D, Gonen M, et al. Actual 10-Year Survivors After Resection of Hepatocellular Carcinoma. Ann Surg Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.