Abstract
The conclusions of the EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, Sweden, and co‐rapporteur Member State, Italy, for the pesticide active substance bifenazate are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012. The conclusions were reached on the basis of the evaluation of the representative uses of bifenazate as an acaricide on strawberry, fruiting vegetables (tomatoes, peppers, aubergines, cucumbers, courgettes, melons, watermelons), flowering and ornamental plants and nursery ornamentals and updated following the request to peer review the exposure and risk assessments for bifenazate. The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: bifenazate, peer review, risk assessment, pesticide, acaricide
Summary
Commission Implementing Regulation (EU) No 844/2012 (hereinafter referred to as ‘the Regulation’) lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. Bifenazate is one of the active substances listed in Regulation (EU) No 686/2012.
In accordance with Article 1 of the Regulation, the rapporteur Member State (RMS), Sweden, and co‐rapporteur Member State (co‐RMS), Italy, received an application from Arysta LifeScience Great Britain Limited for the renewal of approval of the active substance bifenazate. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Italy), the European Commission and the European Food Safety Authority (EFSA) about the admissibility.
The RMS provided its initial evaluation of the dossier on bifenazate in the renewal assessment report (RAR), which was received by EFSA on 29 January 2016. In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, Arysta LifeScience Great Britain Limited, for comments on 5 February 2016. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 10 May 2016.
Following consideration of the comments received on the RAR, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, environmental fate and behaviour and ecotoxicology.
In accordance with Article 13(1) of the Regulation, EFSA should adopt a conclusion on whether bifenazate can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council.
EFSA published its conclusion on the peer review of the pesticide risk assessment of bifenazate on 4 January 2017. On 17 November 2020, the EFSA received from the European Commission the request for an updated peer review concerning the exposure and risk assessments for bifenazate. The conclusions laid down in this report were reached on the basis of the evaluation of the representative uses of bifenazate as an acaricide on strawberry, fruiting vegetables (tomatoes, peppers, aubergines, cucumbers, courgettes, melons, watermelons), flowering and ornamental plants and nursery ornamentals, as proposed by the applicant. Full details of the representative uses can be found in Appendix A of this report.
Data were submitted to conclude that the uses of bifenazate according to the representative uses proposed at EU level result in a sufficient acaricidal efficacy against the target organisms.
A data gap was identified for a search of the scientific peer‐reviewed open literature on the relevant metabolites and more details are needed concerning the available literature search for the active substance.
A data gap was identified for a monitoring method for body fluids. As the residue definitions for soil and groundwater for monitoring were concluded as bifenazate only, a data gap was identified for a monitoring method analysing just bifenazate.
For mammalian toxicology, data gaps are set for the analytical methods used in each of the toxicological studies that are not reported, for a comparative in vitro metabolism study including human material and for the phototoxicity/photomutagenicity potential of bifenazate. In addition, further clarifications are required to assess the endocrine disrupting properties of bifenazate. Moreover, toxicological data are needed (repeated dose toxicity study and genotoxicity data) for three metabolites, D3598, A1530S, carbamate. Finally, some risks are noted for operators during hand‐held applications (all outdoor uses) for the single low application rate.
A series of data gaps were identified in the residue section, and moreover, the finalisation of the toxicological assessment of metabolites including bifenazate‐diazene, part of the residue definition for risk assessment and monitoring, is pending. Therefore, the consumer risk assessment cannot be finalised.
A data gap was identified due to the unknown identity of soil and sediment water metabolites IMH/IBMHC (chromatographic retention time 38 min) and IBMHC/DDC (chromatographic retention time 37.3 min). Furthermore, for these two metabolites, a data gap was identified for the soil adsorption endpoints to be derived. The proposed structures of metabolites DPHPDD and hydroxylated D3598 were not confirmed against authentic reference standards; therefore, a data gap was identified. For the surface water and sediment exposure assessments, several data gaps were identified. In particular, calculations for metabolites D9963 and D9472 were not acceptable, for the highest application rate step 3 calculations for the representative use in strawberries and step 2 and 3 calculations for the representative use in ornamental plants were not available for bifenazate and its metabolites. A data gap was identified for information on the effect of water treatment processes on the nature of residues of metabolites potentially present in surface water, when surface water is abstracted for drinking water. This gap leads to the consumer risk assessment from the consumption of drinking water being not finalised for all the representative uses.
In the area of ecotoxicology, data gaps were identified to further address the risk to birds and mammals, aquatic organisms, honeybees and other non‐target arthropods and soil microorganisms. Critical areas of concerns were identified for birds; a high risk was identified for all representative uses. In the absence of suitable exposure estimates, the aquatic risk assessment for bifenazate and its pertinent metabolites (uses on ornamentals) and for metabolites D9963 and D9472 (uses on strawberries and fruiting vegetables) could not be finalised.
Background
Commission Implementing Regulation (EU) No 844/20121 (hereinafter referred to as ‘the Regulation’) lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/20092. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of up to 8 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3).
In accordance with Article 1 of the Regulation, the RMS Sweden and co‐RMS Italy received an application from Arysta LifeScience Great Britain Limited for the renewal of approval of the active substance bifenazate. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Italy), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on bifenazate in the RAR, which was received by EFSA on 29 January 2016 (Sweden, 2016a).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, Arysta LifeScience Great Britain Limited, for consultation and comments on 5 February 2016. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 10 May 2016. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA and the RMS on 28 June 2016. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, environmental fate and behaviour and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in December 2016.
EFSA published its conclusion on the peer review of the pesticide risk assessment of bifenazate on 4 January 2017 (EFSA, 2017). On 17 November 2020, EFSA received from the European Commission the request for an updated peer review concerning the exposure and risk assessments for bifenazate.
EFSA was requested to peer review the exposure and risk assessments for bifenazate, in particular:
-
1
Risk assessment for operators, workers and residents with a single application using the lowest application rate proposed in the dossier;
-
2
Risk assessment for birds and mammals with a single application using the lowest application rate proposed in the dossier, following a discussion on the determination of the appropriate long‐term endpoint and higher tier refinement with particular consideration of weight of evidence (e.g. extrapolation from invertebrates and other crops) and the information as regards the rapid decline of the active substance in the environment.
-
3
Risk assessment for non‐target arthropods with a single application using the lowest application rate proposed in the dossier by identifying which group of organisms (taxa) are at risk and which not, with particular consideration of weight of evidence (e.g. extrapolation from other invertebrates and/or other crops) and the information as regards a rapid decline of the active substance in the environment. The potential for recovery will be considered.
In addition, EFSA would reconsider the possibility of finalising:
-
4
Assessment of the toxicological profile of the metabolite D3598 based on all available information including studies submitted or referenced in the renewal dossier submitted in 2014;
-
5
A consumer risk assessment with a best‐case (a single application using the lowest application rate) and a worst‐case scenario (two applications using the highest application rate) proposed in the good agricultural practice (GAP) table for the representative use on tomatoes and strawberries;
-
6
Aquatic risk assessment with a single application using the lowest application rate proposed in the dossier for bifenazate and all pertinent metabolites in ornamentals and for the metabolites D9963 and D9472 for all representative uses by assessing the appropriateness of calculations of the RMS with indicative exposure values in the RAR and for the other metabolites.
EFSA was also asked to consider additional risk mitigation measures, in particular for point 1 and point 2, and will clarify that the data gap on metabolites A1530S and carbamate is relevant in case of setting a global plant residue definition for risk assessment and not for the representative uses, given that those are metabolites in cereal metabolism that are not listed as representative uses. EFSA was requested to complete this review by 30 June 2021. Revised assessment was provided by the RMS for the risk assessment for birds, mammals and non‐target arthropods, on aquatic risk assessment, on consumer risk assessment and on risk assessment for operators, workers and residents. EFSA distributed the revised RAR to the Member States and applicant for consultation and comments on 3 March 2021. In addition, an expert consultation in the area of mammalian toxicology and ecotoxicology was considered necessary.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative uses of bifenazate as an acaricide on strawberry, fruiting vegetables (tomatoes, peppers, aubergines, cucumbers, courgettes, melons, watermelons), flowering and ornamental plants and nursery of ornamentals, as proposed by the applicant and updated following request to peer review the exposure and risk assessments for bifenazate. A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
In addition, a key supporting document to this conclusion is the peer review report (EFSA, 2021), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (28 June 2016);
the evaluation table (6 December 2016);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion;
the comments received on the revised RAR;
the comments received on the draft updated EFSA conclusion;
the reports of the additional scientific consultation with Member State experts (where relevant).
Given the importance of the RAR, including its revisions (Sweden, 2016a,b, 2021a,b), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Bifenazate is the ISO common name for isopropyl 3‐(4‐methoxybiphenyl‐3‐yl)carbazate or isopropyl 2‐(4‐methoxybiphenyl‐3‐yl)hydrazinoformate (IUPAC).
The representative formulated product for the evaluation was ‘Floramite 240 SC (UBI 6704‐06)’ a suspension concentrate (SC) containing 240 g/L bifenazate.
The representative uses evaluated were foliar spray applications for the control of Tetranychus urticae in field and protected: strawberry, fruiting vegetables (tomatoes, peppers, aubergines, cucumbers, courgettes, melons, watermelons), flowering and ornamental plants and nursery of ornamentals. Full details of the GAPs can be found in the list of end points in Appendix A.
Data were submitted to conclude that the uses of bifenazate according to the representative uses proposed at EU level result in a sufficient acaricidal efficacy against the target organisms, following the guidance document SANCO/10054/2013 ‐ rev. 3 (European Commission, 2013).
A data gap has been identified for a search of the scientific peer‐reviewed open literature on the relevant metabolites, dealing with side effects on health, the environment and non‐target species and published within the 10 years before the date of submission of the dossier, to be conducted and reported in accordance with EFSA guidance on the submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (EFSA, 2011a). Concerning the active substance, the data available are not reported in sufficient detail in the RAR.
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: SANCO/3029/99‐rev. 4 (European Commission, 2000a), SANCO/3030/99‐rev. 4 (European Commission, 2000b) and SANCO/825/00‐rev. 8.1 (European Commission, 2010).
The reference specification of the first approval was updated. The proposed specification is based on batch data from industrial scale production. The minimum purity of the active substance as manufactured is 980 g/kg. There is no FAO specification available.
The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity, physical, chemical and technical properties of bifenazate or the representative formulation. The main data regarding the identity of bifenazate and its physical and chemical properties are given in Appendix A.
Some of the methods for the generation of pre‐approval data required for the risk assessment did not fully meet the requirements of the guidance document, however they were considered fit for purpose. High‐performance liquid chromatography‐ultraviolet (HPLC‐UV) methods are available for the determination of bifenazate in the technical material and in the representative formulation.
The sum of the residues of bifenazate and bifenazate‐diazene (D3598), expressed as bifenazate can be monitored in food and feed of plant origin by liquid chromatography with tandem mass spectrometry (LC‐MS/MS) with a limit of quantification (LOQ) of 0.01 mg/kg in all plant commodity groups, except hops in which the LOQ is 0.1 mg/kg. Pending on the final residue definition for monitoring in food and feed of plant origin (see Section 3), additional analytical methods might be required. An analytical method for monitoring residues in food and feed of animal origin is not needed as no maximum residue levels (MRLs) were proposed for the animal matrices. A LC‐MS/MS method is available for the determination of the residues of the sum of bifenazate and bifenazate‐diazene, expressed as bifenazate in soil with a LOQ of 0.01 mg/kg. It should be noted, however, that the residue definition for monitoring was concluded as bifenazate only, meaning that a data gap was identified for a monitoring method analysing just bifenazate. The determination of bifenazate and bifenazate‐diazene, expressed as bifenazate in drinking water and surface water can be done by LC‐MS/MS with an LOQ of 0.1 μg/L. The residue definition for monitoring in ground water was concluded bifenazate only, as a consequence, a data gap was identified for a monitoring method in groundwater. Monitoring of the residues of bifenazate in air can be done by LC‐MS/MS with a LOQ of 0.4 μg/m3.
The determination of residues of bifenazate and metabolites bifenazate‐diazene (quantified as bifenazate), A1530 and A1530S (quantified as A1530) in bovine kidney, liver, milk and fat can be done by LC‐MS/MS with a LOQ of 0.01 mg/kg for all commodities. A data gap was, however, identified for a monitoring method in body fluids.
2. Mammalian toxicity
The toxicological profile of the active substance bifenazate and its metabolites was discussed at the Pesticides Peer Review experts’ TC 148 (October 2016) and TC 48 (April 2021) and assessed based on the following guidance documents: SANCO/221/2000 – rev. 10‐final (European Commission, 2003), SANCO/10597/2003 – rev. 10.1 (European Commission, 2012) and Guidance on dermal absorption (EFSA PPR Panel, 2012).
To assess the toxicological profile of the active substance the applicant submitted a complete set of valid toxicity studies. The toxicity studies were representative of the proposed technical specification for the active substance and associated impurities. Toluene is identified as relevant impurity (max of 0.7 g/kg). The analytical methods used in each of the toxicological studies are not reported, a data gap is set.
In the toxicokinetics studies, oral absorption is estimated to be 36%. Bifenazate is widely distributed (highest residues in liver, kidney, whole blood, heart, spleen, red blood cells and lungs) and there is no evidence for accumulation. Excretion of substance is predominantly through the bile at low dose and via faeces for high dose. At low dose, it is extensively metabolised, less at high dose and the metabolites are formed following hydrazine oxidation demethylation, ring hydroxylation, elimination of hydrazine carboxylic acid part and conjugation with glucuronic acid or sulfate. A data gap is set for the comparative in vitro metabolism study including human material that should be performed as required in Regulation (EU) No 283/2013.
In the acute toxicity studies, the substance has a low acute toxicity when administered orally, dermally or by inhalation to rats. It is not a skin or eye irritant but it is a skin sensitiser in Guinea Pig Maximisation Test. A harmonised classification Skin Sens. 1, H3173 (may cause an allergic skin reaction) exists. The available OECD 3T3 NRU‐PT test does not allow concluding properly on the phototoxicity potential of bifenazate (data gap) since the substance as its maximum UV/VIS absorption within UVB range. It is noted however that there is no validated test for UVB absorbers leading to a data gap. The photomutagenicity potential should be reconsidered once phototoxicity potential of bifenazate is addressed.
The most sensitive species is the dog. The relevant short‐ and long‐term oral no observed adverse effect level (NOAEL) is 1 mg/kg body weight (bw) per day. This value is based on liver and haematological effects observed in the 90‐day and 1‐year dog studies (changes in kidney and bone marrow are also observed in the 1‐year study). In the 2‐year rat study supported by the 18‐month mice study, the NOAEL of 1 mg/kg bw per day is based on spleen and haematological effects. Based on the observed effects in the short‐term studies, the harmonised classification STOT RE 2, H3734 (may cause damage to organs through repeated exposure) is supported by the experts.
Bifenazate is unlikely to be genotoxic based on available genotoxicity studies: Ames test, mouse lymphoma mutagenesis assay, in vitro cytogenetic test and in vivo micronucleus test. In a chronic/carcinogenicity study in rat, liver tumours and haematopoietic neoplasia are observed but not dose related. In addition, further data on mammary tumours have been submitted after the Pesticide Peer Review experts’ meeting. There is no dose‐response for mammary carcinomas, multiple mammary carcinomas in unscheduled deaths, interim and terminally sacrificed animals. In addition, when considering the total number of mammary carcinomas, no dose response is observed. In a carcinogenicity study in mice, no treatment‐related increased incidence in tumours is reported. An increase of adenoma in 10 out of 48 males at the highest dose is not statistically significant and is the only effect treatment related. The incidence of haematopoietic neoplasia (spleen, thymus and blood) in female mice is reported as not dose related. To conclude, based on the available data, bifenazate does not show a carcinogenic potential.
In the 2‐generation study in rats, the agreed parental NOAEL is 1.5 mg/kg bw per day based bodyweight and bodyweight gain reduction. EFSA does not support the arguments presented by the RMS to disregard both the body weight effects and delay in vaginal opening as treatment‐related effect relevant to the offspring and the reproduction, even if these effects are observed in the presence of parental toxicity. In EFSA's view, it is considered that the delay in vaginal opening should be taken into consideration for the setting of the reproduction NOAEL and the reduced bodyweight for the offspring's NOAEL. This does not change the risk assessment as parental toxicity is also observed at the same dose level. EFSA proposes to set the both NOAELs at 6.1 mg/kg bw per day based on these effects.
In the developmental toxicity study in rat, developmental toxicity (retro‐oesophageal aortic arch) was observed at a dose eliciting maternal toxicity. The relevant maternal and developmental NOAELs are 10 mg/kg bw per day and 100 mg/kg bw per day for the rat, respectively. In rabbits no maternal or developmental toxicity were observed and both, the maternal and developmental NOAELs are 200 mg/kg bw per day, the highest dose tested.
The substance does not show a neurotoxic potential in an acute neurotoxicity study in rats. The neurotoxicity NOAEL for the short‐term neurotoxicity study is 13 mg/kg bw per day, based on reduced rearing at the lowest observed adverse effect level (LOAEL) of 35 mg/kg bw per day.
No alert on immunotoxicity is observed based on a 28‐day immunotoxicity study in mice and based on the available data package.
During the Pesticide Peer Review experts’ meeting TC 148, the majority of the experts did not see evidence for endocrine disrupting properties for bifenazate, however, some indications are observed for one main metabolite (uterotrophic assay from literature search) and delay in vaginal opening with the parent that may require further clarification (such as level 2/3 of the OECD conceptual Framework for the identification of endocrine disruptors).
The acceptable daily intake (ADI) is the same set during the first review (European Commission, 2005), of 0.01 mg/kg bw per day based on the NOAEL of 1 mg/kg bw per day for spleen, liver and haematological effects observed in the 1‐year dog and 2‐year rat studies, supported by the 18‐month mice study, applying an uncertainty factor (UF) of 100. The AOEL is 0.0036 mg/kg bw per day based on the same effects recorded for the ADI from the 1‐year dog study, applying an UF of 100 and correcting for the limited oral absorption by 36%. The acceptable operator exposure level (AOEL) has been changed compared to that set during the first peer review as the oral absorption value has been increased from 28% to 36%.
An acute reference dose (ARfD) was not set under the first review (European Commission, 2005). An ARfD of 0.1 mg/kg bw and also an acute AOEL (AAOEL) of 0.036 mg/kg bw per day are derived, based on the maternal NOAEL of 10 mg/kg bw per day for early reduced body weight gain from the rat developmental toxicity study and applying an UF of 100, corrected by the limited oral absorption value of 36% for AAOEL.
The RMS estimated non‐dietary exposure (i.e. operator, worker, bystander and resident) as a first Tier with dermal absorption values derived from the in vitro dermal absorption study on human skin, i.e. 2.4% for the concentrate and 75% for the in‐use field dilution. Using these dermal absorption values, all operator exposure estimates exceed the AOEL. As a refinement, it was agreed to derive dermal absorption using a triple pack approach, the resulting dermal absorption values are 1% for the concentrate and 24% for the in‐use field dilutions. Considering these revised dermal absorption values, for a single application at the lowest application rate, the uses with hand‐held equipment (for all outdoor crops) result in exposure estimates above the AOEL for operators. It is noted that impermeable clothing for operators is not part of the personal protective equipment implemented in the EFSA calculator, therefore this is not validated at EU level and could be considered only at MS level. Additionally, with the refined Dislodgeable Foliar Residues (DFR) value and drift reduction equipment, the worker, resident and bystander exposure do not exceed the (A)AOEL for all uses (also for indoor uses, when excluding entry into treated crops for resident and bystander).
Three metabolites (bifenazate‐diazene (D3598), A1530S and carbamate) are found in residues (see Section 3). Based on the discussion during the peer review experts’ meeting TC 48 (April 2021)4 , both genotoxicity and toxicity profile of the metabolite bifenazate‐diazene could not be concluded on the basis of the available data. The QSAR analysis presented some limitations: compound outside of applicability domain in one QSAR model analysis, additional alert to the parent's from the OECD toolbox. In addition, considering the absence of specific toxicokinetic data, and the fact that the hydroxylated form was found in very low amounts in the bile, the postulated presence of D3598 as intermediate metabolite in the rat could not support the conclusion that the systemic exposure was sufficient to be covered by the toxicological profile of the parent. Concerning the metabolite A1530S (biphenyl‐4‐yl sulfate) and carbamate (isopropyl (4‐methoxybiphenyl‐3‐yl)carbamate) no toxicological data are available, therefore, data are required in order to assess the risk for consumer, a repeated dose toxicity study and genotoxicity data.
3. Residues
The assessment in the residue section is based on the OECD guidance document on overview of residue chemistry studies (OECD, 2009), the OECD publication on maximum residue level (MRL) calculations (OECD, 2011), the European Commission guideline document on MRL setting (European Commission, 2011).
Metabolism of bifenazate was investigated after foliar applications in the categories of fruit (oranges, apples and grapes), pulses and oilseeds (cotton), cereals and grass crops (maize) and root crops (radish) using bifenazate 14C‐labelled in the substituted phenyl ring.
In fruits, bifenazate was the main component of the total radioactive residue (TRR) (34–79% TRR) with bifenazate‐diazene (D3598) present as the pertinent metabolite (up to 40% of the TRR). In radish, cotton and maize low translocation of radioactivity to radish roots, cotton seed and maize grain was observed. No identification was attempted in radish roots. Radioactivity in cotton seeds was mostly incorporated into natural compounds. Analysis of radish tops and cotton gin trash confirmed that parent bifenazate was the major residue on the plant parts that were directly exposed to the treatment. Metabolism in maize resulted in a range of metabolites in the different commodities. It is noted that in cereals the metabolic breakdown of bifenazate had progressed further then in the other crops, so that in maize grain parent was not detected while A1530S was the only compound identified, and in maize stover parent was not the major residue (2–6% TRR), but the metabolites bifenazate‐diazene (6–10% TRR), A1530S (8–12% TRR) and carbamate (isopropyl (4‐methoxybiphenyl‐3‐yl)carbamate) (8–14% TRR) were the pertinent residues. However, overall, the metabolite picture is consistent and qualitatively comparable across the different crop categories.
It was previously proposed to define the residue for both enforcement and risk assessment in fruits as the sum of bifenazate and bifenazate‐diazene, expressed as bifenazate, pending confirmation that the same toxicological reference values can be used for bifenazate and bifenazate‐diazene. However, it cannot be established that bifenazate and bifenazate‐diazene share the same toxicological properties and therefore the reference values of bifenazate cannot be applied to bifenazate‐diazene (see Section 2). Therefore, the residue definition for risk assessment should not be expressed as the sum of the two compounds but as (1) bifenazate and (2) bifenazate‐diazene, assessed separately against their individual toxicological reference values (TRVs). This proposal is provisional pending a reevaluation upon availability of toxicological data for bifenazate‐diazene.
Regarding the possibility of setting a global plant residue definition for risk assessment it is necessary to take into account all commodities including feed items and thus to further consider the relevance of the major metabolites A1530S and carbamate in the cereal metabolism. For monitoring, the sum of bifenazate and bifenazate‐diazene, expressed as bifenazate is considered containing compounds that are good markers across all plant commodities. The inclusion of bifenazate‐diazene is also necessary in view of the available analytical method for enforcement. Depending on the toxicological relevance of the metabolite bifenazate‐diazene, the residue definition for monitoring needs to be re‐evaluated (see comment table Residue definition (1)).
In a confined rotational crop study with 14C‐labelled bifenazate residues in succeeding carrots, lettuce and wheat at twice the nominal rate the TRRs in mature food commodities (lettuce, carrot, wheat grain) were < 0.02 mg/kg for all plant‐back intervals, significant residue levels were only found in feed commodities. Identification attempts failed as neither bifenazate nor any of the primary crop plant metabolites could be identified in rotational crops. It is unknown whether the uptake of soil specific metabolites, e.g. with the carbazole structure such as IBMHC/DDC (DT90 up to 154.7 days) was investigated. However, in view of the representative uses under peer review, given the 2‐fold application rate to bare soil in the available study, the data can be used to conclude that significant individual residue compounds are unlikely to be present in rotational crops, while for a more critical use pattern in terms of application rate the issue may have to be reconsidered.
Acceptable storage stability tests are only available with parent bifenazate for up to 6 months in strawberries, showing acceptable stability of residue analysed as the sum of bifenazate and bifenazate‐diazene. Fortification with bifenazate‐diazene was not conducted and thus storage stability of bifenazate‐diazene was not tested over a reproducible period of time. The full validity of the strawberry trials is pending confirmation of storage stability of the metabolite bifenazate‐diazene (data gap).
Significant degradation of both bifenazate and bifenazate‐diazene was observed when studied separately in the fruiting vegetable/cucurbit commodity category, or the number of fortified stored samples was insufficient, respectively. Thus, storage stability of residues in fruiting vegetables is insufficiently addressed. Pending the availability of guideline conforming storage stability studies for both the relevant analytes over the full storage period used in the magnitude‐of‐residue studies (data gap), only the residue trials with storage up to 1 month were considered for further assessment.
Under these circumstances the number of residue trials was insufficient for protected tomatoes (six trials) and field tomatoes in northern Europe (NEU) (four trials) and southern Europe (SEU) (two trials) as a minimum number of 8 trials is required. The data confirm that from a residue perspective the critical use is in protected tomatoes when a comparable good agricultural practice (cGAP) is followed for the protected and non‐protected crop. Three trials each per crop and zone were submitted in peppers and courgettes/cucumbers conducted in the field, but the period of sample storage in these trials exceeded the period for which residue stability could be demonstrated. The pertinent document D.1. in the dossier and the GAP table indicated the applicant intended uses in the fruiting vegetables category and data on tomato as one example crop were submitted that however do not permit extrapolation to the entire category. EFSA acknowledges the RMS position that the scope of intended uses expressed by the applicant is ambiguous in the light of the data submitted in the Residues section on tomato only, yet, as the case may be, the missing residue data to conclude on the entire category of fruiting vegetables were listed for sake of transparency. Therefore, complete data sets of residue trials (indoor and outdoor) supported by acceptable storage stability data are necessary if critical uses in the whole category of fruiting vegetables (i.e. tomatoes, peppers, cucurbits edible peel, cucurbits inedible peel, sweet corn) are intended.
The available residue trials were designed to address the critical GAP (‘worst‐case’) with two applications and residues of bifenazate and bifenazate‐diazene were analysed together as bifenazate. Upon an assessment if the available residue trials could sufficiently address the residue situation for an alternative GAP (‘best‐case’) with only one application of a lower rate, EFSA concluded that the proportionality principle cannot be applied based on the available evidence (see comment table Residue trials in plants and identification of critical GAP (1)). Therefore, a sufficient number of validated residue trials are not available to support the ‘best‐case’ GAP in tomato and strawberry. Moreover, the available trials did not analyse separately for residues of bifenazate and bifenazate‐diazene that would however be necessary to conduct a consumer risk assessment in line with the new provisional residue definition for risk assessment.
A data gap was also set with regard to potential residue levels in pollen and bee products.
In a study simulating food processing conditions bifenazate was hydrolytically stable under all the conditions tested in this study, with bifenazate‐diazene less than 2% applied radioactivity (AR). The behaviour of bifenazate‐diazene under processing was not tested (data gap).
The representative uses do not give rise to significant exposure of food producing animals, and no studies with such animals were submitted and assessed during the peer review. Therefore, a peer reviewed animal residue definition is not available.
In view of a series of issues identified in the residue section that still need clarification or have to be addressed by further data and for the missing TRVs for metabolite bifenazate‐diazene, the consumer risk assessment cannot be finalised. Neither is the magnitude of individual residues of bifenazate and bifenazate‐diazene in strawberry and tomato known for either GAP scenario (‘worst‐case’ and ‘best‐case’), nor is reliable information available on which of the two compounds is driving the residues under field conditions. It is further not known if the TRVs for bifenazate‐diazene would be lower than the ones for bifenazate and if therefore application of the previous assessment method (sum of bifenazate and bifenazate‐diazene, using bifenazate TRVs) as an interim approach would still be sufficiently protective for consumers.
The residue definition for enforcement and monitoring may have to be changed compared to those used in the review of the existing maximum residue levels (MRLs) for bifenazate (EFSA, 2011b). Whether a global plant residue definition for risk assessment can be derived and whether it is confirmed as the sum of both compounds currently included is pending finalisation of both the toxicological and the relevance assessment of major plant metabolites. Therefore, once sufficient data and information is available to finalise the assessment on metabolites with regard to their toxicological properties, a revision of the article 12 reasoned opinion may be necessary. In addition, an ARfD has been derived during the peer review that was not available at the time of the review of the existing MRLs for bifenazate. On the basis of the uses assessed during the review of the existing maximum residue levels (Art. 12) and the residue definitions used in the MRL review, an acute intake concern cannot be excluded for peppers.
4. Environmental fate and behaviour
Bifenazate was discussed at the Pesticides Peer Review TC 141 in October 2016.
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, bifenazate exhibited very low to low persistence forming the major (> 10% applied radioactivity (AR) or > 5% AR in at least two sequential measurements) metabolites bifenazate‐diazene (D3598, max. 85.5% AR), which exhibited very low to low persistence, D1989 (max. 33.3% AR), which exhibited low to moderate persistence, IMH/IBMHC (chromatographic retention time 38 min, max. 12.5% AR) and IBMHC/DDC (chromatographic retention time 37.3 min, max. 6.9% AR), which exhibited moderate persistence. Experts expressed their concerns on the identity of metabolites IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min), however they accepted the kinetic assessment and the pathway scheme proposed. Therefore, a data gap was identified (see Section 7) because the identity of metabolites IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min) is unknown.
Mineralisation of the phenyl ring 14C radiolabel to carbon dioxide accounted for 15.2–23.0% AR after 119 days and 4.0–9.1% AR after 30 days. The formation of unextractable residues (not extracted by acetonitrile/water) for this radiolabel accounted for 64.0–67.3% AR after 119 days and for 53.6–66.8% after 30 days.
In anaerobic soil incubations bifenazate transformation was similar to that under aerobic conditions, forming the major (> 10% AR) metabolites D3598 (max. 50.2% AR) and D1989 (max. 30.6% AR). Additionally, the major metabolite A1530 (max. 20.7% AR) was formed. Metabolite A1530 was only detected under anaerobic conditions. Anaerobic conditions were not considered to be of major importance for the representative uses. The anaerobic DT50 will not be used for the present risk assessment. However, for uses of bifenazate on other crops than the representative uses considered here and/or with altered application timings, the importance of anaerobic conditions would need to be re‐evaluated and metabolites formed under anaerobic conditions (such as A1530) might need to be addressed further. Bifenazate is quickly photodegraded on the soil surface, but the degradation is similar under dark control and irradiated conditions. Photodegradation in soil is not a major transformation pathway of bifenazate.
Bifenazate exhibited medium to low mobility in soil. It was concluded that the adsorption of bifenazate was not pH dependent. Metabolite D3598 exhibited slight mobility or was immobile, metabolite D1989 exhibited slight mobility. It was concluded that the adsorption of these metabolites was not pH dependent. Metabolites IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min) exhibited immobility, but adsorption values were obtained via QSAR and LOQ estimations. This approach was considered acceptable for completing the exposure assessment for the representative uses, however a data gap was identified for deriving adsorption endpoints for both metabolites.
In laboratory incubations in dark aerobic natural sediment water systems, bifenazate exhibited very low persistence, forming the metabolites D3598 (max. 33.6% AR in the total system after 100 days), IMH/IBMHC (retention time 38 min, max. 23.9% AR in the total system after 15 days), D9472 (max. 21.6% AR in the total system after 100 days), IBMHC/DDC (retention time 37.3 min, max. 12.7% AR in the total system after 15 days). The unextractable sediment fraction (not extracted by acetonitrile/water) accounted for 52.4–65.7% AR at study end (15 days) and for 46.9–65.2% AR at study end (100 days) for the phenyl ring 14C radiolabel. Mineralisation accounted for 2.8–12.0% AR at study end (15 days) and for 18.9–33.7% AR at study end (100 days) for the phenyl ring 14C radiolabel.
Chromatographically resolved components accounting for > 10% AR were D3598 (65.7% AR at study end (12 hr)), D9963 (30.4% AR at study end (150 hr)), D9472 (18.6% AR after 2 days), D1989 (13.1% AR after 54 hr) and hydroxylated D3598 (18.0% AR after 4 days).
The metabolite DPHPDD is an unknown component formed in the sterile hydrolysis study and the metabolite hydroxylated D3598 is an unknown component formed in the sterile aqueous photolysis study. A data gap was identified for metabolites DPHPDD and hydroxylated D3598 because the proposed structures were not confirmed against authentic reference standards. However, these metabolites were included in the surface water and sediment exposure assessment based on default parameters.
Considering the highest application rate, the necessary surface water and sediment exposure assessments (predicted environmental concentrations (PEC) calculations) were carried out for bifenazate and its metabolites D3598, D1989, IMH/IBMHC (retention time 38 min), IBMHC/DDC (retention time 37.3 min), D9963, D9472, hydroxylated D3598 and DPHPDD, using the FOCUS (2001) step 1 and step 2 (version 3.2 of the Steps 1–2 in FOCUS calculator), and step 3 approach. A data gap was identified for step 3 calculations for the representative use in strawberries and for steps 2 and 3 calculations for the representative use in ornamental plants for bifenazate and its metabolites D3598, D1989, IMH/IBMHC (retention time 38 min), IBMHC/DDC (retention time 37.3 min), hydroxylated D3598 and DPHPDD. This leads to the surface water and sediment exposure assessment being not finalised for representative use in ornamental plants (see Sections 5 and 9). A data gap was identified for surface water and sediment exposure assessment for all representative uses for metabolites D9963 and D9472, because in the absence of adsorption endpoints conservative values should be used. This leads to the surface water exposure assessment being not finalised for metabolites D9963 and D9472 (see Sections 5 and 9). Adsorption endpoints used for metabolites IMH/IBMHC (retention time 38 min), IBMHC/DDC (retention time 37.3 min), hydroxylated D3598 and DPHPDD were conservative, and thus, in this particular case, the choice of these values for the surface water exposure assessment is acceptable.
Considering the lowest application rate, surface water exposure assessments were carried out for bifenazate and its metabolites D3598, D1989, IMH/IBMHC (retention time 38 min), IBMHC/DDC (retention time 37.3 min), D9963, D9472, hydroxylated D3598 and DPHPDD, using the FOCUS (2001) step 1 and step 2 (version 3.2 of the Steps 1–2 in FOCUS calculator), and step 3 approach for all the representative uses. For metabolite D3598, appropriate step 4 calculations were available for the representative uses on ornamentals. The step 4 calculations appropriately followed the FOCUS (2007) guidance, with no‐spray buffer zones and vegetative buffer strips of up to 10 m (reducing solute flux in run‐off by 60% and erosion runoff by 85%) being implemented for the run‐off scenarios. The SWAN tool (version 5.0) was appropriately used to implement these mitigation measures in the simulations. A data gap was identified for surface water and sediment exposure assessment for all representative uses for metabolites D9963 and D9472, because in the absence of adsorption endpoints conservative values should be used (see Section 9).
Risk managers and others may wish to note that surface water and sediment exposure assessment calculations for the representative use on ornamentals only cover plants less than 50 cm in height.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (2009) scenarios and the models PEARL 4.4.4 and PELMO 5.5.3 for the active substance bifenazate and its metabolites D3598, D1989, IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min). Two sets of calculations were performed for an early and a late application using two applications every year. Due to the uncertainty in their identification, for metabolites IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min) simulations were carried out using adsorption values from QSAR estimation and a worst case estimate based on the LOQ for IBMHC/DDC (retention time 37.3 min) and from worst case estimate based on the LOQ for IMH/IBMHC (retention time 38 min). The potential for groundwater exposure from the representative uses by bifenazate above the parametric drinking water limit of 0.1 μg/L was concluded to be low in geoclimatic situations that are represented by all FOCUS groundwater scenarios for all the representative uses. For metabolites D3598, D1989, IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min) the potential for groundwater exposure above the parametric drinking water limit of 0.1 μg/L was concluded to be low in all geoclimatic situations that are represented by all the relevant FOCUS groundwater scenarios.
For the highest application rate, the applicant made the case that for the glasshouse uses the risk assessment will be covered by the field uses, and then, no specific calculations were performed in accordance with the EFSA guidance (EFSA, 2014). For the lowest application rate, calculations were available for bifenzate and its metabolites using the model GEM (Greenhouse Emission Model – version 3.3.2) (Step 3, EFSA 2014) for soil‐less scenarios. It should be noted that the GEM model reflects Dutch conditions for high technology (permanent) greenhouses, and it may not be representative for the range of these types of structures present in all EU territories. For soil‐bound uses, the assessment is still based on field uses also for the lowest application rate.
The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix A of this conclusion.
The applicant provided information to address the effect of water treatments processes on bifenazate and metabolite D3589 residues that might be present in surface water, however appropriate information to address the effect of water treatments processes on metabolites D1989, IMH/IBMHC (retention time 38 min), IBMHC/DDC (retention time 37.3 min), D9963, D9472, hydroxylated D3598 and DPHPDD residues that might be present in surface water, when surface water is abstracted for drinking water were not provided. This has led to the identification of a data gap (see Section 7) and results in the consumer risk assessment not being finalised (see Section 9).
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002a,b), SETAC (2001), EFSA (2009), EFSA PPR Panel (2013) and EFSA (2013). According to Regulation (EU) No. 283/2013 data should be provided regarding the acute and chronic toxicity to honeybees and data to address the development of honeybee brood and larvae. As the European Commission (2002a) does not provide a risk assessment scheme which is able to use the chronic toxicity data for adult honeybees and the honeybee brood, when performing the risk assessment according to European Commission (2002a), the risk to adult honeybees from chronic toxicity and the risk to bee brood, could not be finalised due to the lack of a risk assessment scheme. Therefore, the EFSA (2013) was used for risk assessment in order to reach a conclusion for the representative uses.
Several aspects of hazard characterisation and risk assessment for bifenazate were discussed at the Pesticides Peer Review Meeting 149 in October 2016 and at the Pesticides Peer Review teleconference 53 in April 2021.
It is noted that the representative uses included uses in greenhouse. Since the use in non‐permanent greenhouse could not be excluded, a risk envelope approach was taken considering the fact that the application pattern is the same for both field and greenhouse uses.
In addition, it must be noted that concerns on the identity of soil and surface water metabolites IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min) were raised (see Section 4).
At tier 1 level, a high risk to birds and mammals via long term exposure via the diet was concluded for all the representative uses (data gap) whilst a low acute risk could be concluded. A low risk to birds and mammals for bifenazate was concluded also for the other exposure routes except for the risk to earthworm‐eating birds from secondary poisoning for all representative uses considering two applications of bifenazate at 0.144 kg bifenazate/ha.
The long‐term risk for all representative uses with a single application at the lowest rate proposed in the GAP (1 × 0.096 kg bifenazate/ha) was further assessed, following a mandate from the European Commission. Several options to refine the risk assessment to birds and mammals and the long‐term endpoint for mammals were discussed and agreed by the experts.5 For birds, a high long‐term risk was concluded for all representative uses. For mammals, a low risk was indicated for a single application of bifenazate at 0.096 kg/ha on strawberries whereas a high risk was concluded for the uses on tomatoes and ornamentals.
Further information is needed to address the risk to bird and mammals via exposure to the plant metabolites, in particular for metabolites bifenazate‐diazene (D3598), carbamate, A1530S, D9472, D9963 and A1530 and to address the risk via exposure through the food chain to the soil metabolites D3598, D1989, IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min) and for the surface water metabolites D3598, D1989, DPHPDD, IBMHC/DDC (retention time 37.3 min), IMH/IBMHC (retention time 38 min), hydroxylated D3598 (data gap).
A low acute and chronic risk to aquatic organisms could be concluded for bifenazate for the uses on fruiting vegetables and strawberries after considering two applications at the maximum rate (0.144 kg bifenazate/ha). For the use on ornamentals, only Step 1 FOCUS exposure estimates were available and a high risk for bifenazate and its pertinent surface water metabolites could not be excluded (data gap), see also Section 4. The risk to aquatic organisms after a single application at the lowest rate was concluded as low for all representative uses (at FOCUS Step 1 PEC values for algae and at FOCUS Step 2 for fish and aquatic invertebrates).
The following metabolites were identified as relevant for surface water: D3598 (relevant also for sediment), D1989, D9963, D9472, DPHPDD, IBMHC/DDC (retention time 37.3 min), IMH/IBMHC (retention time 38 min), hydroxylated D3598. Reliable acute toxicity endpoints were available for metabolite D3598 (all trophic levels) and for metabolite D1989 (aquatic invertebrates only). Chronic toxicity endpoints were not available for these metabolites whilst both acute and chronic toxicity endpoints were not available for the remaining surface water metabolites. Information to address the risk for the pertinent sediment metabolite D3598 was not available (data gap). The outcome of the aquatic risk assessments for the metabolites considering the available toxicity endpoints and the available exposure estimates is presented below:
Two applications at 0.144 kg bifenazate/ha. A high acute risk to aquatic invertebrates and fish and a low risk to algae were concluded for metabolite D3598 for the uses on strawberries and fruiting vegetables (data gap). For metabolite D1989 a high acute risk, for fish only, was concluded for the use on strawberries whilst a low acute risk for all trophic levels was concluded for the uses on fruiting vegetables. By assuming the metabolites as 10 times more toxic than the parent compound, a high acute risk could not be excluded for metabolites DPHPDD (aquatic invertebrates only), hydroxylated D3598 (aquatic invertebrates only), IBMHC/DCC (fish and aquatic invertebrates) and IMH/IBMHC (fish and aquatic invertebrates) and a high chronic risk to fish could not be excluded for metabolites D3598, D1989, DPHPDD and hydroxylated D3598 for the uses on strawberries (data gap). For the uses on fruiting vegetables, a high acute risk to aquatic invertebrates and fish could not be excluded for metabolite IMH/IBMHC and for DPHPDD (aquatic invertebrates) whilst a high chronic risk to fish could not be excluded for D3598, DPHPDD and hydroxylated D3598. A low chronic risk could be concluded for metabolites D1989 and a low acute risk could be concluded for metabolite IBMHC/DDC (with mitigation measures to reduce the run off). A low risk to algae was concluded for all metabolites for all representative uses assessed. Suitable exposure estimates were not available for metabolites D9963 and D9472; therefore, the aquatic risk assessment could not be performed (data gap).
One application at 0.096 kg bifenazate/ha. A low acute and chronic risk to all groups of aquatic organisms were concluded for all metabolites and for all the representative uses.6 For metabolite D3598, a low risk was indicated at FOCUS Step 4 including mitigation measures (i.e. 10 m no spray buffer zone + 10 m vegetative filter strip) for the uses on ornamentals. High risk at FOCUS Step 3 was concluded for situation represented by the FOCUS scenario D6 for the representative use on strawberry (data gap).
Reliable acute contact and oral toxicity studies for honeybees performed with the active substance and the representative formulation were available. By using these data in a screening risk assessment in accordance with EFSA (2013), a low acute risk was concluded for all the representative uses. At tier 1 level a high chronic risk for honeybees was identified for the uses on strawberries and fruiting vegetables for the treated crop scenario (BBCH < 70), weeds and the succeeding crop scenario (data gap). Considering that the use on ornamentals can cover a high variety of plants and include flowering ornamentals, the risk assessment for this use was performed by using the ‘oilseed rape’ and ‘orchard 1’ crop categories of EFSA (2013). For the orchard category, a high risk was concluded for all scenarios except the treated crop (BBCH higher or equal to 70) whilst for the ‘oilseed rape category’ a high risk was concluded for the treated crop (BBCH < 70), the weeds and the succeeding crop scenario (data gap).
A low acute and chronic risk to honeybees was concluded on the basis of the screening assessment for exposure to residues of bifenazate in guttation fluids and surface water. An assessment of the exposure via residues in puddle water was not available. Nevertheless, considering all the available data and assessments including the assessments on guttation fluid and on surface water, a low acute and chronic risk was concluded also for the puddle scenario.
The risk assessment for larvae could not be performed due to the lack of a suitable endpoint (data gap). Other assessments that were not available included sublethal effects (i.e. HPG, data gap), accumulative effects and metabolites occurring in pollen and nectar (data gap).
Data to perform a risk assessment for solitary bees were not available. A literature study on the effects of Floramite 240 mg/L SC on bumblebees (Bombus terrestris) was available (Besard et al., 2010). In this study three different exposure routes were assessed: contact, oral via treated sugar solution and oral via treated pollen. Following oral exposure, especially via treated sugar solutions, effects on mortality and reproduction (no drones produced during the entire exposure period, 11 weeks) at concentrations of 96 mg a.s./L were observed. An individual dose cannot be estimated since no measurements of consumed sugar solutions were reported. Therefore, the potential use of this study in the risk assessment is limited.
A high in‐field risk to non‐target arthropods was concluded for all representative uses at Tier 1 level, for the standard species Typhlodromus pyri. The available higher tier studies were not sufficient to address the risk identified for the representative uses of bifenazate with two applications (data gap). The off‐field risk to NTAs was indicated as low for all representative uses.
Following the mandate from the EC, the risk to non‐target arthropods of a single application of bifenazate at the lowest rate as proposed in the GAP table was assessed. The following lines of evidence were considered: (i) an aged‐residue study from the scientific literature on strawberries with two predatory mites; (ii) a field study on orchards in France also focusing on beneficial mites; (iii) the rapid decline of bifenazate on plants and (iv) the lack of lethal effects reported in the extended laboratory study with T. pyri. The experts considered that the available evidence supports the conclusion that a potential of recovery within 1 year can occur for the representative uses of bifenazate with one application at 0.096 kg bifenazate/ha.7 Therefore, a low risk to non‐target arthropods was concluded for such uses.
A low risk for earthworms and other soil macroorganisms was concluded for bifenazate and its pertinent metabolites. A low risk for soil microorganisms was concluded for bifenazate whilst further data are needed to address the risk for its pertinent soil metabolites (data gap).
A low risk to non‐target terrestrial higher plants was concluded for all the representative uses assessed.
A low risk to biological methods for sewage treatment could be concluded.
With regard to the endocrine disruption potential, it is noted that in the available avian reproduction test on mallard duck, the pathological examinations revealed inactive ovaries in 6/16 female birds in all concentrations tested. No effects on the ovaries were observed in the controls. No other studies were available to address the potential endocrine activity of bifenazate. Pending on the outcome of the data gap in Section 2, further ecotoxicological tests might be necessary to address the potential endocrine disrupting properties of bifenazate.
6. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1, 2, 3–4)
Table 1.
Soil
| Compound (name and/or code) | Persistence | Ecotoxicology |
|---|---|---|
| Bifenazate | Very low to low persistence single first order and bi‐phasic kinetics DT50 0.09–0.40 days (DT90 0.3–12.3 days; laboratory conditions at 20°C, pF2 soil moisture) | Low risk |
| Bifenazate‐diazene (D3598) | Very low to low persistence single first order and bi‐phasic kinetics DT50 0.21–1.45 days (DT90 0.68–28.4 days; laboratory conditions at 20°C, pF2 soil moisture) | Data gap |
| D1989 | Low to moderate persistence single first order kinetics DT50 2.12–11.0 days (DT90 7.04–53.2 days; laboratory conditions at 20°C, pF2 soil moisture) | Data gap |
| IMH/IBMHC (retention time 38 min) | Moderate persistence single first order kinetics DT50 15.4–23.9 days (DT90 51.1–79.2 days; laboratory conditions at 20°C, pF2 soil moisture) | Data gap |
| IBMHC/DDC (retention time 37.3 min) | Moderate persistence single first order kinetics DT50 22.4–46.6 days (DT90 74.3–154.7 days; laboratory conditions at 20°C, pF2 soil moisture) | Data gap |
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| Bifenazate | Medium to low mobility KFoc 243–628 mL/g | No | Yes | Yes |
| Bifenazate‐diazene (D3598) | Slight mobility to immobile KFoc 4,795–22,303 mL/g | No | No | No data, data not required |
| D1989 | Slight mobility KFoc 3,725–3,962 mL/g | No | No | No data, data not required |
| IMH/IBMHC (retention time 38 min) | Immobile KFoc 61,170 mL/g (a worst case estimated adsorption based on the LOQ) | No | No | No data, data not required |
| IBMHC/DDC (retention time 37.3 min) | Immobile KFoc 61,170 mL/g (a worst case estimated adsorption based on the LOQ, if identified as IBMHC) | No | No | No data, data not required |
| Immobile KFoc 71,540 mL/g (QSAR estimation, if identified as DDC) | No | No | No data, data not required |
At least one FOCUS scenario or relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Bifenazate | Low risk to aquatic organisms for the uses at 1 × 0.096 kg bifenazate/ha. Data gap for the uses at 2 × 0.144 kg bifenazate/ha1 |
| Bifenazate‐diazene (D3598) | Low risk to aquatic organisms for the uses at 1 × 0.096 kg bifenazate/ha. Data gap for the uses at 2 × 0.144 kg bifenazate/ha1 , 2 , 3 |
| D1989 | Low risk to aquatic organisms for the uses at 1 × 0.096 kg bifenazate/ha. Data gap for the uses at 2 × 0.144 kg bifenazate/ha1 |
| IMH/IBMHC (retention time 38 min) | Low risk to aquatic organisms for the uses at 1 × 0.096 kg bifenazate/ha. Data gap for the uses at 2 × 0.144 kg bifenazate/ha1 , 3 |
| IBMHC/DDC (retention time 37.3 min) | Low risk to aquatic organisms for the uses at 1 × 0.096 kg bifenazate/ha. Data gap for the uses at 2 × 0.144 kg bifenazate/ha1 , 3 |
| D9963 | Low risk to aquatic organisms for the uses at 1 × 0.096 kg bifenazate/ha. Data gap for the uses at 2 × 0.144 kg bifenazate/ha |
| D9472 | Low risk to aquatic organisms for the uses at 1 × 0.096 kg bifenazate/ha. Data gap for the uses at 2 × 0.144 kg bifenazate/ha |
| Hydroxylated D3598 | Low risk to aquatic organisms for the uses at 1 × 0.096 kg bifenazate/ha. Data gap for the uses at 2 × 0.144 kg bifenazate/ha1 |
| DPHPDD | Low risk to aquatic organisms for the uses at 1 × 0.096 kg bifenazate/ha. Data gap for the uses at 2 × 0.144 kg bifenazate/ha1 |
FOCUS STEP 1, 2 and 3 estimates were available at the lowest application rate for all uses.
FOCUS STEP 4 calculations were available at the lowest application rate for the uses on ornamentals.
At 2 × 0.144 kg bifenazate/ha FOCUS STEP 3 estimates were available only for fruiting vegetables, showing low risk for ¼ scenarios.
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Bifenazate | Rat acute inhalation study: LC50 > 4.4 mg/L air/4 h (no classification required) |
LC50: lethal concentration, median.
7. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of Regulation (EC) No 1107/2009 concerning information on potentially harmful effects).
A data gap has been identified for a search of the scientific peer‐reviewed open literature on the relevant metabolites, dealing with side effects on health, the environment and non‐target species and published within the 10 years before the date of submission of the dossier, to be conducted and reported in accordance with EFSA guidance on the submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (EFSA, 2011a). Concerning the active substance, the data available are not reported in sufficient detail in the RAR.
Monitoring method for the determination of bifenazate in soil and ground water (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown, see Section 1, 4 and 5).
Monitoring method for the determination of the compounds of the residue definition in body fluids (relevant for all representative uses evaluated; submission date proposed by the applicant: end of 2016, see Section 1).
Analytical methods used in each of the toxicological studies are not reported (relevant for all representative uses evaluated, submission date proposed by the applicant: unknown; see Section 2).
A comparative in vitro metabolism study including human material should be performed as required in Regulation (EU) No 283/2013 (relevant for all representative uses evaluated, submission date proposed by the applicant: unknown; see Section 2).
Phototoxicity potential of bifenazate and photomutagenicity are unknown (relevant for all representative uses evaluated, submission date proposed by the applicant: unknown; see Section 2).
Further clarification regarding the endocrine disrupting properties are needed (such as level 2/3 of the OECD conceptual Framework for the identification of EDs) (relevant for all representative uses evaluated, submission date proposed by the applicant: unknown; see Section 2).
Toxicological data (repeated dose toxicity study and genotoxicity data) on metabolites A1530S, carbamate and D3598 are needed to assess the consumer risk (relevant for uses on strawberries and fruiting vegetables, submission date proposed by the applicant: unknown; see Section 2).
Guideline conforming storage stability data over the full period of sample storage under frozen conditions for bifenazate and D3598 in the relevant crops from the category of fruiting vegetables (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
Guideline conforming storage stability data over the full period of sample storage under frozen conditions for D3598 in strawberries (relevant for uses on strawberries, submission date proposed by the applicant: unknown; see Section 3).
Applicant to provide a rational for the observation in the citrus metabolism study that in acidic samples (juice, pulp) there is a significant decrease of the % polar compounds after 14 months of storage and in increase of % D3598, that is not observed in the non‐acidic peel samples (relevant for uses on strawberries and fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A reasoned proposal regarding a global plant residue definition for risk assessment, taking into account the relevance of the major metabolites on the basis of the available plant metabolism studies (relevant for uses on strawberries and fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
Hydrolysis study simulating processing conditions for metabolite D3589 (relevant for uses on strawberries and fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable residue trials in tomato (outdoor) compliant with the residue definitions for risk assessment and monitoring and supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable residue trials in peppers (outdoor) supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable residue trials in cucurbits with edible peel (outdoor) supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable residue trials in cucurbits with inedible peel (outdoor) supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable residue trials in sweet corn (outdoor) supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable residue trials in tomato (indoor) compliant with the residue definitions for risk assessment and monitoring and supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable residue trials in peppers (indoor) supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable residue trials in cucurbits with edible peel (indoor) supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable residue trials in cucurbits with inedible peel (indoor) supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
Clarification on the indoor GAP on fruiting vegetables concerning growing of sweet corn, and if applicable a full data set of acceptable residue trials in sweet corn (indoor) supported by demonstration of acceptable storage stability for NEU and SEU (relevant for uses on fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
A full data set of acceptable strawberry residues trials compliant with the residue definitions for risk assessment and monitoring and supported by demonstration of acceptable storage stability (relevant for uses on strawberries EU outdoor and indoor, submission date proposed by the applicant: unknown; see Section 3)
Submission of data or information against the data requirement on residue levels in pollen and in bee products for human consumption resulting from residues taken up by honeybees from crops at blossom, or in order to support the requested waiver, evidence for the statement that bees are not foraging on flowers of fruiting vegetables and strawberries should be submitted (relevant for uses on strawberries and fruiting vegetables, submission date proposed by the applicant: unknown; see Section 3).
The identity of metabolites IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min) is unknown (relevant for all representative uses evaluated, submission date proposed by the applicant: unknown; see Section 4 and 5).
Adsorption endpoints to be derived for metabolites IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min) (relevant for all representative uses evaluated, submission date proposed by the applicant: unknown; see Section 4).
The proposed structures of metabolites DPHPDD and hydroxylated D3598 were not confirmed against authentic reference standards (relevant for all representative uses evaluated, submission date proposed by the applicant: unknown; see Section 4).
Step 3 calculations for the representative use in strawberries and step 2 and 3 calculations for the representative use in ornamental plants were not performed for bifenazate and its metabolites D3598, D1989, IMH/IBMHC (retention time 38 min), IBMHC/DDC (retention time 37.3 min), D9963, D9472, hydroxylated D3598 and DPHPDD (relevant for representative uses in strawberries and ornamental plants for the highest application rate, submission date proposed by the applicant: unknown; see Section 4 and 5).
The surface water exposure assessment for metabolites D9963 and D9472 is not acceptable considering both the highest and the lowest application rates (relevant for all representative uses evaluated, submission date proposed by the applicant: unknown; see Section 4).
Information on the effect of water treatment processes on the nature of residues of identified metabolites potentially present in surface water (D1989, IMH/IBMHC (retention time 38 min), IBMHC/DDC (retention time 37.3 min), hydroxylated D3598 and DPHPDD), when surface water is abstracted for drinking water, were not sufficient in order to assess the consumer risk from the consumption of drinking water (relevant for all representative uses evaluated, submission date proposed by the applicant: unknown; see Section 4).
Further information to address the long term risk to birds and mammals (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to bird and mammals via exposure to the plant metabolites, in particular for metabolites D3598, carbamate, A1530S, D9472, D9963 and A1530 and to address the risk via exposure through the food chain to the soil metabolites D3598, D1989, IMH/IBMHC (retention time 38 min) and IBMHC/DDC (retention time 37.3 min) and for the surface water metabolites D3598, D1989, DPHPDD, IBMHC/DDC (retention time 38 min), IMH/IBMHC (retention time 37.3 min), hydroxylated D3598 (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
An aquatic risk assessment for the use on ornamentals for bifenazate and its pertinent surface water metabolites (relevant for the uses on ornamentals at 2 × 0.144 kg bifenazate/ha; submission date proposed by the applicant: unknown; see Section 4 and 5).
Further information to address the risk to aquatic organisms for metabolites D9963 and D9472 (relevant for all representative uses at 2 × 0.144 kg bifenazate/ha), the risk to sediment dwellers for metabolite D3598 (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to aquatic organisms for metabolites D3598 (acute and chronic), D1989 (acute and chronic), IMH/IBMHC (retention time 38 min) (acute), IBMHC/DDC (retention time 37.3 min) (acute), DPHPDD (acute and chronic) and hydroxylated D3598 (acute and chronic) (relevant for the uses on strawberries at 2 × 0.144 kg bifenazate/ha). Further information to address the risk to aquatic organisms for metabolites D3598 (acute and chronic), IMH/IBMHC (retention time 38 min) (acute), DPHPDD (acute and chronic) and hydroxylated D3598 (chronic) (relevant for the uses on fruiting vegetables at 2 × 0.144 kg bifenazate/ha, submission date proposed by the applicant: unknown; see Section 5). Further information to address the chronic risk identified to fish to the metabolite D3598 (relevant for the use on strawberry at 1 × 0.096 kg bifenazate/ha, submission date proposed by the applicant: unknown; see Section 5).
Further information to address the chronic risk to honeybees (adult) (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the chronic risk to honeybees (larvae) for all exposure routes (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
Based on EFSA (2013), suitable data to address the risk of sublethal effects (e.g. HPG development effects) to honeybees due to exposure to bifenazate (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
Information to assess the risk to honeybees due to plant metabolites occurring in pollen and nectar (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to non‐target arthropods (relevant for all representative uses at 2 × 0.144 kg bifenazate/ha; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to soil microorganisms for soil metabolites D3598, D1989, IMH/IBMHC (retention time 38 min), IBMHC/DDC (retention time 37.3 min) (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
8. Particular conditions proposed to be taken into account to manage the risk(s) identified
Use of gloves is required for by operators during mixing and loading and application in tractor‐mounted field applications in strawberries to reach exposure estimates below the AOEL (see Appendix A, Section 2).
Use of gloves is required for operators during mixing, loading and application, as well as closed cabin during application, in tractor‐mounted field applications in tomatoes to reach exposure estimates below the AOEL (see Appendix A, Section 2).
Use of gloves, hood and visor is required for operators during mixing and loading and application in tractor‐mounted field applications in ornamentals to reach exposure estimates below the AOEL (see Section 2).
Use of gloves is required for workers re‐entering treated strawberries, fruiting vegetables and ornamentals (also indoor) to reach exposure estimates below the AOEL (see Section 2).
9. Concerns
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/20118 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
-
1
The consumer risk assessment from the consumption of water could not be finalised, whilst satisfactory information was not available to address the effect of water treatment processes on the nature of the residues that might be present in surface water, when surface water is abstracted for drinking water (see Section 4).
-
2
The aquatic risk assessment could not be finalised for metabolites D9963 and D9472 for all representative uses at 2 × 0.144 kg bifenazate/ha (see Sections 4 and 5).
-
3
The aquatic risk assessment for bifenazate and its pertinent metabolites could not be finalised for representative use in ornamental plants at 2 × 0.144 kg bifenazate/ha (see Sections 4 and 5).
-
4
The consumer risk assessment is not finalised due to a number of data gaps that could have an impact on the assessment of residue levels in the different crops and due to missing toxicological reference values for metabolite D3598 (bifenazate‐diazene) included in the residue definition for both monitoring and risk assessment (see Section 3).
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
-
5
A high risk to birds via long term exposure was concluded for all the representative uses (see Section 5).
9.3. Overview of the concerns identified for each representative use considered
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Table 5.
Overview of concerns
| Representative use | Strawberries (Field) | Strawberries (Greenhouse) | Fruiting vegetables (field) | Fruiting vegetables (greenhouse) | Ornamentals (field) | Ornamentals (greenhouse) | |
|---|---|---|---|---|---|---|---|
| Operator risk | Risk identified | For hand‐held application only | For hand‐held application only | For hand‐held application only | |||
| Assessment not finalised | |||||||
| Worker risk | Risk identified | ||||||
| Assessment not finalised | |||||||
| Resident/bystander risk | Risk identified | ||||||
| Assessment not finalised | |||||||
| Consumer risk | Risk identified | ||||||
| Assessment not finalised | X1,4 | X4 | X1,4 | X4 | X1 | ||
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X5 | X5 | X5 | X5 | X5 | X5 |
| Assessment not finalised | |||||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | Xb | Xb | Xb | Xb | Xb | Xb |
| Assessment not finalised | |||||||
| Risk to aquatic organisms | Risk identified | ||||||
| Assessment not finalised | X2 a | X2 a | X2 a | X2 a | X2,3 a | X2,3 a | |
| Groundwater exposure to active substance | Legal parametric value breached | ||||||
| Assessment not finalised | |||||||
| Groundwater exposure to metabolites | Legal parametric value breached | ||||||
| Parametric value of 10 μg/L breached | |||||||
| Assessment not finalised | |||||||
The superscript numbers relate to the numbered points indicated in Sections 9.1 and 9.2. Where there is no superscript number, see Sections 2 to 6 for further information.
For the uses considering two applications at 0.144 kg bifenazate/ha.
High chronic risk identified at Tier 1 using EFSA (2013).
Abbreviations
- 1/n
slope of Freundlich isotherm
- λ
Wavelength
- ɛ
decadic molar extinction coefficient
- a.s.
active substance
- AAOEL
acute acceptable operator exposure level
- ADE
actual dermal exposure
- ADI
acceptable daily intake
- AF
assessment factor
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- ARfD
acute reference dose
- AST
aspartate aminotransferase (SGOT)
- AV
avoidance factor
- BUN
blood urea nitrogen
- bw
body weight
- CAS
Chemical Abstracts Service
- CI
confidence interval
- CL
confidence limits
- DAR
draft assessment report
- DAT
days after treatment
- DFR
dislodgeable foliar residues
- DM
dry matter
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EEC
European Economic Community
- FAO
Food and Agriculture Organization of the United Nations
- FID
flame ionisation detector
- FIR
food intake rate
- FOB
functional observation battery
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GM
geometric mean
- GS
growth stage
- HPLC
high‐pressure liquid chromatography or high‐performance liquid chromatography
- HPLC‐MS
high‐pressure liquid chromatography–mass spectrometry
- HPG
hypopharygeal glands
- HQ
hazard quotient
- HR
hazard rate
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- iv
Intravenous
- KFoc
Freundlich organic carbon adsorption coefficient
- LC
liquid chromatography
- LC50
lethal concentration, median
- LC‐MS
liquid chromatography–mass spectrometry
- LC‐MS-MS
liquid chromatography with tandem mass spectrometry
- LOAEL
lowest observable adverse effect level
- LOQ
limit of quantification
- M/L
mixing and loading
- mm
millimetre (also used for mean measured concentrations)
- MRL
maximum residue level
- MS
mass spectrometry
- NOAEL
no observed adverse effect level
- NOEL
no observed effect level
- OECD
Organisation for Economic Co‐operation and Development
- OM
organic matter content
- Pa
Pascal
- PD
proportion of different food types
- PEC
predicted environmental concentration
- PHI
preharvest interval
- PIE
potential inhalation exposure
- PPE
personal protective equipment
- PT
proportion of diet obtained in the treated area
- QSAR
quantitative structure–activity relationship
- REACH
Registration, Evaluation, Authorisation of Chemicals Regulation
- SC
suspension concentrate
- SMILES
simplified molecular‐input line‐entry system
- TK
technical concentrate
- TRR
total radioactive residue
- TWA
time‐weighted average
- UF
uncertainty factor
- UV
Ultraviolet
- W/S
water/sediment
- w/v
weight per unit volume
- w/w
weight per unit weight
- WBC
white blood cell
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2021.6818
Appendix B – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
| bifenazate‐diazene D3598 | isopropyl (E)‐(4‐methoxybiphenyl‐3‐yl)diazenecarboxylate COc1ccc(cc1/N=N/C(=O)OC(C)C)c1ccccc1 AGTBLMHWQIEASU‐VHEBQXMUSA‐N |
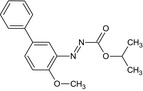
|
| D1989 | 4‐methoxybiphenyl COc1ccc(cc1)c1ccccc1 RHDYQUZYHZWTCI‐UHFFFAOYSA‐N |
|
| DDC IBMHC/DDC (retention time 37.3 min) | 1,8‐dimethoxy‐4,5‐diphenyl‐9H‐carbazole COc1 ccc(c2c1[NH]c1c2c(ccc1OC)c1 ccccc1)c1ccccc1 RWXOASKZSKYKQF‐UHFFFAOYSA‐N or isopropyl 2,2‐bis(4‐methoxybiphenyl‐3‐yl)hydrazinecarboxylate COc1ccc(cc1N(NC(=O)OC(C)C)c1cc(ccc1OC)c1ccccc1)c1ccccc1 UBJNDDBCIGHMDB‐UHFFFAOYSA‐N |
|
| A1530 | biphenyl‐4‐ol Oc1ccc(cc1)c1ccccc1 YXVFYQXJAXKLAK‐UHFFFAOYSA‐N |
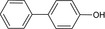
|
| A1530S | biphenyl‐4‐yl hydrogen sulfate OS(=O)(=O)Oc1ccc(cc1)c1ccccc1 JATOIOIIXORRLF‐UHFFFAOYSA‐N |
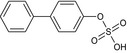
|
| D9963 | 4‐methoxybiphenyl‐3‐ol Oc1cc(ccc1OC)c1ccccc1 HKJUJKFXUPMEKB‐UHFFFAOYSA‐N |
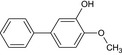
|
| D9472 | biphenyl‐3,4‐diol Oc1ccc(cc1O)c1ccccc1 QDNPCYCBQFHNJC‐UHFFFAOYSA‐N |
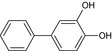
|
| Carbamate | isopropyl (4‐methoxybiphenyl‐3‐yl)carbamate COc1ccc(cc1NC(=O)OC(C)C)c1ccccc1 JPVRHMQFLMLREU‐UHFFFAOYSA‐N |
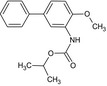
|
| IMH/IBMHC (retention time 38 min) IMH | isopropyl 1,2‐bis(4‐methoxybiphenyl‐3‐yl)hydrazinecarboxylate COc1 ccc(cc1NN(c1 cc(ccc1OC)c1ccccc1)C(=O)OC(C)C)c1ccccc1 LERKVJXCHYRFEY‐UHFFFAOYSA‐N |
|
| D9569 | biphenyl‐4,4′‐diol Oc1ccc(cc1)c1ccc(O)cc1 VCCBEIPGXKNHFW‐UHFFFAOYSA‐N |
|
| DPHDD | 4’’,6′,6’’‐trihydroxy‐1,1’:3’,1’’:3’’,1’’’‐quaterphenyl‐4’,5’‐dione O=C1C(=CC(=C(O)C1 = O)c1ccccc1)c1cc(c2ccccc2)c(O)cc1O YBHHGXGGIMABGH‐UHFFFAOYSA‐N |
|
| Hydroxylated D3598 OH‐D3598 | isopropyl (E)‐(x‐hydroxy‐4‐methoxybiphenyl‐3‐yl)diazenecarboxylate x: 2, 5, 6 |
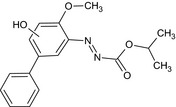
|
The compound name in bold is the name used in the conclusion.
ACD/Name 2019.1.3 ACD/Labs 2019 Release (File version N05E41, Build 111418, 3 September 2019).
ACD/ChemSketch 2019.1.3 ACD/Labs 2019 Release (File version C05H41, Build 111302, 27 August 2019).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation:EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Borroto J, Brancato A, Carrasco Cabrera L, Castoldi AF, Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Gouliarmou V, Ferilli F, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Kienzler A, Leuschner R, Lava R, Linguadoca A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Pedersen R, Reich H, Santos M, Sharp R, Szentes C, Terron A, Tiramani M, Vagenende B and Villamar‐Bouza L, 2021. Conclusion on the updated peer review of the pesticide risk assessment of the active substance bifenazate. EFSA Journal 2021;19(8):6818, 32 pp. 10.2903/j.efsa.2021.6818
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00771
Update: This scientific output, approved on 29 July 2021, supersedes the previous output published on 4 January 2017 (EFSA, 2017).
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgement: EFSA wishes to thank the rapporteur Member State, Sweden, for the preparatory work on this scientific output.
Approved: 29 July 2021
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
For further details, see experts’ consultation in the Report of Pesticide Peer Review Teleconference 48 (EFSA, 2021).
For further details, see experts’ consultation 5.1 and 5.2 in the Report of Pesticide Peer Review Teleconference 53 (EFSA, 2021).
Although the surface water exposure assessment for metabolites D9963 and D9472 did not consider the most conservative values (see Section 4), a low risk was also concluded considering the margin of safety obtained in the risk assessment.
Refer to experts’ consultation point 5.3 in the Report of Pesticide Peer Review Teleconference 53.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Besard L, Mommaerts V, Vandeven J, Cuvelier X, Sterk G and Smagghe G, 2010. Compatibility of traditional and novel acaricides with bumblebees (Bombus terrestris): a first laboratory assessment of toxicity and sublethal effects. Pest Management Science, 66, 786–793. 10.1002/ps.1943 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance on risk assessment for birds and mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009. EFSA Journal 2011;9(2):2092, 49 pp. 10.2903/j.efsa.2011.2092 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Reasoned opinion of EFSA: review of the existing maximum residue levels (MRLs) for bifenazate according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2011;9(12):2484, 48 pp. 10.2903/j.efsa.2011.2484 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. EFSA Guidance Document on clustering and ranking of emissions of active substances of plant protection products and transformation products of these active substances from protected crops (greenhouses and crops grown under cover) to relevant environmental compartments. EFSA Journal 2014;12(3):3615, 43 pp. 10.2903/j.efsa.2014.3615 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Conclusion on the peer review of the pesticide risk assessment of the active substance bifenazate. EFSA Journal 2017;15(1):4693, 27 pp. 10.2903/j.efsa.2017.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2021. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance bifenazate.Available online: www.efsa.europa.eu
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002-rev. 2 final, 17 October 2002
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2005. Review report for the active substance bifenazate. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 3 June 2005 in view of the inclusion of bifenazate in Annex I of Council Directive 91/414/EEC. SANCO/10158/2005-rev.3, 3 June 2005, 27 pp.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9. March 2011. pp. 1–46.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003-rev. 10.1, 13 July 2012.
- European Commission , 2013. Guidance document on data requirements on efficacy for the dossier to be submitted for the approval of new active substances contained in plant protection products. SANCO/10054/2013-rev. 3, 11 July 2013.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.1, March 2012.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005-v. 2.0, 434 pp., as outlined in Generic guidance for estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration, v. 1.0, November 2011.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2007. Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, September 2007.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2009. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010-v. 1, 604 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.0, January 2011.
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: www.oecd.org
- SETAC (Society of Environmental Toxicology and Chemistry), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2.
- Sweden , 2016a. Renewal Assessment Report (RAR) on the active substance bifenazate prepared by the rapporteur Member State Sweden, in the framework of Commission Implementing Regulation (EU) No 844/2012, January 2016. Available online: www.efsa.europa.eu
- Sweden , 2016b. Revised Renewal Assessment Report (RAR) on bifenazate prepared by the rapporteur Member State Sweden in the framework of Commission Implementing Regulation (EU) No 844/2012, November 2016. Available online: www.efsa.europa.eu
- Sweden , 2021a. Revised Renewal Assessment Report (RAR) on bifenazate prepared by the rapporteur Member State Sweden in the framework of Commission Implementing Regulation (EU) No 844/2012, February 2021. Available online: www.efsa.europa.eu
- Sweden , 2021b. Revised Renewal Assessment Report (RAR) on bifenazate prepared by the rapporteur Member State Sweden in the framework of Commission Implementing Regulation (EU) No 844/2012, May 2021. Available online: www.efsa.europa.eu
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


