Abstract
A substance use offense reflects an encounter with law enforcement and the court system in response to breaking the law which may increase risk for substance use problems later in life. Individuals may also be at risk for substance use offending and substance use problems based on genetic predisposition. We examined a mediation model in which polygenic risk for aggression predicted adult substance use disorder diagnoses (SUD) via substance use offending in emerging adulthood. In addition, we explored for potential attenuation of genetic influences on these outcomes by a family-based intervention, the Family Check-Up (FCU). Secondary data analyses based upon the Project Alliance 1 sample was conducted among those with genetic data (n=631; 322 from control and 309 from FCU intervention). The sample was ethnically diverse (30% African American, 44% European American, 6% Latinx, 4% Asian American, 3% Native American, and 13% Other). Greater polygenic risk for aggression was found to increase risk for substance use violations (age 19–23), which in turn was associated with greater likelihood of being diagnosed with SUD at age 27. A gene-by-intervention effect was found in which individuals in the control group had greater risk for SUD with increasing polygenic risk for aggression. Some convergence in results was found when replicating analyses in African American and European American subgroups. Results imply that genetic predisposition may increase risk for problematic substance use later in life via antisocial behavior, such as substance use offending, and that this can be attenuated by a family-centered intervention.
Keywords: Polygenic, Substance use offending, SUD, Longitudinal, Intervention, GxI
Delinquency, aggression, and other antisocial behaviors are common during adolescence. These externalizing behaviors emerge in part due to increased autonomy, decreased parental monitoring, and deviant peer affiliation during adolescence (Dodge, Coie, and Lynam, 2007; Kornienko, Dishion, and Ha, 2018; Wang, Dishion, Stormshak, and Willett, 2011). For most individuals, externalizing behavior subsides during the transition to emerging adulthood as individuals take on more developmentally mature roles, such as entering the workforce, forming romantic relationships, and having children (Sternberg et al., 2018). However, a substantial proportion of individuals continue engaging in antisocial and externalizing behavior into emerging adulthood, which can result in substance use offending (Pelham and Dishion, 2018; Wiesner et al., 2007). Externalizing and antisocial behavior in late adolescence and emerging adulthood, including offending, are associated with substance use disorder (SUD) diagnoses in adulthood (Mulvey and Schubert, 2012; Reef et al., 2011; Wiesner et al., 2005). A separate body of research indicates that there are robust genetic influences on externalizing behavior (see Salvatore and Dick, 2018 for a review), and that antisocial behavior, aggression, conduct disorder, disinhibition, and substance use share genetic etiology (Krueger et al., 2002). Moffitt (2003) and DiLalla and Gottesman (1989) propose that genetic influences may contribute more to antisocial behavior later in life compared to adolescent-limited antisocial behavior. However, important gaps exist in the literature as there is a paucity of evidence regarding genetic influences on offending behavior during emerging adulthood and subsequent influence on SUD in adulthood.
The current study examined whether polygenic risk for aggression increased risk for substance use offending in emerging adulthood and subsequent influences on adult SUD diagnoses. As recent research indicates that genetic predisposition for indices of externalizing behavior can be buffered by psychosocial interventions (Kuo et al., 2019), we also explored gene-by-intervention effects (GxI), based upon the current sample that took part in a family-based intervention in adolescence, the Family Check-Up (FCU). We hypothesized that the intervention would buffer genetic influences on offending and SUD diagnoses.
Externalizing Behavior and Offending in Emerging Adulthood
An offense represents an encounter with law officials and the court systems in response to breaking the law. Externalizing behavior is predictive of delinquency and both can lead to offending with an estimated 62–65% of individuals engaging in adolescent-limited offending (Lussier et al., 2015; Wiesner et al., 2007). Externalizing and offending behaviors are thought to emerge, in part, due to increased autonomy, peer pressures, and lower inhibitory control (Dodge et al., 2007). However, offending often subsides as individuals’ transition into emerging adulthood (Mulvey and Schubert, 2012; Simons et al., 2008). For example, in the Pathways to Desistance study most adolescents reduced or desisted their offending in the transition to emerging adulthood (Wiesner et al., 2007). Theoretically, these individuals may age-out of offending for a number of reasons including increasing social and societal pressure, taking on more mature developmental roles, and normative reductions in externalizing behavior (Dodge et al., 2007; Mulvey and Schubert, 2012; Sternberg et al., 2018). However, approximately 31–37% of individuals persist in offending past adolescence, particularly for substance use violations (Lussier et al., 2015; Wiesner et al., 2007). When substance use offenses persist past adolescence, it greatly increases risk for developing SUD in adulthood, possibly resulting from escalating substance use problems, encounters with the legal system, and underlying biological factors (Mulvey and Schubert, 2012; Wiesner et al., 2005). Researchers have proposed that the presence of antisocial behavior in emerging adulthood, such as offending, may be due to genetic processes compared to adolescent limited antisocial behavior (DiLalla and Gottesman, 1989; Moffitt, 2003).
Genetic Influences on Externalizing Behaviors
There is a robust literature indicating shared genetic etiology underlying substance use, aggression, antisocial behaviors, and disinhibition, as well as genetic prediction of SUD in adulthood (Barr et al.., 2020; Derringer et al., 2015; Elam et al., 2018; Gizer et al., 2016; Kendler et al., 2003; Krueger et al., 2002; McGue et al., 2013; Ronald, de Bode, and Polderman, 2021; Vrieze et al., 2013; Waldman, Rhee, LoParo, and Park, 2018; Young et al, 2000). In accord with this body of work, Iacono’s theoretical model of behavioral disinhibition proposes that early genetic predisposition can manifest as externalizing behavior early in life and substance use later in life when these respective behaviors are most prevalent (e.g., Iacono, 2008; McGue, Irons, and Iacono, 2014). Theoretical and extant evidence indicates that genetic predisposition for externalizing behaviors is primarily shared, rather than specific to individual externalizing behaviors and genetic influences may emerge relative to various behaviors that appear during different developmental periods (e.g., Samek et al., 2017). For example, genetic predisposition for externalizing behavior may be associated with aggression and delinquency during early adolescence prior to patterned substance use (Elam et al., 2018; Narusyte, Andershed, Neiderhiser, and Lichtenstein, 2007; Niv, Tuvblad, Raine, and Baker, 2013) and predictive of substance use in adulthood as it becomes more patterned and normative (Tielbeek, 2018; Zellers et al., 2020).
Research also indicates that genetic predisposition for externalizing behaviors can contribute to offending that is persistent or emerges later in life (Barnes, 2013; Barnes et al., 2011). Offending behavior in emerging adulthood may be one antisocial behavioral outcome resulting from genetic predisposition for externalizing behavior. However, to our knowledge, no research has examined genetic risk for externalizing behavior in predicting offending in pathways to problematic substance use later in life. In the current study we address this by using court records on substance use offending in emerging adulthood and SUD diagnoses in adulthood assessed via diagnostic interviews. It is important to note that these genetic influences should not be viewed through a deterministic lens, as positive family supports and family centered interventions can attenuate genetic predisposition and maladaptive behavior. In particular, emerging research provides evidence of GxI, in which interventions buffer genetic predisposition in longitudinal pathways to substance use (Elam et al., 2020; Kuo et al., 2019).
Emerging GxI Findings
A recent meta-analysis by van Ijzendoorn and Bakermans-Kranenburg (2015) examined GxI studies for a range of developmental outcomes and found empirical support for genetic moderation of intervention effects. Similarly, recent work has found GxI effects on substance use in adolescence and emerging adulthood. For example, Zheng (2018) found a SNP in NR3C1 to moderate intervention effects for African American adolescents’ alcohol abuse trajectories from grade 7 to 2 years post-high school. Intervention effects were strongest for individuals carrying the risk genotype (C alleles). Another study found GxI effects in which risk alleles for two single nucleotide polymorphisms (SNPs) within the ADH1C gene were combined and found to be associated with early adolescent trajectories of alcohol use in the control group but not the intervention group (Cleveland et al., 2018). However, past GxI research has primarily examined single candidate genes (or single variants) which capture little genetic variance and also tend to focus on substance use outcomes compared to problematic substance use or SUDs.
One way to address this is by forming polygenic risk scores (PRSs) that capture cumulative genetic predisposition by leveraging SNPs identified in a discovery genome-wide association study. In the same sample as the current study, Kuo et al. (2019) examined the interaction between a PRS for alcohol dependence and the Family Check-Up intervention (FCU) in predicting lifetime alcohol dependence diagnosis at ages 26–27 for European American and African American individuals. Within the control condition polygenic risk was associated with alcohol dependence diagnosis in the European American sample, while effects were absent in the intervention condition. These findings indicate that the FCU intervention buffered genetic predisposition for alcohol dependence. Polygenic scores can be further strengthened by incorporating bioinformatics approaches that identify SNPs with known biological functions to form functional PRSs. In a separate sample, a study by Elam et al. (2019), applied gene-set enrichment analysis to SNPs from a meta-GWAS on aggression (Pappa et al. 2016). The original meta-GWAS tested associations with behavioral aggression in early adolescence based on the Child Behavior Check List aggression subscale (Achenbach and Rescorla, 2001) or conduct problem scale of the Strengths and Difficulties Questionnaire (Goodman, 1997). Using summary statistics from this meta-GWAS, SNPs were filtered to identify those with known biological functions, for example, those SNPs annotated with a biological function, those that reside in regulatory regions, or those that explain variance in an expression trait (eQTL). Collectively, this approach identified SNPs with an effect either on another genetic loci or directly on a biological function. The resulting SNPs (n = 66) represented multiple biological systems, including glutamate, serotonin, and G-protein coupled receptor activity, among others, all of which are implicated in human and rodent models of aggression (Zhang-James et al., 2019). These SNPs were formed into a functional PRS which explained greater variance in aggression compared to a PRS formed using traditional methods. Of note, a follow-up study to the original meta-GWAS found traditionally formed polygenic scores to predict aggressive behavior from late childhood to early adulthood, demonstrating their prediction of externalizing behaviors later in life (Kretschmer, Ouellet-Morin, Vrijen, Nolte, and Hartman, 2021).
The Current Study
In the present study we extend this research by creating a similar functional PRS for aggression as in Elam et al. (2019) and examining it relative to substance use offending in emerging adulthood and SUD diagnoses in adulthood. As previously reviewed, both SUD in adulthood and offending that exists beyond adolescence may result from underlying genetic risk for externalizing behavior. This is supported by evidence of shared genetic etiology across the externalizing spectrum, and that these genetic influences may emerge for developmentally specific behaviors (e.g., Barr et al., 2020; Iacono, 2008; Vrieze et al., 2013). Based on this, we hypothesize that the PRS for aggression will positively predict substance use offending in emerging adulthood and SUD diagnoses in adulthood, and substance use offending will also positively predict SUD in adulthood. We hypothesize that there will be an indirect effect from the PRS to SUD diagnoses via substance use offending. We also hypothesize a GxI effect whereby genetic predisposition will be related to substance use offending and SUD diagnoses in the control condition but not the intervention condition.
Method
Participants and Procedures
Participants were from the Project Alliance 1 (PAL1) study, a large randomized control trial of 999 adolescents and their families recruited in Portland, Oregon. Participants were randomized to either a control or the FCU intervention condition (Dishion and Kavanagh, 2003). The goal of the intervention was to reduce adolescent problem behavior, substance use, and improve mental health by supporting parenting practices through assessment-driven feedback to motivate parents to change. The FCU is a brief, three-session intervention based on motivational interviewing which consisted of an initial interview, an assessment session, and a feedback session. During the initial interview, a therapist explored parent concerns and stage of change and encouraged the parents to have a family assessment. During the assessment session, the family was videotaped in the home while engaging in a variety of tasks that would help therapists evaluate parent–child interactions. In the feedback session, the therapist systematically summarized the results of the assessment using motivational interviewing strategies. An essential objective of the feedback session was to explore and refer families to services that support family management practices.
All adolescents in 6th grade at three middle schools were invited to participate (90% consented, 50% were randomly assigned to the FCU). Parents’ or guardians’ consent was obtained for all families and adolescents provided assent for participation in the study. Once 18 years old, adolescents provided consent for participation. Court records were available for participants from age 19–23 (Mage = 22.28, SD = .68) and diagnostic interview data were available at ages 19 and 27 (Mage = 27.70; SD = .66), which were used in the present study. The final sample in the present study (n = 631; 322 control, 309 intervention) included participants for whom both court record and genetic data were available.
The current sample (n = 631) did not differ from the larger sample (N = 999) in terms of risk indices (SES or a composite of parental substance use, education, single adult home, overcrowding in the home, parental convictions, neighborhood danger, and poverty), demographic factors (ethnicity), or substance use (p-values ranging from .26 to .89). There was a greater proportion of male participants in the current sample with genetic data (F(1,994) = 22.11, p < .001). All study protocols were approved by the University of Oregon’s Institutional Review board. Data are available upon reasonable request from the principal investigator.
In the present sample, approximately half (49.8%) of the adolescents were female, and 44% European American, 30% African American, 13% multiracial, 6% Hispanic/Latinx, 4% Asian American, and 3% other groups (e.g., Native American, Pacific Islander). The median gross monthly income was $885, with 38% of individuals earning less than $500 per month and 14% earning more than $2000 per month.
DNA was collected using the Oragene saliva collection kits at age 27 and extracted according to Oragene’s recommended procedures. Genotyping was performed at Rutgers University Cell and DNA Repository (RUCDR) using the Affymetrix BioBank Array. Imputation was conducted to 1000 Genomes (Phase 3 reference panel; 1000 Genomes Project Consortium, 2015) using SHAPEIT2 (Delaneau, Zagury, and Marchini, 2013) and then IMPUTE2 (Howie, Donnelly, and Marchini, 2009). Single nucleotide polymorphisms (SNPs) that were palindromic with ambiguous effect directions (A/T or C/G), SNPs with a genotyping rate of < 0.95, SNPs that did not pass Hardy-Weinberg equilibrium (HWE; p < 10−6), or SNPs with a minor allele frequency (MAF) < 0.01 were excluded. In total, 2,067,148 SNPs passed quality control and data cleaning thresholds and were available for analysis.
Measures
Polygenic risk for aggression.
The functional PRS used in this study was originally created and validated as part of previous work in an independent sample. Elam et al. (2019) created the PRS based on summary statistics from a meta-GWAS of aggression (Pappa et al., 2016). Briefly, gene set enrichment analysis using iGSEA4GWASv2 (Zhang et al., 2015) was used to filter summary statistics from the discovery meta-GWAS to identify those that were functional (e.g., annotated, regulatory, eQTLs). This resulted in 766 discovery SNPS which when compared to the sample in Elam et al. (2019) yielded 66 overlapping SNPs in the replication sample and were used to form a polygenic score. In the present sample there were 288 overlapping SNPs in the replication sample which were used to form a functional polygenic score using PRSice v2 (Euesden, Lewis, and O’Reilly, 2015) and the -clump and -score procedures in PLINK to account for nonindependence among SNPs (Purcell et al., 2007). PRS were formed by unit weighting each individual’s total number of minor alleles from these SNPs and dividing by the total number of alleles. The functional PRS for aggression was z-transformed for ease of interpretation.
Population genetic admixture.
Principal Components Analysis were conducted to represent population admixture using snpgdsPCA function from R SNPRelate package (Zheng et al., 2012), after performing LD pruning and filtering using PLINK. The first 20 principal components (PCs) were extracted. When examining PC eigenvalues and the scree plot, the first two PCs were above the elbow cutoff and explained the greatest variance. The first two PCs were found to reliably distinguish European American, African American, and Latinx/Hispanic ancestry. As a further test in the present data, all 20 PCs were examined relative to substance use offending and SUD using stepwise regression. None of the PCs were associated with any of these outcomes but in prior studies in the same sample the first two PCs have been associated with other substance use outcomes. To account for possible variation the first two PCs were residualized from the functional PRS for aggression.
Substance use offending.
With the permission of participants and their parents, court records were searched and obtained for every county where youths reported having resided from age 19 to 23. An offense involved a contact with police enforcement across a number of potential offenses including status violations, misdemeanors, and felonies. A count of substance use offenses encompassed activities such as sale, possession, or use of a controlled or illicit drug and alcohol related offenses such as driving under the influence. 16.7% of individuals in the current sample had one or more substance use offense (one offense: 6.9%, more than one offense: 8.8%). To better reflect the data the count of offenses was recoded to reflect no offenses (0), one offense (1), or more than one offense (2).
SUD diagnoses.
DSM-IV alcohol and other substances (i.e., opiates, cannabis, sedatives, cocaine, amphetamines, hallucinogens, inhalants, PCP/angel dust, and other drugs) abuse and dependence past 12-month diagnoses were assessed at ages 19 and 27 using the computer-assisted personal interview version of the Composite International Diagnostic Interview (CIDI). Individual abuse and dependence diagnoses were combined into a single measure reflecting a count of the number of alcohol/substance diagnoses. 29.9% of individuals had one or more past 12-month abuse or dependence diagnosis (one diagnosis: 16.3%, two diagnoses: 7.2%, three or more diagnoses: 5.4%). To better reflect the data the count of diagnoses was recoded to reflect no diagnosis (0), one diagnosis (1), two diagnoses (2), and three or more diagnoses (3). Age 19 SUD diagnoses were included in the model as a control variable for testing the proposed mediation with age 27 SUD diagnoses as outcome.
Covariates.
Participant gender, age, intervention condition, ethnicity (1 = White, 2 = African American, 3 = Other), and socioeconomic status (z-score) were controlled in all analyses.
Statistical Analyses
After examining descriptive statistics and bivariate correlations among study variables, we examined for significant differences in primary study variables across African American (AA) and European American (EA) subgroups. We next examined a path model in which substance use offending in emerging adulthood was considered a mediator of polygenic risk for aggression and SUD diagnoses in adulthood. SUD diagnoses in emerging adulthood was included as a control for SUD diagnoses in adulthood and as a correlate of substance use offending in emerging adulthood. A GxI interaction term was created for the aggression PRS by intervention condition (0 = control, 1 = intervention) and initially examined as a predictor of SUD at age 19, substance use offending from ages 19–23, and SUD at age 27. To adequately control for confounding effects, PRS by covariate and covariate by intervention interactions were also examined in initial models as recommended by Keller (2014). In the final model, nonsignificant GxI, PRS by covariate, and covariate by intervention interaction terms were removed. Indirect effects were estimated using Rmediation (Tofighi and MacKinnon, 2011), which calculates asymmetric confidence intervals for the distribution of product of alpha (predictor to mediator) and beta (mediator to outcome) paths. This method provides a superior control of Type I error and greater statistical power than the Sobel test (Tofighi and MacKinnon, 2011). The statistical significance of indirect effects was determined when the 95% confidence interval of indirect effects did not include zero. Following the main model, simple slopes for significant interaction terms were probed using interActive (McCabe, Kim, and King, 2018). As a test for sensitivity of PRS effects, we examined the final model in the major AA and EA ethnic subgroups. Full information maximum likelihood was used to handle missing data, based upon the missing at random (MAR) assumption. Descriptive statistics were conducted in SPSS and all other analyses were conducted in Mplus Version 7 using maximum likelihood with robust standard errors to account for non-normal data.
Results
Descriptive statistics
Table 1 presents means, standard deviations, and correlations among study variables for the whole sample. Average levels of offending were low (one offense: 6.9%, two or more offenses: 8.8%) and SUD diagnosis rates were relatively equivalent to national averages (one diagnosis: 16.3%, two diagnoses: 7.2%, three or more diagnoses: 5.4%; McCabe et al., 2017). There were relatively higher levels of SUD diagnoses in the EA sample compared to the AA sample, and higher SES in the EA sample compared to the AA sample (see Table 2). When comparing EA and AA subgroups, the EA subgroup was found to have greater SUD diagnoses in emerging adulthood (F(1, 533) = 5.09, p = .024) and adulthood (F(1, 495) = 4.74, p = .030) when compared to AA participants. No other significant differences were detected.
Table 1.
Means, Standard Deviations, and Correlations among Primary Study Variables for the Entire Sample
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | M (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Polygenic Risk Score | 1 | 0.00 (1.00) | |||||||||
| 2. SUD Diagnoses (age 19) | −0.03 | 1 | 0.13 (0.56) | ||||||||
| 3. SU Offenses (age 19–23) | 0.10* | .13** | 1 | 0.23 (0.59) | |||||||
| 4. SUD Diagnoses (age 27) | 0.01 | .25*** | .11* | 1 | 0.49 (0.86) | ||||||
| 5. Sex | −0.02 | −0.02 | −.18*** | −0.06 | 1 | 0.54 (0.50) | |||||
| 6. Ethnicity | −0.02 | −0.08 | −0.02 | −0.04 | 0.08 | 1 | 1.82 (0.81) | ||||
| 7. Intervention | 0.00 | 0.01 | 0.02 | −0.02 | −0.00 | −0.06 | 1 | 0.49 (0.50) | |||
| 8. SES | 0.05 | −0.00 | −.12** | 0.04 | −0.03 | −.31*** | 0.08 | 1 | −0.01 (0.73) | ||
| 9. PC1 | −0.00 | 0.07 | −0.06 | 0.07 | −0.05 | −.38** | 0.00 | .41*** | 1 | −0.00 (0.04) | |
| 10. PC2 | 0.00 | 0.04 | 0.05 | 0.04 | −0.06 | −.54** | 0.02 | .17*** | −0.01 | 1 | 0.00 (0.03) |
Note. SUD = Substance User Disorder, SU = Substance use, PC = Ancestry Principal Component.
p < .001,
p < .01,
p < .05.
Table 2.
Means, Standard Deviations, and Correlations among Primary Study Variables for African American (above diagonal) and European American (below diagonal)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | AA M (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Polygenic Risk Score | 1 | −0.01 | 0.05 | 0.10 | 0.07 | 0.03 | 0.06 | −0.04 | −0.03 | −0.02 (0.95) |
| 2. SUD Diagnoses (age 19) | −0.04 | 1 | 0.00 | .20* | −0.01 | 0.07 | 0.02 | 0.00 | 0.02 | 0.07 (0.39) |
| 3. SU Offenses (age 19–23) | 0.11 | .24** | 1 | 0.11 | −0.11 | 0.09 | 0.00 | −0.01 | −0.01 | 0.25 (0.62) |
| 4. SUD Diagnoses (age 27) | −0.08 | .28*** | .16* | 1 | −0.06 | −0.09 | −0.04 | 0.02 | 0.07 | 0.38 (0.75) |
| 5. Sex | −0.02 | −0.02 | −.15* | −0.06 | 1 | −0.05 | −0.01 | −.16* | −0.13 | 0.56 (0.50) |
| 6. Intervention | −0.03 | 0.04 | −0.01 | −0.02 | −0.01 | 1 | 0.00 | −0.09 | −0.02 | 0.45 (0.50) |
| 7. SES | 0.03 | −0.07 | −0.11 | 0.10 | −0.01 | 0.07 | 1 | 0.08 | 0.13 | −0.40 (0.71) |
| 8. PC1 | −0.01 | −.17** | −.17** | −0.10 | 0.05 | −0.08 | −0.02 | 1 | .18* | −0.05 (0.02) |
| 9. PC2 | 0.05 | 0.06 | −.22** | −0.02 | 0.12 | −0.04 | −0.04 | .28*** | 1 | 0.01 (0.00) |
| EA M (SD) | 0.02 (1.07) | 0.19 (0.69) | 0.23 (0.61) | 0.56 (0.94) | 0.50 (0.50) | 0.50 (0.50) | 0.32 (0.55) | 0.03 (0.00) | 0.02 (0.00) |
Note. SUD = Substance User Disorder, SU = Substance use, PC = Ancestry Principal Component, AA = African American, EA= European American.
p < .001,
p < .01,
p < .05.
As indicated by the correlations in the entire sample, the PRS was positively associated with substance use offending, which was positively associated with SUD diagnoses in both emerging adulthood and adulthood. In the EA subgroup there was a trend association between the PRS and substance use offending, and offending was positively associated with SUD diagnoses at both ages, but these associations were not present in the AA subgroup (Table 2).
Mediation Model
The final model with the full sample showed good model fit (X2 (8) = 9.61, p = .94, RMSEA = 0.0, CFI/TLI = 1.0). The PRS predicted substance use offenses in emerging adulthood (β = .10, SE β = .04, p = .017), and substance use offenses predicted SUD diagnoses in adulthood (β = .09, SE β = .04, p = .033; see Figure 1). The PRS also directly predicted SUD diagnoses in adulthood (β = .12, SE β = .06, p = .031), as did SUD diagnoses in emerging adulthood (β = .22, SE β = .04, p < .001). The GxI interaction term was not associated with SUD diagnoses or substance use offending in emerging adulthood in initial models so was not included in the final model. The GxI interaction term significantly predicted SUD diagnoses in adulthood (β = −.18, SE β = .06, p = .002). SUD diagnoses and substance use offenses in emerging adulthood were significantly associated (β = .12, SE β = .04, p = .006). An indirect effect was detected from the PRS to SUD via substance use offenses (β = .01, SE β = .006, 95% CI [.00, .02], p = .025). Few covariate effects were detected: female gender (β = −.17, SE β = .04, p < .001) and greater income were associated with fewer substance use offenses (β = −.16, SE β = .05, p = .001).
Figure 1.
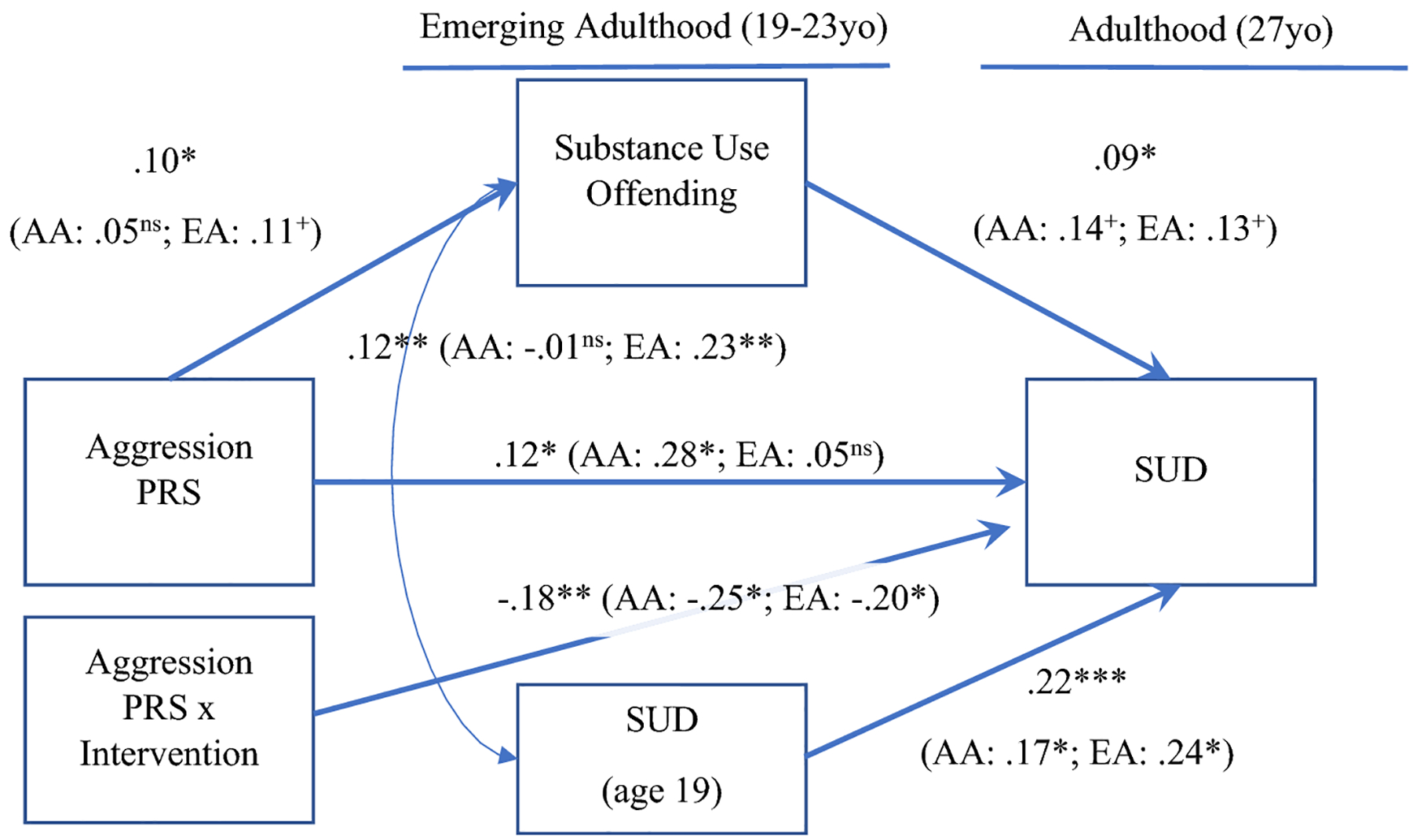
Mediation model between aggression PRS, substance use offending, and SUD. Covariate and nonsignificant paths not pictured. SUD = Substance use disorder diagnoses, PRS = polygenic risk score. +p < .08
The simple slopes for the GxI interaction can be seen in Figure 2. There was a trend towards significance for the simple slope in the intervention condition (β = −.11, 95% CI [−.21, .00], p = .05) and a significant simple slope in the control condition (β = .11, 95% CI [.01, .21], p = .03).
Figure 2.
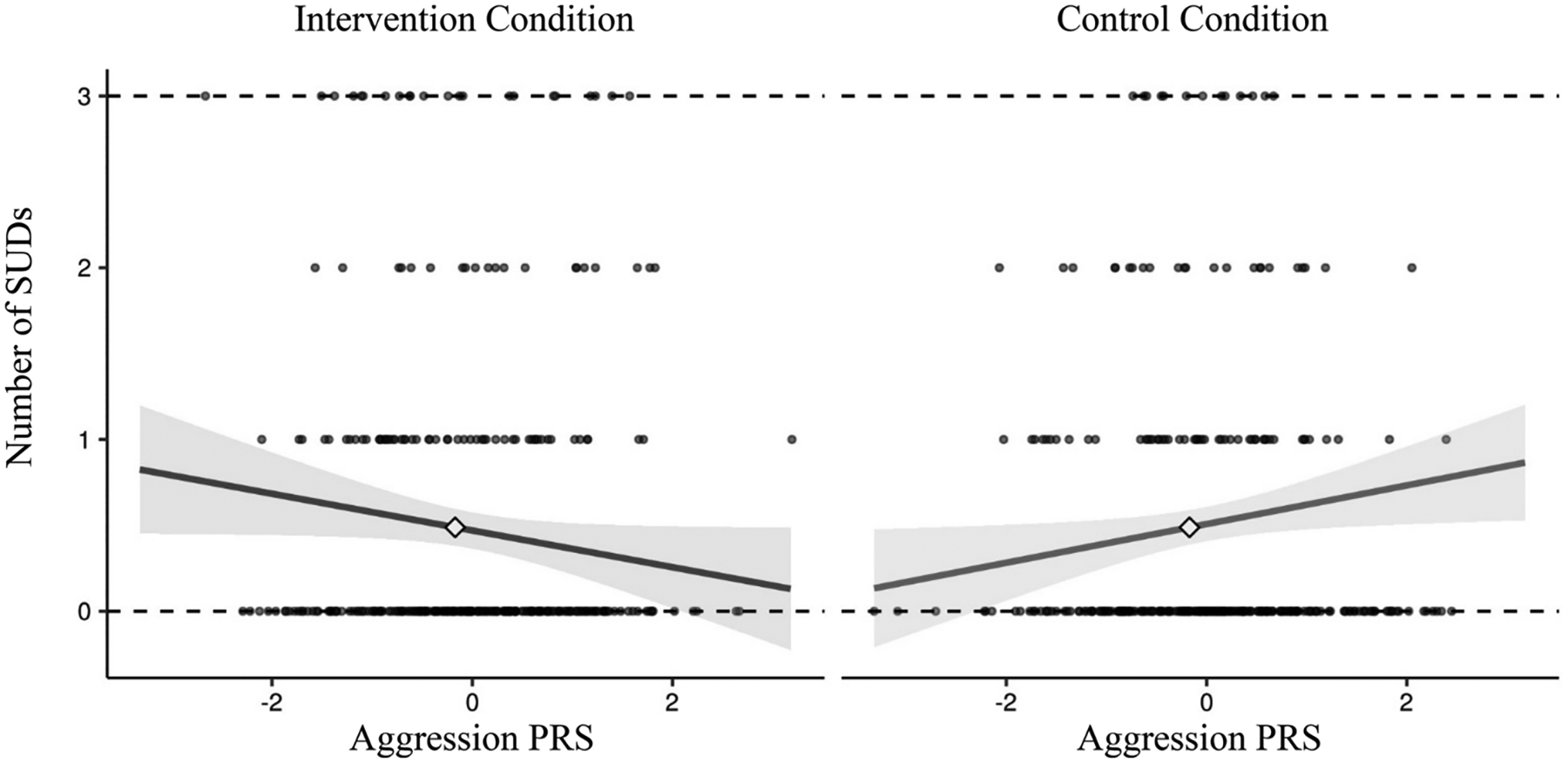
Simple slopes for PRS by intervention condition interaction. The simple slope is significant in the control condition (p = .03) and at a trend level in the intervention condition (p = .05).
AA and EA Subgroups
In the AA subgroup, the PRS did not predict substance use offenses (β = .05, SE β = .08, p = .500; see Figure 1), and there was a trend for substance use offenses to predict SUD diagnoses in adulthood (β = .14, SE β = .07, p = .065). The PRS directly predicted SUD diagnoses in adulthood (β = .28, SE β = .10, p = .005), as did SUD diagnoses in emerging adulthood (β = .17, SE β = .08, p = .029), as well as the GxI interaction term (β = −.25, SE β = .10, p = .010). SUD diagnoses and substance use offenses in emerging adulthood were not correlated (β = −.01, SE β = .04, p = .917). No covariate effects were detected. When examining the GxI interaction (see Figure 3a), the simple slope for the intervention condition was not significant (β = −.05, 95% CI [−.22, .12], p = .57) but the simple slope was significant in the control condition (β = .18, 95% CI [.03, .33], p = .02).
Figure 3.
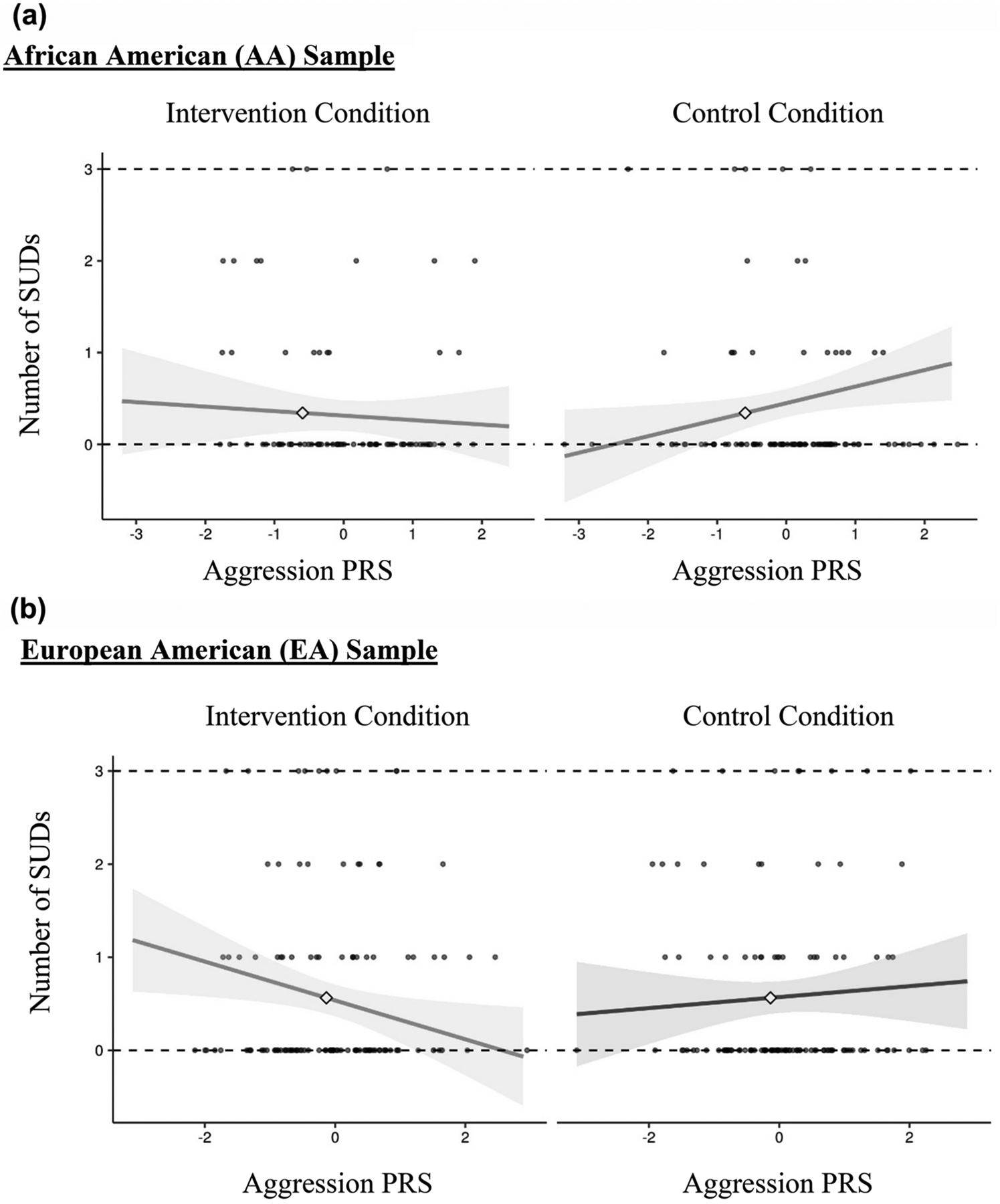
a. Simple slopes for PRS by intervention condition interaction in the AA subgroup. The simple slope is significant in only the control condition.
b. Simple slopes for PRS by intervention condition interaction in the EA subgroup. The simple slope is significant in only the intervention condition.
In the EA subgroup, there was a trend for the PRS to predict substance use offenses (β = .11, SE β = .06, p = .079; see Figure 1), and a trend for substance use offenses to predict SUD diagnoses in adulthood (β = .13, SE β = .07, p = .055). The PRS did not predict SUD diagnoses in adulthood (β = .05, SE β = .09, p = .573) but SUD diagnoses in emerging adulthood did predict SUD diagnoses in adulthood (β = .24, SE β = .04, p < .001). The GxI interaction term predicted SUD diagnoses in adulthood (β = −.20, SE β = .06, p = .024). SUD diagnoses and substance use offenses in emerging adulthood were correlated (β = .23, SE β = .04, p = .001). Greater SES was associated with greater SUD diagnoses in adulthood (β = .13, SE β = .06, p = .036) and female gender was associated with lower substance use offending (β = −.14, SE β = .06, p = .027). Interestingly, when examining the GxI interaction (see Figure 3b), the simple slope for the intervention condition was significant (β = −.23, 95% CI [−.38, −.04], p = .02) but the simple slope was not significant in the control condition (β = −.07, 95% CI [−.19, .05], p = .23).
Discussion
Substance use offending in emerging adulthood is problematic and a significant risk factor for SUD later in life. Offending beyond adolescence and SUD in adulthood may indicate underlying genetic risk for externalizing behavior. Additionally, recent research has demonstrated that genetic influences on substance use can be buffered by psychosocial interventions. In accord with this literature, we hypothesized that a functional PRS for aggression would directly predict SUD diagnoses in adulthood, as well as indirectly via substance use offenses in emerging adulthood. Genetic predisposition for aggression, as indicated by a functional PRS, directly conferred greater risk for adult SUD diagnoses, but also via greater substance use offending in emerging adulthood. In addition, as hypothesized we found the FCU was found to buffer genetic influences on adult SUD diagnoses. We explore these findings below but advise caution in their interpretation given their novelty and some differential effects that we observed in the major ethnic subgroups.
In the current study we found the functional aggression PRS predicted substance use offending in the whole sample. Moffit (2003) as well as DiLalla and Gottesman (1989) propose that genetic influences may contribute more to adult or life-course persistent antisocial behavior compared to adolescent-limited antisocial behavior. In complement, twin studies have found increasing heritability with age for externalizing behavior and substance use (e.g., Bergen et al., 2007; Kendler et al., 2008). Thus, one possibility is that for some individuals, genetic predisposition for externalizing persists or even increases over time. This may be due to new genetic influences that emerge with development. Alternatively, over time genetic associations may emerge for developmentally specific behaviors such as disinhibition and delinquency in adolescence and substance use in adulthood (Samek et al., 2017; Tielbeek et al., 2018; Zellers et al., 2020). It is also possible that transactions between genetic predisposition and negative social environments contribute to increasing heritability and increased antisocial behavior via social mediation of genetic effects (Iacono, 2008; Leve and Reiss, 2007). For example, genetic predisposition can lead to exposure to negative environments via gene-environment correlations, increasing risk for psychopathology later in life, which may also be associated with genotype. Thus, the associations between polygenic risk for aggression and substance use offending, and separately SUD diagnoses, observed in our study may be due to a direct genetic influence, but could also be due to gene-environment interplay, however this study is mute as to this process. Future research should examine for gene-environment correlations with social environments, such as peers and romantic partners, that may also underlie these processes.
Interestingly, when we tested the effects of the PRS on offending separately for EA and AA, the PRS-offending association was only present in the EA subgroup. This may be due to a loss of power in the relatively smaller AA subgroup. It could also be due to greater overall levels of SUD diagnoses in the EA group. Another alternative speculation is that because the PRS was created based on a European descent GWAS, it is more predictive in the EA subgroup. However, this did not appear to be the case for a number of other findings present in the AA subgroup. For instance, in the AA subgroup the PRS was found to directly predict SUD diagnoses in adulthood but not in the EA subgroup. This could indicate stronger genetic effects on SUD diagnoses in the AA subgroup or that there are other environmental mediators not captured in this study. For example, despite lower levels of substance use in AAs, they experience higher rates of some contextual risk factors including discrimination, residential segregation, and limited access to adequate health resources which can increase risk for developing substance use problems (Scott, 2017). Similarly, the influence of discrimination could mask genetic effects on offending for AAs given the possibility that interactions with the legal system are confounded with race rather than actual offending.
We also found that greater substance offending in emerging adulthood predicted SUD diagnoses in adulthood, approximately five years later while controlling for SUD at age 19. When tested within AA and EA subgroups, neither effect was significant but showed trends that were in the same direction. This finding is in line with literature that supports associations between externalizing behaviors (e.g., antisociality, substance use, disinhibition, offending) and substance use problems later in life (Mulvey and Schubert, 2012; Reef et al., 2011; Wiesner et al., 2005). This link may be due to escalation of substance use problems over time, underlying behavioral disinhibition, or possibly that a substance use offense is indicative of more problematic substance use and thus a marker for SUD diagnosis later in life.
In complement to this, these findings emphasize the value of forming functional PRSs. The current functional PRS captured glutamate, serotonin, and G-protein coupled receptor activity, among others, all of which are implicated in human and rodent models of aggression (Zhang-James et al., 2019). It may be that genetic predisposition related to this PRS contributes to greater biological predisposition for aggression, increasing predilection for antisocial and offending behavior in emerging adulthood and SUD diagnoses in adulthood. However, these associations are not deterministic but likely involve a myriad of cascading biological and environmental processes that serve as both risk and resilience factors across development. This is highlighted by our finding that PRS associations with SUD in adulthood were buffered by participating in the FCU intervention in adolescence.
There was a significant GxI effect on SUD diagnoses in adulthood, but not on substance use offending. Closer inspection of these findings indicated that for those in the control condition, greater polygenic risk for aggression was associated with greater risk for a SUD diagnosis in adulthood, which was also found in the AA subgroup. That is, in the absence of the positive supports provided by the FCU genetic risk for aggression contributed to risk for SUD diagnosis. Conversely, in the FCU intervention condition there was decreasing risk for SUD diagnoses with increasing genetic risk for aggression, which was at a trend level in the whole sample and significant in the EA subgroup. This could indicate evidence of differential susceptibility in which genetic predisposition for aggression reflects sensitivity to the environment possibly through improved parenting and family life via the FCU. An alternative explanation is that individuals at greater risk may benefit more from interventions, which is supported by previous research finding greater intervention effects for those individuals at higher demographic risk and higher genetic risk (Wolchik et al., 2000; Zheng et al., 2018). This could be indicated in the present finding such that those at greater genetic risk benefitted more from the FCU. Finally, the absence of GxI effects on substance use offending may indicate offending was less impacted by the intervention, or that such behaviors are more influenced by environmental factors such as peers, which could be indicated by the small effect size observed for the PRS in the current study. More broadly, this illustrates that a family centered intervention administered in adolescence can have long-term impacts by mitigating genetic predisposition on SUD diagnoses in adulthood. This is in accord with previous GxI findings and highlights the importance of providing guidance and positive family supports to at-risk individuals early in life (Elam et al., 2020; Kuo et al., 2019).
The present study had a number of strengths, including use of a functional PRS, examination of longitudinal associations between constructs measured using objective measurements of court records and diagnostic interviews, and the ability to test for long-term intervention effects. However, these findings require replication and limitations should be addressed in future studies. A limitation of this and other polygenic studies is the reliance on discovery GWAS of European descent to examine PRS in other racial/ethnic groups given the lack of discovery GWAS in aligned populations (Martin et al., 2017). In the current study we partially addressed this by controlling for the first two ancestry principal components. Emerging multi-ethnic PRS approaches present a compelling alternative given available GWAS and is an important endeavor for future research (Marquez-Luna et al., 2017). Future research should also pursue other advanced methods for forming PRSs that leverage the whole genome, which continue to evolve. Our functional PRS approach offers one novel method for examining genetic influences but emerging polygenic methods are increasingly able to explain greater variance in outcomes (Pain et al., 2021). Also, many of the effect sizes in the current study were small. This may be related to small genetic effects, which are similar to past polygenic studies (Elam et al., 2018), or low rates of offending and SUD diagnoses from a statistical modeling perspective, but which were broadly in-line or higher than national averages (Grant et al., 2016; 2017). Conversely, these small effects were in the context of controlling for SUD diagnoses in emerging adulthood, which had a relatively larger effect size.
Collectively, these finding can help to inform prevention and intervention strategies designed to reduce substance use offending and SUD. Although not a focus of this study, one strategy could be to leverage endophenotypes reflecting genetic predisposition identified in other studies to help inform intervention strategies (Harold, Leve, Sellers, 2017; Leve et al., 2010). Such approaches could have long term effects on externalizing behaviors and substance use.
Funding:
The research reported in this paper was supported by grants from the National Institute of Drug Abuse (DA07031 to Ha; DA042828 to Elam; DA049393 to Mun) as well as by the Office of the Director and Office of Behavioral and Social Sciences Research (DA042828 to Elam). Additional support was given by the National Institute on Alcoholism and Alcohol Abuse to (AA022071 to Ha). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute on Drug Abuse, the National Institute on Alcoholism and Alcohol Abuse, or the Office of Behavioral and Social Sciences Research.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/Competing interests: None.
Ethics approval: This study was approved by the University of Oregon’s Institutional Review board.
Consent to participate: Informed consent was obtained from all individual participants included in the study.
Availability of data: Data are available upon reasonable request from the study PI.
References
- Achenbach TM, and Rescorla LA (2001). Manual for the ASEBA school age forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Barnes JC, Beaver KM, & Boutwell BB (2011). Examining the genetic underpinnings to Moffitt’s developmental taxonomy: A behavioral genetic analysis. Criminology, 49(4), 923–954. [Google Scholar]
- Barnes JC (2013). Analyzing the origins of life-course-persistent offending: A consideration of environmental and genetic influences. Criminal Justice and Behavior, 40(5), 519–540. [Google Scholar]
- Barr PB, Salvatore JE, Wetherill L, Anokhin A, Chan G, Edenberg HJ, … & Dick DM (2020). A family-based genome wide association study of externalizing behaviors. Behavior Genetics, 50(3), 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, & Kendler KS (2007). Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Research and Human Genetics, 10(3), 423–433. [DOI] [PubMed] [Google Scholar]
- Cleveland HH, Schlomer GL, Vandenbergh DJ, Wolf PS, Feinberg ME, Greenberg MT, … & Redmond C (2018). Associations between alcohol dehydrogenase genes and alcohol use across early and middle adolescence: Moderation× Preventive intervention. Development and Psychopathology, 30(1), 297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derringer J, Corley RP, Haberstick BC, Young SE, Demmitt BA, Howrigan DP, … & McQueen MB (2015). Genome-wide association study of behavioral disinhibition in a selected adolescent sample. Behavior Genetics, 45(4), 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLalla LF, & Gottesman II (1989). Heterogeneity of causes for delinquency and criminality: Lifespan perspectives. Development and Psychopathology, 1, 339–349. [Google Scholar]
- Dishion TJ, & Kavanagh K (2003). Intervening in adolescent problem behavior: A family-centered approach. New York: Guilford. [Google Scholar]
- Dodge KA, Coie JD, & Lynam D (2007). Aggression and antisocial behavior in youth. In Damon W & Lerner R (Eds.) Child and adolescent development: An advanced course. (pp. 437–472). John Wiley & Sons, Inc. [Google Scholar]
- Elam KK, Clifford S, Ruof A, Shaw DS, Wilson MN, & Lemery-Chalfant K (2020). Genotype–environment correlation by intervention effects underlying middle childhood peer rejection and associations with adolescent marijuana use. Development and Psychopathology, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam KK, Clifford S, Shaw DS, Wilson MN, & Lemery-Chalfant K (2019). Gene set enrichment analysis to create polygenic scores: A developmental examination of aggression. Translational Psychiatry, 9(1), 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam KK, Chassin L, & Pandika D (2018). Polygenic Risk, Family Cohesion, and Adolescent Aggression in Mexican-American and European-American Families: Developmental Pathways to Alcohol Use. Development and Psychopathology, 30(5), 1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euesden J, Lewis CM, & O’Reilly PF (2015). PRSice: polygenic risk score software. Bioinformatics, 31(9), 1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Otto JM, & Ellingson JM (2016). Molecular genetics of the externalizing spectrum. In Beauchaine TP & Hinshaw SP (Eds.), Oxford library of psychology. The Oxford handbook of externalizing spectrum disorders (p. 149–169). Oxford University Press. [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, … & Hasin DS (2016). Epidemiology of DSM-5 drug use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions–III. JAMA Psychiatry, 73(1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, … & Hasin DS (2017). Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74(9), 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold GT, Leve LD, & Sellers R (2017). How can genetically informed research help inform the next generation of interparental and parenting interventions? Child Development, 88(2), 446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, & McGue M (2008). Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annual Review of Clinical Psychology, 4, 325–348. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, & Neale MC (2003). The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry, 60(9), 929–937. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, & Prescott CA (2008). Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry, 65(6), 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornienko O, Dishion TJ, & Ha T (2018). Peer network dynamics and the amplification of antisocial to violent behavior among young adolescents in public middle schools. Journal of Emotional and Behavioral Disorders, 26(1), 21–30. [Google Scholar]
- Kretschmer T, Ouellet-Morin I, Vrijen C, Nolte IM, & Hartman C (2021). Polygenic risk for aggressive behaviour from late childhood through early adulthood. PsyArXiv. doi: 10.31234/osf.io/k56q8. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG & McGue M (2002) Etiological connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology, 111, 411–424 [PubMed] [Google Scholar]
- Kuo SI, Salvatore JE, Aliev F, Ha T, Dishion TJ, & Dick DM (2019). The Family Check-up Intervention Moderates Polygenic Influences on Long-Term Alcohol Outcomes: Results from a Randomized Intervention Trial. Prevention Science, 20(7), 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leve LD, Harold GT, Ge X, Neiderhiser JM, & Patterson G (2010). Refining intervention targets in family-based research: Lessons from quantitative behavioral genetics. Perspectives on Psychological Science, 5(5), 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier P, McCuish E, & Corrado RR (2015). The adolescence–adulthood transition and desistance from crime: Examining the underlying structure of desistance. Journal of Developmental and Life-Course Criminology, 1(2), 87–117. [Google Scholar]
- Márquez-Luna C, Loh PR, South Asian Type 2 Diabetes (SAT2D) Consortium, SIGMA Type 2 Diabetes Consortium, & Price AL (2017). Multiethnic polygenic risk scores improve risk prediction in diverse populations. Genetic Epidemiology, 41(8), 811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, … & Kenny EE (2017). Human demographic history impacts genetic risk prediction across diverse populations. The American Journal of Human Genetics, 100(4), 635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Kim D, & King K (2018). Improving Present Practices in the Visual Display of Interactions. Advances in Methods and Practices in Psychological Science, 1(2), 147–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Irons D, & Iacono WG (2014). The adolescent origins of substance use disorders: A behavioral genetic perspective. In Stoltenberg F (Ed.) Genes and the motivation to use substances (pp. 31–50). Nebraska Symposium on Motivation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Zhang Y, Miller MB, Basu S, Vrieze S, Hicks B, … & Iacono WG (2013). A genome-wide association study of behavioral disinhibition. Behavior Genetics, 43(5), 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE (2003). Life-course persistent and adolescence-limited antisocial behavior. In Lahey B, Moffitt T & Caspi A (Eds.) Causes of conduct disorder and juvenile delinquency (pp. 49–75). Guilford Press. [Google Scholar]
- Mulvey EP, & Schubert CA (2012). Some initial findings and policy implications of the pathways to desistance study. Victims & offenders, 7(4), 407–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusyte J, Andershed AK, Neiderhiser JM, & Lichtenstein P (2007). Aggression as a mediator of genetic contributions to the association between negative parent–child relationships and adolescent antisocial behavior. European Child & Adolescent Psychiatry, 16(2), 128–137. [DOI] [PubMed] [Google Scholar]
- Niv S, Tuvblad C, Raine A, & Baker LA (2013). Aggression and rule-breaking: heritability and stability of antisocial behavior problems in childhood and adolescence. Journal of Criminal Justice, 41(5), 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain O, Glanville KP, Hagenaars SP, Selzam SP, Fürtjes AE, Gaspar HA, … & Lewis CM (2021). Evaluation of polygenic prediction methodology within a Reference-Standardized framework. bioRxiv. doi: 10.1101/2020.07.28.224782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa I, St Pourcain B, Benke K, Cavadino A, Hakulinen C, Nivard MG, … Evans DM (2016). A genome-wide approach to children’s aggressive behavior: The EAGLE consortium. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171(5), 562–572. [DOI] [PubMed] [Google Scholar]
- Pelham WE III, & Dishion TJ (2018). Prospective prediction of arrests for driving under the influence from relationship patterns with family and friends in adolescence. Addictive Behaviors, 78, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, … & Sham PC (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics, 81(3), 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reef J, Diamantopoulou S, van Meurs I, Verhulst FC, & van der Ende J (2011). Developmental trajectories of child to adolescent externalizing behavior and adult DSM-IV disorder: results of a 24-year longitudinal study. Social Psychiatry and Psychiatric epidemiology, 46(12), 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, de Bode N, & Polderman TJ (2021). Systematic Review: How the Attention-Deficit/Hyperactivity Disorder Polygenic Risk Score Adds to Our Understanding of ADHD and Associated Traits. Journal of the American Academy of Child & Adolescent Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, & Dick DM (2018). Genetic influences on conduct disorder. Neuroscience & Biobehavioral Reviews, 91, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samek DR, Hicks BM, Keyes MA, Iacono WG, & McGue M (2017). Antisocial peer affiliation and externalizing disorders: Evidence for Gene× Environment× Development interaction. Development and Psychopathology, 29(1), 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MS (2017). Commentary: Perspectives on alcohol-related gene and environment interplay in diverse populations. The American Journal on Addictions, 26(5), 526–531. [DOI] [PubMed] [Google Scholar]
- Simmons C, Rowan Z, Knowles A, Steinberg L, Frick PJ, & Cauffman E (2019). A life history approach to understanding juvenile offending and aggression. Aggression and Violent Behavior, 49, 101317. [Google Scholar]
- Sternberg A, Pandika D, Elam KK, & Chassin L (2018). The relation of parent alcohol disorder to young adult drinking outcomes mediated by parenting: Effects of developmentally limited versus persistent parent alcohol disorder. Drug and Alcohol Dependence, 188, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielbeek JJ, Vink JM, Polderman TJ, Popma A, Posthuma D, & Verweij KJ (2018). Genetic correlation of antisocial behaviour with alcohol, nicotine, and cannabis use. Drug and Alcohol Dependence, 187, 296–299. [DOI] [PubMed] [Google Scholar]
- Tofighi D, & MacKinnon DP (2011). RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods, 43(3), 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, & Bakermans-Kranenburg MJ (2015). Genetic differential susceptibility on trial: Meta-analytic support from randomized controlled experiments. Development and Psychopathology, 27(1), 151–162. [DOI] [PubMed] [Google Scholar]
- Vrieze SI, McGue M, Miller MB, Hicks BM, & Iacono WG (2013). Three mutually informative ways to understand the genetic relationships among behavioral disinhibition, alcohol use, drug use, nicotine use/dependence, and their co-occurrence: twin biometry, GCTA, and genome-wide scoring. Behavior Genetics, 43(2), 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID, Rhee SH, LoParo D, & Park Y (2018). Genetic and environmental influences on psychopathy and antisocial behavior. In Patrick CJ (Ed.), Handbook of psychopathy (pp. 335–353). The Guilford Press. [Google Scholar]
- Wang MT, Dishion TJ, Stormshak EA, & Willett JB (2011). Trajectories of family management practices and early adolescent behavioral outcomes. Developmental Psychology, 47(5), 1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner M, Kim HK, & Capaldi DM (2005). Developmental trajectories of offending: Validation and prediction to young adult alcohol use, drug use, and depressive symptoms. Development and Psychopathology, 17(1), 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner M, Capaldi DM, & Kim HK (2007). Arrest trajectories across a 17-year span for young men: Relation to dual taxonomies and self-reported offense trajectories. Criminology: An Interdisciplinary Journal, 45 (4), 835–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchik SA, West SG, Sandler IN, Tein JY, Coatsworth D, Lengua L, … & Griffin WA (2000). An experimental evaluation of theory-based mother and mother–child programs for children of divorce. Journal of Consulting and Clinical Psychology, 68(5), 843. [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, & Hewitt JK (2009). Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology, 118(1), 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellers SM, Corley R, Thibodeau E, Kirkpatrick R, Elkins I, Iacono WG, … & Vrieze S (2020). Adolescent externalizing psychopathology and its prospective relationship to marijuana use development from age 14 to 30: replication across independent longitudinal twin samples. Behavior Genetics, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang-James Y, Fernàndez-Castillo N, Hess JL, Malki K, Glatt SJ, Cormand B, & Faraone SV (2019). An integrated analysis of genes and functional pathways for aggression in human and rodent models. Molecular Psychiatry, 24(11), 1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Chang S, Guo L, & Wang J (2015). I-GSEA4GWAS v2: A web server for functional analysis of SNPs in trait-associated pathways identified from genome-wide association study. Protein Cell, 6(3), 221–224. doi: 10.1007/s13238-014-0114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Albert D, McMahon RJ, Dodge K, Dick D, & Conduct Problems Prevention Research Group. (2018). Glucocorticoid receptor (NR3C1) gene polymorphism moderate intervention effects on the developmental trajectory of African-American adolescent alcohol abuse. Prevention Science, 19(1), 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


