Abstract
Background and Objectives
Since the onset of the COVID-19 pandemic, a growing number of reports have described cases of acute disseminated encephalomyelitis (ADEM) and acute hemorrhagic leukoencephalitis (AHLE) following infection with COVID-19. Given their relatively rare occurrence, the primary objective of this systematic review was to synthesize their clinical features, response to treatments, and clinical outcomes to better understand the nature of this neurologic consequence of COVID-19 infection.
Methods
Patients with a history of COVID-19 infection were included if their reports provided adequate detail to confirm a diagnosis of ADEM or AHLE by virtue of clinical features, radiographic abnormalities, and histopathologic findings. Cases purported to be secondary to vaccination against COVID-19 or occurring in the context of a preexisting relapsing CNS demyelinating disease were excluded. Case reports and series were identified via PubMed on May 17, 2021, and 4 additional cases from the authors' hospital files supplemented the systematic review of the literature. Summary statistics were used to describe variables using a complete case analysis approach.
Results
Forty-six patients (28 men, median age 49.5 years, 1/3 >50 years old) were analyzed, derived from 26 case reports or series originating from 8 countries alongside 4 patient cases from the authors' hospital files. COVID-19 infection was laboratory confirmed in 91% of cases, and infection severity necessitated intensive care in 67%. ADEM occurred in 31 cases, whereas AHLE occurred in 15, with a median presenting nadir modified Rankin Scale score of 5 (bedridden). Anti-MOG seropositivity was rare (1/15 patients tested). Noninflammatory CSF was present in 30%. Hemorrhage on brain MRI was identified in 42%. Seventy percent received immunomodulatory treatments, most commonly steroids, IV immunoglobulins, or plasmapheresis. The final mRS score was ≥4 in 64% of patients with adequate follow-up information, including 32% who died.
Discussion
In contrast to ADEM cases from the prepandemic era, reported post–COVID-19 ADEM and AHLE cases were often advanced in age at onset, experienced severe antecedent infection, displayed an unusually high rate of hemorrhage on neuroimaging, and routinely had poor neurologic outcomes, including a high mortality rate. Findings are limited by nonstandardized reporting of cases, truncated follow-up information, and presumed publication bias.
Acute disseminated encephalomyelitis (ADEM) is an inflammatory demyelinating disease of the CNS, characterized by fulminant multifocal neurologic injury and distinct neuropathologic findings.1 ADEM is classically thought to be preceded by vaccination or a systemic infection, of which upper respiratory tract infections are most commonly reported.2 Given that ADEM is more common in children, consensus criteria for clinically diagnosing ADEM exist for children only.1 In the absence of a biomarker, the diagnosis of ADEM is largely reliant on the clinical presentation supported by typical MRI findings and the exclusion of competing diagnoses through ancillary testing. Most ADEM cases are clinically monophasic, but a small number are recurrent or subsequently realized to be the initial presentation of a relapsing CNS demyelinating disease such as MS, myelin oligodendrocyte glycoprotein antibody disease, or neuromyelitis optica spectrum disorder.2
Since the onset of the coronavirus (COVID-19) pandemic in November 2019, neurologic complications of SARS-CoV-2 infection are increasingly recognized. Parainfectious neurologic injury is well described, and ageusia and anosmia are components of the primary constellation of symptoms and can be early signs of infection.3,4 Headache, delirium, and dizziness are also common clinical accompaniments.5,6 The neuroinflammatory events following COVID-19, particularly ADEM and its severe variant acute hemorrhagic leukoencephalitis (AHLE and Weston-Hurst disease), occur rarely and have yet to be synthesized in the literature. Most published reports are single cases or small case series. However, the global clinical experience on ADEM and AHLE in the setting of COVID-19 appears to be growing.
In this case series and systematic literature review, we report the collective experience of ADEM and AHLE following COVID-19. We characterize the disease course, diagnostic test findings, patient outcomes, and treatment approaches of both COVID-19 and the subsequent presentations of ADEM and AHLE. Given the novelty of this virus and the ongoing nature of the global pandemic, we address the available data for the phenotypic presentations of ADEM and AHLE, the relationship to COVID-19 disease factors, and highlight issues for future research, data gathering, and clinical care. Finally, we consider whether COVID-associated ADEM and AHLE cases differ from their counterparts that predated the pandemic.
Methods
Overview
We performed a systematic review for cases of post–COVID-19 ADEM and AHLE in the medical literature, adding cases evaluated at our institution. The Massachusetts General Brigham (MGB) Institutional Review Board approved the study.
Information Sources
Cases were principally obtained by systematically searching PubMed. Four cases originating from the MGB health care system were included in the final synthesis of individual cases.
Search Strategy and Screening Process
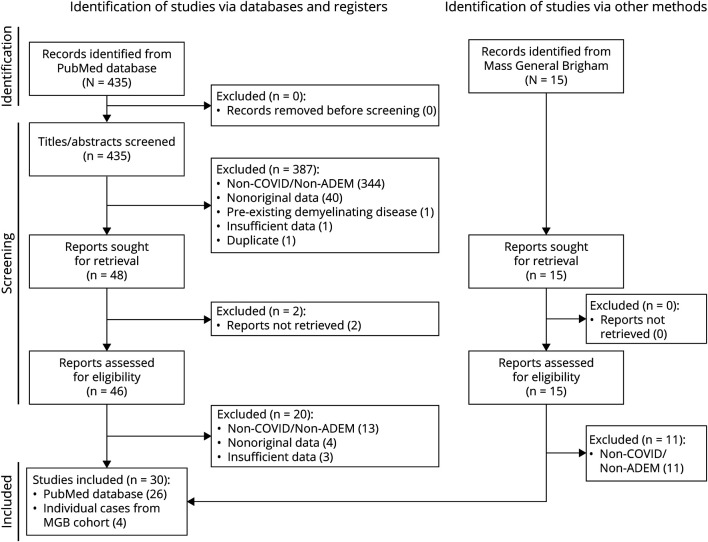
We searched pubmed.gov on May 17, 2021, for cases of COVID-19, followed by ADEM or AHLE. The following search strategy was used: [coronavirus disease OR coronavirus OR COVID OR COVID-19 OR SARS-CoV OR SARS-CoV-2] AND [acute disseminated encephalomyelitis OR ADEM OR acute hemorrhagic leukoencephalitis OR AHLE]. A time filter of reports published between December 1, 2019, and May 17, 2021, was applied; no further restrictions were placed on the search results. Article titles and abstracts indicating a possible central neuroinflammatory relationship to COVID-19 were included for a more detailed review of the full-text article. One neurologist author with neuroimmunology subspecialty expertise (S.K.H.) screened the initial search results without the assistance of automation. Of the 435 results, 46 articles were further reviewed by at least 2 authors with neurology training (G.S.M., C.R.M., and S.K.H.), with a third neuroimmunologist reviewer available to adjudicate disagreements (F.J.M.). A total of 26 articles were retained.
Criteria for Case Selection
Inclusion Criteria
Patients of any age with a history of COVID-19 infection were included in the study if their case descriptions provided sufficient details to confirm a clinical diagnosis of ADEM or AHLE. ADEM was defined as a first multifocal event of fulminant CNS demyelination, supported either histopathologically or radiographically by white matter lesions consistent with demyelination. Encephalopathy was not required for inclusion, in line with multiple studies suggesting its lower prevalence in adult populations.2,7,8 AHLE diagnoses required the additional criteria of hyperacute onset and evidence of hemorrhage (micro- or macro-) on neuroimaging or brain pathology.9,10
We defined COVID-19 infection in a manner consistent with guidance from the United States Centers for Disease Control and Prevention and the Infectious Diseases Society of America: (1) clinical symptoms consistent with COVID-19 without laboratory confirmation in the absence of an alternative explanation, (2) nasopharyngeal swab positive for COVID-19 PCR with or without symptoms, or (3) positive COVID-19 serologies with or without symptoms.11-14 The presence of clinical symptoms without laboratory confirmation of infection was included as a criterion on account of poor testing capacity in many countries early in the pandemic. Asymptomatic infections are known to constitute a significant percentage of patients with COVID-19, and therefore, we elected to include patients with only laboratory evidence of infection to capture the full spectrum of COVID-19-associated ADEM and AHLE.15,16
Exclusion Criteria
To avoid the possibility of coincidental COVID-19 acquisition during hospitalization with ADEM or AHLE, cases with well-established neurologic injury or a preexisting history of a relapsing CNS demyelinating disease before COVID-19 symptom onset were excluded. To ensure strict fidelity to the immunologic reaction to actual viral infection, cases occurring in the context of vaccination for COVID-19 were excluded. Additional exclusion criteria included articles not presenting original data (topical reviews and systematic reviews) and those cases with insufficient information to determine the accuracy of an ADEM or AHLE diagnosis and its relationship to COVID-19 infection.
Data Acquisition and Analysis
The collected cases from the literature review and medical records were each reviewed by at least 2 authors who extracted prespecified variables of interest into a central database using a standardized template. Demographic, clinical, serum and CSF laboratory tests, neuroimaging, treatment course, and patient outcomes were collected. No assumptions were made regarding missing data. Absence of information was noted as not reported or not available.
The geographical origin of a case was based on the location of the original authors' primary practice affiliation. The time delay from onset of COVID-19 infection to neurologic symptoms could not be determined in many cases, often due to discovery of neurologic disease in the setting of persistent encephalopathy following sedation weaning in cases intubated originally for COVID infection. Quantitative reporting of CSF oligoclonal bands (OCBs) was rare in the literature review, so their presence was reported qualitatively to reflect that limitation. Modified Rankin Scale (mRS) scores were retrospectively extrapolated from published case descriptions. In circumstances where a limited follow-up or clinical examination was provided, the mRS score was not calculated and documented as insufficient data.
Summary statistics were used to describe the data from reported cases as a single group. Medians, means, and ranges were reported using a complete case analysis approach. Cases with missing data were not included in the synthesis of the particular variable for which the data point was missing. All analyses were performed using Microsoft Excel.
Mass General Brigham Cases
The Mass General Brigham Research Patient Data Registry was searched for patients with diagnoses of COVID-19 or personal history of COVID-19 and the additional diagnosis of ADEM on March 29, 2021. The Research Patient Data Registry queries the electronic medical records of all hospitals and clinics within the Mass General Brigham system. Four cases meeting the above inclusion and exclusion criteria of the literature review were identified, and 11 were excluded.
Data Availability
Anonymized data will be made available to qualified investigators subject to ethics board approvals. Pertinent data points for the literature review are provided in eTables S2 and S3, links.lww.com/NXI/A584, and the eReferences for incorporated cases reports are included in S4, links.lww.com/NXI/A584.
Results
A PRISMA flow diagram illustrating the case selection process is presented in Figure 1. Twenty-six articles (8 case series and 18 case reports) were identified in the literature review yielding 42 patient cases. Three new cases were identified through query of the MGB electronic medical records along with 1 additional case under review for publication by one of the authors (S.K.H.). A total of 46 patient cases were analyzed (Tables 1–3). Cases originated from the United States (15), United Kingdom (13), Singapore (6), Brazil (5), Iran (3), India (2), Italy (2), and Greece (1). A slight male predominance was noted in cases overall (61% male to 37% female); however, there was a female predominance in reported pediatric cases (<18 years old; 80% female to 20% male).
Figure 1. PRISMA Flow Diagram Demonstrating Case Selection Methodology.
PRISMA flow diagram illustrating source information used, the screening process used and its effects on excluded articles, and final articles and cases selected for study inclusion.
Table 1.
Patient Demographics and Features of Antecedent COVID-19 Infection
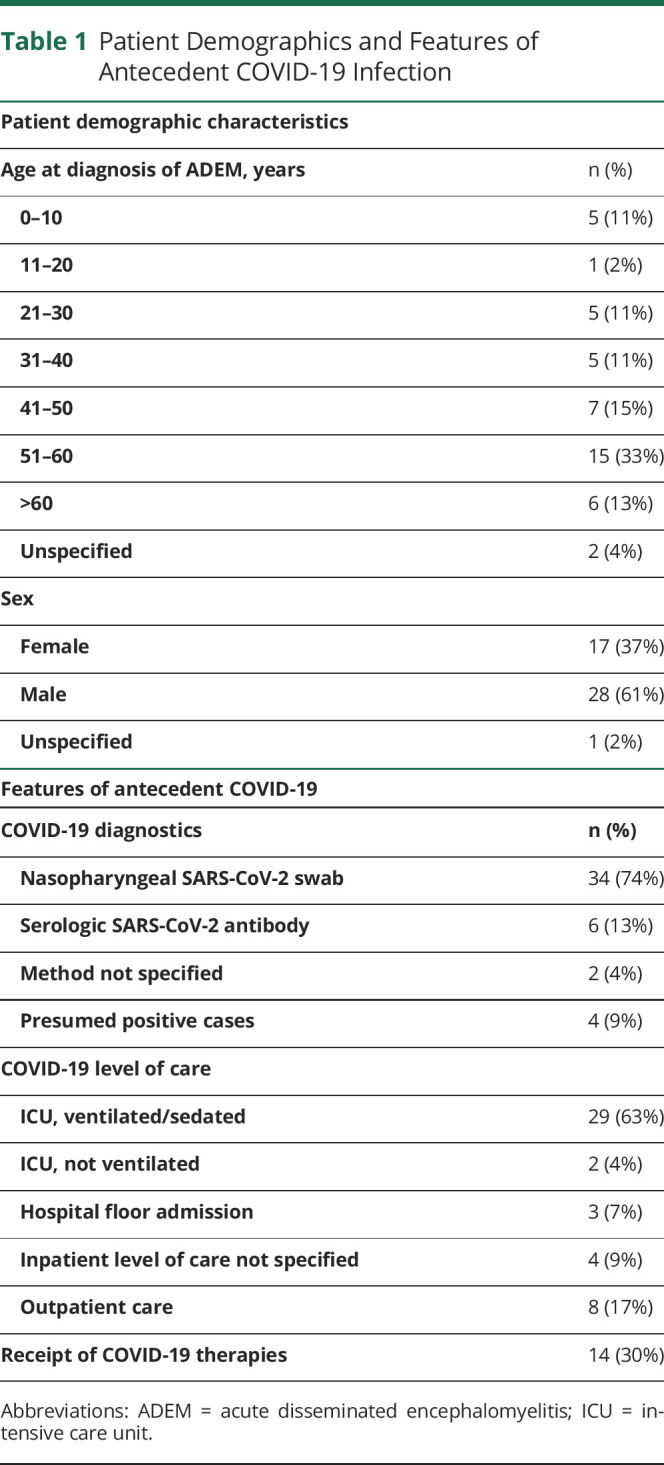
Table 2.
Clinical Features of Post–COVID-19 ADEM
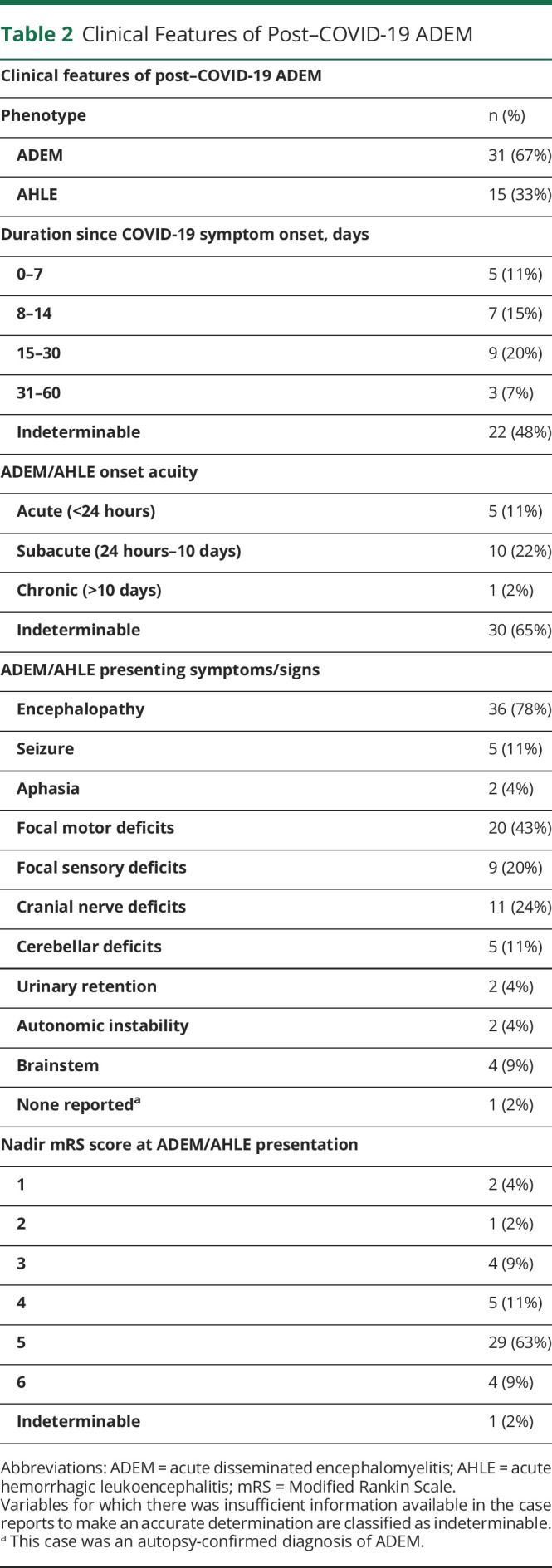
Table 3.
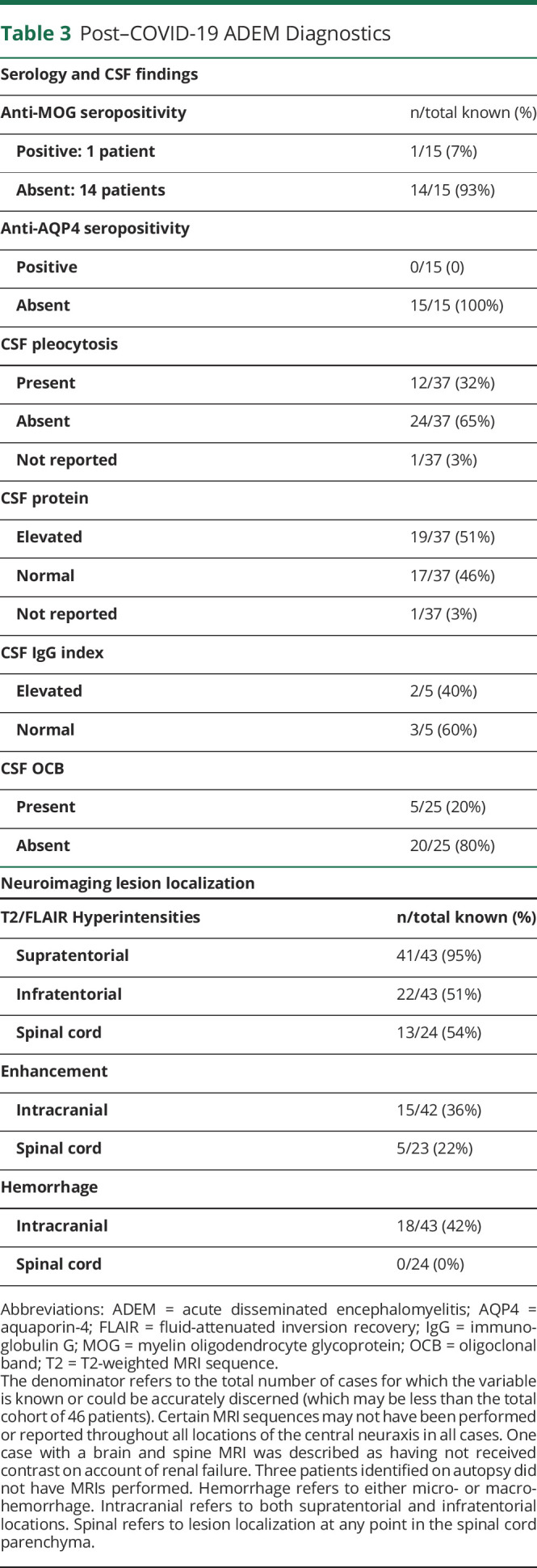
Post–COVID-19 ADEM Diagnostics
Features of Antecedent COVID-19 Infection and/or Exposure
Of 46 total patients included, 39 had a symptomatic respiratory illness before the onset of subsequent neurologic symptoms. The etiology of the preceding respiratory illness was objectively confirmed to be COVID-19 in 42 patients, the majority by nasopharyngeal COVID-19 PCR testing (Table 1). Of those confirmed to have had preceding COVID-19 via serologic testing for SARS-CoV-2 antibodies (14% of 42 confirmed cases), qualitative results were reported. Inpatient hospitalization was warranted for 83% of the patients, with majority necessitating intensive care unit level of care requiring sedation and ventilation (Table 1). Of the 39 patients with symptomatic COVID-19 infection, only 14 patients received COVID-19 directed therapy. These therapies included remdesivir monotherapy (3 patients), convalescent plasma monotherapy (4 patients), remdesivir and convalescent plasma (1 patient), hydroxychloroquine monotherapy (1 patient), hydroxychloroquine and convalescent plasma (1 patient), stress-dose steroids (1 patient), and a combination of multiple therapies inclusive of azithromycin, hydroxychloroquine, lopinavir, ritonavir, and anakinra (1 patient) or a combination of interferon beta-1b, lopinavir, and ritonavir (2 patients).
Included in our literature review are 4 patients from the United Kingdom with presumed COVID-19 preceding ADEM and AHLE (cases 4, 5, 7, and 8). Presumption of prior COVID-19 infection was accepted in these patients due to the development of severe respiratory illness during the early phase of the pandemic when COVID-19 was endemic in the United Kingdom (spring 2020) and routine testing was limited.
Clinical Characteristics of Post–COVID-19 ADEM
As noted in Table 2, the duration from COVID-19 symptom onset to development of ADEM and AHLE varied, with a slight majority occurring within 15–30 days. The acuity of progression of neurologic disease ranged from acute (<24 hours) to subacute (between 24 hours to 10 days) to chronic (>10 days), with 22% of patients demonstrating subacute progression of disease. Notably, the rate of neurologic disease progression could not be determined in 30 patients (65%) due to insufficient reporting and/or patient-specific factors, most commonly being a period of prolonged sedation before neurologic disease discovery. Neurologic signs and symptoms varied, with the majority demonstrating encephalopathy (78%) that encompassed confusion, lethargy, and difficulty waking from sedation. Focal motor deficits were the second most common clinical manifestation of disease (43%). At clinical nadir of ADEM and AHLE, the retrospective mRS score ranged from 1 to 6 (mRS score mean 4.6, median 5). AHLE was diagnosed in 15 cases by the primary authors and affirmed on review. Neuroimaging evidence further confirmed this diagnosis in 14 of these 15 cases. Imaging modalities revealing hemorrhage were brain MRI for 10 cases (5 cases via susceptibility-weighted imaging, 1 case via susceptibility-weighted angiography, and 4 cases without sequence specification), an MRI cervical spine in 1 case (sequence not specified), and noncontrast head CT in 3 cases. In one case, author reporting was the only source of validation of AHLE as no supporting imaging or pathology was made available.
Diagnostic Studies and Radiographic Features
Lumbar puncture was performed in 37 of 46 patients, with a normal profile (cell count, protein, IgG, and OCBs) in 11 of 37 patients (30%). At least 1 marker of inflammation in the CSF (pleocytosis, elevated protein, elevated IgG index, or presence of OCBs) was found in 26 of 37 patients (70%). Serum anti-myelin oligodendrocyte glycoprotein (anti-MOG) antibodies and anti-aquaporin-4 (anti-AQP4) antibodies were each tested in 15 patients, with 1 patient found to have anti-MOG antibody positivity, titer not specified (Table 3).
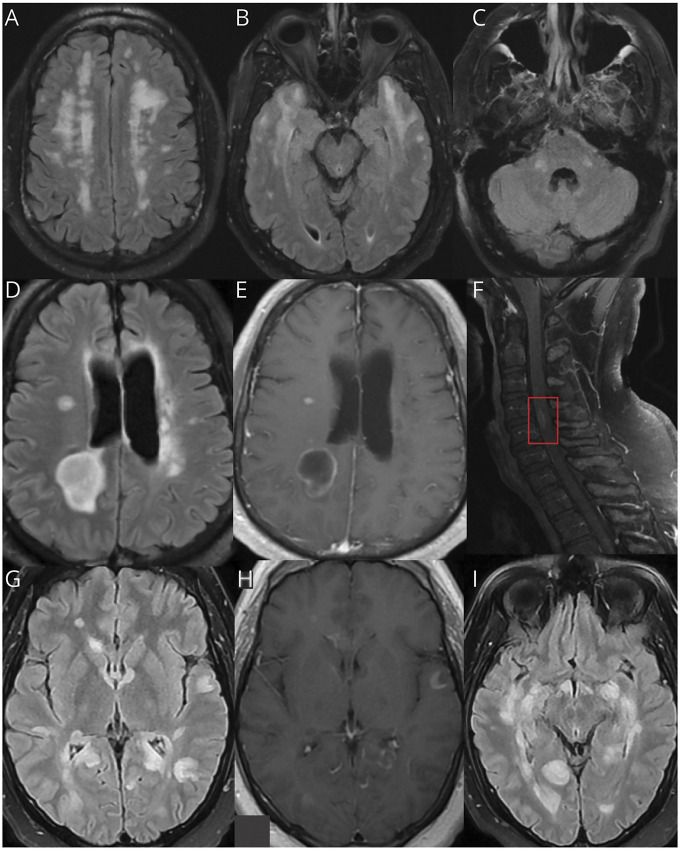
A brain MRI was obtained for 43 cases. Imaging abnormalities are summarized in Table 3, and representative images from the MGB case series are presented in Figure 2. In the 3 cases without brain MRI performed, diagnosis was made via postmortem autopsy. An example of pathology obtained from diagnostic biopsy in 1 living patient is shown in Figure 3. Only 24 patients additionally underwent MRI of the spine, of which 54% had abnormal findings. Spine MRI displayed longitudinal extensive myelitis in 4 patients, short segment lesions in 7 patients, and both long and short segment lesions in 1 patient (details in eTable 2, links.lww.com/NXI/A584). Insufficient detail precluded determination of spinal lesion length in 1 patient.
Figure 2. Neuroimaging Examples From the MGB Case Series.
Axial brain MRI sequences of patients with post-COVID ADEM from the MGB case series. (A–C) Axial brain MRI sequences from case 1 display FLAIR hyperintensities in the bilateral frontal lobes, anterior temporal lobes, and cerebellum. (D and E) Axial brain MRI sequences from case 2 show bilateral FLAIR hyperintensities with a prominent right parietal lesion that enhances, as well as nodular enhancement of the right corona radiata and occipital horn of the right lateral ventricle. (F) Sagittal postcontrast T1 sequence of the cervical spine MRI from case 2 displays a focus of intramedullary enhancement at C4-5 (red box). (G–I) Axial brain MRI sequences from case 3 show diffuse, multifocal T2 FLAIR lesions (G and I) and a predominant open-ring contrast enhancement pattern of several lesions in the T1 postcontrast sequence (H). ADEM = acute disseminated encephalomyelitis; MGB = Massachusetts General Brigham.
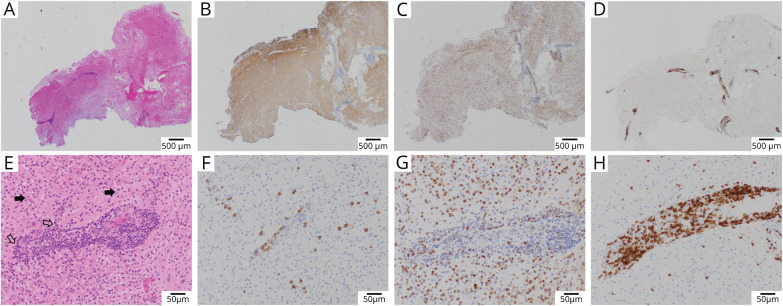
Figure 3. Neuropathology Examples of Post–COVID-19 ADEM.
Brain pathology from case 2 of the MGB series (case details provided in the supplementary files, links.lww.com/NXI/A584). Low-power images (A–D) demonstrate markedly cellular brain parenchyma with prominent perivascular inflammation and reduced myelinated fibers (A, Luxol–H&E stain), with relative preservation of axons (B, neurofilament immunohistochemistry). The cellular population is composed largely of macrophages (C, CD68 immunohistochemistry), whereas the perivascular infiltrates are predominantly T cells (D, CD3 immunohistochemistry). Higher-power views (E–H) characterize the perivascular infiltrates as composed of small mature lymphocytes and focally prominent plasma cells (E, H&E, open arrows), whereas the surrounding tissue is composed of numerous foamy macrophages (solid arrows) and reactive astrocytes. Immunohistochemistry demonstrates scattered perivascular and parenchymal plasma cells (F, CD138), numerous parenchymal foamy macrophages (G, CD68), and marked perivascular T cells (H, CD3). ADEM = acute disseminated encephalomyelitis; MGB = Massachusetts General Brigham.
Only 17 cases had repeat imaging at the time of follow-up. Of these patients, the majority demonstrated radiographic improvement (71%; Table 4). Two patients (cases 32 and 34, 4% of total cases) had normal MRIs initially on days 2 and 6 after neurologic symptom onset and were found to have remarkably abnormal imaging on interval scans obtained on days 4 and 50, respectively. We chose to include these patients because this phenomenon of delayed neuroimaging conversion has been previously noted in 7% of patients in a large ADEM case series with long-term follow-up.17
Table 4.
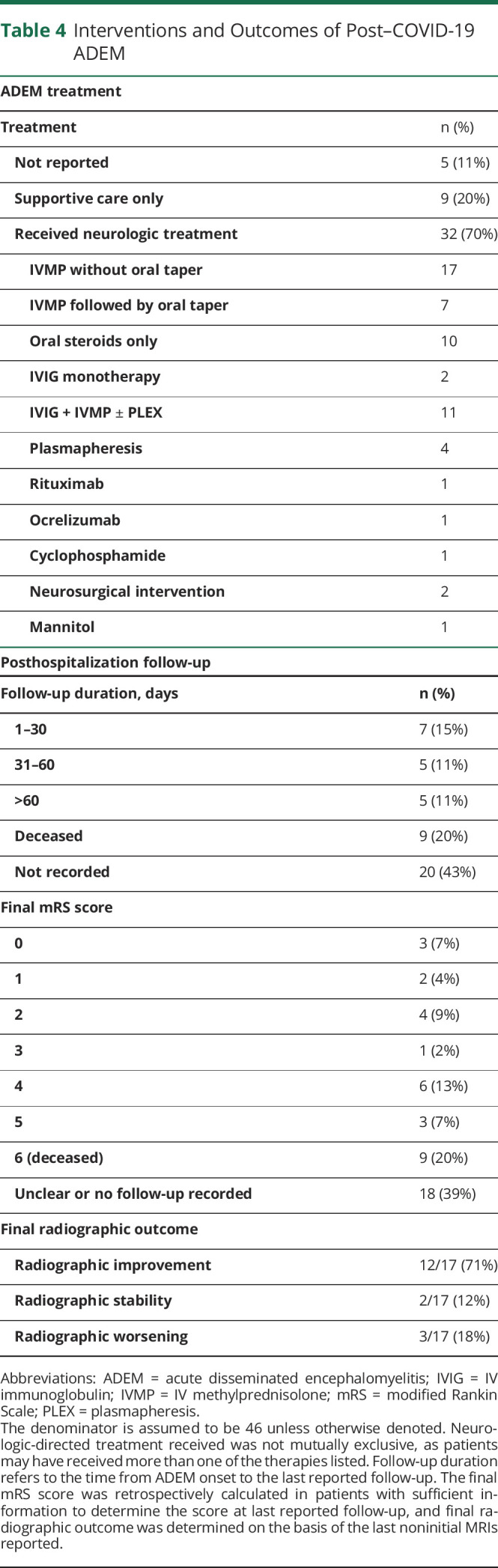
Interventions and Outcomes of Post–COVID-19 ADEM
Neurologic-Directed Treatment and Patient Outcomes
As shown in Table 4, acute neurologic-directed treatments were received by the majority of patients (70%). Treatment regimens varied from corticosteroid or IV immunoglobulin (IVIG) monotherapy to corticosteroids with IVIG and/or plasmapheresis to initiation of long-term immunosuppressants. Two patients required neurosurgical interventions (cases 4 and 7, detailed in eTable 3, links.lww.com/NXI/A584) due to edema and resultant midline shift associated with disease burden.
Duration between ADEM/AHLE diagnosis and follow-up widely varied and was reported for 17 cases (range: 14–240 days; median: 50 days). Duration between ADEM/AHLE diagnosis and date of death was not reliably reported. Insufficient reporting precluded the ability to assess postintervention clinical outcome in 39% of patients. Of the 28 cases with sufficient data to determine a final retrospective mRS score, most remained severely affected by their disease. An mRS score of 4 or greater was noted in 18/28 (64%), and 9 patients died (32% of those with follow-up, 20% of the entire cohort).
International Pediatric Multiple Sclerosis Study Group Criteria for ADEM
The International Pediatric Multiple Sclerosis Study Group (IPMSSG) criteria are the only set of consensus-founded diagnostic criteria for ADEM and created primarily for pediatric patients. Because of a lack of adult-specific diagnostic criteria, IPMSSG criteria are often extrapolated to the adult population. These criteria rely on specified clinical and radiographic features and exclusion of alternative etiologies.1 The size of radiographic lesions was not reliably reported for all reviewed cases. Therefore, we presume that patients who were identified as having ADEM or AHLE by the reporting authors did demonstrate characteristic lesion size and features, as per the IPMSSG criterion. Accounting for this presumption, 38/46 (83%) total cases met the IPMSSG criterion for ADEM, inclusive of pathologically confirmed cases.
Discussion
Since the onset of the pandemic, a growing number of case reports noting the development of ADEM and AHLE following COVID-19 infection have been published. In this systematic review of available cases to date, we synthesize the evidence that ADEM and AHLE occur post–COVID-19, highlighting this temporal association. We clarify the clinical features, ancillary testing results, and outcomes of post–COVID-19 ADEM and AHLE. Although post–COVID-19 ADEM and AHLE cases were similar to those of the prepandemic era in some aspects, such as the clinical features of ADEM and AHLE and the time between systemic infection and demyelination onset, we also found differences in the reported post–COVID-19 ADEM and AHLE cases as a whole: (1) the vast majority of reported cases are adults rather than children, (2) the preceding systemic infection was often severe as opposed to trivial, (3) hemorrhage within lesions was common on neuroimaging, (4) MOG antibody seropositivity was rare, (5) morbidity with ADEM was high despite the use of standard ADEM treatments, and (6) mortality was common even with relatively short follow-up.2,8,18,19
Our findings lend plausibility to COVID-19 acting as a viral trigger for ADEM and AHLE, as has been postulated with other pathogens in the past, including coronaviruses (MERS and OC43).18-22 With the exception of a few early cases occurring before widespread COVID-19 testing availability, almost all (91%) included patients had laboratory-confirmed infection, usually in close temporal association with COVID-19 diagnoses. These findings are biologically supported by mounting evidence that SARS-CoV-2 shares epitopes with neuronal proteins, which may incite subsequent autoimmunity involving the CNS via molecular mimicry.23,24
In contrast to childhood being the traditional vulnerability period of prepandemic ADEM, most post–COVID-19 ADEM and AHLE cases reported to date occurred in adults, usually of advanced age (nearly half >50 years old).2,18 We speculate that this finding is likely multifactorial: (1) all humans were immunologically naive to the virus, thereby massively extending the candidate pool for primary viral infection; (2) COVID-19 on average tends to cause more severe infections in adults, which lends itself to the potential for a more severe inflammatory response; and (3) severe infections will require more intensive prospective medical monitoring, which may lead to a higher detection rate of ADEM diagnosis and enhanced recall bias for subsequent neurologic complications.16,25 It is possible that the extraordinary nature of these cases in adults led to publication bias of more unusual cases, but the very high number of reports in adults compared with children makes the hypothesis that ADEM and AHLE post–COVID-19 exist and are more likely in adults.
The sustained and varying location of now >40 reports demonstrates that ADEM and AHLE are recognized albeit rare potential consequences of severe COVID-19 infection. Nearly two-thirds of patients required intensive care for the antecedent infection. Failure to awaken and resume independent ventilation were the most common clinical scenarios suggesting the presence of coexisting neurologic injury. COVID-19 infections did span the spectrum of disease severity, however, with a smaller fraction of patients managed in less intensive settings. It should be noted that asymptomatic or mildly symptomatic COVID-19 infections commonly occur and the link between them and CNS demyelination may go undetected if serologic testing for COVID-19 is not pursued.15,16
Pediatric ADEM has an association with antibodies against MOG in 35–65% of cases, which carries prognostic value by suggesting a higher likelihood of recurrent demyelinating events in the future.26-29 Although testing was incomplete, only a single MOG-seropositive case was identified in our cohort, which was a 13-month-old female (1/3 children with reported testing were positive, all 12 adults negative).
There was a relatively high rate of noninflammatory CSF in post–COVID-19 ADEM/AHLE, with 30% (11/37) failing to demonstrate at least 1 marker of inflammation, similar to prepandemic ADEM reports where 70–81% had some evidence of inflammation in the CSF.2,30,31 Our finding of CSF-restricted oligoclonal bands in 20% (5/25) of patients is consistent with prepandemic ADEM descriptions in children and adults where the frequency is usually less than 30%.2,32
Forty-two percent of patients had evidence for hemorrhage on brain MRI, significantly higher than that seen for classic ADEM. Prior studies have shown much lower frequencies of hemorrhage, approximating 2% of cases.33 The cause behind this remains uncertain, but it is conceivable that the predilection of COVID-19 to involve the microcirculation may be contributory and therefore could be a unique feature to this viral trigger, either as a result of direct infection or perhaps also secondary to molecular mimicry.34-36 It may also be a byproduct of proinflammatory cytokines promoting endothelial dysfunction in more severe cases of COVID-19 infection.36
The prospect for a favorable outcome (mRS score of ≤2) in cases with reported follow-up was low (20%) and that of mortality quite high (32%). If all patients lost to follow-up are given a favorable mRS score of 0–2, rates for unfavorable outcomes are 41%. This remains quite high in comparison to historical cohorts where typically less than 30% have similarly defined unfavorable outcomes.7,37 In addition to the possibility that post–COVID-19 ADEM and AHLE may inherently portend worse outcomes, additional possible explanations include the direct effects of COVID-19 infection on survival, hesitancy to apply immunotherapy for ADEM or AHLE on account of concurrent COVID-19 infection, and publication bias toward AHLE. The baseline health and comorbidities of these patients may be worse than prior ADEM cohorts, in keeping with their high rate of more severe COVID-19 infections. Extending follow-up over time will be an important tool in clarifying outcomes in this specific patient population.
Included cases mostly originated from countries with high numbers of infections early in the pandemic (United States, United Kingdom, Italy, and Iran) and high numbers of infections overall (United States, Brazil, and India).38,39 Singapore was disproportionately represented in our cohort (6 cases included) despite having only 61,331 cases in total as of May 9, 2021.39 This unexpectedly high contribution most likely reflects the impact of Singapore's prospective surveillance for neurologic complications in all laboratory-confirmed infections since the onset of the pandemic until August 2020.40 As countries continue progressing through the pandemic in their own unique ways, others will likely be able to apply the lessons learned here as they gain their own experience with post–COVID-19 ADEM and AHLE.
ADEM and AHLE remain rare occurrences following COVID-19 infection, but the observations cited here emphasize another reason for the need for primary prevention of infection. Additional recommendations include monitoring for neurologic complications in sedated patients, carefully probing for preceding COVID-19 infection (historically and via laboratory means) in those presenting with ADEM or AHLE, and evaluating for MOG seropositivity, especially in children. Alongside the administration of COVID-19–directed therapies, earlier and more aggressive interventions for ADEM and AHLE may be necessary to avoid a severe final outcome. Coexisting neurologic injury of this type (ADEM/AHLE) could be considered a relatively poor prognostic marker. Although ADEM itself is considered a fixed entity in terms of its clinical features, it may manifest more severely in its course in response to this particular infectious trigger. An international registry of such cases is urgently needed to fully examine the scope of post–COVID-19 ADEM and AHLE and would offer an opportunity to collect and report cases in a standardized manner across all ranges of severity and in all patient settings.
Our findings are limited by the lack of standardized reporting of individual cases and the resultant variability in the quality of such reporting, which contributed to incomplete data for some variables of interest. Given the novelty of COVID-19 and its neurologic associations, many of the reports from earlier in the pandemic were likely prioritized for rapid publication, resulting in truncated follow-up information, and of course, experience with COVID-19 remains in its youth at less than 1.5 years since the first recognized case in humans.
Glossary
- ADEM
acute disseminated encephalomyelitis
- AHLE
acute hemorrhagic leukoencephalitis
- IPMSSG
International Pediatric Multiple Sclerosis Study Group
- IVIG
IV immunoglobulin
- MGB
Massachusetts General Brigham
- mRS
modified Rankin Scale
- OCB
oligoclonal band
Appendix. Authors
Footnotes
COVID-19 Resources: NPub.org/COVID19
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261-1267. [DOI] [PubMed] [Google Scholar]
- 2.Koelman DLH, Chahin S, Mar SS, et al. Acute disseminated encephalomyelitis in 228 patients: a retrospective, multicenter US study. Neurology. 2016;86(22):2085-2093. [DOI] [PubMed] [Google Scholar]
- 3.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol Suppl. 2020;277(8):2251-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y, Min P, Lee S, Kim S. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35(18):e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collantes MEV, Espiritu AI, Sy MCC, Anlacan VMM, Jamora RDG. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48(1):66-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Laurent S, Onur OA, et al. A systematic review of neurological symptoms and complications of COVID-19. J Neurol. 2021;268(2):392-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ketelslegers IA, Visser IER, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ. Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler. 2011;17(4):441-448. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001;56(10):1313-1318. [DOI] [PubMed] [Google Scholar]
- 9.Kuperan S, Ostrow P, Landi MK, Bakshi R. Acute hemorrhagic leukoencephalitis vs ADEM: FLAIR MRI and neuropathology findings. Neurology. 2003;60(4):721-722. [DOI] [PubMed] [Google Scholar]
- 10.Grzonka P, Scholz M, De Marchis GM, et al. Acute hemorrhagic leukoencephalitis: a case and systematic review of the literature. Front Neurol. 2020;11:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing. Infectious Diseases Society of America. Accessed May 17, 2021. idsociety.org/practice-guideline/covid-19-guideline-diagnostics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Serologic Testing. Infectious Diseases Society of America. Accessed May 17, 2021. idsociety.org/COVID19guidelines/serology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. Morbidity Mortality Weekly Re.p 2020;69(24):759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronavirus Disease 2019 (COVID-19) 2020 Interim Case Definition. Centers for Disease Control and Prevention. wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/ (accessed 18 May 2021). [Google Scholar]
- 15.Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021;174(5):655-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol. 2021;93(2):820-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong YYM, van Pelt ED, Ketelslegers IA, Catsman-Berrevoets CE, Hintzen RQ, Neuteboom RF. Evolution of MRI abnormalities in paediatric acute disseminated encephalomyelitis. Eur J Paediatr Neurol. 2017;21(2):300-304. [DOI] [PubMed] [Google Scholar]
- 18.Pavone P, Pettoello-Mantovano M, Le Pira A, et al. Acute disseminated encephalomyelitis: a long-term prospective study and meta-analysis. Neuropediatrics. 2010;41(6):246-255. [DOI] [PubMed] [Google Scholar]
- 19.Iype M, Kunju PAM, Saradakutty G, Anish TS, Sreedharan M, Ahamed SM. Short term outcome of ADEM: results from a retrospective cohort study from South India. Mult Scler Relat Disord. 2017;18:128-134. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi Y, Torisu H, Kira R, et al. A nationwide survey of pediatric acquired demyelinating syndromes in Japan. Neurology. 2016;87(19):2006-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection. 2015;43(4):495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ann YE, Collins A, Cohen M, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;133(1 pt 1):e73-e76. [DOI] [PubMed] [Google Scholar]
- 23.Yapici-Eser H, Koroglu YE, Oztop-Cakmak O, Keskin O, Gursoy A, Gursoy-Ozdemir Y. Neuropsychiatric symptoms of COVID-19 explained by SARS-CoV-2 proteins' mimicry of human protein interactions. Front Hum Neurosci. 2021;15:656313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gammazza AM, Légaré S, Bosco GL, et al. Molecular mimicry in the post-COVID-19 signs and symptoms of neurovegetative disorders? Lancet Microbe. 2021;2(3):e94. [DOI] [PubMed] [Google Scholar]
- 25.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennes EM, Baumann M, Schanda K, et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89(9):900-908. [DOI] [PubMed] [Google Scholar]
- 27.Pröbstel AK, Dornmair K, Bittner R, et al. Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology. 2011;77(6):580-588. [DOI] [PubMed] [Google Scholar]
- 28.Duignan S, Wright S, Rossor T, et al. Myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies are highly specific in children with acquired demyelinating syndromes. Dev Med Child Neurol. 2018;60(9):958-962. [DOI] [PubMed] [Google Scholar]
- 29.Baumann M, Sahin K, Lechner C, et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 2015;86(3):265-272. [DOI] [PubMed] [Google Scholar]
- 30.Krishna Murthy SN, Faden HS, Cohen ME, Bakshi R. Acute disseminated encephalomyelitis in children. Pediatrics. 2002;110(2):e1-e7. [DOI] [PubMed] [Google Scholar]
- 31.Hynson JL, Kornberg AJ, Coleman LT, Shield L, Harvey AS, Kean MJ. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56(10):1308-1312. [DOI] [PubMed] [Google Scholar]
- 32.Dale RC, de Sousa C, Chong WK, Cox TCS, Harding B, Neville BGR. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(pt 12):2407-2422. [DOI] [PubMed] [Google Scholar]
- 33.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224-1231. [DOI] [PubMed] [Google Scholar]
- 34.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905-913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24(1):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CH, Jeng JS, Hsieh ST, Yip PK, Wu RM. Acute disseminated encephalomyelitis: a follow-up study in Taiwan. J Neurol Neurosurg Psychiatry. 2007;78(2):162-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coronavirus Disease (COVID-19) Situation Report 132. World Health Organization. who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed 18 May 2021). [Google Scholar]
- 39.COVID-19 Weekly Epidemiological Update. World Health Organization. who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---11-may-2021 (accessed 18 May 2021). [Google Scholar]
- 40.Koh JS, de Silva DA, Quek AML, et al. Neurology of COVID-19 in Singapore. J Neurol Sci. 2020;418:117118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be made available to qualified investigators subject to ethics board approvals. Pertinent data points for the literature review are provided in eTables S2 and S3, links.lww.com/NXI/A584, and the eReferences for incorporated cases reports are included in S4, links.lww.com/NXI/A584.