Abstract
Background
Isocitrate dehydrogenase (IDH) is an enzyme family involved in cell aerobic metabolism of tricarboxylic acid cycle. However, the landscape of IDH mutations in pan‐cancer has not been fully characterized.
Methods
Tissue or blood samples were subjected to next‐generation sequencing (NGS) for detection the IDH mutation.
Results
A total of 28.868 patients from more than 20 solid tumor species were analyzed. A total of 374 cases (1.30%) with IDH mutations were identified. Among all the IDH mutations cases, 80 (21.4%) were biliary tract cancer (BTC), 80 (21.4%) were lung cancer, 57 (15.2%) were liver cancer, and 42 (11.2%) were colorectal cancer. The most common IDH variant were IDH1 and IDH2 which were discovered in 0.81% cases and 0.47% cases, respectively. However, there were significant differences in IDH1 and IDH2 mutation frequency among different tumor species (p = 0.0003). Of the patients with IDH1 mutations, about 53.0% of these mutations occur in codons 132. Codons 172 (25.4%) was high‐frequency mutation subtypes in IDH2 mutation. TP53, PBRM1, and BAP1 were the most significantly mutated genes in BTC which were different from others cancer. Moreover, TMB were significantly higher in lung cancer, colorectal cancer, and gastric cancer than BTC (p = 0.0164, p < 0.0001, p = 0.0067, respectively) and BTC patients with IDH mutation had lower TMB compared with wild‐type IDH.
Conclusion
Somatic IDH mutation was found in multiple solid tumors and IDH would be a driver gene in BTC.
Keywords: IDH mutation, next‐generation sequencing, pan‐cancer, TMB
Somatic IDH mutation was found in multiple solid tumors and IDH would be a driver gene in biliary tract cancer.
1. INTRODUCTION
Isocitrate dehydrogenase (IDH) is a family of enzymes involved in cell aerobic metabolism of tricarboxylic acid cycle, among which IDH1 and IDH2 are common mutated metabolic genes in human cancers such as glioma and hematologic malignancies (Norsworthy et al., 2019; Yang et al., 2012). Since 2008, when IDH1 missense mutation was first identified in progressive glioblastoma (Parsons et al., 2008), numerous studies have been conducted to describe this gene mutation and understand its biological impact on tumors (Turkalp et al., 2014). And it has been confirmed that IDH1/2 mutation as a biomarker was associated with tumor prognosis and predictive value (Mondesir et al., 2016).
The understanding of the role of IDH1/2 mutations in tumorigenesis and early occurrence has led to the development of IDH1/2 mutation inhibitors. With the clinical development of IDH inhibitors and the entry into phase I trials, IDH inhibitors have shown promising efficacy not only in hematologic malignancies but also in patients with unresectable or metastatic IDH1/2 mutant biliary tract cancer (BTC). In patients with BTC, ivosidenib has been approved in patients with mutant IDH1‐BTC according to NCT02073994 study (Lowery et al., 2019). A recent phase III ClaridHY trial showed that ivosidenib showed a greater advantage over placebo in median PFS and OS in patients with advanced IDH1‐mutated BTC (2.7 vs. 1.4 months, HR 0.37, 95% CI 0.25.0.54, p < 0.0001; Lamarca et al., 2020; Abou‐Alfa et al., 2020). AG‐221 (Enasidenib) and other IDH1/2 inhibitors are currently being assessed in multiple phases I/II clinical trials in subjects with advanced solid tumors, including BTC, who harbor an IDH2 mutation (NCT02273739, NCT02273739, NCT02381886, and NCT02481154). Therefore, the application of IDH inhibitors in BTC looks promising.
However, the landscape of IDH1/2 mutations in pan‐cancer has not been fully characterized. In this study, we invested the IDH1/2 pathologic or likely pathologic mutations landscape in pan‐cancer.
2. MATERIALS AND METHODS
2.1. Ethical compliance
This study was conducted under the approval of the ethics committees of the hospitals and informed consents were obtained from patients.
2.2. Clinical specimens
Formalin‐fixed paraffin‐embedded (FFPE) tumor sample or biopsy (n = 28,868) of pan‐cancer patients between January 2017 and January 2020 was enrolled in this study, including biliary carcinoma (1377 cases), liver cancer (2148 cases), lung cancer (11614 cases), colorectal cancer (4056 cases), etc. Tumor tissue samples (self‐blood negative control) of pan‐cancer were subjected to NGS for detection the IDH1/2 mutation with a well‐designed 733 cancer gene panel on Illumina HiSeq sequencer (Illumina) with 800× sequencing depth in a College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA) certified laboratory (3D Medicine Inc.; Wang et al., 2019)]. The details of IDH1/2 genes, such as the locations and the meaning of the mutations, were showed in supplement. Somatic alterations were identified and clinical information including age, gender, and tumor histology was collected. In addition, the TMB was defined as the number of nonsynonymous somatic SNVs and indels in examined coding regions, with driver mutations excluded. All SNVs and indels in the coding region of targeted genes, including missense, silent, stop gain, stop loss, in‐frame, and frameshift mutations, were considered.
2.3. DNA extraction
FFPE tissue sections were evaluated for tumor cell content using hematoxylin and eosin (H&E) staining. Only samples with a tumor content of ≥20% were eligible for subsequent analyses. FFPE tissue sections were placed in a 1.5 microcentrifuge tube and deparaffinized with mineral oil. Samples were incubated with lysis buffer and proteinase K at 56℃ overnight until the tissue was completely digested. The lysate was subsequently incubated at 80 ℃ for 4 hours to reverse formaldehyde crosslinks. Genomic DNA was isolated from tissue samples using the ReliaPrep™ FFPE gDNA Miniprep System (Promega) and quantified using the Qubit™ dsDNA HS Assay Kit (Thermo Fisher Scientific) following the manufacturer's instructions.
2.4. Library preparation and DNA sequencing
DNA extracts (30–200 ng) were sheared to 250 bp fragments using an S220 focused ultrasonicator (Covaris). Libraries were prepared using the KAPA Hyper Prep Kit (KAPA Biosystems) following the manufacturer's protocol. The concentration and size distribution of each library were determined using a Qubit 3.0 fluorometer (Thermo Fisher Scientific) and a LabChip GX Touch HT Analyzer (PerkinElmer), respectively. Single nucleotide variation (SNV), insertions/deletions, copy number variations (CNV), and gene fusions were assessed and the corresponding criteria are the same as our previous study (Yang et al., 2018). Germline alterations were excluded.
2.5. Statistical analysis
The paired‐end reads were mapped by BWA (Li & Durbin, 2010) MEM algorithm. SNVs were called by MuTect (Cibulskis et al., 2013) with default parameters. Small insertions and deletions were called from the union of Varscan 2 (Koboldt et al., 2012) and Pindel (Ye et al., 2009) with default parameters. Fusions were called by self‐developed scripts with at least 5 pairs of reads spanned over the breakpoints between two partner genes. The CNVs of tumor tissues were calculated by BIC‐seq2 (Xi et al., 2016) with default parameters, and the CNVs of ctDNA samples were called by a method reported by Chabon et al. (2016). All mutations were manually reviewed using integrative genomics viewer (IGV; Robinson et al., 2011) to further eliminate false‐positive results. The probability density distributions of mutant and wild‐type fragments were calculated by Gaussian kernel smoothing using StatsModels 0.8.0.
Categorical variables were described as number and proportions. Categorical relationships were examined by using Pearson's chi‐square test with the Yates continuity correction when applicable and p value <0.05 was considered statistically significant. The SPSS22.0 software (SPSS, Inc.) was carried out for statistical analysis.
3. RESULTS
3.1. Patient characteristics
From January 01, 2017, to October 30, 2019, a total of 28,868 cases of Chinese solid tumor types were included in this study, including biliary carcinoma (4.77%), liver cancer (7.44%), lung cancer (40.23%), and colorectal cancer (14.05%). A total of 374 cases (1.30%) with IDH mutations were identified. Patients with IDH mutation <60 years old were 49.7% (n=186). Among all the IDH mutations cases, 80 (21.4%) were BTC, 80 (21.4%) were lung cancer, 57 (15.2%) were liver cancer, and 42 (11.2%) were colorectal cancer (Table 1).
TABLE 1.
Clinical characteristics of patients
| Characteristics | Total (n = 28.868) | IDH mutated (n, %) |
|---|---|---|
| Gender | ||
| Male | 17140 (59.37) | 234 (1.37%) |
| Female | 11728 (40.63) | 140 (1.19%) |
| Age | ||
| <60 | 13938 (48.28) | 186 (1.33%) |
| ≥60 | 14930 (51.72) | 188 (1.26%) |
| Tumor | ||
| Lung cancer | 11614 (40.23%) | 80 (0.69%) |
| Colorectal cancer | 4056 (14.05%) | 42 (1.04%) |
| Liver cancer | 2148 (7.44%) | 57 (2.65%) |
| Gastric cancer | 1418 (4.91%) | 16 (1.13%) |
| Biliary tract cancer | 1377 (4.77%) | 80 (5.81%) |
| Intestinal cancer | 1117 (3.87%) | 12 (1.07%) |
| Pancreatic cancer | 987 (3.42%) | 7 (0.71%) |
| Kidney cancer | 981 (3.40%) | 5 (0.51%) |
| Breast cancer | 662 (2.29%) | 5 (0.76%) |
| Ovarian cancer | 545 (1.89%) | 7 (1.28%) |
| Prostate cancer | 321 (1.11%) | 9 (2.80%) |
| Endometrial carcinoma | 276 (0.96%) | 6 (2.17%) |
| Other | 270 (0.94%) | 43 (15.93%) |
| Melanoma | 232 (0.8%) | 5 (2.16%) |
3.2. IDH mutation of pan‐cancer
The prevalence of IDH1/IDH2 mutations in 28.868 patients with different cancer types is summarized in Figure 1, with BTC patients having the highest levels of IDH1/IDH2 mutations (80/1377). Across all 28,868 patients, the mutational frequencies of IDH1 and IDH2 were 0.81% and 0.47%, respectively. Furthermore, we analyzed the differences between IDH1 and IDH2 in different tumor species. There was no significant difference in age and sex between the two groups. However, we found that there were significant differences in IDH1 and IDH2 mutation frequency among different tumor species (p = 0.0003, Table 2).
FIGURE 1.
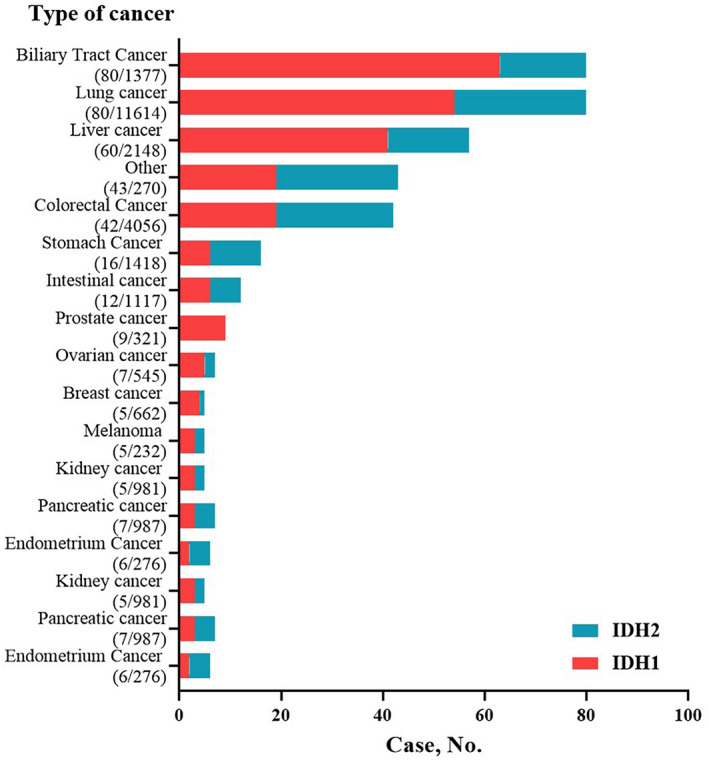
Prevalence of IDH1/IDH2 mutations in 28,868 patients with pan‐cancer
TABLE 2.
Clinicopathological characteristics of IDH mutant tumor
| IDH1 mut (n = 236) | IDH2 mut (n = 138) | p | |
|---|---|---|---|
| Age | |||
| ≥60 | 123 | 64 | 0.2839 |
| <60 | 113 | 74 | |
| Sex | |||
| Male | 149 | 84 | 0.6603 |
| Female | 87 | 54 | |
| Tumor location | |||
| Lung cancer | 53 | 27 | 0.0003 |
| Colorectal cancer | 19 | 23 | |
| Liver cancer | 41 | 16 | |
| Gastric cancer | 6 | 10 | |
| Biliary tract cancer | 63 | 17 | |
| Intestinal cancer | 6 | 6 | |
| Pancreatic cancer | 3 | 4 | |
| Kidney cancer | 3 | 2 | |
| Breast cancer | 4 | 1 | |
| Ovarian cancer | 5 | 2 | |
| Prostate cancer | 9 | 0 | |
| Endometrial carcinoma | 2 | 4 | |
| Other | 19 | 24 | |
| Melanoma | 3 | 2 | |
Of the patients with IDH1 mutations, about 53.0% of these mutations occur in codons 132 (Figure 2A). The proportions of the major other IDH1 mutation were as follows: copy number variations (CNV) loss (3.0%), Y208C (3.0%), R20Q (1.3%), and R49C (1.3%). Codons 172 (25.4%) was high‐frequency mutation subtypes in IDH2 mutation, followed by CNV gain (5.4%), CNV loss (3.9%), and T146Lfs*15 (3.1%; Figure 2B).
FIGURE 2.
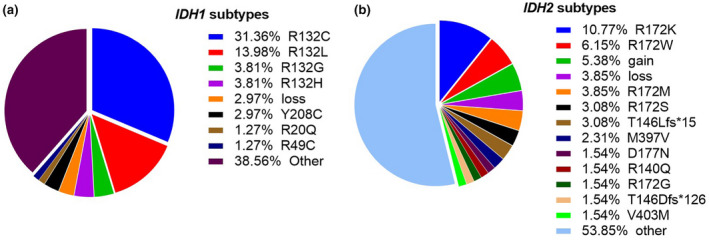
Pie charts of patients with IDH mutation. Pie charts showing the proportions of IDH1 mutation (mut) subtypes (a) and the proportions of IDH2 mutation subtypes (b)
We further investigated the genetic variation spectrum of IDH‐mutated patients (Figure 2). Targeted therapies have been successfully developed to treat lung cancer harboring driver gene mutations. In lung cancer, driver gene mutations EGFR and KRAS were the most significantly mutated genes, whereas TP53, PBRM1, and BAP1 were more frequently observed in BTC (Figure 3a,c). In addition, TP53, PBRM1, and BAP1 were high‐frequency mutated gene in liver cancer (Figure 3b). APC, TP53, and KRAS were among the top mutated genes in colorectal cancer, while TP53, SPTA1, and ACVR2A were more in IDH‐mutated gastric cancer (Figure 3d,e).
FIGURE 3.
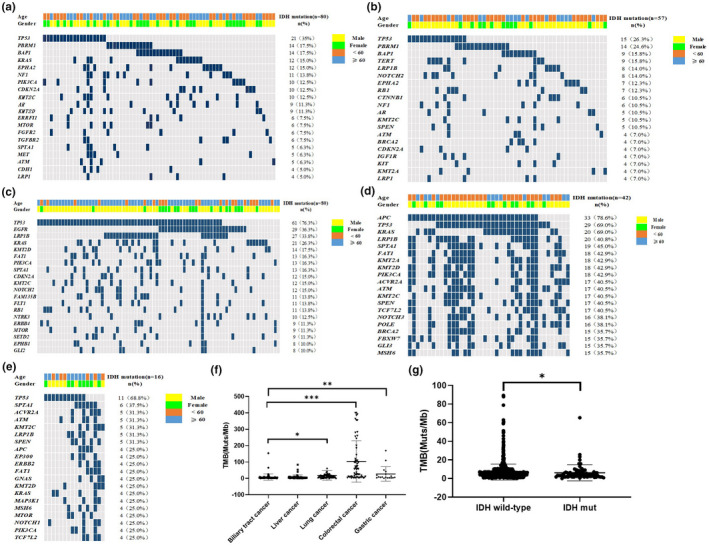
Top 20 significantly mutated genes in IDH1/2‐mutated patients of biliary tract cancer (a), liver cancer (b), lung cancer (c), colorectal cancer (d), and gastric cancer (e). genes were ranked by mutation frequencies (right panel). Age and gender are annotated in the top panel. Tumor mutational load among IDH1/2‐mutated pan‐cancers (f) and in biliary tract cancer (g)
3.3. Associate with TMB and IDH mutation
We also analyzed the association between IDH1/IDH2 mutations and TMB in five type of cancer. As shown in Figure 3f, the TMB were significantly higher in lung cancer, colorectal cancer, and gastric cancer than biliary tract cancer (p = 0.0164, p < 0.0001, p = 0.0067, respectively). In addition, we also analyzed the relationship between IDH mutation and wild‐type with TMB in BTC (Figure 3G). Patients with IDH mutation had lower TMB compared with patients with wild‐type IDH (p = 0.0236).
4. DISCUSSION
From a cohort of 28.868 patients with different types of solid tumors, a high frequency of IDH1/IDH2 mutations was observed not only in BTC but also in liver cancer, lung cancer, colorectal cancer, and others. The reprogramming of cellular metabolism is a fundamental characteristic of cancer, and IDH1/2 mutations represent key therapeutic targets in this arena. Somatic IDH1/2 mutations are found in multiple solid tumors, and mounting evidence indicates that they contribute to premalignant disorders as well as early and late cancers.
In addition, we found that TMB was higher in other gastrointestinal tumors. However, in BTC, IDH mutation accompanied by low TMB indicates that IDH would be a driver gene in BTC. With the continuous emergence of IDH inhibitors, a considerable number of patients with solid tumors carrying IDH1/2 gene mutations may be more likely to benefit from IDH inhibitors, which is worth further expectation and exploration in clinical studies.
Since the heterogeneity of tumor poses a severe challenge to clinical management, it is an urgent need to understand the molecular classification of tumor. For example, although intrahepatic, perihilar, and extrahepatic BTC share the same morphological characteristics and are not subdivided in most clinical trials, there is currently available evidence that there are important biological and genetic differences between tumors at different anatomical sites (Chan‐On et al., 2013). In addition to the IDH1/2 gene, additional recurrent mutations and fusions have been reported in BTC. Therefore, further elucidation of the molecular alterations in these heterogeneous tumors and discovery of meaningful subtypes is a key step in the development of more rational, specific, and effective treatments (Kelley & Bardeesy, 2015). Therefore, we further studied the genetic variation spectrum of patients with IDH mutation. IDH‐mutated lung cancer is accompanied by common co‐mutations in the driver genes EGFR and KRAS. TP53, PBRM1, and BAP1 were more common in IDH‐mutated BTC and liver cancer. It can be seen that IDH is associated with different high‐frequency mutation genes in different cancer types.
In summary, we revealed within the molecular landscape of IDH1/2 pan‐cancer that concurrent IDH1/2 mutation was accompanied by low TMB in BTC.
CONFLICT OF INTEREST
Drs. Junling Zhang, Jing Zhao, Zhengyi Zhao, Longgang Cui, Yuzi Zhang, Guoqiang Wang, Shangli Cai, and Yuezong Bai are employees of 3D Medicines Inc. Other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Shen D, Zhang JL, Li W, and Huang X. Analysis and interpretation of data: Zhang JL. Drafting the article: All authors. Huang X accepts full responsibility for the work and/or the conduct of the study, had access to the data, and oversaw the decision to publish.
Dong Shen and Junling Zhang contributed equally to this work.
Contributor Information
Wei Li, Email: real.lw@163.com.
Xiang Huang, Email: lorelai@njmu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abou‐Alfa, Ghassan K., Macarulla, Teresa, Javle, Milind M., Kelley, Robin K., Lubner, Sam J., Adeva, Jorge, Cleary, James M., Catenacci, Daniel V., Borad, Mitesh J., Bridgewater, John, Harris, William P., Murphy, Adrian G., Oh, Do‐Youn, Whisenant, Jonathan, Lowery, Maeve A., Goyal, Lipika, Shroff, Rachna T., El‐Khoueiry, Anthony B., Fan, Bin, … Zhu, Andrew X. (2020). Ivosidenib in IDH1‐mutant, chemotherapy‐refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Oncology, 21, 796–807. 10.1016/S1470-2045(20)30157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabon, J. J., Simmons, A. D., Lovejoy, A. F., Esfahani, M. S., Newman, A. M., Haringsma, H. J., Kurtz, David M., Stehr, Henning, Scherer, Florian, Karlovich, Chris A., Harding, Thomas C., Durkin, Kathleen A., Otterson, Gregory A., Thomas Purcell, W., Ross Camidge, D., Goldman, Jonathan W., Sequist, Lecia V., Piotrowska, Zofia, Wakelee, Heather A., … Alizadeh, Ash A. Diehn, M. (2016). Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nature Communications, 7, 11815. 10.1038/ncomms11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan‐on, Waraporn, Nairismägi, Maarja‐Liisa, Ong, Choon Kiat, Lim, Weng Khong, Dima, Simona, Pairojkul, Chawalit, Lim, Kiat Hon, McPherson, John R., Cutcutache, Ioana, Heng, Hong Lee, Ooi, London, Chung, Alexander, Chow, Pierce, Cheow, Peng Chung, Lee, Ser Yee, Choo, Su Pin, Tan, Iain Bee Huat, Duda, Dan, Nastase, Anca, … Teh, Bin Tean (2013). Exome sequencing identifies distinct mutational patterns in liver fluke‐related and non‐infection‐related bile duct cancers. Nature Genetics, 45, 1474–1478. 10.1038/ng.2806 [DOI] [PubMed] [Google Scholar]
- Cibulskis, Kristian, Lawrence, Michael S., Carter, Scott L., Sivachenko, Andrey, Jaffe, David, Sougnez, Carrie, Gabriel, Stacey, Meyerson, Matthew, Lander, Eric S., & Getz, Gad (2013). Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature Biotechnology, 31, 213–219. 10.1038/nbt.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, R. K., & Bardeesy, N. (2015). Biliary tract cancers: Finding better ways to lump and split. Journal of Clinical Oncology, 33(24), 2588–2590. 10.1200/JCO.2015.61.6953 [DOI] [PubMed] [Google Scholar]
- Koboldt, D. C., Zhang, Q., Larson, D. E., Shen, D., McLellan, M. D., Lin, L., & Wilson, R. K. (2012). VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Research, 22, 568–576. 10.1101/gr.129684.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarca, A., Barriuso, J., McNamara, M. G., & Valle, J. W. (2020). Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. Journal of Hepatology, 73, 170–185. 10.1016/j.jhep.2020.03.007 [DOI] [PubMed] [Google Scholar]
- Li, H., & Durbin, R. (2010). Fast and accurate long‐read alignment with burrows‐wheeler transform. Bioinformatics, 26, 589–595. 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery, Maeve A., Burris, 3rdH. A., Janku, Filip, Shroff, Rachna T., Cleary, James M., Azad, Nilofer S., Goyal, Lipika, Maher, Elizabeth A., Gore, Lia, Hollebecque, Antoine, Beeram, Muralidhar, Trent, Jonathan C., Jiang, Liewen, Fan, Bin, Aguado‐Fraile, Elia, Choe, Sung, Wu, Bin, Gliser, Camelia, Agresta, Samuel V., … Abou‐Alfa, Ghassan K. (2019). Safety and activity of ivosidenib in patients with IDH1‐mutant advanced cholangiocarcinoma: A phase 1 study. The Lancet Gastroenterology & Hepatology, 4(9), 711–720. 10.1016/S2468-1253(19)30189-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesir, J., Willekens, C., Touat, M., & de Botton, S. (2016). IDH1 and IDH2 mutations as novel therapeutic targets: Current perspectives. Journal of Blood Medicine, 7, 171–180. 10.2147/JBM.S70716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsworthy, Kelly J., Luo, Lola, Hsu, Vicky, Gudi, Ramadevi, Dorff, Sarah E., Przepiorka, Donna, Deisseroth, Albert, Shen, Yuan‐Li, Sheth, Christopher M., Charlab, Rosane, Williams, Gene M., Goldberg, Kirsten B., Farrell, Ann T., & Pazdur, Richard (2019). FDA approval summary: Ivosidenib for relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase‐1 mutation. Clinical Cancer Research, 25(11), 3205–3209. 10.1158/1078-0432.CCR-18-3749 [DOI] [PubMed] [Google Scholar]
- Parsons, D. W., Jones, S., Zhang, X., Lin, J. C., Leary, R. J., Angenendt, P., & Kinzler, K. W. (2008). An integrated genomic analysis of human glioblastoma multiforme. Science, 321(5897), 1807–1812. 10.1126/science.1164382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J. T., Thorvaldsdóttir, H., Winckler, W., Guttman, M., Lander, E. S., Getz, G., & Mesirov, J. P. (2011). Integrative genomics viewer. Nature Biotechnology, 29, 24–26. 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkalp, Z., Karamchandani, J., & Das, S. (2014). IDH mutation in glioma: New insights and promises for the future. JAMA Neurology, 71(10), 1319–1325. 10.1001/jamaneurol.2014.1205 [DOI] [PubMed] [Google Scholar]
- Wang, Zhijie, Duan, Jianchun, Cai, Shangli, Han, Miao, Dong, Hua, Zhao, Jun, Zhu, Bo, Wang, Shuhang, Zhuo, Minglei, Sun, Jianguo, Wang, Qiming, Bai, Hua, Han, Jiefei, Tian, Yanhua, Lu, Jing, Xu, Tongfu, Zhao, Xiaochen, Wang, Guoqiang, Cao, Xinkai, … Wang, Jie (2019). Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non‐small cell lung cancer with use of a next‐generation sequencing cancer gene panel. JAMA Oncology, 5(5), 696–702. 10.1001/jamaoncol.2018.7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, R., Lee, S., & Xia, Y. (2016). Copy number analysis of whole‐genome data using BIC‐seq2 and its application to detection of cancer susceptibility variants. Nucleic Acids Research, 44, 6274–6286. 10.1093/nar/gkw491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H., Ye, D., Guan, K. L., & Xiong, Y. (2012). IDH1 and IDH2 mutations in tumorigenesis: Mechanistic insights and clinical perspectives. Clinical Cancer Research, 18(20), 5562–5571. 10.1158/1078-0432.CCR-12-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Nong, Li, Yi, Liu, Zhidong, Qin, Hao, Du, Duanming, Cao, Xinkai, Cao, Xiaoqing, Li, Jun, Li, Dongge, Jiang, Bo, Duan, Lincan, Yang, Haiyan, Zhang, Zhenghua, Lin, Hao, Li, Jianying, Yang, Zhenhua, Xiong, Lei, Shen, Hua, Lin, Lizhu, & Li, Fugen (2018). The characteristics of ctDNA reveal the high complexity in matching the corresponding tumor tissues. BMC Cancer, 18(1), 319. 10.1186/s12885-018-4199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, K., Schulz, M. H., & Long, Q. (2009). Pindel: A pattern growth approach to detect break points of large deletions and medium sized insertions from paired‐end short reads. Bioinformatics, 25, 2865–2871. 10.1093/bioinformatics/btp394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


