Abstract
Background
Diarrhea is a common occurrence in children below the age of 5 years. In chronic cases, it induces malnutrition that severely stunts growth. Bile acid diarrhea (BAD), caused by malabsorption of bile acid (BA), is a rare form of chronic diarrhea seldom observed in pediatric patients. Here, we present a clinical report on a novel case of chronic BAD, with severe stunting in an infant, induced by a homozygous mutation of SLC10A2.
Methods
We performed DNA extraction, whole‐exome sequencing analysis, and mutation analysis of SLC10A2 to obtain genetic data on the patient. We subsequently analyzed the patient's clinical and genetic data.
Results
The patient's clinical manifestations were chronic diarrhea with increased BAs in the feces and extreme stunting, which was diagnosed as BAD. A homozygous mutation of SLC10A2 at the c.313T>C (rs201206937) site was detected.
Conclusion
Our report reveals the youngest case illustrating the characteristics of BAD induced by genetic variant at 313T>C, and the second case entailing a clear association between a SLC10A2 genetic mutation and the onset of BAD. Our findings expand the mutant spectrum of the SLC10A2 gene and contribute to the refinement of the genotype–phenotype mapping of severe stunting induced by pediatric BAD. Moreover, they highlight the value of molecular genetic screening for diagnosing BAD in young patients.
Keywords: bile acid diarrhea, SLC10A2, stunting, WES
The SLC10A2 313T>C mutation leads to molecular dysfunction, which results in bile acid diarrhea. And the predicted protein stability revealed a decline in Gibbs free energy, indicating an unstable protein structure associated with this mutation.
1. INTRODUCTION
Diarrhea is a common issue in children below the age of 5 years, and it is also one of the main causes of pediatric mortality. Acute diarrhea is generally attributed to community transmission of various kinds of bacteria, viruses, or parasites and requires treatment and sufficient administration of fluids to avoid dehydration. By contrast, chronic diarrhea is associated with various causes, including inflammatory bowel disease, malabsorption of nutrients, gluten intolerance, lactose intolerance, irritable bowel syndrome, and allergies to foods, especially milk protein. Moreover, chronic diarrhea induces malnutrition, leading to severe stunting, which is a key reason for the rise in pediatric morbidity and mortality. Thus, while diarrhea is common in children, it warrants attention.
The causes of diarrhea in adults and children differ significantly. Bile acid diarrhea (BAD, phenotype OMIM #613291) is always discounted as one of the causes of chronic diarrhea in adults (Flores et al., 2021). Recent studies have shown that around 1% of the population suffers from BAD (Hughes et al., 2021), which is primarily caused by ileal Crohn's disease or ileal resections (Habba, 2011; Johnston et al., 2011). Between 25% and 50% of cases of functional diarrhea and diarrhea‐predominant irritable bowel syndrome (IBS‐D) are caused by BAD (Camilleri, 2015). Bile acid malabsorption (BAM) is considered to be the root cause of BAD (Wang et al., 2020). Four types of BAM have been identified: (1) ileal dysfunction and impairment of reabsorption; (2) primary or idiopathic BAD, involving genetic variants; (3) other gastrointestinal disorders; and (4) excessive hepatic bile acid (BA) synthesis (Sadowski et al., 2020a, 2020b; Walters et al., ,,2009, 2020). BAD, however, is rare in pediatric patients and is seldom observed in very young individuals.
Several genetic variations have been identified in BAD patients. Research has confirmed the involvement of seven genes in BA metabolism: SLC10A2, FGFR4, SLC51A, SLC51B, KlothoB, SHP, and CYP7A1 (Camilleri, 2016). SLC10A2 (gene OMIM #601295) codes the apical sodium‐dependent bile acid transporter (ASBT) protein, which help to transport primary BA and plays an important role in BA enterohepatic circulation (Oelkers et al., 1997; Shi et al., 2017). There are only two confirmed variants (c.728T>C and c.785C>T) reported to be associated with BAD (Oelkers et al., 1997). In addition, c.79delT in SLC51B contributes to the rising incidence of this BA enterohepatic disorder (Sultan et al., 2018). The KlothoB gene is thought to influence fecal BA concentration, and FGFR4 expression is associated with colonic transit (Johnston et al., 2016; Wong et al., 2011). Furthermore, TGF5 and GPBAR1 mutations respond to quantitative changes in colonic transit and BA excretion (Alemi et al., 2013; Camilleri et al., 2014).
In this paper, we report on the first clinical case of chronic BAD in an infant associated with severe stunting, which was induced by a homozygous mutation of SLC10A2.
2. METHODS
2.1. Ethical compliance
This study was approved by the ethics committee of the West China Second Hospital of Sichuan University (approval no. 2014‐034). We obtained informed consent from the patient's parents prior to performing whole exon sequencing (WES) and for the inclusion of the patient's clinical and imaging details in subsequent publications.
2.2. DNA extraction and WES analysis
A peripheral blood sample was obtained using an EDTA anticoagulant blood sample tube, which was stored at 4°C for less than 6 h. DNA was extracted using the Blood Genome Column Medium Extraction Kit (Tiangen) according to the manufacturer's instructions. Protein‐coding exome enrichment was performed using the xGen Exome Research Panel v. 1.0 (IDT), comprising 429,826 individually synthesized and quality‐controlled probes, targeting 49.11 Mb protein‐coding regions (>23,000 genes) of the human genome. WES was performed using the Illumina NovaSeq 6000 platform (Illumina Inc.), and the raw data were processed using Fastp for removing adapters and filtering low‐quality reads. The paired‐end reads were performed using the Burrows–Wheeler Aligner to the Ensembl GRCh38/hg38 reference genome. Variant annotation was performed in accordance with database‐sourced minor allele frequencies (MAFs) and practical guidelines on pathogenicity issued by the American College of Medical Genetics. The primary data demonstrated the annotation of MAFs, including 1000 genomes, dbSNP, ESP, ExAC, and Chigene in‐house MAFs database; Provean, Sift, Polypen2_hdiv, Polypen2_hvar, MutationTaster using R software.
2.3. Mutation analysis of SLC10A2
To understand the molecular architecture of the human SLC10A2 gene, we performed comparative modeling using the SWISS‐MODEL (https://swissmodel.expasy.org/ ). We estimated the change in the free energy of the model using the mutation cut off scanning matrix (mCSM) method (http://biosig.unimelb.edu.au/mcsm/) and the Site Directed Mutator (SDM, http://marid.bioc.cam.ac.uk/sdm2). To enable an assessment of the impacts of mutations on the stability of SLC10A2. We also used the DUET server (http://biosig.unimelb.edu.au/duet/ ) that integrates mCSM and SDM to improve the overall prediction accuracy of the mutations under consideration. The signature vector that was ultimately generated was employed to train the predictive classification and regression model used to calculate the change induced by mutations in terms of Gibbs folding free energy (ΔΔG).
3. CLINICAL DESCRIPTION AND MOLECULAR RESULTS
3.1. History of illness and physical examination
The proband in this study was a 3‐month‐old female infant suffering from recurrent fever and chronic diarrhea (>10 times a day) for more than 2 months. Her chronic diarrhea manifested as diarrhea, and no blood or mucus was observed in her stool. Six hours prior to being admitted at our hospital, she suffered one episode of seizure, and her responses to external stimulations remained in poor. This patient presented with severe vomiting during the course of this disease. Compound feeding was provided daily, and no obvious milk protein allergy or lactose intolerance was observed. Moreover, this baby was born full‐term and her birth weight and length at were 2080 g (Z‐score = −3.09) and 44 cm (Z‐score = −3.60), respectively, indicating a small for gestational age. Chronic diarrhea induced extreme stunting; her body weight and length were 3050 g (Z‐score = −5.70) and 49 cm (Z‐score = −4.69), respectively, with limited subcutaneous fat and delayed neuromotor development. During this period, her parents took her to the local hospital several times for consultations, and she received therapies for viral diarrhea. However, her symptoms did not subside and became severe over a short time period. Her brother and sister did not manifest any related clinical manifestations.
3.2. Laboratory results
The results of a blood gas analysis indicated extremely severe metabolic acidosis (pH = 6.9) and electrolyte disturbance (K+ 4.3 mmol/L, Na+123 mmol/L, and Ca2+ 1.4 mmol/L). Peripheral blood counts revealed a hemoglobin level of 70 g/L. Erythrocyte sedimentation rate was normal (4 mm/h; n.v. <8 mm/h). Blood biochemical tests showed elevated levels of alanine aminotransferase (108 U/L; n.v. <50 U/L), aspartate transaminase (89 U/L; n.v. <26 U/L), creatinine (90 µmol/L; n.v. 17.3–54.6 µmol/L), urea (15.49 mmol/L; n.v. 3.2–8.2 µmol/L), ammonia (148 µmol/L; n.v. <20 µmol/L); β‐hydroxybutyrate (1.55 mmol/L; n.v. <0.27 mmol/L), and carboxylate anion (103.4 µmol/l; n.v. 20–100 µmol/L). Besides, the serum bilirubin remains normal. The results of a thyroid function test showed that T3, T4, TSH, FT3, and FT4 levels were globally decreased. A low serum level of total 25‐OH vitamin D (8.4 ng/ml; n.v. 30–100 ng/ml) indicated a vitamin D deficiency. Table 1 presents a summary of all of the related data. The results of a nucleic acid and autoantibody tests ruled out SARS‐Cov‐2, HIV, toxoplasma, rubella virus, herpes simplex virus, Epstein–Barr virus, hepatic virus infections, as well as rotavirus and norovirus. Finally, cranial magnetic resonance imaging, an electroencephalogram, a cerebrospinal fluid test, a bone marrow biopsy, echocardiography, and chest and abdominal contrast‐enhanced computed tomography yielded no significant findings.
TABLE 1.
Laboratory tests findings in the infant with SLC10A2 mutation during hospitalization and follow‐up
| Clinical chemistry | Reference | Administration | Discharge | Follow‐up 6 mo | Follow‐up 12 mo |
|---|---|---|---|---|---|
| PH | 7.35–7.45 | 6.9 | 7.43 | 7.45 | 7.42 |
| BE | −3‐3 mmol/L | −29.1 | −7 | −1.8 | 0.2 |
| Na+ | 135‐145 mmol/L | 123 | 134 | 136 | 138 |
| Cl− | 96‐108 mmol/L | 111 | 109 | 106 | 107 |
| Ca2+ | 1.09–1.30 mmol/L | 1.4 | 1.24 | 1.22 | 1.2 |
| GLU | 3.3–5.3 mmol/L | 4.9 | 4.4 | 4.9 | 3.6 |
| ALT | 9–52 U/L | 108 | 92 | 13 | 13 |
| AST | 14–26 U/L | 89 | 91 | 105 | 43 |
| Total bilirubin | 3‐22 µmol/L | 12 | 7.3 | 86 | 5.1 |
| β‐Hydroxybutyrate | 0–0.27 mmol/L | 1.55 | 1.21 | 0.63 | 0.07 |
| Carboxylate anion | 20‐100 µmol/L | 103.4 | 95.3 | 96.5 | 93.1 |
| Total bile acid | 0‐10 µmol/L | — | — | 3.2 | 5.5 |
| Cholyglycine | <2.7 mg/L | — | — | 1.52 | 1.71 |
| Fecal bile acid | — | — | — | 0.76 mmol/48 h/100 g excrement | — |
3.3. Treatment approaches
Body fluid resuscitation and a noninvasive ventilation nasal mask were administered with acidosis correction. In addition, alternating antibiotics were administered. This baby was sustained with a feed made with complete hydrolyzed milk power. Moreover, anticonvulsants and vitamin D supplements were administered. After 15 days of treatment, the clinical manifestation was partially relieved. The daily episodes of diarrhea were reduced to five or six, but the stool remained watery.
3.4. Molecular results
The patient's diarrhea and stunting were abnormal in the context of general pediatric disease. Therefore, a rare genetic disease was suspected. WES had been performed. And a homozygous mutation of SLC10A2 at c.313T>C site (rs201206937) had been targeted (Figure 1). Moreover, the patient's parents were carriers of the variant. According to researchers at the American College of Medical Genetics, this variant is an uncertain one as PM2+PP3. Furthermore, it is not reported in Asian populations. The PolyPhen score of 1.000 predicted that it is a damaging mutation (sensitivity: 0.00, specificity: 1.00). Moreover, an analysis performed with MutationTaster revealed that it is disease‐causing (probability: 0.999).
FIGURE 1.
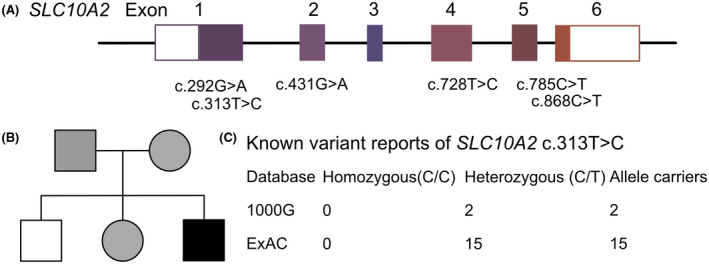
The information of SLC10A2 mutations in population and this family. (a) This demonstrated the known SLC10A2 variants related with bile acid diarrhea and their locations within exons. (b) Family pedigree reveal the parents were carriers of SLC10A2 313T>C, and his sister is also a carrier. This proband presented bile acid diarrhea with a homozygous mutation of SLC10A2 313T>C. (c) Summary of current reports on the individuals of SLC10A2 313T>C
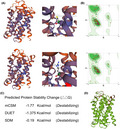
We used the SWISS‐MODEL to predict the variant's wild‐type and mutated protein crystal structures (Figure 2a). Significant structural changes have been identified in its transportation domain (Figure 2a,b). DUET, mCSM, and SDM tools, used to predict protein stability, revealed a decline in Gibbs free energy, indicating an unstable protein structure associated with this mutation (Figure 2c,d).
FIGURE 2.
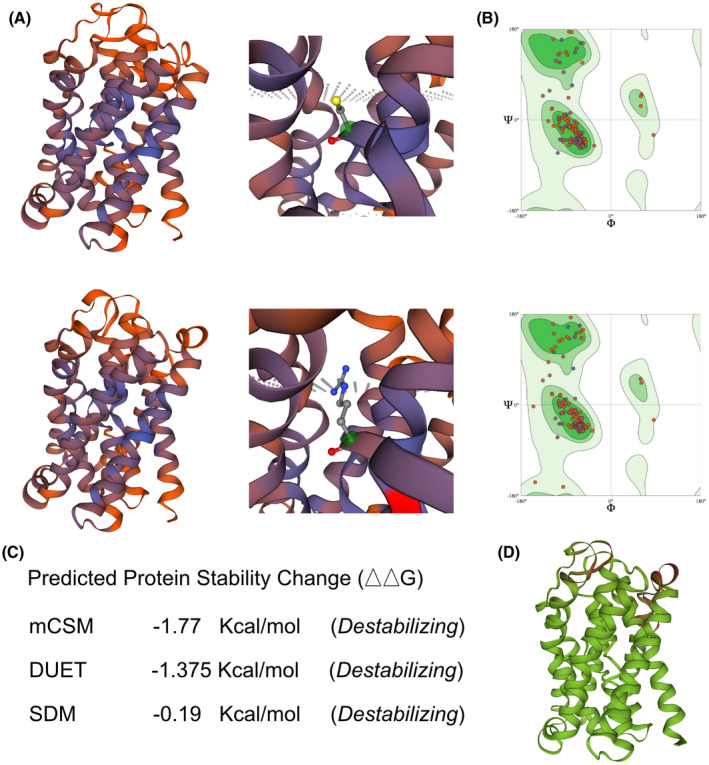
The impacts of SLC10A2 313T>C mutation on the molecular structure of protein. (a) SWISS‐MODEL to predict the variant's wild‐type and mutated protein crystal structure. And changes of structure have been identified in its transporter part. (b) Ramachandran plots of SLC10A2 with or without p.C105R mutation. (c and d) DUET, mCSM, and SDM tools, used to predict protein stability, revealed a decline in Gibbs free energy, indicating an unstable protein structure associated with this mutation. mCSM, mutation cut off scanning matrix
3.5. Follow‐up and clinical outcome
Following the discovery of the homozygous mutation of SLC10A2, BAD was strongly suspected in this case. The total BA and cholyglycine values were 3.2 mmol/L and 1.52 mg/L, respectively. To confirm this hypothesis, fecal samples were collected at 48‐h intervals. As we were unable to obtain a 400 g fecal sample, we normalized fecal BA to 100 g of feces collected at 48‐h intervals. The value recorded was 0.76 mmol/48 h/100 g, which was higher than the cut‐off value which should be normalized to 400 g fecal calculation.
Following the confirmation of a BAD diagnosis, colestyramine was administered in small doses of 0.5 g twice daily. Subsequently, the manifestation of diarrhea subsided. Catch‐up growth was observed when the infant reached the age of 12 months: her body weight increased to 10.06 kg (Z‐score = −2.61) and her body length to 64 cm (Z‐score = −4.59) (Table 2).
TABLE 2.
The growth parameters of this case
| Age (months) | Length (Cm) | Median | Z Score | Percentile | Weight (Kg) | Median | Z Score | Percentile | BMI | Median | Z Score | Percentile |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 44 | 50.38 | −3.60 | <0.1 | 2.08 | 3.32 | −3.09 | 0.1 | 10.74 | 13.07 | −2.10 | 1.7 |
| 3 | 49 | 62.05 | −5.70 | <0.1 | 3.05 | 6.72 | −4.69 | <0.1 | 12.70 | 17.50 | −3.15 | <0.1 |
| 6 | 55 | 68.57 | −5.60 | <0.1 | 5.51 | 8.43 | −3.04 | 0.1 | 18.21 | 17.96 | 0.16 | 56.3 |
| 12 | 64 | 76.58 | −4.59 | <0.1 | 7.10 | 10.06 | −2.61 | 0.4 | 17.33 | 17.19 | 0.11 | 53.9 |
Abbreviation: BMI, body mass index.
4. DISCUSSION AND CONCLUSION
This is the youngest case manifesting the characteristics of BAD induced by a genetic variant, and it is the second case revealing a clear association between an SLC10A2 genetic mutation and the onset of BAD. Primary BAM, which is associated with fecal BA excretion caused by several disorders, was first described in adult patients with idiopathic chronic diarrhea that responded to colestyramine (Hegyi et al., 2018; Oduyebo & Camilleri, 2017). Pediatric BAM usually manifests as a more severe presentation and the dysfunction of BA uptake leads to significant steatorrhea and reduced solubilization of dietary lipids. Consequently, such patients present severe malnutrition, and unabsorbed hydroxyl fatty acids are responsible for inducing recurrent diarrhea (Riba et al., 2020). Although diarrhea is a common disorder in children, BAD is seldom observed and often ignored by pediatricians. A very limited literature has reported on the association between pediatric BAD and genetic variants. And the general reasons to induce pediatric BAD is a consequence of motor abnormalities resulting in intestinal motility with normal intestinal structure on radiological and biopsy examinations.
SLC10A2 codes the ASBT protein, which is located on the luminal membrane in the distal ileum and proximal tubule of the kidneys in humans and rodents. ASBT is crucial for maintaining the normal enterohepatic and renal‐hepatic circulatory function of BA transportation (Döring et al., 2012; Hu et al., 2011; Lionarons et al., 2012). Several studies have demonstrated that ASBT dysfunction interrupts enterohepatic circulation. An in vivo experiment performed on mice showed a reduction in the bile slat pool size by 80%. Medication targeting hypercholesterolemia and diabetes mellitus type 2 is a potentially promising pharmaceutical solution (Yang et al., 2020). The polymorphisms have been characterized. However, few dysfunctional mutations in SLC10A2h with significant clinical features have been documented. Only one study published two decades ago reported on two missense mutations in a family with primary BAM as 728T>C (Leu243Pro) or 785C>T (Thr262Met), leading to an intestinal disorder with congenital diarrhea, steatorrhea, and disrupted enterohepatic circulation (Oelkers et al., 1997). Another cohort study revealed 292G>A and 431G>A variants that were associated with mild and moderately impaired transport function, respectively (Xiao & Pan, 2017). Moreover, 868C>T is associated with complete functional loss of ASBT (Xiao & Pan, 2017). While some whole genome association studies have revealed 313T>C, no clear association with ASBT dysfunction has been confirmed. This report is the first to demonstrate clinical manifestation with the 313T>C variant. Although there is no definitive evidence for flagging it as a pathogenic mutation, all of our predictions indicate that it is a disease‐causing mutation and that this patient had a homozygous genotype mutation, which could positively support the relationship between SLC10A2 313T>C and pediatric BAD with stunted growth.
In conclusion, BAD is rare among infants, but it should be considered etiologically in severe cases of diarrhea after excluding general risk factors. Molecular genetic screening is very useful for diagnosing BAD in young patients. Variants of SLC10A2 demonstrated a significant association with primary BAM‐induced BAD. Our findings expand the mutant spectrum of the SLC10A2 gene and contribute to the refinement of the genotype–phenotype mapping of severe pediatric BAD‐induced stunting.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL STATEMENT
This study was approved by the Ethics Committee of West China Second Hospital of Sichuan University (2014‐034). Informed consent from the patient's parents prior to conducting the WES was obtained, including the patient's clinical and imaging details in the manuscript for the purpose of publication.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81700360, 81971457) and Technology Project of Sichuan Province of China (2020YJ0234, 2020YFS0101, 2020YFS0102, 2020YFS0110).
Qie, D., Zhang, Y., Gong, X., He, Y., Qiao, L., Lu, G., & Li, Y. (2021). SLC10A2 deficiency‐induced congenital chronic bile acid diarrhea and stunting. Molecular Genetics & Genomic Medicine, 9, e1740. 10.1002/mgg3.1740
Di Qie and Yulin Zhang have contributed equally to this article.
Contributor Information
Guoyan Lu, Email: 412562465@qq.com.
Yifei Li, Email: liyfwcsh@scu.edu.cn.
DATA AVAILABILITY STATEMENT
Data sets used in this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alemi, F., Poole, D. P., Chiu, J., Schoonjans, K., Cattaruzza, F., Grider, J. R., Bunnett, N. W., & Corvera, C. U. (2013). The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology, 144(1), 145–154. 10.1053/j.gastro.2012.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri, M. (2015). Bile Acid diarrhea: Prevalence, pathogenesis, and therapy. Gut and Liver, 9(3), 332–339. 10.5009/gnl14397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri, M. (2016). Dissecting molecular mechanisms in bile acid diarrhea. American Journal of Gastroenterology, 111(3), 433–435. 10.1038/ajg.2016.23 [DOI] [PubMed] [Google Scholar]
- Camilleri, M., Shin, A., Busciglio, I., Carlson, P., Acosta, A., Bharucha, A. E., Burton, D., Lamsam, J., Lueke, A., Donato, L. J., & Zinsmeister, A. R. (2014). Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. American Journal of Physiology. Gastrointestinal and Liver Physiology, 307(5), G508–G516. 10.1152/ajpgi.00178.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring, B., Lütteke, T., Geyer, J., & Petzinger, E. (2012). The SLC10 carrier family: Transport functions and molecular structure. Current Topics in Membranes, 70, 105–168. 10.1016/b978-0-12-394316-3.00004-1 [DOI] [PubMed] [Google Scholar]
- Flores, V., Martínez‐Lozano, H., Bighelli, F., Orcajo, J., García‐Lledó, J., Alonso‐Farto, J. C., & Menchén, L. (2021). Prevalence of biliary acid malabsorption in patients with chronic diarrhoea of functional characteristics: A prospective study. BMC Gastroenterology, 21(1), 56. 10.1186/s12876-021-01637-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habba, S. F. (2011). Diarrhea predominant irritable bowel syndrome (IBS‐D): Fact or fiction. Medical Hypotheses, 76(1), 97–99. 10.1016/j.mehy.2010.08.040 [DOI] [PubMed] [Google Scholar]
- Hegyi, P., Maléth, J., Walters, J. R., Hofmann, A. F., & Keely, S. J. (2018). Guts and gall: Bile acids in regulation of intestinal epithelial function in health and disease. Physiological Reviews, 98(4), 1983–2023. 10.1152/physrev.00054.2017 [DOI] [PubMed] [Google Scholar]
- Hu, N. J., Iwata, S., Cameron, A. D., & Drew, D. (2011). Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature, 478(7369), 408–411. 10.1038/nature10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, L. E., Ford, C., Brookes, M. J., & Gama, R. (2021). Bile acid diarrhoea: Current and potential methods of diagnosis. Annals of Clinical Biochemistry, 58(1), 22–28. 10.1177/0004563220966139 [DOI] [PubMed] [Google Scholar]
- Johnston, I. M., Nolan, J. D., Pattni, S. S., Appleby, R. N., Zhang, J. H., Kennie, S. L., Madhan, G. K., Jameie‐Oskooei, S., Pathmasrirengam, S., Lin, J., Hong, A., Dixon, P. H., Williamson, C., & Walters, J. R. F. (2016). Characterizing factors associated with differences in FGF19 blood levels and synthesis in patients with primary bile acid diarrhea. American Journal of Gastroenterology, 111(3), 423–432. 10.1038/ajg.2015.424 [DOI] [PubMed] [Google Scholar]
- Johnston, I., Nolan, J., Pattni, S. S., & Walters, J. R. (2011). New insights into bile acid malabsorption. Current Gastroenterology Reports, 13(5), 418–425. 10.1007/s11894-011-0219-3 [DOI] [PubMed] [Google Scholar]
- Lionarons, D. A., Boyer, J. L., & Cai, S. Y. (2012). Evolution of substrate specificity for the bile salt transporter ASBT (SLC10A2). Journal of Lipid Research, 53(8), 1535–1542. 10.1194/jlr.M025726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduyebo, I., & Camilleri, M. (2017). Bile acid disease: the emerging epidemic. Current Opinion in Gastroenterology, 33(3), 189–195. 10.1097/mog.0000000000000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkers, P., Kirby, L. C., Heubi, J. E., & Dawson, P. A. (1997). Primary bile acid malabsorption caused by mutations in the ileal sodium‐dependent bile acid transporter gene (SLC10A2). Journal of Clinical Investigation, 99(8), 1880–1887. 10.1172/jci119355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba, A., Hassani, K., Walker, A., van Best, N. , von Zeschwitz, D. , Anslinger, T., Sillner, N., Rosenhain, S., Eibach, D., Maiga‐Ascofaré, O., & Rolle‐Kampczyk, U. (2020). Disturbed gut microbiota and bile homeostasis in Giardia‐infected mice contributes to metabolic dysregulation and growth impairment. Science Translational Medicine, 12(565), eaay7019. 10.1126/scitranslmed.aay7019 [DOI] [PubMed] [Google Scholar]
- Sadowski, D. C., Camilleri, M., Chey, W. D., Leontiadis, G. I., Marshall, J. K., Shaffer, E. A., Tse, F., & Walters, J. R. F. (2020a). Canadian association of gastroenterology clinical practice guideline on the management of bile acid diarrhea. Journal of the Canadian Association of Gastroenterology, 3(1), e10–e27. 10.1093/jcag/gwz038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski, D. C., Camilleri, M., Chey, W. D., Leontiadis, G. I., Marshall, J. K., Shaffer, E. A., Tse, F., & Walters, J. R. F. (2020b). Canadian association of gastroenterology clinical practice guideline on the management of bile acid diarrhea. Clinical Gastroenterology and Hepatology, 18(1), 24–41.e21. 10.1016/j.cgh.2019.08.062 [DOI] [PubMed] [Google Scholar]
- Shi, A. X., Zhou, Y., Zhang, X. Y., Zhao, Y. S., Qin, H. Y., Wang, Y. P., & Wu, X. A. (2017). Irinotecan‐induced bile acid malabsorption is associated with down‐regulation of ileal Asbt (Slc10a2) in mice. European Journal of Pharmaceutical Sciences, 102, 220–229. 10.1016/j.ejps.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Sultan, M., Rao, A., Elpeleg, O., Vaz, F. M., Abu‐Libdeh, B., Karpen, S. J., & Dawson, P. A. (2018). Organic solute transporter‐β (SLC51B) deficiency in two brothers with congenital diarrhea and features of cholestasis. Hepatology, 68(2), 590–598. 10.1002/hep.29516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, J. R. F., Arasaradnam, R., & Andreyev, H. J. N. (2020). Diagnosis and management of bile acid diarrhoea: A survey of UK expert opinion and practice. Frontline Gastroenterology, 11(5), 358–363. 10.1136/flgastro-2019-101301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, J. R., Tasleem, A. M., Omer, O. S., Brydon, W. G., Dew, T., & le Roux, C. W. (2009). A new mechanism for bile acid diarrhea: Defective feedback inhibition of bile acid biosynthesis. Clinical Gastroenterology and Hepatology, 7(11), 1189–1194. 10.1016/j.cgh.2009.04.024 [DOI] [PubMed] [Google Scholar]
- Wang, L., Zhou, Y., Wang, X., Zhang, G., Guo, B., Hou, X., Ran, J., Zhang, Q., Li, C., Zhao, X., & Geng, Y. (2020). Mechanism of Asbt (Slc10a2)‐related bile acid malabsorption in diarrhea after pelvic radiation. International Journal of Radiation Biology, 96(4), 510–519. 10.1080/09553002.2020.1707324 [DOI] [PubMed] [Google Scholar]
- Wong, B. S., Camilleri, M., Carlson, P. J., Guicciardi, M. E., Burton, D., McKinzie, S., Rao, A. S., Zinsmeister, A. R., & Gores, G. J. (2011). A Klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology, 140(7), 1934–1942. 10.1053/j.gastro.2011.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, L., & Pan, G. (2017). An important intestinal transporter that regulates the enterohepatic circulation of bile acids and cholesterol homeostasis: The apical sodium‐dependent bile acid transporter (SLC10A2/ASBT). Clinics and Research in Hepatology and Gastroenterology, 41(5), 509–515. 10.1016/j.clinre.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Yang, N., Dong, Y. Q., Jia, G. X., Fan, S. M., Li, S. Z., Yang, S. S., & Li, Y. B. (2020). ASBT(SLC10A2): A promising target for treatment of diseases and drug discovery. Biomedicine & Pharmacotherapy, 132, 110835. 10.1016/j.biopha.2020.110835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sets used in this study are available from the corresponding author upon reasonable request.


