Abstract
Since its first identification in the United Kingdom in late 2020, the highly transmissible B.1.1.7 variant of SARS-CoV-2 has become dominant in several countries raising great concern. We developed a duplex real-time RT-qPCR assay to detect, discriminate, and quantitate SARS-CoV-2 variants containing one of its mutation signatures, the ΔHV69/70 deletion, and used it to trace the community circulation of the B.1.1.7 variant in Spain through the Spanish National SARS-CoV-2 Wastewater Surveillance System (VATar COVID-19). The B.1.1.7 variant was detected earlier than clinical epidemiological reporting by the local authorities, first in the southern city of Málaga (Andalucía) in week 20_52 (year_week), and multiple introductions during Christmas holidays were inferred in different parts of the country. Wastewater-based B.1.1.7 tracking showed a good correlation with clinical data and provided information at the local level. Data from wastewater treatment plants, which reached B.1.1.7 prevalences higher than 90% for ≥2 consecutive weeks showed that 8.1 ± 2.0 weeks were required for B.1.1.7 to become dominant. The study highlights the applicability of RT-qPCR-based strategies to track specific mutations of variants of concern as soon as they are identified by clinical sequencing and their integration into existing wastewater surveillance programs, as a cost-effective approach to complement clinical testing during the COVID-19 pandemic.
Keywords: SARS-CoV-2, COVID-19, B.1.1.7 variant, wastewater-based epidemiology (WBE), RT-qPCR, NGS
Short abstract
The study includes the development of an RT-qPCR assay to discriminate the SARS-CoV-2 B.1.1.7 variant and its implementation on the Spanish sewage surveillance system as a data source to complement clinical pandemic assessment.
Introduction
Environmental surveillance of specimens contaminated by human feces is used to monitor enteric virus disease transmission in the population, and several countries have implemented SARS-CoV-2 wastewater monitoring networks to aid decision making during the COVID-19 pandemic.1−3 In Spain, a nationwide COVID-19 wastewater surveillance project (VATar COVID-19) was launched in June 2020 (https://www.miteco.gob.es/es/agua/temas/concesiones-y-autorizaciones/vertidos-de-aguas-residuales/alerta-temprana-covid19/default.aspx) and has weekly monitored SARS-CoV-2 levels in untreated wastewater from initially 32 wastewater treatment plants (WWTPs) since then. On March 2021, the European Commission adopted a recommendation on a common approach to establish and make greater use of systematic wastewater surveillance of SARS-CoV-2 as a new source of independent information on the spread of the virus and its variants in the European Union.4 In situations with low or absent SARS-CoV-2 circulation in the community, wastewater surveillance has proven to be a useful tool as an early warning system,5−9 and several studies have also tried to infer disease incidence in a community, independent of diagnostic testing availability based on SARS-CoV-2 wastewater concentrations, with considerable uncertainties.10−12
Despite titanic efforts based on confinement measures and mass-vaccination programs, the emergence of novel variants of concern (VOCs), mainly B.1.1.7, B.1.351, B.1.1.28.1, and recently B1.617.2, so far, suggests that continued surveillance is required to control the COVID-19 pandemic in the long run. Since January 2021, countries within and outside Europe have observed a substantial increase in the number and proportion of SARS-CoV-2 cases of the B.1.1.7 variant, first reported in the United Kingdom.13,14 Since the B.1.1.7 variant has been shown to be more transmissible than the previously predominant circulating variants and since its infections may be more severe,15 countries where the variant has spread and become dominant are concerned on whether the occurrence of the variant will result in increases in total COVID-19 incidences, hospitalizations, and excess mortality due to overstretched health systems.
The emergence of SARS-CoV-2 variants that may increase transmissibility and/or immune escape points to an imperative need for the implementation of targeted surveillance methods. While sequencing should be the gold standard for variant characterization, cost-effective molecular assays, which could be rapidly established and scaled up, may offer several advantages and provide valuable quantitative information without delay.
This study included the development and validation of a one-tube duplex quantitative real-time RT-PCR (RT-qPCR) assay to detect, discriminate, and quantitate SARS-CoV-2 variants containing the ΔHV69/70 deletion from variants lacking it, using allelic discrimination probes. Confirmatory sequencing of a subset of samples was performed to be able to ascertain the validity of these assays to trace the community circulation of the B.1.1.7 variant. The RT-qPCR-based assay improved the current variant tracking capability and could be easily implemented for monitoring the emergence of ΔHV69/70-containing SARS-CoV-2 variants (mainly B.1.1.7) in Spain through the nationwide wastewater surveillance network.
Methods
Wastewater Sampling
Influent water grab samples were weekly collected from 32 WWTPs (one weekly sample per site) located in 15 different autonomous communities in Spain, from the middle of December 2020 (week 20_51, year_week number) to the end of March 2021 (week 21_13) (last week of 2020 was not sampled). All samples were transported on ice to one of the four participating laboratories of analysis (A, B, C, and D), stored at 4 °C and processed within 1–2 days upon arrival. The time between sample collection and arrival to the laboratory ranged between 3–24 h.
Sample Concentration, Nucleic Acid Extraction, and Process Control
Wastewater samples (200 mL) were concentrated by the aluminum hydroxide adsorption-precipitation method, as previously described.7,16 Briefly, samples were adjusted to pH 6.0, a 1:100 v:v of 0.9 N AlCl3 solution was added, and samples were gently mixed for 15 min at room temperature. The precipitate was collected by centrifugation at 1700× g for 20 min, and the pellet was resuspended in 10 mL of 3% beef extract (pH 7.4). After a 10 min shaking at 150 rpm, samples were centrifuged at 1900× g for 30 min, and concentrates were resuspended in 1–2 mL of phosphate buffered saline (PBS). All samples were spiked with a known amount of an animal coronavirus used as a process control virus. Animal coronaviruses differed between participant laboratories and included the attenuated PUR46-MAD strain of transmissible gastroenteritis enteric virus (TGEV),17 porcine epidemic diarrhea virus (PEDV) strain CV777 (kindly provided by Prof. A. Carvajal from the University of Leon), and murine hepatitis virus (MHV) strain ATCC VR-764.18 Depending on each laboratory, between 10 and 100 μL of the animal coronavirus stock were added to 200 mL of sample to obtain final concentrations of 2.5 × 104–2.5 × 105 copies/mL (PEDV and MHV) or 6.9 × 103 TCID50/mL (TGEV). Nucleic acid extraction from concentrates was performed from 300 μL of sample using the Maxwell RSC PureFood GMO and Authentication Kit (Promega Corporation, Madison, US) or from 150 μL of sample using the NucleoSpin RNA Virus kit (Macherey-Nagel GmbH & Co., Düren, Germany), following the manufacturer’s instructions. Each extraction included a negative control and a process virus control used to estimate the virus recovery efficiency. RT-qPCR for process control viruses was performed as previously described.19−21 Parallel to ISO 15216-1:201722 for the determination of norovirus and hepatitis A virus in the food chain, samples with a virus recovery ≥1% were considered acceptable.
SARS-CoV-2 RT-qPCR Assays
The N1 assay targeting a fragment of the nucleocapsid gene, as published by US-CDC (US-CDC 2020), was used to quantify SARS-CoV-2 RNA in the sewage samples, using the PrimeScript One-Step RT-PCR Kit (Takara Bio, USA) and 2019-nCoV RUO qPCR Probe Assay primer/probe mix (IDT, Integrated DNA Technologies, Leuven, Belgium). Different instruments were used by different participating labs, including CFX96 BioRad, LightCycler 480 (Roche Diagnostics, Germany), Stratagene Mx3005P (Applied Biosystems, USA), and QuantStudio 5 (Applied Biosystems, USA).
The S gene was analyzed by a duplex gene allelic discrimination TaqMan RT-qPCR assay, using 400 nM of the following primers targeting the S gene (For-S21708 5′ATTCAACTCAGGACTTGTTCTTACCTT3′ and Rev-S21796 5′TAAATGGTAGGACAGGGTTATCAAAC3′) and 200 nM of the following probes (S_Probe6970in 5′FAM- TCCATGCTATACATGTCTCTGGGACCAATG BHQ1–3′ and S_Probe6970del 5′HEX-TTCCATGCTATCTCTGGGACCAATGGTACT BHQ1–3′). The RT-qPCR mastermix was prepared using the PrimeScript One-Step RT-PCR Kit (Takara Bio, USA), and the temperature program was 10 min at 50 °C, 3 min at 95 °C, and 45 cycles of 3 s at 95 °C and 30 s at 60 °C.
RT-qPCR analysis for each target included the analysis of duplicate wells containing undiluted RNA and duplicate wells containing a 10-fold dilution to monitor the presence of inhibitors. Every RT-qPCR assay included four wells corresponding to negative controls (two nuclease-free water and two negative extraction controls). Commercially available Twist Synthetic SARS-CoV-2 RNA Controls (Control 2, MN908947.3 and Control 14, EPI_ISL_710528) were used to prepare standard curves for genome quantitation. Both synthetic RNA controls were quantified by droplet-based digital PCR using the One-Step RT-ddPCR Advanced Kit for probes in a QX200 System (BioRad), to estimate the exact concentration of genome copies (GC)/μl, prior to construction of RT-qPCR standard curves. The limit of detection (LOD) and limit of quantification (LOQ) were determined for each specific target by running a series of dilutions of the target with 4–10 replicates per dilution. Parameters of all standard curves and estimated LOD and LOQ for the four participating laboratories are summarized in Supporting Information Table S1.
RT-qPCR Data Analysis and Interpretation
The following criteria were used to estimate SARS-CoV-2 gene viral titers. For each specific target, Cq values ≤ 40 were converted into GC/L using the corresponding standard curve and volumes tested. Occurrence of inhibition was estimated by comparing average viral titers obtained from duplicate wells tested on undiluted RNA with duplicate wells tested on 10-fold diluted RNA. Inhibition was ascertained when difference in average viral titers was higher than 0.5 log10, and if that occurred, viral titers were inferred from the 10-fold RNA dilution. The percentage of SARS-CoV-2 genomes containing the ΔHV69/70 deletion within the S gene was calculated using the following formula:
In cases with one of the concentrations <LOQ, the percentage was calculated using the corresponding LOQ of the assay. Data with both concentrations <LOQ were not considered.
S Gene Sequencing and Single Nucleotide Polymorphism (SNP) Identification
Full-length S gene sequencing of wastewater samples was performed following the ARTIC Network protocol (https://artic.network/ncov-2019, with minor modifications) with selected v3 primers (Integrated DNA Technologies) for genome amplification and KAPA HyperPrep Kit (Roche Applied Science) for library preparation.23 Libraries were loaded in MiSeq Reagent Kit 600v3 cartridges and sequenced on a MiSeq platform (Illumina). The raw sequenced reads were cleaned from low-quality segments and mapped against the Wuhan-Hu-1 reference genome sequence to find out variant-specific signature mutations (mutations and indels).
Results
Duplex SARS-CoV-2 S Gene Allelic Discrimination RT-qPCR Assay Validation
To confirm the ability of the RT-qPCR assay to discriminate and estimate the proportion of targets containing the ΔHV69/70 deletion in different scenarios, nine preparations containing 90:10, 50:50, or 10:90 proportions of B.1.1.7 and Wuhan-Hu-1 synthetic control RNAs at three different total concentration levels (1 × 104, 1 × 103, and 1 × 102 GC/rxn) were made and analyzed (Figure 1). Assays performed using B.1.1.7 and Wuhan-Hu-1 synthetic control RNAs did not show cross-reactivity (data not shown). Results showed the ability of the method to detect and quantitatively discriminate sequences containing the ΔHV69/70 deletion from wildtype sequences in mixed samples through a wide range of total RNA concentrations often observed in extracted wastewater samples.
Figure 1.
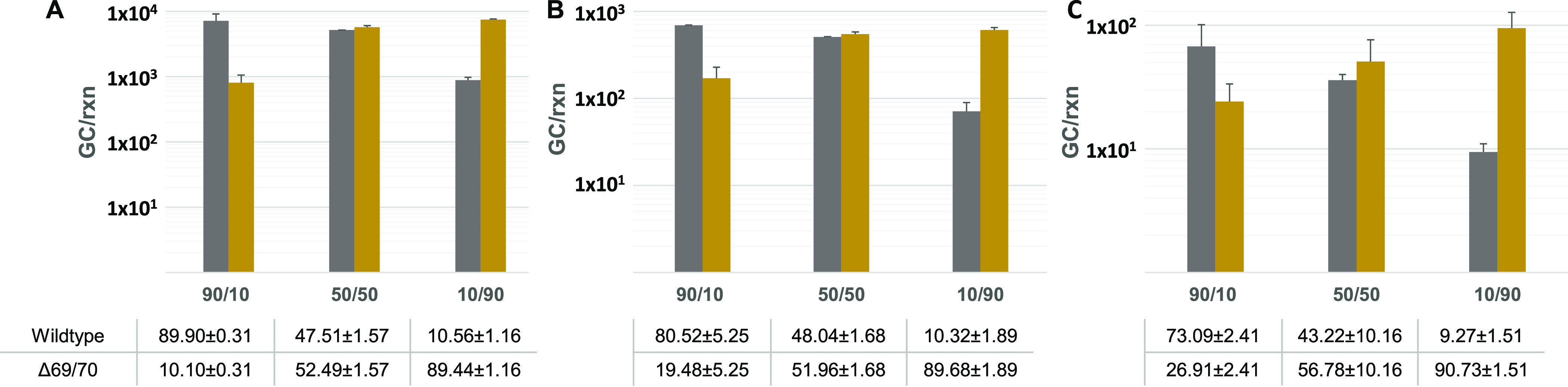
Estimated GC corresponding to wildtype SARS-CoV-2 sequences without ΔHV69/70 deletion (gray bars) and sequences containing ΔHV69/70 deletion in the S gene (yellow bars) from nine preparations at three different total concentration levels ((A): 1 × 104 GC/rxn, (B): 1 × 103 GC/rxn, and (C): 1 × 102 GC/rxn) and three different proportions of Wuhan-Hu-1 and B.1.1.7 GC (90:10, 50:50, and 10:90). Data correspond to mean values ± standard deviations from duplicate samples. Each sample corresponds to an independent preparation containing the indicated proportions of B.1.1.7 and Wuhan-Hu-1 synthetic control RNAs. Samples at different proportions of synthetic control RNAs were prepared in duplicate and were further diluted at the indicated concentration levels.
Additionally, to confirm that sequences detected in natural samples collected during the study period containing the ΔHV69/70 deletion corresponded to the B.1.1.7 variant, a subset of eight samples (four from week 21_11 and four from week 21_12), with B.1.1.7 proportion estimates ranging between 35 and 100% were analyzed by NGS sequencing of the S gene. Variant analysis showed a total of 12 nucleotide substitutions and 4 deletions in comparison with the reference genome of SARS-CoV-2 isolate Wuhan-Hu-1 (MN908947.3), most of them being specific for the B.1.1.7 variant. No strong correlation was observed between coverage and specific amplicons. Between three and seven mutation markers out of nine markers specific for B.1.1.7 variants in the spike region (ΔHV69–70, ΔY144, N501Y, A570D, D614G, P681H, T716I, S982A, and D1118H) were detected in all samples, confirming that the RT-qPCR assay could be used to trace the occurrence of the B.1.1.7 variant, as suggested by the recently published EU recommendation4 (Figure 2). Seven additional amino acid substitutions/deletions, which were not specific for the B.1.1.7 variant, were also detected: G142V, A222V, G257V, S375P, ΔT376, F377L, and K537E. Of these substitutions, as of April 28, G142V and G257V had already been reported in 1128 and 259 sequences published at the GISAID database, respectively, but the others had only been reported at low frequencies. Substitutions S375P/ΔT376/F377L affecting three consecutive residues within the receptor-binding domain (RBD) and identified in 35% of sequences from WWTP-31 in Madrid have been individually reported less than 15 times in several countries, including Spain. However, our data report the occurrence of the three mutations within the same variant. Of note is that mutations in these residues have been related to antigenic drift.24,25 Substitution K537E had been reported once in a nasopharyngeal specimen belonging to B.1.177 (EPI_ISL_1547898; hCoV-19/Slovakia/UKBA-2586/2021). Marker A222V is also present in the B.1.177 variant, which originated in Spain and became the predominant variant in most European countries during the second pandemic wave.26 Sequence data obtained in this study are available at GenBank (SAMN19107574 and PRJNA728923).
Figure 2.
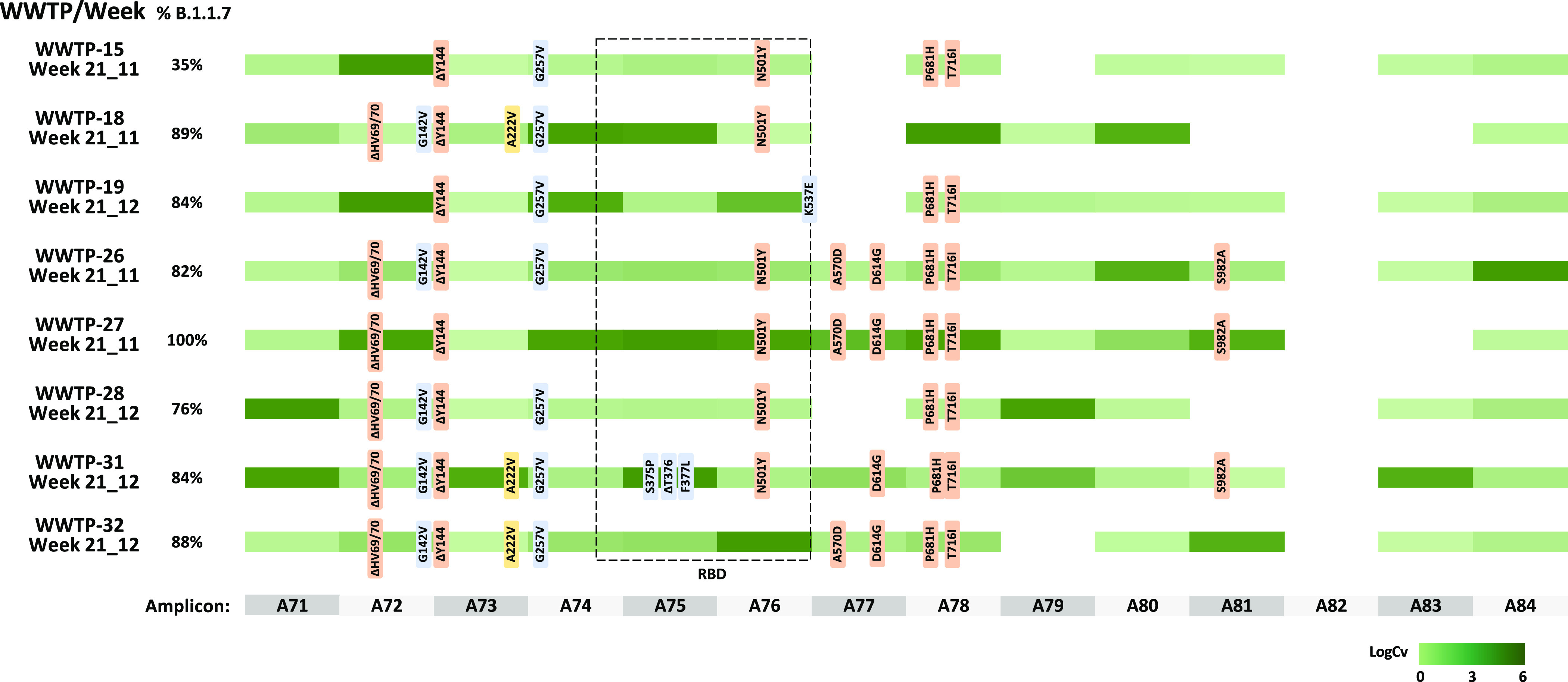
Overview of the nucleotide substitutions detected in SARS-CoV-2 S gene sequences from wastewater samples (n = 8) as compared to the SARS-CoV-2 isolate Wuhan-Hu-1 reference genome (MN908947.3). Percentages before each line indicate the proportion of the B.1.1.7 variant measured in each sample. B.1.1.7-specific markers are shown in light orange, yellow markers show mutations described in the B.1.177 variant, and blue markers indicate others. The RBD is indicated with a dotted square. Amplicon numbers are shown at the bottom. Shaded green colors indicate sequence coverage in a logarithmic scale for each amplicon.
Temporal and Geographical Emergence of the B.1.1.7 Variant in the Spanish Territory
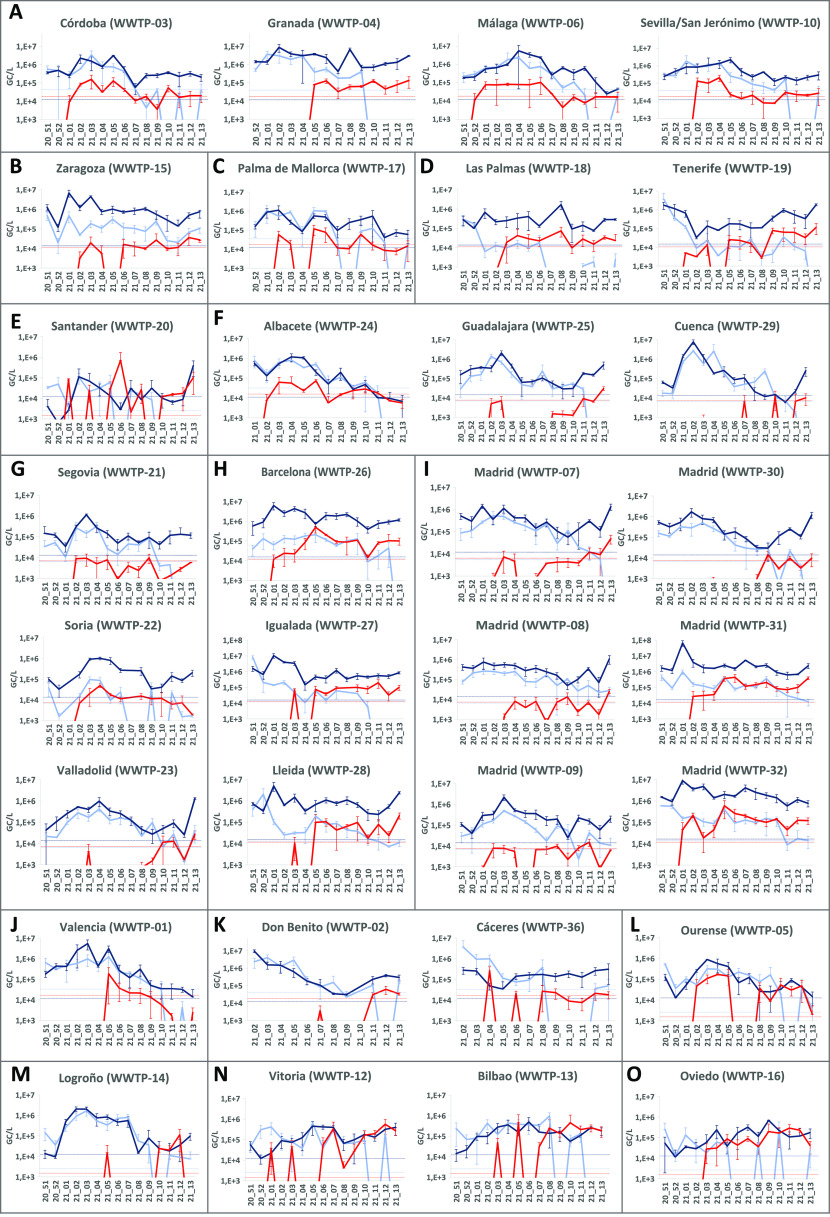
Wastewater samples from 32 Spanish WWTPs from mid-December 2020 to the end of March 2021 were weekly analyzed to monitor the emergence of B.1.1.7 in the territory. Total levels of SARS-CoV-2 RNA were determined by RT-qPCR using N1 target as well as the S discriminatory RT-qPCR, without normalization by the population number (Figure 3). A moderate correlation was observed between both SARS-CoV-2 genome concentration measures between N1 and total S gene titers (R2 = 0.303, data not shown). From all samples analyzed for different SARS-CoV-2 RT-qPCR assays, inhibition was observed in 17.4, 19.5, and 16.1% of samples for N1, S target (wildtype), and S target (B.1.1.7) assays, respectively, without significant differences between targets. Regarding recoveries of the three animal coronaviruses used as process control viruses by different participating laboratories, recovery percentages (mean ± standard deviation) were 23.4 ± 16.0, 24.9 ± 22.6, and 52.9 ± 29.8%, for TGEV, MHV, and PEDV, respectively. The Kruskal–Wallis test showed significant differences for PEDV (p < 0.001).
Figure 3.
Concentration of SARS-CoV-2 RNA in wastewater samples collected in Spain from December 2020 to March 2021, as measured by N1 RT-qPCR (dark blue), and duplex S gene allelic discrimination RT-qPCR [wildtype S (light blue) and B.1.1.7 S (red)]. WWTPs are alphabetically grouped by autonomous communities in Spain ((A): Andalucía, (B): Aragón, (C): Baleares, (D): Canarias, (E): Cantabria, (F): Castilla-La Mancha, (G): Castilla y León, (H): Cataluña, (I): Com. De Madrid, (J): Com. Valenciana, (K): Extremadura, (L): Galicia, M: La Rioja, (N): País Vasco, and (O): Pr. Asturias). Data represent average values, and error bars represent the standard deviation of the RT-qPCR replicates used for calculation. Dotted lines correspond to the LOQ of assays.
Lockdown measures in Spain during the study period were remarkable (mandatory use of face mask, nighttime curfews, restrictions regarding bar and restaurant opening times, social gathering restrictions, restricted opening hours and attendance, municipality of residence confinement implemented in most regions, etc.) and were associated to the nationwide state of alarm, in place since October 2020. As most European countries, at the clinical level, a peak in COVID-19 cases occurred between the end of December 2020 (week 20_52) and early February 2021 (week 21_05). As measured through the N1 target, a peak in SARS-CoV-2 genome levels in wastewater was observed in week 21_01 in nine regions including Zaragoza (Figure 3B; WWTP-15), Las Palmas in Canary Islands (Figure 3D; WWTP-18), three cities in Catalonia (Figure 3H; WWTP-26, -27, and -28), and Madrid (Figure 3I; WWTP-07, -08, -31, and -32); in week 21_02 in regions including Córdoba and Granada in Andalucía (Figure 3A; WWTP-03 and WWTP-04), Palma de Mallorca (Figure 3C; WWTP-17), Santander (Figure 3E; WWTP-20), Cuenca (Figure 3F; WWTP-29), Madrid (Figure 3I; WWTP-30), and Logroño (Figure 3M; WWTP-14); in week 21_03 in Guadalajara (Figure 3F; WWTP-25), Segovia (Figure 3G; WWTP-21), Soria (Figure 3G; WWTP-22), Madrid (Figure 3I; WWTP-09), Valencia (Figure 3J; WWTP-01), and Ourense (Figure 3L; WWTP-05); in week 21_04 in Málaga (Figure 3A; WWTP-06), Albacete (Figure 3F; WWTP-24), Valladolid (Figure 3G; WWTP-23), Bilbao (Figure 3N; WWTP-13), and Oviedo (Figure 3O; WWTP-16), and in week 21_05 in Sevilla (Figure 3A; WWTP-10) and Vitoria (Figure 3N; WWTP-12). SARS-CoV-2 genome titers in Tenerife (Canary Islands) (Figure 3D; WWTP-19) were already at peak titers in the first week of the study (Figure 3).
First detection of the B.1.1.7 variant in wastewater samples occurred in the southern city of Málaga (Andalucía) in week 20_52 (Figure 4). In the first week of January 2021, it could also be detected in the two largest cities (Madrid and Barcelona), in two northern cities (Santander and Vitoria), another city in Andalucía (Córdoba), and in Tenerife (Canary Islands), suggesting that multiple introductions occurred during Christmas holidays in different parts of the Spanish territory and representing 20% of all sampled WWPTs. B.1.1.7 levels detected in week 21_01 were lower than 10% in most WWTPs, with the exception of WWTP-32 in Madrid, which was 22.9%. Percentages of WWTPs with B.1.1.7 detection increased progressively, up to 56% by week 21_04, 91% by week 21_08, and 97% by week 21_13. Our data also showed that while the total level of SARS-CoV-2 RNA genomes measured by the N1 target showed a slight decline for several weeks after the first peak observed during early 2021, this negative trend was slowed or reversed in most WWTPs from the time when the proportion of B.1.1.7 became more abundant (Figure 3). In six cities, a significant increase of N1 RNA titers higher than 1 log10 with respect to the preceding week, as adopted by the VATar COVID-19 Spanish Reporting System (available at https://www.miteco.gob.es/es/agua/temas/concesiones-y-autorizaciones/nota-tecnica-vatar-miterd_tcm30-517518.pdf), was observed in the last week of the study (Figure 3E WWTP-20, Figure 3F WWTP-29, Figure 3G WWTP-23, and Figure 3I WWTP-07, -08, and -30).
Figure 4.
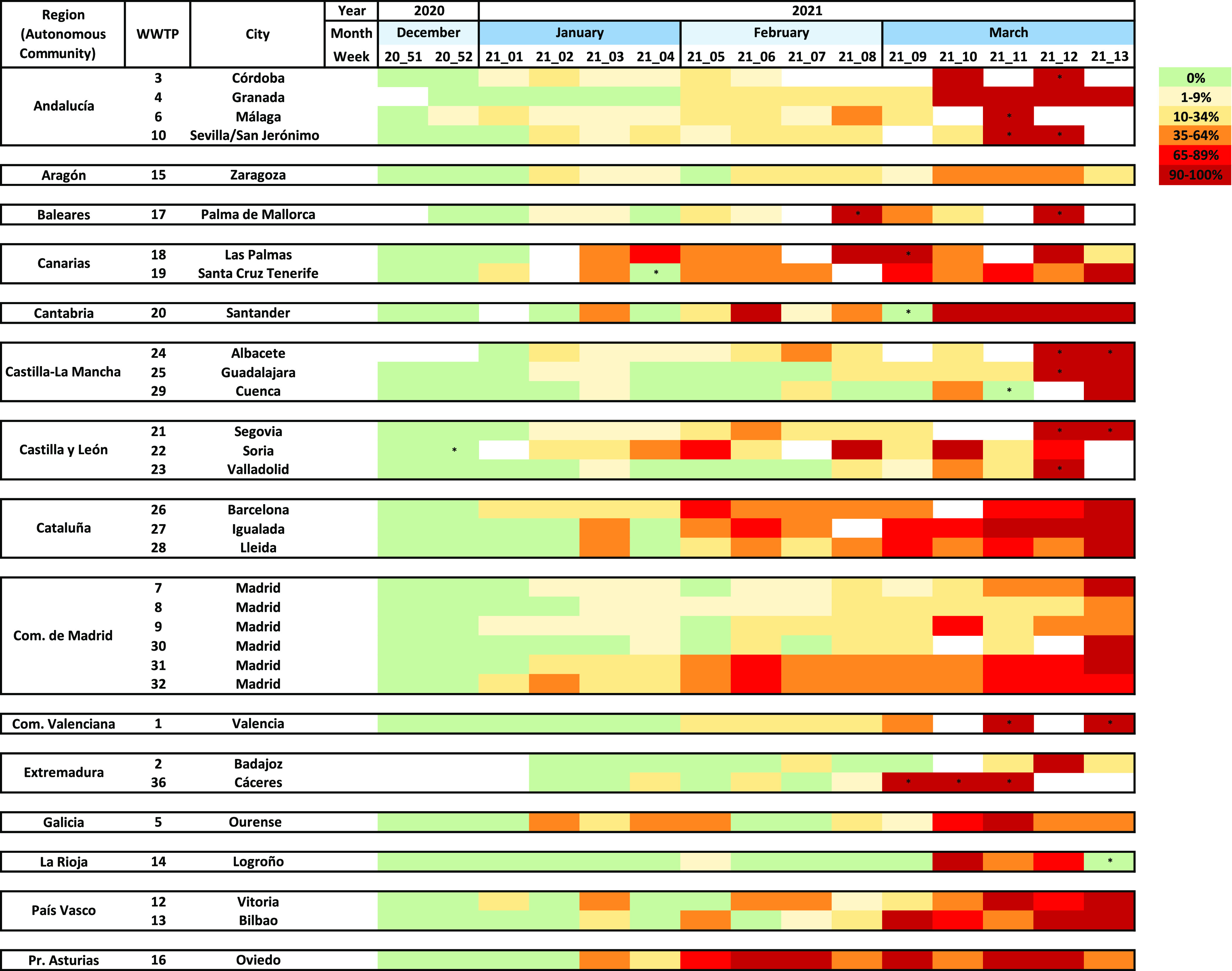
Evolution of B.1.1.7 SARS-CoV-2 prevalence over time, as measured by duplex RT-qPCR in wastewater samples from 32 WWTPs. As in Figure 3, data are alphabetically shown according to autonomous communities. * indicates samples with detection of a single variant but with titers <LOQ.
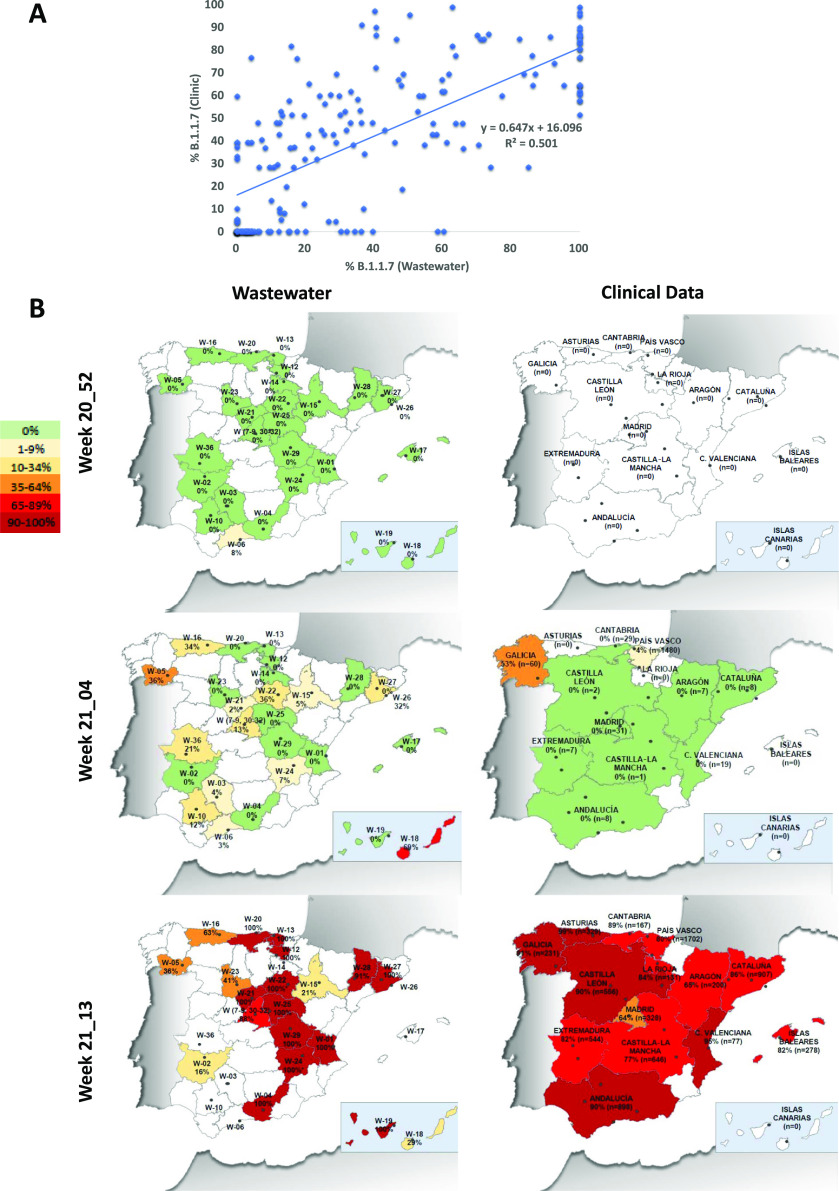
The relative proportion of the B.1.1.7 variant in wastewater could be estimated for 91% of positive samples. Figure 4 shows the heatmap of the evolution of B.1.1.7 prevalence in wastewater over time. Predominance of the B.1.1.7 variant with prevalences ≥ 90% was reached in all WWTPs except in WWTP-08, -09, and -32 in Madrid, and WWTP-15 in Zaragoza. In Madrid, B.1.1.7 reached 48 and 86% and in Zaragoza 51% at the end of the study period, although it kept progressively increasing thereafter (data not shown). When considering data from nine WWTPs, which showed at least 2 consecutive weeks with B.1.1.7 percentages near fixation (90–100%) as a confirmation of predominance (Table S2), we could estimate that approximately 8.1 ± 2.0 weeks’ time was required for the B.1.1.7 variant to become predominant in sewage. This would correspond to an average increase rate of 11.7% (9.6–15.1%) per week.
The proportion of B.1.1.7 was compared to the prevalence detected at the clinical level. The abundance of B.1.1.7, as a fraction of all sequenced clinical specimens by the local authorities in each autonomous community, was obtained from update reports of the epidemiological situation of the variants of SARS-CoV-2 of importance published by the Spanish Ministry of Health.27 When comparing the proportion of B.1.1.7 estimated from wastewater with the proportion estimated from sequencing of clinical isolates, a good correlation was observed (R2 = 0.5012) (Figure 5A). Analysis comparing the B.1.1.7 prevalence estimate with clinical specimens with prevalence estimated from wastewater 1 or 2 weeks before did not increase the correlation determinant (data not shown), suggesting that wastewater analysis did not allow us to anticipate the increase in B.1.1.7 proportion. On a geographical/temporal analysis, wastewater testing allowed the confirmation of circulation of the B.1.1.7 variant before its identification in clinical specimens, especially in part due to a noticeable clinical undertesting in most regions. Data for 3 selected weeks are shown in Figure 5B. By week 21_04 at the end of January, at the clinical level, the variant was only detected on clinical samples in Galicia and País Vasco, mainly due to the low number of sequenced isolates in most regions, while it was detected in 18/32 (56%) WWTPs. At the end of the study, all autonomous communities’ public health departments reported percentages higher than 60%, but while some WWTPs showed very high percentages, some others showed relatively lower proportions, indicating differences at the local level.
Figure 5.
Comparison of B.1.1.7 estimates from wastewater testing and clinical epidemiological surveillance. (A) Correlation between B.1.1.7 proportions estimated by duplex RT-qPCR from wastewater and data reported by local authorities from clinical specimen sequencing. (B) Geographic and temporal evolution of B.1.1.7 SARS-CoV-2 emergence in Spain during the study period, estimated from wastewater samples (left panels) and reported in clinical data (right panels). For wastewater data, percentages are indicated for each WWTP. * indicates samples with detection of a single variant but with titers <LOQ. For clinical data, percentages are indicated for each autonomous community and the number in parenthesis indicates the number of cases under sequence study during that week. Communities in for which data were not available are depicted colorless.
Discussion
As a cost-effective approach to screen thousands of inhabitants, wastewater-based epidemiology (WBE) is a valuable tool to anticipate the circulation of specific pathogens in a community and to closely track their incidence evolution through space and time.28,29 This surveillance has proven to be extremely useful in monitoring the circulation of total SARS-CoV-2 in different parts of the world1−3 and may be effective for tracking novel SARS-CoV-2 VOCs. Despite NGS allowing the definitive identification of specific variants and has already been applied on sewage samples,30,31 it is resource and time-consuming, thus limiting the number of samples that can be processed and the number of labs which can implement this approach on a regular basis. In addition, most deep sequencing protocols allow the identification of signature mutations within short individual reads, which when detected in wastewater samples containing a mixture of different isolates may not be proof of co-occurrence of such mutations within the same genome, thus providing only an indirect evidence of the presence of a certain variant.32 Finally, the use of NGS is also challenged by the presence of inhibitors in samples, limiting its success rate and depth coverage. Given its higher tolerance to inhibitors, droplet digital RT-PCR has been acknowledged as a suitable approach to simultaneously enumerate the concentration of variants with the N501Y mutation and wildtype in wastewater,33 but the widespread use of droplet digital RT-PCR may be limited nowadays because of the high economic investment in instrumentation. On the other hand, the use of RT-qPCR methods offers the advantage of rapid turnaround time, lower cost, and immediate availability in most public health laboratories. In the current study, we validated a duplex RT-qPCR assay to discriminate and enumerate SARS-CoV-2 variants containing the ΔHV69/70 deletion from variants lacking it. Among molecular markers specific for the B.1.1.7 variant, the 6-nucleotide deletion corresponding to residues 69/70 was chosen because it offers the possibility to design highly specific robust probes to be used in wastewater samples, which, unlike clinical specimens, will contain mixed sequences in most cases. Similar to the TaqPath COVID-19 assay (Thermo Fisher Scientific) and other RT-qPCR protocols designed for clinical diagnosis,34 the novel duplex RT-qPCR assay developed in this study proved highly specific and discriminatory.
The ΔHV69/70 deletion is located within the N-terminal domain of the S glycoprotein and has been described to be located at a recurrent deletion region (RDR), and phylogenetic studies showed that it has arisen independently at least 13 times.35 In addition to being a signature mutation of the highly transmissible B.1.1.7 variant, it has also been described in other lineages, including the cluster-5 variant, identified both in minks and humans in Denmark, some isolates belonging to the 20A/S:439 K variant, which emerged twice independently in Europe, and B.1.258 and B.1.525 lineages,35−39 although none of these other lineages have been shown to spread widely. According to GISAID, from a data set of 442,175 sequences collected from 1 December 2020 to 31 March 2021 containing Δ69, as a hallmark of ΔHV69/70 deletion, the proportion of sequences which were classified as B.1.1.7 was 92.4% (for clinical sequences isolated in Spain during the same period, this percentage was 98.1%). Among the sequences containing Δ69 not classified as the B.1.1.7 variant, other lineages including B.1.258, B.1.525, B.1.177, B.1.429 + B.1.427, P1, B.1.351, and B.1.617 were observed in a minority of cases. Among the sequences belonging to the predominant lineage in Spain at the onset of this study, B.1.177, only 0.23% of sequences deposited in GISAID contained Δ69, confirming that detection of ΔHV69/70 is highly indicative of a genome belonging to the B.1.1.7 lineage. Finally, in the NGS analysis performed on eight selected samples, with a high proportion of ΔHV69/70-containing genomes, between 3 and 8 additional B.1.1.7 mutation signatures were identified, confirming that the detected genomes very likely correspond to the B.1.1.7 variant.
During the study period, weekly wastewater estimates of the proportion of B.1.1.7, representing a larger and more comprehensive proportion of typed cases including both symptomatic and asymptomatic cases, well reflected the trends in the reported sequenced clinical cases in most regions. Although the number of clinical specimens sequenced from public health laboratories was not high during the study period and showed strong geographic differences, a correlation was observed between the proportion of B.1.1.7 cases observed at the clinical level and the data estimated from sewage when using samples from the same week (Figure 5A). Of note is that this association was not more robust when using data from 1–2 previous weeks (data not shown). The lack of anticipation ability could be due to several unknown factors, including differences in shedding and kinetic levels between variants, differences in the proportion of asymptomatic infections, and differences in environmental stability. Sewage surveillance allowed the identification of B.1.1.7 circulation in the Spanish territory in the southern city of Málaga before it was confirmed at the clinical level by national public health authorities and allowed us to infer multiple simultaneous introductions during Christmas and New Year’s holidays in distant parts of the country (Madrid, Barcelona, Santander, Vitoria, Córdoba, and Tenerife). By the end of January 2021, only 13% (2/15) autonomous communities had reported B.1.1.7 clinical cases, while circulation in sewage had been confirmed in 67% (10/15) of them, confirming its use as an early warning approach. Data from 9 WWTPs, which reached B.1.1.7 near fixation rates, defined as higher than 90% for ≥2 consecutive weeks, showed that 8.1 ± 2.0 weeks were required to reach B.1.1.7 predominance, which would be a slightly shorter time than what has been locally observed at the clinical level. A research publication reported first detection of imported B.1.1.7 clinical cases in Madrid in week 20_52 (December 2020) and a proportion of 62% of total newly diagnosed COVID-19 cases 10 weeks later.40 Data from other studies are also in the UK reported by ECDC show that B.1.1.7 cases went from less than 5% of all positive cases to more than 60% in less than 6 weeks during November to mid-December 2020,41 and Davies et al. demonstrated that it became dominant throughout the country.15 Estimates from the US indicate that B.1.1.7 would become dominant in most states 4 months after its first identification in late November 2020.14
Our data also showed that predominance of the B.1.1.7 variant appeared to correspond to a slowdown in the negative trend of total SARS-CoV-2 wastewater levels, which had been observed from early 2021 in most cities, probably due to lockdown measures and the mass-vaccination campaign, which was initiated in the last week of 2020. Even in some cities, including Santander (Figure 3E; WWTP-20), Cuenca (Figure 3F; WWTP-29), Valladolid (Figure 3G; WWTP-23), and Madrid (Figure 3I; WWTP-07, -08, and -30), total SARS-CoV-2 levels showed a positive trend at the end of the study. These results suggest that the emergence of B.1.1.7 cases could have produced a higher transmission rate and a slight increase in COVID-19 incidence, as confirmed by clinical epidemiological data reported by the Spanish Ministry of Health, reporting an incidence peak between the end of March and April 2020.42 Despite this positive trend markedly observed in some regions, the smooth running of the mass-vaccination campaign starting on December 27, 2020, in addition to nonpharmaceutical interventions, likely contributed to minimizing the impact of B.1.1.7 emergence. As of the end of March 2021, the percentage of the Spanish population who had been partially immunized or totally vaccinated were of 13.2 and 6.8%, respectively.
Finally, despite the state of alarm decreed by the Spanish government, as a measure to unify confinement and restriction measures across the country, was maintained throughout the study period, predominance of the B.1.1.7 variant was not homogeneous, and dynamics were variable among cities across the country. For instance, a rapid predominance of the B.1.1.7 variant was observed in Granada (WWTP-4) and Cáceres (WWTP-36), while in other cities, B.1.1.7 reached prevalences higher than 90% by week 3–6 after first positive detection and decreased thereafter for 2–3 weeks. Several reasons could explain these waves, including differences in regional social distancing behaviors, repetitive B.1.1.7 case imports, introduction of additional variants, climatic effect on sewage composition, size of the WWTP, or variability related to the use of grab samples instead of composite samples.
This study highlights the use of WBE as a cost-effective, noninvasive, and unbiased approach, which may complement clinical testing during the COVID-19 pandemic, and demonstrates the applicability of duplex RT-qPCR assays on sewage surveillance as a rapid, attractive, and resourceful method to track the early circulation and emergence of the known VOC in a population, especially at times when clinical typing is insufficient and when signature mutations can be unequivocally assigned to a specific VOC. The current strategy could be readily adaptable to track specific mutations of other VOCs as soon as they are identified by clinical genomic sequencing in the future and integrated into existing wastewater surveillance programs.
Acknowledgments
This work was partially supported by the COVID-19 wastewater surveillance project (VATar COVID19), funded by the Spanish Ministry for the Ecological Transition and the Demographic Challenge and the Spanish Ministry of Health, grants from CSIC (202070E101) and MICINN cofounded by AEI FEDER, UE (AGL2017-82909), grant ED431C 2018/18 from the Consellería de Educación, Universidade e Formación Profesional, Xunta de Galicia (Spain), Direcció General de Recerca i Innovació en Salut (DGRIS) Catalan Health Ministry Generalitat de Catalunya through Vall d’Hebron Research Institute (VHIR), and Centro para el Desarrollo Tecnológico Industrial (CDTI) from the Spanish Ministry of Economy and Business, grant number IDI-20200297. P.T. is holding a Ramón y Cajal contract from the Ministerio de Ciencia e Innovación. A.M. is holding a predoctoral fellowship FI_SDUR from Generalitat de Catalunya. We gratefully acknowledge all the staff involved in the VATar COVID-19 project, working with sample collection and logistics. The authors are grateful to Promega Corporation (Madison, US) for technical advice and thank Andrea Lopez de Mota for her technical support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c03589.
Additional data on parameters defining standard curves, LOD and LOQ for RT-qPCR assays used in the study (Table S1), and data used to estimate the time required to reach B.1.1.7 prevalence >90% in wastewater (Table S2) (PDF)
Author Contributions
R.M.P., A.B., S.G., A.A., G.S., and J.L.R. contributed to the study design. Data generation and interpretation was carried out by A.C., A.M.-V., P.T., J.C., M.L., D.P., A.P.-C., A.D.-R., J.G., and D.G.-C. Resources were obtained by M.P. and C.G.R. Funding acquisition was obtained by A.B., A.A., G.S., J.L.R., A.A., and J.Q. Conceptualization of the study was done by R.M.P., A.B., and S.G. R.M.P. and S.G. contributed to manuscript writing. All authors critically revised the manuscript. A.C. and A.M.-V. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Medema G.; Been F.; Heijnen L.; Petterson S. Implementation of Environmental Surveillance for SARS-CoV-2 Virus to Support Public Health Decisions: Opportunities and Challenges. Curr. Opini. Environ. Sci. Health 2020, 17, 49–71. 10.1016/j.coesh.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T.; Fumian T. M.; Mannarino C. F.; Resende P. C.; Motta F. C.; Eppinghaus A. L. F.; Chagas do Vale V. H.; Braz R. M. S.; de Andrade J. d. S. R.; Maranhão A. G.; Miagostovich M. P. Wastewater-Based Epidemiology as a Useful Tool to Track SARS-CoV-2 and Support Public Health Policies at Municipal Level in Brazil. Water Res. 2021, 191, 116810 10.1016/j.watres.2021.116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F.; Xiao A.; Zhang J.; Moniz K.; Endo N.; Armas F.; Bushman M.; Chai P. R.; Duvallet C.; Erickson T. B.; Foppe K.; Ghaeli N.; Gu X.; Hanage W. P.; Huang K. H.; Lee W. L.; Matus M.; McElroy K. A.; Rhode S. F.; Wuertz S.; Thompson J.; Alm E. J. Wastewater Surveillance of SARS-CoV-2 across 40 U.S. States. medRxiv 2021, 21253235 10.1101/2021.03.10.21253235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission . COMMISSION RECOMMENDATION of 17.3.2021 on a Common Approach to Establish a Systematic Surveillance of SARS-CoV-2 and Its Variants in Wastewaters in the EU. 2021.
- Ahmed W.; Angel N.; Edson J.; Bibby K.; Bivins A.; O’Brien J. W.; Choi P. M.; Kitajima M.; Simpson S. L.; Li J.; Tscharke B.; Verhagen R.; Smith W. J. M.; Zaugg J.; Dierens L.; Hugenholtz P.; Thomas K. V.; Mueller J. F. First Confirmed Detection of SARS-CoV-2 in Untreated Wastewater in Australia: A Proof of Concept for the Wastewater Surveillance of COVID-19 in the Community. Sci. Total Environ. 2020, 728, 138764 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Rosa G.; Iaconelli M.; Mancini P.; Bonanno Ferraro G.; Veneri C.; Bonadonna L.; Lucentini L.; Suffredini E. First Detection of SARS-CoV-2 in Untreated Wastewaters in Italy. Sci. Total Environ. 2020, 736, 139652 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W.; Truchado P.; Cuevas-Ferrando E.; Simón P.; Allende A.; Sánchez G. SARS-CoV-2 RNA in Wastewater Anticipated COVID-19 Occurrence in a Low Prevalence Area. Water Res. 2020, 181, 115942 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Miró G.; Anfruns-Estrada E.; Martínez-Velázquez A.; Vázquez-Portero M.; Guix S.; Paraira M.; Galofré B.; Sánchez G.; Pintó R. M.; Bosch A. Time Evolution of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Wastewater during the First Pandemic Wave of COVID-19 in the Metropolitan Area of Barcelona, Spain. Appl. Environ. Microbiol. 2021, 87, e02750-20 10.1128/AEM.02750-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S.; Marechal V.; Mouchel J. M.; Maday Y.; Teyssou R.; Richard E.; Almayrac J. L.; Moulin L. Evaluation of Lockdown Effect on SARS-CoV-2 Dynamics through Viral Genome Quantification in Waste Water, Greater Paris, France, 5 March to 23 April 2020. Eurosurveillance 2020, 25, 2000776 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo D.; Quintela-Baluja M.; Corbishley A.; Jones D. L.; Singer A. C.; Graham D. W.; Romalde J. L. Making Waves: Wastewater-Based Epidemiology for COVID-19 - Approaches and Challenges for Surveillance and Prediction. Water Res. 2020, 186, 116404 10.1016/j.watres.2020.116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C. M.; Moura I. B.; Wilcox M. H. Tracking COVID-19 via Sewage. Curr. Opin. Gastroenterol. 2021, 37, 4. 10.1097/MOG.0000000000000692. [DOI] [PubMed] [Google Scholar]
- Saththasivam J.; El-Malah S. S.; Gomez T. A.; Jabbar K. A.; Remanan R.; Krishnankutty A. K.; Ogunbiyi O.; Rasool K.; Ashhab S.; Rashkeev S.; Bensaad M.; Ahmed A. A.; Mohamoud Y. A.; Malek J. A.; Abu Raddad L. J.; Jeremijenko A.; Abu Halaweh H. A.; Lawler J.; Mahmoud K. A. COVID-19 (SARS-CoV-2) Outbreak Monitoring Using Wastewater-Based Epidemiology in Qatar. Sci. Total Environ. 2021, 774, 145608 10.1016/j.scitotenv.2021.145608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand M.; Hopkins S.; Dabrera G.; Achison C.; Barclay W.; Ferguson N.; Volz E.; Loman N.; Rambaut A. J. B.; Investigation of Novel SARS-COV-2 Variant Variant of Concern 202012/01 (PHE),Public Health England; ; 2020. [Google Scholar]
- Washington N. L.; Gangavarapu K.; Zeller M.; Bolze A.; Cirulli E. T.; Schiabor Barrett K. M.; Larsen B. B.; Anderson C.; White S.; Cassens T.; Jacobs S.; Levan G.; Nguyen J.; Ramirez J. M. 3rd; Rivera-Garcia C.; Sandoval E.; Wang X.; Wong D.; Spencer E.; Robles-Sikisaka R.; Kurzban E.; Hughes L. D.; Deng X.; Wang C.; Servellita V.; Valentine H.; de Hoff P.; Seaver P.; Sathe S.; Gietzen K.; Sickler B.; Antico J.; Hoon K.; Liu J.; Harding A.; Bakhtar O.; Basler T.; Austin B.; MacCannell D.; Isaksson M.; Febbo P. G.; Becker D.; Laurent M.; McDonald E.; Yeo G. W.; Knight R.; Laurent L. C.; de Feo E.; Worobey M.; Chiu C. Y.; Suchard M. A.; Lu J. T.; Lee W.; Andersen K. G. Emergence and Rapid Transmission of SARS-CoV-2 B.1.1.7 in the United States. Cell 2021, 184, 2587–2594.e7. 10.1016/j.cell.2021.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. G.; Jarvis C. I.; van Zandvoort K.; Clifford S.; Sun F. Y.; Funk S.; Medley G.; Jafari Y.; Meakin S. R.; Lowe R.; Quaife M.; Waterlow N. R.; Eggo R. M.; Lei J.; Koltai M.; Krauer F.; Tully D. C.; Munday J. D.; Showering A.; Foss A. M.; Prem K.; Flasche S.; Kucharski A. J.; Abbott S.; Quilty B. J.; Jombart T.; Rosello A.; Knight G. M.; Jit M.; Liu Y.; Williams J.; Hellewell J.; O’Reilly K.; Chan Y.-W. D.; Russell T. W.; Procter S. R.; Endo A.; Nightingale E. S.; Bosse N. I.; Villabona-Arenas C. J.; Sandmann F. G.; Gimma A.; Abbas K.; Waites W.; Atkins K. E.; Barnard R. C.; Klepac P.; Gibbs H. P.; Pearson C. A. B.; Brady O.; Edmunds W. J.; Jewell N. P.; Diaz-Ordaz K.; Keogh R. H.; Increased Mortality in Community-Tested Cases of SARS-CoV-2 Lineage B.1.1.7. Nature 2021, 593, 270–274. 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9510 Detection Of Enteric Viruses . In Standard Methods For the Examination of Water and Wastewater; Standard Methods for the Examination of Water and Wastewater; American Public Health Association, 2018. [Google Scholar]
- Moreno J. L.; Zúñiga S.; Enjuanes L.; Sola I. Identification of a Coronavirus Transcription Enhancer. J. Virol. 2008, 82, 3882–3893. 10.1128/JVI.02622-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaker R. A.; Piczak C. V.; Miller A. A.; Stanton M. F. A Hepatitis Virus Complicating Studies With Mouse Leukemia. JNCI 1961, 27, 29–51. 10.1093/jnci/27.1.29. [DOI] [PubMed] [Google Scholar]
- Raaben M.; Einerhand A. W. C.; Taminiau L. J. A.; van Houdt M.; Bouma J.; Raatgeep R. H.; Büller H. A.; de Haan C. A. M.; Rossen J. W. A. Cyclooxygenase Activity Is Important for Efficient Replication of Mouse Hepatitis Virus at an Early Stage of Infection. Virol. J. 2007, 4, 55. 10.1186/1743-422X-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemulapalli R.; Gulani J.; Santrich C. A Real-Time TaqMan RT-PCR Assay with an Internal Amplification Control for Rapid Detection of Transmissible Gastroenteritis Virus in Swine Fecal Samples. J. Virol. Methods 2009, 162, 231–235. 10.1016/j.jviromet.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.; Zhang T.; Song D.; Huang T.; Peng Q.; Chen Y.; Li A.; Zhang F.; Wu Q.; Ye Y.; Tang Y. Comparison and Evaluation of Conventional RT-PCR, SYBR Green I and TaqMan Real-Time RT-PCR Assays for the Detection of Porcine Epidemic Diarrhea Virus. Mol. Cell. Probes 2017, 33, 36–41. 10.1016/j.mcp.2017.02.002. [DOI] [PubMed] [Google Scholar]
- ISO15216-1:2017 . Microbiology of the Food Chain - Horizontal Method for Determination of Hepatitis A Virus and Norovirus Using Real-Time RT-PCR - Part 1: Method for Quantification. 2017.
- Andrés C.; Garcia-Cehic D.; Gregori J.; Piñana M.; Rodriguez-Frias F.; Guerrero-Murillo M.; Esperalba J.; Rando A.; Goterris L.; Codina M. G.; Quer S.; Martín M. C.; Campins M.; Ferrer R.; Almirante B.; Esteban J. I.; Pumarola T.; Antón A.; Quer J. Naturally Occurring SARS-CoV-2 Gene Deletions Close to the Spike S1/S2 Cleavage Site in the Viral Quasispecies of COVID19 Patients. Emerg. Microb. Infect. 2020, 9, 1900–1911. 10.1080/22221751.2020.1806735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbe S.; Buckland-Merrett G. Data, Disease and Diplomacy: GISAID’s Innovative Contribution to Global Health. Global challenges (Hoboken, NJ) 2017, 1, 33–46. 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A. J.; Starr T. N.; Gilchuk P.; Zost S. J.; Binshtein E.; Loes A. N.; Hilton S. K.; Huddleston J.; Eguia R.; Crawford K. H. D.; Dingens A. S.; Nargi R. S.; Sutton R. E.; Suryadevara N.; Rothlauf P. W.; Liu Z.; Whelan S. P. J.; Carnahan R. H.; Crowe J. E.; Bloom J. D. Complete Mapping of Mutations to the SARS-CoV-2 Spike Receptor-Binding Domain That Escape Antibody Recognition. Cell Host Microbe 2021, 29, 44–57.e9. 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodcroft E. B.; Zuber M.; Nadeau S.; Vaughan T. G.; Crawford K. H. D.; Althaus C. L.; Reichmuth M. L.; Bowen J. E.; Walls A. C.; Corti D.; Bloom J. D.; Veesler D.; Mateo D.; Hernando A.; Comas I.; González Candelas F.; Stadler T.; Neher R. A. Emergence and Spread of a SARS-CoV-2 Variant through Europe in the Summer of 2020. medRxiv 2021, 20219063 10.1101/2020.10.25.20219063. [DOI] [PubMed] [Google Scholar]
- de Sanidad M. Actualización de La Situación Epidemiológica de Las Variantes de SARS-CoV-2 de Importancia En Salud Pública En España; 2021.
- Alfonsi V.; Romanò L.; Ciccaglione A. R.; la Rosa G.; Bruni R.; Zanetti A.; della Libera S.; Iaconelli M.; Bagnarelli P.; Capobianchi M. R.; Garbuglia A. R.; Riccardo F.; Tosti M. E.; Group, C . Hepatitis E in Italy: 5 Years of National Epidemiological, Virological and Environmental Surveillance, 2012 to 2016. Eurosurveillance 2018, 23 (41). 10.2807/1560-7917.ES.2018.23.41.1700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseux M.; Colombet J.; Mirand A.; Roque-Afonso A.-M.; Abravanel F.; Izopet J.; Archimbaud C.; Peigue-Lafeuille H.; Debroas D.; Bailly J.-L.; Henquell C.. Monitoring Human Enteric Viruses in Wastewater and Relevance to Infections Encountered in the Clinical Setting: A One-Year Experiment in Central France, 2014 to 2015. Eurosurveillance 2018, 23 (7), 10.2807/1560-7917.ES.2018.23.7.17-00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Lara R.; Elsinga G.; Heijnen L.; Munnink B. B. O.; Schapendonk C. M. E.; Nieuwenhuijse D.; Kon M.; Lu L.; Aarestrup F.; Lycett S.; Medema G.; Koopmans M. P. G.; de Graaf M. Monitoring SARS-CoV-2 Circulation and Diversity through Community Wastewater Sequencing, the Netherlands and Belgium. Emerg. Infect. Disease J. 2021, 27, 1405. 10.3201/eid2705.204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A.; Nemudraia A.; Wiegand T.; Surya K.; Buyukyoruk M.; Cicha C.; Vanderwood K. K.; Wilkinson R.; Wiedenheft B. Temporal Detection and Phylogenetic Assessment of SARS-CoV-2 in Municipal Wastewater. Cell Report. Med. 2020, 1, 100098 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K.; Dreifuss D.; Topolsky I.; Kull A.; Ganesanandamoorthy P.; Fernandez-Cassi X.; Bänziger C.; Stachler E.; Fuhrmann L.; Jablonski K. P.; Chen C.; Aquino C.; Stadler T.; Ort C.; Kohn T.; Julian T. R.; Beerenwinkel N. Detection of SARS-CoV-2 Variants in Switzerland by Genomic Analysis of Wastewater Samples. medRxiv 2021, 21249379 10.1101/2021.01.08.21249379. [DOI] [Google Scholar]
- Heijnen L.; Elsinga G.; de Graaf M.; Molenkamp R.; Koopmans M. P. G.; Medema G. Droplet Digital RT-PCR to Detect SARS-CoV-2 Variants of Concern in Wastewater. medRxiv 2021, 21254324 10.1101/2021.03.25.21254324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels C. B. F.; Breban M. I.; Ott I. M.; Alpert T.; Petrone M. E.; Watkins A. E.; Kalinich C. C.; Earnest R.; Rothman J. E.; Goes de Jesus J.; Morales Claro I.; Magalhães Ferreira G.; Crispim M. A. E.; Singh L.; Tegally H.; Anyaneji U. J.; Hodcroft E. B.; Mason C. E.; Khullar G.; Metti J.; Dudley J. T.; MacKay M. J.; Nash M.; Wang J.; Liu C.; Hui P.; Murphy S.; Neal C.; Laszlo E.; Landry M. L.; Muyombwe A.; Downing R.; Razeq J.; de Oliveira T.; Faria N. R.; Sabino E. C.; Neher R. A.; Fauver J. R.; Grubaugh N. D. Multiplex QPCR Discriminates Variants of Concern to Enhance Global Surveillance of SARS-CoV-2. PLoS Biol. 2021, 19, e3001236 10.1371/journal.pbio.3001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. R.; Rennick L. J.; Nambulli S.; Robinson-McCarthy L. R.; Bain W. G.; Haidar G.; Duprex W. P. Recurrent Deletions in the SARS-CoV-2 Spike Glycoprotein Drive Antibody Escape. Science 2021, 371, 1139–1142. 10.1126/science.abf6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E. C.; Rosen L. E.; Shepherd J. G.; Spreafico R.; da Silva Filipe A.; Wojcechowskyj J. A.; Davis C.; Piccoli L.; Pascall D. J.; Dillen J.; Lytras S.; Czudnochowski N.; Shah R.; Meury M.; Jesudason N.; de Marco A.; Li K.; Bassi J.; O’Toole A.; Pinto D.; Colquhoun R. M.; Culap K.; Jackson B.; Zatta F.; Rambaut A.; Jaconi S.; Sreenu V. B.; Nix J.; Jarrett R. F.; Beltramello M.; Nomikou K.; Pizzuto M.; Tong L.; Cameroni E.; Johnson N.; Wickenhagen A.; Ceschi A.; Mair D.; Ferrari P.; Smollett K.; Sallusto F.; Carmichael S.; Garzoni C.; Nichols J.; Galli M.; Hughes J.; Riva A.; Ho A.; Semple M. G.; Openshaw P. J. M.; Baillie J. K.; Rihn S. J.; Lycett S. J.; Virgin H. W.; Telenti A.; Corti D.; Robertson D. L.; Snell G. The Circulating SARS-CoV-2 Spike Variant N439K Maintains Fitness While Evading Antibody-Mediated Immunity. bioRxiv 2020, 355842 10.1101/2020.11.04.355842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp S. A.; Meng B.; Ferriera I. A. T. M.; Datir R.; Harvey W. T.; Papa G.; Lytras S.; Collier D. A.; Mohamed A.; Gallo G.; Thakur N.; Carabelli A. M.; Kenyon J. C.; Lever A. M.; de Marco A.; Saliba C.; Culap K.; Cameroni E.; Piccoli L.; Corti D.; James L. C.; Bailey D.; Robertson D. L.; Gupta R. K. Recurrent Emergence and Transmission of a SARS-CoV-2 Spike Deletion H69/V70. bioRxiv 2021, 422555 10.1101/2020.12.14.422555. [DOI] [Google Scholar]
- Bal A.; Destras G.; Gaymard A.; Stefic K.; Marlet J.; Eymieux S.; Regue H.; Semanas Q.; d’Aubarede C.; Billaud G.; Laurent F.; Gonzalez C.; Mekki Y.; Valette M.; Bouscambert M.; Gaudy-Graffin C.; Lina B.; Morfin F.; Josset L.; the COVID-Diagnosis HCL Study Group . Two-Step Strategy for the Identification of SARS-CoV-2 Variant of Concern 202012/01 and Other Variants with Spike Deletion H69–V70, France, August to December 2020. Eurosurveillance 2021, 26 (3), 10.2807/1560-7917.ES.2021.26.3.2100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emma B. H.CoVariants: SARS-CoV-2 Mutations and Variants of Interest https://covariants.org/.
- Pérez-Lago L.; Sola Campoy P. J.; Buenestado-Serrano S.; Pharmacy P. C.; Estévez A.; de la Cueva Technician V. M.; López M. G.; Tecnichian M. H.; Suárez-González J.; Alcalá L.; Comas I.; González-Candelas F.; Muñoz P.; García de Viedma D.; Epidemiological, Clinical and Genomic Snapshot of the First 100 B.1.1.7 SARS-CoV-2 Cases in Madrid. J. Travel Med. 2021, 28, 44. 10.1093/jtm/taab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . SARS-CoV-2 - Increased Circulation of Variants of Concern and Vaccine Rollout in the EU/EEA, 14th Update – 15 February 2021; 2021.
- (ISCIII), E. C.-19. RENAVE. CNE. C. Informe No 77. Situación de COVID-19 En España. Casos Diagnosticados a Partir 10 de Mayo; 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.