To the editor:
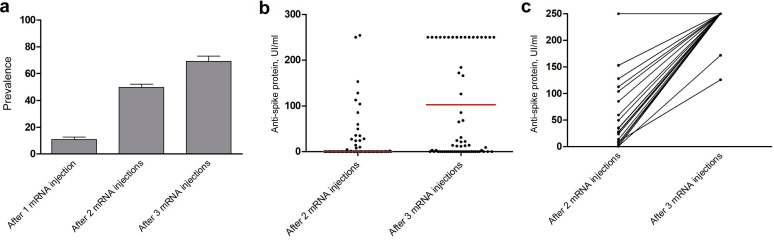
Several reports have highlighted the poor humoral immune response of kidney transplant recipients following coronavirus disease 2019 (COVID-19) mRNA vaccination compared with immunocompetent patients.1, 2, 3 Therefore, the French National Authority for Health has recommended the use of a third vaccine dose for immunosuppressed patients, such as solid organ transplant recipients. We retrospectively assessed the humoral response of all kidney and pancreas transplant recipients vaccinated with the BNT162b2 mRNA (Pfizer BioNTech) COVID-19 vaccine between January and May 2021 in our center. Patients with previous COVID-19 infection or positive prevaccination serology were excluded. The methods for detection of the anti-spike protein responses were dependent on laboratories’ practices and included chemiluminescent microparticle immunoassay (Abbott Architect), chemiluminescence immunoassay (Siemens Atellica), and electrochemiluminescence immunoassay (Roche Elecsys). Anti-spike (IgG) responses were assessed 1 month after the second and third injection, and patients were considered positive if their anti-spike level was above the laboratory threshold. A total of 456 patients had a serologic assessment 1 month after the second injection, of whom 227 were positive, representing 49.7% of our cohort (Table 1 ). A total of 10.7% of these patients had a positive anti-spike protein 1 month after the first injection. Multivariate regression analysis (Table 2 ) revealed that there was an increased likelihood of being a nonresponder after the second mRNA injection for patients treated with antimetabolite drugs (odds ratio [OR], 5.74; 95% confidence interval [CI], 2.99–11.48; P < 0.0001) or steroids (OR, 3.68; 95% CI, 2.10–6.66; P < 0.0001), older recipients (OR, 1.03; 95% CI, 1.01–1.06; P = 0.0010), those with impaired allograft function (OR, 0.98; 95% CI, 0.96–0.99; P = 0.0011), and those with a transplant ≤4 years (OR, 2.91; 95% CI, 1.70–5.08; P = 0.0001). A total of 136 patients had a serologic assessment 1 month after the third injection (median, 30 days; quartile 1, 28 days; quartile 3, 32 days). The average time between the second and the third injection was 50 days. A total of 94 patients were positive, representing a 69.2% serologic conversion following the third mRNA injection (Figure 1 a and Table 3 ). Among patients receiving a third dose, 85 had a serologic assessment after both second and third injections, and 34 of them (40%) became seropositive between the second and the third dose. The magnitude of immune response was investigated in 71 patients who had serologic assessment using electrochemiluminescence immunoassay (Roche Elecsys) after the second and third injections (Figure 1b and c). Nearly all patients with a positive serology after the second mRNA vaccine had a high titer of anti-spike antibody (>250 UI/L). Multivariate regression analysis (Table 4 ) determined that lymphocyte count <1500/mm3 increased the likelihood of being a nonresponder after the third mRNA injection (OR, 3.84; 95% CI, 1.58–19.96; P = 0.0039), as impairment of allograft function (OR, 0.97; 95% CI, 0.94–0.99; P = 0.0232). Of note, the use of antiproliferative drugs and steroids no longer seemed to significantly impact the serologic conversion after the third mRNA vaccine injection. Male recipients were more likely to respond to the third mRNA vaccine injection in our cohort, without any clear explanation so far. Kidney transplant recipients respond poorly to COVID-19 mRNA vaccination, and although cellular responses to the vaccine seem better than humoral responses,4 severe COVID-19 pneumonia can occur following 2 mRNA vaccine injections.5 Our data support the use of a third mRNA injection to improve the humoral response to vaccination from about 50% to 70%, reducing the negative impact of antimetabolite drugs and steroids on seroconversion. Moreover, for previously seropositive patients, the third mRNA vaccine largely improved the intensity of humoral response, reaching titers suggestive of neutralizing antibody activity. Indeed, seropositive assessment (especially weak titers in immunocompromised patients) is not constantly associated with protective antibodies titers.5 However, severe lymphopenia and impaired graft function remained as risk factors for a nonserologic response. In this particular situation, a fourth dose, with or without immunomodulation, could be discussed to improve the humoral response of this highly immunosuppressed population. These data will need confirmation from other series.
Table 1.
Characteristics associated with risk of nonhumoral response to a second dose of mRNA COVID-19 vaccine after univariate analysis (n = 456)
| Characteristics | Global (n = 456) |
Negative (n = 229) |
Positive (n = 227) |
P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NA | n | % | NA | n | % | NA | n | % | ||
| Transplantation ≤4 yr | 0 | 336 | 73.7 | 0 | 146 | 63.8 | 0 | 190 | 83.7 | <0.0001 |
| Male recipient | 0 | 275 | 60.3 | 0 | 130 | 56.8 | 0 | 145 | 63.9 | 0.1455 |
| Transplant rank ≥2 | 0 | 81 | 17.8 | 0 | 40 | 17.5 | 0 | 41 | 18.1 | 0.9653 |
| Transplant type (kidney vs. SPK/pancreas) | 0 | 421 | 92.3 | 0 | 214 | 93.4 | 0 | 207 | 91.2 | 0.3845 |
| Primitive kidney disease | 0 | 0 | 0 | 0.5491 | ||||||
| Unknown | 39 | 8.6 | 15 | 6.6 | 24 | 10.6 | ||||
| Glomerulonephritis | 143 | 31.4 | 71 | 31 | 72 | 31.7 | ||||
| Other | 205 | 45 | 109 | 47.6 | 96 | 42.3 | ||||
| Vascular disease | 28 | 6.1 | 13 | 5.7 | 15 | 6.6 | ||||
| Diabetes | 41 | 9 | 21 | 9.2 | 20 | 8.8 | ||||
| Deceased donor | 0 | 376 | 82.5 | 0 | 196 | 85.6 | 0 | 180 | 79.3 | 0.1002 |
| ABDR incompatibilities >4 | 0 | 102 | 22.4 | 0 | 59 | 25.8 | 0 | 43 | 18.9 | 0.0920 |
| Blood type | 0 | 0 | 0 | 0.4043 | ||||||
| O | 192 | 42.1 | 102 | 44.5 | 90 | 39.6 | ||||
| A | 206 | 45.2 | 103 | 45 | 103 | 45.4 | ||||
| B | 46 | 10.1 | 18 | 7.9 | 28 | 12.3 | ||||
| AB | 12 | 2.6 | 6 | 2.6 | 6 | 2.6 | ||||
| Depleting induction | 1 | 239 | 52.5 | 1 | 129 | 56.6 | 0 | 110 | 48.5 | 0.1009 |
| Lymphocytes <1500/mm3 | 36 | 220 | 52.4 | 20 | 126 | 60.3 | 16 | 94 | 44.5 | 0.0017 |
| Calcineurin inhibitor treatment | 20 | 369 | 84.6 | 11 | 191 | 87.6 | 9 | 178 | 81.7 | 0.1110 |
| Belatacept treatment | 18 | 11 | 2.6 | 10 | 9 | 4.4 | 8 | 2 | 0.9 | 0.0343 |
| mTOR inhibitor treatment | 20 | 68 | 15.6 | 11 | 20 | 9.2 | 9 | 48 | 22 | <0.0001 |
| Antimetabolite treatment | 20 | 325 | 74.5 | 11 | 180 | 82.6 | 9 | 145 | 66.5 | 0.0002 |
| Steroid treatment | 20 | 150 | 34.4 | 11 | 94 | 43.1 | 9 | 56 | 25.7 | 0.0002 |
| Diabetes history | 0 | 79 | 17.3 | 0 | 46 | 20.1 | 0 | 33 | 14.5 | 0.1493 |
| Cardiovascular history | 0 | 179 | 39.3 | 0 | 94 | 41 | 0 | 85 | 37.4 | 0.4890 |
| Neoplasia history | 0 | 89 | 19.5 | 0 | 54 | 23.6 | 0 | 35 | 15.4 | 0.0375 |
| DSA before transplant | 136 | 21 | 6.6 | 47 | 16 | 8.8 | 89 | 5 | 3.6 | 0.1050 |
| DSA de novo | 6 | 56 | 12.4 | 5 | 26 | 11.6 | 1 | 30 | 13.3 | 0.6944 |
| Episode of rejection |
0 |
51 |
11.2 |
0 |
30 |
13.1 |
0 |
21 |
9.3 |
0.2479 |
|
NA |
Mean |
SD |
NA |
Mean |
SD |
NA |
Mean |
SD |
||
| Recipient age, yr | 0 | 61.4 | 12.1 | 0 | 62.7 | 11.1 | 0 | 60.2 | 13.0 | 0.0289 |
| Recipient BMI, kg/m2 | 31 | 25.4 | 4.9 | 16 | 25.5 | 4.8 | 15 | 25.4 | 5.0 | 0.7514 |
| Time from transplantation, yr | 0 | 10.5 | 8.5 | 0 | 8.5 | 7.6 | 0 | 12.4 | 8.9 | <0.0001 |
| Allograft function by MDRD, ml/min | 24 | 48.7 | 19.4 | 14 | 43.4 | 16.8 | 10 | 53.9 | 20.3 | <0.0001 |
| Total leukocyte count, G/L | 30 | 32.1 | 376.2 | 18 | 57.9 | 534.0 | 12 | 6.7 | 2.0 | 0.1653 |
| C0 cyclosporine, ng/ml | 419 | 100.6 | 35.9 | 218 | 99.5 | 50.3 | 201 | 101.1 | 29.0 | 0.9217 |
| C0 tacrolimus, ng/ml | 193 | 5.9 | 1.9 | 83 | 6.1 | 2.0 | 110 | 5.6 | 1.7 | 0.0239 |
BMI, body mass index; C0, trough level; COVID-19, coronavirus disease 2019; DSA, donor-specific antibody; MDRD, Modification of Diet in Renal Disease; mTOR, mechanistic target of rapamycin; NA, not available; SPK, simultaneous pancreas–kidney.
Values in bold are significant (P < 0.05).
Table 2.
Characteristics associated with risk of nonhumoral response to a second dose of mRNA COVID-19 vaccine after multivariate analysis (n = 394)
| Characteristics | OR | 95% CI | P value |
|---|---|---|---|
| Recipient age at vaccination, yr | 1.03 | 1.01–1.06 | 0.0010 |
| Transplantation ≤4 yr | 2.91 | 1.70–5.08 | 0.0001 |
| Allograft function by MDRD, ml/min | 0.98 | 0.96–0.99 | 0.0011 |
| Calcineurin inhibitor treatment | 1.55 | 0.73–3.30 | 0.2555 |
| mTOR inhibitor treatment | 0.73 | 0.33–1.62 | 0.4402 |
| Antimetabolite treatment | 5.74 | 2.99–11.48 | <0.0001 |
| Steroid treatment | 3.68 | 2.10–6.66 | <0.0001 |
| Lymphocytes <1500/mm3 | 1.48 | 0.93–2.35 | 0.0961 |
CI, confidence interval; COVID-19, coronavirus disease 2019; MDRD, Modification of Diet in Renal Disease; mTOR, mechanistic target of rapamycin; OR, odds ratio.
Values in bold are significant (P < 0.05).
Figure 1.
(a) Prevalence of anti-spike IgG seroconversion following 1, 2, and 3 mRNA injections in kidney transplant recipients. (b) Antibodies titers (electrochemiluminescence immunoassay by Roche Elecsys) 1 month after the second and third mRNA injections. (c) The evolution of antibody titers in previously positive patients who received a third mRNA injection.
Table 3.
Characteristics associated with risk of nonhumoral response to a third dose of mRNA COVID-19 vaccine after univariate analysis (n = 136)
| Characteristics | Global (n = 136) |
Negative (n = 42) |
Positive (n = 94) |
P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NA | n | % | NA | n | % | NA | n | % | ||
| Transplantation ≤4 yr | 0 | 46 | 33.8 | 0 | 20 | 47.6 | 0 | 26 | 27.7 | 0.0378 |
| Male recipient | 0 | 86 | 63.2 | 0 | 17 | 40.5 | 0 | 69 | 73.4 | <0.0001 |
| Transplant rank ≥2 | 0 | 20 | 14.7 | 0 | 8 | 19.0 | 0 | 12 | 12.8 | 0.4879 |
| Transplant type (kidney vs. SPK/pancreas) | 0 | 124 | 91.2 | 0 | 38 | 90.5 | 0 | 86 | 91.5 | 1.0000 |
| Primitive kidney disease | 0 | 0 | 0 | 0.6077 | ||||||
| Unknown | 10 | 7.4 | 5 | 11.9 | 5 | 5.3 | ||||
| Glomerulonephritis | 44 | 32.4 | 12 | 28.6 | 32 | 34 | ||||
| Other | 54 | 39.7 | 15 | 35.7 | 39 | 41.5 | ||||
| Vascular disease | 12 | 8.8 | 4 | 9.5 | 8 | 8.5 | ||||
| Diabetes | 16 | 11.8 | 6 | 14.3 | 10 | 10.6 | ||||
| Deceased donor | 0 | 114 | 83.8 | 0 | 37 | 88.1 | 0 | 77 | 81.9 | 0.5142 |
| ABDR incompatibilities >4 | 0 | 33 | 24.3 | 0 | 12 | 28.6 | 0 | 21 | 22.3 | 0.5167 |
| Blood type | 0 | 0 | 0 | 0.4838 | ||||||
| O | 52 | 38.2 | 20 | 47.6 | 32 | 34 | ||||
| A | 66 | 48.5 | 18 | 42.9 | 48 | 51.1 | ||||
| B | 14 | 10.3 | 3 | 7.1 | 11 | 11.7 | ||||
| AB | 4 | 2.9 | 1 | 2.4 | 3 | 3.2 | ||||
| Depleting induction | 0 | 80 | 58.8 | 0 | 29 | 69 | 0 | 51 | 54.3 | 0.1525 |
| Lymphocytes <1500/mm3 | 6 | 65 | 50 | 2 | 29 | 72.5 | 4 | 36 | 40 | 0.0012 |
| Calcineurin inhibitor treatment | 2 | 115 | 85.8 | 1 | 36 | 87.8 | 1 | 79 | 84.9 | 0.8662 |
| mTOR inhibitor treatment | 2 | 20 | 14.9 | 1 | 2 | 4.9 | 1 | 18 | 19.4 | 0.0569 |
| Antimetabolite treatment | 2 | 101 | 75.4 | 1 | 32 | 78 | 1 | 69 | 74.2 | 0.7950 |
| Steroid treatment | 2 | 43 | 32.1 | 1 | 18 | 43.9 | 1 | 25 | 26.9 | 0.0811 |
| Diabetes history | 0 | 28 | 20.6 | 0 | 11 | 26.2 | 0 | 17 | 18.1 | 0.3950 |
| Cardiovascular history | 0 | 56 | 41.2 | 0 | 17 | 40.5 | 0 | 39 | 41.5 | 1.0000 |
| Neoplasia history | 0 | 29 | 21.3 | 0 | 12 | 28.6 | 0 | 17 | 18.1 | 0.2490 |
| DSA before transplant | 35 | 8 | 7.9 | 8 | 4 | 11.8 | 27 | 4 | 6 | 0.4370 |
| DSA de novo | 5 | 17 | 13 | 2 | 6 | 15 | 3 | 11 | 12.1 | 0.8615 |
| Episode of rejection |
0 |
13 |
9.6 |
0 |
6 |
14.3 |
0 |
7 |
7.4 |
0.2207 |
|
NA |
Mean |
SD |
NA |
Mean |
SD |
NA |
Mean |
SD |
||
| Recipient age, yr | 0 | 63.7 | 11.7 | 0 | 65.3 | 10.9 | 0 | 63.0 | 11.9 | 0.2679 |
| Recipient BMI, kg/m2 | 3 | 25.5 | 4.7 | 1 | 24.5 | 3.7 | 2 | 25.9 | 5.0 | 0.0642 |
| Time from transplantation, yr | 0 | 9.4 | 8.1 | 0 | 7.0 | 6.7 | 0 | 10.4 | 8.5 | 0.0143 |
| Allograft function by MDRD, ml/min | 3 | 49.1 | 19.3 | 1 | 41.1 | 15.1 | 2 | 52.6 | 20.0 | 0.0004 |
| Total leukocyte count, G/L | 5 | 6.6 | 2.2 | 1 | 6.7 | 2.4 | 4 | 6.5 | 2.2 | 0.7306 |
| C0 cyclosporine, ng/ml | 125 | 103.0 | 25.0 | 40 | 129.0 | 29.7 | 85 | 97.2 | 21.5 | 0.3526 |
| C0 tacrolimus, ng/ml | 55 | 6.0 | 1.8 | 13 | 6.2 | 1.9 | 42 | 5.9 | 1.8 | 0.5758 |
BMI, body mass index; C0, trough level; COVID-19, coronavirus disease 2019; DSA, donor-specific antibody; MDRD, Modification of Diet in Renal Disease; mTOR, mechanistic target of rapamycin; NA, not available; SPK, simultaneous pancreas–kidney.
Values in bold are significant (P < 0.05).
Table 4.
Characteristics associated with risk of nonhumoral response to a third dose of mRNA COVID-19 vaccine after multivariate analysis (n = 129)
| Characteristics | OR | 95% CI | P value |
|---|---|---|---|
| Male recipient | 0.25 | 0.10–0.61 | 0.0027 |
| Allograft function by MDRD, ml/min | 0.97 | 0.94–0.99 | 0.0232 |
| Antimetabolite treatment | 1.76 | 0.59–5.64 | 0.3237 |
| Steroid treatment | 2.45 | 0.91–6.81 | 0.0795 |
| Lymphocytes <1500/mm3 | 3.84 | 1.58–9.96 | 0.0039 |
CI, confidence interval; COVID-19, coronavirus disease 2019; MDRD, Modification of Diet in Renal Disease; OR, odds ratio.
Values in bold are significant (P < 0.05).
References
- 1.Benotmane I., Gautier-Vargas G., Cognard N. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marion O., Del Bello A., Abravanel F. Safety and immunogenicity of anti–SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021;174:1336–1338. doi: 10.7326/M21-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller L., Andrée M., Moskorz W. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination [e-pub ahead of print]. Clin Infect Dis. Accessed September 14, 2021. [DOI] [PMC free article] [PubMed]
- 4.Cucchiari D., Egri N., Bodro M. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caillard S., Chavarot N., Bertrand D. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021;100:477–479. doi: 10.1016/j.kint.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]