Abstract
Objectives
The treatment for COVID-19 often utilizes immune-modulating drugs. These drugs are also used in immune mediated inflammatory diseases (IMIDs). We performed a systematic review about seroconversion after SARS-CoV-2 vaccination in patients with IMIDs and impact of various drugs on seroconversion rates.
Methods
Electronic databases were searched to identify relevant studies reporting seroconversion rates following SARS-CoV-2 vaccination in IMIDs. We calculated the pooled seroconversion rates after a single or two doses of vaccination, pooled seroconversion rates in patients with specific IMIDs, and rates in patients on various drugs/drug classes.
Results
Twenty-five studies were included in the systematic review. The pooled seroconversion rates after two doses of mRNA vaccination were higher (83.1, 95%CI: 74.9–89.0, I2 = 90%) as compared to a single dose (69.3, 52.4–82.3, I2 = 95%). The odds of seroconversion were lower in IMIDs as compared to healthy controls (0.05, 0.02–0.13, I2 = 21%). The seroconversion rates in patients with inflammatory bowel disease (95.2, 95%CI: 92.6–96.9, I2 = 0%), spondyloarthropathy (95.6, 95% CI: 83.4–98.9, I2 = 35%), and systemic lupus erythematosus (90.7, 95%CI: 85.4–94.2, I2 = 0%) were higher as compared to rheumatoid arthritis (79.5, 95% CI: 65.1–88.9, I2 = 85%), and vasculitis (70.5, 95% CI: 52.9–83.5, I2 = 51%). The seroconversion rates following double dose of mRNA were excellent (>90%) in those on anti-tumour necrosis factor (TNF), anti-integrin (vedolizumab), anti-IL 17 (secukinumab), anti-IL6 (Tocilizumab) and anti-IL12/23 (Ustekinumab) therapies but attenuated (<70%) in patients on anti-CD20 (Rituximab) or anti-cytotoxic T lymphocyte associated antigen (CTLA-4) therapies (Abatacept). The seroconversion rates were good (70–90%) with steroids, hydroxychloroquine, JAK inhibitors, mycophenolate mofetil and leflunomide. Combination of anti-TNF with immunomodulators (azathioprine, 6-meracptopurine, methotrexate) resulted in an attenuated vaccine response as compared to anti-TNF monotherapy.
Conclusion
Seroconversion rates after SARS-CoV-2 vaccination are lower in patients with IMIDs. Certain therapies (anti-TNF, anti-integrin, anti-IL 17, anti-IL6, anti-12/23) do not impact seroconversion rates while others (anti-CD20, anti-CTLA-4) result in poorer responses.
Keywords: Immunization, COVID-19, Adenoviral associated, Inflammatory bowel disease, Rheumatoid arthritis, Vasculitis, Spondyloarthropathy, Systemic lupus erythematosus
1. Introduction
Vaccination has emerged as an important strategy to mitigate the rates and adverse outcomes of COVID-19 infection. Various vaccines approved in different geographic regions have been shown to be efficacious in reducing infection rates and severe disease in randomized studies [[1], [2], [3]]. However, initial randomized studies of SARS-CoV-2 vaccines excluded patients with comorbidities including immune mediated inflammatory diseases (IMIDs) [[1], [2], [3]]. IMIDs may be associated with immune dysfunction related either to the underlying disease or use of immune-modulating drugs. Initially, there were concerns regarding a possible heightened risk of COVID-19 and worse outcomes of COVID-19 in IMIDs which was later refuted [4,5].Certain drugs were also recognized to adversely impact clinical outcomes in IMID patients infected with COVID-19 [[4], [5], [6]].
There is a concern that underlying IMIDs or associated use of immune modifying drugs could attenuate responses to SARS-CoV-2 vaccination. Both antibody and T cell immune responses are considered to be relevant following SARS-CoV-2 vaccination. The development of these responses and their persistence or decay in time may determine the future need for booster dosing schedules. It is unclear if patients with IMIDs (or a subgroup of them) are candidates for monitoring serologic responses. It is important to recognize the subgroups likely to be at risk of suboptimal responses with respect to underlying disease, therapies or vaccine type. Responses to other vaccines like the hepatitis B vaccine and influenza are suboptimal in patients with inflammatory bowel disease (IBD) and IMIDs [7,8]. This is especially true for patients on immunosuppressive medications. This information however may not be directly applicable to SARS-CoV-2 vaccination because of differences with respect to virus and vaccine types. The mRNA and adenoviral vector-based technologies used in SARS-CoV-2 vaccine development are relatively new and the impact of underlying IMID and immune-modulating drugs on serological responses is uncertain. Individual studies, except for a few, may typically describe responses to one type of (single or two doses) vaccine in a particular subset of patients.
In view of these uncertainties, we performed a systematic review on efficacy of SARS-CoV-2 vaccination in patients with IMIDs across the various vaccine platforms. We also attempted to clarify if the use of concomitant drugs (immunomodulators, corticosteroids, biologics, small molecules etc) had an impact on humoral responses following SARS-CoV-2 vaccination in these patients.
2. Methods
This meta-analysis was conducted in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group guidance [9].
3. Database search
We performed a search in electronic databases using PubMed and Embase from inception till 4th July 2021. The keywords used for the search included immune mediated inflammatory diseases, SARS-COV-2 and vaccination which were combined using the Boolean operator ‘AND’. The detailed search strategy is described in the Supplementary Table 1. References of eligible studies were searched for additional papers. We also searched the articles in press or ahead of print papers from major gastroenterology and immunology/ rheumatology journals to identify relevant articles (recent till July 21, 2021). Preprint servers medRxiv and bioRxiv were also searched for any additional papers. The titles retrieved from the search were combined and the duplicates were removed. Two reviewers screened the articles for relevant papers (AJ, SM). Following this, the selected articles were selected for screening of full text (AJ, SM) and any differences were resolved after discussion with a third reviewer (VS).
4. Selection of studies
We included all articles which provided data relevant to questions planned to be addressed in this systematic review. Articles were included irrespective of the format of publication i.e. original paper, abstract, letter, etc. We included studies which reported at least one of the key outcomes.
-
1)
Seroconversion rates after SARS-CoV-2 vaccination in patients with underlying immune mediated inflammatory disorders (IMIDs).
-
2)
Comparison of vaccine seroconversion rates in IMIDs when compared to control group(s).
-
3)
Studies reporting seroconversion rates in patients exposed to drugs used in IMID patients e.g. immunomodulators, corticosteroids, small molecules and/or biologics.
Any study which reported on a patient population of 5 participants or less was excluded. We also excluded studies which only provided the data on patients who did not have seroconversion after the vaccine without providing the denominator (patients vaccinated). The studies which provided only titres of anti-spike antibodies but not the seroconversion rates were also excluded. However, an effort was made to contact the authors on email to provide relevant data.
5. Data extraction
The data were extracted irrespective of the type of vaccine, number of vaccine doses or the timing when the response was measured after vaccination. The data was extracted from the relevant studies by two reviewers (AJ and SM) and any discrepancy was resolved by discussion with the third reviewer (VS). We extracted data including the details of publication (author and location of study), underlying population (type of IMID, number of participants, any healthy controls, age, sex) and current treatment including 5-aminosalicylic acid (5-ASA), hydroxychloroquine, leflunomide, immunomodulators (thiopurines, calcineurin inhibitors and methotrexate), corticosteroids(oral/intravenous), biologics (anti-tumour necrosis factor [TNF], anti-integrins, anti-CD20 etc) and small molecule inhibitors. We recorded the number of individuals who successfully seroconverted. The seroconversion rates in healthy controls and various IMIDs were also extracted where available. For each of the drugs the numbers of individuals who received the vaccination and then successfully seroconverted were also recorded.
6. Outcomes
We calculated the pooled seroconversion rates after COVID vaccination in IMIDs. The responses were calculated for seroconversion rates after single dose or two dose regimens of vaccine respectively, depending on the type of vaccine. The pooled odds for response of vaccine in IMIDs compared to healthy controls were also calculated. Pooled seroconversion rates were estimated for of the specific IMID condition (e.g. IBD, rheumatoid arthritis [RA], systemic lupus arthritis [SLE], spondyloarthropathy and vasculitis). We also calculated the pooled response rates to COVID vaccination in patients who were on a particular drug/ drug class or combinations of drugs.
A pooled analysis was performed only when at least three studies with >5 participants each were available for any individual analysis. The analyses were performed for single or two dose vaccine regimens separately as responses were known to be different depending on the number of doses. We additionally analysed adeno-associated virus based (AAV) and mRNA vaccines individually.
7. Data analysis
R statistical software version 4.0.1 was used for the analysis and in addition to the base package, meta package was used [10,11]. Pooled seroconversion rates and odds ratio were computed by random effect method with inverse variance approach. Logit transformations were made for the individual seroconversion rate before computing pooled summary. Subgroup analyses were conducted for computing pooled seroconversion based on underlying IMID, drug exposure and vaccine dosage (single vs two dose). I2 and p values were used for the assessment of heterogeneity.
8. Methodological quality and risk of bias assessment
Two of the investigators (SM and AJ) independently assessed the methodological quality and risk of bias of studies using the Joanna Briggs Institute (JBI) Critical appraisal checklist. JBI tool for case series was used to assess the studies which described the response to vaccines in patients with IMID only, without any control group or any comparison with a non-vaccinated cohort, for the criteria for inclusion, measurement of condition, reporting of baseline characteristics, reporting of outcomes and appropriateness of the statistical analysis [12]. JBI tool for case control was used to assess the studies which described the response to vaccines in patients with IMID with comparison to a control group for comparability of the two groups, measurement of exposure, identification of confounding factors, measurement of outcome variables, duration of exposure period and appropriateness of statistical analysis [13].Any discordance in quality and risk of bias assessment was resolved by mutual agreement of both the investigators in discussion with a third reviewer (VS).
9. Results
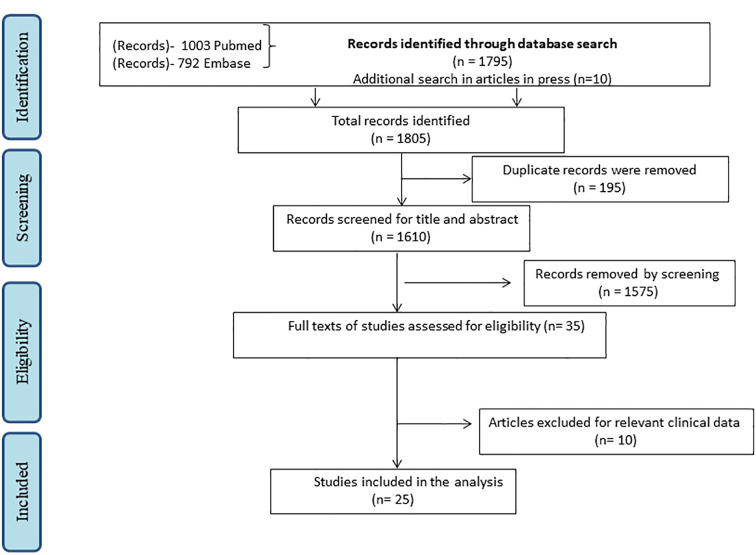
The search of the two databases yielded a total of 1795 citations of which 195 were duplicates. Additional 10 articles were identified by searching relevant journals. Eventually 35 papers underwent full text screening (Fig. 1 , PRISMA flow chart). Out of this, data from 25 studies was used for analysis. Table 1 provides the details of the included studies including site of the study, numbers vaccinated in IMIDs, underlying disease and drugs, the number and type of vaccine received [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]].Supplementary Table 2 lists the reasons for exclusion of studies [[39], [40], [41], [42], [43], [44], [45], [46], [47], [48]].
Fig. 1.
PRISMA flow chart depicting the process of screening and selection of studies for the systematic review.
Table 1.
Details of studies included in the systematic review.
| Author (Place of study) |
N | Age, Gender | Vaccinedose | Diseases | Definition of Response | Response | Response in drugs |
|---|---|---|---|---|---|---|---|
| Al-Janabi A et al.14 (UK) | 120 IMID |
Median age = 53 yrs., Females (n = 49) | mRNA+ AAV First dose |
N = 120 Psoriasis(n = 107) PsA (n = 25) RA (n = 10) SLE (n = 1) Crohn's (n = 1) |
Elecsys SARS-CoV-2 S (Roche) Antibody >0.8 U/mL at 2–12 weeks of first dose | Positive Response (n = 102) Negative Response (n = 18) |
Biological (73/81), Oral IMM (23/31) Combination (6/8) |
| Ammitzbøll C et al [15] (Denmark) |
134 SLE or RA |
Median age = 70 yrs., Female (n = 90) |
mRNA second dose |
N = 134 SLE(n = 61) RA (n = 73) |
double antigen sandwich chemiluminescent immunoassay signal/cutoff (S/CO) of 1 or more was considered positive at 1 week after the second vaccination |
RA (49/73) SLE (54/61) |
Mtx (32/46), TNFi (31/36), JAKi (6/8), Rituximab (4/17), MMF (13/16), HCQ (34/36), Steroid (27/37), Anti IL6 (6/6), abatacept (3/6), Belimumab (3/3) Leflunomide (2/5) |
| Boyarsky et al [16] (USA) | 123 RMD |
Median age = 50 yrs., Female = 117 |
mRNA first dose |
Inflammatory arthritis (n = 34), SLE (n = 24), Sjogren's (n = 16), Myositis (n = 7), Vasculitis (n = 2), Overlap (n = 35). |
Roche Elecsys anti-SARS- CoV- 2 S enzyme immunoassay (EIA) with detectable antibody after a single dose |
Over all (91/123), Inflammatory arthritis (n = 29/34), SLE (n = 16/24), Sjogren's (12/16), Vasculitis (n = 1/2), Overlap (n = 25/35). |
Non biologic AZA (9/13), HCQ (27/37), MMF (3/11), SAAZ (4/5), TAC (0/2), Leflunomide (2/4), MTX (10/13), Biologic Abatacept (3/6), Belimumab (5/10), Interleukin inhibitor (6/6), Rituximab (2/6), TNFi (16/17), Tofacitinib (2/3) |
| Braun-Moscovici et al [17] (Israel) |
264 IRD |
Mean age = 57.6 ± 13.8 yrs., Females (n = 184) | mRNA second dose |
Inflammatory joint diseases (n = 152), CTD (n = 87), Vasculitis (n = 19) |
SARS-CoV- 2 IgG II Quant (Abbott) assay based on a chemiluminescent microparticle immunoassay- test is considered positive above 50 AU/mL at 4–6 weeks after second dose |
Overall (227/264), Inflammatory joint diseases (n = 135/152), CTD (n = 70/87), Vasculitis (n = 17/19), |
MTX (68/78), MMF (17/26), Anti CD20 (24/48), Belimumab (9/11), TNFi (63/63), Anti-interleukin(39/40), Abatacept (5/8), JAKi (9/9), Combined without rituximab (65/70), Steroids (76/92) |
| Bugatti et al [18] (Italy) | 140 Inflammatory arthritis |
Mean age 55.7 ± 14.4 yrs., Females(n = 95) | mRNA first dose |
RA (n = 83), PsA (n = 29), SpA (n = 28) |
using chemiluminescent immu noassay (LIAISON SARS-CoV- 2 S1/S2 IgG; DiaSorin, SARS-CoV- 2 anti-S1 and anti-S2 IgG antibodies, with values >15 AU/mL at 21 days after first dose |
Overall (85/140), RA (n = 40/83), PsA (n = 20/29), SpA (n = 25/28) |
MTX (27/66), SAAZ (10/12), Leflunomide (3/5), Cyclosporine (0/1), TNFi (39/46), Anti-IL6 (8/14), Anti-IL 17/23 (17/19), JAKi (9/12), CTLA4ig (9/30) |
| Dailey et al [19] (USA) |
33 IBD | mRNA (n = 28)/AAV (n = 5) second dose |
IBD (n = 33) | SARS-CoV-2 Spike protein receptor binding domain (S-RBD) IgG positivity at mean of 3.3 weeks after second dose, range 1 to 10 weeks (mRNA) and mean of 3.1 weeks, range 1.6 to 3.6 weeks(AAV) |
Overall (33/33) mRNA (28/28), AAV (5/5) |
Vedolizumab (4/4) Infliximab (22/22) Infliximab+Mtx (3/3), |
|
| Furer V et al [20] (Israel) |
686 IRD and 121 controls |
Median age- 59 yrs., Females (n = 475) |
mRNA second dose |
RA, n = 263 PsA, n = 165 SpA, n = 68 SLE, n = 101 IIM, n = 19 Vasculitis, n = 70 LVV, n = 21 AAV, n = 26, Other vasculitis, n = 23 |
Seropositivity was defined as IgG ≥15 binding antibody units (BAU)/mL. measured 2–6 weeks after the second vaccine dose |
overall IRD (590/686) control (121/121) RA (216/263), PsA (160/165), SpA (67/68), SLE (93/101), IMM (7/19), LVV (20/21), AAV (8/26) other vasculitis (19/21) |
Steroids (86/130), MTX (148/178), HCQ (120/133), Leflunomide (25/28), TNFi (167/172), anti-IL6 (37/37), anti-CD20 (36/87), anti-IL 17 (47/48), Abatacept (10/16), JAKi (41/45), Belimumab (7/9), MMF (18/28) |
| Geisen et al [21] (Germany) |
26 CID and 42 controls | Mean age-50.5 yrs., Females (n = 17) | mRNA second dose |
PsA (n = 2) RA (n = 8), MCTD (n = 1), SpA (n = 3), SLE (n = 2), Psoriasis (n = 4), IBD (n = 3), Myositis (n = 1), Vasculitis (n = 1), Sarcoidosis (n = 1) |
ELISA according to manufacturer's protocol (EUROIMMUN QuantiVac) Antibody titres were assessed by ELISA before initial vaccination and 7 days after secondary vaccination. |
overall 26/26, Control 42/42 Response in all |
Steroid (7/7), Leflunomide (3/3), HCQ (3/3), AZA (1/1), SAAZ (1/1), Infliximab (3/3), Adalimumab (3/3), Golimumab (1/1), Certolizumab (3/3), Etanercept (3/3) Tocilizumab (1/1), Vedolizumab (1/1), Secukinumab (2/2), Ustekinumab (1/1) Ixekizumab (1/1) Belimumab (1/1) |
| Haberman et al [22] (USA) |
51 IMID and 26 control |
Females (n = 36) | mRNA second dose |
In the New York City cohort, direct ELISA:Titre of 5000 units or greater was used as the cut-off to determine an adequate response to vaccine |
Response (42/51) Control (25/26) |
Mtx (18/25), No MTX (24/26) |
|
| Haberman et al [22] (Germany) |
31 ‘ IMID and 179 Controls |
Females (n = 22) | mRNA second dose |
IgG antibodies —S1 domain were tested in Erlangen participants by ELISA from Euroimmun (Lübeck, Germany) on the EUROIMMUN Analyzer I platform. Adequate response was defined as greater than 5.7 nm OD |
Response (20/31) Control (179/179) |
MTX (10/20), TNFi (10/11) |
|
| Kappelman MD et al [23] (USA) |
317 IBD |
Mean age- 50.9 yrs., females (n = 238) |
mRNA second dose |
IBD (n = 317) | IgG RBD antibodies at approximately 8 weeks following completion of the vaccination using LabCorp Cov2Quant IgG™ assay Results of 1.0 g/mL or greater suggest vaccination and/or prior infection with SARS-CoV-2 |
Response in IBD (300/317) | Steroids (2/) StTNFi (101/108), Thiopurines (19/20), combination (21), 5ASA,SAAZ, budesonide and no drugs (61/65) Vedoli (46/46), Ustekinumab (38/39) |
| Kennedy et al [24] (UK) |
IBD 1293 single dose 27 IBD double dose |
Age - 43.8 (32.8–57.6) yrs., Female- 634/1288 |
mRNA/AAV Single dose mRNA double dose |
IBD (n = 1293) IBD (n = 27) |
anti-SARS- CoV- 2 spike (S) antibody concentrations, measured using the Elecsys anti-SARS- CoV- 2 spike (S) antibody assay 3–10 weeks after vaccination, in patients without prior infection. Seroconversion rates was defined by a cut-off of 15 U/mL |
Response in single dose (494/1293) Response after single dose (23/27) |
mRNA vaccine infliximab+IMM(n = 65/240), Infliximab (n = 53/147), Vedolizumab+IMM (n = 20/36), Vedolizumab (n = 124/166) AAV infliximab+IMM(n = 60/297), Infliximab (n = 50/181), Vedolizumab+IMM (n = 28/62), Vedolizumab (n = 94/164) |
| Mahil et al [25] (UK) |
84 patients psoriasis 17 control |
median age of 43 years (IQR 31–52), Females (n = 45) |
mRNA first dose |
Psoriasis (n = 77) | Immunogenicity at day 28 (±2 days) after vaccination seroconversion, assessed using ELISAs for IgG specific for the SARS-CoV-2 spike glycoprotein, and the functional capacity to neutralise both wild-type strain of SARS-CoV-2 and the B.1.1.7 variant |
Response (60/77) Control (17/17) |
methotrexate (7/15), TNF i (19/24), IL-17 i (15/15), IL-23 inhibitors (19/23) |
| Mrak et al [26] (Austria) |
74 IMID on rituximab 10 control |
Mean age 61.7 ± 13.3 years, Females (n = 57) | mRNA second dose |
IgG4-related (n = 2), Connective tissue diseases (n = 22), RA (n = 33), Vasculitis (n = 17). |
Antibodies against RBD were determined after second vaccination |
Response (29/74) IgG4-related (1/2) Connective tissue diseases (5/22), RA (13/33), Vasculitis (10/17). Control (10/10) |
Any csDMARD (16/42) MTX (10/24), MMF (2/8), HCQ (3/7, AZA (1/5), Leflunomide (2/4) SAAZ (1/1) Prednisone (8/22) |
| Deepak P et al [27] (USA) |
133 chronic inflammatory diseases 53 controls |
mean age 45.5 ± 16.0 years, Females (n = 99) |
mRNA second dose |
IBD (n = 43), Inflammatory arthritis (n = 2), RA (n = 35), SpA (n = 6), SLE (n = 13), Sjogren (n = 2), Psoriasis (n = 2), PsA (n = 5) |
anti-S IgG quantification was performed using ELISA and direct ex-vivo ELISpot assays were performed to quantify recombinant S protein-binding IgG secreting cells 96% of blood samples collected within 14 days post-vaccination |
Response (117/133), IBD (42/43), Inflammatory arthritis (2/2), RA (30/35), SpA (5/6), SLE (12/13), Sjogren (2/2), Psoriasis (1/2), PsA (5/5) Response in control (52/53) |
AZA (4/4), MMF (7/9), MTX (26/29), Leflunomide (2/2), Steroid (10/17), TNFi (35/38), Infliximab (6/6), Adalimumab (13/14), Golimumab (2/2), Abatacept (1/2), Vedolizumab (12/12), Ustekinumab (9/9), anti-IL 12/21 (10/10), Tofacitinib (10/10), Rituximab (5/6), anti- IL6(1/1), |
| Rubbert-Roth et al [28] (Switzerland) |
51 RA | mean age 64·6 (11·5) years, Females (n = 29) | mRNA First dose second dose |
RA (n = 51) | Roche Elecsys Anti-SARS-CoV-2 spike subunit 1 (S1) A lower cutoff level of >15 U/mL has been suggested, emphasising the need to establish formal cutoff levels of anti-SARS-CoV-2 antibody titres associated with protection against SARS-CoV-2 infection and severe disease. |
Response in first dose (5/51), Second dose (45/51), RA (45/51) Control (20/20) |
csDMARD (13/16), MTX (24/28), Steroid (16/17), Biologicals (9/9), Abatacept(4/5), JAKi (4/5), |
| Ruddy et al [29] (USA) |
404 RMD |
Females (n = 384) | mRNA second dose |
Myositis (n = 24), | One month after dose 2, SARS-CoV-2 antibody testing on Roche Elecsys anti-SARS-CoV-2 S EIA immunoassay measures total antibody to the SARS-CoV- 2 S RBD protein |
Response (378/404) Myositis (19/24) |
MMF (30/41), Steroid (95/116), TNFi (98/98), Rituximab (5/19) |
| Seyahi et al [30] (Turkey) |
104 IMID Control −347 |
mean age: 42.2 ± 10.0 years, Females (n = 53) |
Inactivated second dose |
RA (n = 19), SpA/IBD(n = 29), Vasculitis (n = 7), Connective tissue disease (n = 17) |
Sera at least 21 days following the second vaccination | Response (93/104) RA (15/19), SpA/IBD(28/29) Vasculitis (5/7) Connective tissue disease (14/17) Response in control (345/347) |
No drug (29/29), csDMARD (22/25), Biologicals (22/25), Rituximab (1/7), |
| Shenoy et al [31] (India) |
102 autoimmune rheumatic diseases 94 Control |
Mean age - 52(12.33) yrs., Females (n = 81) |
AAV/inactivated second dose |
Rheumatoid Arthritis (n = 38), Palindromic Rheumatism (n = 17), Inflammatory Polyarthritis (n = 16) SpA (n = 13) SLE (n = 9), Vasculitis (n = 5), Scleroderma (n = 3), Myositis (n = 1) |
IgG antibody titres to the Spike protein were estimated 1 month after the second dose. | Response (92/102) RA (35/38), Palindromic Rheumatism (16/17), Inflammatory Polyarthritis (16/16) Spondyloarthropathies (13/13), SLE (8/9), Vasculitis (3/5) Scleroderma (1/3) Myositis (0/1) Response in control (86/94) |
MTX (55/58), SAAZ (20/20), Leflunomide (8/9), HCQ, (67/71) Tofacitinib, (6/6), MMF (1/5), Tacrolimus (1/2), Azathioprine (2/2), Iguratimod (2/3), Apremilast (3/3), Rituximab (3/6), Adalimumab (0/1), Steroids (23/27) |
| Simon et al [32] (Germany) |
84 IMID Control 182 |
Mean age - 53.1 ± 17.0 years, Females (n = 55) |
mRNA first dose |
IBD (n = 8) RA (n = 25) SpA (n = 27) Psoriasis (n = 8) |
More than 10 days before serum collection were included. Optical density (OD) was determined at 450 nm (wavelength at 630 nm). A cut-off of =0.8 (OD 450 nm) was considered as positive |
Response (79/84) IBD (8/8), RA (24/25), SpA (26/27), Psoriasis (8/8) Response in control (182/182) |
No drug (23/24), csDMARD (20/20), 5ASA(1/1), HCQ (3/3), MTX (16/16), Steroid (10/10), Biologicals (35/36), TNFi (11/11), anti-IL17i (6/7), anti-IL 23 (6/6), JAKi (5/6), anti- IL6 (3/3), |
| Simon et al [33] (Germany) |
7 patients on rituximab 30 controls |
Mean age - 53.5 ± 7.7 yrs., females(n = 5) |
mRNA second dose |
RA (n = 3), Granulomatosis with polyangiitis (n = 3), Dermatomyositis (n = 1) |
Sera were collected at least 10 days after the second vaccination A cutoff of <0.8 and < 0.2 was considered as negative for IgG antibodies against spike S1 protein and nucleocapsid, respectively |
Response (0/7) Response in controls (30/30) |
Rituximab (0/7) |
| Spiera et al [34] (USA) |
89 rheumatic diseases | mean age- 61.3034 (16.081) years, Female (n = 68) | mRNA first dose |
RA (n = 23), SLE (n = 9), Sjogren (n = 10), Vasculitis (n = 19), Myositis (n = 1), PsA (n = 6), Overlap (n = 1), MCTD (n = 1), Scleroderma (n = 5) |
Sera were collected from patients who had a clinic visit from 24 February 2021 to 8 April 2021 and were serologically screened for antibodies to the SARS-CoV- 2 Spike protein. Roche Elecsys Anti-SARS- CoV-2 |
Response (68/89) RA (21/23), SLE (7/9), Sjogren's (7/10), Vasculitis (11/19), Myositis (1/1), PsA (6/6), Overlap (1/1), MCTD (1/1), Scleroderma (2/5) |
5-ASA (1/1), HCQ (17/19), AZA (3/3), MMF (4/7), MTX (12/13), Leflunomide(2/3), Steroid (12/17), Adalimumab (8/8), Etanercept (1/1), Abatacept (1/1), Secukinumab (2/2), JAKi (6/6), Rituximab (5/15), anti-IL6 (1/2), Belimumab (1/2) |
| Valor-Mendez L et al [35] (Germany) |
10 chronic inflammatory conditions 10 control |
mean age-33 ± 10 years, Females (n = 8) |
mRNA second dose |
Auto-inflammatory diseases (n = 10) | IgG antibodies against the S1 domain of the spike protein of SARS-CoV- 2 were tested by CE-certified ELISA (Euroimmun, Lübeck, Germany). Positive if OD >0.8 units |
Response (9/10) Response in control (10/10) |
Anti-IL1 (9/10) |
| Veenstra et al [36] (USA) |
8 IMID 66 Controls |
Female(n = 7) | mRNA second dose |
IBD (n = 1) RA (n = 3), SLE (n = 4), Psoriasis (n = 1), PsA (n = 1), |
sera after at least 2 weeks were recruited. Individuals with RBD levels below the 0.7 cut-off level were assigned a value of 0. |
Response (7/8) IBD (1/1), RA (2/3), SLE (3/4), Psoriasis (1/1), PsA(1/1) Response in control (66/66) |
HCQ (1/1), AZA (1/1), MMF (1/1), Steroid (1/2), Infliximab (1/1), Tofacitinib (1/1), Ixekizumab (1/1) |
| Westhoff et al [37] (Germany) |
9 14 control |
Median − 64 yrs. Female (n = 3) |
mRNA second dose |
Rituximab treated patients (n = 10) | 3 weeks after the second dose, respectively. |
Response (2/9) Response (14/14) |
Rituximab (2/9) |
| Wong et al [38] (USA) |
26 IBD | – | mRNA second dose |
IBD (n = 26) | using the Siemens Healthineers COV2T and sCOVG assays testing for total immunoglobulins and IgG, respectively, to the receptor binding domain (RBD) of the SARS-CoV-2 S protein and the Roche assay for antibodies to nucleocapsid protein Index value of 1 equals a positive test |
Response (26/26) | No drug (4/4), TNFi (8/8), Vedolizumab (12/12), Ustekinumab (2/2) |
Abbreviations – AAV: Adeno associated vector vaccine, 5ASA: 5 amino-salicylates, AZA: Azathioprine, CTLA4Ig: cytotoxic T lymphocyte associated protein-4 immunoglobulin, EIA: Enzyme Immunoassay, HCQ: Hydroxychloroquine, IBD: Inflammatory bowel disease, IMID: Immune mediated inflammatory diseases, IMM: Immunomodulator, IRD: inflammatory rheumatic diseases, JAKi- Janus kinase inhibitors, MMF: Mycophenolate mofetil, MTX: Methotrexate, PsA: Psoriatic arthritis, RA: Rheumatoid arthritis, RMD: rheumatic and muscular diseases, SAAZ: Sulfasalazine, SLE: Systemic lupus erythematosus, SpA: Spondyloarthropathy, TAC: Tacrolimus, TNFi: Tumour necrosis factor inhibitors.
10. Seroconversion after SARS-CoV-2vaccination
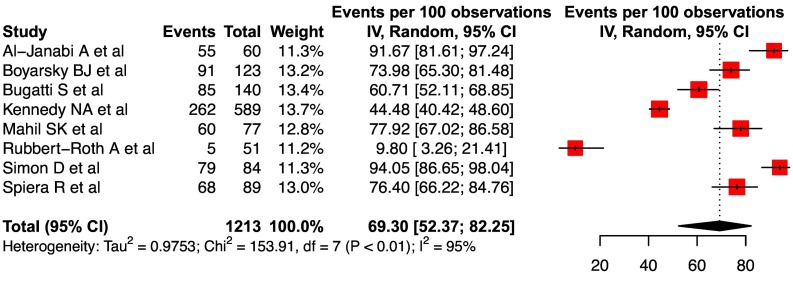
For seroconversion rates to a single dose of vaccine, ten cohorts from eight studies were considered for analysis (two studies provided data for both mRNA and AAV based vaccines) (Supplementary Table 3). However, since only two studies provided data for AAV related responses, these were excluded from analysis. The pooled seroconversion rate after a single dose of vaccine was 69.3 (95% CI, 52.4–82.3, I2 = 95%) ( Fig. 2 ). The high degree of heterogeneity in response to a single dose of mRNA vaccine could be related to various reasons including differences in the baseline population (type of IMIDs and drugs used), assessment of response (definition of seroconversion, laboratory kits used and, timing of assessment after vaccination) (Supplementary Table 3).
Fig. 2.
Forest plot showing the pooled seroconversion rate after a single dose of mRNA vaccine in IMIDs.
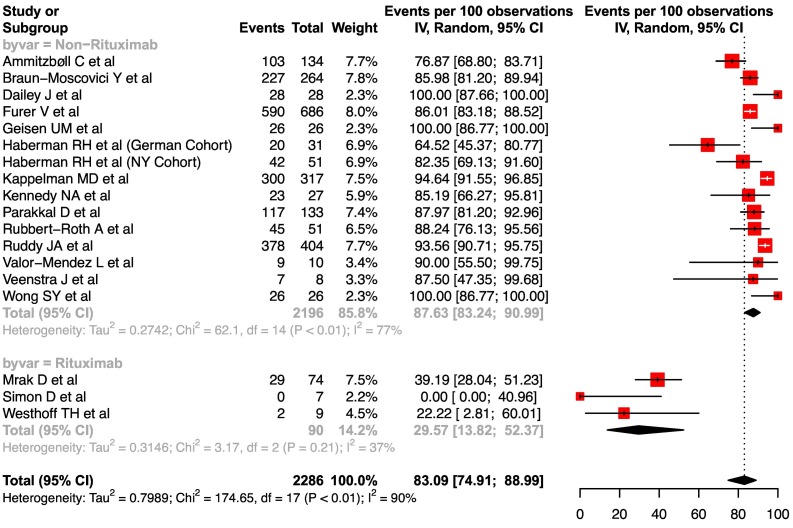
For the response to two doses of vaccination, 22 cohorts identified from 20 studies were considered. However, 4 cohorts (2 AAV related and 2 inactivated vaccines) were excluded from the analysis. Eventually, 18 cohorts with a double dose of mRNA vaccine with more than 5 participants were included for analysis (Supplementary Table 4). The pooled seroconversion rate to two doses of vaccine was 83.1 (95%CI, 74.9–89.0, I2 = 90%) (Fig. 3 ). The pooled response rate in the subgroup of patients who received rituximab was distinctively lower at 29.6 (95%CI, 13.8–52.4, I2 = 37%). The high degree of heterogeneity in response to a two doses of mRNA vaccine could be related to reasons similar to those listed above for the single dose analysis (Supplementary Table 4).
Fig. 3.
Forest plot showing the pooled seroconversion rate after two doses of mRNA vaccine in IMIDs.
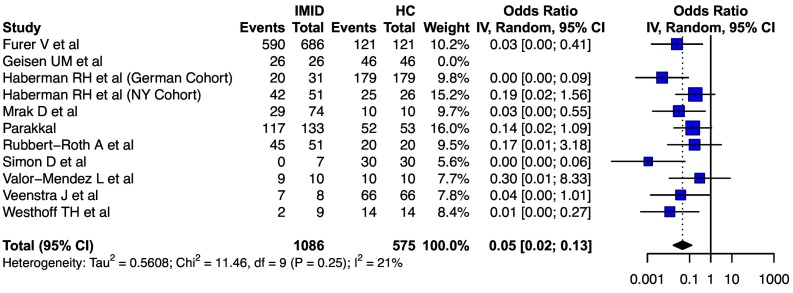
For the comparison of seroconversion rates in patients with IMID vs. healthy controls, 13 cohorts from 12 studies provided relevant data. Two cohorts (inactivated or AAV related) were excluded from analysis (Supplementary Table 5). Of the 11 cohorts included, the pooled odds of seroconversion were significantly lower in individuals with IMIDs (0.05, 95% CI: 0.02–0.13, I2 = 21%) ( Fig. 4 ). For this comparative analysis, all the studies included reported seroconversion rates after the two dose regimen of mRNA vaccine.
Fig. 4.
Forest plot showing the pooled odds ratio of seroconversion after SARS-CoV-2 vaccination in patients with IMIDs as compared to healthy controls.
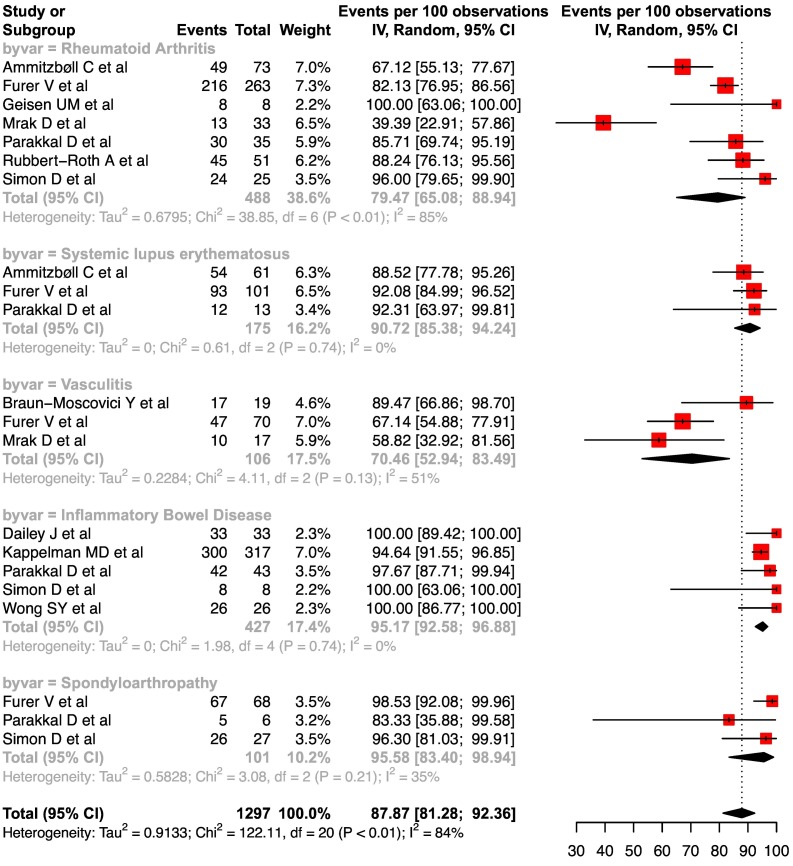
11. Disease specific seroconversion rates
The pooled seroconversion rates for various IMIDs were: rheumatoid arthritis at 79.5 (95%CI, 65.1–88.9, I2 = 85%), systemic lupus erythematosus at 90.7 (95%CI, 85.4–94.2, I2 = 0%), vasculitis at 70.5 (95%CI, 52.9–83.5, I2 = 51%), IBD at 95.2 (95%CI, 92.6–96.9, I2 = 0%) and spondyloarthropathy at 95.6 (95%CI, 83.4–98.9, I2 = 35%), respectively ( Fig. 5 ). For this analysis, the studies which reported use of AAV or inactivated vaccines or response to single dose of mRNA vaccine were excluded.
Fig. 5.
Forest plots depicting the pooled seroconversion rates after two doses of mRNA vaccine in IMID subtypes.
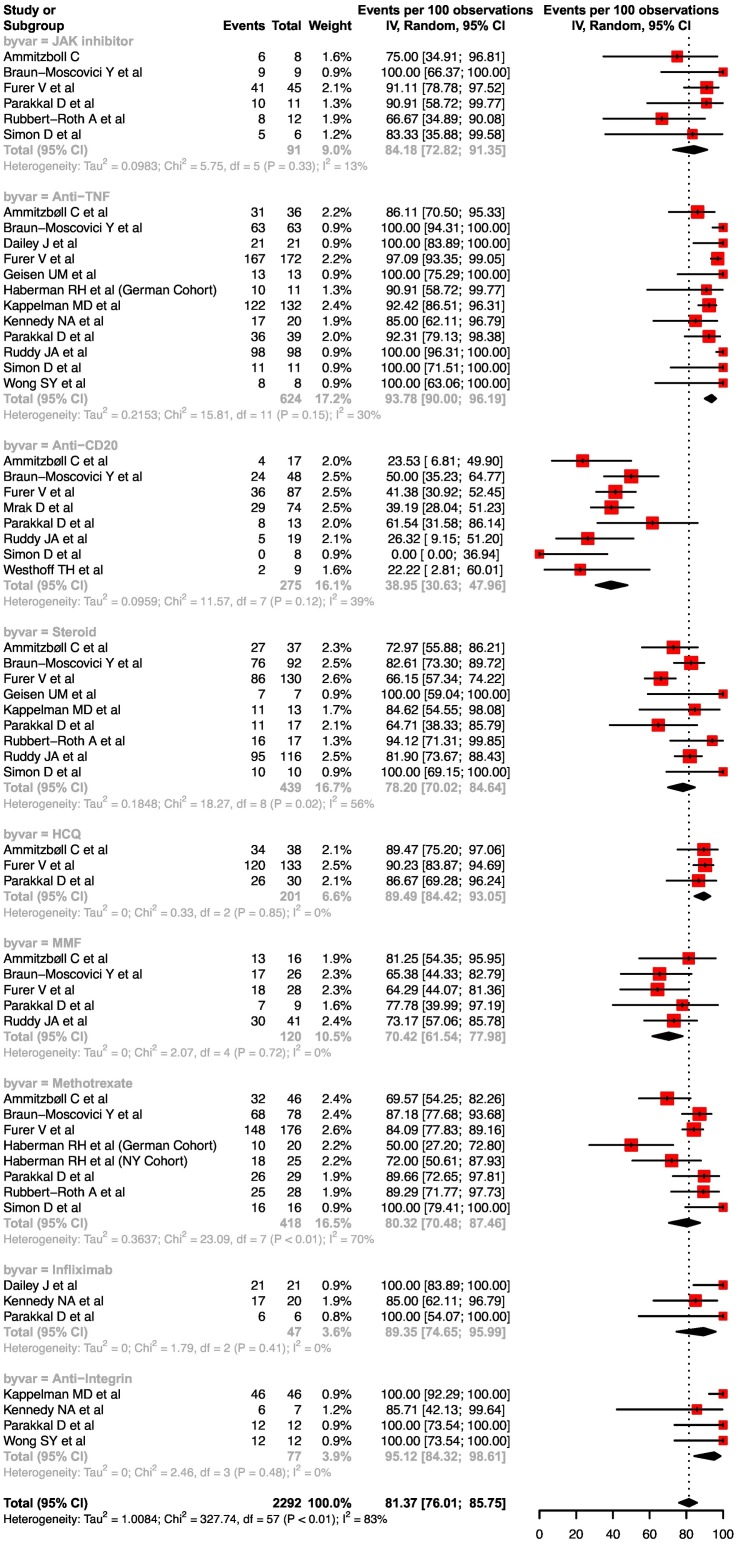
12. Impact of drugs on seroconversion rates
For a single dose of mRNA vaccine, there were only two drugs which had three studies available for analysis for rates of seroconversion- TNF-alpha inhibitors (anti-TNF) and methotrexate. The pooled rates of seroconversion with single dose mRNA vaccine on anti-TNF and methotrexate were 67.4 (95%CI, 36.8–88.0, I2 = 94%) and 62.2 (95%CI, 36.9–82.2, I2 = 73%) respectively (Supplementary Fig. 1).
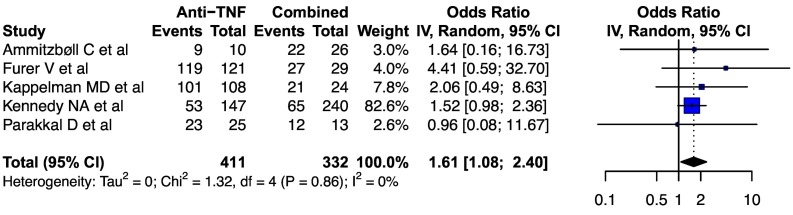
The pooled rates of seroconversion with double dose mRNA vaccine on steroids, mycophenolate mofetil, methotrexate and hydroxychloroquine were 78.2 (95%CI, 70.0–84.6, I2 = 56%), 70.4 (95%CI, 61.5–78.0, I2 = 0%), 80.3 (95%CI, 70.5–87.5, I2 = 70%) and 89.5 (95%CI, 84.4–93.1, I2 = 0%) respectively. For infliximab, the pooled rate of seroconversion with double dose mRNA vaccine was 89.4 (95%CI, 74.7–96.0, I2 = 0%). For TNF inhibitors, the pooled rates of seroconversion with double dose mRNA vaccine were 93.8 (95%CI, 90.0–96.2, I2 = 30%), for anti-CD20 drugs: 39.0 (95%CI, 30.6–48.0, I2 = 39%), anti-integrin: 95.1 (95%CI, 84.3–98.6, I2 = 0%) and for JAK inhibitors: 84.2 (95%CI, 72.8–91.4, I2 = 13%) respectively ( Fig. 6 ).The pooled odds of seroconversion with TNF inhibitor monotherapy were higher than the combination of TNF inhibitor and an immunomodulator [1.61 (95%CI, 1.08–2.40, I2 = 0%)] ( Fig. 7 ).
Fig. 6.
Forest plots depicting the pooled seroconversion rates after two doses of mRNA vaccine after various drug exposures.
Fig. 7.
Forest plot depicting the pooled odds ratio of seroconversion after SARS-CoV-2 vaccination in patients receiving anti-TNF monotherapy as compared to anti-TNF in combination with an immunomodulator.
13. Other drugs
Anti-IL 17 drugs did not appear to adversely affect the seroconversion rates after double dose of mRNA vaccination (Supplementary Table 6). Similarly anti-IL-6 drugs also did not impact seroconversion rates after double dose of COVID-19 vaccine. The response to Belimumab (anti B cell-activating factor) seemed to have a slightly lower response rate. Abatacept (analog of cytotoxic T cell lymphocyte antigen i.e. CTLA-4) was associated with a poor response to vaccination. Data for double dose mRNA response to patients on 5-aminosalicylates was limited but seemed to suggest a good response. Seroconversion rates in patients on leflunomide were slightly impaired. Responses to vaccination in patients on Ustekinumab were not impaired.
Supplementary Table 7 summarizes the expected seroconversion after a two dose regimen of mRNA vaccine across the various drugs used in the treatment of IMIDs.
14. Risk of bias
Risk of bias assessment was performed for the included studies using JBI critical appraisal checklists. Ten studies were assessed using the case series checklist and 15 studies were assessed using the case control checklist, details of which are described in Supplementary Tables 8 and 9. Since the Joanna Briggs guidance suggests against using a score cut off for quality assessment, we also did not score the studies.
15. Discussion
The results of the present systematic review suggest that the seroconversion rates after SARS-CoV-2 vaccination in patients with IMIDs are lower than among the healthy controls. As expected, seroconversion rates are higher after a two-dose regimen of mRNA vaccine platform when compared to a single dose. Among patients with IMIDs who received a two-dose regimen of mRNA vaccine, exposure to anti-CD20 therapies resulted in a much lower seroconversion rate as compared to other groups of drugs. Among the drugs, two doses of mRNA vaccine were associated with good (>90%) seroconversion rates in 5-aminosalicylates, anti-TNF, anti-integrin, anti-IL-6, anti-IL 12/23, and anti-IL 17. Certain other drugs like corticosteroids, hydroxychloroquine, methotrexate, JAK inhibitors, belimumab, leflunomide and mycophenolate mofetil, were associated with slightly lower (70–90%) seroconversion rates. As expected, anti-CTLA-4 therapies were also associated with poor seroconversion rates. Furthermore, a combination of biologics and immunomodulators (anti-TNF and methotrexate or thiopurines) resulted in an attenuation of immunologic response over and above that of anti-TNF monotherapy.
SARS-CoV-2 shares similarities with autoimmune disorders in pathogenesis and immune responses [49]. There is activation of both innate as well as adaptive immune cells [50]. Immune mediated hemolysis, decreased leukocyte counts, cytokine storm, procoagulant state and macrophage activation are similar to both. Various autoantibodies have been detected with SARS-CoV-2 infection. Antigen mimicry might have a role between viral proteins and human proteins. Virus disturbs the self tolerance and accentuates the immune pathways through molecular mimicry with host proteins. Diseases like immune mediated thrombocytopenia, anti-phospholipid syndrome, Guillain-Barre syndrome have all been witnessed secondary to SARS-CoV-2 infection [51]. This similarity with auto-immune diseases is also supported by the fact that some drugs used to treat autoimmune diseases have effect against SARS-CoV-2 infection.
The present systematic review highlights the importance of a two-dose mRNA vaccine regimen in patients with IMIDs. The response to a single dose of either mRNA or AAV based vaccines were attenuated in patients with IMIDs [24].However, the response rates improved following second dose of vaccination. This needs to be considered in policy decisions in relation to the timing of the second dose of vaccines. Due to vaccine shortage, some governments have increased the interval between first and second dose of the vaccine. Our data suggests that this approach may not be appropriate for patients with underlying IMIDs.
Another issue is the attenuated response to even double dose of vaccination in patients on certain immune modulating drugs. Our results make a strong case for assessing seroconversion in patients who are on anti-CD20 or anti-CTLA-4 therapies. Our data do not indicate the need assess antibody responses in patients on TNF inhibitors, anti -integrins or JAK kinase inhibitors. Furthermore, lower response rates were seen in patients with rheumatoid arthritis and vasculitis as compared to SLE, IBD and spondyloarthropathy. It is unclear whether this is attributable to the underlying disease or to the differences in therapies for these diseases.
The systematic review has a few limitations: the heterogeneity of individual therapies and combination of therapies in IMIDs meant comparative effectiveness of vaccinations in different IMIDs could not be ascertained with certainty. Furthermore, there was heterogeneity in respect to type of vaccine (AAV, mRNA based or inactivated), number of doses (single or double) and the definition and time of measurement of seroconversion. We attempted to provide estimates for two doses of mRNA vaccination to ensure homogeneity, but these results may not be applicable to other vaccines. Finally, while the systematic review addresses the question of serological responses, the impact of this on breakthrough SARS-CoV-2 is uncertain particularly since there is sparse data on T cell responses following SARS- CoV-2 vaccination in patients with IMIDs. Furthermore, the antibody decay and thereby the durability of the responses is not clear. A more recent work suggests that anti-TNF therapy may be associated with lower antibody titers even after two doses of COVID-19 vaccine, and the titers decay much faster as compared to anti-integrins [52].
In conclusion, the present systematic review demonstrates a reduced seroconversion to SARS-CoV-2 vaccination in patients with IMIDs. We also identify the subgroup of patients who may require assessment of seroconversion after SARS-CoV-2 vaccination in view of higher risk of non-response.
Funding
None.
Declaration of Competing Interest
None.
PD has served as a consultant or on an advisory board for Janssen, Pfizer, Prometheus Biosciences, Boehringer Ingelheim, AbbVie, Arena Pharmaceuticals and Scipher Medicine Corporation. He has also received funding under a sponsored research agreement unrelated to the data in the paper from Takeda Pharmaceutical, Arena Pharmaceuticals, Bristol Myers Squibb-Celgene, and Boehringer Ingelheim.
AHJK participated in advisory boards and educational speaker events for Exagen Diagnostics Inc. and Aurinia Pharmaceuticals Inc., research grant, advisory boards, and educational speaker events for GlaxoSmithKline, advisory boards for Alexion Pharmaceuticals Inc., and consulting work for Annexon Biosciences.
SS holds research grants from Biogen, Takeda, Pfizer, Janssen,AbbVie, Tillotts Pharma, Ferring and Biohit; served on the advisory boards of Takeda, AbbVie, Merck, Ferring, Pharmacocosmos, Warner Chilcott, Janssen, Falk Pharma, Biohit, TriGenix, Celgene and Tillots Pharma; and has received speaker fees from AbbVie, Takeda, Celltrion, Pfizer, Biogen, AbbVie, Janssen, Merck, Warner Chilcott and Falk Pharma.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.autrev.2021.102927.
Appendix A. Supplementary data
Supplementary material
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416. doi: 10.1056/NEJMoa2035389. Epub 2020 Dec 30. PMID: 33378609; PMCID: PMC7787219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021 Dec 19;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. Epub 2020 Nov 19. PMID: 33220855; PMCID: PMC7674972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh A.K., Jena A., Kumar-M P., Sharma V., Sebastian S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. United European Gastroenterol J. 2021;9(2):159–176. doi: 10.1177/2050640620972602. Epub 2021 Mar 23. PMID: 33210980; PMCID: PMC8250629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attauabi M., Seidelin J.B., Felding O.K., Wewer M.D., Vinther Arp L.K., Sarikaya M.Z., et al. Coronavirus disease 2019, immune-mediated inflammatory diseases and immunosuppressive therapies - A Danish population-based cohort study. J Autoimmun. 2021;118:102613. doi: 10.1016/j.jaut.2021.102613. Epub 2021 Feb 12. PMID: 33592545; PMCID: PMC7879155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungaro R.C., Brenner E.J., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D., et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021 Apr;70(4):725–732. doi: 10.1136/gutjnl-2020-322539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macaluso F.S., Liguori G., Galli M. Vaccinations in patients with inflammatory bowel disease. Dig Liver Dis. 2021 doi: 10.1016/j.dld.2021.05.015. Jun 8:S1590–8658(21)00267-X. Epub ahead of print. PMID: 34116972. [DOI] [PubMed] [Google Scholar]
- 8.Rahier J.F., Moutschen M., Van Gompel A., Van Ranst M., Louis E., Segaert S., et al. Vaccinations in patients with immune-mediated inflammatory diseases. Rheumatology (Oxford) 2010;49(10):1815–1827. doi: 10.1093/rheumatology/keq183. Epub 2010 Jun 29. PMID: 20591834; PMCID: PMC2936949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000 Apr 19;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A language and environment for statistical computing.https://www.R-project.org/ URL. [Google Scholar]
- 11.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019 Nov;22(4):153–160. doi: 10.1136/ebmental-2019-300117. 31563865 Epub 2019 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munn Z., Barker T.H., Moola S., Tufanaru C., Stern C., McArthur A., et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–2133. doi: 10.11124/JBISRIR-D-19-00099. 33038125 [DOI] [PubMed] [Google Scholar]
- 13.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. In: JBI Manual for Evidence Synthesis. Aromataris E., Munn Z., editors. JBI; 2020. Chapter 7: Systematic reviews of etiology and risk. [Google Scholar]
- 14.Al-Janabi A., Littlewood Z., Griffiths C.E.M., Hunter H.J.A., Chinoy H., Moriarty C., et al. Antibody responses to single-dose SARS-CoV-2 vaccination in patients receiving immunomodulators for immune-mediated inflammatory disease. Br J Dermatol. 2021 doi: 10.1111/bjd.20479. May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ammitzbøll C., Bartels L.E., Bøgh Andersen J., RisbølVils S., ElbaekMistegård C., Dahl Johannsen A., et al. Vaccine in patients with systemic lupus Erythematosus and rheumatoid arthritis. ACR Open Rheumatol. 2021 Jul 17 doi: 10.1002/acr2.11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyarsky B.J., Ruddy J.A., Connolly C.M., Ou M.T., Werbel W.A., Garonzik-Wang J.M., et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1098–1099. doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun-Moscovici Y., Kaplan M., Braun M., et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Annals of the Rheumatic Diseases Published Online First. 2021 doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- 18.Bugatti S., De Stefano L., Balduzzi S., Greco M.I., Luvaro T., Cassaniti I., et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220862. [DOI] [PubMed] [Google Scholar]
- 19.Dailey J., Kozhaya L., Dogan M., Hopkins D., Lapin B., Herbst K., et al. Antibody Responses to SARS-CoV-2 after Infection or Vaccination in Children and Young Adults with Inflammatory Bowel Disease. medRxiv [Preprint] 2021 Jun 15 doi: 10.1093/ibd/izab207. 2021.06.12.21258810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 21.Geisen U.M., Berner D.K., Tran F., Sümbül M., Vullriede L., Ciripoi M., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haberman R.H., Herati R., Simon D., et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Annals of the Rheumatic Diseases Published Online First. 25 May 2021 doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kappelman M.D., Weaver K.N., Boccieri M.E., Firestine A., Zhang X., Long M.D., et al. Humoral Immune Response to mRNA COVID019 Vaccines among Patients with IBD. Gastroenterology. 2021 Jun 15 doi: 10.1053/j.gastro.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy N.A., Lin S., Goodhand J.R., et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD [published online ahead of print, 2021 Apr 26] Gut. 2021 doi: 10.1136/gutjnl-2021-324789. [DOI] [PubMed] [Google Scholar]
- 25.Mahil S.K., Bechman K., Raharja A., Domingo-Vila C., Baudry D., Brown M.A., et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021 Jul;8 doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mrak D., Tobudic S., Koblischke M., et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Annals Rheumat Dis Published Online First. 20 July 2021 doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 27.Deepak P., Kim W., Paley M.A., Yang M., Carvidi A.B., El-Qunni A.A., et al. Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of mRNA Vaccines to SARS-CoV-2. medRxiv [Preprint] 2021 Apr 9 2021.04.05.21254656. [Google Scholar]
- 28.Rubbert-Roth A., Vuilleumier N., Ludewig B., Schmiedeberg K., Haller C., von Kempis J. Anti-SARS-CoV-2 mRNA vaccine in patients with rheumatoid arthritis. Lancet Rheumatol. 2021 Jul;3(7):e470–e472. doi: 10.1016/S2665-9913(21)00186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruddy J.A., Connolly C.M., Boyarsky B.J., Werbel W.A., Christopher-Stine L., Garonzik-Wang J., et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220656. [Preprint]; May 24:annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seyahi E., Bakhdiyarli G., Oztas M., Kuskucu M.A., Tok Y., Sut N., et al. Antibody response to inactivated COVID-19 vaccine (CoronaVac) in immune-mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int. 2021 Aug;41(8):1429–1440. doi: 10.1007/s00296-021-04910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shenoy P., Ahmed S., Cherian S., Paul A., Shenoy V., et al. Immunogenicity of the ChAdOx1 nCoV-19 and the BBV152 vaccines in patients with autoimmune rheumatic diseases. Medrxiv. 2021 doi: 10.1101/2021.06.06.21258417. [DOI] [Google Scholar]
- 32.Simon D., Tascilar K., Fagni F., Krönke G., Kleyer A., Meder C., et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon D., Tascilar K., Schmidt K., Manger B., Weckwerth L., Sokolova M., et al. Brief report: Humoral and cellular immune responses to SARS-CoV-2 infection and vaccination in B cell depleted autoimmune patients. Arthritis Rheum. 2021 Jul;1 doi: 10.1002/art.41914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiera R., Jinich S., Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 35.Valor-Méndez L., Tascilar K., Simon D., Distler J., Kleyer A., Schett G., et al. Correspondence on ‘Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort’. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220898. [DOI] [PubMed] [Google Scholar]
- 36.Veenstra J., Wang J., McKinnon-Maksimowicz K., Liu T., Zuniga B., Hamzavi I., et al. Correspondence on ‘Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort’. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westhoff T.H., Seibert F.S., Anft M., Blazquez-Navarro A., Skrzypczyk S., Doevelaar A., et al. Correspondence on ‘SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response’. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220756. [DOI] [PubMed] [Google Scholar]
- 38.Wong S.Y., Dixon R., Martinez Pazos V., Gnjatic S., Colombel J.F., Cadwell K., et al. Serologic response to messenger RNA coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology. 2021;161(2):715–718. doi: 10.1053/j.gastro.2021.04.025. Apr 20:S0016–5085(21)00648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonelli M., Aletaha D. Response to: ’Correspondence on ’SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response by Westhoff et al. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220764. [DOI] [PubMed] [Google Scholar]
- 40.Connolly C.M., Boyarsky B.J., Ruddy J.A., Werbel W.A., Christopher-Stine L., Garonzik-Wang J.M., et al. Absence of Humoral response after two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases: a case series. Ann Intern Med. 2021 May;25:M21–1451. doi: 10.7326/M21-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damiani G., Allocco F., Young Dermatologists Italian Network, Malagoli P. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers [published online ahead of print, 2021 Mar 5] Clin Exp Dermatol. 2021;46(6):1106–1108. doi: 10.1111/ced.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furer V., Zisman D., Kibari A., Rimar D., Paran Y., Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford) 2021 doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan N., Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021;161(3):827–836. doi: 10.1053/j.gastro.2021.05.044. May 25:S0016–5085(21)03066–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramirez G.A., Della-Torre E., Moroni L., Yacoub M.R., Dagna L., OSR-COVAX study group Correspondence on ‘Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort’. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220539. [DOI] [PubMed] [Google Scholar]
- 45.Pacifico A., d’Arino A., Pigatto P.D.M., Malagoli P. Young Dermatologists Italian Network, Damiani G. COVID-19 vaccines do not trigger psoriasis flares in patients with psoriasis treated with apremilast. Clin Exp Dermatol. 2021 May 10 doi: 10.1111/ced.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rimar D., Slobodin G., Paz A., Henig I., Zuckerman T. SARS-COV-2 vaccination after stem cell transplantation for scleroderma. Ann Rheum Dis. 2021 May 28 doi: 10.1136/annrheumdis-2021-220677. [DOI] [PubMed] [Google Scholar]
- 47.Salviani C., Scolari F., Alberici F. Correspondence on ‘Immunogenicity and safety of anti-SARS-Cov-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort’. Ann Rheum Dis. 2021 May 28 doi: 10.1136/annrheumdis-2021-220496. [DOI] [PubMed] [Google Scholar]
- 48.Watad A., De Marco G., Mahajna H., Druyan A., Eltity M., Hijazi N., et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 2021 Apr 29;9(5):435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021 Mar 1;33(2):155–162. doi: 10.1097/BOR.0000000000000776. PMID: 33332890; PMCID: PMC7880581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raucci F., Mansour A.A., Casillo G.M., Saviano A., Caso F., Scarpa R., et al. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmun Rev. 2020 Jul;19(7):102572. doi: 10.1016/j.autrev.2020.102572. Epub 2020 May 3. PMID: 32376393; PMCID: PMC7252120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020 Aug;19(8):102597. doi: 10.1016/j.autrev.2020.102597. Epub 2020 Jun 11. PMID: 32535093; PMCID: PMC7289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simeng Lin, Nicholas A Kennedy, Aamir Saifuddin et al. Covid-19 vaccine-induced antibodies are attenuated and decay rapidly in infliximab treated patients, 30 July 2021, PREPRINT (Version 1) available at Research Square doi:10.21203/rs.3.rs-755879/v1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material