Abstract
Nitric oxide (NO) plays an important role in cardiovascular and immune systems. Quantification of blood nitrite and nitrate, two relatively stable metabolites of NO (generally as NOx), has been acknowledged, in part, representing NO bioactivity. Dysregulation of NOx had been reported in SARS-CoV-2 infected populations, but whether patients recovered from COVID-19 disease present with restored NOx is unknown. In this study, serum NO2− and NO3− were quantified and analyzed among 109 recovered adults in comparison to a control group of 166 uninfected adults. Nitrite or nitrate levels were not significantly different among mild-, common-, severe- and critical-type patients. However, these recovered patients had dramatically lower NO2− and NO2−/NO3− than the uninfected group (p < 0.0001), with significantly higher NO3− levels (p = 0.0023) than the uninfected group. Nitrate and nitrite/nitrate were positively and negatively correlated with patient age, respectively, with age 65 being a turning point among recovered patients. These results indicate that low NO2−, low NO2−/NO3− and high NO3− may be potential biomarkers of long-term poor or irreversible outcomes after SARS-CoV-2 infection. It suggests that NO metabolites might serve as a predictor to track the health status of recovered COVID-19 patients, highlighting the need to elucidate the role of NO after SARS-CoV-2 infection.
Keywords: COVID-19, Nitric oxide, Nitrite, Nitrate, Biomarker, Long-term effect
Graphical abstract
1. Introduction
COVID-19 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused substantial human life losses. As of August 22, 2021, the total number of confirmed infected cases of COVID-19 stood at 211 million worldwide, with a death toll of 4.4 million (from the Johns Hopkins Center for Systems Science and Engineering) [1]. The possibility of developing a severe case of disease and the mortality rate are reported to be influenced substantially by age and the presence of comorbidities, as well as sex [[2], [3], [4]]. Estimates of the influence of age using data from 16 countries suggest that people aged 65 years and older were more susceptible to SARS-CoV-2 infection, with a higher admission rate to intensive care units (ICUs) and a higher fatality rate than younger individuals [5]. Multiple reasons for this age-related susceptibility have been proposed including a link to metabolic, respiratory, or cardiovascular comorbidities [[6], [7], [8], [9], [10], [11]]. For example, COVID-19 patients with existing hypertension have been reported to be more vulnerable to severe disease progression and mortality [7,12], with evidence supported by numerous further larger studies and recent meta-analyses of 24 studies from across the globe [13]. Moreover, substantial organ dysfunction among recovered COVID-19 patients has been increasingly reported, which is both unexpected and concerning [[14], [15], [16]].
The mechanisms underlying these links are hotly debated. However, it is accepted that vascular dysfunction and impaired bioavailability of protective nitric oxide (NO) plays a key role in the progression of relevant co-morbidities including in patients with COPD, hypertension and other vascular diseases [17]. Besides its role as an important vasodilator, NO also functions as a vital immune mediator, exerting non-specific antiviral effects. S-nitroso-N-acetylpenicillamine (SNAP), a NO donor, has been reported to improve host cell survival following infection in one study [18,19], and suppress the replication cycle of severe acute respiratory syndrome (SARS-CoV-1) at RNA and cellular levels and interfere with spike protein activity in another study [20]. More recently, NO has been shown to exert inhibitory effects on SARS-CoV-2 entry into cells and replication [21]. Indeed, SNAP was found to delay or prevent viral cytopathic effects of SARS-CoV-2 in vitro via covalent binding and inhibition by released NO [22]. Currently, a few clinical trials of iNO are underway, for the purpose of prevention or therapy of COVID-19 disease [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32]], but its precise role in COVID-19 pathogenesis has not been fully elucidated yet.
Despite the promise of iNO therapy, systematic evaluations are still needed for safety and efficacy of inhaled nitric oxide (iNO). Targeting NO is a double-edged sword, depending on where, how and how much of it is produced [33]. The effects described above of NO are evidenced with high concentrations of ~30–200 μM. In a small study, detection of nitrite (NO2 −) and nitrate (NO3 −) (commonly referred as NOx) using photometric analysis or the Griess assay was shown to be elevated, suggesting that NO levels were increased, in 11 critically ill non-surviving COVID-19 patients compared to 45 survivors [34]. However, conversely, in several reports, decreased basal NO production or bioavailability (normally in the 100–400 nM range) has been suggested to underlie elevated apoptosis of endothelial cells induced by SARS-CoV-2 infection, increased inflammation, or excessive reactive oxygen species (ROS) [23,24,35]. One recent study reported decreased total NO, nitrite and SNO in COVID-19 patients in hospital [36]. Another study in Spain showed that nitric oxide diffusion was reduced in around 40% patients discharged from hospital, via respiratory function tests with a MasterScreen PFT equipment [37]. To date, the levels of NO in blood among patients discharged from hospitals, i.e. “recovered patients”, remain unknown.
To determine whether alternations in NO metabolite levels might be associated with so-called “long COVID”, we measured serum nitrite and nitrate of COVID-19 recovered patients after being discharged from hospitals for at least 4 months. Since the clinical spectrum of SARS-CoV-2 infection is broad, 113 recruited recovered patients were categorized in this study, according to reported guidelines [38]. Another 175 adults who did not get infected with SARS-CoV-2 were recruited as a control uninfected group. Sex and age factors were evaluated in these two groups. Since food is one source of nitrite and nitrate for human and it is extremely challenging to execute a clinical trial of diet on COVID-19 patients in hospital, we compared serum NOx between two small groups of healthy adults. Another 45 male adults were recruited as a diet-controlled group, who lived for two weeks with the same meal plan as hospitalized patients were, and whose serum NOx levels were compared with age-matched men in the uninfected group. After exclusion of unqualified volunteers, remaining 109 recovered patients, 166 control uninfected adults and 31 diet-controlled men were enrolled in this study.
2. Materials and methods
2.1. Subject recruitment
A total of 113 recovered patients (Recovered), 175 uninfected healthy adults (Uninfected) and 45 healthy diet-regulated male adults (Diet-controlled) were recruited at Hubei Provincial Center for Disease Control and Prevention (Wuhan, Hubei, China). The Case Number is KX200628613 (The Ethical Committee of Chinese Center for Disease Control and Prevention, Beijing, China). Patients were considered as recovered when they met the COVID-19 discharge criteria and were released from the hospital, according to established guidelines [38]. Blood samples were collected for nitrite and nitrate analyses. Each participant gave informed consent prior to participation in the study.
2.2. Meal plans
In order to evaluate the potential effect of diet on serum NOx, healthy male adults were fed the same dining menu for two weeks that COVID-19 patients received in hospitals before blood collection. Breakfast options included boiled eggs, milk, steamed buns, and noodles, which was delivered at 8:00–8:30 a.m. every morning. Lunch and dinner options included pork meat, chicken meat, cabbage, and green beans, and were delivered at 12:00–1:00 p.m. and 5:30–6:00 p.m., respectively.
2.3. Serum sampling
In order to minimize the interference of food and drink on endogenous serum nitrite and nitrate, blood was collected after overnight fasting, and no beverages were allowed overnight except for limited amount of water. Samples were obtained in the morning between 8:00 and 10:00 a.m. The tube containing whole blood remained static on the bench for 50 min, after which serum was isolated from blood via centrifugation at 4000g for 5 min at room temperature (RT). Serum was then put on dry ice and transported to the lab for immediate analysis, or stored at −80 °C for next-step management.
2.4. Pretreatment of serum specimens
Serum specimens were prepared via three different methods [39]: 1) precipitation with sodium hydroxide (NaOH) plus zinc sulfate (ZnSO4): a 0.1 mL serum sample was mixed with 0.2 mL NaOH (0.5 M) and 0.2 mL ZnSO4 (10% in water), the mixture vortexed for 30 s, sat for 15 min at RT and centrifuged at 13,000 g for 5 min before analysis; 2) precipitation with ethanol: a 0.2 mL serum sample was mixed with 0.4 mL cold ethanol, the mixture was kept on ice for 30 min, and then centrifuged at 13,000 g for 5 min before analysis; 3) omission of pre-treatment: serum samples were directly injected for analysis.
2.5. Measurement of serum nitrite and nitrate
Chemiluminescence analysis has been widely recognized as a reliable method for nitrite and nitrate quantification in biological fluids [[40], [41], [42]]. Based on the highly sensitive ozone-chemiluminescence method, its sensitivity reaches 1 pmol (1 nM for a 1 mL injection) for liquid samples. A Nitric Oxide Analyzer 280i (NOA 280i, GE) was applied to measure the levels of nitrite and nitrate in this study. Reducing reagents employed were the triiodide ion for nitrite detection, and vanadium trichloride (VCl3) solution containing 1 M HCl for nitrate quantification. Duplicate injections of standard solutions into the NOA purge vessel were performed. The NOA instrument passed daily calibration when calibration curves were obtained with R2 > 0.999.
Comparison among the three pretreatment methods was performed via injection of each sample six times in order to evaluate the recovery ratio and RSD. Detection of each serum sample was immediately carried out after pretreatment, while samples stayed on ice during measurement. Assays for every group sample were repeated in triplicate.
2.6. Statistical analysis
Comparisons among groups and subgroups were performed using a two-tailed unpaired Student's t-test with OriginPro 9.1 (OriginLab) and Prism 7 (GraphPad). Correlation analysis, multivariate analysis of variance (MANOVA) and independent t-test were performed with SPSS Statistics V22.0 (IBM). Values for all measurements were expressed as mean ± SD for parametric distributions. A p-value of <0.05 was considered statistically significant. A p-value between 0.05 and 0.1was considered borderline significant. All experiments were performed at least three times.
3. Results
3.1. Subjects recruited for this study
All subjects recruited provided informed consent for participation in this research study. Among the recruited 175 adults (18–83 years old) who never got infected by SARS-CoV-2 before the blood collection, 71 were men and 104 were women. Among them, nine people were excluded due to hypertension, diabetes or being under medication, with the remaining 166 volunteers for blood collection to measure nitrite and nitrate. Among the recruited 113 recovered patients (18–83 years old) from COVID-19, there were 3 mild-type, 90 common-type, 9 severe-type, 5 critical-type, 5 unclassified and 1 asymptomatic subjects, according to the established guidelines [38] for clinical types of COVID-19 disease. Four patients out of these 107 classified subjects were excluded due to hypertension or being under medication. The remaining 103 classified, 5 unclassified and 1 symptomatic volunteers were enrolled for blood collection (Fig. S1, Table S1).
The hospital discharge criteria for the COVID-19 subjects were: 1) a return to normal body temperature for more than 3 days; 2) significant improvements in respiratory symptoms; 3) lung imaging showing significant improvement in acute exudative lesions; and 4) two consecutive respiratory tract samples (at least 24 h apart) that returned negative nucleic acid tests for SARS-CoV-2. All these recruited patients were under treatment, and subsequently discharged from hospital for at least four months prior to study participation, who were within the recovered group in this study.
Of the diet-controlled group, only male adults were recruited in order to simplify data analysis by removing factors attributable to female menopause and menstruation. 45 men were recruited, with 14 excluded due to hypertension, diabetes or being under medication. The remaining 31 men were age-matched with 31 men from the uninfected group (Fig. S1, Table S1). These 31 uninfected adults were not under a controlled diet, in order to clarify whether diet causes a difference in serum NO between these two small groups of male participants. For two weeks prior to blood collection, this diet-controlled population followed a meal plan for COVID-19 patients that have been adopted by hospitals in Hubei Province. Volunteers in these two male populations were 28–60 years old.
3.2. Evaluation of serum pretreatment methods
Analyte detection in serum is complicated due to the presence of the serum matrix that includes proteins, lipids and other contents. An efficient pretreatment method is required to reduce matrix interference and achieve high accuracy of NOx levels. In this study, three pretreatment methods were investigated to select the most efficient one for analysis of serum nitrite and nitrate, i.e. the stable metabolites of NO. The results are shown in Fig. 1 , Table S2.
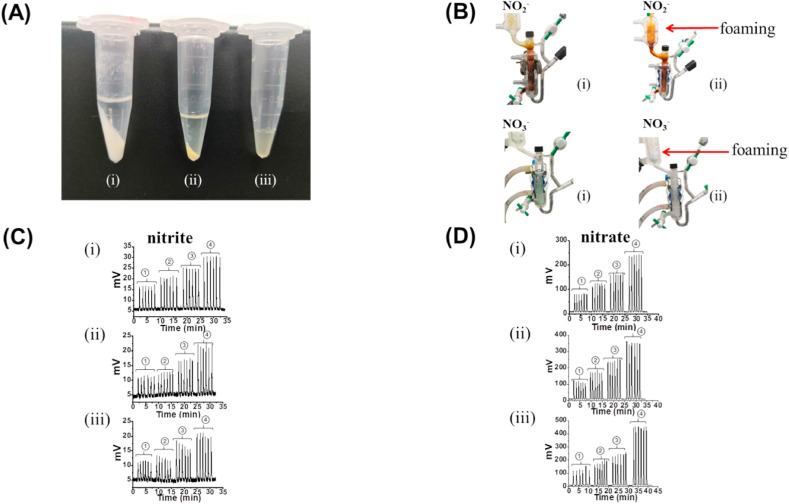
Fig. 1.
Different serum pretreatment methods and their effect on nitrite and nitrate measurements: (A) photographs of serum samples after pretreatment: (i) NaOH/ZnSO4 precipitation, (ii) ethanol precipitation, and (iii) untreated. (B) photographs of the purge vessel during sample measurement after pretreatment i-ii. (C) measurement of nitrite using standard solutions (0, 0.2, 0.5, 1 μM from left to right) as a function of the three pretreatment methods i-iii. (D) measurement of nitrate using standard nitrate solutions (0, 20, 40, 80 μM from left to right) as a function of pretreatment methods i-iii.
We carefully compared the foaming intensity following injection of serum samples after pretreatment. When serum was mixed with NaOH and ZnSO4 in a volumetric ratio of 1:2:2 (serum:NaOH:ZnSO4), a greater amount of precipitation was observed compared to the ethanol pretreatment (serum:ethanol = 1:2), with very little precipitation observed in the absence of the pretreatment step (Fig. 1A). No matter whether serum nitrite or nitrate was detected, samples with the NaOH/ZnSO4 pretreatment had less foaming than those with ethanol pretreatment (Fig. 1B). The measurement of serum without any pretreatment was significantly more challenging, as foaming was too vigorous to perform multiple replicates using a single triiodide or vanadium solution, and the reductive reagents had to be frequently replaced.
As shown in Fig. 1C and D and tabulated in Table S2 (see Supplementary Information), the relative recoveries employing the NaOH/ZnSO4 pretreatment were in the range of 85.2–111.0% with a relative standard deviation (RSD) of no more than 4.2%. The relative recoveries from ethanol pretreatment were in the range of 73.4–110.4% with an RSD in the range of 1.3–11.7%. In the absence of a pretreatment step, the relative recoveries were in the range of 40.1–76.9% with an RSD in the range of 3.9–8.8%. These results confirmed the reliability of NaOH/ZnSO4 pretreatment as our preferred method for quantification of serum nitrate and nitrate in the uninfected and COVID-19 recovered patients.
We also carefully evaluated the association between injected sample volume and area under curve (AUC) with NaOH/ZnSO4 pretreatment. This procedure was performed to minimize systematic errors during detection (Fig. S2). The results showed that the chemiluminescent signal was linear with increasing sample volume in the range of 10–100 μL (R2 > 0.999), which covered the injected volumes used for nitrite or nitrate measurement.
3.3. Quantification of serum nitrite and nitrate in the uninfected and recovered group
In order to investigate whether NOx levels in the recovered population were different from uninfected adults, blood was collected from 166 uninfected adults and 109 recovered patients without hypertension, diabetes or being under medication after overnight fasting.
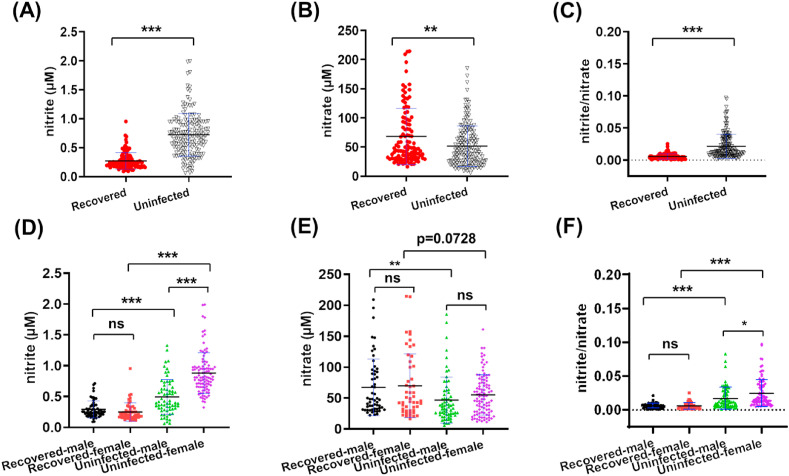
Nitrite levels (Fig. 2 A) were 0.72 ± 0.37 μM (range of 0.06–1.99 μM) for the uninfected group, a statistically significant difference (p < 0.0001) when compared to the average of 0.27 ± 0.14 μM (range of 0.09–0.95 μM) for the recovered group. Similarly, a statistically significant (p = 0.0023) difference in nitrate levels was observed between the uninfected group 51.70 ± 34.74 μM (range of 5.64–185.40 μM; Fig. 2B) and the recovered group 68.25 ± 48.17 μM (range of 16.46–214.6 μM). The ratio of nitrite versus nitrate in the uninfected group (0.0214 ± 0.0189; Fig. 2C) was statistically higher (p < 0.0001) than in the recovered group (0.0057 ± 0.0041) (Table S3). The opposite trends observed for serum nitrite versus nitrate levels in recovered COVID-19 patients led us to perform further analysis.
Fig. 2.
Serum nitrite levels (A, D), nitrate levels (B, E) and the nitrite/nitrate ratio (C, F) in the recovered and uninfected groups (A–C), and in the gender differentiated recovered and uninfected groups (D–F). ***: p ≤ 0.001; **: p ≤ 0.01; *: p ≤ 0.05; value listed: 0.05 < p < 0.1; ns: p > 0.1, not significant.
3.4. Effect of sex, age and clinical severity on serum nitrite and nitrate
Since vascular tone, as well as immune responses, may be regulated by sex, age and pathophysiological status [43], the data were further analyzed to see if serum nitrite and nitrate levels were impacted by the factors of sex, age, and clinical severity before recovery. Between the two groups, recovered men had lower nitrite levels than men in the uninfected group (p < 0.0001), a similar finding to that observed for women (p < 0.0001) (Fig. 2D). Within the recovered group, no difference was found between male and female patients, but male nitrite levels were lower than those of females inside the uninfected group. As for serum nitrate, recovered men had higher nitrate levels than men in the uninfected group (p = 0.0066), a similar trend to that observed for women with borderline significance (p = 0.0728) (Fig. 2E). No significant difference of nitrate levels was observed between men and women in the same group. As for the ratio of nitrite versus nitrate, the same sex in two groups had statistically different values, but the two sexes in the recovered group had similar levels and women had higher nitrite/nitrate levels than men in the uninfected group (Fig. 2F, Table S3). It may suggest that infection attenuates the sex-related nitrite difference among healthy subjects, as reported in literature [44].
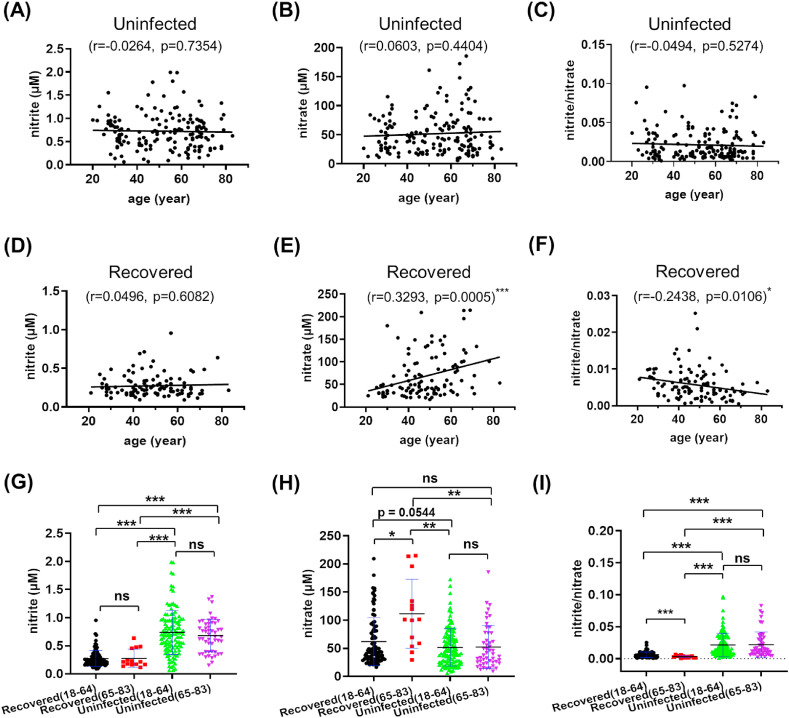
Age is another factor influencing viral infection and disease progression during the COVID-19 pandemic [3]. Therefore, the association between nitrite and nitrate (NOx) and age were analyzed in these two groups (Fig. 3 , Fig. S3). In the recovered group, there existed positive correlation between nitrate and age, and negative correlation between the ratio of nitrite/nitrate and age (Fig. 3D–F). Such correlations were not observed in the uninfected group (Fig. 3A–C). Since an age of 65 years or older is considered as a high-risk factor for COVID-19 [5], we further split each group into 18–64 and 65–83 years old subgroups and performed multivariate analysis of variance (MANOVA) and a simple effect analysis. As for nitrite and nitrite/nitrate, recovered adults in the same-age ranges still had dramatically lower levels than the uninfected peers (Fig. 3G and I), with the opposite trend of nitrate levels (Fig. 3H). In addition, nitrate and nitrite/nitrate levels were significantly higher and lower in the recovered (65–83) than in the recovered (18–64) subgroup (Fig. 3H–I), respectively. The results indicate that high NO3 − and low NO2 −/NO3 − may serve a promising marker for long-term consequences among aged people infected by SARS-CoV-2.
Fig. 3.
Correlation between age and serum nitrite (A, D), nitrate (B, E), nitrite/nitrate ratio (C, F) among the recovered (A–C) and uninfected (D–F) groups. Serum nitrite levels (G), nitrate levels (H) and the nitrite/nitrate ratio (I) in the age-differentiated (18–64, 65–83 years old) recovered and uninfected groups. ***: p ≤ 0.001; **: p ≤ 0.01; *: p ≤ 0.05; value listed: 0.05 < p < 0.1; ns: p > 0.1, not significant.
Whether the clinical classification during infection impacts NOx levels in blood stays unknown. In this study, we performed a comparison of serum NOx among patients from four categories. The clinical types (mild, common, severe, and critical) of COVID-19 patients were categorized based on their symptoms according to established guidelines [38]. As the name implies, mild-type COVID-19 patients presented with mild clinical symptoms and no evidence of pneumonia in imaging. Patients categorized as common-type COVID-19 presented with fever and respiratory symptoms, with pneumonia visible on imaging. Severe type criteria in adults included one of the following symptoms: shortness of breath with a respiratory rate (RR) ≥30 times/min; upon air inhalation, oxygen saturation was ≤93% at rest; arterial partial pressure of oxygen (PaO2)/inhaled oxygen concentration (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa); or progressively worsening of clinical symptoms, with >50% significant progress of lesions within 24–48 h by pulmonary imaging. The criteria for critical-type COVID-19 disease include one of the following: respiratory failure occurs and mechanical ventilation is required; shock occurs; respiratory failure is combined with other organ failure, and ICU monitoring and treatment are required.
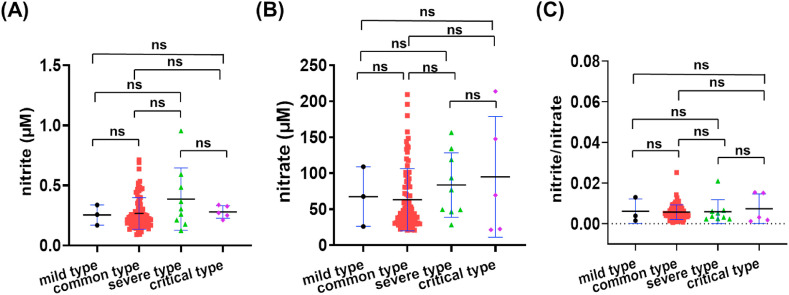
Among the 103 classified recovered patients, there were 3 mild type, 86 common type, 9 severe type and 5 critical type patients when they were in hospital. As shown in Fig. 4 and Table S3, among the 3 mild-type patients, the nitrite level was 0.25 ± 0.08 μM, with a range of 0.17–0.34 μM. Among the 86 common-type patients, the nitrite level was 0.27 ± 0.13 μM (0.09–0.71 μM range), while the 9 severe-type patients had a nitrite level of 0.39 ± 0.26 μM (0.13–0.95 μM range). Finally, the 5 critical-type patients had a nitrite level of 0.28 ± 0.05 μM with a range of 0.21–0.34 μM.
Fig. 4.
Serum nitrite and nitrate levels in patients recovered from COVID-19. (A) nitrite levels, (B) nitrate levels, and (C) the nitrite/nitrate ratio in patients presenting mild, common, severe and critical COVID-19 disease.
Among mild-, common-, severe- and critical-type patients, the serum nitrate levels were, respectively, 67.50 ± 41.44 μM (26.00–108.90 μM range), 63.08 ± 43.14 μM (20.72–209.20 μM range), 83.64 ± 44.80 μM (28.23–156.50 μM range), 94.89 ± 84.01 μM (21.26–213.80 μM range). Though the clinical symptoms among these subtypes were different, no significant difference was found for either nitrite, nitrate or nitrite/nitrate levels in this study (Fig. 4A–C, Table S3).
In short, nitrite and nitrite/nitrate were significantly lower in the recovered patients than in the uninfected control group. In the recovered population, high nitrate seemed a marker for poor long-term effects for patients aged 65 or older, as well as for COVID-19 patients in general. Sex appeared to only impact serum nitrite levels in the uninfected group.
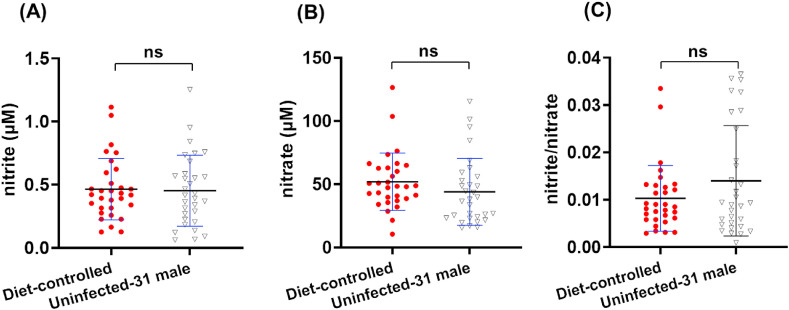
3.5. Quantification of serum nitrite and nitrate in the diet-controlled group
Nitrite and nitrate from diet has been suggested a source of endogenous NO in humans [45]. Since the role of sex in NO circulation and metabolism has not yet been fully elucidated, and female menopause and menstruation may affect detected analytes, only healthy male volunteers (n = 31, 28–60 years old) were recruited in order to investigate the potential effect of hospital meals on serum NO. After two weeks of fixed meal plans, blood samples were collected for nitrite and nitrate analysis (Fig. 5 ). Among the diet-controlled men, serum nitrite and nitrate levels were 0.46 ± 0.24 μM (0.13–1.11 μM) and 51.88 ± 22.63 μM (10.52–126.40 μM), respectively, with a nitrite/nitrate ratio of 0.0103 ± 0.0069 (0.0029–0.0335). Among 31 age-matched men in the uninfected group, serum nitrite and nitrate levels were 0.45 ± 0.28 μM (0.06–1.25 μM) and 43.88 ± 26.39 μM (15.37–115.40 μM), respectively, with a nitrite/nitrate ratio of 0.0140 ± 0.0116 (0.0009–0.0365). No statistically significant difference was found between these two groups of healthy men, suggesting that hospital meals did not influence serum NOx level in uninfected adults, at least for a short-term period of two weeks. Whether diet affects NOx in COVID-19 patients in hospital and recovered patients, a well-designed study is highly needed.
Fig. 5.
Serum nitrite levels (A), nitrate levels (B) and the nitrite/nitrate ratio (C) in the diet-controlled and uninfected-31 male groups. ***: p ≤ 0.001; **: p ≤ 0.01; *: p ≤ 0.05; value listed: 0.05 < p < 0.1; ns: p > 0.1, not significant.
4. Discussion
The pathogenesis of COVID-19 disease has been linked to oxidative stress or redox imbalance, including higher nitrate and cytokine storm in patients, and the use of antioxidants has been proposed as a potential therapy [46]. Taking advantage of the sensitive and precise properties of the chemiluminescent technique, this study systematically measured serum nitrite and nitrate in recovered COVID-19 patients discharged from hospital for more than four months, in order to clarify whether different metabolites of NO may serve as a potential biomarker for long-term effects of SARS-CoV-2 infection.
To date, there has been no parallel comparison assessingthe optional method for chemiluminescence measurements of NOx, even though multiple serum preparation methods have been described [[47], [48], [49]]. After evaluating the recovery ratio and foaming conditions during sample preparation, we thereafter established a NaOH/ZnSO4 pretreatment method for the reproducible and quantifiable determination of nitrite and nitrate levels in serum.
We found that patients presenting different clinical types of COVID-19 disease did not have statistically different levels of serum NOx, indicating the damage caused by SARS-CoV-2 infection is not correlated to disease severity but appears to be irreversible even after 4 months of recovery time. This phenomenon might suggest that the damage caused by SARS-CoV-2 virus is chronic and long lasting.
In this study, opposite changes were observed for serum nitrite and nitrate in the recovered group compared to the uninfected group, suggesting potential differences in the synthesis/metabolism of NO in the different scenarios in vivo. In one study by Joshi et al., in 2002, NO was predominantly and irreversibly consumed by oxy-hemoglobin to form nitrate under normoxia, instead of being conserved as nitrite or S-nitrosohemoglobin (one type of S-nitrosothiol, SNO) [50]. Less than 1% of homogenously delivered NO from its donor was converted into nitrite or SNO [50]. As reported for recovered COVID-19 patients [51], they usually encounter shortness of breath or difficulty breathing, which might suggest that their blood hemoglobin needs to work more efficiently to deliver enough oxygen and therefore consume more NO. This speculation of a compensatory effect of hemoglobin needs experimental confirmation.
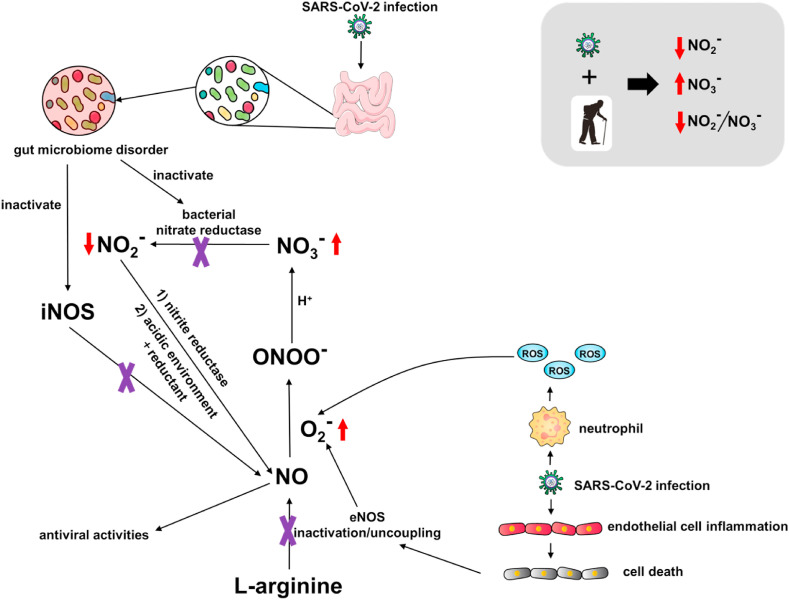
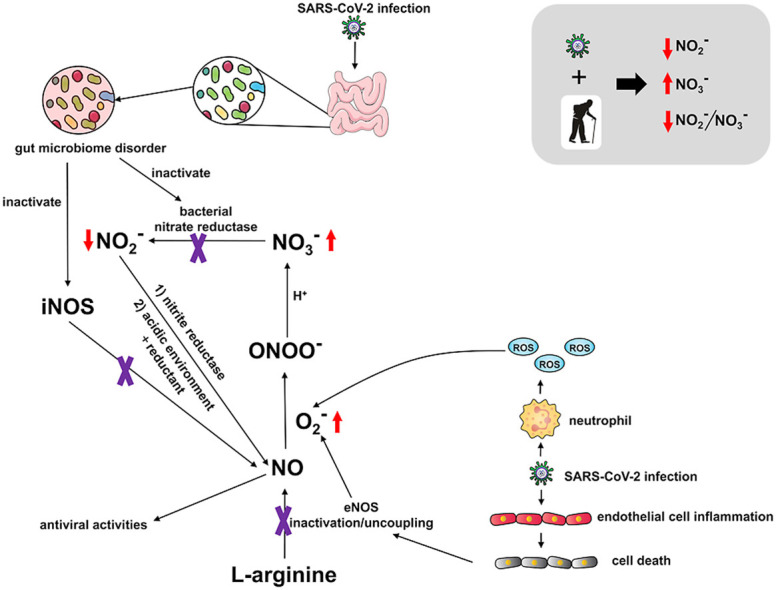
In 2001, Lauer et al. reported that plasma nitrite alone, instead of nitrate or the sum of nitrite and nitrate, reflected regional endothelial NO synthase (eNOS) activity in one study of 24 volunteers, and was positively correlated with forearm blood flow [52]. In the study herein, we speculate that decreased nitrite might be linked to eNOS dysfunction in impaired endothelium during SARS-CoV-2 infection [23], as shown in Scheme 1 . In addition, dysfunction of iron metalism was reported among COVID-19 patients [53]. Hyperferritinemia was associated with severe COVID-19 disease, which also existed in recovered patients, especially those with severe or critical COVID-19 [54]. Whether and how the dysregulation of iron metabolism, such as release of free toxic circulating heme, is linked to the pathogenesis of COVID-19 and NO remains unknown and warrants further investigation.
Scheme 1.
Potential pathways of dysregulated NO among patients recovered from COVID-19.
Furthermore, a positive association between nitrate and age in the recovered COVID-19 patients was observed. Aging has been linked to detrimental NO production and bioavailability [55], which seems in accordance with lower NO2 −/NO3 − and higher NO3 − in patients 65 years or older in this study. One potential cause for the decreased nitrite levels in recovered group may lie in perturbed gut microbiota of COVID-19 patients [56]. In one study of 100 patients after clearance of SARS-CoV-2, the level of a gut bacterium with immunomodulatory potential, Faecalibacterium prausnitzii, was reported to be diminished when compared to a healthy control group. A summary of the above factors that influence NOx serum levels as they relate to COVID-19 disease are presented in Scheme 1. The commensal microbe F. prausnitzii has been shown to affect NO production via iNOS induction [57]. Commensal gut bacteria also modulate nitrate reduction to nitrite [58], and might be depleted in COVID-19 patients. These additive effects may then cause lower NO availability, which triggers more nitrite reduction to NO in order to remedy the loss of NO among patients.
On the other hand, endothelial cells are damaged during SARS-CoV-2 entry and progression, which either inactivates eNOS or uncouples eNOS to release reactive oxygen species (ROS) (Scheme 1) [59]. Excessive ROS can consume NO to form peroxynitrite (ONOO−). In the presence of protons, i.e. neutral or acidic pH, the stability of peroxynitrite is compromised and peroxynitrous acid (ONOOH) will decomposed to form nitrate. Either in blood under physiological condition (~pH 7.35–7.45) or blood from COVID-19 patients (~pH 7.40–7.51), the degradation of peroxynitrous acid and formation of nitrate is plausible. A similar phenomenon was observed in older people, who are thought to have dysfunctional vascular function and decreased NO bioavailability [60]. It is highly possible that the combined effect of aging and viral infection leads to low nitrite, low nitrite/nitrate and high nitrate among subjects recovered from COVID-19.
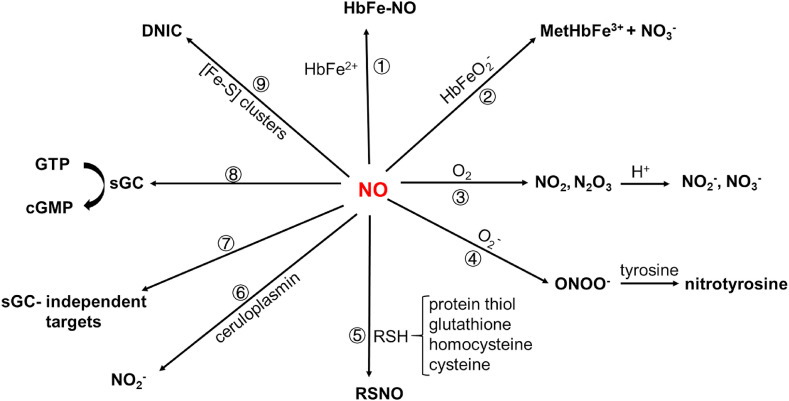
However, there exists other pathways to inactivate NO, such as s-nitrosylation, nitration, etc. As shown in Scheme 2 , nine patwhays are known to inactivate or transform NO into other N-containing forms [50,61,62]: (1) NO reacts with reduced hemoglobin (HbFe2+) to generate S-nitroso-hemoglobin (HbFe-NO); (2) oxygen-containing hemoglobin (HbFeO2 -) scavenges NO to form met-hemoglobin (MetHbFe3+) and nitrate; (3) NO is oxidized by oxygen to form NO2 and N2O3, that is converted to NO2 − and NO3 −; (4) NO directly reacts with superoxide anion (O2 −) to form peroxynitrite anion (ONOO−), and the latter causes nitration of tyrosine on proteins; (5) NO reacts with various thiols from low-molecular-weight molecules or proteins to produce S-nitrosothiol (SNO) (Equation (1)&2, Equilibrium 1); (6) NO is oxidized by copper protein ceruloplasmin to form NO2 −; (7) NO reacts with targets independently of guanylyl cyclase 1 (GC-1 also known as soluble guanylate cyclase), such as cytochrome c oxidase to pass signaling; (8) NO targets at GC-1 to perform classic NO signaling; (9) NO reacts with iron-sulfur ([Fe–S]) clusters to form nitrosyl iron complex (DNIC). Among these possibilitis, S-nitrosation might play a role during SARS-CoV-2 infection.
Scheme 2.
Potential inactivation or signaling pathways of NO in vivo.
As is a covalent post-translational oxidative modification, S-nitrosylation, also called S-nitrosation (SNO), is though to be an important modification in physiology and diseases [61,62]. Cellular S-nitrosylation is a regulated equilibrium between low-molecular-weight SNOs (LMW-SNO, such as S-nitrosolglutathione, S-nitroso-coenzyme A) and SNO-cysteine-proteins [61,62], as shown in Equilibrium 1. Several studies have suggested that NO exerts its pathophysiological function mainly via S-nitrosation, instead of via other pathways as shown in Scheme 2. In one recent study by Dominic et al., S-nitrosothiol was significantly lower in COVID-19 patients than in the control group [36]. It indicated that blood SNO levels changed during SARS-CoV-2 infection via an unknown mechanims as of yet.
| NO+ + RSH → RSNO | (1) |
| R1SNO + R2SH → R1SH + R2SNO | (2) |
| LMW-SNO ↔ Protein1-SNO ↔ Protein2-SNO Equilibrium 1 |
On the other hand, S-nitrosation may serve as a defense against viral attack during this pandemic [22]. Using the NO donor SNAP, Akaberi et al. identified that reduced activity of SARS-CoV-2 main protease was consistent with S-nitrosylation of cysteine in the active site of this protease [22], though the mechanism needs to be clarified. This is not the first time that S-nitrosylation was suggested as the molecular base of NO's antiviral effects [20,63]. Whether the decreased level of NO (in this case, the level of S-nitrosolthiol) is due to cellular defense against SARS-CoV-2 certainly needs further comprehensive investigation from both analytic measurement and mechanistic studies.
To date, mounting evidence suggests that COVID-19 survivors may encounter long-term health issues [64,65]. Chronic unexplained medical symptoms of so called “long COVID” has been reported in both adults and children [[66], [67], [68], [69], [70]], even among initially mild-infected patients, which included fatigue, headache, insomnia, dizziness, lung damage, erectile dysfunction, as well as other psychiatric and neurological symptoms. In a recent cohort study of COVID-19 patients discharged from hospitals for 6 months, most survivors were still troubled by a number of symptoms, including fatigue or muscle weakness, sleep difficulties, anxiety or depression [15]. These findings are consistent with another follow-up study performed 3 months after hospital discharge [71,72], and are similar to that observed for SARS patients, suggesting long-term sequelae due to coronavirus infections [73]. In addition, as endothelial dysfunction diminishes NO bioavailability, it also causes erectile dysfunction, which was observed with significantly higher occurrence among men with SARS-CoV-2 infection [70,74]. Unfortunately, validated biomarkers are still missing to quantify these abnormalities for most of these post-infectious symptoms [75].
As a key immune, cardiovascular and neurological mediator, altered circulating NO levels may represent some unknown long-term consequence of SARS-CoV-2 infection. Our data presented herein supports association between levels of NOx, SARS-CoV-2 infection and age and intimates that NO is involved in COVID-19 pathogenesis. Comparison of serum NOx between two healthy adult groups in this study is certainly not an ideal method to investigate the effect of food-source NOx on SARS-CoV-2 infected patients in hospital. More strictly designed trials are necessary to elucidate the influence of diet, which contains both antioxidants and oxidants. One recent study in primary human lung cells and intestinal organoids showed that SARS-CoV-2 dysregulated cellular metabolism and reduced autophagy [69], which might indirectly modulate NO pathways due to interaction between them [76]. There is an urgent need to understand how pro-oxidants and antioxidants function in patients with COVID-19 disease. Combined with chest imaging and pathological diagnosis, potential biomarkers or predictors like NOx may help elucidate persisting symptoms and substantial organ dysfunction [65].
5. Conclusion
This is the first time that NO levels have been reported among patients recovered from COVID-19 disease after discharge from hospitals for at least 4 months. Lower nitrite and nitrite/nitrate, and higher nitrate levels were found among these patients. In addition, nitrate and nitrite/nitrate were positively and negatively correlated with patient age, respectively, with age 65 being a turning point.
Funding
This work was funded by the National Natural Science Foundation of China (91954001), National Natural Science Foundation of China TUBITAK (Turkey)-NSFC (China) Bilateral Cooperation Grant (82061138005) (Xueji Zhang), and Hubei Provincial Department of Education Innovation Group Grant (6101/12267) (Jun Wang).
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgement
We thank Dr. Rong Zhang of the Neuroscience Research Institute at Peking University for her insightful comments during experimental design.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.freeradbiomed.2021.08.237.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.https://coronavirus.jhu.edu/map.html (2021.08.22)
- 2.Ghisolfi S., Almås I., Sandefur J.C., von Carnap T., Heitner J., Bold T. Predicted COVID-19 fatality rates based on age, sex, comorbidities and health system capacity. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin J.M., Bai P., He W., Wu F., Liu X.F., Han D.M., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanez N.D., Weiss N.S., Romand J.A., Treggiari M.M. COVID-19 mortality risk for older men and women. BMC Publ. Health. 2020;20:1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020:1–8. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. Jama. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R., Liang W., Jiang M., Guan W., Zhan C., Wang T., et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158:97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 12.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8 doi: 10.1016/s2213-2600(20)30116-8. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y., Zhou N., Zha W., Lv Y. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: a meta-analysis. Nutr. Metabol. Cardiovasc. Dis. 2021;31:745–755. doi: 10.1016/j.numecd.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortinovis M., Perico N., Remuzzi G. Long-term follow-up of recovered patients with COVID-19. Lancet. 2021;397:173–175. doi: 10.1016/s0140-6736(21)00039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/s0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Augustin M., Schommers P., Stecher M., Dewald F., Gieselmann L., Gruell H., et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamin N., Vane J. Nitric oxide and hypertension. Circulation. 1996;94:1197–1198. doi: 10.1161/01.CIR.94.6.1197. [DOI] [PubMed] [Google Scholar]
- 18.Akerström S., Mousavi-Jazi M., Klingström J., Leijon M., Lundkvist A., Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/jvi.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akerström S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefano G.B., Esch T., Kream R.M. Potential immunoregulatory and antiviral/SARS-CoV-2 activities of nitric oxide. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/msm.925679. e925679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akaberi D., Krambrich J., Ling J., Luni C., Hedenstierna G., Järhult J.D., et al. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020;37:101734. doi: 10.1016/j.redox.2020.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang W., Jiang J., Su L., Shu T., Liu H., Lai S., et al. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2021;163:153–162. doi: 10.1016/j.freeradbiomed.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ignarro L.J. Inhaled NO and COVID-19. Br. J. Pharmacol. 2020;177:3848–3849. doi: 10.1111/bph.15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotz C., Muellenbach R.M., Meybohm P., Mutlak H., Lepper P.M., Rolfes C.B., et al. Effects of inhaled nitric oxide in COVID-19-induced ARDS - is it worthwhile? Acta Anaesthesiol. Scand. 2021;65:629–632. doi: 10.1111/aas.13757. [DOI] [PubMed] [Google Scholar]
- 26.Swathi Krishna S., Thennavan A., Kanthlal S.K. Dietary foods containing nitric oxide donors can be early curators of SARS-CoV-2 infection: a possible role in the immune system. J. Food Biochem. 2021 doi: 10.1111/jfbc.13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi J. Lifestyle-mediated nitric oxide boost to prevent SARS-CoV-2 infection: a perspective. Nitric Oxide. 2021;115:55–61. doi: 10.1016/j.niox.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laghlam D., Rahoual G., Malvy J., Estagnasié P., Brusset A., Squara P. Use of almitrine and inhaled nitric oxide in ARDS due to COVID-19. Front. Med. 2021;8:655763. doi: 10.3389/fmed.2021.655763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziehr D.R., Alladina J., Wolf M.E., Brait K.L., Malhotra A., La Vita C., et al. Respiratory physiology of prone positioning with and without inhaled nitric oxide across the coronavirus disease 2019 acute respiratory distress syndrome severity spectrum. Crit Care Explor. 2021;3 doi: 10.1097/cce.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava S., Garg I., Hembrom A.A., Kumar B. Assessment of nitric oxide (NO) potential to mitigate COVID-19 severity. Virusdisease. 2021:1–6. doi: 10.1007/s13337-021-00702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prakash A., Kaur S., Kaur C., Prabha P.K., Bhatacharya A., Sarma P., et al. Efficacy and safety of inhaled nitric oxide in the treatment of severe/critical COVID-19 patients: a systematic review. Indian J. Pharmacol. 2021;53:236–243. doi: 10.4103/ijp.ijp_382_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinciroli R., Traeger L., Fischbach A., Gianni S., Morais C.C.A., Fakhr B.S., et al. A novel inhalation mask system to deliver high concentrations of nitric oxide gas in spontaneously breathing subjects. JoVE. 2021 doi: 10.3791/61769. [DOI] [PubMed] [Google Scholar]
- 33.Yamagishi S., Matsui T. Nitric oxide, a janus-faced therapeutic target for diabetic microangiopathy-Friend or foe? Pharmacol. Res. 2011;64:187–194. doi: 10.1016/j.phrs.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Lorente L., Gómez-Bernal F., Martín M.M., Navarro-Gonzálvez J.A., Argueso M., Perez A., et al. High serum nitrates levels in non-survivor COVID-19 patients. Med. Intensiva. 2020 doi: 10.1016/j.medin.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassiliou A.G., Zacharis A., Keskinidou C., Jahaj E., Pratikaki M., Gallos P., et al. Soluble angiotensin converting enzyme 2 (ACE2) is upregulated and soluble endothelial nitric oxide synthase (eNOS) is downregulated in COVID-19-induced acute respiratory distress syndrome (ARDS) Pharmaceuticals. 2021;14:695. doi: 10.3390/ph14070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dominic P., Ahmad J., Bhandari R., Pardue S., Solorzano J., Jaisingh K., et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021;43:101982. doi: 10.1016/j.redox.2021.101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Núñez-Fernández M., Ramos-Hernández C., García-Río F., Torres-Durán M., Nodar-Germiñas A., Tilve-Gómez A., et al. Alterations in respiratory function test three months after hospitalisation for COVID-19 pneumonia: value of determining nitric oxide diffusion. J. Clin. Med. 2021;10:2119. doi: 10.3390/jcm10102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guideline on diagnosis and treatment of novel coronavirus pneumonia (interim eighth ed.). 10.3969/j.issn.1007-8134.2020.04.001. [DOI]
- 39.Romitelli F., Santini S.A., Chierici E., Pitocco D., Tavazzi B., Amorini A.M., et al. Comparison of nitrite/nitrate concentration in human plasma and serum samples measured by the enzymatic batch Griess assay, ion-pairing HPLC and ion-trap GC-MS: the importance of a correct removal of proteins in the Griess assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:257–267. doi: 10.1016/j.jchromb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 40.MacArthur P.H., Shiva S., Gladwin M.T. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Girgis R.E., Champion H.C., Diette G.B., Johns R.A., Permutt S., Sylvester J.T. Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am. J. Respir. Crit. Care Med. 2005;172:352–357. doi: 10.1164/rccm.200412-1684OC. [DOI] [PubMed] [Google Scholar]
- 42.Baksu B., Davas I., Baksu A., Akyol A., Gulbaba G. Plasma nitric oxide, endothelin-1 and urinary nitric oxide and cyclic guanosine monophosphate levels in hypertensive pregnant women. Int. J. Gynaecol. Obstet. 2005;90:112–117. doi: 10.1016/j.ijgo.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Orshal J.M., Khalil R.A. Gender, sex hormones, and vascular tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 44.Reckelhoff J.F., Hennington B.S., Moore A.G., Blanchard E.J., Cameron J. Gender differences in the renal nitric oxide (NO) system: dissociation between expression of endothelial NO synthase and renal hemodynamic response to NO synthase inhibition. Am. J. Hypertens. 1998;11:97–104. doi: 10.1016/s0895-7061(97)00360-9. [DOI] [PubMed] [Google Scholar]
- 45.Raat N.J., Noguchi A.C., Liu V.B., Raghavachari N., Liu D., Xu X., et al. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic. Biol. Med. 2009;47:510–517. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelletier M.M., Kleinbongard P., Ringwood L., Hito R., Hunter C.J., Schechter A.N., et al. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic. Biol. Med. 2006;41:541–548. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Miranda K.M., Espey M.G., Wink D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 49.Liddle L., Monaghan C., Burleigh M.C., McIlvenna L.C., Muggeridge D.J., Easton C. Changes in body posture alter plasma nitrite but not nitrate concentration in humans. Nitric Oxide. 2018;72:59–65. doi: 10.1016/j.niox.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Joshi M.S., Ferguson T.B., Jr., Han T.H., Hyduke D.R., Liao J.C., Rassaf T., et al. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10341–10346. doi: 10.1073/pnas.152149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salamanna F., Veronesi F., Martini L., Landini M.P., Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front. Med. 2021;8:653516. doi: 10.3389/fmed.2021.653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lauer T., Preik M., Rassaf T., Strauer B.E., Deussen A., Feelisch M., et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavezzi A., Troiani E., Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020;10:1271. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonnweber T., Boehm A., Sahanic S., Pizzini A., Aichner M., Sonnweber B., et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients' performance: a prospective observational cohort study. Respir. Res. 2020;21:276. doi: 10.1186/s12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sverdlov A.L., Ngo D.T., Chan W.P., Chirkov Y.Y., Horowitz J.D. Aging of the nitric oxide system: are we as old as our NO? J Am Heart Assoc. 2014;3 doi: 10.1161/jaha.114.000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roychowdhury S., Cadnum J., Glueck B., Obrenovich M., Donskey C., Cresci G.A.M. Faecalibacterium prausnitzii and a prebiotic protect intestinal health in a mouse model of antibiotic and Clostridium difficile exposure. JPEN - J. Parenter. Enter. Nutr. 2018;42:1156–1167. doi: 10.1002/jpen.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiso M., Schechter A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PloS One. 2015;10 doi: 10.1371/journal.pone.0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herrera M.D., Mingorance C., Rodríguez-Rodríguez R., Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res. Rev. 2010;9:142–152. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Stomberski C.T., Hess D.T., Stamler J.S. Protein S-nitrosylation: determinants of specificity and enzymatic regulation of S-Nitrosothiol-Based signaling. Antioxidants Redox Signal. 2019;30:1331–1351. doi: 10.1089/ars.2017.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evangelista A.M., Kohr M.J., Murphy E., S-nitrosylation specificity, occupancy, and interaction with other post-translational modifications. Antioxidants Redox Signal. 2013;19:1209–1219. doi: 10.1089/ars.2012.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colasanti M., Persichini T., Venturini G., Ascenzi P. S-nitrosylation of viral proteins: molecular bases for antiviral effect of nitric oxide. IUBMB Life. 1999;48:25–31. doi: 10.1080/713803459. [DOI] [PubMed] [Google Scholar]
- 64.Cohen J.I., Burbelo P.D. Reinfection with SARS-CoV-2: implications for vaccines. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dimeglio C., Herin F., Miedougé M., Martin-Blondel G., Soulat J.M., Izopet J. Protection of healthcare workers against SARS-CoV-2 reinfection. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- 67.Marshall M. How COVID-19 can damage the brain. Nature. 2020;585:342–343. doi: 10.1038/d41586-020-02599-5. [DOI] [PubMed] [Google Scholar]
- 68.Sterky E., Olsson-Åkefeldt S., Hertting O., Herlenius E., Alfven T., Ryd Rinder M., et al. Persistent symptoms in Swedish children after hospitalisation due to COVID-19. Acta Paediatr. 2021;110:2578–2580. doi: 10.1111/apa.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gassen N.C., Papies J., Bajaj T., Emanuel J., Dethloff F., Chua R.L., et al. SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nat. Commun. 2021;12:3818. doi: 10.1038/s41467-021-24007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sansone A., Mollaioli D., Ciocca G., Colonnello E., Limoncin E., Balercia G., et al. Mask up to keep it up": preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021;9:1053–1059. doi: 10.1111/andr.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao Y.M., Shang Y.M., Song W.B., Li Q.Q., Xie H., Xu Q.F., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang P., Li J., Liu H., Han N., Ju J., Kou Y., et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sansone A., Mollaioli D., Ciocca G., Limoncin E., Colonnello E., Vena W., et al. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J. Endocrinol. Invest. 2021;44:223–231. doi: 10.1007/s40618-020-01350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burke M.J., Del Rio C. Long COVID has exposed medicine's blind-spot. Lancet Infect. Dis. 2021;21:1062–1064. doi: 10.1016/s1473-3099(21)00333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarkar S., Korolchuk V.I., Renna M., Imarisio S., Fleming A., Williams A., et al. Complex inhibitory effects of nitric oxide on autophagy. Mol Cell. 2011;43:19–32. doi: 10.1016/j.molcel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.