Abstract
The term non-melanoma skin cancer (NMSC) refers to skin cancer different from melanoma, and it is usually restricted to basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and their pre-cancerous lesions, e.g., actinic keratosis. These conditions represent the most frequent tumors in Caucasians and are characterized by an increasing incidence worldwide and a high socio-economic impact. The term Integrated Care Pathway (ICP) refers to “a complex intervention for the mutual decision making and organization of care processes for a well-defined group of patients during a well-defined period”. The purpose of this paper is to present a proposal from the Italian Association of Hospital Dermatologists (ADOI) for an ICP organization of care of NMSC, considering the hub-and-spoke model in the different geographical areas.
This proposal is based on the most recent literature and on documents from the Italian Association of Medical Oncology (AIOM), the European consensus-based interdisciplinary guidelines from the European Association of Dermato- Oncology (EADO), and the National Comprehensive Cancer Network (NCCN).
We initially discuss the NMSC outpatient clinic, the role of the multidisciplinary working groups, and the hub-and-spoke model regarding this topic. Then, we define the ICP processes specific for BCC and SCC.
The ICP for NMSC is an innovative strategy to guarantee the highest possible quality of health care while the hub-andspoke model is crucial for the organization of different health care structures. Considering the importance on this topic, it is essential to create a valid ICP together with an efficient organization within the different geographical areas.
Key words: Integrated care pathway, Nonmelanoma skin cancer, Actinic keratosis, Basal cell carcinoma, Squamous cell carcinoma, hub and spoke model
Introduction
The term non-melanoma skin cancer (NMSC) traditionally comprises skin cancer that arise from keratinocytes of the epidermis and mainly includes basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and actinic keratosis (AK). The incidence of NMSC is constantly growing worldwide and, therefore, the management and treatment of the aforementioned neoplasms has become an important challenge for the health system.1
The cellular origin of BCC has not been completely elucidated but it is thought to arise from follicular and interfollicular epidermal basal keratinocytes.2 BCC is predominantly locally invasive and rarely metastasizes (0.05-0.1% of cases).3 The incidence of a first BCC in Italy has been estimated at about 87.6 per 100,000 inhabitants/ year and represents 15% of all cancers. 4
SCC originates from the squamous cells of the epidermis. SCC includes “invasive” and other “non-invasive” forms such as keratoacanthoma, Bowen’s disease, Bowenoid papulosis and Queyrat’s erythroplasia.5 SCC can arise from a previous AK or de novo and is more often localized in photoexposed areas but can also affect the mucous membranes and genitalia. SCC represents the second most frequent skin cancer (20%), after BCC, with an incidence of about 28.9 per 100,000 inhabitants/year. In 1-5% of cases, it can give distant metastases and such metastases are associated with an average survival of two years.5
The main risk factors related to the onset of NMSC are exposure to UV radiation, advanced age, light skin type, chronic immunosuppression, ionizing radiation, arsenic exposure, human papillomavirus infections, burns, chronic inflammatory processes, and specific genodermatoses.3,5
The Integrated Care Pathway (ICP) is, according to the definition adopted by the European Pathway Association (EPA), “a complex intervention for the mutual decision making and organization of care processes for a well-defined group of patients during a well-defined period”.6 The aim of an ICP is to enhance the quality of care across the continuum by improving riskadjusted patient outcomes, promoting patient safety, increasing patient satisfaction, and optimizing the use of resources.6
The ICP of the patient affected by NMSC is multidisciplinary. A multidisciplinary working group (MWG), integrating specific skills, includes professional figures such as dermatologists, medical oncologists, general surgeons, plastic surgeons, radiation oncologists, radiologists, pathologists, and epidemiologists. This MWG allows the optimization of the care process for the management of the patient suffering from NMSC.
Limited data have been published on ICPs focusing on NMSC, but, considering the importance of this topic, it is essential to create a dedicated ICP.7 The purpose of this study was to present a proposal for an ICP of NMSC from the Italian Association of Hospital Dermatologists (ADOI) in order to ensure uniformity of behavior in the management of NMSC.
Materials and methods
The following ICP of patients affected by NMSC is based on the most recent literature regarding NMSC, and make reference to documents from the Italian Association of Medical Oncology (AIOM) the National Guidelines on BCC (2020) and SCC (2019),8,9 the European consensus-based interdisciplinary guidelines from the European Association of Dermato- Oncology (EADO) related to BCC (2019) and SCC (2020),10,11 and from the National Comprehensive Cancer Network (NCCN) on BCC and SCC, both from 2021.12,13
The hub-and-spoke model
The increasing number of NMSC, the severity of some cases and the possibility to utilize recent treatments for advanced or metastatic cases of BCC (i.e., sonidegib and vismodegib) and SCC (i.e., cemiplimab) require an integration of community-based health care services with specialized hospital clinics that provide the optimal environment to address the complex needs of the cases and improve outcomes. This approach requires coordination across different levels and sites of care within and beyond the health sector. The hub-and-spoke organization design is a model characterized by service delivery assets into a network involving an anchor establishment (hub) offering a full array of services, complemented by secondary establishments (spokes) offering more limited service arrays, routing patients needing more intensive services to the hub for treatment (Figure 1).14 The hub-andspoke model favours a healthcare network involving a main campus and one or more satellite campuses and this model is more efficient than organization designs organized in multiple sites.15 Hub-and-spoke network structure could vary with satellites that could be increased as needed or desired.16 If geographic distance makes satellite-to-hub access difficult, an extra hub could be added. Therefore, the huband- spoke model represents an organization of care that works collaboratively with the primary care sector and is greatly integrated with community-based multidisciplinary teams of health care professionals and specialty care.17 Strategic centralization brings many benefits to the hub-and-spoke organization design. Telemedicine, particularly teledermatology (TD), represent a valid and well-established tool to help to coordinate the hub-and-spoke workflow. TD has been proven in various studies to be a valid triage system for skin cancer detection.18
Integrated care pathway for nonmelanoma skin cancer
The ICP for NMSC should consider different institutional levels and the risk of the BCC or SCC as shown in Figure 2 (Tables 1 and 2 for BCC and SCC risk classification). Local dermatology outpatient clinic should treat patients affected by low risk BCC or SCC, while, general hospital with a Dermatology Unit could manage intermediate and high-risk BCC or high and very high-risk SCC. More complicated cases (i.e., locally advanced or metastatic BCC or SCC, high risk BCC, or very high-risk SCC) should be managed by tertiary node with skin cancer centres and oncologic services organised in multidisciplinary working group (MWG). The NMSC outpatient clinic is the clinic in which the patient suffering from this neoplasm is checked by a specialist dermatologist in order to treat and follow-up the patient. This dermatological outpatient clinic is configured as a central part in the ICP process for NMSC because it is dedicated to the diagnosis, management and follow-up of patients affected by most of these tumors.
The MWG is essential for locally advanced forms of BCC and/or SCC for which there is no indication for surgery or radiotherapy or for metastatic NMSC (Figure 3). The MWG has been introduced to reduce the variation in decision-making between different specialists and for patients and their carers to access the best care possible. Considering the ageing population with multiple comorbidities, the number of treatment options becoming available, and the complexity of some NMSC, the MWG is crucial in order to offer the gold standard oncologic services. The MWG should periodically meet to discuss specific NMSC cases. In such meetings, clinicians should select appropriate cases to be discussed and these cases should be treated following specific ICP for NMSC, if possible. Routine cases should not be discussed in MWG meetings and should be treated as per protocol. Finally, the MWG meetings offer a source of support, education and management updates for the clinicians and trainees in a constantly and rapidly changing area. Clinical diagnosis of NMSC could be enough in many cases but the use of dermoscopy may improve diagnostic accuracy. In the diagnostic process, it is important to consider medical history (advanced age, higher phototype, previous diagnosis of NMSC, excessive sun exposure, immunosuppression therapy) and physical examination (location, size, infiltration and margins of the lesion). In case of diagnostic doubt, it is essential to perform a skin biopsy and histological examination.
Figure 1.
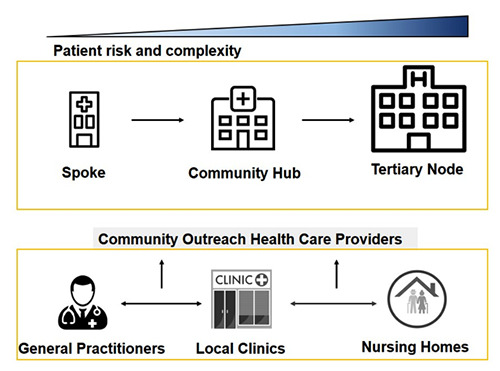
The hub-and-spoke model. The hub-and-spoke organization design is a model characterized by service delivery assets into a network involving an anchor establishment (hub) offering a full array of services, complemented by secondary establishments (spokes) offering more limited service arrays, routing patients needing more intensive services to the hub for treatment.
Surgery represents the first-line therapy for BCC and SCC because it allows histopathological analysis and low risk of relapsing. Other alternative techniques are indicated in case of low-risk BCC or in case of in situ SCC and they are discussed below.
The histological examination reports the local staging (pT) following the excisional biopsy of the NMSC which allows to define and plan any subsequent instrumental or surgical procedures. Furthermore, it must include the following information: patient data, report number/year, report date, site of tumor, macroscopic finding, histological subtype (in case of high-risk neoplasm), histological grade (for SCC: well differentiated - G1 moderately differentiated - G2, poorly differentiated - G3), state of lateral and deep margins / complete excision, tumor thickness (related to NMSC), possible perineural invasion and lymphatic / vascular invasion.7
Any instrumental examinations will be planned based on the type of NMSC and the clinical assessment. These examinations include ultrasound of the loco-regional lymph nodes which is indicated in case of high-risk invasive SCC. Advanced forms of SCC or BCC may require the use of diagnostic imaging techniques such as computed tomography and magnetic resonance imaging that allow evaluation of the local extension of the neoplasm, the infiltration of adjacent anatomical areas and the possible presence of nodal or distant organ metastasis.7
Basal cell carcinoma
Risk stratification for basal cell carcinoma
BCC can be classified into low-risk and high-risk according to prognostic factors such as tumor size, definition of clinical margins (poorly-defined lesions are at higher risk), histological subtype (morpheaform, and metatypical BCC represent high-risk lesions), histological features (perineural and/or perivascular invasion is a marker of higher risk), recurrence, and tumor location (Table 1).19 With regards to the location, high-risk zones are the nose, periorificial areas of the head and neck; intermediaterisk zones are the forehead, cheek, chin, scalp, and neck; low-risk zones are the trunk and limbs. Low-risk BCCs are superficial BCC, Pinkus tumor, and small nodular BCC on intermediate or low-risk areas while high-risk BCCs present at least one poor prognostic factor (Table 1).19 Furthermore, French guidelines also added an intermediate-risk to classify recurrent superficial BCC from other recurrent BCC, and some nodular BCCs according to size and location (Table 1). This classification has been used by the most recent AIOM guidelines regarding BCC (2020).8
Therapy of basal cell carcinoma
The first-line therapy for BCC is surgery because it allows the complete excision of the skin cancer and the preservation of the cosmetic and functional aspects.3 An evidence-based review regarding the interventions for BCC reported that the best results have been obtained with surgery.20
Figure 2.
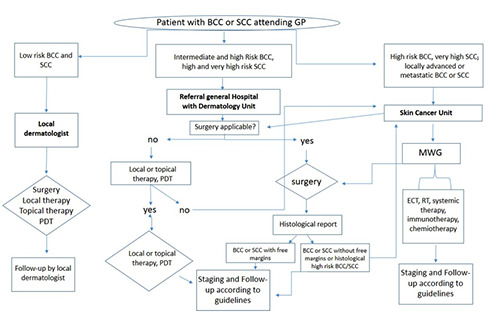
The integrated care pathway for non-melanoma skin cancers between different institutional centres. Local dermatology outpatient clinic should treat patients affected by low risk BCC or SCC, while, general hospital with a Dermatology Unit could manage intermediate and high-risk BCC or high and very high risk SCC. More complicated cases (i.e., locally advanced or metastatic BCC or SCC, high risk BCC, or very high-risk SCC) should be managed by tertiary node with skin cancer centres and oncologic services organised in multidisciplinary working group (MWG). GP: general practitioner; PDT: photodynamic therapy; MWG: Multidisciplinary working group; RT: radiotherapy; ECT: electrochemotherapy.
Figure 3.
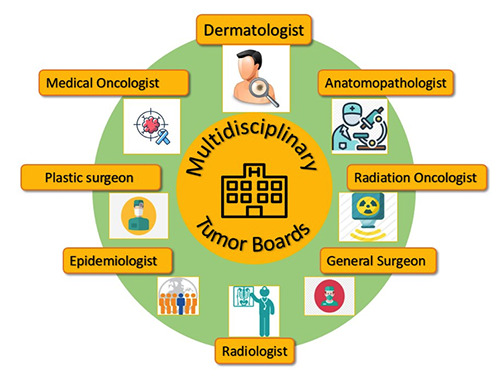
Multidisciplinary working group. The multidisciplinary working group (MWG), integrating their specific skills, include professional figures such as dermatologist, medical oncologist, general surgeon, plastic surgeon, radiation oncologist, radiologist, anatomopathologist, and epidemiologist.
Surgery and Mohs micrographic surgery
Standard excision is the primary treatment of BCC while the Mohs technique, not always feasible in medical institutions, could be preferable for the high-risk BCC. Standard excision with postoperative margin assessment (SEPMA) must guarantee a post-operative histological evaluation with free margins. Regarding the extension of free margins, the National Comprehensive Cancer Network (NCCN) recommends clinical margins of at least 4 mm for lowrisk BCC treated with SEPMA.12 Indeed, Brodland et al., showed that for well-circumscribed BCC <2 cm in diameter, excision with 4-mm clinical margins guarantees a complete removal in more than 95% of cases.21 Furthermore, in case of SEPMA for high-risk BCC, wider surgical margins compared to low-risk BCC are needed. A greater recurrence rate is predictable in case of high-risk BCC (Table 1).12
MMS (also known as chemosurgery, microscopically controlled excision, or histographic surgery) is a surgical approach consisting in a complete excision of the tumor followed by an examination of the microscopic margins. This technique could be considered as the treatment of choice for high-risk and recurrent BCCs because it showed a higher long-term cure rates compared to other surgical approaches and a maximum preservation of normal tissue in relation to conventional surgery. It has been reported that 5-year recurrence rates for primary and recurrent BCCs treated with MMS are 1% and 5.6%, respectively, compared with 10.1% and 17.4%, respectively, for SEPMA.22 Unfortunately, limited medical institutions in Italy have all the necessary facilities to implement this specific practice.
Table 1.
Prognostic groups for BCC and therapeutic strategies (Trakatelli et al. 2014; Dandurand 2006). BCC can be classified into low-risk, intermediate-risk, and high-risk according to prognostic factors as indicated by the AIOM guidelines on BCC (2020). Therapeutic strategies are indicated into three main groups of BCC: low-risk, intermediate and high-risk, and locally advanced.
| Low-Risk | Intermediate-risk | High-Risk | |
|---|---|---|---|
| Prognostic groups for BCC | Superficial primary BCC Nodular primary BCC when: <1 cm in intermediate risk area* <2 cm in low risk area* Pinkus tumor | Superficial recurrent BCC Nodular primary BCC when: >1 cm in intermediate risk area* >2 cm in low risk area* <1 cm in high risk area* | Morpheaform or poor-defined types Nodular primary BCC when: >1 cm in high risk area* |
| Histological forms: aggressive,* recurrent forms (apart from superficial BCC) | |||
| Low-Risk | Intermediate and high-risk | Locally advanced | |
| Therapeutic strategies | Suspicious superficial BCC: Surgery Defined superficial BCC: surgery, topical therapies, local therapies, PDT Nodular BCC: surgery | Surgery Mohs micrographic surgery | Sonidegib Vismodegib |
* High-risk zones are the nose, periorificial areas of the head and neck; intermediate-risk zones are the forehead, cheek, chin, scalp, and neck; low-risk zones are the trunk and limbs. Aggressive histological forms include micronodular, morpheaform, and metatypical basosquamous forms. Perineural invasion also seems to be a histological sign of aggressiveness.
Table 2.
Prognostic groups of SCC and therapeutic strategies.
| Prognostic groups for SCC (NCCN SCC version 1.2021) | |||
|---|---|---|---|
| Risk group1 | Low-risk | High-risk | Very high-risk |
| Location/size2 | Trunk, extremities < 2 cm | Trunk, extremities > 2 cm < 4 cm Head, neck, hands, feet, pretibia, and anogenital (any size)5 | ≥ 4 cm Any location |
| Primary vs recurrent | Primary | Recurrent | |
| Immunosuppression | (-) | (+) | |
| Site of prior RT or chronic inflammatory process | (-) | (+) | |
| Rapidly growing tumor | (-) | (+) | |
| Neurologic symptoms | (-) | (+) | |
| Histopathology | |||
| Degree of differentiation | Well or moderated differentiation | Poorly differentiation | |
| Adenoid, adenosquamous, or metaplastic subtypes | (-) | (+) | Desmoplastic SCC |
| Depth: thickness or level of invasion3,4 | ≤ 6 mm or no invasion beyond | > 6 mm or invasion | |
| subcutaneous fat | beyond subcutaneous fat | ||
| Perineural involvement | (-) | (+) | Tumor cells within th nerve sheath of a nerve lying deeper than the dermis or measuring ≥ 0.1 mm |
| Lymphatic or vascular involvement | (-) | (-) | (+) |
| Therapeutic strategies | |||
| Low-risk | High-risk and very high-risk | Locally advanced or metastatic | |
| Surgery | Surgery | ||
| Mohs micrographic surgery | Cemiplimab | ||
1Risk stratification should be based on the highest risk factor present.
2Preoperative clinical tumor diameter.
3If clinical evaluation of incisional biopsy suggests that microstaging is inadequate, consider narrow margin excisional biopsy.
4Deep invasion is defined as invasion beyond the subcutaneous fat OR>6 mm.
5Location on the head, neck, hands, feet, pretibia, or anogenital area constitutes high risk based on location, independent of size.
Furthermore, it could be possible to consider the excision with complete circumferential peripheral and deep margin assessment (CCPDMA), that is an advanced surgical treatment using intraoperative frozen section assessment of all deep and peripheral margins, as an efficient alternative to MMS.12
Local therapy with curettage, electrodessication, cryosurgery and lasertherapy
These therapies must be limited to lowrisk BCCs because generally they lack histological examination. Furthermore, in case of doubt regarding the diagnosis, an incisional biopsy must be performed before the treatment is performed.
Curettage and electrodessication are considered as fast and cost-effective technique, as indicated by the NCCN, for selected low-risk BCCs.12
Cryosurgery is an easy, fast, and costeffective technique able to destroy the skin cancer by freeze-thaw cycles. Some large case series report cure rates from 94% to 99% for BCC.23
Laser therapy has been used for BCC treatment as monotherapy and adjunct therapy. 24 The most utilized lasers reported are superpulsed carbon dioxide and pulsed neodymium-based laser therapy.25,26 Reactive hyperemia, edema, scarring, and soreness could occur as adverse effects (AEs) of these therapies.3
Topical therapies
Imiquimod 5% cream and 5-fluorouracil (5-FU) 5% cream are used for the treatment of superficial BCC but their use should be limited to BCCs that could not be treated with other regimens or when cosmetic results are of major concern.27,28
Imiquimod 5% cream is an immune response modifier approved for the treatment of non-facial superficial BCC. Treatment regimen for BCC is five times weekly for six weeks. In a randomized controlled trial (RCT) that used twice daily imiquimod 5% for 12 weeks for superficial BCC, a 100% histologic clearance after 6 weeks of treatment has been reported.29 It has been shown that the application of imiquimod 5% cream once daily for 12 weeks for nodular BCCs led to a 76% clinical clearance.30 Imiquimod 5% cream is also used in case of basal cell nevus syndrome or Gorlin-Goltz syndrome.31 AEs of imiquimod 5% cream are local erythema and irritation of the skin and systemic effects include fatigue, fever and exfoliative dermatitis.
5-FU is recommended for superficial BCC but not for nodular forms.3 A statistically equivalent efficacy between 5-FU and imiquimod 5% in treating superficial BCC at a 12-month follow-up has been reported in a RCT.32 Other studies with longer follow- up showed a superiority of imiquimod compared to 5-FU.33 AEs of 5-FU could be erythema, swelling, and erosions.34
Photodynamic therapy
Photodynamic therapy (PDT) is a technique to mainly treat superficial BCCs or thinner nodular subtype, generally in patients affected by extensive or multifocal disease or multiple AKs.3 It consists in the application of a photosensitizing agent, aminolevulinic acid or methyl aminolevulinate, followed by irradiation with a light source. Studies reported that cure rates for BCC range from 70% to 90% but some of these studies had short follow-up periods.35 The PDT treatment protocol for BCC generally consists of two separate sessions, interrupted by a week, repeatable after three months in case of recurrence of the cancer.
Intralesional therapy
Intralesional chemotherapy is rarely used and mainly for high-risk BCC in patients not candidates for surgical therapy. It consists in the application of drugs such as 5-FU, interferons, interleukin-2, and bleomycin, and it has been used to treat BCC with variable results. AEs are unusual and mainly dose-dependent consisting of local effects at the treatment site and flulike symptoms.36,37
Elettrochemotherapy
Elettrochemotherapy (ECT) is a cancer therapy that combines the administration of a chemotherapy agent to the delivery of permeabilizing pulses released singularly or as bursts. It is based on the principle of electroporation of the cell membrane. This therapy is used for the local treatment of cutaneous metastases or for the treatment of primary cutaneous tumors such as BCC in patients not candidates for surgical therapy. In a single-center study, the complete response rate for 84 BCC patients, ineligible for conventional treatments, treated with ECT was overall 50%.38
Radiation
If surgery is contraindicated or the tumor is unresectable, radiation (RT) should be considered. RT should obtain a complete eradication of the BCC with preservation of the healthy tissue. Both teletherapy (external beam RT) and brachytherapy could be utilized to treat BCC.36 RT is contraindicated in patients affected by genetic syndromes, such as Gorlin-Goltz syndrome and xeroderma pigmentosum, or in case of lupus erythematosus or systemic scleroderma because it could increase the risk to induce other malignancies due to ionizing radiation.39 AEs include tissue necrosis, radiodermatitis, pigmentation, skin atrophy, telangiectasias, alopecia and the onset of radio-induced secondary skin cancers.8
Systemic therapy for locally advanced and metastatic basal cell carcinoma
Systemic therapy in the treatment of BCC is indicated in locally advanced and metastatic lesions, when surgical intervention and radiotherapy have been excluded.3 Historically, locally advanced or metastatic BCC was treated by chemotherapy after exclusion of surgical and radiotherapeutic options. Cisplatin-based monotherapy or combination regimens have been most frequently used. Despite reports of partial or complete responses, the therapeutic benefit of chemotherapy has never been demonstrated in prospective randomized clinical trials.40 Thus, chemotherapy is currently not recommended for the treatment of advanced BCC by international guidelines.12
Selecting patients appropriate for systemic therapy requires consideration of their functional status, comorbidities, social support and likely compliance with treatment and follow-up. Therefore, patients are best treated by a multidisciplinary team, including oncologists, dermatologists, surgeons (e.g. maxillofacial surgeon) and radiotherapist and medical oncologists, in order to identify the best therapeutic process. The clinicians have to discuss potential side effects of proposed treatment with the patient and ensure their appropriate clinical support.
The targeted systemic therapy of BCC consists of inhibitors of the Hedgehog (HH) pathway. The HH pathway plays a crucial role in organogenesis during early development, and is largely inactive in adults, except for its function in tissue repair and maintenance. Extracellular HH ligands bind to PTCH1 receptor relieving the inhibition of smoothened (SMO) by PTCH1 itself. SMO activates a signaling cascade of interacting proteins, including suppressor of fused (SUFU), resulting in activation of GLI family of transcription factors. The HH target genes include GLI1, PTCH1 and HH interacting protein (HHIP1) that regulate the pathway itself. The outcome of the HH signaling depends on transcription of several cell-specific targets mediating different cellular responses, including proliferation, differentiation, cell survival, self-renewal, angiogenesis, epithelial-mesenchymal transition. Aberrant activation of HH pathway is a tumor-driver in BCC pathogenesis. Therefore, the inhibition of the HH pathway can be considered a valid strategy to counteract neoplastic growth. The most frequent alterations in BCCs are the loss or inactivating mutations in PTCH1 or SUFU, as well as activating mutations in SMO or GLI. HH pathway activity can be inhibited through several mechanisms, including inhibition of the receptor-ligand interaction, direct binding to SMO, and inhibition of GLI transcription factors. Currently, in Italy, two targeted drugs are indicated and approved for the treatment of advanced BCC: vismodegib and sonidegib. The mechanism of action of both drugs consists in the inhibition of SMO and, in turn, in the prevention of the signal cascade, maintaining the suppression of the transcription factors GLI. Thus, the two molecules have both a cytostatic and cytotoxic action on tumoral cells.
Vismodegib is indicated in both locally advanced and metastatic BCC. Historically, it was the first member of the HH pathway inhibitors (HPIs) class that is now considered to be a first-line treatment option. The drug is administrated orally (150mg/d), every day at the same time. No dose reductions are provided for in the technical data sheet.
The approval of vismodegib by Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) in 2013 was based on the results of a phase II, open-label, noncomparative, international trial (ERIVANCE BCC), which showed high rates of tumor control in the indicated patient populations, including individuals with or without Gorlin syndrome.41,42 The pivotal ERIVANCE BCC study had enrolled 104 patients with locally advanced and metastatic BCC. The primary endpoint of the study was the ORR (Objective Response Rate), assessed through RECIST Criteria (Central Review), and results indicated 47,6% for locally advanced BCC and 33,3% for metastatic BCC. When assessed through RECIST criteria and Investigator Review, the ORR was 60,3% for locally advanced BCC and 48,5% metastatic BCC.41 The long-term update of the study demonstrated the durability of the response, the efficacy and the long-term safety. However, adverse events were significant. Indeed, discontinuation rate due to adverse effects was 21.2%. The deaths were 31.7% but none were related to vismodegib.43 The observational open-label STEVIE study was aimed at assessing the safety profile of vismodegib as primary endpoint in a more representative population. Thus, 1215 patients were enrolled. Most patients showed treatment-related side effects, including muscle spasms, alopecia, dysgeusia, weight loss, and asthenia. Secondary endpoint was efficacy. The ORR assessed through RECIST 1.1 Criteria and Investigator Review was 68.5% for locally advanced BCC and 36.9% for metastatic BCC.42 Primary and secondary resistance to vismodegib has been reported, albeit at a low rate compared with some other targeted therapies. Vismodegib is therefore an effective and generally well tolerated systemic therapy and, since its regulatory approval, has become an established treatment option in clinical practice for patients with locally advanced and metastatic BCC that can no longer be suitably controlled with surgery and/or radiotherapy. However, some limitations of vismodegib treatment should be kept in mind. The inevitably occurring side effects of vismodegib lead to a significant rate of treatment discontinuation limiting overall drug exposure. Hence, long-term continuous treatment with vismodegib is not feasible in most patients.
Sonidegib is indicated in locally advanced BCC. The drug is administrated orally (200mg/d), every day at the same time, away from food.
The results of the pivotal multicenter phase II study (BOLT) which evaluated efficacy and safety of Sonidegib, led to the approval of the drug as a first-line treatment for locally advanced BCC. This trial enrolled 230 patients with advanced BCC and compared two dosing regimens (200 vs. 800 mg per day) of Sonidegib in a doubleblinded, 1:2 randomized fashion.44 Sonidegib 200 mg demonstrated a better safety-risk profile than 800 mg at 30 months, with lower rates of grade 3/4 adverse events (43.0% vs. 64.0%) and adverse events leading to discontinuation (30.4% vs. 40.0%). Treatment-related side effects included muscle spasms, alopecia, dysgeusia, weight loss, and asthenia. Adverse events were managed with dose adjustments or interruptions, since Sonidegib offers in label the option for dose reduction of the drug. Patients receiving 200 mg of therapy had an ORR assessed through the stringent mRECIST criteria of 56,1% and of 71.2% assessed through central and investigator review respectively for locally advanced BCC, with a mDOR and a PFS of 26.1 and 22.1 months respectively (central review). On the contrary, objective response rates with 200mg were 7.7% (Central Review) and 23.1% (Investigator Review) in metastatic BCC.44,45 A group of clinical experts in the management of locally advanced BCC summarized in a recent paper the clinical and pharmacological profiles of sonidegib and vismodegib based on published data and their own clinical experience. 46 They highlighted that one key difference between the two pivotal studies was the criteria used to assess BCC severity. ERIVANCE used the conventional Response Evaluation Criteria in Solid Tumors (RECIST), while the more recent double-blind randomized BOLT trial used the more stringent modified RECIST (mRECIST). A preplanned analysis presented the outcomes from BOLT with RECISTlike criteria, and this enabled the experts to discuss relative efficacy outcomes for the two treatments. Centrally reviewed objective response rate (ORR) for vismodegib was 47.6% (95% CI: 35.5-60.6) at 21- month follow-up using RECIST. Using RECIST-like criteria, the ORR for sonidegib according to central review at 18-month follow-up was higher, at 60.6% (95% CI: 47.8-72.4).46 Both treatments were associated with similar patterns of adverse events. However, sonidegib demonstrated a longer time to adverse events onset (except for fatigue), with less frequent and less severe adverse events compared with vismodegib. 46 The pharmacokinetic profile of sonidegib and vismodegib shows several differences, such as volume of distribution and half-life. The consensus among the experts is that these pharmacokinetic differences could related to the differences seen in tolerability and efficacy between the two drugs.46 Although the efficacy and side effect profile of Sonidegib trial appear generally comparable to results from largescale studies with vismodegib for locally advanced BCC, direct comparative clinical studies would be necessary to thoroughly assess differences.42,46 Therefore, currently the only drug approved for the treatment of metastatic BCC is vismodegib whereas, for locally advanced BCC, two aforementioned therapeutic possibilities are possible.
Next landscape in advanced systemic treatment in case of progression or intolerance to HH inhibitors
Cemiplimab-rwlc is an anti-PD-1 antibody approved for treatment of advanced cutaneous SCC. On February 9, 2021, the Food and Drug Administration (FDA) granted regular approval to cemiplimabrwlc for patients with locally advanced BCC previously treated with a HH inhibitor or for whom a HH inhibitor is not appropriate. Furthermore, FDA granted accelerated approval to cemiplimab-rwlc for patients with metastatic BCC previously treated with a HH inhibitors or for whom a HH inhibitors is not appropriate.47 Recently has been reported an open-label, multicentre, single-arm, phase 2 trial across 38 outpatient clinics, that analyzed the data of cemiplimab in patients with locally advanced BCC after HP inhibitor therapy. An OR per independent central review was observed in 26 (31%; 95% CI 21-42) of 84 patients, confirming that cemiplimab exhibited clinically meaningful anti-tumor activity in patients with locally advanced BCC after HH inhibitor therapy.48 Based on these data, on May 2021 the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion regarding cemiplimab indicated, as monotherapy, for the treatment of adult patients with locally advanced or metastatic BCC who have progressed on or are intolerant to a HH inhibitor.49
Squamous cell carcinoma
Risk stratification for squamous cell carcinoma
A risk assessment for SCC has to be made to determine the treatment and follow- up for patients. Table 2 shows SCC risk stratification according to the most recent NCCN Guidelines considering SCC risk factors associated with recurrence and metastasis.13 Risk factors for SCC stratification include location and size of the tumor, primary versus recurrent disease, immunosuppression of the patient, site of prior RT or chronic inflammatory process, neurologic symptoms, and histopathological features (Table 2). Concerning the actual histopathological staging systems, it is worth to notice that the American Joint Committee on Cancer (AJCC) 8th edition SCC staging system expanded the criteria for upstaging to T3 and also included extranodal extension as a risk factor for upstaging of the N classification (Tables 3 and 4). Otherwise, the AJCC 8th edition is limited to head and neck tumors. An alternative T staging system (the Brigham and Women’s Hospital [BWH] Tumor Staging for SCC) proved in its ability to stratify low-risk versus highrisk tumors and it can be applied to tumors across all body sites.50
Therapy of squamous cell carcinoma
The primary goal for SCC therapy is the complete removal of the skin cancer with the maximal functional and cosmetic preservation. Surgical excision alone guarantees a successful treatment for SCC with a good prognosis and cure rates greater than 90%.51 Besides surgical therapy, traditional techniques such as curettage, electrodessication, cryosurgery, lasertherapy, and PDT are available for non-invasive SCC forms like Bowen disease. Furthermore, RT is a valid and curative treatment strategy for SCC.
Surgery and Mohs micrographic surgery
Traditional surgery and MMS are the two different surgical approaches that may be utilized in patients with primary SCC. It has been reported that SEPMA guarantees a 5-year disease-free rates of 91% or higher for SCC.52,53 Safety excision margins have to be defined according to the risk of subclinical extensions, recurrence or and metastasis of the skin cancer depending on the low or high-risk factors for SCC.54 Considering low-risk SCC, AIOM and NCCN guidelines recommend free-margins of at least 4-mm for low-risk types treated with SEPMA.9,13 The European consensus group proposed a 5-mm margin for low-risk SCC.11 Otherwise, for high-risk SCC, NCCN guidelines recommend wider surgical margins compared to low-risk SCC and postoperative margin assessment.13 NCCN guidelines does not recommend a defined margin for standard excision for high-risk SCC due to the wide variability of clinical characteristics that defined this type of SCC.13 The European consensus group recommends 6-10 mm safety margins for highrisk SCC.13 In case of positive margins, it should be performed a re-excision, for operable cases.11 In case of margins that appear more limited than the recommended safety margins due to the tissue shrinkage a wider excision should be considered.11 We also recommend, as alternative to surgery, a close follow-up.
MMS offers the highest rate of R0 resection (i.e., no cancer cells seen microscopically at the primary tumor site), above 90%, with lower recurrence rates (0-4%) compared to traditional surgery (3.1-8.0%).55,56 MMS is mainly considered for patients with high-risk SCC to obtain a complete tumor resection with optimal anatomic and functional preservation. Otherwise, MMS is performed in limited centers and more time-consuming, labourintensive, and expensive compared to traditional surgery. Van Lee et al., confirmed in a retrospective study that MMS could be superior to standard excision for SCC of the head and neck for the lower rate of recurrence. 57
Alternative treatment for low-risk squamous cell carcinoma: curettage, electrodessication, cryotherapy, and PDT
Curettage and electrodessication may be considered for small and low-risk primary SCC, according to NCCN guidelines.5,13
As indicated by NCCN guidelines, cryotherapy could be a treatment option in selected cases of low-risk SCC while there is scarce evidence regarding efficacy of PDT for invasive SCC and it should not be performed in these cases.13,58
Figure 4.
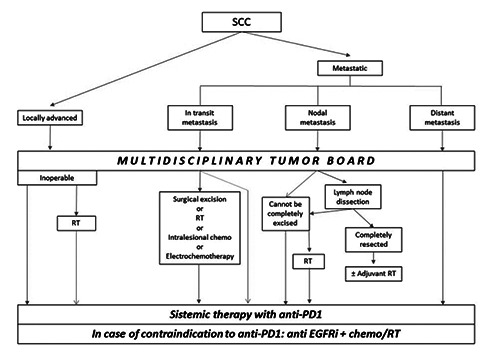
Main therapeutic indications for locally advanced and metastatic cutaneous SCC as indicated in the “European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2” in 2020. SCC, cutaneous squamous cell carcinoma; RT, radiotherapy; EGFRi, epidermal growth factor receptor inhibitors.
Surgery for regional nodal disease
Patients affected by SCC nodal metastases should be surgically treated similarly to patients affected by melanoma or Merkel cell carcinoma. If surgery is not possible due to patient-related factors, a non-surgical approach such as immunotherapy with cemiplimab should be considered by the MWG. The regional lymph node dissection is the surgical treatment of choice in case of nodal metastasis.11,59,60
Considering patients affected by SCC and negative lymph node, an elective or prophylactic lymph node dissection is not recommended due to the low rate of nodal metastases, the high morbidity and the limited evidence in patients with mucosal head and neck SCC.61,62
Elettrochemotherapy
ECT is an alternative treatment for unresectable SCC consisting of an intravenous injection of a chemotherapy agent, such as cisplatin or bleomycin, combined with local electric pulses that permeabilize tumor cell membranes to increase its cytotoxicity. 63
In some retrospective studies and one meta-analysis, it has been reported that 20-70% of patients treated with ECT presented a good local response and disease control, while, in a prospective study EURECA on SCC patients the rate of complete response at 2-months follow-up was 55% with 4% rate of progression only.64-67
Radiation
RT could be an alternative treatment in case of patients affected by SCC who are not eligible for surgery such as locally advanced tumor, multi-morbidity or frail elderly patient at high risk for surgery, or patient that refuse surgery. Otherwise, surgery must be chosen wherever possible because RT presents lower cure rates and many cases of aggressive post-treatment recurrence have been observed. In a metaanalysis of 14 observational studies, a 6.4% average rate of local tumor recurrence after the first-line RT in 1018 primary SCC has been reported.68
RT could also be considered as an esthetic option for SCC localized on neck and head or as a functional option for SCC localized on sensitive areas such as lips or eyelids. Furthermore, radical primary RT could be used for small SCC.69,70
AEs related to RT are rare and consist of radiodermatitis, hypo/hyperpigmentation, and telangiectasia. Furthermore, RT is not recommended in patients with genetic disorders such as Gorlin syndrome or ataxia telangiectasia and others for the higher risk of radiosensitivity.71
Systemic treatment
Most advanced SCC, including locally advanced or metastatic lesions, may be nonresectable. Locally advanced SCC has been defined as a non-metastatic SCC, not amenable to either surgery or RT with reasonable hope for cure, because of multiple recurrences, large extension, bone erosion or invasion, or deep infiltration beyond subcutaneous tissue into muscle or along nerves, or else tumors in which curative resection would result in unacceptable complications, morbidity or deformity.11 Metastatic SCC includes loco-regional metastatic SCC with in-transit metastases or metastasis to regional lymph nodes, or distant metastatic SCC.11 Until recently, treatment options have been off-label chemotherapy or anti-epidermal growth factor receptor (EGFR) therapies. However, the clinical evidence for these options is limited and chemotherapy is associated with a high risk of significant adverse events, especially in older patients.72
The therapy with immune checkpoint inhibitors (ICI), which block programmed cell death protein-1 (PD-1), up to now represents the first-line therapy for advanced SCC (Figure 4). Suppression of the immune system appears to be a significant event in cutaneous SCC development, as evidenced by immunosuppression being a significant risk factor and the efficacy of immunomodulators in AK. The immunogenicity of cutaneous SCC is due to the exposition of neoantigens by tumor cells. Indeed, they display a high tumor mutational burden (TMB) caused by chronic UV exposure. Notably, the response to ICI treatment in several tumors is correlated to TMB.74
Table 3.
TNM Staging Classification for Cutaneous Carcinoma of the Head and Neck according to the American Joint Committee on Cancer (AJCC) (8th ed., 2017). Definitions for T and clinical N.
| T | Primary Tumor | |
|---|---|---|
| TX | Primary tumor cannot be assessed | |
| Tis | Carcinoma in situ | |
| T1 | Tumor smaller than or equal to 2 cm in greatest dimension | |
| T2 | Tumor larger than 2 cm, but smaller than or equal to 4 cm in greatest dimension | |
| T3 | Tumor larger than 4 cm in maximum dimension or minor bone erosion or perineural invasion or deep invasion* | |
| T4 | Tumor with gross cortical bone/marrow, skull base invasion and/or skull base foramen invasion | |
| T4a | Tumor with gross cortical bone/marrow invasion | |
| T4b | Tumor with skull base invasion and/or skull base foramen involvement | |
| cN | Regional Lymph Nodes | |
| NX | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | |
| N1 | Metastasis in a single ipsilateral lymph node, 3 cm or smaller in greatest dimension and ENE(−) | |
| N2 | Metastasis in a single ipsilateral node larger than 3 cm but not larger than 6 cm in greatest dimension and ENE(−);or metastases in multiple ipsilateral lymph nodes, none larger than 6 cm in greatest dimension and ENE(−); or in bilateral or contralateral lymph nodes, none larger than 6 cm in greatest dimension and ENE(−) | |
| N2a | Metastasis in a single ipsilateral node larger than 3 cm but not larger than 6 cm in greatest dimension and ENE(−) | |
| N2b | Metastases in multiple ipsilateral nodes, none larger than 6 cm in greatest dimension and ENE(−) | |
| N2c | Metastases in bilateral or contralateral lymph nodes, none larger than 6 cm in greatest dimension and ENE(−) | |
| N3 | Metastasis in a lymph node larger than 6 cm in greatest dimension and ENE(−);or metastasis in any node(s) and clinically overt ENE [ENE(+)] | |
| N3a | Metastasis in a lymph node larger than 6 cm in greatest dimension and ENE(−) | |
| N3b | Metastasis in any node(s) and ENE (+) |
*Deep invasion is defined as invasion beyond the subcutaneous fat or >6 mm (as measured from the granular layer of adjacent normal epidermis to the base of the tumor); perineural invasion for T3 classification is defined as tumor cells within the nerve sheath of a nerve lying deeper than the dermis or measuring 0.1 mm or larger in caliber, or presenting with clinical or radiographic involvement of named nerves without skull base invasion or transgression. Note: A designation of “U” or “L” may be used for any N category to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L). Similarly, clinical and pathological extranodal extension (ENE) should be recorded as ENE(−) or ENE(+).
Cemiplimab (Libtayo®) is a fully human IgG4 antibody against PD-1, which blocks the signaling between PD-1 and its ligands PD-L1 and PD-L2. It is the first approved systemic treatment for advanced SCC, including locally advance and metastatic lesions, in patients who are not candidates for curative surgery or radiotherapy in the USA and Europe.73-76 Up to now, cemiplimab represents the only approved systemic therapy for cutaneous SCC in Europe. The approved fixed dose regimen is 350 mg intravenously every three weeks. Other inhibitors of PD-1/PDL-1-axis are under investigation and Pembrolizumab, a PD-1 inhibitor, has been recently approved by FDA on the basis of a phase II trial for patients with recurrent or metastatic cutaneous SCC that is not curable by surgery or radiation.77
The safety and the efficacy of intravenous cemiplimab was investigated in a phase I study for expansion cohorts of patients with locally advanced or metastatic cutaneous squamous-cell carcinoma (NCT02383212), as well as in a pivotal phase II study for a cohort of patients with metastatic disease (metastatic-disease cohort or group 1) (NCT02760498 EMPOWER-CSCC 1).75 Patients who had undergone organ transplantation and patients with hematologic malignancies or any immunosuppressive conditions were excluded in both studies. The patients were treated with cemiplimab (3 mg per kilogram of body weight) intravenously every 2 weeks for up to 48 weeks (phase I) or 96 weeks (phase II) unless stopped due to disease progression or non-tolerable toxic effects. Patients were assessed for a response every 8 weeks. The primary outcomes of the phase I study were the safety and adverse event profile, while the response rate assessed by independent central review was the primary outcome of the phase II study. In the phase 1 study, a response to cemiplimab was observed in 13 of 26 patients (50%; 95% CI = 30 to 70). In the metastatic-disease cohort of the phase II study, a response was observed in 28 of 59 patients (47%; 95% CI = 34 to 61). The median follow-up was 11.1 months in the phase I and 7.9 months in the metastaticdisease cohort of the phase II study. Among the 28 patients who had a response, the duration of response exceeded 6 months in 57%, and 82% continued to have a response and to receive cemiplimab at the time of data cutoff. The treatment was generally well tolerated with only 7% of patients stopping therapy due to adverse events. The most commonly reported adverse events (15%) usually occur with immune checkpoint inhibitors, such as diarrhea, fatigue, nausea, constipation and rash. In 3 of 11 patients in the phase II cohort who died during the study, death was associated with a non-treatment emergent adverse event. Based on this study, cemiplimab was approved in September 2018 by the FDA. In 2020, Migden et al.75 reported the clinical activity of cemiplimab from the primary analysis of patients with locally advanced cutaneous SCC (group 2) from the pivotal phase II study (NCT02760498). Between 2016 and 2018, 78 patients were enrolled and treated with cemiplimab (3 mg/kg) intravenously over 30 min every 2 weeks for up to 96 weeks. Tumor measurements were done every 8 weeks. An objective response was observed in 34 (44%; 95% CI 32-55) of 78 patients. The best overall response was ten (13%) patients with a complete response and 24 (31%) with a partial response. Grade 3-4 treatment-emergent adverse events occurred in 34 (44%) of 78 patients. The most common were hypertension in six (8%) patients and pneumonia in four (5%). Serious treatment-emergent adverse events occurred in 23 (29%) of 78 patients. One treatment-related death was reported that occurred after onset of aspiration pneumonia. Thus, cemiplimab showed antitumor activity and an acceptable safety profile in patients with locally advanced cutaneous squamous cell carcinoma for whom there was no widely accepted standard of care.75 In the meantime, Rischin et al.76 published outcomes of the primary analysis of fixed dose cemiplimab 350 mg intravenously treatment every 3 weeks (Group 3) and provide a long-term followup after the primary analysis of weightbased cemiplimab 3 mg/kg intravenously every 2 weeks (Q2W) (Group 1) among metastatic SCC patients in the pivotal study phase II. For Group 3 (n=56) and Group 1 (n=59), median follow-up was 8.1 and 16.5 months, respectively. ORR per ICR was 41.1% (95% CI, 28.1% to 55.0%) in Group 3, 49.2% (95% CI, 35.9% to 62.5%) in Group 1, and 45.2% (95% CI, 35.9% to 54.8%) in both groups combined. Per ICR, Kaplan-Meier estimate for DOR at 8 months was 95.0% (95% CI, 69.5% to 99. 3%) in responding patients in Group 3, and at 12 months was 88.9% (95% CI, 69.3% to 96.3%) in responding patients in Group 1. Per INV, ORR was 51.8% (95% CI, 38.0% to 65.3%) in Group 3, 49.2% (95% CI, 35.9% to 62.5%) in Group 1, and 50.4% (95% CI, 41.0% to 59.9%) in both groups combined. Overall, the most common adverse events regardless of attribution were fatigue (27.0%) and diarrhea (23.5%). Therefore, durable responses and similar safety profile have been observed in both weight-based and fixed-dosing groups.76 A further update of this trial has been presented at 10th World Congress of Melanoma in 2021 with a prolonged follow-up at 43 months. Compared to previous analyses, the 43-month follow-up data demonstrated incremental improvements in DOR with cemiplimab treatment across all locally advanced SCC study groups, as well as improvements in ORR and complete response rate on the cemiplimab 350 mg every 3 weeks regimen. Furthermore, there were no new safety signals compared with previous reports on cemiplimab in advanced cutaneous SCC.
Table 4.
TNM Staging Classification for Cutaneous Carcinoma of the Head and Neck according to the American Joint Committee on Cancer (AJCC) (8th ed., 2017). Definitions for Pathological N and metastasis.
| pN | Regional Lymph Nodes | |
|---|---|---|
| NX | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | |
| N1 | Metastasis in a single ipsilateral lymph node, 3 cm or smaller in greatest dimension and ENE(−) | |
| N2 | Metastasis in a single ipsilateral lymph node, 3 cm or smaller in greatest dimension and ENE(+); or larger than 3 cm but not larger than 6 cm in greatest dimension and ENE(−); or metastases in multiple ipsilateral lymph nodes, none larger than 6 cm in greatest dimension and ENE(−); or in bilateral or contralateral lymph node(s), none larger than 6 cm in greatest dimension, ENE(−) | |
| Metastasis in single ipsilateral node 3 cm or smaller in greatest dimension and ENE(+); or a single ipsilateral node larger than 3 cm but not larger than 6 cm in greatest dimension and ENE(−) | ||
| N2a | Metastases in multiple ipsilateral nodes, none larger than 6 cm in greatest dimension and ENE(−) | |
| N2b | Metastases in bilateral or contralateral lymph node(s), none larger than 6 cm in greatest dimension and ENE(−) | |
| N3 | N2c | Metastasis in a lymph node larger than 6 cm in greatest dimension and ENE(−); or in a single ipsilateral node larger than 3 cm in greatest dimension and ENE(+); or multiple ipsilateral, contralateral, or bilateral nodes, any with ENE(+); or a single contralateral node of any size and ENE(+) |
| N3a | Metastasis in a lymph node larger than 6 cm in greatest dimension and ENE(−) | |
| N3b | Metastasis in a single ipsilateral node larger than 3 cm in greatest dimension and ENE(+); or multiple ipsilateral, contralateral, or bilateral nodes, any with ENE(+); or a single contralateral node of any size and ENE(+) | |
| M | Distant Metastasis | |
| M0 | No distant metastasis | |
| M1 | Distant metastasis | |
| G | Histologic Grade | |
| GX | Grade cannot be assessed | |
| G1 | Well differentiated | |
| G2 | Moderately differentiated | |
| G3 | Poorly differentiated | |
| G4 | Undifferentiated |
Staging
TNM staging classification for head and neck BCC and cutaneous SCC and prognostic stage groups according to the AJCC 8th edition is represented in Tables 3-5.
Follow up
The patient should be regularly monitored to recognize any new NMSC or relapses of the tumor and, mainly for locally advanced or metastatic cases, to manage the HPI therapy for BCC or immunotherapy for SCC. Furthermore, it is essential to educate the patient regarding the correct photoprotection and the periodic self-control of skin lesions.
Basal cell carcinoma follow-up
Considering that a patient with a previous diagnosis of BCC has a 15% higher risk to develop another BCC in one year and 35% in 5 years and that the risk increases in case of multiple previous BCCs, it is important to advice a periodic follow-up to the patient. This follow-up should be performed by dermatologists and should include the inspection of the entire body. Monitoring in the first two years is essential. Furthermore, dermatological examination should be performed every 6-12 months (Table 6).12
Squamous cell carcinoma follow-up
Considering that 95% of relapses and the same percentage of metastases occur within the first five years from SCC diagnosis and that 30-50% of patients may present a second SCC within 5 years, patients affected by SCC requires a long-term follow- up.
SCC patients should be monitored with regular physical exams that include complete skin and regional lymph node examination. The frequency of follow-up depends on the risk of SCC and it is indicated in Table 6.
Conclusions
The ICP for NMSC represents an innovative strategy to support the highest quality health care system, favouring all necessary procedures for the patients, optimizing the necessary timing, and guaranteeing an updated clinical knowledge of the various health professionals involved. The hub-andspoke model is essential for the organization of different health care structures to guarantee the best management and treatment for patients affected by NMSC. Considering the increasing incidence of these very common tumors and the high impact on the sanitary system, it is crucial to create an efficient and dedicated ICP with a valid organization within the different geographical areas.
Table 5.
Prognostic stage groups according to the American Joint Committee on Cancer (AJCC) (8th ed., 2017)
| T | N | M | |
|---|---|---|---|
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1 | N1 | M0 | |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | |
| Stage IV | T1 | N2 | M0 |
| T2 | N2 | M0 | |
| T3 | N2 | M0 | |
| Any T | N3 | M0 | |
| T4 | Any N | M0 | |
| Any T | Any N | M1 |
Table 6.
BCC and SCC recommended follow-up (NCCN BCC and SCC version 2021).
| BCC | 6-12 months |
|---|---|
| Low-risk SCC | Every 3-12 months for 2 years, then every 6-12 months for 3 years, then annually for life |
| High-risk SCC | Every 3-6 months for 2 years, then every 6-12 months for 3 years, then annually for life |
| Very high-risk SCC | Every 3-6 months for 2 years, then every 6 months for 3 years, then every 6-12 months for life |
Funding Statement
Funding: None.
References
- 1.Guy GP, Ekwueme DU. Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: A systematic review of the literature. Pharmacoeconomics 2011;29:863-74. [DOI] [PubMed] [Google Scholar]
- 2.Wang GY, Wang J, Mancianti M-L, Epstein EH. Basal Cell Carcinomas Arise from Hair Follicle Stem Cells in Ptch1+/− Mice. Cancer Cell 2011;19:114-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fania L, Didona D, Morese R, et al. Basal Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2020;8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boi S, Cristofolini M, Micciolo R, et al. Epidemiology of Skin Tumors: Data from the Cutaneous Cancer Registry in Trentino, Italy. J Cutan Med Surg Inc Med Surg Dermatology 2003;7:300-5. [DOI] [PubMed] [Google Scholar]
- 5.Fania L, Didona D, Di Pietro FRFR, et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EUROPEAN ASSOCIATION P. Percorsi diagnostici terapeutici e assistenziali (PDTA) [Internet]. Available from: http://e-p-a.org/sito-internet-e-pa/percorsi-diagnostici-terapeutici-eassistenziali-pdta/ [Google Scholar]
- 7.Fania L, Morese R, Di Lella G, et al. The integrated care pathway for non melanoma skin cancer: the Istituto Dermopatico dell’Immacolata - IRCCS experience in Rome. Recenti Prog Med 2020;111:749-60. [DOI] [PubMed] [Google Scholar]
- 8.Aiom. Linee guida: Tumori Cutanei Non Melanoma. 2020. [Google Scholar]
- 9.Queirolo P. Linee Guida Tumori Cutanei Non Melanoma Carcinoma squamocellulare cutaneo. 2019 [Google Scholar]
- 10.Peris K, Fargnoli MC, Garbe C, et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer 2019;118:10-34. [DOI] [PubMed] [Google Scholar]
- 11.Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur J Cancer 2020;128:60-82. [DOI] [PubMed] [Google Scholar]
- 12.NCCN Guidelines Guidelines N.Basal cell skin cancer Version 1.2021. Medlin Med Encycl 2021. Available from: https://www.nlm.nih.gov/medlineplus/ency/article/000829.htm [Google Scholar]
- 13.NCCN Guidelines. Squamous cell skin cancer Version 1.2021. Medlin Med Encycl. 2021. [Google Scholar]
- 14.Elrod JK, Fortenberry JL. The hub-andspoke organization design: An avenue for serving patients well. BMC Health Serv Res 2017;17:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devarakonda S.Hub and spoke model: Making rural healthcare in India affordable, available and accessible. Rural Remote Health 2016;16:1-8. [PubMed] [Google Scholar]
- 16.Ahlquist G, Saxena SB, Belokrinitsky I, Kapur A.Charting a clear course in rough seas: a new view on hospital and health systems strategy. New York Strateg 2012. [Google Scholar]
- 17.Huitema AA, Harkness K, Heckman GA, McKelvie RS. The Spoke-Huband- Node Model of Integrated Heart Failure Care. Can J Cardiol 2018;34:863-70. [DOI] [PubMed] [Google Scholar]
- 18.Tensen E, van der Heijden JP, Jaspers MWM, Witkamp L.Two Decades of Teledermatology: Current Status and Integration in National Healthcare Systems. Curr Dermatol Rep 2016;5:96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trakatelli M, Morton C, Nagore E, et al. Update of the European guidelines for basal cell carcinoma management: Developed by the guideline subcommittee of the European Dermatology Forum. Eur J Dermatol 2014;24:312-29. [DOI] [PubMed] [Google Scholar]
- 20.Bath-Hextall F, Bong J, Perkins W, Williams H.Interventions for basal cell carcinoma of the skin: Systematic review. Br Med J 2004;329:705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol 1992;27:241-8. [DOI] [PubMed] [Google Scholar]
- 22.Rowe D, Carroll RJ, Day CL.Mohs Surgery Is the Treatment of Choice for Recurrent (Previously Treated) Basal Cell Carcinoma. J Dermatol Surg Oncol 1989;15:424-31. [DOI] [PubMed] [Google Scholar]
- 23.Kuflik EG. Cryosurgery for Skin Cancer: 30-Year Experience and Cure Rates. Dermatologic Surg 2004;30:297-300. [DOI] [PubMed] [Google Scholar]
- 24.Lanoue J, Goldenberg G.Basal Cell Carcinoma: A Comprehensive Review of Existing and Emerging Nonsurgical Therapies. J Clin Aesthet Dermatol 2016;9:26-36. [PMC free article] [PubMed] [Google Scholar]
- 25.Campolmi P, Brazzini B, Urso C, et al. Superpulsed CO2 laser treatment of basal cell carcinoma with intraoperatory histopathologic and cytologic examination. Dermatologic Surg 2002;28:909-12. [DOI] [PubMed] [Google Scholar]
- 26.Moskalik K, Kozlov A, Demin E, Boiko E.The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg 2009;27:345-9. [DOI] [PubMed] [Google Scholar]
- 27.Geisse J, Caro I, Lindholm J, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J Am Acad Dermatol 2004;50:722-33. [DOI] [PubMed] [Google Scholar]
- 28.Micali G, Lacarrubba F, Nasca MR, Schwartz RA. Topical pharmacotherapy for skin cancer: Part I. Pharmacology. J Am Acad Dermatol 2014;70:965. [DOI] [PubMed] [Google Scholar]
- 29.Geisse JK, Rich P, Pandya A, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: A double-blind, randomized, vehicle-controlled study. J Am Acad Dermatol 2002;47:390-8. [DOI] [PubMed] [Google Scholar]
- 30.Shumack S, Robinson J, Kossard S, et al. Efficacy of topical 5% imiquimod cream for the treatment of nodular basal cell carcinoma: Comparison of dosing regimens. Arch Dermatol 2002;138:1165-71. [DOI] [PubMed] [Google Scholar]
- 31.Micali G, Lacarrubba F, Nasca MR, De Pasquale R.The use of imiquimod 5% cream for the treatment of basal cell carcinoma as observed in Gorlin’s syndrome. Clin Exp Dermatol 2003;28:19-23. [DOI] [PubMed] [Google Scholar]
- 32.Arits AHMM, Mosterd K, Essers BAB, et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: a single blind, non-inferiority, randomised controlled trial. Lancet Oncol 2013;14:647-54. [DOI] [PubMed] [Google Scholar]
- 33.Roozeboom MH, Arits AHMM, Mosterd K, et al. Three-Year Follow-Up Results of Photodynamic Therapy vs. Imiquimod vs. Fluorouracil for Treatment of Superficial Basal Cell Carcinoma: A Single-Blind, Noninferiority, Randomized Controlled Trial. J Invest Dermatol 2016;136:1568-74. [DOI] [PubMed] [Google Scholar]
- 34.Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: A systematic review. Arch Dermatol 2009;145:1431-8. [DOI] [PubMed] [Google Scholar]
- 35.Savoia P, Deboli T, Previgliano A, Broganelli P.Usefulness of photodynamic therapy as a possible therapeutic alternative in the treatment of basal cell carcinoma. Int J Mol Sci 2015;16:23300-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: Contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol 2019;80:321-39. [DOI] [PubMed] [Google Scholar]
- 37.Good LM, Miller MD, High WA. Intralesional agents in the management of cutaneous malignancy: A review. J Am Acad Dermatol 2011;64:413-22. [DOI] [PubMed] [Google Scholar]
- 38.Campana LG, Marconato R, Valpione S, et al. Basal cell carcinoma: 10-year experience with electrochemotherapy. J Transl Med 2017;15:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neville JA, Welch E, Leffell DJ. Management of nonmelanoma skin cancer in 2007. Nat Clin Pract Oncol 2007;4:462-9. [DOI] [PubMed] [Google Scholar]
- 40.Moeholt K, Aagaard H, Pfeiffer P, Hansen O.Platinum-Based Cytotoxic Therapy in Basal Cell Carcinoma a review of the literature. Acta Oncol (Madr) 1996;35:677-82. [DOI] [PubMed] [Google Scholar]
- 41.Sekulic A, Migden MR, Oro AE, et al. Efficacy and Safety of Vismodegib in Advanced Basal-Cell Carcinoma. N Engl J Med 2012;366:2171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur J Cancer 2017;86:334-48. [DOI] [PubMed] [Google Scholar]
- 43.Sekulic AA, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017;17:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol 2015;16:716-28. [DOI] [PubMed] [Google Scholar]
- 45.Lear JT, Migden MR, Lewis KD, et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J Eur Acad Dermatology Venereol 2018;32:372-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatology Venereol 2020;34:1944-56. [DOI] [PubMed] [Google Scholar]
- 47.U.S Food & Drug Administration. FDA approves cemiplimab-rwlc for locally advanced and metastatic basal cell carcinoma 2021. Available from: https://www.fda.gov/drugs/resourcesinformation-approved-drugs/fdaapproves-cemiplimab-rwlc-locallyadvanced-and-metastatic-basal-cellcarcinoma [Google Scholar]
- 48.Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021. [DOI] [PubMed] [Google Scholar]
- 49.European Medicine Agency. Libtayo as monotherapy is indicated for the treatment of adult patients with metastatic or locally advanced cutaneous squamous cell carcinoma. 2021. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/libtayo [Google Scholar]
- 50.Ruiz ES, Karia PS, Besaw R, Schmults CD. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs the Brigham and Women’s Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol 2019;155:819-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brougham NDLS, Dennett ER, Cameron R, Tan ST. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol 2012;106:811-5. [DOI] [PubMed] [Google Scholar]
- 52.Rowe D, Carroll R, Day C.Prognostic factors for local recurrence, metastasis and survival rates in SCC of the skin, ear and lip. JAMA Dermatol 1992;26:976-90. [DOI] [PubMed] [Google Scholar]
- 53.Griffiths RW, Suvarna SK, Feeley K.Audit of clinical and histological prognostic factors in primary invasive squamous cell carcinoma of the skin: Assessment in a minimum 5-year follow- up study after conventional excisional surgery. Br J Plast Surg 2002;55:287-92. [DOI] [PubMed] [Google Scholar]
- 54.Motaparthi K, Kapil JP, Velazquez EF. Cutaneous Squamous Carcinoma 8Th. 2017;24:171-94. [DOI] [PubMed] [Google Scholar]
- 55.Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamouscell carcinoma: a prospective study. Lancet Oncol 2008;9:713-20. [DOI] [PubMed] [Google Scholar]
- 56.Chren MM, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol 2013;133:1188-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Lee CB, Roorda BM, Wakkee M, et al. Recurrence rates of cutaneous squamous cell carcinoma of the head and neck after Mohs micrographic surgery vs. standard excision: a retrospective cohort study. Br J Dermatol 2019;181:338-43. [DOI] [PubMed] [Google Scholar]
- 58.Morton C, Szeimies R-M, Sidoroff A, et al. European Dermatology Forum guidelines on topical photodynamic therapy. Eur J Dermatology 2015;25:296-311 [DOI] [PubMed] [Google Scholar]
- 59.Skulsky SL, O’Sullivan B, McArdle O, et al. Review of high-risk features of cutaneous squamous cell carcinoma and discrepancies between the American Joint Committee on Cancer and NCCN Clinical Practice Guidelines In Oncology. Head Neck 2017;39:578-94. [DOI] [PubMed] [Google Scholar]
- 60.Stratigos A, Garbe C, Lebbe C, et al. European Dermatology Forum (EDF); European Association of Dermato- Oncology (EADO); European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interd. Eur J Cancer 2015;51:1989-2007. [DOI] [PubMed] [Google Scholar]
- 61.Xiao Y, Yuan S, Liu F, et al. Comparison between wait-and-see policy and elective neck dissection in clinically N0 cutaneous squamous cell carcinoma of head and neck. Medicine (Baltimore) 2018;97:e10782-e10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cannon RB, Dundar Y, Thomas A, et al. Elective Neck Dissection for Head and Neck Cutaneous Squamous Cell Carcinoma with Skull Base Invasion. Otolaryngol Head Neck Surg 2017;156:671-6. [DOI] [PubMed] [Google Scholar]
- 63.Gehl J, Sersa G, Matthiessen LW, et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol 2018;57:874-82. [DOI] [PubMed] [Google Scholar]
- 64.Testori A, Tosti G, Martinoli C, et al. Electrochemotherapy for cutaneous and subcutaneous tumor lesions: a novel therapeutic approach. Dermatol Ther 2010;23:651-61. [DOI] [PubMed] [Google Scholar]
- 65.Di Monta G, Caracò C, Simeone E, et al. Electrochemotherapy efficacy evaluation for treatment of locally advanced stage III cutaneous squamous cell carcinoma: a 22-cases retrospective analysis. J Transl Med 2017;15:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seyed Jafari SM, Jabbary Lak F, Gazdhar A, et al. Application of electrochemotherapy in the management of primary and metastatic cutaneous malignant tumours: a systematic review and meta-analysis. Eur J Dermatol 2018;28:287-313. [DOI] [PubMed] [Google Scholar]
- 67.Bertino G, Sersa G, De Terlizzi F, et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur J Cancer 2016;63:41-52. [DOI] [PubMed] [Google Scholar]
- 68.Babington S, Veness MJ, Cakir B, et al. Squamous cell carcinoma of the lip: is there a role for adjuvant radiotherapy in improving local control following incomplete or inadequate excision? ANZ J Surg 2003;73:621-5. [DOI] [PubMed] [Google Scholar]
- 69.Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: Systematic review and pooled analysis of observational studies. BMJ 2013;347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Porceddu SV. Prognostic Factors and the Role of Adjuvant Radiation Therapy in Non-Melanoma Skin Cancer of the Head and Neck. Am Soc Clin Oncol Educ B 2015:e513-8. [DOI] [PubMed] [Google Scholar]
- 71.Likhacheva A, Awan M, Barker CA, et al. Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: Executive Summary of an American Society for Radiation Oncology Clinical Practice Guideline. Pract Radiat Oncol 2020;10:8-20. [DOI] [PubMed] [Google Scholar]
- 72.Guminski A, Stein B.Immunotherapy and other systemic therapies for cutaneous SCC. Oral Oncol 2019;99:104459. [DOI] [PubMed] [Google Scholar]
- 73.Migden MR, Rischin D, Schmults CD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med 2018:341-51. [DOI] [PubMed] [Google Scholar]
- 74.Patel R, Chang ALS. Immune Checkpoint Inhibitors for Treating Advanced Cutaneous Squamous Cell Carcinoma. Am J Clin Dermatol 2019;20:477-82. [DOI] [PubMed] [Google Scholar]
- 75.Migden MR, Khushalani NI, Chang ALS, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol 2020;21:294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rischin D, Migden MR, Lim AM, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer 2020;8:e000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grob JJ, Gonzalez R, Basset-Seguin N, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase II trial (KEYNOTE-629). J Clin Oncol 2020;38:2916-25. [DOI] [PMC free article] [PubMed] [Google Scholar]


