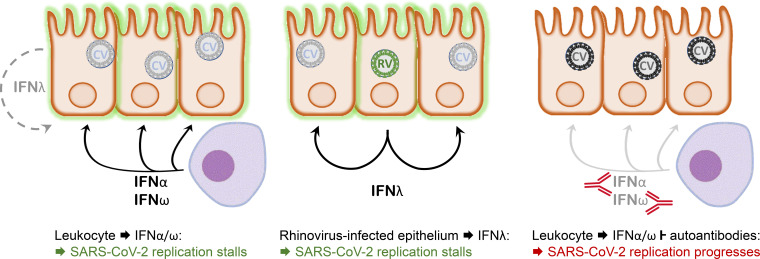
Interferon responses in the nasopharyngeal epithelium are critical to prevent severe COVID-19. Epithelial cells alone are unable to stop SARS-CoV-2 growth, but sufficient early interferon can be provided by non-epithelial cells or a co-infecting virus. Autoantibodies block early interferon, enhancing the risk of severe COVID-19.
Abstract
Interferons establish innate antiviral immunity. Two recent papers in JEM by Lopez et al. (2021. J. Exp. Med. https://doi.org/10.1084/jem.20211211) and Cheemarla et al. (2021. J. Exp. Med. https://doi.org/10.1084/jem.20210583) show that an appropriate supply of antiviral interferon enables epithelial cells of the nasopharyngeal mucosa to inhibit SARS-CoV-2 growth and that interferon-induced mucosal genes serve as biomarkers of infection.
IFNs provide innate protection from otherwise life-threatening viral disease. Type I IFN (IFN-I, including IFNα, β, and ω) and type III IFN (IFN-III or IFNλ) are of particular importance for antiviral immunity (Lazear et al., 2019; Park and Iwasaki 2020). IFN-I receptors appear on all somatic cells, but receptors for IFN-III show tissue-restricted expression. Epithelia are among the tissues expressing IFN-III receptors, and IFN-III are thought to reinforce innate immunity of this viral entry site. In line with their shared antiviral activity, signaling by both IFN-I and IFN-III receptors culminates in the activation of a master transcription factor, ISGF3, composed of the subunits STAT1, STAT2, and IRF9. ISGF3 controls the induction of IFN-induced genes (ISG) that are required to establish a cell-autonomous antiviral state.

Insights from Thomas Decker.
IFNs have been considered a promising option for clinical treatment of COVID-19 from the beginning, but success has so far been moderate (Peiffer-Smadja and Yazdanpanah, 2021). To define a potential window of opportunity for IFN treatment and to better understand the role of endogenous IFN production for the course of disease, much research was devoted to IFN in the host–SARS-CoV-2 relationship. Factors emerged that limit the efficacy of IFN action and reduce the benefit of their production. Like other coronaviruses, the SARS-CoV-2 genome was shown to express several gene products with the ability to block IFN synthesis and response (Kim and Shin, 2021; Park and Iwasaki 2020). On the host side, genetic risk factors were defined, which include genes controlling IFN production and the emergence of IFN-neutralizing autoantibodies (Bastard et al., 2020; Zhang et al., 2020). The proinflammatory character of IFN-I may exacerbate the course of advanced disease, mainly through activation of immune cells such as monocytes (Blanco-Melo et al., 2020). Since infection starts in the upper respiratory tract and SARS-CoV-2 replication at this site is correlated with transmission (Cevik et al., 2020), there is an urgent need to understand whether and how the limiting factors imposed by both virus and host impinge on IFN-related protective mechanisms at the nasopharyngeal mucosae, and whether they predict the course of infection. The two recent papers in JEM address early events of SARS-CoV-2 infection using patient material derived from nasal swabs and cellular models of nasopharyngeal epithelium. An important message conveyed by the two papers, resulting from both a comparison of infected and noninfected healthcare workers and longitudinal analyses of infected individuals, is a generally good correlation between ISG expression and the amount and kinetic changes of viral genomes, assessed by PCR or cell-based assays. In this issue, Lopez et al. (2021) further demonstrated that the ISG signature and its dynamics in blood leukocytes reflected that observed in nasal swab material in the early stage of infection. Both studies emphasize the suitability of measuring expression of selected ISGs in nasal swab material as a good correlative to early nasopharyngeal virus replication. Whereas Lopez et al. (2021) employed an ISG score set by expression of four ISGs (IFI27, IFI44L, RSAD2, IFIT1), Cheemarla et al. (2021) found that measurement of the chemokine CXCL10 alone is sufficient to report viral loads.
These observations provide the basis for simplified assessment of early SARS-CoV-2 infection of the upper respiratory tract using ISG biomarkers. Replication rates may be calculated to precisely assess differences in viral loads caused by exponential growth of different viral variants. However, both studies also deepen our knowledge of the basics of mucosal innate immunity to SARS-CoV-2, which can help physicians decide when and how treatment of infected individuals is indicated. Lopez et al. (2021) investigated disease parameters in eight critically ill individuals with autoantibodies to IFN-I. Whenever their sera completely neutralized the IFN-I species IFNα2 and IFNω, the nasopharyngeal epithelia presented with low ISG scores despite high viral loads. This disparity may more generally identify patients with an impaired innate response to SARS-CoV-2 and thus may serve as an important indicator for the administration of antiviral drugs. This result also demonstrates the surprising ability of anti-IFN autoantibodies to penetrate and act at mucosal sites. Since the antibodies specifically neutralized IFNα and IFNω, another puzzling conclusion is that epithelia-derived IFNλ are unable to compensate for the lack of these IFN-I. To corroborate their observations, the authors made use of a human airway epithelial cell model permissive for SARS-CoV-2 replication. Treatment of infected cells with IFNα2 inhibited viral replication, and serum containing anti-IFN autoantibodies blocked its antiviral effect and ISG induction. The authors further tested the ability of the sera to block the antiviral effects of recombinant IFN-I species IFNβ and IFNω or those of IFN-III members IFNλ1-3. A specific inhibition of IFNα2 and IFNω was recorded, whereas no blocking antibodies against the other IFN were present in the autoimmune sera. In conclusion, IFNα2 and IFNω are special in the antiviral response of respiratory tract epithelia to SARS-CoV-2 and/or the ability to cause autoantibody production. The antiviral role of IFNα is well documented, but the production of IFNω in humans is poorly explored and its contribution to innate antiviral activity unclear (Li et al., 2017). Its prominent role during SARS-CoV-2 infection raises the need for further investigation of IFNω producer cells and the role of this cytokine in innate immunity against other respiratory viruses. The surprising inability of IFNβ or the IFNλ to compensate for the neutralized IFN-I species demonstrates another gap in our understanding of innate antiviral responses of human mucosae. However, the lack of neutralizing autoantibodies may also provide an opportunity to treat autoimmune patients with IFNβ or IFNλ.
In their efforts to understand the importance of the IFN system in the nasopharyngeal epithelium, Cheemarla et al. (2021) took a different approach. These authors argued that the occurrence of multiple viruses and nonviral microbes in the upper respiratory tract is a frequent occurrence in asymptomatic subjects. Hence, diseases caused by pathogenic viruses such as SARS-CoV-2 may be better understood in the context of potential coinfections. Based on this, the authors used human respiratory tract epithelial organoids to interrogate how rhinovirus infection impacts a subsequent infection with SARS-CoV-2. Infection with SARS-CoV-2 alone led to exponential replication and generated small amounts of IFNλ1. The synthesis of the ISG CXCL10 trailed virus replication by ∼24 h. Rhinovirus replication induced much higher levels of IFNλ1, and ISG mRNA expression showed no delay compared with viral RNA synthesis. At the single cell level, only a small number of organoid cells contained viral RNA, but a very large fraction expressed ISG. This suggests a large impact of the innate response to rhinovirus on noninfected bystander cells. Despite a twofold-increased expression of the full-length form of ACE2, preinfection of the organoids with rhinoviruses blocked subsequent SARS-CoV-2 replication, an outcome accompanied by high levels of IFNλ1, but not IFNβ synthesis, and by rapid ISG induction. Suppression of SARS-CoV-2 replication by rhinovirus was abrogated by inhibition of the IFN regulatory factor (IRF) pathway, which controls both IFN-I and IFN-III synthesis (Fitzgerald et al., 2003). Importantly, IRF pathway blockade had very little impact on viral replication upon single infection with SARS-CoV-2 under the conditions used in the coinfection model, and only a change to very low multiplicities of infection revealed an impact of inhibiting IFN synthesis by reducing the doubling time of viral growth from 5.1 to 3.6 h.
Model depicting the interaction between SARS-CoV-2 and the nasopharyngeal epithelium. Left: In a situation of asymptomatic infection or mild disease, mucosal leukocytes provide the IFN-I IFNα and IFNω for an inhibitory antiviral state. IFNλ production by the infected cells alone is insufficient in this situation. Middle: Infection with an RNA virus such as rhinovirus causes epithelial cells to produce sufficient IFNλ to cause an antiviral state in bystander cells that subsequently lose permissiveness for SARS-CoV-2 replication. Right: As in the left panel, but autoantibodies inhibit the leukocyte-derived IFN. Consequently, the epithelium remains permissive for SARS-CoV-2 replication, allowing for virus spread to the lower respiratory tract and favoring the development of severe pulmonary disease.
Which conclusions can be drawn from these experiments, and what are their limitations? First, the patient data suggest that the IFN response of the mucosae of the upper respiratory tract can curb SARS-CoV-2 replication, and that genetic factors favoring severe illness act at least in part at this early infection stage. However, the cell culture models indicate that the infected epithelial cells, if fending for themselves, are inefficient in inhibiting the growth of SARS-CoV-2. In line with previous findings (Galani et al., 2017), the dominant IFN produced by epithelial cells was IFNλ, but both studies agree that the epithelial IFNλ production is insufficient to inhibit the growth of SARS-CoV-2. Cultured airway epithelia do not recapitulate the cell composition of infected mucosae, lacking leukocytes in particular. The identification of IFNα2 and IFNω as predominant mediators of innate antiviral effects suggests that leukocytes are needed to produce the antiviral state in epithelial cells. That said, the rhinovirus coinfection model shows that epithelia are not generally poor IFN producers, and that viruses less efficient in suppressing the IFN system are on one hand vulnerable to its antiviral capacity, and on the other hand able to cause enough bystander effect to protect against subsequent infection with a different virus. Whether this scenario is relevant for COVID-19 requires future investigation. However, the coinfection model clearly demonstrates that SARS-CoV-2 is vulnerable to a preexisting antiviral state in the airway epithelium without any input from leukocytes. While this may be good news for IFN therapies, an upshot of the two papers is that such treatment may have to be administered at a very early stage of infection to block viral spread to the lower respiratory tract and to prevent it from causing pulmonary disease.
Acknowledgments
Critical reading of the manuscript by Manuela Baccarini is gratefully acknowledged.
Research in the author’s laboratory is funded by the Austrian Science Fund through projects SFB F6103 and W1261 (signaling in cellular homeostasis).
The author declares no competing financial interests.
References
- Bastard, P., et al. 2020. Science. 10.1126/science.abd4585 [DOI] [Google Scholar]
- Blanco-Melo, D., et al. 2020. Cell. 10.1016/j.cell.2020.04.026 [DOI] [Google Scholar]
- Cevik, M., et al. 2020. BMJ. 10.1136/bmj.m3862 [DOI] [Google Scholar]
- Cheemarla, N.R., et al. 2021. J. Exp. Med. 10.1084/jem.20210583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, K.A., et al. 2003. Nat. Immunol. 10.1038/ni921 [DOI] [Google Scholar]
- Galani, I.E., et al. 2017. Immunity. 10.1016/j.immuni.2017.04.025 [DOI] [Google Scholar]
- Kim, Y.M., and Shin E.C.. 2021. Exp. Mol. Med. 10.1038/s12276-021-00592-0 [DOI] [Google Scholar]
- Lazear, H.M., et al. 2019. Immunity. 10.1016/j.immuni.2019.03.025 [DOI] [Google Scholar]
- Li, S.-F., et al. 2017. Int. Immunopharmacol. 10.1016/j.intimp.2017.08.028 [DOI] [PubMed] [Google Scholar]
- Lopez, J., et al. 2021. J. Exp. Med. 10.1084/jem.20211211 [DOI] [Google Scholar]
- Park, A., and Iwasaki A.. 2020. Cell Host Microbe. 10.1016/j.chom.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer-Smadja, N., and Yazdanpanah Y.. 2021. Lancet Respir. Med. 10.1016/S2213-2600(20)30523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., et al. 2020. Science. 370:eabd4570. 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]