Abstract
Porphyromonas gingivalis (P. gingivalis) is a gram‐negative bacterium and an important etiologic agent of periodontitis. P. gingivalis releases outer membrane vesicles containing lipopolysaccharides (LPS), which can penetrate periodontal tissues. Once in the periodontal tissues and in contact with immune cells, it may participate in the destructive innate host response associated with the disease. The exact mechanism of P. gingivalis LPS in the disease process is not clear, but it is known to affect a variety of immune responses.
Objectives
To investigate how LPS from P. gingivalis affect neutrophil extracellular trap (NET) formation, cell death and production of cytokines from human neutrophils and peripheral mononuclear blood mononuclear cells (PBMCs).
Materials and methods
Isolated neutrophils and PBMCs were cultured with LPS from P. gingivalis or Escherichia coli (E. coli) (control). The NET formation was measured using Sytox green stain. Cell death of neutrophils and PBMCs was analyzed using flow cytometry or Sytox green stain. Cytokine production was measured using enzyme‐linked immunosorbent assay (ELISA) kit or Bio‐Plex assay.
Results
Exposure to LPS from P. gingivalis and E. coli caused significantly lower cell death in neutrophils. NETs were formed after exposure to the two different LPS. In PBMCs, exposure to P. gingivalis and E. coli LPS caused increased levels of IL‐1β and IL‐6 compared to unstimulated controls. Increased cell death in PBMCs after exposure to LPS from E. coli in comparison to LPS from P. gingivalis and unstimulated controls was also observed.
Conclusions
LPS from P. gingivalis has the ability to affect both human neutrophils and PBMCs with regard to cytokine production, cell death and production of NETs. LPS from P. gingivalis could be involved in the pathogenesis of periodontitis, and our results may contribute information regarding possible markers for diagnosis and targets for treatment of periodontal disease.
Keywords: Escherichia coli, lipopolysaccharide, mononuclear leukocytes, neutrophils, Porphyromonas gingivalis
Saliva contains 107–9 bacteria/ml (Martin, 2009) consisting of more than 600 different oral bacterial strains (Aas, Paster, Stokes, Olsen, & Dewhirst, 2005; Dewhirst et al., 2010). These bacteria cover all surfaces of the oral cavity including the gingiva and the teeth. The bacteria can be classified in many different ways, for example, based on their cell wall structure as either Gram‐positive or Gram‐negative. In healthy conditions, the oral microbiota maintains a community stability referred to as homeostasis. However, dysbiosis of the microflora caused by changes in the environmental factors may result in different oral diseases such as periodontal disease (Kilian et al., 2016). Periodontal disease is a group of inflammatory diseases that leads to inflammation of the gingiva and destruction of periodontal tissues. Only some bacteria have periodontopathic potential and can initiate periodontal diseases when a critical concentration is reached (Wang & Ohura, 2002). Porphyromonas gingivalis (P. gingivalis) has been called a “keystone pathogen” associated with periodontitis (How et al., 2016) which leads to disruption of the homeostatic balance with the host tissue, causing destructive inflammation (Hajishengallis & Lambris, 2012).
Porphyromonas gingivalis is a proteolytic, gram‐negative bacterium that produces a multitude of different metabolites including various acids, ammonia and hydrogen sulfide (Greabu et al., 2016). These metabolites may induce gingival inflammation which can progress to loss of dental attachment and periodontitis (Hajishengallis, Darveau, & Curtis, 2012). P. gingivalis produces other virulence factors such as endotoxins that may cause damage to the host cells. Lipopolysaccharide (LPS) is an endotoxin that is found on the outer membrane of gram‐negative bacteria, and is a potent inducer of immune response by various cell types (Schmalz, Krifka, & Schweikl, 2011). P. gingivalis LPS has a critical role in mediating inflammation and inducing cells to secrete pro‐inflammatory cytokines that in turn affect, for example, bone resorption (Diya et al., 2008). Thus, P. gingivalis LPS has been considered to be an important pathogenic component in the initiation and development of periodontal disease (Wang & Ohura, 2002).
Gingival crevicular fluid (GCF), flows into the gingival crevice through the junctional epithelium thereby transporting cells into the oral cavity. The population of cells in the GCF comprises mainly neutrophils but also lymphocytes, with fewer T cells than B cells, and monocytes (Attstrom, 1970). It is therefore important to study the effect LPS from P. gingivalis may have on target immune cells.
The aim of the present study was to investigate how LPS from P. gingivalis affect Interleukin (IL)‐8 production, neutrophil extracellular trap (NET) formation and cell death from human neutrophils. Also, to study the production of IL‐1 receptor antagonist (IL‐1RA), interferon gamma‐induced protein 10 (IP‐10), monocyte chemoattractant protein‐1 (MCP‐1), IL‐1β, IL‐6 and tumor necrosis factor alfa (TNF‐α) and cell death in peripheral mononuclear blood cells. For this purpose, we used LPS from Escherichia coli (E. coli) as control since it is a potent inducer of immune response by various cell types (Alexander et al., 2001).
1. MATERIALS AND METHODS
1.1. Isolation of polymorphonuclear neutrophils (PMNs) and peripheral blood mononuclear cells (PBMCs) from human blood
Fresh blood samples from healthy blood donors were obtained from Sahlgrenska University Hospital in Gothenburg, Sweden. Peripheral blood neutrophil isolation was performed as first described by Boyum (Boyum, 1968). After removal of red blood cells in a sedimentation step, the suspension was centrifuged on Ficoll‐Paque Plus (GE Healthcare Bio‐Sciences, Uppsala, Sweden). The pelleted neutrophils and the layer of PBMCs above the Ficoll‐Paque Plus were collected and the remaining erythrocytes were lysed by hypotonic treatment. All the washing of the neutrophils was performed using Krebs Ringer Phosphate (KRG) buffer. In the end, the isolated neutrophils were resuspended in KRG supplemented with Ca2+ (1 mM) while the PBMCs were resuspended in phosphate‐buffered saline (PBS), centrifuged, and resuspended in Dulbecco's Modified Eagle's Medium (D‐MEM) (Invitrogen, Lidingö, Sweden) supplemented with 5% heat‐inactivated human AB serum (Sigma Chemical Co., St. Louis, MO), and 100 U/ml of penicillin and 100 μg/ml of streptomycin (Invitrogen, Lidingö, Sweden). Cell viability was determined by staining with 0.4% Trypan Blue (Sigma‐Aldrich) and cells were counted under a microscope using a Bürker chamber.
1.2. DNA release measurements from PMNs and cell death measurements in PBMCs using Sytox Green DNA stain
Sytox Green DNA stain (Thermo Fisher Scientific, Gothenburg, Sweden) is a membrane impermeable dye that can be used to measure extracellular DNA from ruptured cells (Gupta, Chan, Zaal, & Kaplan, 2018). For the measurements of NETosis with Sytox Green DNA stain (Thermo Fisher Scientific, Gothenburg, Sweden), cells at a concentration of 0.5 × 106 cells/ml (n = 6) in RPMI (without phenol red: Thermo Fisher Scientific) and the Sytox Green DNA stain (2.5 μM; Molecular Probes) were added to a black 96‐well plate. LPS from P. gingivalis (InvivoGen, San Diego, CA) and LPS from E. coli (serotype O127:B8, Sigma‐Aldrich) at a concentration of 0.1 or 1 μg/ml were added to the wells and the plate was incubated at 37°C and 5% CO2. Phorbol 12‐myristate 13‐acetate (PMA; Sigma‐Aldrich) at a concentration of 50 nM/well and 1% Triton X‐100 were used as controls. Sytox Green fluorescence was measured after 0 and 3 hr of incubation at 485/535 nm using a CLARIOstar plate reader.
The same method as mentioned above for measuring NETosis in PMNs, can also be used for measuring cell death in mononuclear cells. For this purpose, Sytox Green DNA stain, mononuclear cells at 0.5 × 106 cells/ml (n = 5) together with LPS, 0.1 μg/ml from P. gingivalis or E. coli, were incubated and measured as mentioned. The cell death was monitored by an increase in fluorescence intensity of the cell‐impermeable dye (Sytox Green) after plasma membrane disintegration.
1.3. Microscopic visualization of PMNs
Another method commonly used to confirm the production of NETs is staining the samples with antibodies against myeloperoxidase (MPO). MPO is an antimicrobial protein found on the chromatin fibers released during NETosis.
Isolated human neutrophils (5.5 × 105 cells/ml) were suspended in RPMI and added to poly‐lysine‐coated 24 well glass bottom plates (Cellvis, CA) and incubated at 37°C in 5% CO2 for 10 min. After stimulation with PMA (50 nM) or LPS from P. gingivalis or E. coli (1 μg/ml), the cells were further incubated for 3 hr (the incubation time was chosen from the results received after Sytox Green DNA stain). Cells were fixed in 4% paraformaldehyde for 30 min at room temperature and permeabilized with cold acetone and methanol (1:1) for 5 min. To visualize NETs, the samples were stained with antibodies against MPO (DAKO), followed by secondary antibody staining (Donkey Anti‐Rabbit IgG H&L Alexa Fluor 488 purchased from ThermoFisher). Finally, the coverslips were mounted with ProLong Gold antifade mountant with DAPI (ThermoFisher). The cells were visualized using an Olympus BX41 epifluorescent microscope with the CellSens software.
1.4. Evaluation of cell death in PMNs using FACS analyses
For the FACS analyses, 450 μl isolated human neutrophils (5 × 106 cells/ml) (n = 6) were suspended in RPMI complemented with 10% FCS and 1% PEST and incubated for 30 min at 37°C in 5% CO2. Next, 50 μl of LPS from P. gingivalis or E. coli (0.1 or 1 μg/ml) (concentrations chosen based on previous studies) were added, the pro‐apoptotic anti‐CD95 (10 μg/ml) were used as positive control.
After 20 hr incubation, 200 μl cell free supernatant from each sample was saved for cytokine analysis, while 200 μl from each cultured sample was washed in 2 ml Annexin buffer [1 mM Hepes, 14 mM NaCl, 0.25 mM CaCl2, (pH 7.4)]. The pellets were resuspended in 100 μl Annexin buffer, supplemented with 2 μl Annexin V‐FLUOS and 5 μl 7‐amino‐actinomycin D (7‐AAD) and incubated for 10 min in the dark. Thereafter, another 300 μl Annexin buffer was added, and samples were subjected to FACS analysis using an Accuri C6 (Becton Dickinson, Mountain View, CA). At least 10,000 events were acquired, and neutrophils were gated on the basis of side‐ and forward‐scatter. Apoptosis was assessed on the basis of Annexin V‐FLUOS fluorescence and necrosis was on the basis of membrane permeability to 7‐AAD. All data were analyzed using CFlow and GraphPad Prism software.
1.5. Measuring IL‐8 released from PMNs
A DuoSet ELISA Development kit from R&D Systems (Abingdon, UK) was used according to the manufacturer's instructions to measure the levels of IL‐8 in the supernatant fluids of the LPS‐exposed PMN (n = 7) cultures.
1.6. Bio‐Plex Pro human cytokine assay for cytokine production from PBMCs
The samples (supernatants from PBMCs (n = 6) exposed to LPS) were added, and thereafter the color‐coded beads coupled to antibodies. The antibodies reacted with the biomarkers of interest present in the sample. After a series of repeated washes in order to remove un‐bound proteins, a biotinylated detection antibody (used to create a sandwich complex) was added. The final detection complex was formed when streptavidin‐phycoerythrin (SA‐PE) conjugate was added to bind to the biotinylated antibody. Finally, the samples were analyzed using a BioPlex 200 instrument equipped with BioManager analysis software using red and green lasers to detect the different colors on the beads while measuring the fluorescence intensity using a standard curve. The red (635 nm) laser and the green (532 nm) laser measured concentration (pg/ml) and median fluorescence intensity (MFI) respectively. The concentration of the analyte bound to each bead was proportional to the MFI of the reporter signal.
1.7. Statistical analysis
All analyses were performed using GraphPad Prism. For all tests, a p‐value of <.05 was considered statistically significant. Statistical comparisons between paired samples were made using the Wilcoxon matched‐pairs signed‐rank test.
2. RESULTS
2.1. Measurement of DNA release from PMNs with Sytox Green DNA stain
As the Sytox Green dye only reacts with extracellular DNA, it has previously, and in combination with microscopy, been used as a proxy for evaluations of NET formation (Makarov et al., 2013). Neutrophils where exposed to LPS (E. coli and P. gingivalis) for 3 hr. It was observed that 0.1 μg/ml LPS from E. coli significantly (p = .0312) increased the NETs formation, while exposure to LPS in the same concentration from P. gingivalis did not (Figure 1). However, if the LPS concentration was increased to 1 μg/ml, the LPS from both P. gingivalis (p = .0312) and E. coli (p = .0156) it significantly increased the amount of NETosis compared to unstimulated cells. LPS from E. coli was more potent, indicating towards a higher level of NETosis, as compared to LPS from P. gingivalis (p = .0312) (Figure 1).
FIGURE 1.

Measurement of DNA release from PMNs after exposure to lipopolysaccharides (LPS) from Porphyromonas gingivalis (PG) and Escherichia coli (EC) Sytox Green fluorescence was measured from neutrophils cultured with or without LPS (0.1 or 1 μg) from PG or EC for 3 hr. PMA, 50 nM was used as control. Results are from seven independent experiments. *, p < .05
2.2. Microscopic visualization of PMNs
The results from the Sytox Green plate reader assay was confirmed by using immunofluorescence microscopy and quantifying neutrophils with different nuclear morphology and extracellular DNA after 0 and 3 hr of exposure to LPS (1 μg/ml) (Figure 2).
FIGURE 2.

Microscopic visualization of NETs produced by PMNs. Neutrophils were exposed to PMA, 50 nM or LPS from Porphyromonas gingivalis (PG) or Escherichia coli (EC) for 3 hr incubated at 37°C. The cells were fixed and stained for DNA (blue) and MPO (red). The images are from one representative experiment out of two independent experiments performed. The cells were visualized using an epifluorescence microscope
2.3. FACS analyses of cell death in PMNs
Out of the cells that were incubated for 20 hr in the absence of stimuli, nearly 50% were apoptotic (Annexin V+/7‐AAD–), while necrosis (Annexin V+/7‐AAD+) was consistently below 5%. The total cell death was significantly lower when cells were exposed to LPS from E. coli 0.1 μg/ml (p = .0002) and 1 μg/ml (p = .0002) compared to unstimulated cells (Figure 3). However, only the highest concentration (1 μg/ml) of LPS from P. gingivalis showed anti‐apoptotic effect (Figure 3).
FIGURE 3.

Evaluation of cell death in PMNs using FACS analyses. Neutrophils were exposed to the pro‐apoptotic (anti‐CD95 antibody, 10 μg/ml) and 1 μg/ml LPS from Porphyromonas gingivalis (PG) or Escherichia coli (EC) for 20 hr and incubated at 37°C. The cell death of the neutrophils was evaluated by flow cytometry. Apoptotic cells labeled with Annexin V are presented as blue parts of the bars, while necrotic cells positive for both Annexin V and 7‐AAD are seen as black parts of the bars. Results are from six independent experiments shown as mean ± SD. *, p < .05; **, p < .01; ***, p < .005 and ****, p < .001
2.4. Production of IL‐8 from PMNs
In order to study IL‐8 production after exposure to the different LPS, supernatants were collected from neutrophils cultured with or without LPS from P. gingivalis or E. coli for 20 hr. There were significantly higher levels of IL‐8 in the supernatants of cultures exposed to LPS from E. coli compared to LPS from P. gingivalis 0.1 μg/ml (p = .0177), and also between LPS from E. coli 0.1 μg/ml (p = .0007) and 1 μg/ml (p = .0014) and the supernatants of unexposed cells. The release of IL‐8 in response to LPS from P. gingivalis did not differ from the unstimulated control (Figure 4).
FIGURE 4.
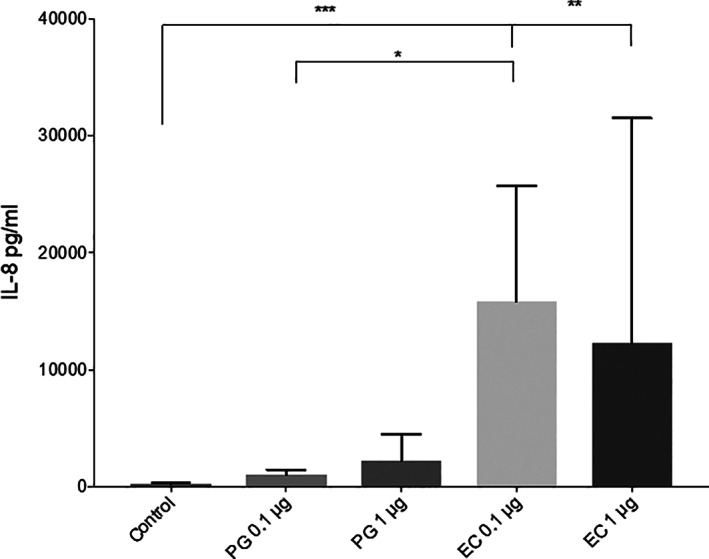
IL‐8 released from PMNs neutrophils were exposed to 0.1 or 1 μg/ml LPS from Porphyromonas gingivalis (PG) or Escherichia coli (EC) for 20 hr and incubated at 37°C. Total IL‐8 content in the cultures were measured by ELISA. Results are from seven independent experiments. *, p < .05; **, p < .01; ***, p < .005
2.5. Cytokine production from PBMCs
The levels of six different cytokines were measured in the culture supernatants of PBMCs exposed to 0.1 μg LPS from P. gingivalis or E. coli. After 24 hr of exposure to the two different types of LPS, the levels of the cytokines IL‐1RA, IP‐10 and MCP‐1 were suppressed, while the levels of IL‐1β, IL‐6 and were significantly increased compared to the unstimulated cells (Figure 5). TNF‐α was significantly increased only in the presence of E coli LPS. LPS from E. coli more potently increased the levels of IL‐1β and IL‐6 in the PBMC cultures compared to LPS from P. gingivalis (Figure 5).
FIGURE 5.

Cytokine production from PBMCs. The levels of six different cytokines were measured in the culture supernatants of PBMCs exposed to 0.1 μg/ml LPS from Porphyromonas gingivalis (PG) or Escherichia coli (EC) for 20 hr and incubated at 37°C. Results are from six independent experiments. *, p < .05; **, p < .01
2.6. Cell death in mononuclear leukocytes using Sytox Green DNA stain
After 24 hr exposure to LPS from P. gingivalis and LPS from E. coli, PBMC death was measured using Sytox Green DNA stain. The results showed significantly increased cell death in PBMCs after exposure to LPS from E. coli in comparison to the LPS from P. gingivalis (p = .0312) and to unstimulated cells (p = .0312) (Figure 6).
FIGURE 6.

Cell death measurements in PBMCs the PBMCs were exposed to 0.1 μg/ml LPS from Porphyromonas gingivalis (PG) or Escherichia coli (EC) for 20 hr and incubated at 37°C. Sytox Green DNA stain was used to measure cell death in PBMCs. Results are from six independent experiments. *, p < .05
3. DISCUSSION
Porphyromonas gingivalis is a Gram‐negative bacterium recognized as an important etiological agent of chronic periodontitis and can be found in a large proportion of the human population (Holt, Kesavalu, Walker, & Genco, 1999). A major constituent of the outer membrane of P. gingivalis is LPS which plays a critical role in mediating inflammation and inducing various immune cells to secrete pro‐inflammatory cytokines (Yucel‐Lindberg et al., 2013), including IL‐1β, TNF‐α and IL‐6 (Diya et al., 2008). It has been demonstrated that most LPS stimulate the production of pro‐inflammatory cytokines mainly through Toll‐like receptor 4 and nuclear factor‐κB (Diya et al., 2008). However, P. gingivalis LPS has been shown to differ from E. coli in structure and various functional activities (Diya et al., 2008; Holt et al., 1999). In PMNs stimulated with E. coli LPS there has been a greater production of IL‐1β, IL‐1RA, IL‐8 and TNF‐α compared to exposure to P. gingivalis LPS (Yoshimura, Hara, Kaneko, & Kato, 1997). However, in most publications LPS from E. coli is used to investigate immunological responses in various cell types and inflammatory conditions, but in oral infections LPS from oral microbes as P. gingivalis is of more interest. Some of the responses that have been studied in the present study are cell death processes in PMNs and PBMCs, the effect on NETosis, the release of IL‐8 from PMNs and the production of pro‐inflammatory cytokines produced by PBMCs.
Periodontal diseases are multifactorial, perhaps beginning with the activation of the immune system at the cellular level by the LPS from a potential pathogen such as P. gingivalis. Neutrophils are the most abundant cells in the gingival pockets in periodontal disease (Scott et al., 2012), but it is obvious that there are also multiple other cells involved in the immune responses caused by LPS. Therefore, it was of interest for us to investigate the effects caused by LPS from P. gingivalis on neutrophils and other leukocytes that are responsible for the immune system activation by P. gingivalis LPS. Neutrophils are primary effectors of the innate immune system against microbial pathogens. In addition to phagocytic killing, neutrophils can catch and kill microbes via an alternative mechanism known as NET formation also called NETosis. NETosis is known to be a violent, pro‐inflammatory type of death where NETs are being thrown out from the cells in order to capture the pathogen (Remijsen et al., 2011). NETs are networks composed of chromatin and neutrophil granule proteins with high bactericidal potential. NETs are thought to neutralize pathogens and create a barrier that prevents the spread of bacteria (Brinkmann et al., 2004). It has previously been shown that LPS from E. coli has the potential to induce NETosis (Brinkmann et al., 2004). There have been several studies investigating the role of NETosis in periodontitis (Vitkov, Hartl, Minnich, & Hannig, 2017; White, Chicca, Cooper, Milward, & Chapple, 2016). The proteolytic enzymes, called gingipains from P. gingivalis have been shown to have a huge role in the NETosis triggered by this bacterium (Bryzek et al., 2019). However, no previous studies have been conducted on LPS from P. gingivalis and its effect on NET formation. In the present study, it has been shown that neutrophil exposure to LPS from P. gingivalis significantly increased the release of extracellular DNA and the formation of NETs was confirmed microscopically. However, LPS from E. coli was observed to be a more potent NET inducer as compared to LPS from P. gingivalis.
Another observed effect of by LPS exposure on the neutrophils was the increased IL‐8 production. IL‐8 is a chemokine with proinflammatory abilities inducing neutrophil recruitment (Baggiolini, Loetscher, & Moser, 1995). This recruitment might result in a prolonged inflammation and may play a role in periodontal disease that is a chronic inflammatory condition. As previously shown, neutrophils exposed to LPS from E. coli release high levels of IL‐8 (Christenson et al., 2013; Yoshimura et al., 1997) while the results of our study show that exposure to LPS from P. gingivalis only tended to slightly increase the level of IL‐8, however none of statistically significance value.
Previous studies conducted on monocytes/macrophages have shown that exposure to LPS from P. gingivalis induce production of IL‐1β, TNF‐α and IL‐6 (Baqui et al., 1998; Diya et al., 2008). These cytokines are usually increased once monocytes or macrophages are stimulated with LPS. However, it has been reported in most studies that P. gingivalis LPS is less potent than E. coli LPS in inducing the release of inflammatory cytokines in various cells (Diya et al., 2008; Martin, Katz, Vogel, & Michalek, 2001). This is in concordance with the results in the present study. It was also observed that after 24 hr exposure of the two different LPS, the levels of the cytokines IL‐1RA, IP‐10 and MCP‐1 were suppressed, while the levels of IL‐1β, IL‐6 and TNF‐α were significantly increased. There were also significantly higher levels of IL‐1β, IL‐6 and a tendency towards higher level of TNF‐α in the PBMC cultures exposed to LPS from E. coli compared to LPS from P. gingivalis. TNF‐α is a proinflammatory cytokine that is involved in upregulation of inflammatory reactions. This may explain why LPS from E. coli is known to be more potent than LPS from P. gingivalis. Exploring the effects of the two LPS on human PBMCs, the viability of these cells was measured using Sytox Green DNA stain. After 24 hr exposure, the results showed significantly increased fluorescence intensity in cells exposed to LPS from E. coli in comparison to the LPS from P. gingivalis and cells alone. In summary, after 24 hr exposure, LPS from E. coli caused higher cell death in human PBMCs compared to LPS from P. gingivalis.
The immunomodulating properties of LPS from P. gingivalis were not as strong as the those of LPS from E. coli. The ability of P. gingivalis LPS to affect the different parts of the immune system may explain its possible participance in the pathogenesis of periodontitis. While the viability of mononuclear cells was unaffected by exposure to LPS from P. gingivalis, the proinflammatory cytokine production (IL‐1β, IL‐6 and TNF‐α) was elevated and the anti‐inflammatory cytokine IL‐1RA was decreased. The change in the balance of the cytokines might promote the inflammation caused by LPS from P. gingivalis. This can be related to the previously reported ability of P. gingivalis to cause inflammation of the gingiva (gingivitis) that might result in chronic destruction of connective tissues and thereby the formation of periodontal pockets and ultimately result in loss of teeth (Hajishengallis et al., 2012). Another interesting finding in this study was the ability of LPS from P. gingivalis to cause NETosis in human PMNs. As summarized by Rajendran et al., the literature has recently shown that NET production has a significant role in periodontal infections (Rajendran et al., 2018). The changes in the normal functions of neutrophils and PBMCs caused by LPS from P. gingivalis exposure may lead to an altered inflammatory response.
In the present study we have put focus at describing some of the effects caused by LPS P. gingivalis exposure, and a limitation of the study is that we cannot describe the exact mechanism behind these effects. However, in order to be able to describe an exact mechanism, the present study was required as a first step.
In conclusion, LPS from P. gingivalis is an immunomodulator that has the ability to affect both human neutrophils and PBMCs. These effects included modulated cytokine production, affected cell death and production of NETs. Although the results showed a clear change in immunological response due to LPS from P. gingivalis, the changes were less compared to those caused by LPS from E. coli. The present findings may contribute as future targets for treatment of immune reactions caused by LPS from P. gingivalis.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
The peripheral blood cells obtained from Sahlgrenska University Hospital blood bank are de‐identified, and according to the Swedish legislation section code 4§ 3 p SFS2003:460, no informed consent is needed.
ACKNOWLEDGMENTS
TUA Research Funding; The Sahlgrenska Academy at University of Gothenburg/Region Västra Götaland, Sweden is gratefully acknowledged.
Alizadehgharib S, Östberg A‐K, Dahlstrand Rudin A, Dahlgren U, Christenson K. Immunological response of human leucocytes after exposure to lipopolysaccharides from Porphyromonas gingivalis . Clin Exp Dent Res. 2021;7:531–538. 10.1002/cre2.388
Funding information TUA Research Funding
DATA AVAILABILITY STATEMENT
Data available on request from the authors
REFERENCES
- Aas, J. A., Paster, B. J., Stokes, L. N., Olsen, I., & Dewhirst, F. E. (2005). Defining the normal bacterial flora of the oral cavity. Journal of Clinical Microbiology, 43, 5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, C., & Rietschel, E. T. (2001). Bacterial lipopolysaccharides and innate immunity. Journal of Endotoxin Research, 7, 167. [PubMed] [Google Scholar]
- Attstrom, R. (1970). Presence of leukocytes in crevices of healthy and chronically inflamed gingivae. Journal of Periodontal Research, 5, 42–47. [DOI] [PubMed] [Google Scholar]
- Baggiolini, M., Loetscher, P., & Moser, B. (1995). Interleukin‐8 and the chemokine family. International Journal of Immunopharmacology, 17, 103–108. [DOI] [PubMed] [Google Scholar]
- Baqui, A. A., Meiller, T. F., Chon, J. J., Turng, B. F., & Walker, W. A. Jr. (1998). Granulocyte‐macrophage colony‐stimulating factor amplification of interleukin‐1beta and tumor necrosis factor alpha production in THP‐1 human monocytic cells stimulated with lipopolysaccharide of oral microorganisms. Clinical and Diagnostic Laboratory Immunology, 5, 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum, A. (1968). Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scandinavian Journal of Clinical and Laboratory Investigation. Supplementum, 97, 77. [PubMed] [Google Scholar]
- Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, D. S., Weinrauch, Y., & Zychlinsky, A. (2004). Neutrophil extracellular traps kill bacteria. Science, 303, 1532–1535. [DOI] [PubMed] [Google Scholar]
- Bryzek, D., Ciaston, I., Dobosz, E., Gasiorek, A., Makarska, A., Sarna, M., Eick, S., Puklo, M., Lech, M., Potempa, B., Potempa, J., & Koziel, J. (2019). Triggering NETosis via protease‐activated receptor (PAR)‐2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathogens, 15, e1007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson, K., (2013). Endogenous acute phase serum amyloid a lacks pro‐inflammatory activity, contrasting the two recombinant variants that activate human neutrophils through different receptors. Frontiers in Immunology, 4, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst, F. E., Chen, T., Izard, J., Paster, B. J., Tanner, A. C. R., Yu, W. H., Lakshmanan, A., & Wade, W. G. (2010). The human oral microbiome. Journal of Bacteriology, 192, 5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diya, Z., (2008). Lipopolysaccharide (LPS) of Porphyromonas gingivalis induces IL‐1beta, TNF‐alpha and IL‐6 production by THP‐1 cells in a way different from that of Escherichia coli LPS. Innate Immunity, 14, 99–107. [DOI] [PubMed] [Google Scholar]
- Greabu, M., Totan, A., Miricescu, D., Radulescu, R., Virlan, J., & Calenic, B. (2016). Hydrogen sulfide, oxidative stress and periodontal diseases: A concise review. Antioxidants (Basel), 5, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S., Chan, D. W., Zaal, K. J., & Kaplan, M. J. (2018). A high‐throughput real‐time imaging technique to quantify NETosis and distinguish mechanisms of cell death in human neutrophils. Journal of Immunology, 200, 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis, G., Darveau, R. P., & Curtis, M. A. (2012). The keystone‐pathogen hypothesis. Nature Reviews. Microbiology, 10, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis, G., & Lambris, J. D. (2012). Complement and dysbiosis in periodontal disease. Immunobiology, 217, 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, S. C., Kesavalu, L., Walker, S., & Genco, C. A. (1999). Virulence factors of Porphyromonas gingivalis . Periodontol 2000, 20, 168–238. [DOI] [PubMed] [Google Scholar]
- How, K. Y., Song, K. P., & Chan, K. G. (2016). Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Frontiers in Microbiology, 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian, M., Chapple, I. L. C., Hannig, M., Marsh, P. D., Meuric, V., Pedersen, A. M. L., Tonetti, M. S., Wade, W. G., & Zaura, E. (2016). The oral microbiome—An update for oral healthcare professionals. British Dental Journal, 221, 657–666. [DOI] [PubMed] [Google Scholar]
- Makarov, R., Geserick, P., Feoktistova, M., & Leverkus, M. (2013). Cell death in the skin: How to study its quality and quantity? Methods in Molecular Biology, 961, 201. [DOI] [PubMed] [Google Scholar]
- Martin, M., Katz, J., Vogel, S. N., & Michalek, S. M. (2001). Differential induction of endotoxin tolerance by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli . Journal of Immunology, 167, 5278–5285. [DOI] [PubMed] [Google Scholar]
- Martin, P. M. A. M. (2009). Oral microbiology (5th ed.). Churchill Livingstone. [Google Scholar]
- Rajendran, V., & Uppoor, A. (2018). A perspective on NETosis in diabetes and periodontal diseases. Journal of Indian Society of Periodontology, 22, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijsen, Q., Kuijpers, T. W., Wirawan, E., Lippens, S., Vandenabeele, P., & vanden Berghe, T. (2011). Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death and Differentiation, 18, 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalz, G., Krifka, S., & Schweikl, H. (2011). Toll‐like receptors, LPS, and dental monomers. Advances in Dental Research, 23, 302–306. [DOI] [PubMed] [Google Scholar]
- Scott, D. A., & Krauss, J. (2012). Neutrophils in periodontal inflammation. Frontiers of Oral Biology, 15, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkov, L., Hartl, D., Minnich, B., & Hannig, M. (2017). Janus‐faced neutrophil extracellular traps in periodontitis. Frontiers in Immunology, 8, 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. L., & Ohura, K. (2002). Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts‐CD14 and toll‐like receptors. Critical Reviews in Oral Biology and Medicine, 13, 132–142. [DOI] [PubMed] [Google Scholar]
- White, P. C., Chicca, I. J., Cooper, P. R., Milward, M. R., & Chapple, I. L. C. (2016). Neutrophil extracellular traps in periodontitis: A web of intrigue. Journal of Dental Research, 95, 26–34. [DOI] [PubMed] [Google Scholar]
- Yoshimura, A., Hara, Y., Kaneko, T., & Kato, I. (1997). Secretion of IL‐1 beta, TNF‐alpha, IL‐8 and IL‐1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. Journal of Periodontal Research, 32, 279–286. [DOI] [PubMed] [Google Scholar]
- Yucel‐Lindberg, T. (2013). Inflammatory mediators in the pathogenesis of periodontitis. Expert Reviews in Molecular Medicine, e7, 15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors


