Abstract
Objective:
We sought to determine whether an accessory maxillary ostium (AMO) is a congenital or acquired condition and we investigated concomitant sinus pathologies associated with this structure.
Methods:
Paranasal sinus CT examinations of individuals aged ≥13 years and <13 years were compared retrospectively. In total, 552 sinuses of 276 patients aged ≥13 years (Group 1) and 284 maxillary sinuses of 142 children aged <13 years (Group 2) were evaluated. Patients were classified as AMO-positive or -negative. The following features were evaluated in Group 1: AMO presence, mucus retention cysts, mucosal thickening, sinusitis of the maxillary sinus, nasal septum deviation, concha hypertrophy, concha bullosa, primary ostium obstruction, uncinate process atelectasis, paradox concha, Agger nasi and Haller cells, and sinus hypoplasia. The sizes and locations of AMOs were also evaluated. The presence of an AMO and sinusitis were evaluated in Group 2.
Results:
AMOs were detected in 122 sinuses in Group 1. In the AMO-positive group, sinusitis, mucosal thickening, and primary ostium obstruction were significantly more common than in the AMO-negative group (p < 0.00001). Statistically significant associations were not observed between AMO presence and other parameters. AMOs were present in two sinuses in Group 2.
Conclusion:
Our results suggest that AMOs are acquired defects caused by sinus diseases. The rare occurrence of these structures in patients aged <13 years suggests that they may be a perforation or secondary drainage pathway in patients with sinusitis or primary ostium obstruction.
Keywords: Accessory maxillary ostium, maxillary sinus, sinusitis, computed tomography, variation
Introduction
The maxillary sinus is a pyramid-shaped cavity and the largest paranasal sinus.1 The medial wall (i.e. lateral nasal wall) separates the maxillary sinus from the nasal cavity.2 Maxillary sinus development begins in the 10th week of gestation, occurs rapidly between the ages of 1 and 8 years, and then reaches an adult form during adolescence.3–5 The primary ostium (i.e. the maxillary sinus drainage pathway) is located at the top of the medial wall of the sinus. Thus, mucus drains against gravity. Moreover, this drainage travels into the narrow ethmoid infundibulum, rather than directly into the nasal cavity.4 The primary ostium, ethmoid infundibulum, and hiatus semilunaris are maxillary sinus drainage pathways. Obstructions in any of these structures can cause maxillary sinus diseases. Treatment involves functional endoscopic sinus surgery (FESS), which aims to open the primary ostium and provide mucus circulation.
An accessory maxillary ostium (AMO) was long considered an incidental finding and a physiologically normal structure.6 It was presumed to be an anatomic variation located on the lateral nasal wall.7 The fontanelle is a membranous area, covered solely by mucoperiosteum between the uncinate process and inferior turbinate. The ethmoid process of the inferior turbinate separates the fontanelle into anterior and posterior regions. AMOs may be located anywhere within the lateral nasal wall, but are most commonly found in the posterior fontanelle, where the nasal airflow is greatest.8,9 Although the prevalence of AMOs is reportedly near 30% in patients with chronic sinusitis and 10–20% in healthy individuals, there is no consensus concerning whether AMOs are congenital or acquired.7,10,11
This study was performed to test three hypotheses: (1) AMOs constitute an acquired defect and are therefore rare in children (<13 years). (2) This phenomenon is associated with sinus pathologies or variations. (3) Sinus pathologies vary by AMO location and size. This study evaluated the difference in the prevalence of an AMO between two different age groups to infer that AMO is an acquired defect rather than a congenital variation.
Methods and materials
Paranasal sinus CT examinations performed between January 2020 and March 2020 were reviewed retrospectively. Institutional ethical board approval was obtained prior to this study. Paranasal sinus CTs performed for any reason (suspected sinusitis, polyposis, maxillofacial trauma, sinus headache, pre-surgical evaluation, or a dental pathology) were evaluated. Paranasal CT was performed in children suspected of bacterial sinusitis complications, those with sinusitis unresponsive to medical treatment, and maxillofacial trauma. The exclusion criteria were as follows: age <1 year, sinus wall destruction, sinonasal polyposis, history of FESS (antrostomy, uncinectomy, and/or turbinectomy defects), new or previous fractures in maxillary sinus walls, the presence of an ethmomaxillary sinus, inadequate image quality (Figure 1 [flow chart]).
Figure 1.
Flowchart of the study (n; number of patients). AMO, accessory maxillary ostium; FESS, functional endoscopic sinus surgery.
In total, 547 patients underwent paranasal sinus CT scans and 129 patients were excluded from the study. Of the remaining patients, 276 were aged ≥13 years (Group 1) and 142 were aged <13 years (Group 2). The following features were evaluated in Group 1: AMO presence, mucous retention cysts, mucosal thickening, sinusitis of the maxillary sinus, nasal septum deviation, concha hypertrophy, concha bullosa, primary ostium obstruction, uncinate process atelectasis, paradox concha, Agger nasi and Haller cells, and maxillary sinus hypoplasia (Figure 2). All parameters were evaluated on both sides (right and left). Furthermore, Group 1 was divided into two subgroups (AMO-positive and -negative) to evaluate the relationships of AMOs with other parameters. AMO and sinusitis status were evaluated in Group 2. A Schneiderian membrane thickness >2 mm was considered to indicate mucosal thickening.12 Maxillary sinusitis featured a gas–fluid meniscus in the sinus, gas bubbles in the fluid, opacification of the (normally aerated) sinus lumen, and/or obstruction of the osteomeatal complex. Polypoid soft tissue densities filling the nasal cavity and/or sinus with bone remodeling were evaluated as polyposis. Mucosal thickening, mucous retention cysts, and sinusitis were defined as “sinus disease”.13
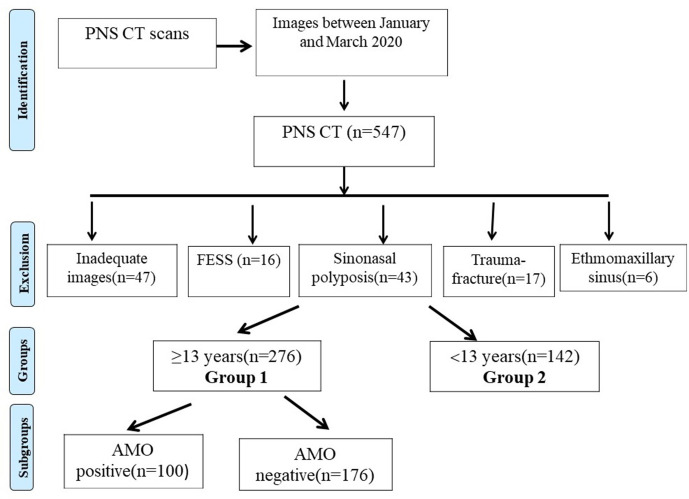
Figure 2.
Variations and pathologies evaluated separately for all sinuses. (a) Right maxillary sinus hypoplasia and uncinate process atelectasis (arrow); (b) right-sided concha bullosa (dotted arrow) and concha hypertrophy (arrow); (c) bilateral mucus retention cysts (arrows) in the maxillary sinuses and obliterated primary ostia (dotted arrows); (d) nasal septal deviation to the left (thick arrow), right-sided paradox concha (dotted arrow), and left-sided mucosal thickening (arrow) in the maxillary sinus; (e) Haller cell in the left side (arrow); (f) Agger nasi cell on the left side (arrow); (g) bilateral maxillary sinus disease (stars) and left-sided AMO (arrow); and (h) a patient excluded from the study because of a left-sided ethmomaxillary sinus (star). AMO, accessory maxillary ostium.
For AMO-positive patients, mean AMO diameters in the coronal plane were measured to determine whether a significant difference in AMO size was evident according to sinus disease status. The maxillary sinus medial wall was divided into three equal parts at the level where it appears longest in the coronal plane. AMO location was then designated as upper, middle, or lower (Figure 3). The relationships between the frequency of sinus disease (mucosal thickness, mucous retention cyst, and sinusitis) and both AMO location and size were evaluated.
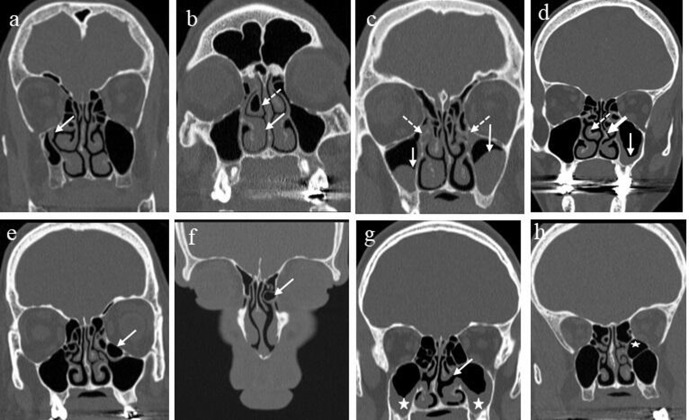
Figure 3.
(a) AMO in an upper location in the medial wall of the maxillary sinus on the left side (arrow) identified on coronal CT; (b) AMO in a middle location (arrow) in the medial wall of the maxillary sinus on the left side identified on coronal CT, combined with bilateral sinus disease; (c) AMO in a lower location in the medial wall of the maxillary sinus on the left side (arrow) identified on coronal CT. AMO, accessorymaxillary ostium.
After all had been evaluated by both observers, a consensus meeting on debatable images was held. A consensus was attained for every debatable qualitative parameter. The mean values of quantitative parameters were calculated. To assess intrarater reliabilities in terms of both qualitative and quantitative data evaluation, all images were re-examined by both observers 1 month after the first evaluations.
CT imaging protocol
All paranasal sinus CT scans in this study were performed on a GE Optima CT660 device (GE Healthcare, Milwaukee, WI) at our university hospital using an axial-plane bone window and reformatted images of the paranasal sinuses. The CT acquisition parameters were as follows: 100-mAs tube current, 100 kV, 0,6 s rotation time, table speed of 1 mm/rotation (pitch, 0,984), 1,25 mm slice thickness (0,625 mm reformatted), 2,8 s scan time, 200 mm field of view, and matrix of 1,024 × 1,024. CT scans were performed with patients in the supine position such that their head position oriented the hard palate parallel to the floor. Low-dose CT (80 kV tube voltage, iterative reconstruction) was performed in children; multiplanar thin-section images were obtained.
Statistical analyses
Data were analyzed using SPSS software (v. 26,0; IBM Corp., Armonk, NY). Inter- and intrarater reliabilities in terms of qualitative data evaluation were assessed using the κ coefficient (κ). The intraclass correlation coefficient (ICC) was used to analyze the extents of inter- and intrarater agreements when evaluating quantitative data (AMO diameters). The Mann–Whitney U test was used to compare quantitative data between two independent groups. Comparisons of categorical variables were performed using the Pearson χ2 test. Data are expressed as numbers (n), medians, ranges, or percentages. p-values < 0,05 were considered to indicate statistical significance.
Results
In total, 552 right and left sides of 276 individuals were included in Group 1 (13–99 years; median age 39 years; 139 female and 137 males). The 284 right and left sides of 142 individuals were included in Group 2 (1–9 years, median age 4 years; 57 females and 85 males) (Table 1). Substantial interobserver agreement was achieved in terms of paradox concha and sinusitis (κ: 0,61–0,80). Perfect interobserver agreement was achieved for all of other parameters (κ: 0,823–1,000). Good interobserver agreement (ICC; 0,75–0,90) and excellent intraobserver agreement (ICC; 0,90–1,00) were achieved when AMO diameters were measured.
Table 1.
Comparison of Groups 1 and 2 in terms of demographic distribution and presence of sinusitis
| Group 1 | Group 2 | ||
|---|---|---|---|
| n = 552 | n = 284 | ||
|
Age (years)
Median (IQR) (min/max) |
39 (27)(13 - 95) | 4 (3)(1 - 9) | |
| Gender (n/%) Female | 278 (50,3%) | 114 (40,1%) | |
| Male | 274 (49,6%) | 170 (59%) | 0,472 |
| AMO (n/%) | 122 (22,1%) | 2 (0,7%) | *<0,001 |
| Sinusitis (n/%) | 88 (15,9%) | 84 (29,5%) | *<0,001 |
AMO; Accessory maxillary ostium, n; number of sinuses, IQR; interquartile range, Max; maximum,
Min; minimum, *; significant.
AMOs were detected in 122 sinuses (22,1%) of 100 patients in Group 1 and 2 sinuses of 2 patients in Group 2 (p < 0,00001). In Group 1, there were 35 right-sided, 43 left-sided and 22 bilateral AMOs (Figure 4). Furthermore, one patient who had bilateral AMO, also had two AMOs on the left side (one on the right and two on the left side). For statistical analyses, AMOs on the left side were counted as a single AMO because they were present in a single sinus. The demographic distribution did not significantly differ between the AMO-positive and -negative groups (Table 2). The presence of an AMO was significantly associated with sinusitis (p = 0,003), mucosal thickening (p = 0,046), and primary maxillary ostium obstruction (p = 0,019). There were no significant associations between other parameters and the presence of an AMO (Table 2).
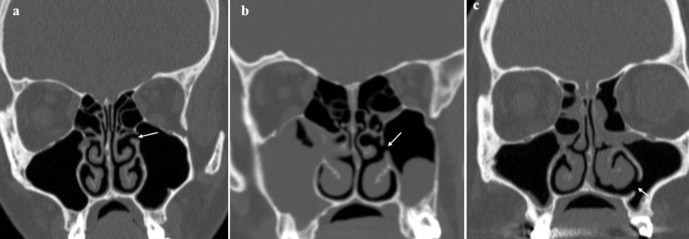
Figure 4.
(a) AMOs in the medial wall of both maxillary sinuses (arrows); (b) normal primary maxillary ostia (arrows) in the same patient. AMO, accessorymaxillary ostium.
Table 2.
Demographic distribution of AMO positive and negative groups and comparison of accompanying variations-pathologies
| AMO positive | AMO negative | P | |
|---|---|---|---|
| n = 122 | n = 430 | ||
|
Age (years)
Median (IQR) (min/max) |
34 (24,2)(13 - 95) | 39 (27) (13 - 95) | 0,07 |
| Gender (n/%) Female | 53 (43,4%) | 225 (52,3%) | |
| Male | 69 (56,5%) | 205 (47,6%) | 0,344 |
| 30 (24,5%) | 58 (13,4%) | *0,003 | |
| 56 (45,9%) | 154 (35,8%) | *0,046 | |
| Primary ostium obstruction(n/%) | |||
| 27 (22,1%) | 58 (13,4%) | *0,019 | |
| Mucous retention cyst(n/%) | |||
| 18 (14,7%) | 43 (10,0%) | 0,13 | |
| Agger nasi cell(n/%) | |||
| 6 (4,9%) | 13 (3,02%) | 0,308 | |
| Haller cell(n/%) | |||
| 3 (2,4%) | 18 (4,1%) | 0,38 | |
| Septum deviation(n/%) | |||
| 54 (44,2%) | 212 (49,3%) | 0,33 | |
| MS hypoplasia(n/%) | |||
| 5 (4,09%) | 34 (7,9%) | 0,14 | |
| Paradox concha(n/%) | |||
| 15 (12,2%) | 34 (7,9%) | 0,13 | |
| Atelectatic uncinate process(n/%) | 4 (4,09%) | 10 (2,3%) | 0,55 |
| 34 (27,8%) | 103 (23,9%) | 0,36 | |
| 8 (6,5%) | 29 (6,7%) | 0,94 | |
AMO; Accessory maxillary ostium, n; number of sinuses. Max; maximum, Min; minimum, MS; maxillary sinus, IQR; interquartile range, *; significant.
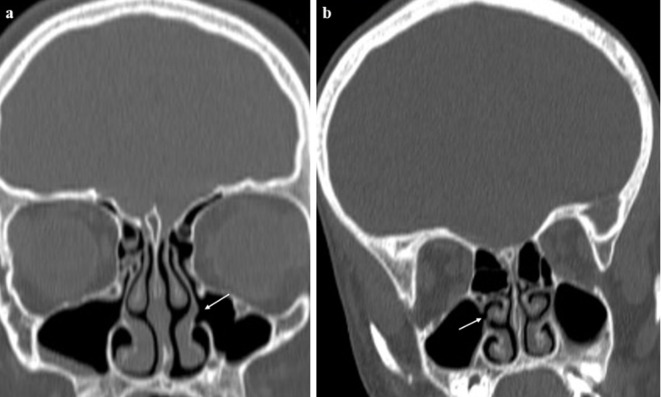
AMOs were located in the upper region in 45 maxillary sinuses (36,8%), in the middle region in 74 maxillary sinuses (60,6%), and in the lower region in 3 maxillary sinuses (2,4%). There was no significant association between AMO localization and sinus disease (Table 3). The median AMO diameter in the coronal plane was 2,2 mm in the sinus disease-positive group and 2,5 mm in the sinus disease-negative group (range, 0,9–8,8 mm). Although the mean AMO size was smaller in patients with sinus disease, this difference was not statistically significant (Table 3). In Group 2, AMOs were detected in two sinuses of two different patients both aged 8 years (0,7%). In Group 2, sinusitis was detected in 84 sinuses of 49 children (Table 1 and Figure 5). In two patients, the antrochoanal polyp passed through the AMO (Figure 6).
Table 3.
The relationship between the size and location of AMO and sinus disease
| AMO location | Sinus disease positive (n = 75) | Sinus disease negative (n = 47) | P |
|---|---|---|---|
| Upper (n/%) | 26 (34,6%) | 19 (40,4%) | 0,206 |
| Middle (n/%) | 47 (62,6%) | 27 (57,4%) | |
| Lower (n/%) | 2 (2,6 %) | 1 (2,2%) | |
| Diameter (mm) Median (IQR)(min/max) |
2,2 (1,6)(0,9–8,8) | 2,5 (1,7)(1 - 6,4) | 0,928 |
AMO; accessory maxillary ostium, n; number of sinuses, IQR; interquartile range, Max; maximum, Min; minimum, mm; millimeters
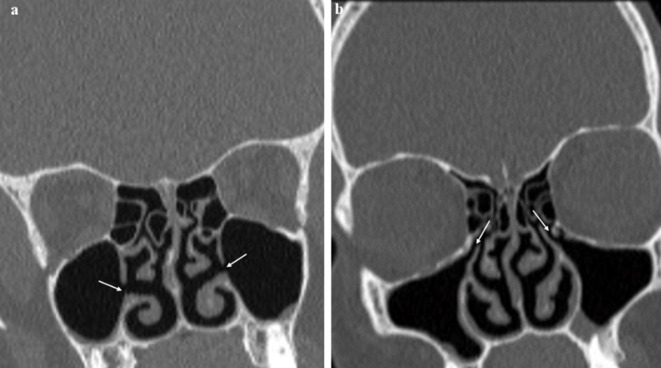
Figure 5.
(a) Images showing left-sided (arrow) and (b) right-sided (arrow) AMOs in two 8-year-old children. AMO, accessory maxillary ostium.
Figure 6.
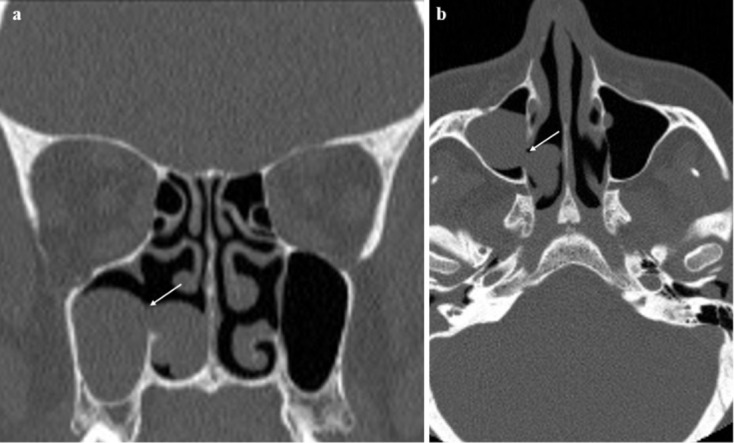
(a) Coronal and (b) axial CT images showing right-sided AMO (arrows) and polyp passing through the AMO; this patient excluded from the study. AMO, accessory maxillary ostium.
Discussion
To the best of our knowledge, this is the first study to evaluate the presence of AMOs in patients under the age of 13 years. In addition, this study evaluated the largest number of parameters that might influence the relationship between the presence of an AMO and many sinus variations and pathologies. The prevalences of AMOs were evaluated in patients aged <13 years and ≥13 years. AMOs were detected in only 2 sinuses of 2 patients aged <13 years (0,7%) and in 122 sinuses of 100 patients aged ≥13 years (22,1%). These findings indicate that AMOs are more likely to occur after the completion of sinus development3,14,15 imply that AMOs are acquired formations. This confirms our first hypothesis. Furthermore, maxillary sinusitis, mucosal thickening, and primary maxillary ostium obstruction were significantly associated with the presence of an AMO. No significant associations were found between the presence of an AMO and mucous retention cyst, nasal septum deviation, concha hypertrophy, concha bullosa, paradox concha, uncinate process atelectasis, sinus hypoplasia, and the presence of Agger nasi and Haller cells (p > 0,005). Although these findings suggest that our second hypothesis is correct, our third hypothesis was rejected because AMO location and size were not associated with sinus disease.
Paranasal sinuses are reservoirs for nitric oxide (NO). The quantity of NO is much greater in exhaled nasal respiration than in oral respiration, suggesting large-scale NO production within nasal cavities.16,17 Enzymes that ensure continuous NO production are produced by the maxillary sinus mucosa.18 AMOs change the airflow pattern and increase maxillary antrum ventilation. Furthermore, AMOs can prevent normal high NO accumulation in the antrum and allow colonization by nasal pathogens. NO is toxic for most pathogens and its normal current might offer protection against sinus disease.19,20 Nasal air flow is highest in the posterior fontanelle where AMOs most frequently occur.8,9 Because the posterior fontanelle is a membranous area, it may deteriorate with elevated pressure.4
During mucus recirculation, mucociliary activity drains mucus in the maxillary antrum from the primary ostium toward the nasal cavity (against gravity). This implies that AMOs do not participate in mucociliary transport in the maxillary antrum.21 However, viscous mucus may re-enter the antrum, from the nasal cavity, via the AMO. After recovery from sinusitis, the primary ostium begins to drain the sinus once more. However, if an AMO is present, mucus drained from the primary ostium may return to the antrum via the AMO. This vicious circle prohibits mucus clearance from the maxillary sinus. Mucus viscosity increases, inflammatory agents accumulate, and mucosal inflammatory disease develops.21 The condition is termed the ‘two-hole syndrome’ and is one of the major complications of an AMO and one cause of chronic sinusitis (Figure 7).6,21–23 Also, AMOs may play role in FESS failure. Penttila24 repaired such defects using inferior turbinate flaps in a series of 116 patients. However, we lack evidence indicating that an AMO may drain the maxillary ostium during an episode of sinus pathology or compromise the resolution of sinusitis. Should an AMO be surgically repaired to prevent failure of sinusitis treatment? If future, randomized clinical trials reveal that surgical repair is in fact required, radiological data will be crucial.
Figure 7.
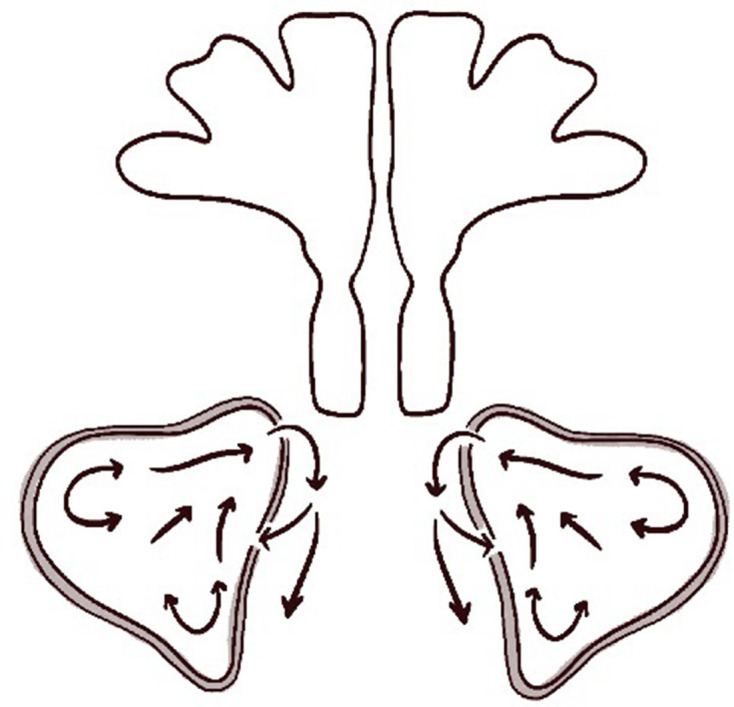
Mucociliary transport in the maxillary sinus and nasal cavity in subjects with the two-hole syndrome. When the AMO and primary ostium are simultaneously open, mucus draining from the primary ostium to the nasal cavity returns from that cavity to the maxillary sinus through the AMO (arrows indicate direction of mucus transport). AMO, accessory maxillary ostium.
Some studies have evaluated the relationships of AMOs with sinus pathologies, but it remains unclear whether AMOs are congenital.6,7,25 Because of the aforementioned mechanisms, AMOs are considered acquired defects that compensate for sinus disease but subsequently impede mucus circulation.6 Notably, our results revealed that AMOs were uncommon in patients under the age of 13 years, which supports the notion that AMOs are acquired defects. However, some holes in the medial wall of the maxillary sinus are not AMOs. A variant ethmomaxillary sinus and opening, and iatrogenically opened defects, have been reported.12,24,26 Therefore, we excluded patients with an ethmomaxillary sinus or a history of FESS.
While some previous studies have reported that AMOs are more common on one side in male patients with ethmoid sinusitis,27 others have demonstrated a relationship involving Haller cells, septum deviation, and AMOs.9,28 However, our study did not reveal any significant associations between the presence of an AMO and parameters other than mucosal thickening, sinusitis, and primary maxillary ostium obstruction. In agreement with our findings, Yenigün et al7 reported significant associations between the existence of an AMO and both mucosal thickening and sinusitis. Furthermore, Arslan et al29 found a significant association between the presence of an AMO and primary maxillary ostium obstruction. Although our findings suggest that AMOs were associated with sinusitis, mucosal thickness, and obstruction of the primary ostium, we found no associations between AMO location or size and sinus disease. This is presumably either due to the relatively small number of individuals included in our study or to potential changes in AMO size and location during and after sinusitis. Because we did not use cone beam CT images, we could not evaluate defect shapes. In a cone beam CT study, the authors found associations between AMO length and morphological sinus changes, as well as between AMO area and morphological sinus changes.30
Since some physiological opacification can be found in the sinus and mucosal thickness may increase during and after crying in children, only sinusitis was evaluated to prevent confusion.31 The higher rate of sinusitis in Group 2 than Group 1 may reflect the limited CT indications in children and the need to avoid irradiation. Paranasal sinus CT is performed more commonly in children than adults to detect sinusitis and associated complications. In Group 1, the sinusitis incidence may have been relatively low because of the large number of indications including septal deviation, conchal pathology, and evaluation prior to septoplasty and rhinoplasty. The significantly lower AMO rate in Group 2, despite the higher rate of sinusitis in this group, supports the idea that an AMO may be an acquired defect that develops after chronic or recurrent sinusitis.
Because of differences in techniques and study design, it is difficult to compare the prevalences observed in this study with the findings of previous studies. AMOs were detected in 22,1% of the sinuses in our study, while previous studies have demonstrated prevalences of 0 to 56% in cadaveric, CT, and endoscopic analyses.7,28 The 0% rate reported might have been due to the low number of patients in that cadaveric study or the difficulty of detecting this small membranous defect within a cadaver. Studies that reported higher rates might have been biased by difficulties involved in distinguishing between AMOs and additional openings in the ethmomaxillary sinus.26
There were some limitations in this study. The exclusion of patients with polyposis because of sinus wall destruction may have interfered with the evaluation of the relationship between true chronic sinusitis and AMOs. Moreover, because this was a radiological study, clinical manifestations of AMOs were not evaluated. Additional evaluations of both radiological appearances and clinical manifestations of AMOs in large patient cohorts are needed to confirm our findings and provide sufficient guidance for clinicians.
Conclusion
The results of our study support the hypothesis that an AMO is an acquired defect associated with maxillary sinus pathologies. Evaluation of a large prospective patient series is required to reveal whether an AMO is a cause or a result of a sinus pathology.
Contributor Information
Umut Percem Orhan Soylemez, Email: umutpercem@gmail.com.
Basak Atalay, Email: basak_hosgoren@yahoo.com.
REFERENCES
- 1.Standring S. Gray’s anatomy: the anatomical basis of clinical practice.. : 41st. London: Elsevier Health Sciences; 2015. [Google Scholar]
- 2.Iwanaga J, Wilson C, Lachkar S, Tomaszewski KA, Walocha JA, Tubbs RS. Clinical anatomy of the maxillary sinus: application to sinus floor augmentation. Anat Cell Biol 2019; 52: 17–24. doi: 10.5115/acb.2019.52.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nuñez-Castruita A, López-Serna N, Guzmán-López S. Prenatal development of the maxillary sinus: a perspective for paranasal sinus surgery. Otolaryngol Head Neck Surg 2012; 146: 997–1003. [DOI] [PubMed] [Google Scholar]
- 4.Prasanna LC, Mamatha H. The location of maxillary sinus Ostium and its clinical application. Indian J Otolaryngol Head Neck Surg 2010; 62: 335–7. doi: 10.1007/s12070-010-0047-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarna A, Hayman LA, Laine FJ, Taber KH. Coronal imaging of the osteomeatal unit: anatomy of 24 variants. J Comput Assist Tomogr 2002; 26: 153–7. doi: 10.1097/00004728-200201000-00027 [DOI] [PubMed] [Google Scholar]
- 6.Mladina R, Vuković K, Poje G. The two holes syndrome. am j rhinol allergy 2009; 23: 602–4. doi: 10.2500/ajra.2009.23.3375 [DOI] [PubMed] [Google Scholar]
- 7.Yenigun A, Fazliogullari Z, Gun C, Uysal II, Nayman A, Karabulut AK. The effect of the presence of the accessory maxillary ostium on the maxillary sinus. Eur Arch Otorhinolaryngol 2016; 273: 4315–9. doi: 10.1007/s00405-016-4129-8 [DOI] [PubMed] [Google Scholar]
- 8.Kane KJ. Recirculation of mucus as a cause of persistent sinusitis. Am J Rhinol 1997; 11: 361–70. doi: 10.2500/105065897781286034 [DOI] [PubMed] [Google Scholar]
- 9.Ozel HE, Ozdogan F, Esen E, Genc MG, Genc S, Selcuk A. The association between septal deviation and the presence of a maxillary accessory Ostium. Int Forum Allergy Rhinol 2015; 5: 1177–80. doi: 10.1002/alr.21610 [DOI] [PubMed] [Google Scholar]
- 10.Joe JK, Ho SY, Yanagisawa E. Documentation of variations in sinonasal anatomy by intraoperative nasal endoscopy. Laryngoscope 2000; 110(2 Pt 1): 229–35. doi: 10.1097/00005537-200002010-00008 [DOI] [PubMed] [Google Scholar]
- 11.Jones NS. Ct of the paranasal sinuses: a review of the correlation with clinical, surgical and histopathological findings. Clin Otolaryngol Allied Sci 2002; 27: 11–17. doi: 10.1046/j.0307-7772.2001.00525.x [DOI] [PubMed] [Google Scholar]
- 12.Hung K, Montalvao C, Yeung AWK, Li G, Bornstein MM, Frequency BMM. Frequency, location, and morphology of accessory maxillary sinus ostia: a retrospective study using cone beam computed tomography (CBCT. Surg Radiol Anat 2020; 42: 219–28. doi: 10.1007/s00276-019-02308-6 [DOI] [PubMed] [Google Scholar]
- 13.Momeni AK, Roberts CC, Chew FS. Imaging of chronic and exotic sinonasal disease: review. American Journal of Roentgenology 2007; 189(6 Suppl): S35–45. doi: 10.2214/AJR.07.7031 [DOI] [PubMed] [Google Scholar]
- 14.Kilic C, Kamburoglu K, Yuksel SP, Ozen T. An assessment of the relationship between the maxillary sinus floor and the maxillary posterior teeth root tips using dental cone-beam computerized tomography. Eur J Dent 2010; 04: 462–7. doi: 10.1055/s-0039-1697866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncavage J. The maxillary sinus: medical and surgical management.. , 2011New yorkThieme Medical Publishers. Available from: http://site.ebrary.com/id/10658297.
- 16.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 1993; 6: 1368–70. [PubMed] [Google Scholar]
- 17.Jankowski R, Nguyen DT, Poussel M, Chenuel B, Gallet P, Rumeau C. Sinusology. Eur Ann Otorhinolaryngol Head Neck Dis 2016; 133: 263–8. doi: 10.1016/j.anorl.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 18.Lundberg JON, Weitzberg E, Rinder J, Rudehill A, Jansson O, Wiklund NP, et al. Calcium-Independent and steroid-resistant nitric oxide synthase activity in human paranasal sinus mucosa. Eur Respir J 1996; 9: 1344–7. doi: 10.1183/09031936.96.09071344 [DOI] [PubMed] [Google Scholar]
- 19.Rennie CE, Hood CM, Blenke EJSM, Schroter RS, Doorly DJ, Jones H, et al. Physical and computational modeling of ventilation of the maxillary sinus. Otolaryngol Head Neck Surg 2011; 145: 165–70. doi: 10.1177/0194599811401202 [DOI] [PubMed] [Google Scholar]
- 20.Na Y, Kim K, Kim SK, Chung S-K. The quantitative effect of an accessory ostium on ventilation of the maxillary sinus. Respir Physiol Neurobiol 2012; 181: 62–73. doi: 10.1016/j.resp.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 21.Gutman M, Houser S. Iatrogenic maxillary sinus recirculation and beyond. Ear Nose Throat J 2003; 82: 61–3. doi: 10.1177/014556130308200118 [DOI] [PubMed] [Google Scholar]
- 22.Yanagisawa E, Yanagisawa K. Endoscopic view of recirculation phenomenon of the maxillary sinus. Ear Nose Throat J 1997; 76: 196–8. doi: 10.1177/014556139707600404 [DOI] [PubMed] [Google Scholar]
- 23.Chung SK, Dhong HJ, Na DG, . Mucus circulation between accessory Ostium and natural Ostium of maxillary sinus. J. Laryngol. Otol. 1999; 113: 865–7. doi: 10.1017/S002221510014544X [DOI] [PubMed] [Google Scholar]
- 24.Penttilä M. Accessory maxillary ostium repair using middle turbinate flap: a case series of 116 patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2018; 8: 1204–10. doi: 10.1002/alr.22159 [DOI] [PubMed] [Google Scholar]
- 25.Sahin C, Ozcan M, Unal A. Relationship betweendevelopment of accessory maxillary sinusand chronic sinusitis. Medical Journal of Dr. D.Y. Patil Vidyapeeth 2015; 8: 606–8. [Google Scholar]
- 26.Liu J. Comments about the article “frequency, location, and morphology of accessory maxillary sinus ostia: a retrospective study using cone beam computed tomography (CBCT)”. Surg Radiol Anat 2020; 42: 557–8(letter). doi: 10.1007/s00276-019-02370-0 [DOI] [PubMed] [Google Scholar]
- 27.Bani-Ata M, Aleshawi A, Khatatbeh A, Al-Domaidat D, Alnussair B, Al-Shawaqfeh R, et al. Accessory Maxillary Ostia: Prevalence of an Anatomical Variant and Association with Chronic Sinusitis]]>. Int J Gen Med 2020; 13: 163–8. doi: 10.2147/IJGM.S253569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali IK, Sansare K, Karjodkar FR, Vanga K, Salve P, Pawar AM. Cone-Beam computed tomography analysis of accessory maxillary ostium and Haller cells: prevalence and clinical significance. Imaging Sci Dent 2017; 47: 33–7. doi: 10.5624/isd.2017.47.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.İB A, Uluyol S, Demirhan E, Kozcu SH, Pekçevik Y, İ Çukurova. Paranasal sinus anatomic variations accompanying maxillary sinus retention cysts: a radiological analysis. Turk Arch Otorhinolaryngol 2017; 55: 162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung AWK, Colsoul N, Montalvao C, Hung K, Jacobs R, Bornstein MM, Visibility BMM. Visibility, location, and morphology of the primary maxillary sinus Ostium and presence of accessory ostia: a retrospective analysis using cone beam computed tomography (CBCT. Clin Oral Investig 2019; 23: 3977–86. doi: 10.1007/s00784-019-02829-9 [DOI] [PubMed] [Google Scholar]
- 31.Ahmed A. Imaging of the paediatric paranasal sinuses. South African Journal of Radiology 2013; 17: 91–7. doi: 10.7196/sajr.778 [DOI] [Google Scholar]