Abstract
Although extracellular host DNA (ecDNA) levels in CF airways were linked to airflow obstruction and recombinant DNAse therapy is beneficial for CF patients, it remains incompletely understood whether ecDNA also leads to an autoimmune response. Here we hypothesized that chronic presence of DNA in CF airways triggers the production of autoantibodies targeting host human DNA. We measured the levels of IgA autoantibodies recognizing host double-stranded (ds) DNA in the blood and sputum samples of CF patients and only sera of controls subjects and patients suffering from rheumatoid arthritis and systemic lupus erythematosus (SLE) that served as non-CF, autoimmune disease cohorts. We found that concentrations of anti-dsDNA IgA, but not IgG, autoantibodies in the circulation were significantly elevated in adult CF patients compared to age-matched, control subjects. Systemic levels of anti-dsDNA IgA antibodies negatively correlated with FEV1% predicted, a measure of lung function, in CF patients. Anti-dsDNA IgA autoantibodies were also detected in CF sputa but sputum levels did not correlate with the degree of airway obstruction or sputum levels of DNA. We also found elevated autoantibody levels in CF children as 76.5% of CF patients younger than 10 years and 87.5% of CF patients 10–21 years had higher blood anti-dsDNA IgA levels than the highest value found in healthy control adults. Overall, our results detect elevated systemic anti-dsDNA IgA autoantibody levels in CF adults, teenagers and young children. We speculate that the appearance of an autoimmune response against host DNA in CF is an early event potentially contributing to disease pathogenesis.
Keywords: Cystic fibrosis, autoimmunity, DNA, autoantibody, IgA
Introduction
Early studies found that DNA is present in large amounts in the airways of CF patients (1–4). Interestingly, the host was identified as the main source of airway extracellular DNA (ecDNA) in CF, not bacteria (5). EcDNA mainly originates from neutrophils recruited to the lungs of CF patients and DNA levels in CF airway secretions correlate with airflow obstruction (1, 5–8). Although the primary pathological mechanism by which ecDNA is thought to contribute to CF lung disease is its viscous nature hindering mucociliary clearance (9, 10), it remains to be determined whether ecDNA also drives CF lung disease by additional mechanisms. Chronic presence of ecDNA is thought to contribute to autoimmune responses in several diseases, most prominently in systemic lupus erythematosus (SLE). Antibodies generated against host double-stranded DNA (anti-dsDNA) have been described early in SLE (11), and they have been shown to correlate with disease activity, especially nephritis [reviewed in (12)]. Anti-dsDNA antibodies are known to be produced in large quantities in SLE (13). Since ecDNA is chronically present in CF airways, we hypothesized that autoantibodies directed against host DNA could also be generated and detected in CF patients. In fact, prior observations reported already the presence of anti-neutrophil cytoplasmic antibodies (ANCA) that target cytoplasmic PMN components: MPO (MPO-ANCA (14, 15)), bacterial permeability increasing protein (BPI-ANCA (16–20)) and proteinase 3 (Pr3-ANCA (14, 15)). Appearance of anti-dsDNA autoantibodies would suggest an increasingly complex autoimmune component of CF and association of anti-dsDNA autoantibodies with lung disease or other clinical symptoms would indicate their potential role in CF disease pathogenesis.
Materials and Methods
Control subjects
All the human subject studies were performed according to the guidelines of the World Medical Association’s Declaration of Helsinki. Control human subjects recruited at the University of Georgia provided informed consent before blood donation according to the protocol UGA# 2012-10769-06. Healthy subjects were chosen to match the sex and age distributions of CF patients and did not suffer from CF, rheumatoid arthritis (RA) or SLE based on self-report (Table 1). Eight to ten milliliters of blood was collected by venipuncture, allowed to clot for thirty minutes and centrifuged. Serum aliquots were stored frozen until used.
Table 1.
Age and sex distribution of patient cohorts used in this study.
| Patient cohorts | Cystic Fibrosis (CF) | Healthy controls (HC) | Rheumatoid arthritis (RA) | Systemic Lupus Erythematosus (SLE) | |||
|---|---|---|---|---|---|---|---|
| CF1 | CF2 | CF3 | HC1 | HC2 | |||
| Collection site | Emory | UGA | Emory | UAB | UMI | ||
| Specimen | Sputum | Serum | |||||
| n = | 70 | 32 | 66 | 23 | 4 | 20 | 10 |
| Gender | |||||||
| % male | 54.2% (n=39) | 56.2% (n=18) | 66.2% (n=44) | 69.6% (n=16) | 0% (n=0) | 40.0% (n=8) | 10.0% (n=1) |
| Age (yrs, mean+/−S.D.) (age range, min.–max.) |
31.0+/−9.1 (19 – 61) |
31.5+/−9.3 (18 – 55) |
20.6+/−14.1 (1 – 61) |
29.2+/−11.6 (18 – 66) |
17.5+/−0.6 (17 – 18) |
32.5+/−4.8 (27 – 44) |
37.7+/−8.5 (21 – 50) |
| Adults | Adults, children | Adults | Children | Adults | |||
| FEV1%pred | 66.4+/−23.7% (20.4–117.5%) |
78.2+/−20.8% (35.4–110.8%) |
- | - | - | - | - |
FEV1%pred: forced expiratory volume in one percent predicted; CF: cystic fibrosis; HC: healthy controls; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; UGA: University of Georgia; UAB: University of Alabama; UMI: University of Michigan; S.D.: standard deviation.
CF patients
CF subjects were patients recruited from the Children’s + Emory CF Care Center which follows approximately 700 CF patients from the newborn period to old age. CF patients or their parent signed informed consent to provide blood and expectorated sputum (adults only) samples (IRB00042577). Samples were tested from three cohorts of CF patients (Table 1): cohort 1 (CF1) consisting of 70 CF adults who provided sputum samples only; cohort 2 (CF2) consisting of 32 CF adults who provided serum while cohort 3 (CF3) was composed of 66 CF children and adults who gave serum to expand the observations made on the CF2 cohort. CF diagnosis was confirmed by pilocarpine iontophoresis sweat testing and/or CFTR gene mutation analysis showing the presence of two disease causing mutations. CF participants were selected for blood draw or sputum collection only if they were clinically stable and on no new medications within the previous three weeks of the clinic visit. For cohort 2, sputum cultures were taken on the same day when the blood was drawn and the presence or absence of Pseudomonas aeruginosa (P. aeruginosa) or Staphylococcus aureus (S. aureus) as identified by the clinical microbiology laboratory was noted. Baseline lung function was defined according to the guidelines of the CF Foundation Patient Registry which is the average of the best percent predicted forced expiratory volume in one second (ppFEV1) for each quarter of the calendar year. Blood was drawn into a silicone coated tube and processed as above until shipped to UGA for analysis. Sputum samples were diluted by 6 ml sterile PBS and kept on ice throughout the entire procedure. Clean sputum supernatants were generated by centrifugation at 3,000g for 10 minutes. Sputum supernatants were stored frozen immediately for future analysis at −80 °C. No protease inhibitors were added. Based on measurements performed on sputum samples obtained from a small cohort of adult CF patients, there were no appreciable differences in autoantibody levels with or without protease inhibitor treatment in sputum samples.
SLE patients
SLE patients meeting four or more American College of Rheumatology lupus criteria (21) were recruited from the University of Michigan lupus clinic under IRB-Med 00066116. All patients underwent written, informed consent. Five mL of blood was collected in a serum separator tube and processed as above.
RA patients
Sera from RA patients were obtained from the University of Alabama at Birmingham (UAB) Rheumatology Arthritis Database and Repository (RADAR). All patients met the 1987 ARA (now ACR) or 2010 ACR/EULAR classification criteria. All data and samples were obtained in accordance with the UAB Institutional Review Board for Human Use (IRB). Standard techniques for venipuncture and isolation of serum were used as detailed above, and aliquots were shipped on dry ice for analysis.
Anti-dsDNA IgA and IgG ELISA
The amount of antibodies binding to dsDNA was quantitated with a commercial assay “ds-DNA Ab IgA ELISA Kit” (Abnova, cat#: KA1098). Human recombinant dsDNA is bound to microwells and exposed to human serum or sputum supernatant samples diluted 25–100–fold. Samples are next exposed to horseradish peroxidase (HRP) conjugated anti-human IgA antibodies and colorimetric signal is measured by a microplate photometer using TMB substrate. With the help of calibrators with IgA class anti-dsDNA antibodies, the results are expressed as U/ml. The detection limit of the assay has been determined at 1.0 U/ml. As the manufacturer states, no interference has been observed with haemolytic (up to 1000 mg/dL), lipemic (up to 3 g/dL triglycerides) or bilirubin-(up to 40 mg/dL) containing sera, nor have any interfering effects been observed with the use of anticoagulants. No details are provided whether the assay distinguishes monomeric from secretory IgA molecules.
Similarly, the amount of IgG autoantibodies binding to dsDNA was quantitated with a commercial “ds-DNA Ab IgG ELISA Kit” (Abnova, cat#: KA1100). Human serum or sputum supernatant samples were diluted 10–100–fold for the measurement. Samples were exposed to horseradish peroxidase (HRP) conjugated anti-human IgG antibodies and colorimetric signal was measured by microplate photometer using TMB substrate as described above. With the help of calibrators with IgG class anti-dsDNA antibodies, the results are expressed as U/ml. The detection limit of the assay has been determined at 20.0 U/ml.
DNA quantification
The concentration of cell-free dsDNA in clear supernatants of sputum samples was quantitated with the Quant-iT™ PicoGreen™ dsDNA Assay Kit (ThermoFisher Scientific, Grand Island, NY, USA) as previously described (22). Sputum samples were diluted 10 to 100–fold in sterile PBS and PicoGreen reagent was added according to the manufacturer’s instructions. DNA levels were determined using a known standard and are expressed as ng/ml in undiluted human sputum. The limit of detection of this assay is 5 ng/ml DNA.
Quantification of neutrophil extracellular traps (NETs)
NETs in human serum and sputum samples was quantitated as complexes of human neutrophil elastase and DNA using a non-commercial ELISA kit as described (23, 24). Briefly, ELISA plates coated with a capture antibody against human neutrophil elastase were blocked, exposed to diluted samples and a detection antibody against double-stranded DNA conjugated with horseradish peroxidase was applied. The signal was developed using TMB substrate and measured at 450 nm using a microplate photometer (Eon, Biotek Instruments, Winooski, VT, USA). NET results are expressed as percentage of a semi-quantitative NET-standard composed of NETs of 100 nM PMA-stimulated human neutrophils pooled from at least five different healthy human donors (23).
Statistical analysis
Results between two subject cohorts were analyzed by Mann-Whitney test while data among more than two cohorts were compared by Kruskal-Wallis test. Correlation between two parameters was evaluated with Spearman’s rank-order correlation. Data are expressed as mean plus-minus standard deviation (SD). The correlation coefficient (r) and two-tailed p values were calculated. Statistically significant differences were considered as *, p<0.05; **, p<0.01; ***, p<0.001. Statistical analysis was carried out with GraphPad Prism version 6.07 for Windows software.
Results
Anti-dsDNA IgA autoantibodies are detectable in CF sputum
Since ecDNA is mainly present in the airways in CF, the primary site for production of DNA-targeting autoantibodies is likely the respiratory mucosa. The IgA antibody subclass is dominant at mucosal sites. Therefore, expectorated sputum was collected from 70 adult CF patients (CF1 cohort) and sputum supernatants were tested for the presence of anti-dsDNA IgA autoantibodies by ELISA (Table 1). The results shown in Fig. 1A indicate that anti-dsDNA IgA autoantibodies are detectable in CF sputum supernatants up to a concentration of 441.5 U/ml. Only 2 out of 70 CF patients had undetectable levels of anti-dsDNA IgA autoantibodies in their sputa.
Figure 1. Sputum levels of anti-dsDNA IgA autoantibodies do not correlate with airway obstruction in adult CF.

A) Sputum anti-dsDNA IgA antibody levels assessed in CF patients (CF1 cohort, n=70) by ELISA were correlated with lung function measured as FEV1%pred (Spearman correlation coefficient, r). B) Anti-dsDNA IgA autoantibody levels were compared in the blood of CF patients (CF1 cohort) with mild (FEV1%>80%, n=24) or severe (FEV1%<50%, n=19) lung disease (Mann-Whitney test). Each dot represents a separate patient. Mean±S.D. is also indicated. Ns, not significant.
Sputum anti-dsDNA IgA autoantibody levels do not correlate with the degree of airflow obstruction
We next asked whether anti-dsDNA IgA autoantibodies present in CF sputa correlate with airway obstruction measured as baseline ppFEV1 for the year when the sputum was collected. Anti-dsDNA IgA autoantibody concentrations did not show any significant correlation with airflow obstruction (Fig. 1A). When CF adults with normal lung function (ppFEV1>80%, n=24) or with severe airflow obstruction (ppFEV1<50%, n=19) were compared for their sputum anti-dsDNA IgA autoantibody levels, no significant difference (p=0.6672, Mann-Whitney test) was found either (Fig. 1B).
Anti-dsDNA IgA autoantibody levels do not correlate with ecDNA or NETs in CF sputum
Since ecDNA present in CF airways (8, 25) is the likely autoantigen for the production of anti-dsDNA autoantibodies, we explored a potential correlation between sputum levels of extracellular DNA and anti-dsDNA IgA autoantibodies. As shown in Figure 2A, no correlation was observed between these two parameters in CF sputum. Activated neutrophils in the CF airways form neutrophil extracellular traps (NETs) which are large extracellular complexes extruded and composed of a DNA scaffold associated with histones and granule components, such as neutrophil elastase. NETs are present in CF airways in large quantities and we have recently shown that circulating autoantibodies directed against a specific component of NETs is associated with worse lung disease in CF (22). However, measuring ecDNA levels is insufficient to prove that NET formation is the sole source of the ecDNA as it may originate from neutrophils by mechanisms other than NET formation (i.e. by necrosis) or from cells other than neutrophils. Using an assay assessing levels of NET-specific, neutrophil elastase-DNA complexes (23) we failed to show a correlation between sputum levels of NETs and anti-dsDNA IgA autoantibodies either (Fig. 2B). A strong correlation was, however, seen between sputum DNA and NETs supporting the notion that ecDNA in CF airways mainly originates from NETs (Fig. 2C).
Figure 2. Sputum levels of anti-dsDNA IgA autoantibodies do not correlate with sputum DNA or NET levels in adult CF patients.

Anti-dsDNA IgA autoantibody concentrations were measured by ELISA in sputa of 70 CF patients (CF1 cohort) and correlated with levels of A) ecDNA or B) NETs. EcDNA was quantitated using the PicoGreen fluorescent kit while NETs were determined by ELISA recognizing NET-specific human neutrophil elastase-DNA complexes (HNE-DNA). C) Sputum levels of ecDNA and NETs demonstrated strong correlation with each other in CF sputum. Spearman correlation coefficient, r. Results are shown on a log2 scale. ***, p<0.001; ns, not significant.
Systemic levels of anti-dsDNA IgA antibodies are elevated in CF
Previously, we reported that autoantibodies directed against a NET-specific protein, peptidylarginine deiminase 4, are elevated in the circulation of CF patients and are associated with worse lung disease (22). Therefore, we also aimed at detecting anti-dsDNA IgA autoantibodies in the blood of adult CF patients. Serum samples of 32 CF patients (CF2 cohort) were compared with serum samples of 23 control subjects (HC1 cohort), 20 subjects with RA and 10 with SLE (Table 1). RA and SLE served as non-CF, autoimmune disease controls. The age and sex distributions of the enrolled adult patients were comparable except for the SLE sex ratio that is due to SLE mainly affecting women (Table 1) (12). As results in Figure 3A show, CF patients have significantly elevated levels of anti-dsDNA IgA autoantibodies in their blood (238.9+/−210.7 U/ml, mean+/−S.D.) compared to healthy controls (47.9+/−21.8 U/ml, mean+/−S.D.) (p<0.001). More than two-thirds (69.7%) of the CF patients had higher anti-dsDNA IgA blood levels than any of the control subjects (Figure 3A). Anti-dsDNA IgA serum levels in RA patients (216.5+/−287.1 U/ml, mean+/−S.D.) were significantly higher than healthy control subjects but were not significantly different from CF (Fig. 3A). As expected, SLE patients had serum anti-dsDNA IgA levels (1,244.0+/−2,387.0 U/ml, mean+/−S.D.) significantly higher than those of HC patients (Fig. 3A.). No statistically significant difference was observed between the three disease cohorts - SLE, RA, and CF - in terms of their anti-dsDNA IgA blood levels (Fig. 3A). However, the extreme levels seen in some SLE subjects emphasize variability in the biologic response. For example, the highest anti-dsDNA IgA serum level in CF (1,017.3 U/ml) was in the mid-range of the SLE cohort (Figure 3A). On the opposite end, the highest anti-dsDNA IgA serum concentration in SLE patients (7,920.9 U/ml) is more than 7.8-fold larger than the highest value in the CF cohort (Figure 3A).
Figure 3. Serum anti-dsDNA IgA autoantibody levels are elevated in adult CF patients.
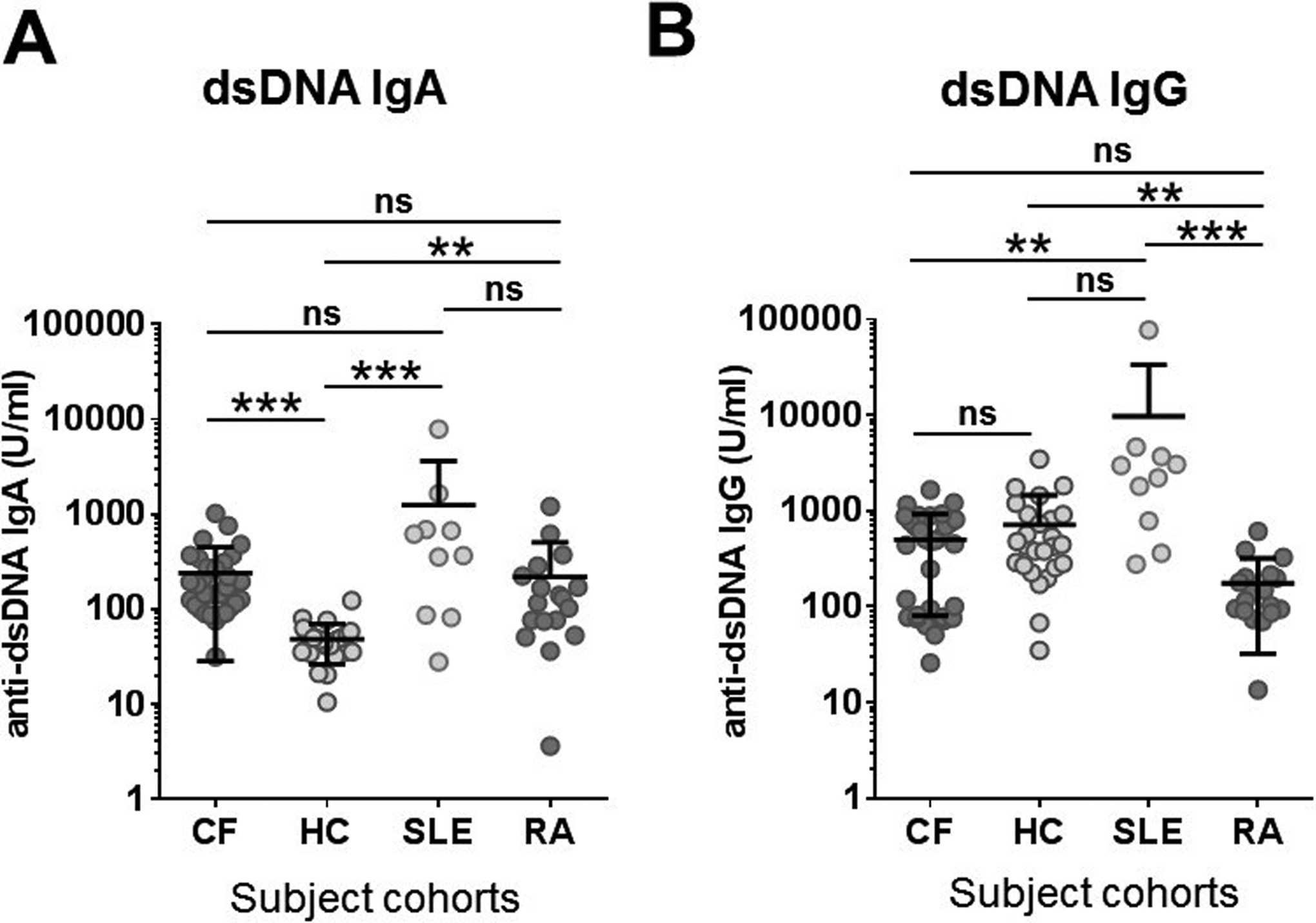
Concentrations of A) anti-dsDNA IgA and B) anti-dsDNA IgG autoantibodies were determined by ELISA in the sera of CF (n=32, CF2 cohort), rheumatoid arthritis (RA, n=20) and systemic lupus erythematosus (SLE, n=10) patients and control subjects (n=23, HC1 cohort). Results were compared by ANOVA and Kruskal-Wallis test. Each symbol represents a separate human subject. Mean±S.D. is also indicated. ***, p<0.001; ns, not significant. HC, healthy controls; CF, cystic fibrosis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; ds, double-stranded; Ab, antibody.
The most common antibody class in the blood is represented by IgG antibodies. Therefore, we also measured anti-dsDNA IgG blood levels in the same patient cohorts by ELISA. No significant difference was found between CF patients and healthy controls in their systemic anti-dsDNA IgG levels (p>0.999, Fig. 3B). This observation is in stark contrast with the significant difference seen in anti-dsDNA IgA autoantibody levels between the same cohorts emphasizing the importance of the IgA autoantibody class targeting host DNA. Anti-dsDNA IgG autoantibodies are diagnostic markers in SLE (26). SLE results in our hands are higher than healthy control values but the difference is not significant (Fig. 3B). The p-value is close, though, to the level of significance (p=0.086) and only a limited number of SLE samples were utilized (Fig. 3B). Overall, this is the first report to our knowledge to detect significantly higher serum levels of IgA, not IgG, autoantibodies produced against dsDNA in CF.
Serum levels of IgA autoantibodies targeting dsDNA negatively correlate with lung function in CF
To explore a potential link between the autoimmune response against ecDNA and lung disease in CF, we correlated blood levels of anti-dsDNA IgA autoantibodies with lung function data of CF adults (CF2 cohort, see Table 1). As shown in Figure 4A, we observed a significant, negative correlation between serum levels of anti-dsDNA IgA autoantibodies and lung function measured as ppFEV1 (r=−0.560, p=0.0009, Spearman’s correlation). The lung function of CF patients with dsDNA IgA serum levels higher than control subjects was significantly (p=0.0044, Mann-Whitney test) worse (ppFEV1=71.8+/−20.2%, mean+/−S.D.) than the lung function of CF patients with dsDNA IgA levels overlapping with the healthy control cohort (ppFEV1=92.8+/−12.0%, mean+/−S.D.) (Fig. 4B). This further emphasizes that higher autoantibody levels associate with worse lung function.
Figure 4. Serum anti-dsDNA IgA levels correlate with airway obstruction in CF but are not associated with lung infections by main respiratory pathogens.
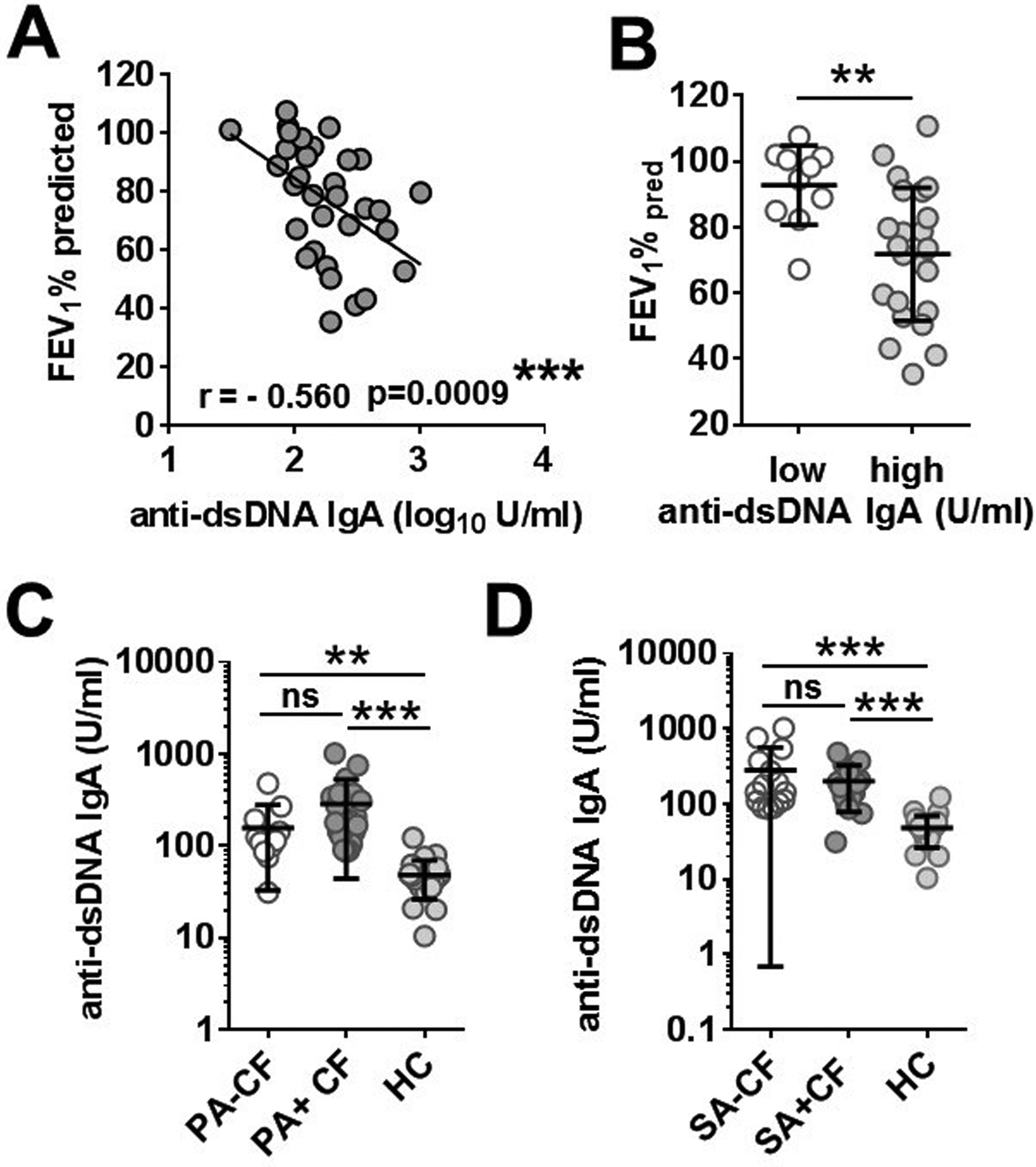
A) Serum anti-dsDNA IgA antibody levels measured in CF patients (n=32, CF2 cohort) by ELISA were correlated with lung function measured as FEV1%pred (Spearman correlation coefficient, r). B) FEV1%pred values were compared between CF patients with systemic anti-dsDNA IgA autoantibody levels that overlaps with healthy donors (low) or are higher than controls subjects (high) (Mann-Whitney test). Anti-dsDNA IgA blood levels in CF patients were also compared in the absence or the presence of P. aeruginosa (C) or S. aureus (D) lung infections. Each dot represents a separate CF patient. Mean±S.D. is also indicated. **, p<0.01; ***, p<0.001; ns, not significant; CF, cystic fibrosis; PA, P. aeruginosa; SA, S. aureus; FEV1%pred forced expiratory volume in 1 per cent predicted; Ab, antibody.
Anti-dsDNA IgA autoantibody levels in serum are not linked to clinical covariates in CF
Since CF lungs are infected by a select group of microorganisms and previously reported autoantibodies (anti-PAD4, BPI-ANCA) have been linked to P. aeruginosa lung infection in CF (20, 22, 27, 28), we next asked whether blood anti-dsDNA IgA autoantibody levels are linked to any of the two main bacterial respiratory pathogens in CF, P. aeruginosa and S. aureus (29). We grouped the CF2 adult cohort according to the presence or absence of P. aeruginosa in sputum cultures taken on the day of blood draw and found that there was no significant difference in anti-dsDNA IgA autoantibody levels between CF patients with or without P. aeruginosa (Fig 4C). Similarly, there was no significance difference in antibody levels in those with or without S. aureus (Fig 4D). No association between systemic anti-dsDNA IgA autoantibody levels and other variables of CF patients (sex, copy number of ΔF508 CFTR allele, presence of CF-related diabetes, and CF-related joint disease) have been noted either (data not shown).
Anti-dsDNA IgA autoantibodies are detectable in CF children and their blood levels correlate with age
To gain insight into the development of the DNA-related systemic autoimmune response in younger CF patients and to also expand our observations made on 32 adult CF patients to a larger group of adult CF individuals, we measured serum levels of anti-dsDNA IgA autoantibodies in an additional 66 CF patients aged 1 to 61 years (CF3 cohort Table 1.). The primary research question utilizing the CF3 cohort was to reveal any age-related changes in serum autoantibody levels. We also had access to sera of four healthy adolescent controls (HC2 cohort, Table 1) that serve as a reflection of levels in healthy adolescents. As results in Figure 5A show, serum anti-dsDNA IgA autoantibody concentrations significantly correlated with the age of the CF patients (r = 0.4091, p= 0.0005). Interestingly, autoantibodies were already detectable in the youngest toddlers in our cohort (age=1 to 2 years) (Fig. 5A). When we grouped CF patients and healthy individuals according to their age into age groups of young children (0–10 years, n=17), adolescents (10–21 years, n=24) and adults (> 21 years, n=25), a significant difference was observed in anti-dsDNA IgA autoantibody levels between the CF adult cohort vs young children (p=0.0032) or teenagers (p=0.0137) (Fig. 5B). Autoantibody levels in adolescent (p=0.0018) and adult (p<0.001) CF patients of the CF3 cohort were significantly elevated compared to their age group-matched healthy cohort (Fig. 5B). The highest level of anti-dsDNA IgA autoantibody among the healthy controls of any age was 122.96 U/ml (Fig. 3A and 6A). Reinforcing the fact that circulating autoantibodies form early in CF, and to a significant level is the observation that 76.5% of CF patients younger than 10 years and 87.5% of CF patients 10–21 years had higher blood anti-dsDNA IgA levels than the highest healthy control value. Furthermore, all CF adults in the CF3 cohort had levels above the highest control value. Thus, DNA-targeting autoantibodies appear very early in life, continue to increase with age and reach high levels in CF adults similar to the classic autoimmune diseases of RA and SLE.
Figure 5. Serum levels of anti-dsDNA IgA autoantibodies in CF children.

A) Serum anti-dsDNA IgA concentrations were measured by ELISA and correlated with airway obstruction (FEV1%pred) in a larger CF cohort also including children (CF3 cohort). Spearman correlation coefficient, r. B) Serum anti-dsDNA IgA levels were compared between different age groups of CF patients (CF3 cohort) and healthy individuals (HC1 and HC2 cohorts) by ELISA. Each dot represents a separate patient. Mean±S.D. is also indicated. Results were compared by ANOVA and Kruskal-Wallis test. *, p<0.05; **, p<0.01; ***, p<0.001; ns, not significant. HC, healthy control; CF, cystic fibrosis.
Conclusions
Our report detects elevated levels of autoantibodies targeting human host dsDNA in CF. While the presence of these antibodies in CF is novel, SLE, a systemic and poly-etiological autoimmune disease, is characterized by high titers of autoantibodies directed against nuclear components including DNA (13). Antibodies targeting host dsDNA are prognostic markers in SLE (13). Generation of anti-dsDNA antibodies requires first that dsDNA normally found inside of cells hidden from the immune system is released into the extracellular environment. Different routes of cell death have been proposed as the main mechanism of dsDNA release in SLE. Although impaired clearance of apoptotic cells has been proposed originally as its main mechanism in SLE (30), recently neutrophils and formation of NETs have gained most of the attention. Rather than being a protective mechanism, a long line of evidence suggests the relevance of neutrophils and NETs in SLE pathogenesis (31).
In CF, it was recognized decades ago that patients’ lungs contain large amounts of extracellular DNA (3, 4). DNA was detected in the bronchoalveolar lavage fluid of both adults and infants with CF (1). DNA levels in CF airway fluid samples negatively correlate with lung function or lung function decline (32). This suggest that DNA found in CF airway fluids could contribute from early on in life to lung disease. Similarly to ecDNA present in SLE, DNA found in CF airways is mainly thought to originate from host neutrophils (5). While the classical mechanism by which neutrophils were thought to release their DNA in CF airways is apoptosis and secondary necrosis by neglect, recent data suggest NET formation, as an alternative mechanism. NETs are abundant in CF airways (33). NETs could also persist longer in CF airways because of impaired mechanisms of NET degradation. Overall, NET formation is the most likely mechanism to deliver autoantigens, including ecDNA, for the generation of anti-dsDNA autoantibodies in CF. Our results found a negative correlation between systemic, but not airway, levels of anti-dsDNA IgA autoantibodies and airflow obstruction in CF. DNA is one of the main host factors increasing the viscosity of CF airway secretions and thereby inhibiting efficient mucociliary clearance (34). This is the major mechanism by which ecDNA is thought to contribute to CF lung disease. In addition to its viscosity-enhancing effect, neutrophil-derived ecDNA was also suggested to support biofilm growth of P. aeruginosa, a feature that is associated with severe lung disease in CF (35). It remains to be explored, however, whether these processes are the only ones by which ecDNA contributes to CF lung damage or additional, ecDNA-mediated mechanisms exists. Our data here propose that release of ecDNA by netting neutrophils could contribute to CF lung disease via a novel mechanism, e.g. generation of anti-dsDNA autoantibodies.
The findings presented here provide new information related to autoimmunity in CF. Autoreactive mononuclear immune cells have already been detected in CF (36). We add here a new autoantibody to the increasing list of autoantibodies detected in CF. Cytosolic components of PMNs have been described to be targeted by autoantibodies in CF (37). The best described autoantibody target in CF is the bacterial permeability-increasing protein (BPI-ANCA) and BPI autoantibodies have been associated with P. aeruginosa infection in CF (16–20). MPO (MPO-ANCA) and proteinase 3 (Pr3-ANCA) have also been reported as autoimmune targets in CF (14, 15). We have recently described the presence of autoantibodies in the CF blood targeting peptidyl arginine deiminase type IV (PAD4), a PMN-specific enzyme essential for histone citrullination and subsequent NET release (38). Similar to BPI-ANCA autoantibodies, anti-PAD4 autoantibodies were also associated with P. aeruginosa infection in CF patients (20, 22). On the contrary, we failed to detect any link between anti-dsDNA IgA levels and P. aeruginosa lung infection. Interestingly, our previously published results also indicate that CF patients do not have elevated blood levels of autoantibodies targeting nucleosomes or citrullinated proteins (22).
The importance of distinguishing the mucosal from the systemic autoimmune response in CF has recently been highlighted in relation to anti-bacterial permeability-increasing protein (BPI) autoantibodies (39). The co-occurrence of anti-BPI and anti-P. aeruginosa IgA in the airways, but not serum, of CF patients was demonstrated (39). It has been suggested that BPI tolerance is lost in the P. aeruginosa-infected airway and serologic IgG autoantibodies are later induced (39). While our study identifies the second IgA-specific autoantibody in CF, the results propose a different dissociation of the DNA-targeting mucosal and systemic autoimmune responses than in the case of anti-BPI IgA (39). Serum, and not sputum, anti-dsDNA IgA (and not IgG) autoantibodies correlate with lung disease in CF. Whether these autoantibodies are pathogenic and contribute to CF lung disease, remains to be determined (40, 41). Anti-dsDNA IgA autoantibodies in the blood could bind DNA attached to endothelial cells and promote local inflammation in the lung or could bind to immune cells in the blood (neutrophils, macrophages) and drive their activation in an Fc receptor-mediated fashion after binding to the corresponding autoantigen targets in CF airways.
One of the most intriguing findings in our study is the detection of anti-dsDNA IgA autoantibodies in young CF children, in many cases at levels that are higher than seen in healthy older children and adults. This suggests that autoimmunity to host DNA released by neutrophils may be an early event in CF rather than a secondary event related to advanced lung disease. Adding credence to this concept is our observation that autoantibody levels are not associated with the presence of what is considered the major CF pathogen, P. aeruginosa, which is found with much lower prevalence in young CF children compared to CF adults. In other words, neutrophil release of DNA in young CF children may not require the presence of bacterial pathogens but rather happen independently without provocation, much like the low-density granulocyte (LDG) subset of neutrophils in SLE. Finally, studies of CF infants and toddlers identified through newborn screening before airway disease has advanced, provide ample evidence that release of elastase by the airway neutrophil is a central and early event driving progression of airway disease. This progression is accentuated in the presence of infection but also appears to occur independent of the usual external stimuli. We now add to this body of evidence the possibility that mucosal autoimmunity to extracellular host DNA is associated with disease progression.
In summary, we identified IgA autoantibodies targeting double-stranded host DNA in CF and show their correlation with lung dysfunction. These mucosal autoantibodies appear very early in life, continue to increase with age, and reach high levels in CF adults similar to the classic autoimmune diseases of RA and SLE. Future studies will investigate the mechanisms by which these autoantibodies are made and potentially contribute to CF disease pathogenesis.
Highlights.
CF serum contains elevated levels of anti-dsDNA IgA, but not anti-dsDNA IgG, autoantibodies
Anti-dsDNA IgA autoantibody levels in serum correlate with airflow obstruction in CF
Anti-dsDNA IgA autoantibodies are detected in CF sputum but do not correlate with airflow obstruction
Anti-dsDNA IgA autoantibodies are also elevated in the blood of the majority of CF toddlers and youth
Acknowledgements
Human CF patient samples were provided by the CF Biospecimen Registry at the Children’s Healthcare of Atlanta and Emory University CF Discovery Core. We would like to thank Jane Wei and Julie Flores, data managers of the Biospecimen Registry and the entire team of CF research coordinators, for collecting the samples and verifying the clinical data for the subjects enrolled in this study. We also thank the staff of the University of Georgia Clinical and Translational Research Unit (CTRU) for their continued support and collaboration to collect blood from healthy donors.
Funding sources
This work was supported by grants provided by the Cystic Fibrosis Foundation (grant ID: 438903, to B.R. and RDP Center McCart15R0 to A.S) and the National Institutes of Health (R21 AI130504-01 and 5R01HL136707 to B.R.). This project was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 to B.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CF
cystic fibrosis
- dsDNA
double-stranded DNA
- ecDNA
extracellular DNA
- SLE
systemic lupus erythematosus
- RA
rheumatoid arthritis
- NET
neutrophil extracellular traps
Footnotes
Conflict of interest statement
The authors have no conflict of interest related to this work.
References
- 1.Kirchner KK, Wagener JS, Khan TZ, Copenhaver SC, and Accurso FJ. 1996. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am J Respir Crit Care Med 154: 1426–1429. [DOI] [PubMed] [Google Scholar]
- 2.Mrsny RJ, Daugherty AL, Short SM, Widmer R, Siegel MW, and Keller GA. 1996. Distribution of DNA and alginate in purulent cystic fibrosis sputum: implications to pulmonary targeting strategies. J Drug Target 4: 233–243. [DOI] [PubMed] [Google Scholar]
- 3.Chernick WS, and Barbero GJ. 1959. Composition of tracheobronchial secretions in cystic fibrosis of the pancreas and bronchiectasis. Pediatrics 24: 739–745. [PubMed] [Google Scholar]
- 4.Matthews LW, Spector S, Lemm J, and Potter JL. 1963. Studies on Pulmonary Secretions. I. The over-All Chemical Composition of Pulmonary Secretions from Patients with Cystic Fibrosis, Bronchiectasis, and Laryngectomy. Am Rev Respir Dis 88: 199–204. [DOI] [PubMed] [Google Scholar]
- 5.Lethem MI, James SL, Marriott C, and Burke JF. 1990. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur Respir J 3: 19–23. [PubMed] [Google Scholar]
- 6.Regelmann WE, Siefferman CM, Herron JM, Elliott GR, Clawson CC, and Gray BH. 1995. Sputum peroxidase activity correlates with the severity of lung disease in cystic fibrosis. Pediatr Pulmonol 19: 1–9. [DOI] [PubMed] [Google Scholar]
- 7.Sagel SD, Wagner BD, Anthony MM, Emmett P, and Zemanick ET. 2012. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med 186: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcos V, Zhou-Suckow Z, Onder Yildirim A, Bohla A, Hector A, Vitkov L, Krautgartner WD, Stoiber W, Griese M, Eickelberg O, Mall MA, and Hartl D. 2015. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediators Inflamm 2015: 408935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picot R, Das I, and Reid L. 1978. Pus, deoxyribonucleic acid, and sputum viscosity. Thorax 33: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puchelle E, de Bentzmann S, and Zahm JM. 1995. Physical and functional properties of airway secretions in cystic fibrosis--therapeutic approaches. Respiration 62Suppl 1: 2–12. [DOI] [PubMed] [Google Scholar]
- 11.Ceppellini R, Polli E, and Celada F. 1957. A DNA-reacting factor in serum of a patient with lupus erythematosus diffusus. Proc Soc Exp Biol Med 96: 572–574. [DOI] [PubMed] [Google Scholar]
- 12.Stannard JN, and Kahlenberg JM. 2016. Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus. Curr Opin Rheumatol 28: 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blatt NB, and Glick GD. 1999. Anti-DNA autoantibodies and systemic lupus erythematosus. Pharmacol Ther 83: 125–139. [DOI] [PubMed] [Google Scholar]
- 14.Chiappini E, Taccetti G, Campana S, Turchini S, and Marianelli L. 2001. [Anti-Pseudomonas aeruginosa antibodies, circulating immune complexes, and anticytoplasm antibodies of neutrophils in patients with cystic fibrosis with and without Pseudomonas aeruginosa colonization]. Pediatr Med Chir 23: 27–30. [PubMed] [Google Scholar]
- 15.Noel LH 2000. [Antineutrophil cytoplasm antibodies (ANCA): description and immunopathological role]. Ann Med Interne (Paris) 151: 178–183. [PubMed] [Google Scholar]
- 16.Carlsson M, Eriksson L, Erwander I, Wieslander J, and Segelmark M. 2003. Pseudomonas-induced lung damage in cystic fibrosis correlates to bactericidal-permeability increasing protein (BPI)-autoantibodies. Clin Exp Rheumatol 21: S95–100. [PubMed] [Google Scholar]
- 17.Schultz H, and Weiss JP. 2007. The bactericidal/permeability-increasing protein (BPI) in infection and inflammatory disease. Clin Chim Acta 384: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotschild M, Elias N, Berkowitz D, Pollak S, Shinawi M, Beck R, and Bentur L. 2005. Autoantibodies against bactericidal/permeability-increasing protein (BPI-ANCA) in cystic fibrosis patients treated with azithromycin. Clin Exp Med 5: 80–85. [DOI] [PubMed] [Google Scholar]
- 19.Skopelja-Gardner S, Theprungsirikul J, Meagher RE, Beliveau CM, Bradley KE, Avery M, Henkle E, Siegel S, Gifford AH, Winthrop KL, and Rigby WFC. 2019. Autoimmunity to bactericidal/permeability-increasing protein in bronchiectasis exhibits a requirement for Pseudomonas aeruginosa IgG response. Eur Respir J 53. [DOI] [PubMed] [Google Scholar]
- 20.Skopelja S, Hamilton BJ, Jones JD, Yang ML, Mamula M, Ashare A, Gifford AH, and Rigby WF. 2016. The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight 1: e88912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochberg MC 1997. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725. [DOI] [PubMed] [Google Scholar]
- 22.Yadav R, Yoo DG, Kahlenberg JM, Bridges SL Jr., Oni O, Huang H, Stecenko A, and Rada B. 2019. Systemic levels of anti-PAD4 autoantibodies correlate with airway obstruction in cystic fibrosis. J Cyst Fibros. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sil P, Yoo DG, Floyd M, Gingerich A, and Rada B. 2016. High Throughput Measurement of Extracellular DNA Release and Quantitative NET Formation in Human Neutrophils In Vitro. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo DG, Floyd M, Winn M, Moskowitz SM, and Rada B. 2014. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes. Immunol Lett 160: 186–194. [DOI] [PubMed] [Google Scholar]
- 25.Bai Y, Tong Y, Liu Y, and Hu H. 2018. Self-dsDNA in the pathogenesis of systemic lupus erythematosus. Clin Exp Immunol 191: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisetsky DS 2016. Anti-DNA antibodies--quintessential biomarkers of SLE. Nat Rev Rheumatol 12: 102–110. [DOI] [PubMed] [Google Scholar]
- 27.Carlsson M, Shukla S, Petersson AC, Segelmark M, and Hellmark T. 2011. Pseudomonas aeruginosa in cystic fibrosis: pyocyanin negative strains are associated with BPI-ANCA and progressive lung disease. J Cyst Fibros 10: 265–271. [DOI] [PubMed] [Google Scholar]
- 28.Lindberg U, Carlsson M, Hellmark T, and Segelmark M. 2015. BPI-ANCA Provides Additional Clinical Information to Anti-Pseudomonas Serology: Results from a Cohort of 117 Swedish Cystic Fibrosis Patients. J Immunol Res 2015: 947934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crull MR, Somayaji R, Ramos KJ, Caldwell E, Mayer-Hamblett N, Aitken ML, Nichols DP, Rowhani-Rahbar A, and Goss CH. 2018. Changing rates of chronic Pseudomonas aeruginosa infections in cystic fibrosis: a population-based cohort study. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michlewska S, McColl A, Rossi AG, Megson IL, and Dransfield I. 2007. Clearance of dying cells and autoimmunity. Autoimmunity 40: 267–273. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan MJ 2011. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol 7: 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JS, Okamoto K, and Rubin BK. 2006. Pulmonary function is negatively correlated with sputum inflammatory markers and cough clearability in subjects with cystic fibrosis but not those with chronic bronchitis. Chest 129: 1148–1154. [DOI] [PubMed] [Google Scholar]
- 33.Manzenreiter R, Kienberger F, Marcos V, Schilcher K, Krautgartner WD, Obermayer A, Huml M, Stoiber W, Hector A, Griese M, Hannig M, Studnicka M, Vitkov L, and Hartl D. 2012. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros 11: 84–92. [DOI] [PubMed] [Google Scholar]
- 34.Shak S, Capon DJ, Hellmiss R, Marsters SA, and Baker CL. 1990. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A 87: 9188–9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitchurch CB, Tolker-Nielsen T, Ragas PC, and Mattick JS. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295: 1487. [DOI] [PubMed] [Google Scholar]
- 36.Stechova K, Kolouskova S, Sumnik Z, Cinek O, Kverka M, Faresjo MK, Chudoba D, Dovolilova E, Pechova M, Vrabelova Z, Bohmova K, Janecek L, Saudek F, and Vavrinec J. 2005. Anti-GAD65 reactive peripheral blood mononuclear cells in the pathogenesis of cystic fibrosis related diabetes mellitus. Autoimmunity 38: 319–323. [DOI] [PubMed] [Google Scholar]
- 37.Forde AM, Feighery C, and Jackson J. 1998. Anti-phagocyte antibodies and infection. Autoimmunity 28: 5–14. [DOI] [PubMed] [Google Scholar]
- 38.Yadav R, Yoo DG, Kahlenberg JM, Bridges SL Jr., Oni O, Huang H, Stecenko A, and Rada B. 2019. Systemic levels of anti-PAD4 autoantibodies correlate with airway obstruction in cystic fibrosis. J Cyst Fibros 18: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theprungsirikul J, Skopelja-Gardner S, Meagher RE, Clancy JP, Zemanick ET, Ashare A, and Rigby WFC. 2019. Dissociation of systemic and mucosal autoimmunity in cystic fibrosis. J Cyst Fibros. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkin T 1989. Autoimmunity: attack, or defence? (The case for a primary lesion theory). Autoimmunity 3: 57–73. [DOI] [PubMed] [Google Scholar]
- 41.Conrad FJ, Rice JS, and Cambier JC. 2007. Multiple paths to loss of anergy and gain of autoimmunity. Autoimmunity 40: 418–424. [DOI] [PubMed] [Google Scholar]


