Abstract
Objectives:
To study the effect of lymph node dissection (LND) at the time of nephrectomy and tumor thrombectomy on oncological outcomes in patients with renal cell carcinoma (RCC) and tumor thrombus.
Patients and Methods:
The records of 1,978 patients with RCC and tumor thrombus who underwent radical nephrectomy and tumor thrombectomy from 1985 to 2014 at 24 centers were analyzed. None of the patients had distant metastases. Extent and pathologic results of LND were compared with respect to cancer-specific survival (CSS). Multivariable Cox regression models were used to quantify the effect of multiple covariates.
Results:
LND was performed in 1,026 patients. In multivariable analysis, the presence of LN metastasis, the number of positive LNs, and LN density were independently associated with cancer-specific mortality (CSM). Clinical node-negative (cN−) disease was documented in 573 patients, 447 of them underwent LND with 43 cN− patients (9.6%) revealing positive LNs at pathology. LN positive cN− patients showed significantly better CSS when compared to LN positive cN+ patients. In multivariable analysis, positive cN status in LN positive patients was a significant predictor of CSM (HR, 2.923; P = 0.015).
Conclusions:
The number of positive nodes harvested during LND and LN density was strong prognostic indicators of CSS, while number of removed LNs did not have a significant effect on CSS. The rate of pN1 patients among clinically node-negative patients was relatively high, and LND in these patients suggested a survival benefit. However, only a randomized trial can determine the absolute benefit of LND in this setting. © 2018 Elsevier Inc. All rights reserved.
Keywords: Renal cell carcinoma, Lymph node metastasis, Vena cava tumor thrombectomy, Lymphadenectomy, Survival, Inferior vena cava
1. Introduction
With the introduction of minimally invasive surgery, management of localized renal cell carcinoma (RCC) has undergone significant shifts in the last decade. For locally advanced and metastatic RCC with tumor thrombus, however, the standard remains open radical nephrectomy.
Although lymphadenectomy has become an integral part of management for most other genitourinary malignancies, this has not been standardized in the management of RCC. Even though recent surgical series confirmed prior anatomic studies demonstrating predictable lymphatic drainage patterns of RCC to primary landing zones, those same studies revealed that perihilar lymph nodes are often skipped (45% of the time), and in patients with metastatic disease, 40% to 60% will have no lymph node involvement [1,2]. This, in combination with the uncertain survival benefit of lymphadenectomy has limited its usage. In the EORTC randomized phase 3 trial 30881, the only prospective randomized trial to assess the role of lymphadenectomy at the time of nephrectomy, only 4% of patients were found to have nodal disease at the time of nephrectomy, and there was no survival benefit. However, the study was limited by patient selection, as most patients included had localized or low-grade RCC [3]. Smaller series have found oncologic benefit to lymphadenectomy at the time of nephrectomy, particularly if there is clinical evidence of nodal disease [2,4–8]. At this time, lymphadenectomy appears to be of benefit in patients with certain high-risk features, including nuclear grade 3 to 4, presence of sarcomatoid histology, tumor size ≥10 cm, tumor stage T3 or T4, and the presence of tumor necrosis [9–11]. Of note, however, Delacroix et al. [6] found that the absence of sarcomatoid features was associated with better overall survival in the setting of lymph node dissection. In the absence of prospective randomized data, the benefit of lymphadenectomy in high-risk patients can only be extrapolated.
Up to 10% of patients can present with tumor thrombus extending into the renal vein and inferior vena cava (IVC) [12,13]. These patients, if left untreated, have a very poor prognosis—Reese et al. [14] demonstrated a 29% 1-year cancer-specific survival. However, with treatment, 5-year CSS approaches 40% to 65% in nonmetastatic patients, and 6% to 29% in metastatic patients [15–17]. By TNM classification, these patients are, by definition, pT3. However, due to the relative rarity of this presentation, there is little large volume data to support lymphadenectomy in this population. Prior studies infer benefit to this patient population, by including them with other pT3 patients [9,18]. However, none specifically look at tumor thrombus patients in the absence of metastatic disease.
As such, using the International Renal Cell Carcinoma-Venous Thrombus Consortium (IRCC-VTC), a multi-institutional database of patients with tumor thrombus managed with surgery [13], we aim to assess the clinical benefit of lymphadenectomy in this patient population.
2. Patients and methods
2.1. Patient selection and data collection
This was an institutional review board approved study with all participating sites providing institutional data sharing agreements prior to initiation of the study. A total of 24 centers from the United States and Europe provided data (International Renal Cell Carcinoma-Venous Thrombus Consortium [IRCC-VTC]). A computerized databank was generated for data transfer. The database was frozen prior to final analysis, and the final dataset was produced for the current analysis. Patients with distant metastasis were excluded from the study. The records of 1,978 patients with RCC and venous thrombus who underwent radical nephrectomy and complete tumor thrombectomy between 1985 and 2014 were reviewed. None of the patients had distant metastases.
2.2. Surgical procedure and pathologic evaluation
Approach (open or minimally invasive), the decision to pursue lymphadenectomy at the time of nephrectomy, and the extent of lymphadenectomy were at the discretion of the primary surgeon. All surgical specimens were processed according to standard pathological procedures. Tumor size was evaluated on fixed pathologic specimens. Histological subtype was determined according to the 1997 World Health Organization Heidelberg classification [19]. Tumor nuclear grade was determined according to the Fuhrman system. Pathologic staging was designated according to the 2009 TNM classification of American Joint Committee on Cancer (AJCC) [20].
2.3. Tumor thrombus (TT) level
The Mayo classification was used for the macroscopic vascular involvement [21]. Level I: TT is either at the entry of the renal vein or within the inferior vena cava (IVC) <2 cm from the confluence of the renal vein and the IVC. Level II: Thrombus extends within the IVC >2 cm above the confluence of the renal vein and IVC but still remains below the hepatic veins. Level III: Thrombus involves the intrahepatic IVC. The size of the thrombus ranges from a narrow tail that extends into the IVC to one that fills the lumen and enlarges the IVC. Level IV: Thrombus extends above the diaphragm or into the right atrium.
2.4. Follow-up
Follow-up was performed according to institutional protocols. Follow-up visits consisted of a physical examination and serum chemistry evaluation, including liver function tests and alkaline phosphatase. Diagnostic imaging (e.g., ultrasonography, chest radiography, CT abdomen/pelvis with IV contrast) were also performed according to institutional protocols and at the discretion of the treating physician when clinically indicated.
2.5. Outcomes
Primary outcome was cancer-specific survival stratified by clinical and pathologic node status. When patients died, the cause of death was determined by the treating physicians, by chart review corroborated by death certificates, or by death certificates alone. Perioperative mortality (death within 30 days of surgery) was censored at the time of death for cancer-specific survival analyses. Secondary analyses included predictors of cancer-specific mortality.
2.6. Statistical analysis
The Kaplan-Meier method was used to calculate survival functions, and differences were assessed with the log rank statistic. Univariable and multivariable survival analyses were performed using the Cox proportional hazard regression model. The P values were calculated with t-tests, Chi-squared tests, and Kruskal Wallis tests. All reported P values are two-sided, and statistical significance was set at P < 0.05. Data were analyzed using STATA 14 for Windows (StataCorp LP, College Station, TX).
3. Results
3.1. Clinical and pathological characteristics
A total of 1,978 patients with RCC and venous thrombus underwent radical nephrectomy and tumor thrombectomy. The clinical and pathological features for these patients are summarized in Table 1. Mean age in the entire group was 64.0 years (IQR: 56.2–72.1). Of the 1,978 patients, lymph node dissection was performed in 1,026 patients (51.9%). Further, 223 (21.7%) of these patients had pathologically confirmed lymph node metastases. In the entire cohort of patients undergoing lymphadenectomy, mean (median) number of removed LN was 11.2 (7) and mean (median) number of positive LN was 4.1 (2). In the lymph node positive patients, mean (median) number of removed LN was 13.1 (9). Clinical node-negative (cN−) disease was documented in 573 patients; 447 of these received a lymphadenectomy with 43 cN− patients (9.6%) revealing positive lymph nodes at pathology (Table 2).
Table 1.
Clinical and pathological characteristics of patients harboring renal cell carcinoma with tumor thrombus and without distant metastases treated at 24 international institutions
| All 1978 | pN1 223 | pN0 803 | pNx 952 | P value pN1 vs. pN0 | P value pN1 vs. pNx | P value pN0 vs. pNx | |
|---|---|---|---|---|---|---|---|
| Year of surgery, % | |||||||
| 1985–2004 | 53.8 | 51.6 | 53.6 | 54.6 | 0.600 | 0.410 | 0.653 |
| 2005–2014 | 46.2 | 48.3 | 46.5 | 45.4 | |||
| Total number of LN harvested, mean (median) | 11.2 (7) | 13.1 (9) | 10.6 (7) | – | 0.012 | – | – |
| Total number of positive LN, mean (median) | 4.1 (2) | 4.1 (2) | – | – | – | – | – |
| Age, yr mean (SD) | 64.0 (12.4) | 62.7 (14.0) | 63.0 (13.0) | 65.2 (11.4) | 0.732 | 0.004 | <0.001 |
| Sex, % | |||||||
| Female | 34.7 | 24.6 | 32.0 | 39.3 | 0.035 | <0.001 | 0.002 |
| Male | 65.3 | 75.4 | 68.0 | 60.7 | |||
| TT level (Mayo), % | |||||||
| 0 | 8.3 | 3.7 | 8.8 | 9.6 | 0.005 | 0.001 | 0.315 |
| I | 30.5 | 26.1 | 30.0 | 33.5 | |||
| II | 26.2 | 28.4 | 27.2 | 23.5 | |||
| III | 20.1 | 23.3 | 19.5 | 19.6 | |||
| IV | 14.9 | 18.5 | 14.5 | 13.8 | |||
| Tumor size, % | |||||||
| ≤4 cm | 7.6 | 4.2 | 6.1 | 9.6 | <0.001 | <0.001 | <0.001 |
| 4–7 cm | 30.1 | 15.8 | 25.8 | 36.9 | |||
| 7–10 cm | 31.8 | 31.6 | 35.6 | 28.7 | |||
| >10 cm | 30.5 | 48.4 | 32.5 | 24.8 | |||
| Histology (%) | |||||||
| Clear cell | 84.4 | 65.9 | 85.2 | 88.0 | <0.001 | <0.001 | 0.055 |
| Papillary | 5.2 | 16.6 | 3.5 | 4.0 | |||
| Chromophobe | 1.7 | 0.9 | 1.5 | 2.0 | |||
| Othera | 8.8 | 16.6 | 9.8 | 6.0 | |||
| Sarcomatoid features, % | |||||||
| Yes | 9.3 | 20.2 | 6.9 | 8.9 | <0.001 | <0.001 | 0.144 |
| No | 90.7 | 79.8 | 93.1 | 91.1 | |||
| Fat invasion, % | |||||||
| Yes | 56.9 | 76.3 | 52.8 | 54.4 | <0.001 | <0.001 | 0.635 |
| No | 43.1 | 23.7 | 47.2 | 45.6 | |||
Other histology types include very rare histology types such as collecting duct carcinoma and mixed histological types (such as clear cell + papillary type, clear cell + chromophobe)
Table 2.
Distribution of clinical and pathological N-stage
| cN | pN |
|||
|---|---|---|---|---|
| pN0 | pN1 | pNx | Overall | |
| cN0 | 404 | 43 | 126 | 573 |
| cN1 | 80 | 93 | 35 | 208 |
| cNx | 319 | 87 | 791 | 1197 |
| Overall | 803 | 223 | 952 | 1978 |
3.2. Clinical outcomes and association of lymph node dissection and lymph node counts with survival
Median follow-up was 80.7 months (IQR: 32.6–149.2). A total of 996 patients (50.4%) were deceased at the time of analysis, including 652 patients (33.0%) who died of RCC, and 5-year CSS was 60.9% (CI: 58.1%–63.5%) in the entire patient group.
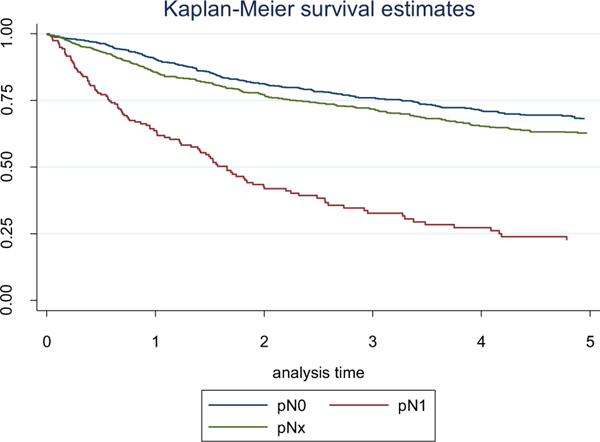
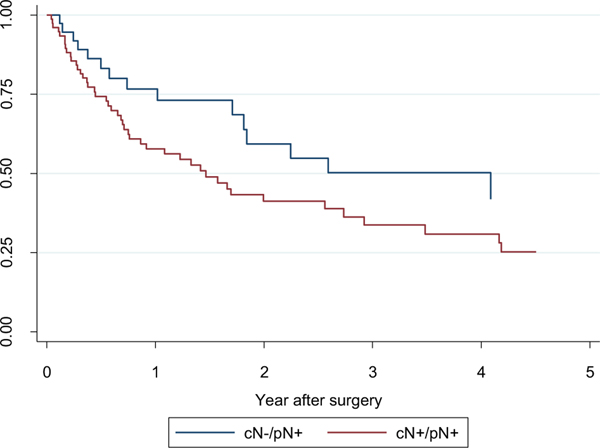
Five-year CSS estimates in the patients without LN metastases (pN0), with pathologically confirmed lymph node metastases (pN1) and with unknown pathological LN status (pNx) were 68.3% (95% CI: 64.0%–71.9%), 22.6% (95% CI: 15.3%–30.8%), and 62.5% (95% CI: 58.4%–66.4%), respectively (Fig. 1). LN positive cN−patients (pN1/cN0) showed significantly better CSS when compared to LN positive cN+ patients (pN1/cN1) (5-year CSS 33.5% (95% CI: 12.9%–55.9%) vs. 25.3% (95% CI: 13.8%–38.4%); P = 0.047; Fig. 2). In multivariable analysis, the presence of lymph node metastasis, the number of positive LNs, and lymph node density were independently associated with cancer-specific mortality (CSM) (Tables 3 and 4). Moreover, positive cN status in LN positive patients was a significant predictor of CSM (HR, 2.923; P = 0.015; Table 5).
Fig. 1.
Probability estimates of cancer-specific survival in patients with renal cell carcinoma and tumor thrombus stratified by pN-status (P < 0.001).
Fig. 2.
Probability estimates of cancer-specific survival in patients with positive LNs stratified by cN status (P = 0.047).
Table 3.
Impact of number of positive LNs in N+ patients on CSM (multivariable)
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Number of pos. LN | 1.055 | 1.00–1.11 | 0.049 |
| Age at surgery | 1.003 | 0.98–1.03 | 0.769 |
| Sex | |||
| Female | Ref. | ||
| Male | 0.647 | 0.38–1.10 | 0.110 |
| Tumor size | 1.019 | 0.96–1.09 | 0.544 |
| Tumor thrombus level | |||
| TT0/1 | Ref. | ||
| TT2 | 0.904 | 0.48–1.71 | 0.756 |
| TT3 | 1.109 | 0.52–2.39 | 0.791 |
| TT4 | 1.736 | 0.79–3.79 | 0.167 |
| Fat invasion | |||
| No | Ref. | ||
| Yes | 1.069 | 0.58–1.96 | 0.830 |
| Sarcomatoid features | |||
| No | Ref. | ||
| Yes | 2.232 | 1.00–5.00 | 0.051 |
| Year of surgery | 0.996 | 0.95–1.05 | 0.870 |
Table 4.
Impact of LN density in N+ patients on CSM (multivariable)
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Lymph density | |||
| <0.20 | Ref. | ||
| ≥0.20 | 1.993 | 1.06–3.74 | 0.031 |
| Age at surgery | 1.007 | 0.98–1.03 | 0.561 |
| Sex | |||
| Female | Ref. | ||
| Male | 0.671 | 0.38–1.18 | 0.169 |
| Tumor size | 1.036 | 0.97–1.10 | 0.275 |
| Tumor thrombus level | |||
| TT0/1 | Ref. | ||
| TT2 | 0.867 | 0.44–1.71 | 0.680 |
| TT3 | 1.461 | 0.67–318 | 0.339 |
| TT4 | 2.219 | 1.03–4.77 | 0.041 |
| Fat invasion | |||
| No | Ref. | ||
| Yes | 1.343 | 0.71–2.55 | 0.368 |
| Sarcomatoid features | |||
| No | Ref. | ||
| Yes | 2.200 | 1.04–4.64 | 0.038 |
| Year of surgery | 0.997 | 0.94–1.05 | 0.913 |
Table 5.
Impact of cN status in N+ patients on CSM (multivariable)
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Clinical nodal stage | |||
| Negative | Ref. | ||
| Positive | 2.923 | 1.23–6.96 | 0.015 |
| Age at surgery | 0.999 | 0.97–1.03 | 0.939 |
| Sex | |||
| Female | Ref. | ||
| Male | 0.417 | 0.23–0.77 | 0.005 |
| Tumor size | 1.014 | 0.94–1.10 | 0.737 |
| Tumor thrombus level | |||
| TT0/1 | Ref. | ||
| TT2 | 0.790 | 0.38–1.64 | 0.527 |
| TT3 | 1.070 | 0.40–2.86 | 0.893 |
| TT4 | 1.872 | 0.81–4.33 | 0.143 |
| Fat invasion | |||
| No | Ref. | ||
| Yes | 1.123 | 0.53–2.37 | 0.760 |
| Sarcomatoid | |||
| No | Ref. | ||
| Yes | 2.2625 | 1.07–6.43 | 0.035 |
| Year of surgery | 0.972 | 0.92–1.03 | 0.325 |
The number of removed lymph nodes did not have a significant effect on cancer-specific survival in pN0 or pN1 patients.
4. Discussion
Locally advanced RCC with TT represents a unique population of patients. Owing to the rarity of these patients, there are few data focused on this population alone. The International Renal Cell Carcinoma-Venous Thrombus Consortium (IRCC-VTC) dataset provides an opportunity to learn more about this specific subset of patients [13,22,23].
Node-positive disease in renal cell carcinoma has been established as a poor prognostic indicator [24,25]. Despite ongoing clinical trials, currently approved management paradigms do not recommend adjuvant therapy in the absence of proven metastatic disease [26]. For patients with either clinically evident or micrometastatic nodal involvement at the time of surgical resection, as there is no approved adjuvant therapy, any treatment that can enhance survival is warranted.
Although lymphadenectomy has become an integral part of management for most other genitourinary malignancies, the role of lymphadenectomy for RCC continues to be debated, and unfortunately, there is still no consensus on its benefit. This is complicated by the fact that, despite the presence of predictable landing zones for RCC, perihilar nodes are skipped 45% of the time, and 40% to 60% of patients with metastatic disease will have no nodal involvement [1,2]. In combination with the currently undefined survival benefit of lymphadenectomy, the utilization of lymphadenectomy remains low [27]; this is demonstrated by the fact that, even at expert centers within the IRCC-VTC, only 51.9% underwent lymphadenectomy.
The indications and extent for lymphadenectomy remain fluid, and more data are needed to help shape these guidelines. In the EORTC study, 4% of patients were found to have nodal disease at the time of nephrectomy, and there was no survival benefit noted. Critics of the study note that the majority of patients included had localized or low-grade RCC, and therefore were unlikely to have nodal involvement or benefit from lymphadenectomy [3]. Currently, the EAU guidelines state that in patients with localized disease and no clinical evidence of lymph node metastases, lymphadenectomy is not recommended (level 1 evidence). In patients with localized disease and clinically enlarged lymph nodes, however, the survival benefit of lymphadenectomy was not clearly demonstrated (level 3 evidence) [28,29]. The AUA, however, does not have any specific guidelines regarding lymphadenectomy at the time of nephrectomy for renal cell carcinoma. Although there are no prospective randomized data to support lymphadenectomy, smaller series have identified a survival benefit from lymphadenectomy, particularly in the presence of clinically evident lymphadenopathy [2,4–8]. In these series, lymphadenectomy in patients with the following high-risk features appeared to provide survival advantage—nuclear grade 3 to 4, tumor size ≥10 cm, tumor stage T3 or T4, and presence of tumor necrosis [11]. Based on these indications, patients with tumor thrombus, by definition pT3, may also benefit from lymphadenectomy at the time of radical nephrectomy and tumor thrombectomy. However, until now, there has been no literature in this particular subset to support this practice.
Of the 447 patients who were cN0 on preoperative evaluation and underwent lymphadenectomy in the present study, 43 were pN1 (9.6%). In prior series, when all RCC patients were assessed, the rate ranged from 4% to 10% [4,30,31]. This suggests that the presence of tumor thrombus alone increases the risk of LN involvement, further corroborating the indications for lymphadenectomy at the time of initial resection. Indeed, a higher level of thrombus is associated with a higher risk of pN1 disease in the present study (P = 0.005).
Cancer-specific survival (CSS) for the entire cohort at 5 years was 60.9%, consistent with prior studies. As expected, pN1 patients had significantly worse 5-year CSS (22.6%) compared to pN0 (68.3%) and pNx (62.5%). Interestingly, cN0/pN1 showed significantly better CSS when compared to cN1/pN1 (33.5% vs. 25.3%; P = 0.047; Fig. 2), and was confirmed in the multivariable analysis; this corroborates findings by Babaian et al [32]. This may reflect the difference in amount of disease in involved LNs. As 10% of cN0 patients were identified to be pN1 due to micrometastatic disease burden, and since this population does better with lymphadenectomy than if they become cN1, in this specific cohort of RCC patients, lymphadenectomy may be warranted even in clinically node-negative patients. Looking specifically at patients with pN1 disease, total nodal count removed did not have a significant effect on cancer-specific survival. Although this may suggest that pure extent and volume of node dissection is not the driving force of survival benefit, due to the limitations of the current database, the total nodal count remains a weak surrogate marker for extent of lymph node dissection. More importantly, however, the number of positive LNs and LN density were independently associated with cancer-specific mortality (CSM). However, as alluded to by Bekema et al. [28], the true nature of the survival benefit from lymphadenectomy remains unclear—it may derive either from the direct removal of grossly positive lymph node metastatic disease and clinically undiagnosed micrometastatic disease, or it may be due to removal of the pathways of future lymphatic spread. As only the total number of positive lymph nodes removed and LN density were associated with survival benefit, this appears to support the former rather than the latter; this is further supported by recent data by Gershman et al [33].
The limitations of the study include those inherent to a large multi-institutional retrospective chart review, including missing data and confounding variable and selection bias for which we could not control. Additionally, due to the time frame of the study, from 1985 to 2014, clinical and radiographic abilities have changed during the study period, which may contribute to some variability in clinical staging. However, a specific limitation is the lack of standardization of lymphadenectomy—lymphadenectomy was at the discretion of the primary surgeon, and the database was not equipped to accrue details regarding extent of dissection. As such, lymphadenectomy was highly variable among institutions. This precludes the ability to determine the benefit of extended vs. targeted lymphadenectomy.
Despite these limitations, our study utilizes the largest multinational multi-institutional database of patients with renal cell carcinoma and tumor thrombus who have undergone radical nephrectomy and tumor thrombectomy, thereby providing access to a large volume cohort to specifically answer the question of the role of lymphadenectomy in the setting of tumor thrombus. No prior studies have ever specifically addressed this clinical question in this patient population.
In our multi-institutional series of patients with RCC who underwent radical nephrectomy and tumor thrombectomy, the number of positive nodes harvested during LND and LN density was strong prognostic indicators of cancer-specific survival. The number of removed lymph nodes was not prognostic of cancer-specific survival. The rate of pN1 patients among clinically node-negative patients was relatively high (9.6%), and as the removal of positive nodal disease appears to provide survival benefit, lymphadenectomy may be warranted in this patient population. A randomized trial, however, would be required to ascertain this benefit.
References
- [1].Crispen PL, Breau RH, Allmer C, Lohse CM, Cheville JC, Leibovich BC, et al. Lymph node dissection at the time of radical nephrectomy for high-risk clear cell renal cell carcinoma: indications and recommendations for surgical templates. Eur Urol 2011;59(1):18–23. [DOI] [PubMed] [Google Scholar]
- [2].Vasselli JR, Yang JC, Linehan WM, White DE, Rosenberg SA, Walther MM, et al. Lack of retroperitoneal lymphadenopathy predicts survival of patients with metastatic renal cell carcinoma. J Urol 2001;166(1):68–72. [PubMed] [Google Scholar]
- [3].Blom JH, van Poppel H, Maréchal JM, Jacqmin D, Schröder FH, de Prijck L, et al. ; EORTC Genitourinary Tract Cancer Group. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol 2009;55(1):28–34. [DOI] [PubMed] [Google Scholar]
- [4].Pantuck AJ, Zisman A, Dorey F, Chao DH, Han KR, Said J, et al. Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. J Urol 2003;169(6):2076–83. [DOI] [PubMed] [Google Scholar]
- [5].Whitson JM, Harris CR, Reese AC, Meng MV. Lymphadenectomy improves survival of patients with renal cell carcinoma and nodal metastases. J Urol 2011;185(5):1615–20. [DOI] [PubMed] [Google Scholar]
- [6].Delacroix SE Jr., Chapin BF, Chen JJ, Noqueras-Gonzalez GM, Tamboli P, Matin SF, et al. Can a durable disease-free survival be achieved with surgical resection in patients with pathological node positive renal cell carcinoma? J Urol 2011;186(4):1236–41. [DOI] [PubMed] [Google Scholar]
- [7].Karakiewicz PI, Trinh QD, Bhojani N, Bensalah K, Salomon L, de la Taille A. Renal cell carcinoma with nodal metastases in the absence of distant metastatic disease: prognostic indicators of disease-specific survival. Eur Urol 2007;51(6):1616–24. [DOI] [PubMed] [Google Scholar]
- [8].Canfield SE, Kamat AM, Sánchez-Ortiz RF, Detry M, Swanson DA, Wood CG. Renal cell carcinoma with nodal metastases in the absence of distant metastatic disease (clinical stage TxN1–2M0): the impact of aggressive surgical resection on patient outcome. J Urol 2006; 175(3 Pt 1):864–9. [DOI] [PubMed] [Google Scholar]
- [9].Capitanio U, Montorsi F. Renal cancer. Lancet 2016;387:894–906. [DOI] [PubMed] [Google Scholar]
- [10].Barrisford GW, Gershman B, Blute ML Sr. The role of lymphadenectomy in the management of renal cell carcinoma. World J Urol 2014;32(3):643–9. [DOI] [PubMed] [Google Scholar]
- [11].Blute ML, Leibovich BC, Cheville JC, Lohse CM, Zincke H. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J Urol 2004;172(2):465–9. [DOI] [PubMed] [Google Scholar]
- [12].Psutka SP, Leibovich BC. Management of inferior vena cava tumor thrombus in locally advanced renal cell carcinoma. Ther Adv Urol 2015;7(4):216–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Martínez-Salamanca JI, Linares E, González J, Bertini R, Carballido Ja, Chromecki T, et al. Lessons learned from the International Renal Cell Carcinoma-Venous Thrombus Consortium (IRCC-VTC). Curr Urol Rep 2014;15(5):404. [DOI] [PubMed] [Google Scholar]
- [14].Reese AC, Whitson JM, Meng MV. Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urol Oncol 2013;31(7): 1305–9. [DOI] [PubMed] [Google Scholar]
- [15].Haddad AQ, Wood CG, Abel EJ, Krabbe LM, Darwish OM, Thompson RH, et al. Oncologic outcomes following surgical resection of renal cell carcinoma with inferior vena caval thrombus extending above the hepatic veins: a contemporary multicenter cohort. J Urol 2014;192(4):1050–6. [DOI] [PubMed] [Google Scholar]
- [16].Whitson JM, Reese AC, Meng MV. Population based analysis of survival in patients with renal cell carcinoma and venous tumor thrombus. Urol Oncol 2013;31(2):259–63. [DOI] [PubMed] [Google Scholar]
- [17].Pouliot F, Shuch B, Larochelle JC, Pantuck A, Belldegrun AS. Contemporary management of renal tumors with venous tumor thrombus. J Urol 2010;184(3):833–41:quiz 1235. [DOI] [PubMed] [Google Scholar]
- [18].Studer UE, Birkhauser FD. Lymphadenectomy combined with radical nephrectomy: to do or not to do? Eur Urol 2009;55(1):35–7. [DOI] [PubMed] [Google Scholar]
- [19].Störkel S, Eble JN, Edlakha K, Amin M, Blute ML, Bostwick DG, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 1997;80(5):987–9. [DOI] [PubMed] [Google Scholar]
- [20].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17(6):1471–4. [DOI] [PubMed] [Google Scholar]
- [21].Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol 1987;59(5):390–5. [DOI] [PubMed] [Google Scholar]
- [22].Tilki D, Hu B, Nguyen HG, Dall’Era MA, Bertini R, Caballido JA, et al. Impact of synchronous metastasis distribution on cancer specific survival in renal cell carcinoma after radical nephrectomy with tumor thrombectomy. J Urol 2015;193(2):436–42. [DOI] [PubMed] [Google Scholar]
- [23].Tilki D, Nguyen HG, Dall’Era MA, Bertini R, Caballido JA, Chromeck T, et al. Impact of histologic subtype on cancer-specific survival in patients with renal cell carcinoma and tumor thrombus. Eur Urol 2014;66(3):577–83. [DOI] [PubMed] [Google Scholar]
- [24].Leibovich BC, Blute ML. Lymph node dissection in the management of renal cell carcinoma. Urol Clin North Am 2008;35(4):673–8:[viii]. [DOI] [PubMed] [Google Scholar]
- [25].Russo P, Jang TL, Pettus JA, Huang WC, Eggener SE, O’Brien MF, et al. Survival rates after resection for localized kidney cancer: 1989 to 2004. Cancer 2008;113(1):84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pinto A. Adjuvant therapy for renal cell carcinoma. Clin Genitourin Cancer 2014;12(6):408–12. [DOI] [PubMed] [Google Scholar]
- [27].Filson CP, Miller DC, Colt JS, Ruterbusch J, Linehan WM, Chow WH, et al. Surgical approach and the use of lymphadenectomy and adrenalectomy among patients undergoing radical nephrectomy for renal cell carcinoma. Urol Oncol 2012;30(6):856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bekema HJ, MacLennan S, Imamura M, Lam TB, Stewart F, Scott N, et al. Systematic review of adrenalectomy and lymph node dissection in locally advanced renal cell carcinoma. Eur Urol 2013;64(5):799–810. [DOI] [PubMed] [Google Scholar]
- [29].Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67(5):913–24. [DOI] [PubMed] [Google Scholar]
- [30].Studer UE, Scherz S, Schneidegger J, Kraft R, Sonntag R, Ackermann D, et al. Enlargement of regional lymph nodes in renal cell carcinoma is often not due to metastases. J Urol 1990;144(2 Pt 1): 243–5. [DOI] [PubMed] [Google Scholar]
- [31].Chapman TN, Sharma S, Zhang S, Wong MK, Kim HL. Laparoscopic lymph node dissection in clinically node-negative patients undergoing laparoscopic nephrectomy for renal carcinoma. Urology 2008;71(2):287–91. [DOI] [PubMed] [Google Scholar]
- [32].Babaian KN, Kim DY, Kenney PA, Wood CG Jr., Wong J, Sanchez C, et al. Preoperative predictors of pathological lymph node metastasis in patients with renal cell carcinoma undergoing retroperitoneal lymph node dissection. J Urol 2015;193(4):1101–7. [DOI] [PubMed] [Google Scholar]
- [33].Gershman B, Moreira DM, Thompson RH, Boorjian SA, Lohse CM, Costello BA, et al. Renal cell carcinoma with isolated lymph node involvement: long-term natural history and predictors of oncologic outcomes following surgical resection. Eur Urol 2017;72(2):300–6. [DOI] [PubMed] [Google Scholar]