Aplastic anaemia (AA), pure red cell aplasia (PRCA), pure white cell aplasia (PWCA) and immune cytopenias (IC) associated with T-cell large-granular lymphocytic leukaemia (T-LGL) share certain aspects of immune-pathogenesis. Polyclonal, clonally skewed or clonal cytotoxic T-lymphocytes (CTLs) mediate cytopenias (Young & Maciejewski, 1997; Risitano et al, 2010; Dumitriu et al, 2016). Immunosuppressive therapy (IST) has been used to ameliorate cytopenias and, in some cases, restore normal haematopoiesis. Refractoriness to front-line therapies results from insufficient IST, or exhaustion of the haematopoietic stem/progenitor cells, necessitating therapeutic alternatives (Risitano & Schrezenmeier, 2013). Alemtuzumab, a humanized IgG1 anti-CD52 monoclonal antibody, profoundly depletes B- and T-cells. Applied initially as a high-dose intravenous regimen for chronic lymphocytic leukaemia (CLL), alemtuzumab has subsequently been adopted as a form of IST (Willis et al, 2001). Alemtuzumab is currently marketed for autoimmune diseases like multiple sclerosis, although its potential therapeutic utility in refractory IC has been noted (Willis et al, 2001). Early applications as an IST for acquired bone marrow failure (aBMF) adopted dosing analogous to CLL. With overall response-rates (ORR) of 37–80%, administration of intravenous alemtuzumab dose-intense regimens had significant infusional and IST-related adverse events (AEs) (Willis et al, 2001). Due to lack of therapeutic superiority to anti-thymocyte globulin (ATG) in frontline settings, alemtuzumab has not been widely adopted for upfront IST in AA (Scheinberg et al, 2012). Subcutaneous (sc) administration at lower doses has better tolerability and acceptable AEs (Gomez-Almaguer et al, 2010; Risitano et al, 2010). Consequently, alemtuzumab became a viable therapeutic option in patients intolerant or refractory to ciclosporin A (CsA) or/and not fit to receive ATG.
Long-term follow-up of alemtuzumab therapy in aBMF is lacking; optimal dosing and utility continue to be refined. We present real-world experience and the long-term outcomes of sc alemtuzumab in aBMF. This study is approved by Institutional Review Boards. We identified aBMF patients in a pilot study of sc alemtuzumab (NCT00895739) and those treated outside the trial for CsA/ATG-intolerability or refractoriness to multiple lines of IST. Initial experience from the pilot trial was previously reported (Risitano et al, 2010); we present its long-term follow-up at 72 months. Patients in the trial received a single course of sc alemtuzumab over 4–5 consecutive days [103 mg in severe AA (SAA) and 73 mg for PRCA/PWCA with CsA]. Patients treated outside the trial received 10 mg once/twice weekly for 4–8 consecutive weeks for a median total dose of 70 mg. Some patients received a longer course with maintenance treatment, and/or intermittent “boosts” to prevent/treat relapse [10 mg every 2–8 weeks]. All patients received prophylactic trimethoprim-sulfamethoxazole, acyclovir and cytomegalovirus monitoring. Complete response was defined as neutrophil count >1·0 × 109/l, haemoglobin >100 g/l, platelet count >100 × 109/l and partial response defined patients achieving transfusion-independence and/or no longer meeting criteria for severe disease.
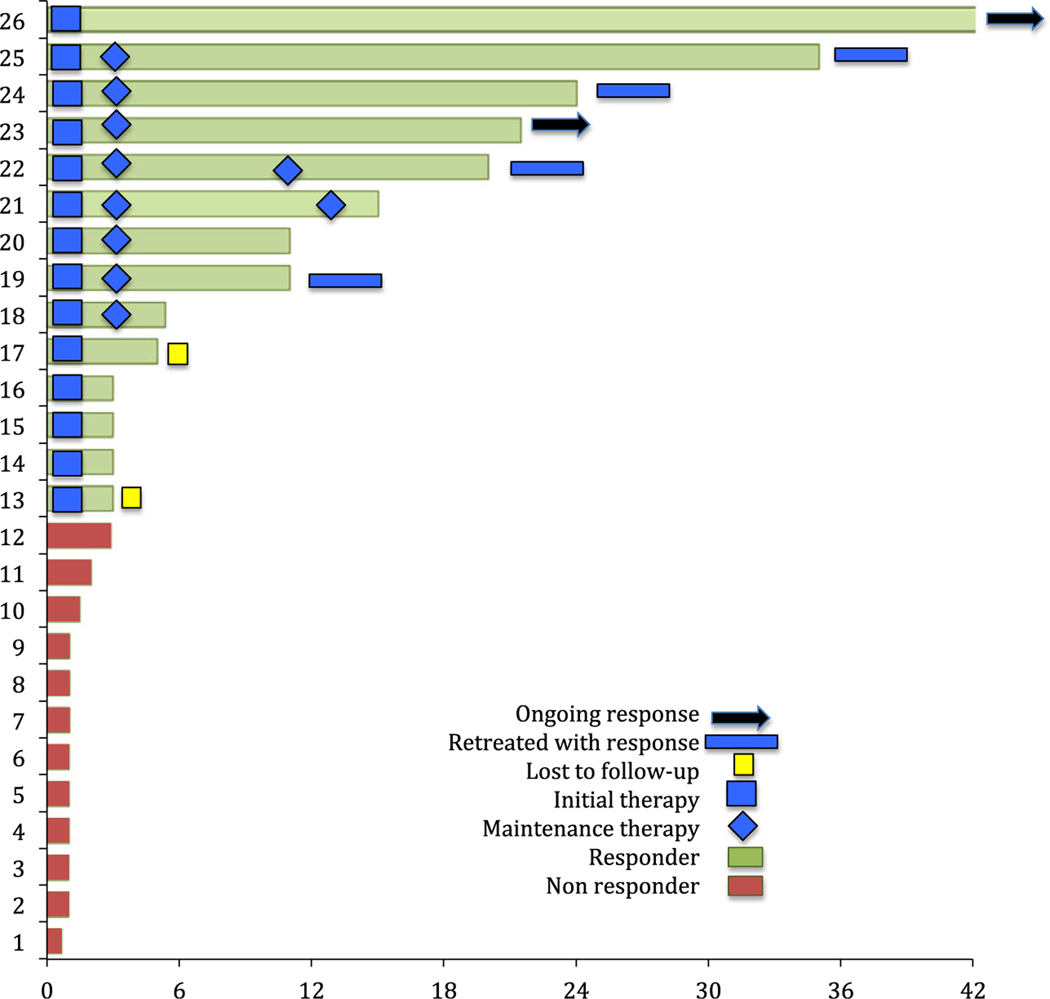
In this 59-patient aBMF cohort (median age: 59 years) included 20 with SAA, 15 with T-LGL, 21 with PRCA and three with PWCA (Table SI). Eleven AA patients received up-front alemtuzumab therapy, including elderly patients with multiple comorbidities and and Eastern Cooperative Oncology Group performance status >2 (N = 6). Paroxysmal nocturnal haemoglobinurea (PNH) clones were found in 8/20 cases with 3 patients on concomitant eculizumab therapy. Patients with IC (8 PRCA, 1 PCWA and 15 T-LGL) treated outside the trial (NCT00895739) that had received an average 3 and 4·5 prior therapies, respectively, received sc alemtuzumab as salvage therapy. PRCA cases were idiopathic (N = 13) or associated with thymoma (N = 3) or lymphoproliferative disease (N = 4). Treatment with low-dose sc alemtuzumab was well tolerated, with an ORR of 63%: 4/59 patients (7%) discontinued treatment. The ORR was 60% for AA and T-LGL patients, 62% for PRCA and 100% for PWCA (Table I). Responses were noted in 71%, 65% and 52% of treatment-naïve, relapsed and refractory aBMF groups, respectively (Table I). Responses were durable in 30% of the responders, with responses lasting beyond 60 months. Treatment duration and outcomes of patients treated outside NCT00895739 are depicted in Fig 1. Maintenance treatment with (10 mg alemtuzumab every 2–8 weeks) was administered to 8 patients, until last follow-up or loss of response. At relapse, 15 responders were retreated with alemtuzumab, with the majority (93%) responding. The median overall survival was 60 months (range: 1–136) (Figure S1).
Table I.
Treatment outcomes and adverse events from sc alemtuzumab therapy in patients with acquired bone marrow failure.
| N = 59 | Treatment outcomes (%) |
|---|---|
| ORR | 63 |
| CR | 36 |
| PR | 27 |
| Age | |
| <60 years | 64·5 |
| >60 years | 61 |
| Diagnosis | |
| AA | 60 |
| PRCA | 62 |
| T-LGL | 60 |
| IN/PWCA | 100 |
| Disease status | |
| Treatment na€ıve | 71 |
| Relapsed | 65 |
| Refractory | 52 |
| Therapy discontinuation* | |
| Injection reaction | 1 |
| Patient preference | 3 |
| Adverse events | |
| Injection reactions | 20 |
| Infections | 29 |
| Clonal events | 6 |
AA, aplastic anaemia; CR, complete response; IC, immune cytopenia; ORR, overall response rate; PR, partial response; PRCA, pure red cell aplasia; PWCA, pure white cell aplasia; T-LGL, T-cell large-granular lymphocytic leukaemia.
4 discontinued treatment.
Fig 1.
Response duration and treatment details of acquired bone marrow failure patients in the retrospective cohort.
Late clonal events were noted in 3 AA patients (15%), at a similar rate to historical controls (Young & Maciejewski, 1997) and 4 PRCA cases (20%). Patients in NCT00895739 were screened for clonal evolution with fluorescence in situ hybridisation for del5q−. In contrast to a previous misreport (Cerchione et al, 2015) no additional cases with del5q− were detected. Injection-related AEs of all grades were recorded in 20% of patients. Immunosuppressive complications due to lymphodepletion occurred in 3 patients. Cytomegalovirus reactivation was noted in 8 cases, with only one patient having symptomatic disease; 4 had herpes-simplex reactivation, and there was one case of varicella and hepatitis-B reactivation (Table SII). Two fatal infections were recorded: 1 fungaemia and 1 JC virus-related progressive multifocal leucoencephalopathy in neutropenic, heavily pretreated patients. Mechanisms of resistance to alemtuzumab have been noted with proliferation of CD52(−) CTLs (Mohan et al, 2009). This, and the expansion of PNH clones, which are intrinsically CD52(−), did not emerge in our cohort. Possibly, chronic low-dose IST with a maintenance strategy might mitigate this resistance pathway.
The ORR of 63% reported here is consistent with previous experiences of alemtuzumab therapy in aBMF cohorts (Kim et al, 2009; Scheinberg et al, 2012) (Table SIII). The results are particularly noteworthy for the patients studied, i.e., SAA with multiple comorbidities ineligible for other intense-therapies (N = 6) and heavily pre-treated relapsed/refractory PRCA/T-LGL patients (N = 23) without an increase in opportunistic infections. Weekly or twice a week dosing of sc alemtuzumab was better tolerated than previous dosing strategies, with 20% injection-related AEs. A lower median dose of 70 mg was used in IC patients and achieved an ORR of 63%, comparable to other cohorts (Mohan et al, 2009; Dumitriu et al, 2016). Long-term responses were seen in 30% of the responders and maintenance ‘boosts’ could optimize this; late-clonal complications were rare.
In conclusion this study emphasizes the acceptable side-effect profile and key clinical applications of low-dose sc alemtuzumab in aBMF patients.
Supplementary Material
Table SI. Patient and disease characteristics of the cohort receiving sc alemtuzumab.
Table SII. Infectious complications from alemtuzumab therapy.
Table SIII. Review of therapeutic outcomes of alemtuzumab therapy in aBMF conditions.
Table SIV. Primary data.
Figure S1. Kaplan–Meier survival curve (Median OS: 60 months).
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Conflict-of-interest statements
The authors declare no conflict of interest and no competing financial interests.
References
- Cerchione C, Catalano L, Cerciello G, Avilia S, Picardi M, Risitano AM, Pisano I, Alfinito F. & Pane F. (2015) Role of lenalidomide in the management of myelodysplastic syndromes with del(5q) associated with pure red cell aplasia (PRCA). Annals of Hematology, 94, 531–534. [DOI] [PubMed] [Google Scholar]
- Dumitriu B, Ito S, Feng X, Stephens N, Yunce M, Kajigaya S, Melenhorst JJ, Rios O, Scheinberg P, Chinian F, Keyvanfar K, Battiwalla M, Wu CO, Maric I, Xi L, Raffeld M, Muranski P, Townsley DM, Young NS, Barrett AJ & Scheinberg P. (2016) Alemtuzumab in T-cell large granular lymphocytic leukaemia: interim results from a single-arm, open-label, phase 2 study. The Lancet Haematology, 3, e22–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Almaguer D, Jaime-Perez JC, Garza-Rodriguez V, Chapa-Rodriguez A, Tarin-Arzaga L, Herrera-Garza JL, Ruiz-Arguelles GJ, Lopez-Otero A, Gonzalez-Llano O. & Rodriguez-Romo L. (2010) Subcutaneous alemtuzumab plus cyclosporine for the treatment of aplastic anemia. Annals of Hematology, 89, 299–303. [DOI] [PubMed] [Google Scholar]
- Kim H, Min YJ, Baek JH, Shin SJ, Lee EH, Noh EK, Kim MY & Park JH (2009) A pilot dose-escalating study of alemtuzumab plus cyclosporine for patients with bone marrow failure syndrome. Leukemia Research, 33, 222–231. [DOI] [PubMed] [Google Scholar]
- Mohan SR, Clemente MJ, Afable M, Cazzolli HN, Bejanyan N, Wlodarski MW, Lichtin AE & Maciejewski JP (2009) Therapeutic implications of variable expression of CD52 on clonal cytotoxic T cells in CD8+ large granular lymphocyte leukemia. Haematologica, 94, 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano AM & Schrezenmeier H. (2013) Alternative immunosuppression in patients failing immunosuppression with ATG who are not transplant candidates: Campath (alemtuzumab). Bone Marrow Transplantation, 48, 186–190. [DOI] [PubMed] [Google Scholar]
- Risitano AM, Selleri C, Serio B, Torelli GF, Kulagin A, Maury S, Halter J, Gupta V, Bacigalupo A, Socie G, Tichelli A, Schrezenmeier H, Marsh J, Passweg J. & Rotoli B; Working Party Severe Aplastic Anaemia (WPSAA) of the European Group for Blood and Marrow Transplantation (EBMT). (2010) Alemtuzumab is safe and effective as immunosuppressive treatment for aplastic anaemia and single-lineage marrow failure: a pilot study and a survey from the EBMT WPSAA. British Journal of Haematology, 148, 791–796. [DOI] [PubMed] [Google Scholar]
- Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Wu CO & Young NS (2012) Activity of alemtuzumab monotherapy in treatment-naive, relapsed, and refractory severe acquired aplastic anemia. Blood, 119, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis F, Marsh JC, Bevan DH, Killick SB, Lucas G, Griffiths R, Ouwehand W, Hale G, Waldmann H. & Gordon-Smith EC (2001) The effect of treatment with campath-1H in patients with autoimmune cytopenias. British Journal of Haematology, 114, 891–898. [DOI] [PubMed] [Google Scholar]
- Young NS & Maciejewski J. (1997) The pathophysiology of acquired aplastic anemia. The New England Journal of Medicine, 336, 1365–1372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Patient and disease characteristics of the cohort receiving sc alemtuzumab.
Table SII. Infectious complications from alemtuzumab therapy.
Table SIII. Review of therapeutic outcomes of alemtuzumab therapy in aBMF conditions.
Table SIV. Primary data.
Figure S1. Kaplan–Meier survival curve (Median OS: 60 months).