Abstract
Frankliniella occidentalis is an invasive insect pest that incites damage to ornamental and agronomic crops on a global scale. In this study, the effects of temperature on gene expression and enzyme activity were studied for superoxide dismutase (SOD), peroxidase (POD), and glutathione-S-transferase (GST) in F. occidentalis. SOD, POD and GST enzyme activity increased significantly at 35–37 °C but declined as the temperature increased to 41 °C. In a time course study at 35 °C, SOD, POD and GST activities were significantly elevated at 0.5, 1 and 2 h in comparison to the control at 26 °C. Expression patterns were evaluated for the three antioxidant genes under high and low temperature stress. In a time course study at –4 °C, SOD, POD and GST expression peaked at 1 h and declined at 2 h of exposure. In contrast, when transcription was monitored at 35 °C, expression was lowest at 1 h and increased at 2 h. The results provide data that will be useful in deciphering the role of antioxidant enzymes in the adaptation of F. occidentalis to climate change.
Keywords: Frankliniella occidentalis, Thermal stress, Oxidative defense, Enzymatic activity, Gene expression
Introduction
Temperature impacts the reproduction, development, and distribution of insects (Cossins & Bowler, 1987; Worner, 1998; Bale et al., 2002), and extreme temperatures are known elicitors of reactive oxygen species (ROS) in invertebrates. The excessive generation of ROS can damage cellular constituents, including lipids, proteins, and nucleic acids (Halliwell, 1989; Kamata & Hirata, 1999; Foyer & Noctor, 2005; Lopez-Martinez et al., 2008). In order to survive, insects reduce or detoxify ROS through the action of antioxidants; these function as enzymatic and non-enzymatic scavengers that reduce lipid peroxidation and decrease damage to nucleic acids and proteins (Felton & Summers, 1995; Lyakhovich et al., 2006; Krishnan et al., 2007). Peroxidase (POD), superoxide dismutase (SOD), and glutathione-S-transferase (GST) are antioxidant enzymes that defend cells from excessive levels of ROS (Felton & Summers, 1995; Wang, Oberley & Murhammer, 2001; Dubovskiy et al., 2008; Liu et al., 2020). SOD functions by degrading superoxide anions to hydrogen peroxide (H2O2) and oxygen, and H2O2 is subsequently converted to H2O by POD (Kashiwagi et al., 1997; Wang & Li, 2002; Liu & Ma, 2007). GSTs function to detoxify compounds that are produced from lipid peroxidation (Ahmad et al., 1991; Kono & Shishido, 1992; Dubovskiy et al., 2008).
The western flower thrips (WFT), Frankliniella occidentalis, damages both vegetables and ornamental plants on a global scale and is especially problematic in greenhouses (Morse & Hoddle, 2006; Kirk & Terry, 2015; Mouden et al. 2017). In addition to direct damage, WFT causes serious damage to plants by transmitting plant viruses such as the Tomato Spotted Wilt Virus (Pappu, Jones & Jain, 2009; Tomitaka, 2019). WFT is endemic to the western region of North America and has spread globally due to the transportation of agricultural products (Reitz, 2009; Kirk & Terry, 2015). According to the CABI Invasive Species Compendium, F. occidentalis has been discovered on all continents except Antarctica (https://www.cabi.org/isc/datasheet/24426). In mainland China, F. occidentalis was initially found in Beijing in 2003 (Zhang et al., 2003) and has since been discovered in at least ten provinces (Wu et al., 2017).
Previous studies indicated that temperature impacts development, sex ratios, reproduction, population growth, and mortality of F. occidentalis (Li et al., 2007; Li et al., 2011a; Zhang et al., 2012). During the hot summers in subtropical China, high temperatures may cause oxidative stress to F. occidentalis, particularly in greenhouses (Wang et al., 2014). Previous studies demonstrated that the expression of genes encoding catalase (CAT) and subsequent enzymatic activity were altered in F. occidentalis exposed to hot and cold stress (Shi et al., 2013; Qin et al., 2017). However, the impact of high and low temperatures on other antioxidant enzymes in F. occidentalis is unclear.
In this study, we investigated the effect of temperature stress on POD, SOD, and GST in F. occidentalis. The results provide important data on how antioxidant enzymes counteract oxidative damage in the WFT and provide a more comprehensive framework for understanding thermal tolerance in F. occidentalis.
Materials & Methods
Insects and temperature treatments
Frankliniella occidentalis populations were collected in Hangzhou, China, in 2008 and were reared with kidney bean, Phaseolus vulgaris Linn, at 25 ± 0.5 °C and 70 ± 5% relative humidity with a 16:8 h light:dark photoperiod as outlined by Li et al. (2011b). Newly emerged 2nd instar larvae were collected, and pools of 100 were exposed to high (31, 33, 35, 37, 39 or 41 °C) or low (0, –2, –4, –6, –8 and –10 °C) temperatures for 1 h in glass tubes as described (Chang et al., 2017). Through the results of the pre-experiment, 35 and –4 °C were decided as the model temperature on F. occidentalis, which was further explored by subjecting groups of individuals to 0, 0.5, 1, and 2 h of thermal stress; controls were maintained at 26 °C (0 h time point). Following thermal stress, larvae were incubated at 26 °C for 30 min and used a brush to touch it gently, thrips would be identified as surviving if it respond to the stimulus. Survivors were frozen in liquid nitrogen and stored at –80 °C for future use. Four replicate pools were used for each temperature and time period.
Determination of enzyme activity
The assay kit used for protein extraction was from Nanjing Jiancheng Bioengineering Institute, Jiangsu, China. Treated samples were homogenized in 0.9% saline and then centrifuged at 2,500 × rpm for 10 min (Jia et al., 2011). Supernatants containing the enzyme fractions were collected, and protein content was determined using the Bradford (1976) method.
POD and SOD activities were assessed with commercially available kits (Qin et al., 2017). Absorbance values were obtained using the BioTek PowerWave HT Microplate Spectrophotometer (Bio-Tek Instruments Inc., Winooski, Vt., USA). GST activity was measured as a function of reduced glutathione (GSH) using 10 mg of cytosolic protein and 1-chloro-2,4-dinitrobenzene (CDNB; Shanghai Chem, Shanghai, China) as a substrate (Habig, Pabst & Jakoby, 1974; Attig et al., 2014). GST activity was determined at A340 with a microplate spectrophotometer (Shanghai Xinmao Instrument, Shanghai, China), and results are shown as μmol GSH-CDNB/min/mg protein.
RNA isolation, partial cloning of SOD, POD and GST1, and qRT-PCR
The SV Total RNA Isolation System was used to isolate RNA from F. occidentalis as recommended by the manufacturer (Promega, San Luis Obispo, CA, USA). RNA quality and concentration were determined, and cDNA was generated from total RNA with the First Strand cDNA Synthesis Kit (Clontech, Mountain View, CA, USA) as outlined previously (Zhang et al., 2019).
Transcriptome sequencing was performed on F. occidentalis exposed to low temperature (–13 °C), high temperature (40 °C) and normal temperature control (26 °C) at the Shanghai Biotechnology corporation by Illumina sequencing platform. The RNA-seq data were deposited with the Sequence Read Archives PRJNA73493 at NCBI. To remove adapter contamination, low-quality bases and bases artificially introduced during library construction, we trimmed all raw reads using Trimmomatic 0.32 (http://www.usadellab.org/cms/index.php?page=trimmomatic) before transcript assembly, while the unpaired reads were discarded (Bolger, Lohse & Usadel, 2014). The clean reads were mapped to the sequences in the rRNA database of all published insects downloaded from NCBI to discard rRNAs using SOAP. Only the clean reads with the standard of Q30 > 85% and processed with Trimmomatic 0.32 and SOAP were used for further analysis (Gu et al., 2019). Clean data were assembled with Trinity to obtain a high-quality unigene library (Grabherr et al., 2011). The unigene library of three species were first assembled to obtain individual UniGene databases; the general UniGene library was obtained by clustering the three individual databases through CD-Hit to facilitate comparison of expression patterns (Fu et al., 2012). The transcripts selected in the clustering united as unigenes using the De Bruijn graph algorithm; CD-Hit was used to reduce sequence redundancy and improve the performance of other sequence analyses (Fu et al., 2012; Yang & Smith, 2013). For functional annotation, we obtained information on unigenes using BLAST (cut-off e-value of 10–5) with protein databases such as NR (NCBI nonredundant database), Swiss-Prot, GO (Gene Ontology), COG (Clusters of Orthologous Groups), KOG (euKaryotic Orthologous Groups), eggNOG, Pfam (Protein family) and KEGG (Kyoto Encyclopedia of Genes and Genomes). “Superoxide Dismutase”, “Peroxidase” and “glutathione-s-transferase” were used as key words to search related gene fragments in the transcriptome database of F. occidentalis, respectively. The fragment gene cDNA of the SOD, GST1 and POD was submitted to GenBank (accession no. MZ364120, MZ364118 and MZ364119, respectively). According to the obtained gene fragments, the corresponding primers (Table 1) were designed for fragment verification. PCR products were cloned and sequenced as described (Zhang et al., 2019).
Table 1. Primers used in the study.
| Primer name | Primer sequences | Tm (°C) | Length (bp) | Ec (%) |
|---|---|---|---|---|
| DP-SOD-F | AATGCTGCGTTCTCTGTTGTG | 58.7 | 335 | |
| DP-SOD-R | TCTGGTTTTGTTGTTTCAGGAGT | 58.4 | ||
| DP-POD-F | CAACCCCGACCAGCCCTAC | 62.3 | 600 | |
| DP-POD-R | AAAAGGGGAAATCGGTGTCG | 61.4 | ||
| DP-GST-F | TGACCGTGAACCAGACCGAG | 61.3 | 431 | |
| DP-GST-R | GATGCCGAAAATACTGAGTGTGG | 61.4 | ||
| qPCR-SOD-F | GAAATAACTGGTTCCAAGGCACT | 59.6 | 125 | 91.8 |
| qPCR-SOD-R | AATGCTGCGTTCTCTGTTGTG | 58.7 | ||
| qPCR-POD-F | CCGCACTGGGACGACGAGAC | 65.8 | 235 | 96.4 |
| qPCR-POD-R | CGATGAGCGAGTGGAAGTATCTGAA | 64.8 | ||
| qPCR-GST-F | GCTGCTGCTGTGCTGGATTA | 59.7 | 170 | 90.0 |
| qPCR-GST-R | ACCGTGAACCAGACCGAGAC | 59.4 | ||
| EF-1-F | TCAAGGAACTGCGTCGTGGAT | 58.6 | 130 | 95.4 |
| EF-1-R | ACAGGGGTGTAGCCGTTAGAG | |||
| 18S-F | AACACGGGAAACCTCACCA | 55.4 | 116 | 108.9 |
| 18S-R | CAGACAAATCGCTCCACCAA | |||
| RPL32-F | CAACATCGGTTATGGAAGCA | 55.0 | 141 | 100.1 |
| RPL32-R | ACAGCGTGGGCAATTTCAGC | |||
| GAPDH-F | AAGGGTGCTCAGGTTGTTGCT | 56.5 | 89 | 104.4 |
| GAPDH-R | CGACCGTGGGTGGAGTCATAT |
Quantitative real-time reverse transcriptase PCR (qRT-PCR) was conducted using the protocols described by Zhang et al. (2019). Specific primers (Table 1) were designed according to the above verified fragments for qRT-PCR. Melting curve analysis was executed to analyze the specificity of PCR products. According to the evaluation results of Zheng et al. (2014) on the reliability of reference genes in F. occidentalis, expression levels were normalized using reference genes GAPDH, RPL32 and EF-1, 18S for high and low temperature stress, respectively.
Statistical analyses
qRT-PCR data analyzing was conducted in Bio-Rad CFX Manager 3.1 software. The average Ct values of biological replicates were used to calculate the relative expression levels. The results of qPCR were analyzed with the 2–ΔΔCt method (Livak & Schmittgen, 2001). Firstly, for all test samples and calibration samples, the Ct value of the housekeeping gene were used to normalize the Ct value of the target gene. Normalized results were ΔCt(test) and ΔCt (calibrator), respectively. And using ΔCt (calibrator) to normalize ΔCt (test), ΔΔCt was obtained. The ratio of expression level was calculated by 2–ΔΔCt. The lg(X) method was used to transform the expression level data for normality and homogeneity of variance. Significant differences were detected by one-way analysis of variance (ANOVA) and Duncan’s multiple comparisons test. Data were analyzed with SPSS v. 16.0 and considered significant at P < 0.05.
Results
Effect of high temperature stress on antioxidant activity
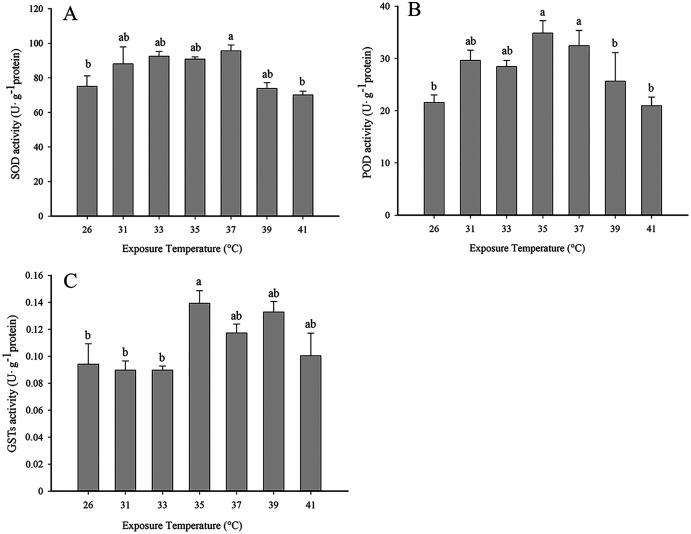
SOD activity increased with rising temperature from 31 to 37 °C and was highest at 37 °C. The activity of SOD activity began to decline at 39 °C, and the level at 41 °C was significantly lower than 37 °C (F6,19 = 4.245, P < 0.05) (Fig. 1A). A similar pattern was observed with POD, where activity rose with increasing temperature, peaked at 35 °C and was significantly lower at 41 °C than 35 °C (F6,21 = 7.089, P < 0.05) (Fig. 1B). GST activity was highest at 35 °C (Fig. 1C) and began to decline with increasing temperature (F6,21 = 8.312, P < 0.05).
Figure 1. Effect of high temperature stress on antioxidant enzyme activity in 2nd instar larvae of F. occidentalis.
(A) SOD, superoxide dismutase; (B) POD, peroxidase; (C) GST, glutathione-S-transferase. Larvae were exposed to 31, 33, 35, 37, 39, and 41 °C for 1 h in glass tubes; 26 °C was used as the control. Each value represents the mean (±SE) of four replications. Columns labeled with different letters indicate significance at P < 0.05 using ANOVA (Ducan’s test).
Temporal changes in antioxidant enzyme activity at 35 °C
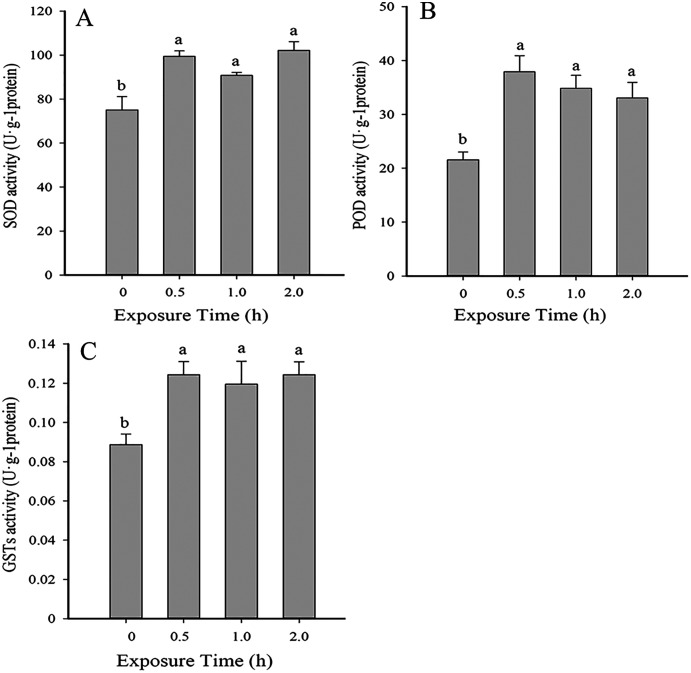
Antioxidant enzyme activity was significantly higher than the control (0 h, 26 °C) when insects were exposed to 35 °C for 0.5, 1 and 2 (SOD: F3,10 = 10.005, P < 0.05; POD: F3,12 = 8.037, P < 0.05; GSTs: F3,10 = 5.815, P < 0.05). No significant differences in antioxidant activity were detected between 0.5, 1 and 2 h of exposure (Fig. 2).
Figure 2. Temporal changes in antioxidant enzyme activity in 2nd instar larvae of F. occidentalis exposed to 35 °C.
(A) SOD, superoxide dismutase; (B) POD, peroxidase; (C) GST, glutathione-S-transferase. F. occidentalis was exposed to 35 °C for 0.5, 1, and 2 h and then analyzed for enzyme activity. The control group was maintained at 26 °C (0 h time point). Columns show the mean (±SE) of four replications, and columns labeled with different letters indicate significance at P < 0.05 in ANOVA (Ducan’s test).
Expression of antioxidant genes in response to heat and cold stress
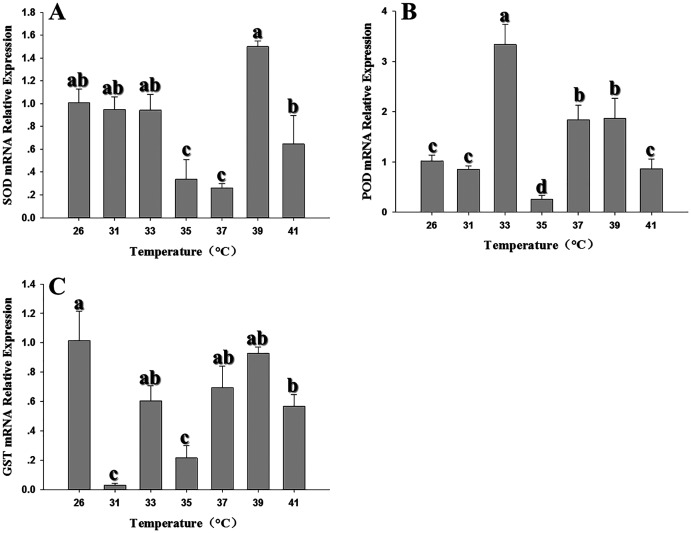
The expression of antioxidant genes was evaluated at 31, 33, 35, 37, 39 and 41 °C; 26 °C served as a control. SOD expression showed significant decreases in expression at 35–37 °C; however, expression peaked at 39 °C and was comparable to the control (26 °C) (Fig. 3A). With the exception of 35–37 °C, SOD expression was not significantly changed by high temperatures (F6,18 = 29.203, P < 0.05). In contrast, POD expression levels at 33, 37 and 39 °C were significantly higher than the control at 26 °C; however, except that the expression level was significantly decreased at 35 °C, there was no significant difference in the expression level of 31 °C compared with the control (F6,18 = 51.745, P < 0.05) (Fig. 3B). GST1 expression was suppressed or unaffected relative to the control at all elevated temperatures (F6,17 = 32.682, P < 0.05) (Fig. 3C). All three antioxidant genes shared a common insensitivity in response to high temperature. Even under some temperatures, the expression level was higher than that of the control, but the relative expression level was not very high.
Figure 3. Effect of high temperature stress on expression of antioxidant genes in 2nd instar larvae of F. occidentalis.
(A) SOD, superoxide dismutase; (B) POD, peroxidase; (C) GST, glutathione-S-transferase. Larvae were exposed to 31, 33, 35, 37, 39, and 41 °C for 1 h in glass tubes; 26 °C was used as the control. Expression levels were normalized with respect to GAPDH. Values represent the mean (±SE) of four replications, and columns labeled with different letters indicate significance at P < 0.05 in ANOVA (Ducan’s test).
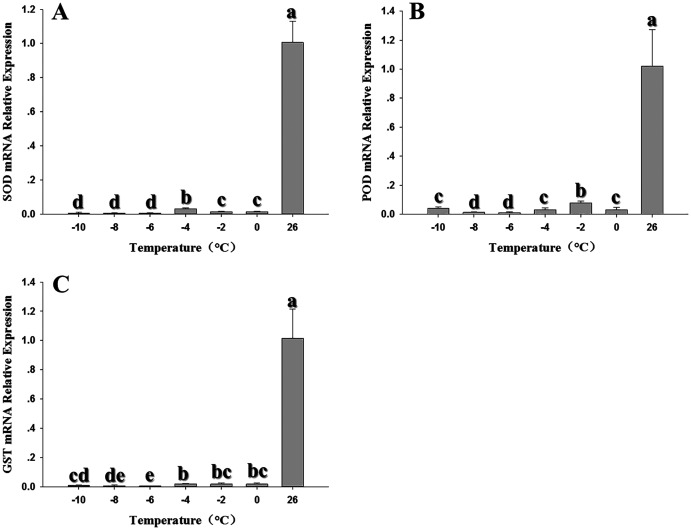
Expression of the three antioxidant genes was also evaluated in response to low temperature stress at 0, –2, –4, –6, –8 and –10 °C. SOD expression showed a significant decline at all temperatures relative to the control at 26 °C; although the expression of –4 °C was higher than other temperatures, it was also inhibited by low temperature (Fig. 4A) (F6,20 = 243.607, P < 0.05). POD expression was also strongly inhibited, with the lowest expression appeared at –6 °C. (F6,18 = 51.909, P < 0.05) (Fig. 4B). Like SOD and POD, the GST1 expression were decreased compared with the control relative with low temperature. (F6,17 = 32.682, P < 0.05) (Fig. 4C).
Figure 4. Effect of low temperature stress on expression of antioxidant genes in 2nd instar larvae of F. occidentalis.
(A) SOD, superoxide dismutase; (B) POD, peroxidase; (C) GST, glutathione-S-transferase. Larvae were exposed to 0, −2, −4, −6, −8 and −10 °C for 1 h in glass tubes; 26 °C was used as the control. Expression levels were normalized with respect to EF-1. Values represents the mean (±SE) of four replications, and columns labeled with different letters indicate significance at P < 0.05 in ANOVA (Ducan’s test).
Temporal changes in the expression of antioxidant genes
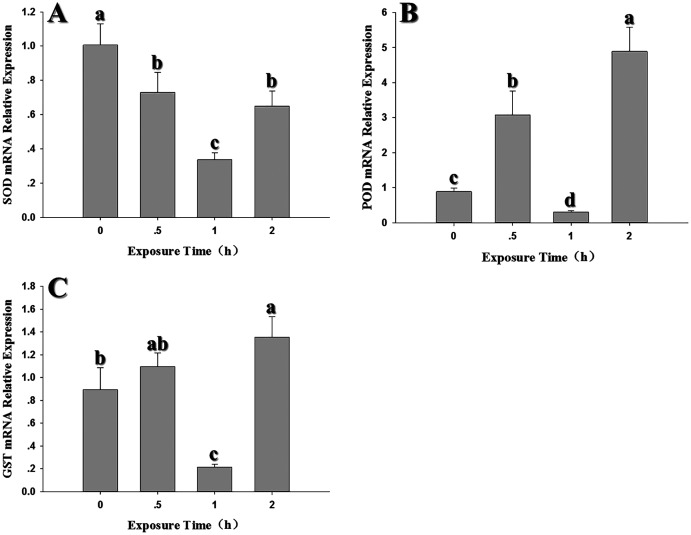
Compared to the control (0 h, 26 °C), SOD expression decreased significantly when 2nd instar larvae were exposed to 35 °C for 0.5, 1 and 2 h (SOD: F3,12 = 31.689, P < 0.05) and was lowest at the 1 h exposure period (Fig. 5A). POD expression was significantly upregulated at 0.5 and 2 h and was higher than expression levels at 0 (control) and 1 h, with the peak appeared at 2 h. (F3,12 = 72.243, P < 0.05) (Fig. 5B). GST1 expression pattern was similar to POD (Fig. 5C). Although there was no significant difference at 0.5 h compared to the control (GST1: F3,11 = 1709.476, P < 0.05).
Figure 5. Temporal changes in the expression of antioxidant genes in 2nd instar larvae of F. occidentalis exposed to 35 °C.
(A) SOD, superoxide dismutase; (B) POD, peroxidase; (C) GST, glutathione-S-transferase. F. occidentalis was exposed to 35 °C for 0.5, 1, and 2 h and then analyzed for gene expression; the control group was maintained at 26 °C (0 h time point). Expression levels were normalized with respect to GAPDH. Columns show the mean (±SE) of four replications, and columns labeled with different letters indicate significance at P < 0.05 in ANOVA (Ducan’s test).
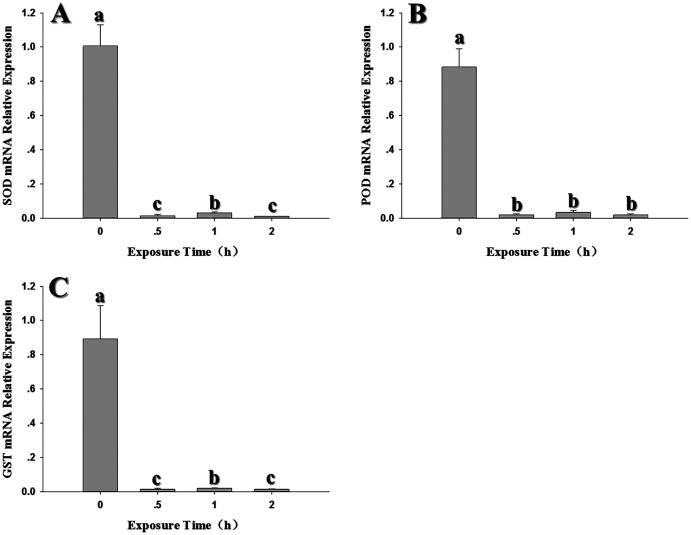
After exposure to −4 °C, the expression levels of the three antioxidant genes decreased significantly when compared to the control (SOD: F3,10 = 201.898, P < 0.05; POD: F3,11 = 204.420, P < 0.05; GST1: F3,10 = 72.835, P < 0.05). Interestingly, all three genes showed a peak in expression after a 1 h exposure to −4 °C; however, it should be noted that expression at 1 h was lower than the control (Fig. 6).
Figure 6. Temporal changes in the expression of antioxidant genes in 2nd instar larvae of F. occidentalis exposed to −4 °C.
(A) SOD, superoxide dismutase; (B) POD, peroxidase; (C) GST, glutathione-S-transferase. F. occidentalis was exposed to 4 °C for 0.5, 1, and 2 h and then analyzed for gene expression; the control group was maintained at 26 °C (0 h time point). Expression levels were normalized with respect to EF-1. Columns show the mean (±SE) of four replications, and columns labeled with different letters indicate significance at P < 0.05 in ANOVA (Ducan’s test).
Discussion
Insects are poikilotherms that are greatly impacted by temperature fluctuations (Cossins & Bowler, 1987; Worner, 1998; Bale et al., 2002). When exposed to thermal stress, insects sustain oxidative damage at the cellular level and respond with surplus levels of ROS (Lopez-Martinez et al., 2008; Cui et al., 2011; Li & Sattar, 2019). ROS can cause direct damage to biological macromolecules and can also incite genetic mutations and cell death (Ryter et al., 2007). Antioxidant enzymes function to eliminate or reduce ROS levels in insects. Previous studies showed that SOD, POD and GST play important roles in the response of insects to ROS (Abele et al., 1998; An & Choi, 2010; Celino et al., 2011; Liu et al., 2020). In this study, SOD, POD and GST activity increased significantly in response to high temperatures, which suggests that these enzymes function to remove excess ROS during thermal stress. Thus, our results are consistent with those reported for Bactrocera dorsalis, Bombyx mori, Mononychellus mcgregori, Diaphorina citri and Neoseiulus cucumeris (Lee et al., 2005; Jia et al., 2011; Marutani-Hert, Hunter & Hall, 2010; Lu et al., 2014; Zhang et al., 2014). In a previous report, low temperature stress significantly altered SOD, POD, CAT and GST activity in F. occidentalis (Shi et al., 2013). The increase in POD activity was likely the result of elevated levels of SOD activity in response to H2O2. Although increased levels of antioxidant enzymes suggests a defensive function of these enzymes in counteracting the negative effect of ROS, there were no significant differences in SOD, POD or GST activity at 0.5, 1.0 and 2.0 h of exposure to 35 °C (Fig. 2). This might indicate that antioxidant enzyme activity is very sensitive to high temperature stress and reached a threshold level at 0.5 h or earlier.
Many researchers have shown that temperature stress can lead to changes in antioxidant gene expression in insects (Yang et al., 2019; Xia et al., 2019; Lu et al. 2017). Previous results showed that temperature stress inhibited the transcription of SOD, POD, GST1 and related enzymes in Mythimna separate, Apis cerana cerana and Helicoverpa armigera (Shen et al., 2016; Yang et al., 2019; Xia et al., 2019). These results reflect the diversity of molecular responses in organisms exposed to external stress. In addition to recruiting antioxidant enzymes to remove ROS in response to thermal stress, insects also respond by synthesizing osmoprotectants, altering membrane lipid content, and expressing heat shock proteins (Chen & Kang, 2005). A previous study demonstrated that both high and low thermal stress induced CAT expression in F. occidentalis (Qin et al., 2017); therefore, the down-regulation of POD in this study might be attributed to increased expression of CAT. In the case of SOD and GST1, thermal stress may induce the synthesis of unknown substances that could inhibit transcription. Further research is needed to validate or disprove these conjectures.
Differential regulation of antioxidant genes and enzymes has been reported in insects; for example, POD, CAT and SOD expression patterns were not necessarily correlated with enzyme activity during high temperature stress in Mononychellus mcgregori (Lu et al. 2017). In larvae of Bombyx mori, carboxylesterase activity was not correlated with gene expression (Liu et al., 2010). Elevated protein levels can be stressful for the organism, and the organism may inhibit gene transcription to maintain homeostasis. Conversely, if protein levels fall to a suboptimal level, the cell may respond by promoting transcription. Furthermore, transcription is often followed by post-transcriptional processing, degradation of transcription products, translation, post-translational processing and further modifications that impact protein levels. Further research is needed to understand the mechanisms that control the response of F. occidentalis to thermal stress.
Conclusions
This study reveals differential regulation of antioxidant gene expression and enzyme production in response to thermal stress. The results confirm the importance of antioxidant enzymes in modulating the response to thermal stress in F. occidentalis, and provide new avenues for further study of antioxidant mechanisms and physiological responses of F. occidentalis. The inconsistencies between gene expression and enzyme activity further illustrate the complexity of thermal adaptation in F. occidentalis. Future multidisciplinary research in genomics, transcriptomics, proteomics, and metabolomics will help explain the underlying mechanisms of thermal adaptation in F. occidentalis.
Supplemental Information
Acknowledgments
We express our deep gratitude to the Testing Center of Yangzhou University.
Funding Statement
This work was supported by the Special Fund for Agro-Scientific Research in the Public Interest of China (201103026, 200803025). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Jia-Wen Yuan conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Yutao Zheng conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Ya-Wen Chang conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jing Bai performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jing Qin conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Yu-Zhou Du conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Abele et al. (1998).Abele D, Burlando B, Viarengo A, Pörtnera HO. Exposure to elevated temperatures and hydrogen peroxide elicits oxidative stress and antioxidant response in the Antarctic intertidal limpet Nacella concinna. Comparative Biochemistry and Physiology B: Biochemistry and Molecular Biology. 1998;120:425–435. [Google Scholar]
- Ahmad et al. (1991).Ahmad S, Duval DL, Weinhold LC, Pardini RS. Cabbage looper antioxidant enzymes: tissue specificity. Insect Biochemistry. 1991;21:563–572. [Google Scholar]
- An & Choi (2010).An MI, Choi CY. Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comparative Biochemistry and Physiology B: Biochemistry and Molecular Biology. 2010;155(1):34–42. doi: 10.1016/j.cbpb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Attig et al. (2014).Attig H, Kamel N, Sforzini S, Dagnino A, Jamel J, Boussetta H, Viarengo A, Banni M. Effects of thermal stress and nickel exposure on biomarkers responses in Mytilus galloprovincialis (Lam) Marine Environmental Research. 2014;94:65–71. doi: 10.1016/j.marenvres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Bale et al. (2002).Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Symrnioudis I, Watt AD, Whittaker JB. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biology. 2002;8:1–16. [Google Scholar]
- Bolger, Lohse & Usadel (2014).Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford (1976).Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Celino et al. (2011).Celino FT, Yamaguchi S, Miura C, Ohta T, Tozawa Y, Iwai T, Miura T. Tolerance of spermatogonia to oxidative stress is due to high levels of Zn and Cu/Zn superoxide dismutase. PLOS ONE. 2011;6(2):e16938. doi: 10.1371/journal.pone.0016938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang et al. (2017).Chang Y-W, Chen J-Y, Lu M-X, Gao Y, Tian Z-H, Gong W-R, Zhu W, Du Y-Z. Selection and validation of reference genes for quantitative real-time PCR analysis under different experimental conditions in the leafminer Liriomyza trifolii (Diptera: Agromyzidae) PLOS ONE. 2017;12(7):e0181862. doi: 10.1371/journal.pone.0181862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen & Kang (2005).Chen B, Kang L. Adaptation of insects to environmental temperature stress and population differentiation. Progress in Natural Science. 2005;3:11–17. [Google Scholar]
- Cossins & Bowler (1987).Cossins AR, Bowler K. Temperature biology of animals. New York: Chapman and Hall; 1987. pp. 125–157. [Google Scholar]
- Cui et al. (2011).Cui YD, Du YZ, Lu MX, Qiang CK. Antioxidant responses of Chilo suppressalis (Lepidoptera: Pyralidae) larvae exposed to thermal stress. Journal of Thermal Biology. 2011;36:292–297. [Google Scholar]
- Dubovskiy et al. (2008).Dubovskiy IM, Martemyanov VV, Vorontsova YL, Rantala MJ, Gryzanova EV, Glupov VV. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae) Comparative Biochemistry and Physiology Part C Toxicology and Pharmacology. 2008;148(1):1–5. doi: 10.1016/j.cbpc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Felton & Summers (1995).Felton GW, Summers CB. Antioxidant systems in insects. Archives of Insect Biochemistry and Physiology. 1995;29:187–197. doi: 10.1002/arch.940290208. [DOI] [PubMed] [Google Scholar]
- Foyer & Noctor (2005).Foyer CH, Noctor G. Oxidant and antioxidant signalling in plant: a reevaluation of the concept of oxidative stress in a physiological context. Plant Cell and Environment. 2005;28:1056–1071. [Google Scholar]
- Fu et al. (2012).Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28(23):3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr et al. (2011).Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu et al. (2019).Gu XY, Zhao Y, Su Y, Wu JJ, Wang ZY, Hu JT, Liu LJ, Zhao ZH, Hoffmann AA, Chen B, Li ZH. A transcriptional and functional analysis of heat hardening in two invasive fruit fly species, Bactrocera dorsalis and Bactrocera correcta. Evolutionary Applications. 2019;12(6):1147–1163. doi: 10.1111/eva.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig, Pabst & Jakoby (1974).Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. Journal of Biological Chemistry. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Halliwell (1989).Halliwell B. Free-radicals, reactive oxygen species and human disease-acritical evaluation with special reference to atherosclerosis. British Journal of Experimental Pathology. 1989;70:737–757. [PMC free article] [PubMed] [Google Scholar]
- Jia et al. (2011).Jia FX, Dou W, Hu F, Wang JJ. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) Florida Entomologist. 2011;94:956–963. [Google Scholar]
- Kamata & Hirata (1999).Kamata H, Hirata H. Redox regulation of cellular signalling. Cellular Signalling. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Kashiwagi et al. (1997).Kashiwagi A, Kashiwagi K, Takase M, Hanada H, Nakamura M. Comparison of catalase in diploid and haploid Rana rugosa using heat and chemical inactivation techniques. Comparative Biochemistry and Physiology B: Biochemistry and Molecular Biology. 1997;118:499–503. doi: 10.1016/s0305-0491(97)00216-2. [DOI] [PubMed] [Google Scholar]
- Kirk & Terry (2015).Kirk WDJ, Terry LI. The spread of the western flower thrips Frankliniella occidentalis (Pergande) Agricultural and Forest Entomology. 2015;5:301–310. [Google Scholar]
- Kono & Shishido (1992).Kono Y, Shishido T. Distribution of glutathione S-transferase activity in insect tissues. Applied Entomology and Zoology. 1992;27:391–397. [Google Scholar]
- Krishnan et al. (2007).Krishnan N, Kodrík D, Turanli F, Sehnal F. Stage-specific distribution of oxidative radicals and antioxidant enzymes in the midgut of Leptinotarsa decemlineata. Journal of Insect Physiology. 2007;53(1):67–74. doi: 10.1016/j.jinsphys.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2005).Lee KS, Kim SR, Park NS, Kim I, Kang PD, Sohn BH, Choi KH, Kang SW, Je YH, Lee SM, Sohn HD, Jin BR. Characterization of a silkworm thioredoxin peroxidase that is induced by external temperature stimulus and viral infection. Insect Biochemistry and Molecular Biology. 2005;35(1):73–84. doi: 10.1016/j.ibmb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Li & Sattar (2019).Li LJ, Sattar A. Effect of temperature stress on the main antioxidant enzymes in the pupa of Carpomya vesuviana Costa. Xinjiang Agricultural Sciences. 2019;56(11):2062–2071. [Google Scholar]
- Li et al. (2011a).Li HB, Shi L, Lu MX, Wang JJ, Du YZ. Impact of temperature hardening on thermal tolerance and reproduction in Frankliniella occidentalis. Chinese Journal of Applied Entomology. 2011a;36:437–442. [Google Scholar]
- Li et al. (2011b).Li HB, Shi L, Lu MX, Wang JJ, Du YZ. Thermal tolerance of Frankliniella occidentalis: effects of temperature, exposure time and gender. Journal of Thermal Biology. 2011b;36:437–442. [Google Scholar]
- Li et al. (2007).Li JZ, Zhi JR, Yuan CM, Wang H. The effect of temperature on the development of Frankliniella occidentalis. Guizhou Agricultural Science. 2007;5:1–5. [Google Scholar]
- Liu et al. (2020).Liu L, Hou XL, Yue WB, Xie W, Zhang T, Zhi JR. Response of protective enzymes in western flower thrips (Thysanoptera: Thripidae) to two leguminous plants. Environmental Entomology. 2020;49(5):1191–1197. doi: 10.1093/ee/nvaa090. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2010).Liu HT, Li B, Zhao GD, Zhang T, Gao RN, Wei ZG, Shen WD. Sexual differences in main detoxification enzymes and their gene expression in different instars of Bombyx mori larvae. Acta Entomologica Sinica. 2010;53(5):479–486. [Google Scholar]
- Liu & Ma (2007).Liu CM, Ma JQ. Effects of different temperatures on cultivating and protection enzymes of Polyrhachis dives. Journal of Xuzhou Normal University. 2007;25:72–74. [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez-Martinez et al. (2008).Lopez-Martinez G, Elnitsky MA, Benoit JB, Lee RE, Denlinger DL. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase, and heat shock proteins. Insect Biochemistry and Molecular Biology. 2008;38(8):796–804. doi: 10.1016/j.ibmb.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2014).Lu FP, Chen Q, Chen ZH, Lu H, Xu XL, Jing FL. Effects of heat stress on development, reproduction and activities of protective enzymes in Mononychellus mcgregori. Experimental and Applied Acarology. 2014;63(2):267–284. doi: 10.1007/s10493-014-9784-0. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2017).Lu YH, Bai Q, Zheng XS, Lu ZX. Expression and enzyme activity of catalase in chilo suppressalis (Lepidoptera: Crambidae) is responsive to environmental stresses. Journal of Economic Entomology. 2017;110(4):1803–1812. doi: 10.1093/jee/tox117. [DOI] [PubMed] [Google Scholar]
- Lyakhovich et al. (2006).Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry Biokhimiia. 2006;71(9):962–974. doi: 10.1134/s0006297906090033. [DOI] [PubMed] [Google Scholar]
- Marutani-Hert, Hunter & Hall (2010).Marutani-Hert M, Hunter WB, Hall DG. Gene response to stress in the Asian citrus psyllid (Hemiptera: Psyllidae) Florida Entomologist. 2010;93:519–525. [Google Scholar]
- Morse & Hoddle (2006).Morse JG, Hoddle MS. Invasion biology of thrips. Annual Review of Entomology. 2006;51:67–89. doi: 10.1146/annurev.ento.51.110104.151044. [DOI] [PubMed] [Google Scholar]
- Mouden et al. (2017).Mouden S, Sarmiento KF, Klinkhamer PG, Leiss KA. Integrated pest management in western flower thrips: past, present and future. Pest Management Science. 2017;73(5):813–822. doi: 10.1002/ps.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu, Jones & Jain (2009).Pappu HR, Jones RA, Jain RK. Global status of tospovirus epidemics in diverse cropping systems: successes achieved and challenges ahead. Virus Research. 2009;141(2):219–236. doi: 10.1016/j.virusres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Qin et al. (2017).Qin J, Lu MX, Zheng YT, Du YZ. Molecular cloning, characterization and functional analysis of catalase in Frankliniella occidentalis. Annals of the Entomological Society of America. 2017;110:212–220. [Google Scholar]
- Reitz (2009).Reitz SR. Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): The making of a pest. Florida Entomologist. 2009;92:7–13. [Google Scholar]
- Ryter et al. (2007).Ryter SW, Kim HP, Hoetzel A, Park JW, Choi AMK. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2016).Shen S, Wang M, Li X, Li S, van Oers MM, Vlak JM, Braakman I, Hu Z, Deng F, Wang H. Mutational and functional analysis of N-linked glycosylation of envelope fusion protein F of Helicoverpa armigera nucleopolyhedrovirus. Journal of General Virology. 2016;97(4):988–999. doi: 10.1099/jgv.0.000404. [DOI] [PubMed] [Google Scholar]
- Shi et al. (2013).Shi L, Li HB, Kim HJ, Wang JJ, Du YZ. Effect of low temperature stress on antioxidase activity of western flower thrips, Frankliniella occidentalis. Chinese Journal of Applied Entomology. 2013;50:1062–1067. [Google Scholar]
- Tomitaka (2019).Tomitaka Y. Studies on the interaction between tomato spotted wilt tospovirus and thrips. Journal of General Plant Pathology. 2019;85:465–467. [Google Scholar]
- Wang & Li (2002).Wang M, Li ZZ. Studies on the activities of enzymes of protective system during diapause of sawfly Chinolyda flagellicorni. Scientia Silvae Sinicae. 2002;38:100–104. [Google Scholar]
- Wang, Oberley & Murhammer (2001).Wang Y, Oberley LW, Murhammer DW. Evidence of oxidative stress following the viral infection of two lepidopteran insect cell lines. Free Radical Biology and Medicine. 2001;30:1254–1262. doi: 10.1016/s0891-5849(01)00728-6. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang JC, Zhang B, Li HG, Wang JP, Zheng CY. Effects of exposure to high temperature on Frankliniella occidentalis (Thysanoptera: Thripidae), under arrhenotoky and sexual reproduction conditions. Florida Entomologist. 2014;97:504–510. [Google Scholar]
- Worner (1998).Worner SP. Ecoclimatic assessment of potential establishment of exotic pests. Journal of Economic Entomology. 1998;81:973–983. [Google Scholar]
- Wu et al. (2017).Wu SY, Tang LD, Zhang XR, Xing ZL, Lei ZR, Gao YL. A decade of a thrips invasion in China: lessons learned. Ecotoxicology. 2017;27(7):1032–1038. doi: 10.1007/s10646-017-1864-6. [DOI] [PubMed] [Google Scholar]
- Xia et al. (2019).Xia ZY, Qin M, Wang HF, Liu ZG, Wang Y, Zhang WX, Xu BH. Effect of low temperature stress on antioxidant indexs and cold tolerance gene expression in Apis cerana cerana during overwintering period. Chinese Journal of Animal Nutrition. 2019;31(3):1250–1258. [Google Scholar]
- Yang & Smith (2013).Yang Y, Smith SA. Optimizing de novo assembly of short-read RNA-seq data for phylogenomics. BMC Genomic. 2013;14:328. doi: 10.1186/1471-2164-14-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2019).Yang H, Wang X, Pei H, Fan D. Cloning a peroxidase cDNA sequence from the oriental armyworm, Mythimna separate walker and its induction to different temperature stress. Chinese Journal of Biological Control. 2019;35(01):44–52. [Google Scholar]
- Zhang et al. (2014).Zhang GH, Liu H, Wang JJ, Wang ZY. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of the predatory mite, Neoseiulus cucumeris (Acari: Phytoseiidae) Enperimental and Applied Acarology. 2014;64(1):73–85. doi: 10.1007/s10493-014-9806-y. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2019).Zhang X, Qin J, Yuan J, Lu M, Du Y. Cloning of a new HSP70 gene from western flowerthrips, Frankliniella occidentalis, and expression patterns during thermal stress. PeerJ. 2019;7:e7687. doi: 10.7717/peerj.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2003).Zhang YJ, Wu QJ, Xu BY, Zhu GR. The occurrence and damage of Frankliniella occidentalis (Thysanoptera: Thripidae): a dangerous alien invasive pest in Beijing. Plant Protection. 2003;4:58–59. [Google Scholar]
- Zhang et al. (2012).Zhang ZJ, Zhang YJ, Xu BY, Zhu GR, Wu QJ. Effects of temperature on development, reproduction and population growth of the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae) Acta Entomologica Sinica. 2012;55(10):1168–1177. [Google Scholar]
- Zheng et al. (2014).Zheng YT, Li HB, Lu MX, Du YZ. Evaluation and validation of reference genes for qRT-PCR normalization in Frankliniella occidentalis (Thysanoptera: Thripidae) PLOS ONE. 2014;9(10):e111369. doi: 10.1371/journal.pone.0111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.