Abstract
Background:
Postoperative delirium (POD) is a common complication in cardiac surgery especially in elderly population which can lead to a delay of weaning from ventilator and extubation. Cardiopulmonary bypass (CPB)-induced inflammation is related to POD. Anti-inflammatory effect of anesthetic agent might attenuate POD.
Aims:
The present study was primarily aimed to compare within-24-h POD between ketamine-based anesthesia and propofol-based anesthesia during CPB. The secondary objective was to identify risk factors associated with within-24-h POD.
Setting and Design:
Our study was a randomized controlled trial in patients undergoing cardiac surgery with CPB. Enrolling patients were aged >65 years, and able to comprehensive communication. Exclusion criteria were aortic surgery, cognitive disorders, cerebrovascular and carotid disease, and positive result of preoperative CAM-ICU.
Materials and Methods:
Patients were randomly assigned to group Ketamine infusion of 1 mg/kg/h and group Propofol infusion of 1.5-6 mg/kg/h during CPB. POD was evaluated by validated Thai version CAM-ICU at 8-24 hour after ICU arrival.
Statistics:
Chi-square, Fisher exact and t-test tests, univariate analysis and multivariate logistic regression were utilized. Results: Total 82 patients entered this study and 64 patients remained after exclusion (Group Ketamine = 32 and Group Propofol = 32). Within-24-h POD were 31.25% and 56.25% (P = 0.04) and mean arterial pressure (MAP) were 71.45 and 65.53 mmHg (P = 0.01) respectively in Ketamine and Propofol group. Postoperative leukocytosis was a significant risk to POD (adjusted OR 124.5).
Conclusion:
With limitations of the study, prevention of 24-h POD in general by ketamine was inconclusive. In comparison with propofol, ketamine leaded to less events of 24-h POD and kept higher MAP. Severity of postoperative inflammation was a significant prediction of 24-h POD.
Keywords: Cardiac surgery, ketamine, postoperative delirium, propofol
INTRODUCTION
Postoperative delirium (POD) is a common complication in elderly patient and cardiac surgery.[1,2] Incidence of POD after cardiac surgery can reach as high as 60%.[1] Consequences of POD vary from minor disturbances to severe physical harms, such as displacement of catheters, self-extubation, long-term postoperative cognitive dysfunction, and high 1-year mortality rate.[2] Delirium can lead to a delay of weaning from ventilator or extubation[3] and contribute 14% in fast track failure cause.[4] Therefore, proper management to reduce POD especially in the first postoperative day is mandatory.
Despite the fact that cause of POD is multifactorial, inflammation is one of predominant factors.[5,6] High surgical stress from cardiac surgery, cardiopulmonary bypass (CPB), organ ischemia, and high dose adrenergic drugs can trigger systemic inflammation response (SIR).[7] Anesthetic agents have anti-inflammatory effect which can attenuate SIR, and as the result, may decrease incidence of POD.
Ketamine has shown strong anti-inflammatory effect in human and animal studies and potential effect to reduce POD.[8,9] In cardiac surgery, only one study demonstrated that ketamine added to routine anesthetics could reduce POD to 3%, in comparison with 31% of placebo group.[10] Moreover, ketamine was associated with reduction of POD in cardiac intensive care unit.[11] On the contrary, a large-scale study in both cardiac and non-cardiac surgery using only single dose of ketamine during induction did not affect the outcome.[12] Therefore, ketamine should be reinvestigated for its prevention effect of POD in specific situation of cardiac surgery in elderly patients. The main objective of study was aimed to compare within-24-h POD after cardiac surgery between ketamine-based anesthesia and propofol-based anesthesia during CPB. The secondary objective was to identify factors associated with within-24-h POD.
MATERIALS AND METHODS
We conducted a randomized control trial followed CONSORT guideline. The study was approved by our institutional ethics committee (IRB No. 788/61, at Nov, 6, 2018) and registered clinical trials (TCTR No. 20200324003). We enrolled patients at the cardiac center of King Chulalongkorn Memorial Hospital and gave all patients written informed consent.
Inclusion and exclusion
The inclusion criteria were patients undergoing cardiac surgery with CPB, aged >65 years, be able to comprehensive communication (no language barrier), and Euroscore <4.
Before-randomization, exclusion criteria were patients having (1) aortic surgery, (2) previous cognitive disorders (dementia, cognitive impairment, psychiatric or mental disorder) (3) cerebrovascular disease, (4) carotid disease, and (5) positive result of Thai version of the confusion assessment method (Thai-CAM) on the day before surgery.
After-randomization, exclusion criteria were (1) death, (2) reoperation, and (3) stroke in 24 hours, postoperatively.
Randomization and intervention protocol
The investigator (SS) performed preoperative CAM evaluation in the evening day before surgery and excluded patients who had positive result of Thai-CAM test. After induction of anesthesia, anesthesiologist used a four-block, sealed envelope method to randomly allocated patients into 2 groups with ratio 1:1.
Group Ketamine received infusion of ketamine at 1 mg/kg/h,[13] fentanyl at 0.5-1 mcg/kg/h and cisatracurium at 1.5 mcg/kg/min during CPB. Group Propofol received infusion of propofol at 1.5-6 mg/kg/h,[14] fentanyl at 0.5-1 mcg/kg/h and cisatracurium at 1.5 mcg/kg/min.
Anesthesia and monitoring
We induced all patients with etomidate 0.2-0.3 mg/kg, fentanyl 1-2 mcg/kg, and cisatracurium 0.15 mg/kg and then infused cistatracurium 1.5 mcg/kg/min after intubation. Before and after CPB, sevoflurane 1-2% was allowed to maintain depth of anesthesia guided by keeping Bispectral index (BIS) between 40-60. If BIS was >60, 0.02-0.05 mg/kg of midazolam could be added intermittently and total dose should not be higher than 0.1 mg/kg.
We applied standard monitoring and maintained cardiovascular parameters within baseline range. Cerebral oximetry was monitored and maintained not lower than 20% from baseline. Blood glucose was controlled in range of 140-180 mg/dl by institutional protocol.
During CPB, MAP 65-80 mmHg, PaO2 150-250 mmHg, PaCO2 35-40 mmHg, pump flow ≥2.4 L/min/m2, and hematocrit 25-30% were maintained. If mean arterial pressure (MAP) during CPB was >80 mmHg, 1-3 mcg/kg/min of nitroglycerine was started, and additional nicardipine 0.2-04 mg was given intravenously as needed.
For wean-off CPB, anesthesiologists made decision to use any inotropic and vasoactive agents (dopamine, dobutamine, epinephrine, amrinone, and norepinephrine) in order to maintain cardiovascular stability and adequate circulation.
In ICU, cardiovascular management, ventilatory support, and extubation were provided based on institutional protocols. Fentanyl infusion was administered for postoperative sedation and pain control at 0.5-1.5 mcg/kg/h adjusted to keep VAS pain score <5.
Primary outcome and measurement
We evaluated POD within 24 h as the primary outcome by using Thai version of CAM-ICU. Thai version of CAM-ICU was developed and validated with good interrater reliability (Cohen’k = 0.81) and good validity (94.7%) of specificity testing with clinical experts’ diagnosis.[15,16]
Richmond Agitation-Sedation Scale was applied together with CAM-ICU to assess sedation level by observation.[17]
The investigator (SS) who did not know patients’ interventions conducted post-CAM-ICU at 8-24 hours after ICU arrival. The results of evaluation were recorded and determined as positive or negative for delirium.
Secondary outcomes
We recorded demographic data (age, body mass index, gender), underlying diseases, cardiovascular parameters (BP, HR), intraoperative events (aortic cross clamp time, CPB time, operation time and inotropic drug infusion), laboratory data (WBC, PaO2, and PaCO2) and postoperative event. Postoperative blood sampling was done at 30 mins after ICU arrival. All of the above mentions were analyzed to identify risk factors of POD.
Sample size and statistical analysis
Based on the study result of Hudetz JA, et al.,[10] the incidence of postoperative delirium in cardiac surgery was 3% in patients receiving ketamine compared with 31% in placebo. Calculated sample size was 28.22 (95% of confidence interval and 80% of power). As a result, the calculated sample size was at least 29 samples per group.
Descriptive statistics (frequencies and percentages) were displayed for categorical variables. Chi-square test was performed to compare percentage of POD and categorical variables between groups. Fisher exact test was applied where observed/expected number was <5. Univariate analysis then multivariate logistic regression analysis were performed to identify risks of POD using adjusted odds ratio (ORs) with 95% confidence interval (CI). Statistically significance was considered as P < 0.05 (two-sided). We used SPSS Statistics for Windows to analyze all statistical tests.
RESULTS
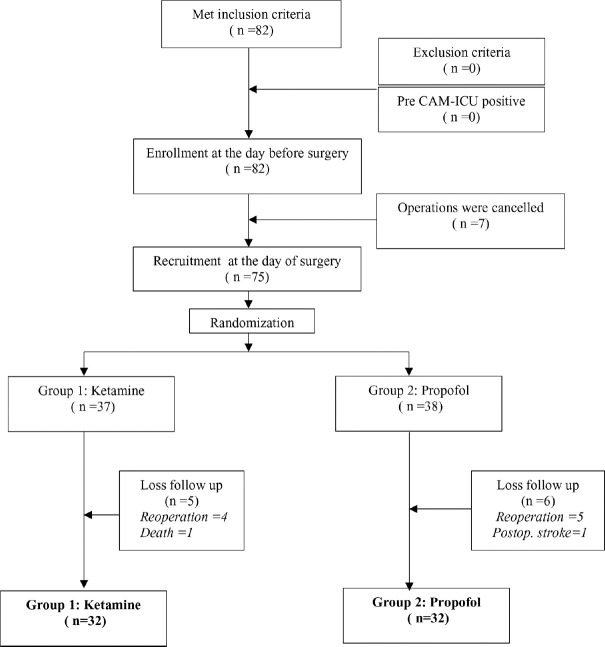
During June 2019 to February 2020, we enrolled total eighty-two patients. There was no positive result of pre-CAM-ICU. Seven patients got cancellation because of unavailable ICU bed. Seventy-five patients were assigned into study groups on the day of surgery. We lost data of 5 patients in Group Ketamine and 6 patients in Group Propofol. Finally, data of 32 patients in each group remained for analysis. A flow of allocation was showed in Figure 1.
Figure 1.
Flow of enrollment, recruitment and randomization
Demographic data and preoperative conditions were comparable as demonstrated in Table 1. Types of operations were CABG (n = 40, 62.5%) and valvular surgery (n = 24,37.5%). There was no statistical different of operation type between the two groups (P = 0.30).
Table 1.
Compared demographic data, preoperative characteristics, and type of operation
| Patients characteristics | Group Ketamine (n=32) | Group Propofol (n=32) | P | |
|---|---|---|---|---|
| Age (Y) | 65-75 | 20 (62.5%) | 24 (75%) | 0.28 |
| 75-85 | 12 (37.5%) | 8 (25%) | ||
| Gender (n,%) | Male | 14 (43.7%) | 21 (65.6%) | 0.79 |
| BMI (n,%) (kg.m-2) | 25 | 24 (75%) | 24 (75%) | 0.99 |
| >=25 | 8 (25%) | 8 (25%) | ||
| Euroscore (n, %) | <2 | 24 (75%0 | 20 (62.5%) | 0.28 |
| 2-4 | 8 (25%) | 12 (37.5%) | ||
| Smoking (n, %) | Yes | 11 (34.4%) | 10 (31.2%) | 0.79 |
| Hypertension (n, %) | Yes | 30 (93.8%) | 31 (96.8%) | 0.55 |
| DM (n, %) | Yes | 14 (43.7%) | 13 (40.6%) | 0.80 |
| CKD (n, %) | Yes | 14 (43.7%) | 9 (28.1%) | 0.19 |
| Atrial fibrillation (n, %) | Yes | 9 (28.1%) | 7 (21.8%) | 0.56 |
| Preop O2 saturation (n, %) | <90% | 0 (0%) | 2 (6.3%) | 0.15 |
| >=90% | 32 (100%) | 30 (93.7%) | ||
| Preop WBC (n,%) | <10,000 | 32 (100%) | 32 (100%) | 1.00 |
| Preop BG (n,%) (mg%) | >200 | 24 (75%) | 27 (84.4%) | 0.35 |
| <=200 | 8 (25%) | 5 (15.6%) | ||
| Type of operation (n, %) | Valve surgery | 14 (43.8%) | 10 (31.3%) | 0.30 |
| CABG | 18 (56.2%) | 22 (68.8%) |
Remark: CKD=Glomerular filtration rate <60 ml.kg.1.73/m2, Preop=Preoperative, BG=Blood glucose, WBC=White blood cell count (cell/mm3)
Intraoperative variables were not significantly different except for mean arterial pressure (MAP) (Group ketamine 71.45, 95% CI = 69.66-73.10 mmHg VS Group Propofol 65.53, 95%CI = 63.27-67.67, P = 0.01), [Table 2]. PaO2 <200 mmHg was found in 8 patients (Group Ketamine = 4, Group Propofol = 4). Cerebral oxygen saturation was well maintained within 20% in all patients. BIS score could be maintained between 40 and 60 in all cases. Total midazolam used were 5.97 (95% CI: 5.09-6.83) and 6.78 (95% CI: 5.91-7.62) mg respectively with no statistical difference (P = 0.21).
Table 2.
Compared intraoperative dependent variables
| Intraoperative dependent variables | Group Ketamine (n=32) | Group Propofol (n=32) | P | ||
|---|---|---|---|---|---|
| Pre CPB | SBP (mmHg) (n,%) | <80 | 5 (15.6%) | 5 (15.6%) | 0.66 |
| 80-120 | 9 (28.1%) | 6 (18.8%) | |||
| >120 | 18 (56.3%) | 21 (65.6%) | |||
| DBP (mmHg) (n, %) | 40-60 | 13 (40.6%) | 9 (28.1%) | 0.12 | |
| 61-80 | 18 (56.2%) | 17 (53.1%) | |||
| >80 | 1 (3.2%) | 6 (18.8%) | |||
| Mean (mmHg) (n, %) | <60 | 0 (0%) | 1 (3.2%) | 0.23 | |
| 60-75 | 22 (68.8%) | 16 (50.0%) | |||
| >75 | 10 (31.2%) | 15 (46.8%) | |||
| CPB | MAP (mmHg) (95% CI) | 71.45 | 65.53 | 0.01 | |
| (69.66-73.10) | (63.27-67.67) | ||||
| Sympathetic | Number of | 1 | 3 (9.4%) | 2 (6.2%) | 0.83 |
| stimulation | sympathomimetic drug (n,%) | 2 | 14 (43.7%) | 16 (50.0%) | |
| >2 | 15 (46.9%) | 14 (43.8%) | |||
| Operation | Op time (h) (n,%) | <6 | 26 (81.2%) | 22 (68.8%) | 0.28 |
| >=6 | 6 (18.8%) | 10 (31.2%) | |||
| CPB time (min) (n,%) | <120 | 12 (37.5%) | 10 (31.2%) | 0.59 | |
| >=120 | 20 (62.5%) | 22 (68.8%) | |||
| Clamp time (min) (n,%) | <60 | 26 (81.2%) | 22 (68.8%) | 0.25 | |
| >=60 | 6 (18.8%) | 10 (31.2%) | |||
Remark: SBP=Systolic blood pressure, DBP=Diastolic blood pressure, MAP=Mean arterial pressure, Op time=Operation time, Clamp time=Aortic cross clamp time, sympathomimetic drug=Dopamine, dobutamine, epinephrine, norepinephrine, or milrinone
Postoperative conditions were shown in Table 3. SIR-related conditions, oxygenation, and ventilation were not significantly different between two groups. There were less patients with VAS pain score >5 in Group Ketamine (n = 7, 21.9%) than Group Propofol (n = 15, 46.9%) with statistical significance (P = 0.03).
Table 3.
Compared SIR, oxygenation, ventilation and others
| Dependent variables | Group Ketamine (n=32) (n,%) | Group Propofol (n=32) (n,%) | P | ||
|---|---|---|---|---|---|
| Post SIR | Post WBC | <10,000 | 23 (71.9%) | 19 (59.4%) | 0.29 |
| >=10,000 | 9 (28.1%) | 13 (40.6%) | |||
| Temperature | <=37.5 ºC | 27 (84.4%) | 27 (84.4%) | 0.99 | |
| >37.5 ºC | 5 (15.6%) | 5 (15.6%) | |||
| Post HR | <100 | 20 (62.5%) | 18 (56.3%) | 0.61 | |
| >=100 | 12 (37.5%) | 14 (43.8%) | |||
| Post SBP | <80 | 7 (21.9%) | 12 (37.5%) | 0.39 | |
| 80-120 | 21 (65.6%) | 17 (3.1%) | |||
| >120 | 4 (12.5%) | 3 (9.4%) | |||
| Oxygenation | Post PaO2 | 100-200 | 6 (18.6%) | 9 (28.1%) | 0.37 |
| Ventilation | Post PaCO2 | 30-40 | 30 (93.8%) | 25 (78.1%) | 0.07 |
| >=40 | 2 (6.2%) | 7 (21.9%) | |||
| Others | Post BG | <200 | 27 (84.4%) | 27 (84.4%) | 0.99 |
| >=200 | 5 (15.6%) | 5 (15.6%) | |||
| VAS Pain | 1-5 | 25 (78.1%) | 17 (53.1%) | 0.03 | |
| >5 | 7 (21.9%) | 15 (46.9%) | |||
| Time to extubation | < 8 h | 2 (6.2%) | 4 (12.5%) | 0.68 | |
| 8-24 h | 24 (75%) | 23 (71.9%) | |||
| >24 h | 6 (18.8%) | 5 (15.6%) | |||
Remark: Post=Postoperative, WBC=White blood cell count (cell/mm3), HR=Heart rate (beat per minute), SBP=Systolic blood pressure (mmHg), Intra=Intraoperative, PaO2=Blood oxygen tension (mmHg), Cerebral O2=Intraoperative cerebral oximetry variation (%), PaCO2=Blood carbon dioxide tension (mmHg), BG=Blood glucose (mg/dl), VAS=Visual analog scale
Post-CAM-ICU assessment showed positive for delirium in total 28 (43.75%) patients, in group Ketamine 10 (31.25%) patients, and in group Propofol 18 (56.25%) patients with statistical difference (P = 0.04).
We performed univariate analysis for positive CAM-ICU patients. Risk of POD was higher in Group Propofol (OR = 2.67); however, it was not statistical significance (P = 0.061). All of demographic data and preoperative variables revealed as non-significant risk factors. CABG was in higher risk than valvular surgery (OR = 1.90, P = 0.23). For intraoperative events, MAP 60-75 mmHg (OR = 3.70, P = 0.02) and CPB time >=120 mins (OR = 3.30, P = 0.03) were identified as significant risk factors. For postoperative events, WBC >10,000 cell/mm3 (OR = 47.34, P = 0.001), and VAS pain score >5 (OR = 18.17, P = 0.001) were identified as significant independent risk factors. After multivariate logistic regression analysis, only significant risk factor was postoperative WBC >10,000 cell/mm3 (adjusted OR = 124.5, P = 0.001) [Table 4].
Table 4.
Multivariate logistic regression analysis and risk of POD
| Dependent variables | Post CAM-ICU | Adjusted | P | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Positive (n=28) | Negative (n=36) | Odd ratio | 95% CI | |||
| Intervention | Propofol | 18 (64%) | 14 (39%) | 2.11 | 0.29-26.42 | 0.47 |
| Ketamine | 10 (36%) | 22 (61%) | ||||
| CPB time | >=120 min | 22 (79%) | 18 (50%) | 1.92 | 0.08-46.97 | 0.88 |
| <120 min | 6 (21%) | 18 (50%) | ||||
| MAP in CPB | 60-75 | 26 (93%) | 31 (86%) | 1.46 | 0.14-15.32 | 0.45 |
| >75 | 7 (7%) | 5 (14%) | ||||
| Post WBC | >10,000 | 8 (29%) | 34 (94%) | 124.5 | 8.36-1,857 | 0.001* |
| <=10,000 | 20 (71%) | 21 (6%) | ||||
| VAS Pain | >5 | 18 (64%) | 4 (11%) | 12.53 | 1.43-97.28 | 0.06 |
| 1-5 | 10 (36%) | 32 (89%) | ||||
Remark: 95%CI=95% confidence interval, *significant level at P<0.05
During postoperative 24 h, we detected no event of nightmare or hallucination in both groups.
DISCUSSION
Delirium is defined as an acutely fluctuating mental status change with features of inattention and altered level of consciousness.[1] Assessment tool is immensely important to confirm POD diagnosis. The standard of POD assessment has been the Confusion Assessment Method-Intensive Care Unit (CAM-ICU) which was established reliability and validity for assessing delirium.[3,18] To assess the level of sedation, the CAM-ICU utilizes the Richmond Agitation and Sedation Score which is a 10 point scale that provides discrete criteria for levels of sedation and agitation.[17] The CAM-ICU can be performed with good inter-rater reliability by both physician and nurse[18] and can be assessed in intubated patients. Thai version CAM-ICU was developed based on the CAM-ICU and well verified with good validity[16] and supposed to be the most appropriate tool for POD assessment in Thai context.
POD is more common in elderly patient and can cause declining in functional level, which might change ability from self-care to dependence in the year of surgery.[1] The beneficial effects of ketamine on postoperative delirium is enhanced by evidence of its anti-inflammatory effects,[9,10,19] neuroprotective actions,[2] and rapid and lasting anti-depressant actions.[12,20] Despite of a negative result from previous large-scale study of subanesthetic dose of ketamine,[12] we still believed in anti-inflammatory effect of ketamine especially in specific condition. Unlike non-cardiac surgery, cardiac surgery is well known for causing a high degree of inflammation by CPB, organ ischemia, high sympathetic state, etc.[7] Ketamine acts differently from other anti-inflammatory drugs by attacking at local inflammation in addition to systemic regulator of inflammation process which involves both inflammation and anti-inflammation.[9] Furthermore, higher dose of ketamine has been reported of its effectiveness to prevent delirium. Perbet S, et al. studied ketamine infusion at rate 2 mg/kg/h in ICU patients and revealed significant reduction of delirium.[21] McLean RF, et al. studied ketamine of 1-2 mg/kg IV bolus and then 50 mcg/kg/min infusion during CPB and found that it was appropriate dosage to reach neuroprotective effect.[13] As a result, reinvestigation of anesthetic dose of ketamine for prevention POD only in cardiac surgery was motivated.
We decided to give ketamine of 1 mg/kg/h infusion during CPB so that it can reach anti-inflammatory and neuroprotective effects[13,21] and avoid side effects from higher dose.[12] In addition, we compare with one of common anesthetic techniques during CPB, propofol infusion rather than placebo, since either ketamine or propofol can be used as a sole anesthetic agent. We designed to control other reported risks of POD such as BIS <40,[22] cerebral oxygen saturation <50%,[23] poor oxygenation,[24] and some anesthetics.[25]
The result of our study demonstrated that there is a significant lower of POD incidence in ketamine group than propofol group, but without significant relative risk. Based on POSCAST,[12] history of depression that is common occur in elderly is a significant risk to POD. It was possible that anti-depression effect of ketamine might be an explanation for this favorable result. Due to 25% difference of delirium events in our results, type II error might involve in the result. Therefore, increased sample size is needed for confirming advantage of ketamine over propofol for POD reduction in cardiac surgery.
Based on PODCAST,[12] ketamine does not increase nor prevent POD comparing with placebo, and it will need a very large-scale sample size for future investigation. Our study revealed a rather high incidence of POD from ketamine (31.25%) and higher than that of PODCAST (major surgery, 19% of ketamine),[12] the study of Hudetz JA, et al. (cardiac surgery, 3% of ketamine)[10] and a study of Burkhart CS, et al. (cardiac surgery, 30% of overall).[26] Despite the fact that we intended to study in high risk group, elderly patients in cardiac surgery, it might imply that ketamine in spite of administering in anesthetic dose could not reduce POD.
For the secondary outcome, we found MAP >75 mmHg, and VAS pain score 1-5 were associated with receiving ketamine and significant risk-reduction by univariate analysis. Nevertheless surgical pain was associated with POD,[6,27] we found that VAS pain score >5 was not a significant risk by regression analysis. Maintaining higher MAP during CPB can lead to higher cerebral blood flow then higher cerebral oxygenation. However, we found that MAP 60-75 mmHg was neither a significant risk by regression analysis. Moreover, based on a recent study,[28] relative cerebral hyper perfusion during CPB was significantly associated with POD.
In addition, CPB time >=120 mins, and postoperative WBC >10,000 cell/mm3 were revealed as significant risks by univariate analysis. Long CPB time has been reported as a risk factor to POD.[29] Recently, POD has been proved associated with inflammation.[30] Leukocytosis (WBC >10,000 cell/mm3) is highly related to process of inflammation or infection.[31] Interestingly, postoperative WBC >10,000 cell/mm3 was the only significant risk factor by multiple regression. We postulates that (1) long CPB time leads to POD via inducing postoperative inflammation and (2) neither anti-inflammation of ketamine nor propofol is sufficient for reduction of inflammation from cardiac surgery.
The advantage of the present study was the first investigation conducting in elderly patient undergoing cardiac surgery with anesthetic maintenance dose of ketamine. Second, we controlled other major risks of POD (cerebral oxygenation, depth of anesthesia and anesthetics). Lastly, POD assessment in the present study was a single assessment within 24 hours in order to exclude confounding factor of long-term ICU-related delirium.
The first limitation of our study was a small sample size. The second limitation was no long-term observation of ketamine side effects. We recorded postoperative 24 h hallucination and nightmare events, but they can occur increasingly for 3 postoperative days.[12] Thirdly, we did not actually compare total consumption of fentanyl. As opioids are associated with POD.[26,27,32] Since administration of fentanyl was strictly commenced in the study protocol, we assumed fentanyl consumption of both groups were comparable. Moreover, past studies regarding opioids induced POD were less qualitive,[26] therefore it remained inconclusive.[26,33]
CONCLUSION
With limitations of the study, prevention of 24-h POD by ketamine in elderly patient underwent cardiac surgery with CPB was inconclusive. In comparison with propofol, ketamine leaded to less incident of 24-h POD and maintained higher MAP during CPB. Severity of postoperative inflammation was a significant prediction of 24-h POD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Authors would like to express our gratitude to Dr. Pakorn Urusopone for English grammatical correction. Material support was by Department of Anesthesiology, Faculty of Medicine, Chulalongkorn University.
REFERENCES
- 1.Kotfis K, Szylinska A, Listewnik M, Strzelbicka M, Brykczynski M, Rotter I, et al. Early delirium after cardiac surgery: An analysis of incidence and risk factors in elderly (≥65 years) and very elderly (≥80 years) patients. Clin Interv Aging. 2018;13:1061–70. doi: 10.2147/CIA.S166909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glumac S, Kardum G, Karanovic N. Postoperative cognitive decline after cardiac surgery: A narrative review of current knowledge in 2019. Med Sci Monit. 2019;25:3262–70. doi: 10.12659/MSM.914435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeon K, Jeong BH, Ko MG, Nam J, Yoo H, Chung CR, et al. Impact of delirium on weaning from mechanical ventilation in medical patients. Respiratory. 2016;21:313–22. doi: 10.1111/resp.12673. [DOI] [PubMed] [Google Scholar]
- 4.Lee A, Mu JL, Chiu CH, Gin T, Underwood MJ, Joynt GM. Effect of motor subtypes of delirium in the intensive care unit on fast-track failure after cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:268–75. doi: 10.1016/j.jtcvs.2017.08.139. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph JL, Ramlawi B, Kuchel GA, McElhaney JE, Xie D, Sellke FW, et al. Chemokines are associated with delirium after cardiac surgery. J Gerontol A Bio Sci Med Sci. 2008;63:184–9. doi: 10.1093/gerona/63.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuura Y, Kamidaira M, Tamura A. Risk factors for postoperative delirium after cardiac surgery. Int Arch Nurs Health Care. 2018;4:103. [Google Scholar]
- 7.Corral-Velez V, Lopez-Delgado JC, Bentancur-Zambrano NL, Lopez-Sune N, Rojas-Lora M, Torrado H, et al. The inflammatory response in cardiac surgery: An overview of the pathophysiology and clinical implications. Inflamm Allergy Drug Targets. 2015;13:357–70. doi: 10.2174/1871528114666150529120801. [DOI] [PubMed] [Google Scholar]
- 8.Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: State of the art. Acta Anaesthesiol Belg. 2011;62:47–58. [PubMed] [Google Scholar]
- 9.Dale O, Somogi AA, Li Yibai, Sullivan T, Shavit , Y Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg. 2012;115:934–43. doi: 10.1213/ANE.0b013e3182662e30. [DOI] [PubMed] [Google Scholar]
- 10.Hudetz JA, Patterson KM, Iqbal Z, Gandi SD, Byrne AJ, Hudetz AG, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009;23:651–7. doi: 10.1053/j.jvca.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Mazzeffi M, Johnson K, Paciullo C. Ketamine in adult cardiac surgery and the cardiac surgery Intensive Care Unit: An evidence-based clinical review. Ann Card Anaesth. 2015;18:202–9. doi: 10.4103/0971-9784.154478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: An international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390:267–75. doi: 10.1016/S0140-6736(17)31467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLean RF, Baker AJ, Walker SE, Mazer D, Wong BI, Harrington EM. Ketamine concentrations during cardiopulmonary bypass. Can J Anaesth. 1996;43:580–4. doi: 10.1007/BF03011770. [DOI] [PubMed] [Google Scholar]
- 14.Samir A, Gandreti N, Madhere M, Khan A, Brown M, Loomba V. Anti-inflammatory effects of propofol during cardiopulmonary bypass: A pilot study. Ann Card Anaesth. 2015;18:495–501. doi: 10.4103/0971-9784.166451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 16.Pipanmekaporn T, Wongpakaran N, Mueankwan S, Dendumrongkul P, Chittawata narat K, Khongpheng N, et al. Validity and reliability of the Thai version of the confusion assessment method for the intensive care unit (CAM-ICU) Clin Interv Aging. 2014;9:879–95. doi: 10.2147/CIA.S62660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 19.Hirsiger S, Simmen H-P, Werner C, Wanner G, Rittirsch D. Danger signals activating the immune response after trauma. Mediators Inflamm 2012. 2012 doi: 10.1155/2012/315941. 315941. doi: 10.1155/2012/315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–60. doi: 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perbet S, Verdonk F, Godet T, Jabaudon M, Chartier C, Cayot S, et al. Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: A randomised double-blind control trial. Anaesth Crit Care Pain Med. 2018;37:589–95. doi: 10.1016/j.accpm.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Chan MT, Cheng BC, Lee TM, Gin T. CODA Trial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 23.Lei L, Katznelson R, Fedorko L, Carroll J, Poonawala H, Machina M, et al. Cerebral oximetry and postoperative delirium after cardiac surgery: A randomised, controlled trial. Anesthesiology. 2017;73:1456–66. doi: 10.1111/anae.14056. [DOI] [PubMed] [Google Scholar]
- 24.Imai R, Tsugitomi R, Jinta T, Yumada U, Aoki K, Tamura T. Delirium as a predictor of high flow nasal cannula failure in patients with acute respiratory failure. Eur Resp J. 2018;52:PA2295. [Google Scholar]
- 25.Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiol. 2011;77:448–56. [PMC free article] [PubMed] [Google Scholar]
- 26.Swart LM, van der Zanden V, Spies PE, de Rooij SE, van Munster BC. The Comparative risk of delirium with different opioids: A systematic review. Drugs Aging. 2017;34:437–43. doi: 10.1007/s40266-017-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan R, Inouye SK, Meagher D. Delirium and depression: Inter-relationship and clinical overlap in elderly people. Lancet Psychiatry. 2014;1:303–11. doi: 10.1016/S2215-0366(14)70281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thudium M, Ellerkmann , RK , Heinze I, Hilber T. Relative cerebral hyperperfusion during cardiopulmonary bypass is associated with risk for postoperative delirium: A cross-sectional cohort study. BMC Anesthesiol. 2019;19:35. doi: 10.1186/s12871-019-0705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’neal JB, Billings FT, Lui X, Shotwell MS, Liang Y, Shah AS, et al. Risk factors for delirium after cardiac surgery: A historical cohort study outlining the influence of cardiopulmonary bypass. Can J Anaesth. 2017;64:1129–37. doi: 10.1007/s12630-017-0938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcantonio ER, Vasunilashorn S, Dillon S, Ngo L, Jones RN, Arnold S, et al. The role of inflammation in postoperative delirium. Innov Aging. 2017;1(Suppl 1):1326. [Google Scholar]
- 31.Willems JW, Trompet S, Blauw GJ, Westendorp RGJ, de Craen AJM. White blood cell count and C-reactive protein are independent predictors of mortality in the oldest old. J Gerontol A Bio Sci Med Sci. 2010;65:764–8. doi: 10.1093/gerona/glq004. [DOI] [PubMed] [Google Scholar]
- 32.Burkhart CS, Dell-Kuster S, Gamberini M, Moeckli A, Grapow M, Filipovic M, et al. Modifiable and nonmodifiable risk factors for postoperative delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2010;24:555–9. doi: 10.1053/j.jvca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Tse L, Schwarz SK, Bowering JB, Moore RL, Burns KD, Richford CM, et al. Pharmacological risk factors for delirium after cardiac surgery: A review. Curr Neuropharmacol. 2012;10:181–96. doi: 10.2174/157015912803217332. [DOI] [PMC free article] [PubMed] [Google Scholar]