SUMMARY
Urinary tract infections (UTI) are one of the most common indications for antibiotic prescriptions in the outpatient setting. Given rising rates of antibiotic resistance among uropathogens, antibiotic stewardship is critically needed to improve outpatient antibiotic use, including in outpatient clinics (primary care and specialty clinics) and emergency departments. Outpatient clinics are in general a neglected practice area in antibiotic stewardship programs, yet most antibiotic use in the United States is in the outpatient setting. This article provides a comprehensive review of antibiotic stewardship strategies for outpatient UTI in the adult population, with a focus on the “five Ds” of stewardship for UTI, including right diagnosis, right drug, right dose, right duration, and de-escalation. Stewardship interventions that have shown success for improving prescribing for outpatient UTI are discussed, including diagnostic stewardship strategies, such as reflex urine cultures, computerized decision support systems, and modified reporting of urine culture results. Among the many challenges to achieving stewardship for UTI in the outpatient setting, some of the most important are diagnostic uncertainty, increasing antibiotic resistance, limitations of guidelines, and time constraints of stewardship personnel and front-line providers. This article presents a stewardship framework, built on current evidence and expert opinion, that clinicians can use to guide their own outpatient management of UTI.
KEYWORDS: antibiotic stewardship, emergency department, outpatient, primary care, urinary tract infections
INTRODUCTION
The Need for Antibiotic Stewardship for UTI
The World Health Organization identified antimicrobial resistance as one of the top 10 threats to global health in 2019 (1). In the United States, it is estimated that over 2.8 million antibiotic-resistant infections occur annually, causing more than 35,000 deaths (2). Overuse and misuse of antibiotics contribute to antimicrobial resistance, high health care expenditures, avoidable adverse drug events, and Clostridioides difficile (formerly Clostridium difficile) infection (2).
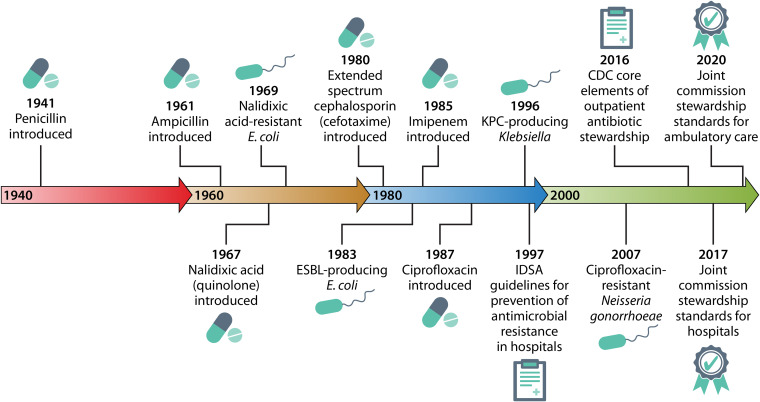
Urinary tract infections (UTI) and urinary pathogens have particular relevance to the emergence of antimicrobial resistance and the need for antimicrobial stewardship. Since ampicillin was introduced in 1961, ampicillin resistance in human Escherichia coli isolates increased significantly, by 0.59% per year, ranging from 0% to 66.7% during the period from 1950 to 2002 (3) (Fig. 1). By 1999, guidelines recommended against using β-lactams for cystitis and pyelonephritis due to rising resistance, high recurrence rates, and lower eradication rates than for trimethoprim-sulfamethoxazole and fluoroquinolones (4). Soon after the introduction of second-generation penicillins, quinolones (nalidixic acid), cephalosporins, and carbapenems, antibiotic-resistant urinary pathogens emerged (Fig. 1) (5–7).
FIG 1.
Timeline of antibiotic development, antimicrobial resistance, and the advent of antibiotic stewardship.
Although antibiotics are frequently prescribed in every health care setting, outpatient antibiotic use is substantial and is often unnecessary (8–11). From 2010 to 2015, $56.0 billion was spent on antibiotics in the United States, of which 59.1% occurred in the outpatient setting, usually in community pharmacies (12). UTI are one of the most common indications for antibiotics at outpatient visits to physician offices and emergency departments (EDs) (13, 14). In 2016, there were 3.7 million office visits and 2.6 million ED visits for UTI in the United States, representing 0.4% and 1.8% of all office visits and ED visits, respectively (13, 14). Among infectious disease-related ED visits, upper and lower UTI account for 12.6% of visits by persons of all ages (15) and 25.3% of visits by elderly adults (16). In a database study of antibiotic prescribing behavior in primary care practices in England, 22.7% of over 3.1 million antibiotic prescriptions were for UTI (17).
International clinical practice guidelines from the Infectious Diseases Society of America (IDSA), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and the U.S. Preventive Services Task Force (USPSTF) provide recommendations for the management of UTI and asymptomatic bacteriuria (ASB) (18–22). Despite widespread dissemination of these guidelines, adherence remains low (19–25). Numerous studies have outlined the high prevalence of inappropriate antibiotic prescribing in the outpatient setting, particularly for UTI (23–28). Studies based in the United States, Europe, Asia, the Middle East, and Africa have described guideline-discordant treatment of outpatient UTI, including unnecessary prescriptions, excessive duration of therapy, and misuse of broad-spectrum antimicrobials (26–33). Within the Veterans Affairs (VA) system, a random sample of outpatient antibiotic prescriptions revealed that primary care providers (PCPs) prescribed 84 antibiotic prescriptions per 1,000 patients per year (34). UTI was the indication for 23% of prescriptions; of these, 30% were not indicated, 28% were for guideline-discordant agents, and 13% were guideline discordant in duration. Inappropriate therapy for UTI has also been documented in the home-based (35) and ED (36–40) settings.
The Era of Antibiotic Stewardship
In response to rising rates of resistance, the field of antimicrobial stewardship developed in the late 1990s as a strategy for slowing and preventing the emergence of resistant bacteria (Fig. 1) (41, 42). Antibiotic stewardship is defined as “coordinated interventions designed to improve and measure the appropriate use of antimicrobial agents by promoting the selection of the optimal antimicrobial drug regimen including dosing, duration of therapy, and route of administration” (43). In 2014 and 2015, the Centers for Disease Control and Prevention (CDC) released core elements of antibiotic stewardship programs in hospitals (44) and nursing homes (45). Although a significant body of literature has focused on antibiotic stewardship in the hospital setting, the importance of stewardship in primary care clinics, urgent care, and EDs is increasingly recognized (46). Accordingly, the 2016 report “Core Elements of Outpatient Antibiotic Stewardship” outlines key strategies for improving outpatient antibiotic use, including commitment, action for policy and practice, tracking and reporting, and education and expertise (47). The Joint Commission established new antimicrobial stewardship requirements for hospitals in 2017 and ambulatory health care organizations in 2020 (48) (Fig. 1). The ambulatory antibiotic stewardship program standards include identifying an antimicrobial stewardship leader, establishing an annual antimicrobial stewardship goal, implementing evidence-based guidelines, providing educational resources, and reporting stewardship data.
The 5-Ds Model of Stewardship for UTI
Given the urgency of implementing antibiotic stewardship programs in ambulatory care, including both outpatient primary care clinics and EDs, educational resources and comprehensive reviews are helpful to guide best practices for common infections. Although much of the stewardship literature in the outpatient setting is focused on respiratory tract infections (46), there is growing evidence for the effectiveness of stewardship programs targeting outpatient UTI (49–51). This article reviews current approaches to implementing antibiotic stewardship for UTI in the adult population in outpatient settings, with an emphasis on successful interventions that have led to measurable improvements in antibiotic prescribing practices. Our literature review included interventions from the year 2000 or later, given that the field of antibiotic stewardship gained momentum after the publication of IDSA guidelines for prevention of antimicrobial resistance in 1997 (42). The target audience is anyone interested in antibiotic stewardship for UTI, which may include clinicians, nurses, health services researchers, microbiologists, and clinical laboratory directors, among other stewardship stakeholders.
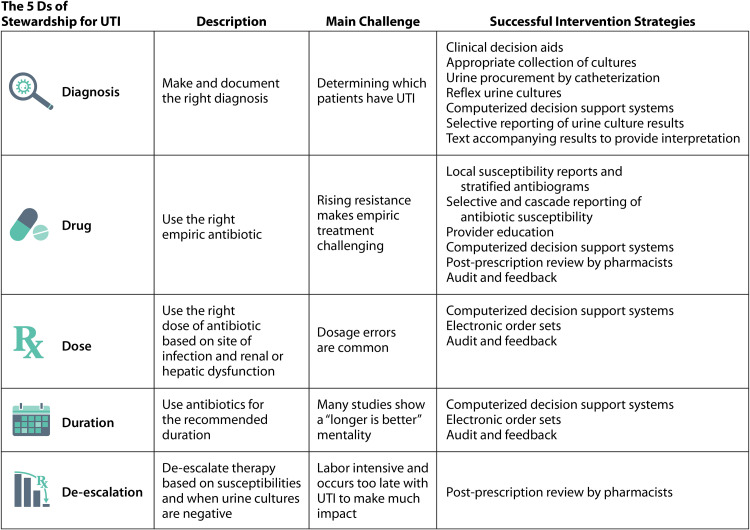
Antibiotic stewardship can be framed by the “six Ds of antimicrobial stewardship”: diagnosis, drug, dose, duration, de-escalation, and debridement/drainage (52, 53). For outpatient management of UTI, debridement/drainage is not usually relevant; therefore, we focus on the first five Ds (Fig. 2). Applying the 5-Ds model of antibiotic stewardship to outpatient UTI provides a very useful framework for understanding the challenges and opportunities unique to stewardship for UTI in the outpatient setting. For the purposes of this review, outpatient settings include outpatient clinics (primary care and specialty clinics) and EDs. Some of the evidence presented is not specific to ambulatory care, but the strategies employed are still applicable to primary care and ED settings. There is a paucity of literature addressing antibiotic stewardship in urgent care, and we do not address the unique antibiotic stewardship challenges of urgent care clinics in this review. The focus is on how to diagnose and treat nonpregnant adults seen as outpatients, so UTI in pregnant women, children, and residents of long-term-care facilities is not discussed. Management of recurrent UTI is also outside the scope of this review; recent guidelines from the American Urologic Association provide detailed recommendations for the diagnosis and treatment of recurrent UTI in women (54).
FIG 2.
The 5 Ds of antibiotic stewardship for UTI.
RIGHT DIAGNOSIS
The biggest challenge for antibiotic stewardship in the outpatient setting is diagnostic uncertainty, particularly for patients with nonspecific symptoms. Symptomatic UTI merits immediate treatment with antibiotics to relieve symptoms, shorten duration of symptoms, and prevent progression to pyelonephritis (55). However, many patients receive unnecessary treatment for ASB that was incorrectly diagnosed as UTI. Symptomatic UTI must be distinguished from ASB, sexually transmitted infections (STI), and overactive bladder, among other conditions (Table 1). Although fever may be a sign of complicated UTI and pyelonephritis, outpatients presenting with fever, in the absence of urinary tract symptoms, should be evaluated for other sources of infection (e.g., upper respiratory infection, cellulitis, and sinusitis). Given that most patients present to clinic or the ED due to bothersome symptoms, the art of diagnostic medicine is to distinguish symptoms of UTI (e.g., dysuria) from those that most likely do not originate in the urinary tract (e.g., falls and abdominal pain).
TABLE 1.
Strategies to achieve the right diagnosis of urinary tract infection
| Diagnostic task | What works | What might work | What does not work |
|---|---|---|---|
| Differentiate UTI from asymptomatic bacteriuria | Careful history to elicit symptoms of UTI | Physical examination | Urinalysis and urine culture (both will be positive in ASB) |
| Differentiate UTI from sexually transmitted infection | Careful history to elicit symptoms of STI (vaginal discharge); test for STI | Limited pelvic exam (discharge, lesions); rapid UTI diagnostics (in development) | Urinalysis (pyuria likely) and urine culture (contamination likely) |
| Differentiate UTI from overactive bladder | Careful history; urine culture (if negative) | Urologic evaluation | Urinalysis and urine culture in patients with high prevalence of baseline bacteriuria |
| Determine etiology of delirium in older adults | Explore nonurinary etiologies of acute mental status change | Observe patient without prescribing antibiotics; encourage oral fluids | Urinalysis and urine culture (both may be positive in nonurinary etiologies) |
| Differentiate UTI from nonurinary conditions | Careful history, explore nonurinary etiologies | Rapid UTI diagnostics (in development), particularly in younger adults | Urinalysis and urine culture in patients with high prevalence of baseline bacteriuria |
Differentiate UTI from Asymptomatic Bacteriuria
IDSA guidelines from 2005 and reinforced in 2019 recommend that ASB should be screened for and treated only in pregnant women or prior to invasive urologic procedures; treatment of ASB in other adult populations is discouraged (18, 19). The 2019 statement from the USPSTF reaffirms the recommendation against screening for ASB in nonpregnant adults (D recommendation) (20). Despite the evidence that the risks of ASB treatment outweigh the benefits for most populations, guideline-discordant treatment remains common. In the outpatient and ED settings, ASB is treated inappropriately in 20 to 52% of cases (56–61).
Careful history taking, physical examination, and clinical judgment all contribute to making an accurate diagnosis of UTI in the outpatient setting. Table 1 summarizes common clinical scenarios in which the diagnosis of UTI can be particularly challenging and offers suggestions for strategies that may guide clinicians in arriving at the right diagnosis. In a study of the diagnostic accuracy of history taking and physical examination, four symptoms and one sign significantly increased the posttest probability of UTI; these were dysuria, frequency, hematuria, back pain, and costovertebral angle tenderness (62). Patients with vaginal discharge on examination, history of vaginal irritation, and absence of dysuria or back pain had a lower probability of UTI (62). Misleading signs and symptoms, including foul-smelling urine and cloudy urine, are not diagnostic of UTI (18). Similarly, laboratory findings, such as pyuria, organism type and number, and systemic leukocytosis cannot distinguish between ASB and UTI yet are often interpreted as evidence of UTI (63). Ignoring a positive urinalysis for a patient with vague symptoms makes many providers uneasy, and this perception that untreated ASB is dangerous to the patient is a major barrier to antibiotic stewardship (64).
Utility and Limitations of Urine Cultures
In the outpatient setting, primary care and ED providers typically treat UTI empirically based on a clinical diagnosis, without sending a urine culture. In general, outpatient urine culture results are not available in time to impact clinical decision making or to guide antibiotic choice at the point of care. However, urine culture should be performed for patients with suspected pyelonephritis, catheter-associated UTI, relapse or recurrent infection, complicated UTI, and suspected multidrug-resistant infection (21, 65, 66).
Although urine culture is currently regarded as the gold standard for diagnosis of UTI (albeit often retrospectively), midstream-voided urine colony counts are an imperfect diagnostic criterion for UTI (67–69). Contamination and colony counts below the laboratory’s threshold for reporting can both lead to a “negative” culture for a patient who actually has a symptomatic UTI (70). Using catheterized urine as the reference, growth of Escherichia coli in midstream urine cultures was highly predictive of bladder bacteriuria in symptomatic women with acute uncomplicated cystitis (67). Even colony counts as low as 10 to 102 CFU per milliliter in midstream urine were sensitive and specific for the subsequent recovery of E. coli from a bladder (catheterized) urine sample. However, for Gram-positive organisms, including enterococci and group B streptococci, growth in voided midstream urine had low positive predictive values (PPV) for being recovered from the catheterized urine specimen from the bladder. Among paired specimens with enterococcus and/or group B streptococci in midstream urine, E. coli grew in bladder cultures in 25/41 (61%). Therefore, the recovery of these Gram-positive organisms from a midstream-voided urine culture does not necessarily prove that the etiologic agent of the UTI has been isolated (67), and the provider may consider choosing an antibiotic that also covers E. coli.
The most important limitation of urine cultures from a stewardship perspective is that culture results in themselves tell us nothing about whether the patient has urinary symptoms (and thus true UTI) or symptoms unrelated to the urinary tract (ASB). Although a positive urine culture is often a trigger to prescribe antibiotics, a positive culture is not in itself diagnostic of UTI. Given the high prevalence of ASB, and keeping in mind that the presence of bacteria does not equate with symptomatic UTI, urine cultures should be sent only for patients with symptoms attributable to the urinary tract (71). The key point is that accurate diagnosis through urine culture requires a high pretest probability of UTI. The specificity of urine cultures varies across different patient populations, depending on the prevalence of chronic bacterial colonization (72). Due to the high rate of ASB in patients with chronic indwelling catheters, the specificity of urine cultures is very low for diagnosis of symptomatic UTI in this population (19, 72). Similarly, the specificity of a positive urine culture for patients with spinal cord injury with catheter use (intermittent or condom catheter) for symptomatic UTI ranges from 43 to 54% (19, 72). For populations in which positive urine cultures have low specificity for symptomatic UTI (because the population has high rates of asymptomatic bladder colonization), urine cultures are more useful for antibiotic selection than for diagnosis of UTI (72). Diagnosis must rely on the clinician’s assessment of symptoms, and a positive urine culture can be a misleading finding.
New rapid diagnostic tests have been proposed as a solution to the diagnostic delays created by the typical 48-h turnaround time for urine culture results. Novel technologies, including mass spectrometry, biosensor-based platforms, and microfluidics, are being developed to rapidly detect pathogens directly from urine samples (73). Unfortunately, if such rapid diagnostic tools are mainly used to detect the absence or presence of bacteriuria (also providing identification of the organism), they may do more harm than good for outpatient stewardship programs. The identification of a named organism, particularly a uropathogen, through a rapid diagnostic test may encourage the provider to prescribe antibiotics, regardless of whether the patient has urinary symptoms. As rapid UTI diagnostics are rolled out into clinical practice, we hope that well-designed research studies will delineate their role in UTI diagnosis and antibiotic stewardship.
Differentiate UTI from STI
UTI should be differentiated from STIs such as gonorrhea, chlamydial urethritis and cervicitis, genital herpes simplex, and trichomoniasis. Evidence-based symptoms of UTI, including dysuria, urinary frequency, and urinary urgency, may overlap the clinical presentation of STIs. In the ED, adult women are often overdiagnosed with UTI and underdiagnosed with STI (39, 74, 75). A stewardship study in an academic urban ED found that 40 to 42% of female patients (aged 18 to 65) diagnosed with UTI by clinicians had unlikely or incorrect diagnoses (39). Of 245 female patients incorrectly diagnosed with UTI, 20.4% had a definite genital tract infection, and 28.2% had a probable non-genital tract alternative diagnosis. In an observational cohort study of adult women (median age 27) presenting to the ED with genitourinary symptoms, 175/264 (66.3%) were diagnosed with UTI; of these, only 84/175 (48.0%) had a positive urine culture and 91/175 (52.0%) had negative or contaminated cultures (74). Among 22 patients with a missed diagnosis of STI (no treatment for STI within 7 days of ED visit), 63.6% were inappropriately treated for UTI instead of STI (74).
Sterile pyuria is prevalent among women with STI and may lead to an incorrect diagnosis of UTI (Table 1) (76). In a retrospective review of women aged 18 to 50 who had a urinalysis and tested positive for Neisseria gonorrhoeae, Chlamydia trachomatis, and/or Trichomonas vaginalis, 394/1,052 (37.5%) had pyuria (more than 5 leukocytes per high-power field [HPF]) on urinalysis (77). Among patients with pyuria, 293/394 (74.4%) had negative urine cultures. Despite having a documented STI, 295/1,052 (28.0%) women were also prescribed antibiotics for suspected UTI, and of these, only 100 (33.9%) had positive urine cultures, defined as growth of >105 CFU/ml of a known uropathogen. Although pyuria does not distinguish between UTI and STI, other components of urinalysis may have diagnostic utility. In a cross-sectional study of sexually active females with genitourinary symptoms, positive nitrites or protein predicted UTI, and the presence of urinary leukocytes or blood predicted STI (76). These studies suggest that clinicians should inquire about vaginal discharge (a symptom more consistent with STI) and strongly consider testing for both UTI and STI in sexually active females presenting with urinary symptoms (39, 74, 76).
Differentiate UTI from Overactive Bladder and Interstitial Cystitis
Nearly 30 million adults aged ≥40 years in the United States have symptoms of overactive bladder, including 27% of men and 43% of women (78). This syndrome of lower urinary tract dysfunction is defined by symptoms, including “urgency, with or without urge incontinence, usually with frequency and nocturia,” in the absence of underlying pathology (79). Given the overlap in symptomatology, overactive bladder is often misdiagnosed as UTI (80). While dysuria is not a symptom of overactive bladder and may be suggestive of UTI, dysuria may also be seen in patients with other noninfectious conditions, including vaginal atrophy and interstitial cystitis (80). The chronicity of symptoms and limited response to previous courses of antibiotics can be important clues in the patient’s history that point away from UTI and toward overactive bladder or interstitial cystitis. In patients with chronic (>6 weeks) lower urinary tract symptoms, a negative urine culture can distinguish between interstitial cystitis or overactive bladder and UTI (80, 81).
Determine Etiology of Delirium in Older Adults
The diagnosis of UTI is particularly challenging in special populations such as persons with chronic indwelling urinary catheters, dementia, and spinal cord injury. Among older adults, UTI is often diagnosed based on nonspecific symptoms such as falls, generalized weakness, and delirium. In a recent systematic review of 22 studies on the association between confusion and UTI in older adults, no valid conclusions could be drawn from the evidence (82). The 2019 IDSA guidelines on ASB emphasize the importance of seeking nonurinary causes of delirium in elderly patients that lack localizing symptoms of UTI (19). Rather than providing immediate empirical treatment with antibiotics, stable patients with altered mental status and no systemic signs of infection should be observed while undergoing evaluation for alternative diagnoses (19, 83). The aforementioned systematic review and the IDSA guidelines together emphasize the importance of not relying on UTI as a scapegoat for nonspecific symptoms, including delirium, in older adults.
Strategies in Diagnostic Stewardship
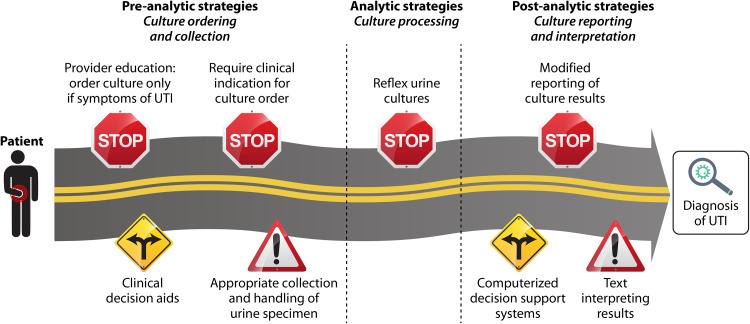
A key component of antibiotic stewardship for UTI is diagnostic stewardship, which involves “modifying the process of ordering, performing, and reporting diagnostic tests to improve the treatment of infections” (84). Since urinalysis and urine culture can be misleading, diagnostic stewardship is essential to reduce overdiagnosis of UTI and can be accomplished in three phases: (i) preanalytical phase (ordering and collection of the specimen), (ii) analytical phase (specimen processing), and (iii) postanalytical phase (reporting of the results) (Table 2) (84, 85). When a patient arrives in the clinic or ED with symptoms, there are multiple steps in the pathway between patient evaluation and diagnosis of UTI. Diagnostic stewardship strategies can have a positive impact at each step along this pathway, functioning as road signs that increase the likelihood that providers reach the correct destination (right diagnosis) (Fig. 3). A stewardship “stop sign” causes clinicians to pause to rethink a diagnosis of UTI, thus decreasing unnecessary antibiotics in patients with alternative diagnoses. A stewardship “fork in the road” helps providers to interpret clinical information and reach the right diagnosis. A stewardship “caution sign” improves the collection and interpretation of urine tests.
TABLE 2.
Strategies in diagnostic stewardship to reduce unnecessary testing, optimize collection of urine specimens, and improve providers’ interpretation of urine test results
| Strategy | Description | Advantage(s) | Disadvantage(s) | Reference(s) describing successful outpatient interventions |
|---|---|---|---|---|
| Preanalytical | ||||
| Provider education | Review processes and policies; educate providers to increase adherence to guidelines and order urine cultures only for symptomatic patients | Essential foundation | Education alone may not change behavior | 93 |
| Delayed urine culture processing | Hold cultures for up to 48 h before processing; process cultures only upon request by providers | Decreases unnecessary urine cultures and antibiotic prescriptions | Requires special media and storage of unprocessed urine specimens | 94 |
| Clinical decision aids | Use diagnostic criteria and decision rules to improve diagnostic accuracy | Decrease unnecessary antibiotics | Rigorous validation is time-consuming | 98, 99 |
| Appropriate collection of urine culturesa | Refrigerate cultures after collection; provide patients with instructions for cleansing procedures; consider straight catheterization | Decreases contamination and thus false positives | How to best avoid contamination is unclear | 106, 107 |
| Removal of urine cultures from order sets | Prechecked order sets for abdominal pain and psychiatric patients have high rates of negative urine cultures | Reduces urine cultures in patients with nonspecific symptoms | May miss opportunity to collect urine culture before antibiotics started | 117, 118 |
| Requirement of symptom-based clinical indication for culture ordering | Providers must select evidence-based symptoms before ordering urine culture | Decreases urine cultures performed and unnecessary antibiotics | Providers find ways to work around the order set | 119 |
| Analytical | ||||
| Reflex urine culturesa | Limit urine culture processing to samples that meet criteria of pyuria | Decreases unnecessary urine cultures | Will yield positive cultures for patients with ASB | 116 |
| Postanalytical | ||||
| Computerized decision support systemsa | CDSS incorporates extracted clinical information with evidence-based guidelines | Decrease treatment of ASB | Requires information technology investment and local validation; alert fatigue is likely if poorly designed | 122 |
| Modified reporting of culture resultsa | Identification and antibiotic susceptibilities are not provided unless providers call to request the culture result | Decreases treatment of ASB | Providers may not know how to access results when needed; creates an extra step for providers | 123, 124 (both studies were in inpatient setting; not studied in outpatients) |
| Interpretation of results | Text facilitates interpretation of culture results such as contaminated cultures and skin flora | Provides point-of-care education | Text interpretations are easily ignored; alert fatigue is likely if poorly designed | 125 (inpatient setting; not studied in outpatients) |
Worth future study—highly promising strategy.
FIG 3.
Diagnostic stewardship strategies for outpatient UTI. Preanalytical, analytical, and postanalytical stewardship strategies function as road signs (stop signs, caution signs, and forks in the road) that give instruction and guidance to health care providers, increasing the likelihood that providers reach the correct destination (right diagnosis). A stewardship stop sign causes clinicians to pause to rethink a diagnosis of UTI, a stewardship fork in the road helps providers to interpret clinical information and reach the right diagnosis, and a stewardship caution sign improves the collection and interpretation of urine tests.
Preanalytical Strategies
Strategies to improve diagnostic stewardship in the preanalytical phase include provider education, delayed urine culture processing, appropriate collection/handling of urine samples, requiring documentation of evidence-based symptoms, decision aids, and removing urine cultures from order sets (Table 2; Fig. 3).
Provider education: order urine cultures only for symptomatic patients.
Urine testing is frequently ordered in the outpatient setting for patients who lack symptoms of UTI (86). Abnormal urinalysis often leads to urine culture collection and initiation of antibiotics (56, 57). Among patients with ASB, unnecessary urine cultures trigger unnecessary antibiotics (56, 87, 88). In a retrospective analysis of 676 ED patients with positive cultures, 27.2% had ASB and 72.8% had symptomatic UTI (56). Among these ED patients with ASB, 60% had no indication for urine testing, and 20% were treated with antibiotics. Pyuria (>20 cells/HPF) and positive nitrites were significantly associated with antibiotic use in multivariate analyses (56). In a prospective observational study of ED patients with urinalyses, 121/195 (62.1%) had no symptoms or nonspecific symptoms (88). Positive urinalysis and positive urine culture were associated with new antibiotic prescriptions, but the presence of signs and symptoms (specific or nonspecific) was not associated with antibiotic use in a multivariable model.
Antibiotic use can be decreased by limiting urinalysis and urine culture to guideline-concordant clinical indications, including screening pregnant women, screening prior to urologic procedures, and testing nonpregnant adults with symptoms of UTI (18, 19). Although there are few studies evaluating the impact of provider education on stewardship in the outpatient setting, multifaceted interventions that include an educational component have successfully reduced urine culture ordering and overtreatment of ASB in the hospital and long-term care settings (89, 90). Drawbacks to education as a stewardship intervention are that education alone often does not change provider behavior (91), and providing education in an interactive format (more effective than passive education) requires a time investment (92).
In the ED, urine cultures are often ordered preemptively (typically as part of an order set) for nonspecific indications in an effort to increase efficiency and avoid delays in care; these preemptive culture orders and order sets represent a potential target for stewardship interventions (Table 2) (93, 94). Understanding ED staff practice patterns can uncover other opportunities for diagnostic stewardship (93, 95). For example, a single-site ED quality improvement study of ED staff workflow found the following factors that contributed to inappropriate urine culture collection: poor adherence to guidelines, outdated nursing policies, and the presence of sterile urine containers in catheterization kits (93). Collaborative thinking sessions engaging front-line ED staff resulted in behavioral and cultural changes and a reduction in the rate of urine culture ordering (93). A diagnostic stewardship intervention in a large community ED utilized a novel collection system containing a preservative that allowed cultures to be held at room temperature for up to 48 h before processing in the microbiology lab (94). Urine cultures collected in the ED were processed only if requested by ED physicians following clinical assessment of signs and symptoms of UTI. Implementation of this two-step model using delayed culture processing led to reductions in the number of processed urine cultures and antibiotic prescriptions for urinary indication among admitted patients.
Clinical decision aids (CDA).
Urine cultures often prove to be negative for women treated empirically for cystitis, thus calling into question the ability to diagnose UTI based on clinical judgment. In a study of 186 women who were prescribed antibiotics for suspected cystitis, urine cultures were negative for 39.8% (96). Decision rules and clinical scoring algorithms may improve diagnostic accuracy (97).
CDA are a recommended component of outpatient antibiotic stewardship programs (47) and can be utilized as a preanalytical strategy, to guide urine test ordering, or as a postanalytical strategy, to improve interpretation of culture results. A few UTI-focused CDA have been developed and validated in the outpatient setting. One acute cystitis decision aid that was tested in a family practice setting included three diagnostic criteria: dysuria, a positive urine for leukocytes (more than a trace amount), and urinary nitrites (any present) (96, 98, 99). Initial validation of the decision aid showed that >70% of urine cultures were positive if two or more criteria were present (98). The CDA recommended empirical antibiotics if at least two criteria were present, and culture only (with subsequent antibiotic prescription based on culture results) if one or no criteria were present. Based on a validation cohort of family physicians, the sensitivity of the CDA for positive urine cultures was 82.5%, compared to 97.6% for clinical diagnosis by physicians (99). Importantly, although the specificity of the CDA was low (35.5%), it was significantly higher than physician specificity (6.6%). Compared to usual care by family physicians, using the CDA would result in reductions in urine culture testing (64.0% total reduction), total antibiotics (20.4% absolute reduction), and unnecessary antibiotics (11.1% absolute reduction).
Appropriate collection of urine cultures.
Once a provider has decided to order a urine culture, accurate diagnosis of UTI requires appropriate collection and handling of urine samples (Table 2). The contamination rate of urine culture specimens is high in the outpatient setting, including the ED and ambulatory clinics (100, 101). The 2008 College of American Pathologists Q-Probes Study was a peer comparison quality assurance program that reviewed results for 14,739 outpatient urine culture specimens from 127 institutions (100). Overall, 15.0% of cultures were contaminated, defined as growth of more than 2 isolates at ≥10,000 CFU/ml, with an average contamination rate of 41.7% in low-performing laboratories (those in the 10th percentile for highest contamination rates). The median contamination rates were 17.3% for females and 7.4% for males. Factors associated with lower contamination rates included refrigeration of urine culture specimens after collection (rather than holding specimens at room temperature) and providing written collection instructions for male and female ED patients. A recent study in primary care clinics also found a high rate of urine culture contamination: among 678 urine cultures collected from symptomatic adults (79% female) presenting to family medicine clinics, 48.7% grew mixed urogenital flora (101). Contaminated urine cultures are often interpreted as evidence of UTI, triggering prescriptions for unnecessary antibiotics. In a study of 131 patients with contaminated urine cultures, defined as growth of at least 2 organisms from a single specimen, 44.3% received antibiotics and may not have had true infection based on urine culture (102).
For noncatheterized outpatients, a midstream clean-catch specimen is preferred to a random specimen (103). Laboratory guidelines from the IDSA and the American Society for Microbiology recommend skin cleansing prior to collection of midstream urine to reduce the risk of specimen contamination (104). However, whether cleansing makes a difference is unclear, as a recent meta-analysis found no difference in the odds of contamination between midstream urine cultures collected with or without cleansing in women (high strength of evidence) and men (low strength of evidence due to large imprecision in the data) (68). Using straight catheterization as the reference standard, there is low evidence strength that midstream clean-catch urine collection has high diagnostic sensitivity (98 to 100% in women; 82 to 100% in men) and specificity (95 to 100% in women; 92 to 100% in men) (68). A retrospective study of elderly women with ED-diagnosed UTI found that urine procurement by catheterization yielded a lower proportion of false-positive urinalysis than clean-catch specimens (105). Although catheterized urine specimens may be more accurate than midstream clean-catch specimens, catherization is not always practical or desirable (from both the patient and workload perspective) in the outpatient setting.
For patients with suspected catheter-associated UTI (CAUTI), the indwelling catheter should be replaced prior to collection of a urine specimen if the catheter has been in place for >2 weeks (21, 103). Cultures should be collected from the catheter sampling port, not the drainage port or collection bag, as the flora in the collection bag may not be representative of the organisms in the bladder.
Few interventional studies to improve urine specimen collection have been successful in decreasing rates of contamination in the outpatient and ED settings (106, 107). In a multifaceted nursing intervention that targeted outpatient and inpatient areas, practice changes included new collection instructions for patients, nursing education, and contacting the physician to change chronic indwelling catheters prior to urine collection (106). Within a year of implementation, the incidence of coagulase-negative Staphylococcus (CoNS) in urine cultures decreased, and this reduction in contaminated specimens conferred a cost savings of $35,250 per year (106). In an Australian, pseudorandomized controlled trial in the ED, providing illustrated patient instructions for urine collection decreased the contamination rate from 40% to 25% (107). However, many interventions, including novel urine collection devices (108, 109) and patient education (110, 111), failed to decrease urine contamination. These studies did not include stakeholder engagement and were not preceded by a feasibility study, a critical step in the process of intervention development (112). Future efforts to decrease urine contamination should include stakeholders most central to this process: nurses, medical assistants, and patients.
Analytical Strategies
Analytical strategies in diagnostic stewardship utilize urinalysis-based criteria to determine whether to process the urine culture (Table 2; Fig. 3).
Reflex urine cultures.
Reflex urine culture testing is a diagnostic stewardship strategy that limits urine culture processing to those urine samples that meet prespecified laboratory criteria of inflammation or presence of bacteria based on urinalysis, such as nitrites, white blood cells (WBCs), and leukocyte esterase. In the appropriate clinical population, the absence of pyuria has a high negative predictive value (NPV) for the diagnosis of UTI. Although the degree of pyuria does not differentiate between ASB and UTI, the absence of pyuria makes UTI very unlikely (19, 21, 113). In a retrospective database study of symptomatic and asymptomatic patients (>90% outpatients) with simultaneous orders for urinalysis and urine culture, 758/32,998 (2.3%) urine cultures were positive, defined as one or two predominant organisms (114). Cultures with growth of at least three different organisms were considered contaminated and excluded. The NPV of <5 WBCs per HPF for negative culture was >98% (114). Using a higher cutoff of >10 WBCs/HPF to define pyuria, the NPV of pyuria was 92.4% in an ED population with a high prevalence of bacteriuria (20% of cultures were positive) (115).
One example of reflex urine culturing is using pyuria as a screening tool prior to culture processing in patients with symptoms of UTI. In an intervention at three urban EDs, electronic medical record (EMR) order options were changed from separate orders for urinalysis and urine culture to ordering urine culture through reflex from a positive urinalysis (116). The new orders included urinalysis with reflex culture (UTI symptoms present) and urinalysis without culture (no UTI symptoms present). Among symptomatic patients, reflex urine cultures were performed if any of the following laboratory criteria was present on urinalysis: positive leukocyte esterase, positive nitrite, ≥6 WBCs, few yeasts, or moderate bacteria. Implementation of reflex urine culture ordering decreased the mean number of urine culture orders by 6.0 per 100 ED visits. Diagnostic yield improved, with a decrease in negative cultures by 2.4 per 100 ED visits postimplementation and an increase in the positive proportion of cultures from 24% to 33%.
Important caveat: order reflex cultures only for symptomatic patients.
It should be emphasized that reflex urine cultures can be impactful as a stewardship strategy only when providers order urine testing exclusively for patients with UTI symptoms. Pyuria alone should not be used to guide whether urine needs to be cultured. Among patients with ASB, pyuria is present in 32% of young women, 90% of elderly institutionalized patients, and 50 to 100% of patients with long-term catheters (18). The PPV of urinalysis for positive urine culture can be low, depending on the patient population (71, 72). In a recent study of healthy premenopausal women at high risk of recurrent UTI, pyuria was common in asymptomatic women, but the PPV of pyuria for E. coli ASB was only 4% (71). In one study of 195 ED patients (70% female; median age, 56 years), the PPV of urinalysis for positive urine culture was 38%, defining urinalysis as positive if it contained nitrites, leukocyte esterase, bacteria, or >10 white blood cells per high-power field (88).
If urinalysis with reflex culture is ordered inappropriately for asymptomatic patients, then automatic reflex to culture based on positive urinalysis may paradoxically lead to increased urine cultures and unnecessary treatment of ASB. Inappropriate urine culture testing contributes to laboratory waste and high health care costs (117). In an urban ED in Illinois, urinalysis with reflex to culture was prechecked in psychiatric and abdominal pain order sets, leading to a high rate of negative urine cultures, with 6,020/8,721 (69.0%) having no growth or nondiagnostic growth (117). Modification of prechecked order sets (with removal of prechecked urinalysis with reflex culture) and increasing the reflex to culture thresholds from >3 WBCs to >4 WBCs resulted in a significant decrease in urinalysis with reflex culture orders, from 92 to 49 orders per day (117). The rate of negative urine cultures decreased from 13 to 6 cultures per day postintervention, with an estimated cost savings of $71,350 per year. In one VA system, the antibiotic stewardship team observed that automatic reflex culture for all urinalyses with pyuria (≥8 per HPF) led to inappropriate treatment of asymptomatic patients who had an incidental finding of bacteriuria (118). After eliminating universal reflex cultures and requiring separate urine culture orders based on patient symptoms, rates of urine cultures per month decreased from 90.1 to 61.3 in the outpatient setting (118). Given that pyuria is common in patients with ASB and cannot be used to distinguish between ASB and UTI, urinalysis with reflex to a urine culture should be ordered only for patients with evidence-based symptoms of UTI.
Require a clinical indication for reflex cultures.
A promising strategy to ensure that reflex urine cultures are not ordered indiscriminately is to require the provider to choose a clinical indication (from among a menu of evidence-based symptoms) to complete the reflex order. A recent quasiexperimental before-and-after intervention in inpatients and EDs illustrated the impact of requiring symptom-based clinical indications for ordering urine tests (119). Prior to the addition of the indication requirement, providers could order urinalysis with reflex to urine culture for any patient, regardless of symptoms. A new order set in the EMR directed providers to select a reason (from among a menu of evidence-based symptoms) for ordering urinalysis with reflex to culture in patients with suspected UTI. For patients with “noninfectious indication” for urine testing, practitioners were directed to order urinalysis alone (no reflex to culture). During the same intervention period, criteria for reflex urine culture were modified from any abnormality in the urinalysis to requiring >10 WBCs/high-power field before reflexing to culture. Implementation of the new order set resulted in a 40.4% decrease in the number of urine cultures performed. Among patients with a UTI indication, antibiotic days of therapy decreased from 102.5 to 86.9 per 1,000 patient days.
These studies demonstrate the importance of incorporating evidence-based symptoms into algorithms that utilize reflex urine cultures. Symptom-based reflex urine culture algorithms may decrease the number of urine specimens that progress to culture. On the other hand, indiscriminate reflex urine culture ordering (whenever a urinalysis is desired) may increase the total number of cultures, subsequently leading to increased antibiotic use. Ultimately, the key to successful diagnostic stewardship for UTI is reducing the number of unnecessary urine cultures ordered.
Postanalytical Strategies
Once a urine culture has been processed, positive cultures often result in antibiotic prescriptions. Antibiotic stewardship strategies are needed to improve interpretation of urine culture results. Computerized decision support systems, text providing interpretation of the results, and selective reporting of antibiotic susceptibilities are postanalytical strategies that may decrease inappropriate treatment once the provider sees the urine culture result (Table 2; Fig. 3).
CDSS.
The 2016 IDSA guidelines for inpatient antibiotic stewardship interventions suggest the incorporation of computerized decision support systems (CDSS) (120). In the inpatient setting, implementation of CDSS has been associated with reduced use of broad-spectrum antibiotics and reduced antibiotic costs. Innovative machine learning models that utilize artificial intelligence may increase diagnostic efficiency and produce cost savings (121). Studies assessing the implementation of CDSS for management of UTI in the outpatient setting are scarce. A multicenter prospective interventional study in three French EDs assessed the impact of a clinical algorithm-based CDSS that incorporated diagnostic and therapeutic recommendations, individualized using patient-specific data (122). The UTI-focused CDSS led to modification of the initial diagnosis in 42/182 (23%) of cases. A UTI diagnosis was changed to ASB in 20/182 (11%) of cases when the CDSS was used. In multivariate analysis, use of the CDSS was associated with more appropriate antibiotics based on international UTI guidelines.
Modified reporting of urine culture results.
In selective or restricted reporting of urine culture results, susceptibility reports are tailored to display only the antibiotics recommended by treatment guidelines or those that the local stewardship program endorses (44). Inappropriate treatment is discouraged by hiding some components of the full microbiology report, or even limiting availability of urine culture results unless requested by providers. A randomized, parallel, unblinded trial assessed the impact of a laboratory reporting intervention on inappropriate treatment of ASB (123). Inpatient urine cultures were randomized to modified reporting versus standard reporting. Standard reporting provided bacterial identification and a wide range of antibiotic susceptibilities. In the modified report, providers were informed that bacterial growth was detected, but identification and antibiotic susceptibilities were not provided unless providers called for the full report. For 110 randomized cultures, 44/55 (80.0%) patients in the modified reporting arm versus 29/55 (52.7%) in the standard reporting arm received appropriate treatment, defined as no antibiotics for ASB or any antibiotic for UTI. In another nonrandomized before-after study of noncatheterized inpatients, positive culture results were not reported unless a request was made by telephone call (124). Compared to contemporary catheterized patients that served as controls, the rate of inappropriate treatment of ASB in noncatheterized patients decreased significantly, from 15/31 (48.4%) to 4/33 (12.1%) after implementation of modified culture reporting. Although these proof-of-concept studies did not include outpatients, the stewardship strategy of modified reporting is very likely applicable to the outpatient setting.
Interpretative reporting of culture results (text interpreting results).
Another strategy for modified reporting of cultures is interpretative reporting, or the inclusion of text that facilitates correct interpretation of results (125). For example, some laboratories include a note stating that “three or more organisms represent contamination.” For urine cultures that grow skin flora, a note may be added that “coagulase-negative staphylococci and diphtheroids are not uropathogens.” Clinical laboratories may also consider hiding the antibiotic susceptibilities of organisms like coagulase-negative staphylococci to decrease the clinician’s interest in treating this organism.
RIGHT DRUG
Patients with suspected UTI should be prescribed the right drug at the point of care, ideally one that will kill the causative pathogen but is not an excessively broad-spectrum agent. These empirical prescribing decisions can be guided by local resistance patterns, side effects, and patient-specific factors, including drug allergies, renal function, previous culture susceptibilities (individual antibiogram), and drug-drug interactions (52). In the 2010 IDSA international clinical practice guidelines, trimethoprim-sulfamethoxazole (TMP-SMX), nitrofurantoin, and fosfomycin were recommended as first-line empirical therapy for acute uncomplicated cystitis in women (22), although these guidelines are currently undergoing update. TMP-SMX was recommended as empirical therapy if local resistance rates of pathogens causing uncomplicated cystitis do not exceed 20%. For acute pyelonephritis in patients not requiring hospitalization, treatment options include oral TMP-SMX for susceptible isolates or oral ciprofloxacin as empirical therapy if the local prevalence of resistance does not exceed 10%. For empirical treatment of patients in areas with a high prevalence of TMP-SMX or fluoroquinolone resistance, an initial intravenous (i.v.) dose of ceftriaxone or an aminoglycoside is recommended (22).
Limitations of Treatment Guidelines
Importantly, the current guidelines focus on the treatment of nonpregnant, premenopausal women with uncomplicated UTI, in the absence of known urological abnormalities or comorbidities (22). They do not address clinical management of UTI in pregnant or postmenopausal women, recurrent cystitis, or UTI in males. Despite the significant burden of UTI in postmenopausal women, men, and patients with urological abnormalities, optimal treatment in these groups is not well defined because many clinical trials have excluded such subjects. The heterogeneity of complicated UTI and the narrow scope of current guidelines, as dictated by available evidence, create inherent challenges to antibiotic stewardship in the outpatient setting. Treatment options must be individualized, based on the patient’s comorbidities, age, sex, and local resistance patterns.
Fluoroquinolone Risks and Overuse
Although fluoroquinolones are effective for treatment of infection with susceptible organisms, fluoroquinolones should be reserved for more invasive infections when alternative therapies are not applicable (22, 65, 126). Boxed warnings from the U.S. Food and Drug Administration (FDA) highlight serious potential risks associated with fluoroquinolones, including tendinitis and tendon rupture, peripheral neuropathy, central nervous system effects, exacerbation of myasthenia gravis, and possibly aortic aneurysm (126, 127). In addition to the risks outlined by the FDA, fluoroquinolone exposure significantly increases the risk of community-acquired Clostridioides difficile infection (128), although this problem is relevant to many other classes of antibiotics as well. Outpatient fluoroquinolone treatment for UTI also contributes to the emergence and spread of resistant strains of Enterobacteriaceae, both in treated patients and in their household contacts (129, 130).
Despite high rates of resistance and the potential for serious and irreversible side effects, fluoroquinolones remain one of the most frequently prescribed antibiotic classes for acute uncomplicated cystitis in the outpatient setting (26, 28, 131–134). Between 2006 and 2010, 47.3% of ED visits for UTI and 35.4% of clinic UTI visits included broad-spectrum fluoroquinolones in the United States (32). More recent ambulatory prescription data from 2014 revealed that fluoroquinolones were prescribed for 40.3% of uncomplicated UTI and 74.3% of complicated UTI, accounting for more prescriptions than any other antibiotic class (132). Of 31.5 million outpatient fluoroquinolone prescriptions dispensed in 2014, 15% were for uncomplicated UTI (132). Retrospective cohort studies showed that fluoroquinolone prescribing for uncomplicated UTI did not significantly decrease after the recent 2016 FDA expanded boxed warning (135, 136), demonstrating the need for active interventions to improve outpatient UTI treatment.
Choosing the Right Drug in the Era of Increasing Antibiotic Resistance
Since the publication of the last IDSA guidelines in 2010, antibiotic resistance among uropathogens has increased (15, 137–139). In the setting of widespread antibiotic misuse and overuse, rising resistance rates are a major threat to successful outpatient treatment of acute uncomplicated cystitis and pyelonephritis. Using data from The Surveillance Network in the United States, susceptibility patterns were analyzed for 305,749 urinary isolates from female outpatients in 2012, of which 64.9% were E. coli (138). Between 2003 and 2012, the prevalence of E. coli TMP-SMX resistance increased from 17.2% to 22.2% in isolates from adults aged 18 to 64 years and from 18.5% to 26.7% in isolates from older adults (≥65 years). E. coli resistance to ciprofloxacin increased from 3.6% in 2003 to 11.8% in 2012 among isolates from adults and from 11.8% to 29.1% among isolates from older adults.
In contrast to rising resistance rates among other treatment options, fosfomycin and nitrofurantoin remain effective against most uropathogens (139–142). National data on fosfomycin susceptibilities are limited, as this drug is absent from the standard susceptibility test panel, so susceptibility tests are rarely performed or reported (138). Based on surveillance data among urine isolates from U.S. female outpatients in 2012, E. coli resistance to nitrofurantoin and fosfomycin was low (0.9% and 4.2%, respectively) (138). A recent UTI-focused antibiogram for a diverse urban population revealed that 100% of tested Gram-negative isolates were susceptible to fosfomycin, and E. coli susceptibility to nitrofurantoin was 96.1% (101).
Although fosfomycin is available, FDA approved, and effective, its use in the United States in outpatient settings is extremely low (27, 28). In a study of national outpatient antibiotic prescribing practices for uncomplicated UTI, fosfomycin was prescribed in only 0.01% of visits, both before and after the release of the 2010 IDSA guidelines (27). Fosfomycin was not prescribed in any of 1,546 visits for uncomplicated cystitis in 2 private family medicine clinics in the period from 2011 to 2014 (28). Barriers to fosfomycin use in the United States include its exclusion from the standard antibiogram, high cost, limited insurance coverage of costs, and provider unfamiliarity with fosfomycin as a first-line treatment option (64).
Outpatient Treatment of ESBL-Producing Enterobacteriaceae
Multidrug resistance, including production of extended-spectrum beta-lactamases (ESBLs), is also increasingly prevalent among community-associated Enterobacteriaceae worldwide (143–148). In a recent national cohort study of 890 U.S. hospitals in the period from 2012 to 2017, the incidence of infections decreased for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multidrug-resistant (MDR) Pseudomonas aeruginosa (147). However, between 2012 and 2017, the incidence of infections due to ESBL-producing Enterobacteriaceae increased from 37.6 to 57.1 cases per 10,000 hospitalizations, with a 5-year change of 64.1% in community onset infection versus 4.1% in hospital-onset infection (147). While antibiotic stewardship efforts are making headway, concerted effort is needed to control the spread of resistance in the organisms most likely to cause UTI.
ESBL production by the organism causing UTI is a risk factor for treatment failure (143), at least in part because outpatient treatment options for UTI due to ESBL-producing uropathogens are limited. Outpatient management often requires outpatient parenteral antimicrobial therapy (OPAT) with carbapenems such as ertapenem. Risks of OPAT are well described and include vascular access complications such as phlebitis, infection, thrombosis, infiltration, and line dislodgement (149, 150). Although data are limited, fosfomycin (151–157) and nitrofurantoin (158–160) may be effective oral alternatives for outpatient treatment of lower UTI due to ESBL-producing uropathogens (161). In the 2020 IDSA treatment guidelines for antimicrobial-resistant Gram-negative bacterial infections, nitrofurantoin and trimethoprim-sulfamethoxazole are recommended as preferred treatment for uncomplicated cystitis due to ESBL-producing Enterobacteriaceae, assuming that in vitro susceptibility has been confirmed (162). Amoxicillin-clavulanate, single-dose aminoglycosides, and oral fosfomycin (for E. coli only) are listed as alternative agents in the guidelines (162). Fosfomycin was noninferior to intravenous ertapenem for outpatient UTI due to ESBL-producing organisms in a retrospective cohort study (153). An important caveat to interpreting the results of that study is that 83.7% of patients had E. coli UTI and only 14.6% had infection due to Klebsiella spp. The fosA gene, which confers intrinsic resistance to fosfomycin, is rarely identified in E. coli but is widely distributed among non-E. coli Gram-negative bacteria, including Klebsiella, Enterobacter, and Pseudomonas (163). Additionally, nitrofurantoin and oral fosfomycin should not be used in patients with suspected or confirmed pyelonephritis because these drugs may not achieve therapeutic tissue levels (162). The bottom line is that when patients have risk factors for ESBL-producing urinary organisms, a urine culture is essential to guide antibiotic choice, both for the immediate need and for future UTI in the same patient (164).
Strategies To Improve Drug Choice
Tools for prediction of antibiotic resistance.
Opportunities for stewardship interventions related to empirical drug choice include resistance prediction scores, local susceptibility reports, selective and cascade reporting of culture results, and postprescription culture review to target drug-bug mismatch (Table 3). Empirical antibiotic selection for outpatient UTI may be optimized by clinical tools for prediction of antibiotic resistance. In a case-control study of 351 patients with community onset UTI in South Carolina, 20% of isolates were resistant to TMP-SMX (165). A multivariate model identified predictors of TMP-SMX resistance, including prior infection or colonization with TMP-SMX-resistant Enterobacteriaceae and TMP-SMX use within the past 12 months. Similarly, a score model for fluoroquinolone resistance accurately predicted the probability of fluoroquinolone resistance among outpatients and inpatients diagnosed with complicated UTI (166). In this model, risk factors for fluoroquinolone resistance included male sex, diabetes mellitus, residence in a skilled nursing facility, and prior fluoroquinolone use within the past 12 months. A fluoroquinolone resistance score of ≥3 had a PPV of 56% and NPV of 90%. Accurate empirical treatment of MDR UTI can be increased by selecting an antibiotic based on previous microbiological data within 6 months to 2 years from the current infection (164). In a VA database study, empirical treatment for MDR UTI was accurate in 40/52 (76.9%) patient episodes if concordant with the previous microbiological data, versus 14/43 (32.6%) if empirical therapy was discordant from prior results (164).
TABLE 3.
Strategies to choose the right drug for urinary tract infection
| Strategy | Description | Advantage(s) | Disadvantage(s) | Reference(s) describing successful outpatient interventions |
|---|---|---|---|---|
| Guideline implementationa | Multifaceted interventions to educate providers on current treatment guidelines | Guide antibiotic choice using evidence-based recommendations; decrease fluoroquinolone prescribing | How to best implement guidelines in outpatient settings is unclear | 39, 181, 182, 188, 189 |
| Tools for prediction of antibiotic resistancea | Select empirical antibiotic based on previous microbiological data and prior antibiotic use | Increase accuracy of empirical choice | May require local validation | 164 |
| Local susceptibility report and stratified antibiogramsa | Stratify local antibiograms by patient location (outpatient vs inpatient), age, infection site, or specific patient groups | Increase accuracy of empirical choice | Representative outpatient antibiograms are typically unavailable; requires resources to generate antibiogram | 169–172, 182 |
| Selective and cascade reporting | Limit the no. and type of susceptibility results that are released in culture reports | Decreases use of broad-spectrum agents | Unlikely to decrease overall antibiotic use | 176, 178 |
| Postprescription culture review | Pharmacist or provider reviews culture results and modifies the treatment plan | Corrects drug-bug mismatches; may decrease treatment failure | Labor-intensive; results may not be available until after completion of antibiotic course | 184, 186 |
Worth future study—highly promising strategy.
Local susceptibility reports and stratified antibiograms.
Given rising rates of resistance, utilization of local susceptibility reports (antibiograms) is increasingly important for empirical treatment of UTI in the ambulatory setting. When feasible, stratified antibiograms should be used to tailor appropriate empirical antibiotics (120). If at least 30 isolates are available for each organism, antibiograms can be stratified by multiple variables, including patient location (inpatient, outpatient, ED, or ward), gender, age (pediatric or adult), or infection site (blood, urine, or respiratory) (120). Depending on local resistance patterns, demographics, and other clinical risk factors, urinary antibiograms may differ significantly between inpatients and outpatients and between specific patient groups (101, 167). In a study of urinary pathogen susceptibility patterns at a community ED in California, urinary E. coli antibiograms were stratified by gender, age, and residence prior to admission (home versus skilled nursing facility) (168). Among 145 ED isolates, susceptibilities for ciprofloxacin and TMP-SMX were both under 80% (71% and 66%, respectively), and ESBL was detected in 11/145 (7.6%). Compared to the hospital-wide antibiogram that excluded outpatients, the ED-specific E. coli antibiogram showed lower susceptibilities to TMP-SMX (66% in ED versus 74% hospital-wide) and cefazolin (67% in ED versus 86% hospital-wide). In the ED antibiogram, fluoroquinolone susceptibility was also lower among urinary isolates from patients aged ≥65 years and those residing in skilled nursing facilities.
If available, antibiotic stewardship interventions can use stratified antibiograms to improve guideline-adherent treatment of UTI (169–171). A stewardship intervention in a large ED used an ED-specific antibiogram to implement institution-specific guidelines for cystitis and pyelonephritis (169). After deployment of the antibiograms, antibiotic prescriptions for recommended first-line agents increased from 44.8% to 83%, largely through an increase in nitrofurantoin use for cystitis. Similarly, ED physician education on local urinary pathogen resistance patterns led to changes in their prescribing behavior (171). In that study, physician education included emails with antibiotic resistance data and recommendations to consider nitrofurantoin for uncomplicated cystitis. Postintervention, prescriptions for TMP-SMX and ciprofloxacin significantly decreased, while nitrofurantoin prescriptions increased from 20% to 30% (P = 0.003). Prescriptions for ineffective antibiotics (bug-drug mismatch) decreased from 7.6% to 4.1% (odds ratio [OR], 0.51; 95% confidence interval [CI], 0.17 to 1.52).
A clinic-specific antibiogram can also be the foundation of a CDSS tool. Significant improvements in antibiotic prescribing practices in a family medicine clinic were observed after implementation of a clinical decision support tool that took into account a clinic-specific antibiogram (172). When the CDSS was used, empirical use of nitrofurantoin increased, while ciprofloxacin and TMP-SMX prescriptions decreased. Although utilization of the tool clinic-wide was only 29%, the overall rate of fluoroquinolone prescribing for uncomplicated UTI decreased from 42% to 15% after implementation. This study suggests that combining both CDSS and local antibiograms into an antibiotic stewardship intervention may be impactful.
Despite the utility of stratified antibiograms, at this time resources are inadequate in most outpatient settings to provide a urinary outpatient-specific antibiogram. Antibiograms in the outpatient setting may also be subject to selection bias given that outpatient urine cultures are typically obtained from patients with complicated UTI, relapse, or suspected multidrug-resistant infection. This sampling bias may yield an outpatient antibiogram that demonstrates higher resistance rates than are present in the general community population with uncomplicated cystitis.
Selective and cascade reporting.
Guidelines from the IDSA and CDC recommend that antibiotic stewardship programs use selective and cascade reporting of antibiotic susceptibility reports to reduce inappropriate use of broad-spectrum antimicrobials (44, 120). As a tool for antibiotic stewardship, selective reporting limits the number and type of antibiotic susceptibility results that are routinely released in culture reports. In other words, if the laboratory’s automated testing checks 20 antibiotics for their ability to inhibit a specific organism, only 15 of the antibiotics may be reported, to nudge providers toward use of these 15 agents (and presumably away from newer, very-broad-spectrum antibiotics). A cross-sectional survey by ESCMID revealed that selective reporting was implemented or partially implemented in only 15/36 (41.7%) of European countries that completed the survey (173). In the inpatient setting, selective reporting for uropathogens has been shown to decrease targeted antibiotic consumption and improve Gram-negative susceptibility (174).
Selective reporting has been shown in several studies to improve appropriate treatment of UTI in the outpatient setting. A cohort study in the United Kingdom found that general practitioners were more likely to prescribe nitrofurantoin in areas where laboratories reported nitrofurantoin susceptibilities versus those that did not include nitrofurantoin susceptibilities in their reports (175). In a randomized controlled clinical case vignette study, general practice residents selected intended antibiotic prescriptions based on UTI vignettes with susceptibility results and were randomized to two groups: a control group evaluated cases with full susceptibility data, and an intervention group received cases with selective reporting of cultures (176). Intended antibiotic choices were more appropriate in the intervention group with selective reporting of antibiotic susceptibility data than in the control group (176). Another randomized controlled case vignette study assessed the impact of selective reporting on the intended treatment of UTI by French general practitioners (177). Based on four case vignettes of community-acquired UTI, selective reporting of susceptibilities for uropathogens significantly increased the rate of guideline-adherent treatment while decreasing prescriptions of broad-spectrum antibiotics such as amoxicillin-clavulanate, fluoroquinolones, and cephalosporins (177). A prospective study also demonstrated that changes in antibiotic susceptibility reporting had a direct effect on choice of antibiotic prescribed for UTI by primary care physicians (178). However, the impact of selective reporting of antimicrobial susceptibilities of urinary pathogens may be muted in primary care, where empirical prescriptions are typically given before culture results are available.
Interventions targeting fluoroquinolone usage.
Multiple stewardship interventions, including education, stratified antibiograms, and selective reporting, have been successful in decreasing outpatient fluoroquinolone usage (170, 179–181). An ED-based intervention used pharmacist-led provider education and an ED-specific antibiogram to decrease fluoroquinolone use for the treatment of acute uncomplicated cystitis (182). Another stewardship intervention utilized local susceptibility data to create a best-practice algorithm for treatment of uncomplicated UTI in outpatients seen in the ED (170). After education of emergency physicians, ciprofloxacin use decreased from 32% to 11% and nitrofurantoin use increased from 30% to 50% of UTI cases. A region-wide stewardship intervention in France implemented prescription guidelines and used education of general practitioners to restrict fluoroquinolone prescribing for UTI (181). Through this multimodal approach, nitrofurantoin and fosfomycin prescriptions increased, while norfloxacin prescriptions decreased by 9.1%.
Postprescription culture review to select the right drug.
Postprescription culture review has a role in addressing drug-bug mismatch and in achieving de-escalation (addressed below). ED pharmacists play an important role in optimizing treatment of outpatient UTI in the ED population, particularly through follow-up of cultures after patients are discharged from the ED (183–187). A retrospective review of ED urine cultures revealed that 42/180 (23.3%) of empirical discharge antibiotics were inappropriate; cultures from 31 patients grew organisms that were resistant to the prescribed empirical antibiotic (drug-bug mismatch) (184). After review of cultures and clinical data by the ED pharmacist, 83% of inappropriate treatment plans were corrected through intervention by the ED pharmacist, which meant that the ED pharmacist had to notify the ED physician, create a modified treatment plan, prescribe a new antibiotic, and notify the patient.
Outpatient culture review and intervention may also be performed by infectious diseases pharmacists. In a prospective study of ID pharmacist-led review of outpatient cultures, 194/965 (20.1%) of antibiotic prescriptions required intervention, of which 42.3% were from the ED and 38.7% were from primary care (186). Drug-bug mismatch was more common among Gram-negative and mixed cultures. Of 194 interventions, 138 (71.1%) were for UTI, and the treating provider accepted the recommended changes in 76.8% of UTI encounters. Overall, when interventions were accepted, pharmacist intervention was associated with decreased rates of 30-day treatment failure and admission. Although these are effective interventions, the downside to postprescription culture review and subsequent intervention is that such activities require substantial time from the stewardship team.
Multifaceted outpatient interventions.
Multifaceted interventions have been successful in the ED setting (39, 188). A collaborative ED stewardship intervention used a multidisciplinary working group of pharmacists and physicians to develop an ED UTI treatment algorithm based on national guidelines and local resistance rates (188). Implementation of the algorithm was multifaceted, including dissemination through a pocket card, an educational campaign, and case-based audit and feedback. After implementation, empirical nitrofurantoin use increased and was associated with reduced 30-day return visits; bug-drug mismatches remained stable. In another ED-focused stewardship intervention, fluoroquinolone prescriptions for uncomplicated cystitis decreased from 44% (n = 200) to 13% (n = 200) after implementation of an electronic UTI order set followed by a 2-month period of audit and feedback (39). In a family medicine setting, a multifaceted intervention including case-based audit and feedback and a clinical decision aid increased the adherence to guidelines for antibiotic choice and duration for uncomplicated cystitis (189). The audit and feedback intervention incorporated individualized, active education of primary care providers to improve prescriptions for the right drug with right duration (189).
RIGHT DOSE AND DURATION
Appropriate dosing and duration are key targets of antibiotic stewardship for UTI. Importantly, recommendations for dose, dose interval, and antibiotic duration vary between national UTI guidelines in Europe and the United States (21, 22, 190). Both underdosing and excessive duration of therapy may contribute to the emergence of resistance, although the evidence base to support this concern is limited (191–193). Longer durations of therapy increase the risk for adverse side effects and Clostridioides difficile infection (194, 195).
Compared to longer duration of therapy, shorter courses of antibiotics have clinical efficacy for treatment of acute cystitis in women (66, 196), including older women (195). In a meta-analysis of 32 trials for uncomplicated cystitis in adult nonpregnant women, symptomatic failure rates were similar between 3 days and ≥5 days of therapy for fluoroquinolones, beta-lactams, and TMP-SMX (194). Although prolonged therapy was associated with lower risk of long-term bacteriological failure, patients who received prolonged therapy had more adverse effects overall. Among elderly women with uncomplicated symptomatic lower UTI, a Cochrane database systematic review showed no difference in efficacy between short-course (3 to 6 days) and long-course (7 to 14 days) therapy (197). Although the optimal duration of therapy for men with UTI is less clear, there are limited data to support shorter courses for male UTI (198, 199). In a recently published randomized controlled trial, afebrile men with UTI were randomized to 7 versus 14 days of TMP-SMX or ciprofloxacin (200). Symptom resolution occurred in 122/131 (93.1%) who received 7 days versus 111/123 (90.2%) who received 14 days of therapy (difference, 2.9% [1-sided 97.5% CI, −5.2 to ∞]), confirming noninferiority (200). Notably, most European guidelines still recommend a longer duration of 7 to 14 days for male UTI (190).
Duration Errors
Multiple studies have demonstrated that PCPs and ED physicians prescribe longer durations of antibiotics than recommended for uncomplicated cystitis (27, 28, 201–203). In a retrospective cohort study of outpatient and ED encounters for UTI in 654,432 younger nonpregnant women, more than 75% of antibiotic prescriptions were written for guideline-discordant treatment durations (longer courses) (27). Fluoroquinolones were prescribed in 284,744/665,120 (43%) of cases; of these, 35% received 7 days of antibiotics. Similarly, 31% of TMP-SMX prescriptions and 66% of nitrofurantoin prescriptions were written for 7 days, despite guideline-recommended durations of 3 days and 5 days, respectively. In an outpatient cohort of 1,845 women with acute cystitis, diabetics received longer treatment courses, with duration of >5 days in 119/150 (79.3%) of postmenopausal diabetic women versus 370/571 (64.8%) of those without diabetes (201). In that study, treatment duration of >5 days was independently associated with higher risk of early UTI recurrence, but not late recurrence, in multivariate analyses.
Dosing Errors
Dose errors have been described for fluoroquinolones, nitrofurantoin, and trimethoprim-sulfamethoxazole used to treat outpatient UTI (204). Inappropriate dosing includes incorrect adjustment for renal function and guideline-discordant doses (both higher and lower than recommended). A prospective observational cohort of outpatient VA patients showed that among 193 UTI cases, the correct dose was prescribed in 70.5%, with the correct duration of antibiotics selected in only 52.9% (205). In that study, among 138 ciprofloxacin prescriptions for any type of infection (mostly UTI and prostatitis), the dose was incorrect in 24% of cases. In a prospective study of antibiotic prescriptions for UTI in France, 16/185 (8.6%) of patients were prescribed antibiotics with guideline-discordant dosage and/or duration (204). Underdosing of ciprofloxacin and nitrofurantoin was also observed, including dosages at half the recommended dose.
Strategies To Improve Dose and Duration
Provider education with feedback.
Antibiotic stewardship strategies that target dosing and duration include CDSS tools, electronic order sets, and education with audit and feedback (Table 4). The European Drug Education Project randomized general practitioner peer groups in the Netherlands to an educational program for asthma or UTI (206). The educational intervention included self-learning and case-based feedback, with a focus on prescribing short courses of antibiotics in the UTI program. Based on prescribing data before and after the intervention, the average duration of treatment decreased from 6.07 days to 4.29 days per prescription postintervention. A similar randomized controlled trial in Norway found a significant reduction in duration of UTI treatment after the feedback intervention (207).
TABLE 4.
Strategies to prescribe the right dose and duration of therapy for urinary tract infection
| Strategy | Description | Advantage(s) | Disadvantage(s) | References describing successful outpatient interventions |
|---|---|---|---|---|
| Provider education | Self-guided learning, case-based audit with feedback | Essential foundation | Education alone may not change behavior; can be time-consuming | 39, 189, 206, 207 |
| Computerized decision support | Embeds defaulted dose and duration in electronic medical record; electronic order sets | Provides point-of-care guidance; once in place, less effort needed to maintain and update | Providers may find work arounds to poorly designed CDSS; requires upfront time investment | 39, 208, 209 |
Computerized decision support systems to improve antibiotic dose and duration.
Well-designed EMR-based CDSS is an excellent option to improve antibiotic dose and duration for UTI. A multicenter, pre-/postintervention assessed the impact of an EMR-based CDSS that utilized defaulted dose and dispense quantities for common infections (including UTI) in the ED (208). Among 10,921 patients, UTI was the most common indication for antibiotics (60% preintervention and 56.9% postintervention). Utilizing the CDSS, guideline-concordant antibiotic dosage improved from 79.3% to 92.7% (P < 0.001), and correct duration increased from 38.5% to 71.1% (P < 0.001). In another quasiexperimental study that targeted treatment of women with uncomplicated cystitis in primary care, the addition of prefilled default prescribing instructions in the EMR resulted in increased adherence to recommended durations from 231/787 (29.4%) to 658/862 (76.3%), and the average duration of therapy decreased by 1.5 days (209). Multifaceted stewardship interventions can further boost the impact of the CDSS. For example, ED physicians’ adherence to the recommended duration of treatment for uncomplicated UTI increased from 62% to 75% after implementation of an electronic order set, and adherence increased further from 75% to 88% after audit and feedback (39). One advantage of CDSS is that after the initial time investment for CDSS design, the strategy does not require considerable effort to maintain. If designed well, the CDSS can streamline clinician workload while also promoting guideline-concordant prescribing.
DE-ESCALATION
De-escalation of antibiotics is a pillar of inpatient antibiotic stewardship. Antibiotics may be stopped or narrowed in response to culture results, susceptibility testing, and clinical response (44, 120). Hospital-based interventions targeting antibiotic de-escalation were successful in decreasing median hospital stay (210) and the use of total and broad-spectrum antibiotics (211). In the outpatient setting, de-escalation is more challenging given that acute uncomplicated cystitis is often treated empirically without urine culture testing. For patients in whom pretreatment urine culture is indicated (pyelonephritis, complicated UTI, CAUTI, or suspected resistant organism), empirical therapy should be de-escalated to pathogen-directed therapy based on culture results and susceptibilities (212). In the setting of negative culture growth, the diagnosis of UTI should be re-evaluated. A retrospective review of 153 elderly women with ED-diagnosed UTI found that only 87 (57%) had UTI confirmed by cultures (105). Of 66 patients with negative cultures, 95% received antibiotics, meaning that these women’s ED visits represented opportunities for de-escalation.
Strategies To Improve De-escalation
Overtreatment of presumed UTI may be corrected through postprescription review of cultures (Table 5). In a prospective study of outpatient (primary care and ED) antibiotic utilization, pharmacist-led culture review identified 194 encounters (the majority of which were UTI) that required intervention for inappropriate initial antibiotic choice (186). Based on culture results, pharmacists recommended discontinuation of therapy in 65/194 (33.5%) of encounters that required intervention. Notably, recommended interventions were accepted by providers more often when the pharmacist’s recommendation was to change to a different antibiotic or modify dose/duration, rather than stopping antibiotics altogether. Cultures were negative in 23/148 (15.5%) of encounters with accepted interventions versus 13/46 (28.3%) of those with rejected interventions. A major limitation of de-escalation as a stewardship strategy for outpatient UTI is that by the time culture results are available and the patient can be contacted, the patient often has already completed the course of antibiotics. The time commitment required from pharmacists to achieve de-escalation is also substantial. Given these limitations, UTI-focused stewardship interventions may derive more benefit through prioritizing upfront interventions, such as CDSS, rather than backwards-looking strategies, such as de-escalation.
TABLE 5.
Strategies to de-escalate antibiotics for urinary tract infection
| Strategy | Description | Advantage | Disadvantages | Reference describing successful outpatient interventions |
|---|---|---|---|---|
| Postprescription culture review | Narrow antibiotics based on culture results; stop antibiotics if results of urinalysis or urine culture is negative | May decrease unnecessary antibiotics | Labor-intensive; results may not be available until after completion of antibiotic course; providers may not accept stewardship team recommendations | 186 |
NUDGING IN ANTIBIOTIC STEWARDSHIP
Several antibiotic stewardship interventions discussed in this review take advantage of the concepts of behavioral nudging and human factors engineering (213–215). As a theory in behavioral economics, a nudge is “when the choice architecture is designed to influence behavior in a predictable way but without restricting choice” (215). The principles of behavioral economics and nudging have been applied in diverse industries, including marketing and government. By guiding decision making while maintaining prescriber autonomy, nudging can be used to improve health care delivery, quality, and efficiency (213). In antibiotic stewardship, nudging can be utilized by clinical microbiology laboratories and embedded in the design of EMRs. For example, providers can be “nudged” to select guideline-concordant antibiotics by presenting recommended options at the top of the order options or by hiding options that are less desirable from a stewardship point of view (213). Interpretative reporting, selective or cascade reporting, and CDSS are all examples of nudging strategies that may be used to improve guideline-concordant treatment of outpatient UTI (213, 214). Other nudging approaches focus on counteracting risk perceptions and erroneous prescribing norms through peer comparison, in which providers are informed of their performance in antibiotic prescribing compared to their peers (216). In primary care settings, several randomized trials showed significant improvement in appropriate antibiotic prescribing through nudging, but all were focused on respiratory infections (217–219). Although observational studies have demonstrated successful outcomes through nudging approaches for outpatient UTI (123–125, 175, 178), prospective, randomized trials are needed to understand the impact of nudging strategies in stewardship for outpatient UTI.
CONCLUSIONS, CHALLENGES, AND FUTURE DIRECTIONS
In this review, we have focused on opportunities and strategies to achieve antibiotic stewardship for UTI in outpatient settings, chiefly primary care practices and EDs. While much of the literature on antibiotic stewardship is focused on the inpatient setting, rising antimicrobial resistance in the community has driven the need to develop robust outpatient stewardship programs. In the last 5 years, CDC guidelines and accreditation requirements marked the advent of the era of outpatient antimicrobial stewardship (47, 48). In this new era, the 5-Ds model provides a framework for targeting key outcomes in antibiotic stewardship for UTI (Fig. 2).
To summarize our key recommendation, arriving at the right diagnosis is the step that offers potentially the greatest benefit in terms of antibiotic stewardship. Unfortunately for both clinicians and patients, accurately diagnosing “simple” UTI in the outpatient clinic or ED is anything but simple. UTI must be differentiated from ASB, STI, and other noninfectious conditions in a short time span using diagnostic tools that are either nonspecific (urinalysis) or not timely (urine culture). Rapid diagnostic tests on the horizon offer the promise of being able to rule out UTI (no bacteriuria) or help narrow antibiotic choice (providing rapid data on susceptibilities), but they will not be able to substitute for clinician judgment on whether the patient’s presentation is consistent with UTI. Clinician awareness that all genitourinary complaints are not UTI is essential to prevent overdiagnosis and overtreatment of UTI.
Numerous programs have developed innovative tools and interventions that successfully improved antibiotic use for UTI in one or more of the 5Ds of antibiotic stewardship. Many of these interventions relied on action and effort from dedicated health care professionals, particularly pharmacists. One reason that a personalized, in-person approach is so vital to successful antibiotic stewardship in outpatient settings is the providers’ pervasive fear of sending their patients out with inadequate treatment for UTI (64). Person-to-person communication from a trusted colleague is one means to overcome this fear of withholding antibiotics or of choosing a drug perceived as “weaker” (such as nitrofurantoin). Unfortunately, sustainability becomes a challenge with any labor-intensive intervention that requires extra, often unpaid, effort from health care professionals.
Many aspects of the 5 Ds of antibiotic stewardship for UTI can and should be supported by information technology. CDSS can improve diagnostic accuracy, reduce unnecessary urine culture orders, and support choice of the optimal drug and duration. Information technology can be used to create clinic- or ED-specific antibiograms for local urinary pathogens, thus reducing drug-bug mismatch. Upfront investment in the software and information technology needed to support antibiotic stewardship seems well justified by the potential benefits, particularly as automation can reduce the burden on the antibiotic stewards.
Future research should address some of the key gaps in knowledge uncovered by this review. Studies of the comparative effectiveness of in-person approaches versus automation via the EMR and CDSS would be welcome, particularly as randomized controlled trials. Rapid diagnostics for UTI have great potential to improve drug choice but could also lead to overdiagnosis and overtreatment of UTI in patients who actually have ASB. Clinical studies of the sensitivity and specificity of rapid diagnostics for clinically diagnosed UTI in various outpatient populations (young women, older women, older men, patients with dementia, etc.) will be essential to determine their role as diagnostic tools. EMR-based nudges and CDSS are helpful only if they are intuitive to use and perceived positively by the end user. How to optimize and customize EMR-based approaches to support antibiotic stewardship for outpatient UTI will be a fruitful area for research discovery for years to come. Other areas for future research include the effectiveness and safety of selective reporting and de-escalation of antibiotics for outpatient UTI.
Another important gap that undermines outpatient antibiotic stewardship for UTI is the lack of specific or validated performance measures relevant to this clinical issue. The Joint Commission antibiotic stewardship standards for ambulatory care include a requirement that the organization “sets at least one annual antimicrobial stewardship goal” (48). However, specific performance metrics to target for improvement might help organizations focus their stewardship efforts, and allotment of resources to support stewardship might become a higher priority. Ideally, performance metrics would address multiple clinical scenarios in which antibiotics are typically overused, as well as drill down on specific misused antibiotics (e.g., unnecessary antibiotics for ASB, inappropriate fluoroquinolone use, and excess duration of therapy) (220). For example, a recent multihospital cohort study developed a multifaceted metric for antibiotic overuse after discharge in patients treated for pneumonia or UTI (220). In that model, unnecessary antibiotics and excess duration contributed to over half of fluoroquinolone overuse. Additional research is needed to develop and validate comprehensive performance metrics for outpatient antibiotic stewardship.
We hope that this comprehensive summary of the successes and challenges in antibiotic stewardship for outpatient UTI will guide providers in their clinical practice, motivate researchers in their investigations, and inspire all stakeholders toward future innovation in the quest to improve antibiotic use.
ACKNOWLEDGMENTS
We thank Patrick Lane (ScEYEnce Studios) for his graphic assistance during preparation of the illustrations for this article.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector. Barbara W. Trautner’s work is supported in part by the Houston Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413) and by Veterans Affairs funding (HSR&D 16-025, RR&D RX002595 and CSR&D 1I01CX000830-01A2). Larissa Grigoryan’s work is supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number UM1Al104681.
Biographies

Melanie C. Goebel, M.D., is an Assistant Professor in the Department of Medicine, Section of Infectious Diseases at Baylor College of Medicine. Her training includes medical school at Baylor College of Medicine and an internal medicine/pediatrics internship and internal medicine residency at Duke University Medical Center. She completed an infectious diseases fellowship at Baylor College of Medicine with a focus on implementation science and antimicrobial stewardship for urinary tract infections. Dr. Goebel provides care to patients with HIV and general infectious diseases in Harris Health System in Houston, TX. She is the Associate Clinical Director of the Houston AIDS Education and Training Center.

Barbara W. Trautner, M.D., Ph.D., is an infectious diseases clinician-investigator at Baylor College of Medicine and the Michael E. DeBakey Veterans Affairs Medical Center, affiliated with the Center for Innovations in Quality, Effectiveness, and Safety (IQuESt). Dr. Trautner obtained her Ph.D. in clinical investigation from Baylor College of Medicine Graduate School of Biomedical Sciences. Her primary research interest is the development of new strategies for the prevention of catheter-associated urinary tract infection (CAUTI). Dr. Trautner’s frustration with seeing her patients’ bladder flora become more resistant after courses of unnecessary antibiotics led to her current research interests: (i) novel strategies for prevention and management of UTI, including bacteriophage, and (ii) implementation of antibiotic stewardship guidelines, particularly for UTI.

Larissa Grigoryan, M.D., Ph.D., is an Assistant Professor in the Department of Family and Community Medicine at Baylor College of Medicine and the Veterans Affairs Center for Innovations in Quality, Effectiveness and Safety (IQuESt). She obtained her Ph.D. in epidemiology from the University of Groningen in The Netherlands and completed a primary care research fellowship at Baylor College of Medicine in Houston, TX. Dr. Grigoryan uses her expertise in epidemiology and background in antimicrobial stewardship to develop and test antimicrobial stewardship interventions in primary care.
REFERENCES
- 1.Akbar R, World Health Organization. 2019. Ten threats to global health in 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019. Accessed 13 September 2019.
- 2.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 19 November 2020.
- 3.Tadesse DA, Zhao S, Tong E, Ayers S, Singh A, Bartholomew MJ, McDermott PF. 2012. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg Infect Dis 18:741–749. 10.3201/eid1805.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. 1999. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis 29:745–758. 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2020. About antibiotic resistance. https://www.cdc.gov/drugresistance/about.html#anchor_1552062951754. Accessed 30 April 2021.
- 6.Gould K. 2016. Antibiotics: from prehistory to the present day. J Antimicrob Chemother 71:572–575. 10.1093/jac/dkv484. [DOI] [PubMed] [Google Scholar]
- 7.Emmerson AM, Jones AM. 2003. The quinolones: decades of development and use. J Antimicrob Chemother 51(Suppl 1):13–20. 10.1093/jac/dkg208. [DOI] [PubMed] [Google Scholar]
- 8.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, Finkelstein JA, Gerber JS, Hyun DY, Linder JA, Lynfield R, Margolis DJ, May LS, Merenstein D, Metlay JP, Newland JG, Piccirillo JF, Roberts RM, Sanchez GV, Suda KJ, Thomas A, Woo TM, Zetts RM, Hicks LA. 2016. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 315:1864–1873. 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion. 2018. Outpatient antibiotic prescriptions—United States, 2018. https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-local-activities/outpatient-antibiotic-prescriptions-US-2018.html. Accessed 27 July 2020.
- 10.Public Health England. 2019. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR), report 2018-2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/843129/English_Surveillance_Programme_for_Antimicrobial_Utilisation_and_Resistance_2019.pdf. Accessed 27 July 2020.
- 11.Public Health Agency of Sweden. 2018. Consumption of antibiotics and occurrence of resistance in Sweden, on Swedres-Svarm 2018. https://www.sva.se/media/jzdlctnk/rapport_swedres-svarm_2018.pdf. Accessed 27 July 2020.
- 12.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Matusiak LM, Schumock GT. 2018. Antibiotic expenditures by medication, class, and healthcare setting in the United States, 2010–2015. Clin Infect Dis 66:185–190. 10.1093/cid/cix773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rui P, Okeyode T. 2016. National Ambulatory Medical Care Survey: 2016 national summary tables. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf. Accessed 18 February 2021.
- 14.Rui P, Kang K, Ashman J. 2016. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf. Accessed 18 February 2021.
- 15.O’Neal F, Kramer J, Cooper M, Septimus E, Sharma S, Burgess LH. 2019. Analysis of antibiotic use in a large network of emergency departments. Am J Health Syst Pharm 76:1753–1761. 10.1093/ajhp/zxz193. [DOI] [PubMed] [Google Scholar]
- 16.Goto T, Yoshida K, Tsugawa Y, Camargo CA, Jr, Hasegawa K. 2016. Infectious disease-related emergency department visits of elderly adults in the United States, 2011-2012. J Am Geriatr Soc 64:31–36. 10.1111/jgs.13836. [DOI] [PubMed] [Google Scholar]
- 17.Dolk FCK, Pouwels KB, Smith DRM, Robotham JV, Smieszek T. 2018. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother 73:ii2–ii10. 10.1093/jac/dkx504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM, American Geriatric Society. 2005. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 40:643–654. 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 19.Nicolle LE, Gupta K, Bradley SF, Colgan R, DeMuri GP, Drekonja D, Eckert LO, Geerlings SE, Köves B, Hooton TM, Juthani-Mehta M, Knight SL, Saint S, Schaeffer AJ, Trautner B, Wullt B, Siemieniuk R. 2019. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis 68:e83–e110. 10.1093/cid/ciy1121. [DOI] [PubMed] [Google Scholar]
- 20.US Preventive Services Task Force. 2019. Screening for asymptomatic bacteriuria in adults: US Preventive Services Task Force recommendation statement. JAMA 322:1188–1194. 10.1001/jama.2019.13069. [DOI] [PubMed] [Google Scholar]
- 21.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE, Infectious Diseases Society of America. 2010. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 50:625–663. 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 22.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE, European Society for Microbiology and Infectious Diseases. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 23.Al Salman J, Alawi SS, Alyusuf EY. 2017. Antibiotic appropriateness for urinary tract infection in the emergency room. Bahrain Med Bull 39:38–42. 10.12816/0047440. [DOI] [Google Scholar]
- 24.Alanazi MQ. 2018. An evaluation of community-acquired urinary tract infection and appropriateness of treatment in an emergency department in Saudi Arabia. Ther Clin Risk Manag 14:2363–2373. 10.2147/TCRM.S178855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindbäck H, Lindbäck J, Melhus Å. 2017. Inadequate adherence to Swedish guidelines for uncomplicated lower urinary tract infections among adults in general practice. APMIS 125:816–821. 10.1111/apm.12718. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi M, Shapiro DJ, Hersh AL, Sanchez GV, Hicks LA. 2016. Outpatient antibiotic prescribing practices for uncomplicated urinary tract infection in women in the United States, 2002–2011. Open Forum Infect Dis 3:ofw159. 10.1093/ofid/ofw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durkin MJ, Keller M, Butler AM, Kwon JH, Dubberke ER, Miller AC, Polgreen PM, Olsen MA. 2018. An assessment of inappropriate antibiotic use and guideline adherence for uncomplicated urinary tract infections. Open Forum Infect Dis 5:ofy198. 10.1093/ofid/ofy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grigoryan L, Zoorob R, Wang H, Trautner BW. 2015. Low concordance with guidelines for treatment of acute cystitis in primary care. Open Forum Infect Dis 2:ofv159. 10.1093/ofid/ofv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagen TL, Hertz MA, Uhrin GB, Dalager-Pedersen M, Schonheyder HC, Nielsen H. 2017. Adherence to local antimicrobial guidelines for initial treatment of community-acquired infections. Dan Med J 64:A5381. [PubMed] [Google Scholar]
- 30.Chang Y, Chusri S, Sangthong R, McNeil E, Hu J, Du W, Li D, Fan X, Zhou H, Chongsuvivatwong V, Tang L. 2019. Clinical pattern of antibiotic overuse and misuse in primary healthcare hospitals in the southwest of China. PLoS One 14:e0214779. 10.1371/journal.pone.0214779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alanazi MQ, Al-Jeraisy MI, Salam M. 2015. Prevalence and predictors of antibiotic prescription errors in an emergency department, Central Saudi Arabia. Drug Healthc Patient Saf 7:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May L, Mullins P, Pines J. 2014. Demographic and treatment patterns for infections in ambulatory settings in the United States, 2006–2010. Acad Emerg Med 21:17–24. 10.1111/acem.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M, Lloyd A, Condren M, Miller MJ. 2015. Beyond antibiotic selection: concordance with the IDSA guidelines for uncomplicated urinary tract infections. Infection 43:89–94. 10.1007/s15010-014-0659-4. [DOI] [PubMed] [Google Scholar]
- 34.Shively NR, Buehrle DJ, Clancy CJ, Decker BK. 2018. Prevalence of inappropriate antibiotic prescribing in primary care clinics within a Veterans Affairs health care system. Antimicrobial Agents & Chemotherapy 62:e00337-18. 10.1128/AAC.00337-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gee ME, Ford J, Conway EL, Ott MC, Sellick JA, Mergenhagen KA. 2018. Proper antibiotic use in a home-based primary care population treated for urinary tract infections. Consult Pharm 33:105–113. 10.4140/TCP.n.2018.105. [DOI] [PubMed] [Google Scholar]
- 36.Lim M, Petty L, Dillman N, Walker P, Nagel J. 2018. Evaluation of antibiotic prescribing practices for lower urinary tract infections in the emergency department. Open Forum Infect Dis 5:S470. 10.1093/ofid/ofy210.1346. [DOI] [Google Scholar]
- 37.Lee A, Guirguis M. 2016. A closer look at urinary tract infection prescribing patterns in a suburban emergency room department. Open Forum Infect Dis 3(suppl_1):1908. 10.1093/ofid/ofw172.1456. [DOI] [Google Scholar]
- 38.Vieira AL, Capela C. 2013. Appropriateness of antibiotic prescriptions for hospital emergency department patients. Eur J Intern Med 24:e198–e199. 10.1016/j.ejim.2013.08.508. [DOI] [Google Scholar]
- 39.Hecker MT, Fox CJ, Son AH, Cydulka RK, Siff JE, Emerman CL, Sethi AK, Muganda CP, Donskey CJ. 2014. Effect of a stewardship intervention on adherence to uncomplicated cystitis and pyelonephritis guidelines in an emergency department setting. PLoS One 9:e87899. 10.1371/journal.pone.0087899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takhar SS, Moran GJ. 2014. Diagnosis and management of urinary tract infection in the emergency department and outpatient settings. Infect Dis Clin North Am 28:33–48. 10.1016/j.idc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion. U.S. national action plan for combating antibiotic-resistant bacteria. https://www.cdc.gov/drugresistance/us-activities/national-action-plan.html#anchor_1534867320. Published 2015. Accessed 13 September 2019.
- 42.Shlaes DM, Gerding DN, John JF, Jr, Craig WA, Bornstein DL, Duncan RA, Eckman MR, Farrer WE, Greene WH, Lorian V, Levy S, McGowan JE, Jr, Paul SM, Ruskin J, Tenover FC, Watanakunakorn C. 1997. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the prevention of antimicrobial resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis 25:584–599. 10.1086/513766. [DOI] [PubMed] [Google Scholar]
- 43.Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society. 2012. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 33:322–327. 10.1086/665010. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. 2014. Core elements of hospital antibiotic stewardship programs. US Department of Health and Human Services, CDC, Atlanta, GA. [Google Scholar]
- 45.Centers for Disease Control and Prevention. 2015. The core elements of antibiotic stewardship for nursing homes. US Department of Health and Human Services, CDC, Atlanta, GA. [Google Scholar]
- 46.Drekonja DM, Filice GA, Greer N, Olson A, MacDonald R, Rutks I, Wilt TJ. 2015. Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol 36:142–152. 10.1017/ice.2014.41. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. 2016. Core elements of outpatient antibiotic stewardship. MMWR Recommend Rep 65:1–12. 10.15585/mmwr.rr6506a1. [DOI] [PubMed] [Google Scholar]
- 48.The Joint Commission. 2019. Antimicrobial stewardship in ambulatory health care. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/r3_23_antimicrobial_stewardship_amb_6_14_19_final2.pdf. Accessed 11 September 2020.
- 49.Ranji SR, Steinman MA, Shojania KG, Sundaram V, Lewis R, Arnold S, Gonzales R. 2006. Closing the quality gap: a critical analysis of quality improvement strategies, vol 4. Antibiotic prescribing behavior. Agency for Healthcare Research and Quality, Rockville, MD. [PubMed] [Google Scholar]
- 50.Santarossa M, Kilber EN, Wenzler E, Albarillo FS, Sterk EJ. 2019. BundlED up: a narrative review of antimicrobial stewardship initiatives and bundles in the emergency department. Pharmacy (Basel) 7:145. 10.3390/pharmacy7040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinman MA, Ranji SR, Shojania KG, Gonzales R. 2006. Improving antibiotic selection: a systematic review and quantitative analysis of quality improvement strategies. Med Care 44:617–628. 10.1097/01.mlr.0000215846.25591.22. [DOI] [PubMed] [Google Scholar]
- 52.Morency-Potvin P, Schwartz DN, Weinstein RA. 2017. Antimicrobial stewardship: how the microbiology laboratory can right the ship. Clin Microbiol Rev 30:381–407. 10.1128/CMR.00066-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joseph J, Rodvold KA. 2008. The role of carbapenems in the treatment of severe nosocomial respiratory tract infections. Expert Opin Pharmacother 9:561–575. 10.1517/14656566.9.4.561. [DOI] [PubMed] [Google Scholar]
- 54.Anger J, Lee U, Ackerman AL, Chou R, Chughtai B, Clemens JQ, Hickling D, Kapoor A, Kenton KS, Kaufman MR, Rondanina MA, Stapleton A, Stothers L, Chai TC. 2019. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol 202:282–289. 10.1097/JU.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 55.Gupta K, Grigoryan L, Trautner B. 2017. Urinary tract infection. Ann Intern Med 167:ITC49–ITC64. 10.7326/AITC201710030. [DOI] [PubMed] [Google Scholar]
- 56.Khawcharoenporn T, Vasoo S, Ward E, Singh K. 2011. Abnormal urinalysis finding triggered antibiotic prescription for asymptomatic bacteriuria in the ED. Am J Emerg Med 29:828–830. 10.1016/j.ajem.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Rowan N, Pflugeisen BM, Alajbegovic S. 2017. Urine culture guided antibiotic interventions: a pharmacist driven antimicrobial stewardship effort in the ED. Am J Emerg Med 35:594–598. 10.1016/j.ajem.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 58.James D, Lopez L. 2019. Impact of a pharmacist-driven education initiative on treatment of asymptomatic bacteriuria. Am J Health Syst Pharm 76:S41–S48. 10.1093/ajhp/zxy081. [DOI] [PubMed] [Google Scholar]
- 59.Hermida Pérez JA, Loro Ferrer JF. 2004. Asymptomatic bacteriuria in women. Epidemiological, pathologic and therapeutic study. Arch Esp Urol 57:784–804. (In Spanish.) [PubMed] [Google Scholar]
- 60.Cai T, Mazzoli S, Migno S, Malossini G, Lanzafame P, Mereu L, Tateo S, Wagenlehner FM, Pickard RS, Bartoletti R. 2014. Development and validation of a nomogram predicting recurrence risk in women with symptomatic urinary tract infection. Int J Urol 21:929–934. 10.1111/iju.12453. [DOI] [PubMed] [Google Scholar]
- 61.Flokas ME, Andreatos N, Alevizakos M, Kalbasi A, Onur P, Mylonakis E. 2017. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis 4:ofx207. 10.1093/ofid/ofx207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. 2002. Does this woman have an acute uncomplicated urinary tract infection? JAMA 287:2701–2710. 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 63.Trautner BW, Bhimani RD, Amspoker AB, Hysong SJ, Garza A, Kelly PA, Payne VL, Naik AD. 2013. Development and validation of an algorithm to recalibrate mental models and reduce diagnostic errors associated with catheter-associated bacteriuria. BMC Med Inform Decis Mak 13:48. 10.1186/1472-6947-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grigoryan L, Nash S, Zoorob R, Germanos GJ, Horsfield MS, Khan FM, Martin L, Trautner BW. 2019. Qualitative analysis of primary care provider prescribing decisions for urinary tract infections. Antibiotics 8:84. 10.3390/antibiotics8020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grigoryan L, Trautner BW, Gupta K. 2014. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA 312:1677–1684. 10.1001/jama.2014.12842. [DOI] [PubMed] [Google Scholar]
- 66.Gupta K, Hooton TM, Roberts PL, Stamm WE. 2007. Short-course nitrofurantoin for the treatment of acute uncomplicated cystitis in women. Arch Intern Med 167:2207–2212. 10.1001/archinte.167.20.2207. [DOI] [PubMed] [Google Scholar]
- 67.Hooton TM, Roberts PL, Cox ME, Stapleton AE. 2013. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med 369:1883–1891. 10.1056/NEJMoa1302186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LaRocco MT, Franek J, Leibach EK, Weissfeld AS, Kraft CS, Sautter RL, Baselski V, Rodahl D, Peterson EJ, Cornish NE. 2016. Effectiveness of preanalytic practices on contamination and diagnostic accuracy of urine cultures: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev 29:105–147. 10.1128/CMR.00030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lifshitz E, Kramer L. 2000. Outpatient urine culture: does collection technique matter? Arch Intern Med 160:2537–2540. 10.1001/archinte.160.16.2537. [DOI] [PubMed] [Google Scholar]
- 70.Chu CM, Lowder JL. 2018. Diagnosis and treatment of urinary tract infections across age groups. Am J Obstet Gynecol 219:40–51. 10.1016/j.ajog.2017.12.231. [DOI] [PubMed] [Google Scholar]
- 71.Hooton TM, Roberts PL, Stapleton AE. 16 March 2020. Asymptomatic bacteriuria and pyuria in premenopausal women. Clin Infect Dis 10.1093/cid/ciaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan-Tack KM, Trautner BW, Morgan DJ. 10 February 2020. The varying specificity of urine cultures in different populations. Infect Control Hosp Epidemiol 10.1017/ice.2020.16:1-2. [DOI] [PubMed] [Google Scholar]
- 73.Davenport M, Mach KE, Shortliffe LMD, Banaei N, Wang TH, Liao JC. 2017. New and developing diagnostic technologies for urinary tract infections. Nat Rev Urol 14:296–310. 10.1038/nrurol.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tomas ME, Getman D, Donskey CJ, Hecker MT. 2015. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 53:2686–2692. 10.1128/JCM.00670-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shapiro T, Dalton M, Hammock J, Lavery R, Matjucha J, Salo DF. 2005. The prevalence of urinary tract infections and sexually transmitted disease in women with symptoms of a simple urinary tract infection stratified by low colony count criteria. Acad Emerg Med 12:38–44. 10.1197/j.aem.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 76.Huppert JS, Biro F, Lan D, Mortensen JE, Reed J, Slap GB. 2007. Urinary symptoms in adolescent females: STI or UTI? J Adolesc Health 40:418–424. 10.1016/j.jadohealth.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shipman SB, Risinger CR, Evans CM, Gilbertson CD, Hogan DE. 2018. High prevalence of sterile pyuria in the setting of sexually transmitted infection in women presenting to an emergency department. West J Emerg Med 19:282–286. 10.5811/westjem.2017.12.35605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. 2011. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 77:1081–1087. 10.1016/j.urology.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 79.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A, Standardisation Sub-Committee of the International Continence Society. 2003. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61:37–49. 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 80.Nik-Ahd F, Lenore Ackerman A, Anger J. 2018. Recurrent urinary tract infections in females and the overlap with overactive bladder. Curr Urol Rep 19:94. 10.1007/s11934-018-0839-3. [DOI] [PubMed] [Google Scholar]
- 81.McLennan MT. 2014. Interstitial cystitis: epidemiology, pathophysiology, and clinical presentation. Obstet Gynecol Clin North Am 41:385–395. 10.1016/j.ogc.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Mayne S, Bowden A, Sundvall PD, Gunnarsson R. 2019. The scientific evidence for a potential link between confusion and urinary tract infection in the elderly is still confusing—a systematic literature review. BMC Geriatr 19:32. 10.1186/s12877-019-1049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colgan R, Jaffe GA, Nicolle LE. 2020. Asymptomatic bacteriuria. Am Fam Physician 102:99–104. [PubMed] [Google Scholar]
- 84.Morgan DJ, Malani P, Diekema DJ. 2017. Diagnostic stewardship—leveraging the laboratory to improve antimicrobial use. JAMA 318:607–608. 10.1001/jama.2017.8531. [DOI] [PubMed] [Google Scholar]
- 85.Claeys KC, Blanco N, Morgan DJ, Leekha S, Sullivan KV. 2019. Advances and challenges in the diagnosis and treatment of urinary tract infections: the need for diagnostic stewardship. Curr Infect Dis Rep 21:11. 10.1007/s11908-019-0668-7. [DOI] [PubMed] [Google Scholar]
- 86.Yin P, Kiss A, Leis JA. 2015. Urinalysis orders among patients admitted to the general medicine service. JAMA Intern Med 175:1711–1713. 10.1001/jamainternmed.2015.4036. [DOI] [PubMed] [Google Scholar]
- 87.Sloane PD, Kistler CE, Reed D, Weber DJ, Ward K, Zimmerman S. 2017. Urine culture testing in community nursing homes: gateway to antibiotic overprescribing. Infect Control Hosp Epidemiol 38:524–531. 10.1017/ice.2016.326. [DOI] [PubMed] [Google Scholar]
- 88.Pallin DJ, Ronan C, Montazeri K, Wai K, Gold A, Parmar S, Schuur JD. 2014. Urinalysis in acute care of adults: pitfalls in testing and interpreting results. Open Forum Infect Dis 1:ofu019. 10.1093/ofid/ofu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trautner BW, Grigoryan L, Petersen NJ, Hysong S, Cadena J, Patterson JE, Naik AD. 2015. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med 175:1120–1127. 10.1001/jamainternmed.2015.1878. [DOI] [PubMed] [Google Scholar]
- 90.Irfan N, Brooks A, Mithoowani S, Celetti SJ, Main C, Mertz D. 2015. A controlled quasi-experimental study of an educational intervention to reduce the unnecessary use of antimicrobials for asymptomatic bacteriuria. PLoS One 10:e0132071. 10.1371/journal.pone.0132071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arnold SR, Straus SE. 2005. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005(4): CD003539. 10.1002/14651858.CD003539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohl CA, Luther VP. 2014. Health care provider education as a tool to enhance antibiotic stewardship practices. Infect Dis Clin North Am 28:177–193. 10.1016/j.idc.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Chironda B, Clancy S, Powis JE. 2014. Optimizing urine culture collection in the emergency department using frontline ownership interventions. Clin Infect Dis 59:1038–1039. 10.1093/cid/ciu412. [DOI] [PubMed] [Google Scholar]
- 94.Stagg A, Lutz H, Kirpalaney S, Matelski JJ, Kaufman A, Leis J, McCready J, Powis J. 2018. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf 27:140–147. 10.1136/bmjqs-2016-006250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zimmerman B, Reason P, Rykert L, Gitterman L, Christian J, Gardam M. 2013. Front-line ownership: generating a cure mindset for patient safety. Healthc Pap 13:6–22. 10.12927/hcpap.2013.23299. [DOI] [PubMed] [Google Scholar]
- 96.McIsaac WJ, Low DE, Biringer A, Pimlott N, Evans M, Glazier R. 2002. The impact of empirical management of acute cystitis on unnecessary antibiotic use. Arch Intern Med 162:600–605. 10.1001/archinte.162.5.600. [DOI] [PubMed] [Google Scholar]
- 97.Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, Smith H, Hawke C, Mullee M. 2006. Developing clinical rules to predict urinary tract infection in primary care settings: sensitivity and specificity of near patient tests (dipsticks) and clinical scores. Br J Gen Pract 56:606–612. [PMC free article] [PubMed] [Google Scholar]
- 98.McIsaac WJ, Moineddin R, Ross S. 2007. Validation of a decision aid to assist physicians in reducing unnecessary antibiotic drug use for acute cystitis. Arch Intern Med 167:2201–2206. 10.1001/archinte.167.20.2201. [DOI] [PubMed] [Google Scholar]
- 99.McIsaac WJ, Moineddin R, Gagyor I, Mazzulli T. 2017. External validation study of a clinical decision aid to reduce unnecessary antibiotic prescriptions in women with acute cystitis. BMC Fam Pract 18:89. 10.1186/s12875-017-0660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bekeris LG, Jones BA, Walsh MK, Wagar EA. 2008. Urine culture contamination: a College of American Pathologists Q-Probes study of 127 laboratories. Arch Pathol Lab Med 132:913–917. 10.5858/2008-132-913-UCCACO. [DOI] [PubMed] [Google Scholar]
- 101.Grigoryan L, Goebel M, Willis S, Danek L, Matas J, Kachalia N, Katta A, Muldrew K, Zare M, Hudson F, Atmar R, Trautner B. 2020. Creating an outpatient-specific antibiogram to guide treatment for urinary tract infections. Infect Control Hosp Epidemiol 41:s182–s183. 10.1017/ice.2020.717. [DOI] [Google Scholar]
- 102.Klausing BT, Tillman SD, Wright PW, Talbot TR. 2016. The influence of contaminated urine cultures in inpatient and emergency department settings. Am J Infect Control 44:1166–1167. 10.1016/j.ajic.2016.03.055. [DOI] [PubMed] [Google Scholar]
- 103.Garcia R, Spitzer ED. 2017. Promoting appropriate urine culture management to improve health care outcomes and the accuracy of catheter-associated urinary tract infections. Am J Infect Control 45:1143–1153. 10.1016/j.ajic.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 104.Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB, Jr, Bourbeau P, Carroll KC, Kehl SC, Dunne WM, Robinson-Dunn B, Schwartzman JD, Chapin KC, Snyder JW, Forbes BA, Patel R, Rosenblatt JE, Pritt BS. 2013. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis 57:e22–e121. 10.1093/cid/cit278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gordon LB, Waxman MJ, Ragsdale L, Mermel LA. 2013. Overtreatment of presumed urinary tract infection in older women presenting to the emergency department. J Am Geriatr Soc 61:788–792. 10.1111/jgs.12203. [DOI] [PubMed] [Google Scholar]
- 106.Dolan VJ, Cornish NE. 2013. Urine specimen collection: how a multidisciplinary team improved patient outcomes using best practices. Urol Nurs 33:249–256. 10.7257/1053-816X.2013.33.5.249. [DOI] [PubMed] [Google Scholar]
- 107.Eley R, Judge C, Knight L, Dimeski G, Sinnott M. 2016. Illustrations reduce contamination of midstream urine samples in the emergency department. J Clin Pathol 69:921–925. 10.1136/jclinpath-2015-203504. [DOI] [PubMed] [Google Scholar]
- 108.Lough ME, Shradar E, Hsieh C, Hedlin H. 2019. Contamination in adult midstream clean-catch urine cultures in the emergency department: a randomized controlled trial. J Emerg Nurs 45:488–501. 10.1016/j.jen.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 109.Collier S, Matjiu F, Jones G, Harber M, Hopkins S. 2014. A prospective study comparing contamination rates between a novel mid-stream urine collection device (Peezy) and a standard method in renal patients. J Clin Pathol 67:139–142. 10.1136/jclinpath-2013-201686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maher PJ, Brown AEC, Gatewood MO. 2017. The effect of written posted instructions on collection of clean-catch urine specimens in the emergency department. J Emerg Med 52:639–644. 10.1016/j.jemermed.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 111.Jacob MS, Kulie P, Benedict C, Ordoobadi AJ, Sikka N, Steinmetz E, McCarthy ML. 2018. Use of a midstream clean catch mobile application did not lower urine contamination rates in an ED. Am J Emerg Med 36:61–65. 10.1016/j.ajem.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 112.Kistin C, Silverstein M. 2015. Pilot studies: a critical but potentially misused component of interventional research. JAMA 314:1561–1562. 10.1001/jama.2015.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Humphries RM, Dien Bard J. 2016. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol 54:254–258. 10.1128/JCM.03021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kayalp D, Dogan K, Ceylan G, Senes M, Yucel D. 2013. Can routine automated urinalysis reduce culture requests? Clin Biochem 46:1285–1289. 10.1016/j.clinbiochem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 115.Jones CW, Culbreath KD, Mehrotra A, Gilligan PH. 2014. Reflect urine culture cancellation in the emergency department. J Emerg Med 46:71–76. 10.1016/j.jemermed.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 116.Coughlin RF, Peaper D, Rothenberg C, Golden M, Landry ML, Cotton J, Parwani V, Shapiro M, Ulrich A, Venkatesh AK. 2020. Electronic health record-assisted reflex urine culture testing improves emergency department diagnostic efficiency. Am J Med Qual 35:252–257. 10.1177/1062860619861947. [DOI] [PubMed] [Google Scholar]
- 117.Jaeger C, Waymack J, Sullivan P, Lankala S, Petersen L, Coultas C, Griffen D. 2019. Refining reflex urine culture testing in the ED. Am J Emerg Med 37:1380–1382. 10.1016/j.ajem.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 118.Dietz J, Lo TS, Hammer K, Zegarra M. 2016. Impact of eliminating reflex urine cultures on performed urine cultures and antibiotic use. Am J Infect Control 44:1750–1751. 10.1016/j.ajic.2016.04.232. [DOI] [PubMed] [Google Scholar]
- 119.Watson KJ, Trautner B, Russo H, Phe K, Lasco T, Pipkins T, Lembcke B, Al Mohajer M. 2020. Using clinical decision support to improve urine culture diagnostic stewardship, antimicrobial stewardship, and financial cost: a multicenter experience. Infect Control Hosp Epidemiol 41:564–570. 10.1017/ice.2020.37. [DOI] [PubMed] [Google Scholar]
- 120.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. 2016. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51–e77. 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burton RJ, Albur M, Eberl M, Cuff SM. 2019. Using artificial intelligence to reduce diagnostic workload without compromising detection of urinary tract infections. BMC Med Inform Decis Mak 19:171. 10.1186/s12911-019-0878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Demonchy E, Dufour JC, Gaudart J, Cervetti E, Michelet P, Poussard N, Levraut J, Pulcini C. 2014. Impact of a computerized decision support system on compliance with guidelines on antibiotics prescribed for urinary tract infections in emergency departments: a multicentre prospective before-and-after controlled interventional study. J Antimicrob Chemother 69:2857–2863. 10.1093/jac/dku191. [DOI] [PubMed] [Google Scholar]
- 123.Daley P, Garcia D, Inayatullah R, Penney C, Boyd S. 2018. Modified reporting of positive urine cultures to reduce inappropriate treatment of asymptomatic bacteriuria among nonpregnant, noncatheterized inpatients: a randomized controlled trial. Infect Control Hosp Epidemiol 39:814–819. 10.1017/ice.2018.100. [DOI] [PubMed] [Google Scholar]
- 124.Leis JA, Rebick GW, Daneman N, Gold WL, Poutanen SM, Lo P, Larocque M, Shojania KG, McGeer A. 2014. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis 58:980–983. 10.1093/cid/ciu010. [DOI] [PubMed] [Google Scholar]
- 125.Cunney R, Aziz HA, Schubert D, McNamara E, Smyth E. 2000. Interpretative reporting and selective antimicrobial susceptibility release in non-critical microbiology results. J Antimicrob Chemother 45:705–708. 10.1093/jac/45.5.705. [DOI] [PubMed] [Google Scholar]
- 126.US Food and Drug Administration. 2016. FDA updates warnings for fluoroquinolone antibiotics. https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics. Accessed 11 September 2020.
- 127.US Food and Drug Administration. 2018. FDA updates warnings for fluoroquinolone antibiotics on risks of mental health and low blood sugar adverse reactions. https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse. Accessed 11 September 2020.
- 128.Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Sferra TJ, Hernandez AV, Donskey CJ. 2013. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 68:1951–1961. 10.1093/jac/dkt129. [DOI] [PubMed] [Google Scholar]
- 129.Stewardson AJ, Vervoort J, Adriaenssens N, Coenen S, Godycki-Cwirko M, Kowalczyk A, Huttner BD, Lammens C, Malhotra-Kumar S, Goossens H, Harbarth S, SATURN WP3 Study Group. 2018. Effect of outpatient antibiotics for urinary tract infections on antimicrobial resistance among commensal Enterobacteriaceae: a multinational prospective cohort study. Clin Microbiol Infect 24:972–979. 10.1016/j.cmi.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 130.Trautner BW. 2018. Fluoroquinolones for urinary tract infection and within-household spread of resistant Enterobacteriaceae: the smoking gun. Clin Microbiol Infect 24:929–930. 10.1016/j.cmi.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 131.St-Jean A, Chateau D, Dahl M, Ernst P, Daneman N, Sketris IS, Zhang J, Lalji F, Quail J, Bugden S. 2019. Regional variation in the potentially inappropriate first line use of fluoroquinolones in Canada: a key to antibiotic stewardship? Pharmacoepidemiol Drug Saf 28:506–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kabbani S, Hersh AL, Shapiro DJ, Fleming-Dutra KE, Pavia AT, Hicks LA. 2018. Opportunities to improve fluoroquinolone prescribing in the United States for adult ambulatory care visits. Clin Infect Dis 67:134–136. 10.1093/cid/ciy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zweigner J, Meyer E, Gastmeier P, Schwab F. 2018. Rate of antibiotic prescriptions in German outpatient care—are the guidelines followed or are they still exceeded? GMS Hyg Infect Control 13:Doc04. 10.3205/dgkh000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. 2014. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 69:234–240. 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 135.Cowart K, Worley M, Rouby NE, Sando K. 2019. Evaluation of FDA boxed warning on prescribing patterns of fluoroquinolones for uncomplicated urinary tract infections. Ann Pharmacother 53:1192–1199. 10.1177/1060028019865224. [DOI] [PubMed] [Google Scholar]
- 136.Bratsman A, Mathias K, Laubscher R, Grigoryan L, Rose S. 2020. Outpatient fluoroquinolone prescribing patterns before and after US FDA boxed warning. Pharmacoepidemiol Drug Saf 29:701–707. 10.1002/pds.5018. [DOI] [PubMed] [Google Scholar]
- 137.Leski TA, Taitt CR, Bangura U, Stockelman MG, Ansumana R, Cooper WH, III, Stenger DA, Vora GJ. 2016. High prevalence of multidrug resistant Enterobacteriaceae isolated from outpatient urine samples but not the hospital environment in Bo, Sierra Leone. BMC Infect Dis 16:167. 10.1186/s12879-016-1495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sanchez GV, Babiker A, Master RN, Luu T, Mathur A, Bordon J. 2016. Antibiotic resistance among urinary isolates from female outpatients in the United States in 2003 and 2012. Antimicrob Agents Chemother 60:2680–2683. 10.1128/AAC.02897-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heytens S, Boelens J, Claeys G, DeSutter A, Christiaens T. 2017. Uropathogen distribution and antimicrobial susceptibility in uncomplicated cystitis in Belgium, a high antibiotics prescribing country: 20-year surveillance. Eur J Clin Microbiol Infect Dis 36:105–113. 10.1007/s10096-016-2776-8. [DOI] [PubMed] [Google Scholar]
- 140.Falagas ME, Vouloumanou EK, Togias AG, Karadima M, Kapaskelis AM, Rafailidis PI, Athanasiou S. 2010. Fosfomycin versus other antibiotics for the treatment of cystitis: a meta-analysis of randomized controlled trials. J Antimicrob Chemother 65:1862–1877. 10.1093/jac/dkq237. [DOI] [PubMed] [Google Scholar]
- 141.Hirsch EB, Raux BR, Zucchi PC, Kim Y, McCoy C, Kirby JE, Wright SB, Eliopoulos GM. 2015. Activity of fosfomycin and comparison of several susceptibility testing methods against contemporary urine isolates. Int J Antimicrob Agents 46:642–647. 10.1016/j.ijantimicag.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 142.Seitz M, Stief C, Waidelich R. 2017. Local epidemiology and resistance profiles in acute uncomplicated cystitis (AUC) in women: a prospective cohort study in an urban urological ambulatory setting. BMC Infect Dis 17:685. 10.1186/s12879-017-2789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Anesi JA, Lautenbach E, Nachamkin I, Garrigan C, Bilker WB, Omorogbe J, Dankwa L, Wheeler MK, Tolomeo P, Han JH, for the CDC Prevention Epicenters Program. 2018. Poor clinical outcomes associated with community-onset urinary tract infections due to extended-spectrum cephalosporin-resistant Enterobacteriaceae. Infect Control Hosp Epidemiol 39:1431–1435. 10.1017/ice.2018.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fatima S, Muhammad IN, Usman S, Jamil S, Khan MN, Khan SI. 2018. Incidence of multidrug resistance and extended-spectrum beta-lactamase expression in community-acquired urinary tract infection among different age groups of patients. Indian J Pharmacol 50:69–74. 10.4103/ijp.IJP_200_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thaden JT, Fowler VG, Sexton DJ, Anderson DJ. 2016. Increasing incidence of extended-spectrum beta-lactamase-producing Escherichia coli in community hospitals throughout the southeastern United States. Infect Control Hosp Epidemiol 37:49–54. 10.1017/ice.2015.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. 2013. Community-associated extended-spectrum beta-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis 56:641–648. 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jernigan JA, Hatfield KM, Wolford H, Nelson RE, Olubajo B, Reddy SC, McCarthy N, Paul P, McDonald LC, Kallen A, Fiore A, Craig M, Baggs J. 2020. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012–2017. N Engl J Med 382:1309–1319. 10.1056/NEJMoa1914433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. 2016. Fecal colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis 63:310–318. 10.1093/cid/ciw283. [DOI] [PubMed] [Google Scholar]
- 149.Tice AD, Rehm SJ, Dalovisio JR, Bradley JS, Martinelli LP, Graham DR, Gainer RB, Kunkel MJ, Yancey RW, Williams DN, IDSA. 2004. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 38:1651–1672. 10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 150.Norris AH, Shrestha NK, Allison GM, Keller SC, Bhavan KP, Zurlo JJ, Hersh AL, Gorski LA, Bosso JA, Rathore MH, Arrieta A, Petrak RM, Shah A, Brown RB, Knight SL, Umscheid CA. 2019. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis 68:e1–e35. 10.1093/cid/ciy745. [DOI] [PubMed] [Google Scholar]
- 151.Pullukcu H, Tasbakan M, Sipahi OR, Yamazhan T, Aydemir S, Ulusoy S. 2007. Fosfomycin in the treatment of extended spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infections. Int J Antimicrob Agents 29:62–65. 10.1016/j.ijantimicag.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 152.Senol S, Tasbakan M, Pullukcu H, Sipahi OR, Sipahi H, Yamazhan T, Arda B, Ulusoy S. 2010. Carbapenem versus fosfomycin tromethanol in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related complicated lower urinary tract infection. J Chemother 22:355–357. 10.1179/joc.2010.22.5.355. [DOI] [PubMed] [Google Scholar]
- 153.Veve MP, Wagner JL, Kenney RM, Grunwald JL, Davis SL. 2016. Comparison of fosfomycin to ertapenem for outpatient or step-down therapy of extended-spectrum β-lactamase urinary tract infections. Int J Antimicrob Agents 48:56–60. 10.1016/j.ijantimicag.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 154.Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, Tórtola T, Mirelis B, Navarro G, Cuenca M, Esteve M, Peña C, Llanos AC, Cantón R, Pascual A. 2008. Community infections caused by extended-spectrum β-lactamase–producing Escherichia coli. Arch Intern Med 168:1897–1902. 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 155.Reffert JL, Smith WJ. 2014. Fosfomycin for the treatment of resistant gram-negative bacterial infections. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 34:845–857. 10.1002/phar.1434. [DOI] [PubMed] [Google Scholar]
- 156.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. 2010. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 10:43–50. 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 157.Hirsch EB, Zucchi PC, Chen A, Raux BR, Kirby JE, McCoy C, Eliopoulos GM. 2016. Susceptibility of multidrug-resistant gram-negative urine isolates to oral antibiotics. Antimicrob Agents Chemother 60:3138–3140. 10.1128/AAC.02961-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Puerto AS, Fernández JG, del Castillo JDDL, Pino MJS, Angulo GP. 2006. In vitro activity of beta-lactam and non-beta-lactam antibiotics in extended-spectrum beta-lactamase-producing clinical isolates of Escherichia coli. Diagn Microbiol Infect Dis 54:135–139. 10.1016/j.diagmicrobio.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 159.Qiao LD, Chen S, Yang Y, Zhang K, Zheng B, Guo HF, Yang B, Niu YJ, Wang Y, Shi BK, Yang WM, Zhao XK, Gao XF, Chen M, Tian Y. 2013. Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ Open 3:e004152. 10.1136/bmjopen-2013-004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tasbakan MI, Pullukcu H, Sipahi OR, Yamazhan T, Ulusoy S. 2012. Nitrofurantoin in the treatment of extended-spectrum β-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents 40:554–556. 10.1016/j.ijantimicag.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 161.Walker E, Lyman A, Gupta K, Mahoney MV, Snyder GM, Hirsch EB. 2016. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis 63:960–965. 10.1093/cid/ciw396. [DOI] [PubMed] [Google Scholar]
- 162.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2021. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis 72:e169–e183. 10.1093/cid/ciaa1478. [DOI] [PubMed] [Google Scholar]
- 163.Ito R, Mustapha MM, Tomich AD, Callaghan JD, McElheny CL, Mettus RT, Shanks RMQ, Sluis-Cremer N, Doi Y. 2017. Widespread fosfomycin resistance in Gram-negative bacteria attributable to the chromosomal fosA gene. mBio 8:e00749-17. 10.1128/mBio.00749-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Linsenmeyer K, Strymish J, Gupta K. 2015. Two simple rules for improving the accuracy of empiric treatment of multidrug-resistant urinary tract infections. Antimicrob Agents Chemother 59:7593–7596. 10.1128/AAC.01638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.DeMarsh M, Brandon Bookstaver P, Gordon C, Lim J, Griffith N, Bookstaver NK, Justo JA, Kohn J, Al-Hasan MN. 2019. Prediction of sulfamethoxazole/trimethoprim resistance in community-onset urinary tract infections. J Glob Antimicrob Resist 21:218–222. 10.1016/j.jgar.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 166.Shah A, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. 2017. Application of fluoroquinolone resistance score in management of complicated urinary tract infections. Antimicrob Agents Chemother 61:e02313-16. 10.1128/AAC.02313-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rosa R, Abbo LM, Raney K, Tookes HE, Supino M. 2017. Antimicrobial resistance in urinary tract infections at a large urban ED: factors contributing to empiric treatment failure. Am J Emerg Med 35:397–401. 10.1016/j.ajem.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 168.Jorgensen S, Zurayk M, Yeung S, Terry J, Dunn M, Nieberg P, Wong-Beringer A. 2017. Emergency department urinary antibiograms differ by specific patient group. J Clin Microbiol 55:2629–2636. 10.1128/JCM.00481-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Percival KM, Valenti KM, Schmittling SE, Strader BD, Lopez RR, Bergman SJ. 2015. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med 33:1129–1133. 10.1016/j.ajem.2015.04.067. [DOI] [PubMed] [Google Scholar]
- 170.Landry E, Sulz L, Bell A, Rathgeber L, Balogh H. 2014. Urinary tract infections: leading initiatives in selecting empiric outpatient treatment (UTILISE). Can J Hosp Pharm 67:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hudepohl NJ, Cunha CB, Mermel LA. 2016. Antibiotic prescribing for urinary tract infections in the emergency department based on local antibiotic resistance patterns: implications for antimicrobial stewardship. Infect Control Hosp Epidemiol 37:359–360. 10.1017/ice.2015.283. [DOI] [PubMed] [Google Scholar]
- 172.Eudaley ST, Mihm AE, Higdon R, Jeter J, Chamberlin SM. 2019. Development and implementation of a clinical decision support tool for treatment of uncomplicated urinary tract infections in a family medicine resident clinic. J Am Pharm Assoc (2003) 59:579–585. 10.1016/j.japh.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 173.Pulcini C, Tebano G, Mutters NT, Tacconelli E, Cambau E, Kahlmeter G, Jarlier V, EUCIC-ESGAP-EUCAST Selective Reporting Working Group. 2017. Selective reporting of antibiotic susceptibility test results in European countries: an ESCMID cross-sectional survey. Int J Antimicrob Agents 49:162–166. 10.1016/j.ijantimicag.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 174.Langford BJ, Seah J, Chan A, Downing M, Johnstone J, Matukas LM. 2016. Antimicrobial stewardship in the microbiology laboratory: impact of selective susceptibility reporting on ciprofloxacin utilization and susceptibility of gram-negative isolates to ciprofloxacin in a hospital setting. J Clin Microbiol 54:2343–2347. 10.1128/JCM.00950-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tan TY, McNulty C, Charlett A, Nessa N, Kelly C, Beswick T. 2003. Laboratory antibiotic susceptibility reporting and antibiotic prescribing in general practice. J Antimicrob Chemother 51:379–384. 10.1093/jac/dkg032. [DOI] [PubMed] [Google Scholar]
- 176.Coupat C, Pradier C, Degand N, Hofliger P, Pulcini C. 2013. Selective reporting of antibiotic susceptibility data improves the appropriateness of intended antibiotic prescriptions in urinary tract infections: a case-vignette randomised study. Eur J Clin Microbiol Infect Dis 32:627–636. 10.1007/s10096-012-1786-4. [DOI] [PubMed] [Google Scholar]
- 177.Bourdellon L, Thilly N, Fougnot S, Pulcini C, Henard S. 2017. Impact of selective reporting of antibiotic susceptibility test results on the appropriateness of antibiotics chosen by French general practitioners in urinary tract infections: a randomised controlled case-vignette study. Int J Antimicrob Agents 50:258–262. 10.1016/j.ijantimicag.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 178.McNulty CA, Lasseter GM, Charlett A, Lovering A, Howell-Jones R, Macgowan A, Thomas M. 2011. Does laboratory antibiotic susceptibility reporting influence primary care prescribing in urinary tract infection and other infections? J Antimicrob Chemother 66:1396–1404. 10.1093/jac/dkr088. [DOI] [PubMed] [Google Scholar]
- 179.Mang N, Wei W, Ortwine J, Prokesch B. 2018. Fluoroquinolone usage reduction in the outpatient setting. Open Forum Infect Dis 5:S525–S526. 10.1093/ofid/ofy210.1498. [DOI] [Google Scholar]
- 180.Ferren R, Lee H, Park S, Thrupp L, Hsieh L. 2017. Impact of education on fluoroquinolone use in uncomplicated cystitis. Open Forum Infect Dis 4:S489. 10.1093/ofid/ofx163.1258. [DOI] [Google Scholar]
- 181.Slekovec C, Leroy J, Vernaz-Hegi N, Faller JP, Sekri D, Hoen B, Talon D, Bertrand X. 2012. Impact of a region wide antimicrobial stewardship guideline on urinary tract infection prescription patterns. Int J Clin Pharm 34:325–329. 10.1007/s11096-012-9606-6. [DOI] [PubMed] [Google Scholar]
- 182.Toy C, Peksa G, Wang S, Varughese C, Won S. 2017. Impact of an antimicrobial stewardship intervention on antibiotic prescribing practices for community-acquired acute uncomplicated cystitis in the emergency department. Open Forum Infect Dis 4:S270. 10.1093/ofid/ofx163.596. [DOI] [Google Scholar]
- 183.Davis LC, Covey RB, Weston JS, Hu BB, Laine GA. 2016. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm 73:S49–S56. 10.2146/sp150036. [DOI] [PubMed] [Google Scholar]
- 184.Lingenfelter E, Drapkin Z, Youngquist S, Fritz K, Fix M. 2014. Emergency department pharmacist monitoring of provider antibiotic selection for empiric treatment of outpatient urinary tract infections. Ann Emerg Med 64:S35. 10.1016/j.annemergmed.2014.07.121. [DOI] [PubMed] [Google Scholar]
- 185.Dumkow LE, Kenney RM, MacDonald NC, Carreno JJ, Malhotra MK, Davis SL. 2014. Impact of a multidisciplinary culture follow-up program of antimicrobial therapy in the emergency department. Infect Dis Ther 3:45–53. 10.1007/s40121-014-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wattengel BA, Sellick JA, Mergenhagen KA. 2020. Outpatient antimicrobial stewardship: optimizing patient care via pharmacist led microbiology review. Am J Infect Control 48:189–193. 10.1016/j.ajic.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 187.Bishop BM. 2016. Antimicrobial stewardship in the emergency department: challenges, opportunities, and a call to action for pharmacists. J Pharm Pract 29:556–563. 10.1177/0897190015585762. [DOI] [PubMed] [Google Scholar]
- 188.Jorgensen SCJ, Yeung SL, Zurayk M, Terry J, Dunn M, Nieberg P, Pallares J, Wong-Beringer A. 2018. Leveraging antimicrobial stewardship in the emergency department to improve the quality of urinary tract infection management and outcomes. Open Forum Infect Dis 5:ofy101. 10.1093/ofid/ofy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Grigoryan L, Zoorob R, Germanos G, Sidani M, Horsfield M, Khan F, Zare M, Goebel M, Atmar R, Trautner B. 2021. Case-based audit and feedback around a decision aid improved antibiotic choice and duration for uncomplicated cystitis in primary care clinics. Fam Med Com Health 9:e000834. 10.1136/fmch-2020-000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Malmros K, Huttner BD, McNulty C, Rodríguez-Baño J, Pulcini C, Tängdén T, ESGAP UTI Working Group. 2019. Comparison of antibiotic treatment guidelines for urinary tract infections in 15 European countries: results of an online survey. Int J Antimicrob Agents 54:478–486. 10.1016/j.ijantimicag.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 191.Tam VH, Louie A, Fritsche TR, Deziel M, Liu W, Brown DL, Deshpande L, Leary R, Jones RN, Drusano GL. 2007. Impact of drug-exposure intensity and duration of therapy on the emergence of Staphylococcus aureus resistance to a quinolone antimicrobial. J Infect Dis 195:1818–1827. 10.1086/518003. [DOI] [PubMed] [Google Scholar]
- 192.Sumi CD, Heffernan AJ, Lipman J, Roberts JA, Sime FB. 2019. What antibiotic exposures are required to suppress the emergence of resistance for gram-negative bacteria? A systematic review. Clin Pharmacokinet 58:1407–1443. 10.1007/s40262-019-00791-z. [DOI] [PubMed] [Google Scholar]
- 193.Nakano M, Yasuda M, Yokoi S, Takahashi Y, Ishihara S, Deguchi T. 2001. In vivo selection of Pseudomonas aeruginosa with decreased susceptibilities to fluoroquinolones during fluoroquinolone treatment of urinary tract infection. Urology 58:125–128. 10.1016/s0090-4295(01)01110-4. [DOI] [PubMed] [Google Scholar]
- 194.Katchman EA, Milo G, Paul M, Christiaens T, Baerheim A, Leibovici L. 2005. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med 118:1196–1207. 10.1016/j.amjmed.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 195.Vogel T, Verreault R, Gourdeau M, Morin M, Grenier-Gosselin L, Rochette L. 2004. Optimal duration of antibiotic therapy for uncomplicated urinary tract infection in older women: a double-blind randomized controlled trial. CMAJ 170:469–473. [PMC free article] [PubMed] [Google Scholar]
- 196.Arredondo-Garcia JL, Figueroa-Damian R, Rosas A, Jauregui A, Corral M, Costa A, Merlos RM, Rios-Fabra A, Amabile-Cuevas CF, Hernandez-Oliva GM, Olguin J, Cardenosa-Guerra O, uUTI Latin American Study Group. 2004. Comparison of short-term treatment regimen of ciprofloxacin versus long-term treatment regimens of trimethoprim/sulfamethoxazole or norfloxacin for uncomplicated lower urinary tract infections: a randomized, multicentre, open-label, prospective study. J Antimicrob Chemother 54:840–843. 10.1093/jac/dkh414. [DOI] [PubMed] [Google Scholar]
- 197.Lutters M, Vogt-Ferrier NB. 2008. Antibiotic duration for treating uncomplicated, symptomatic lower urinary tract infections in elderly women. Cochrane Database Syst Rev 2008:CD001535. 10.1002/14651858.CD001535.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Drekonja DM, Rector TS, Cutting A, Johnson JR. 2013. Urinary tract infection in male veterans: treatment patterns and outcomes. JAMA Intern Med 173:62–68. 10.1001/2013.jamainternmed.829. [DOI] [PubMed] [Google Scholar]
- 199.Germanos GJ, Trautner BW, Zoorob RJ, Salemi JL, Drekonja D, Gupta K, Grigoryan L. 2019. No clinical benefit to treating male urinary tract infection longer than seven days: an outpatient database study. Open Forum Infect Dis 6:ofz216. 10.1093/ofid/ofz216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Drekonja DM, Trautner B, Amundson C, Kuskowski M, Johnson JR. 2021. Effect of 7 vs 14 days of antibiotic therapy on resolution of symptoms among afebrile men with urinary tract infection: a randomized clinical trial. JAMA 326:324–331. 10.1001/jama.2021.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Grigoryan L, Zoorob R, Wang H, Horsfield M, Gupta K, Trautner BW. 2017. Less workup, longer treatment, but no clinical benefit observed in women with diabetes and acute cystitis. Diabetes Res Clin Pract 129:197–202. 10.1016/j.diabres.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 202.Sigler M, Leal JE, Bliven K, Cogdill B, Thompson A. 2015. Assessment of appropriate antibiotic prescribing for urinary tract infections in an internal medicine clinic. South Med J 108:300–304. 10.14423/SMJ.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 203.Kahan NR, Chinitz DP, Kahan E. 2004. Physician adherence to recommendations for duration of empiric antibiotic treatment for uncomplicated urinary tract infection in women: a national drug utilization analysis. Pharmacoepidemiol Drug Saf 13:239–242. 10.1002/pds.862. [DOI] [PubMed] [Google Scholar]
- 204.Denes E, Prouzergue J, Ducroix-Roubertou S, Aupetit C, Weinbreck P. 2012. Antibiotic prescription by general practitioners for urinary tract infections in outpatients. Eur J Clin Microbiol Infect Dis 31:3079–3083. 10.1007/s10096-012-1668-9. [DOI] [PubMed] [Google Scholar]
- 205.White AT, Clark CM, Sellick JA, Mergenhagen KA. 2019. Antibiotic stewardship targets in the outpatient setting. Am J Infect Control 47:858–863. 10.1016/j.ajic.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 206.Veninga CC, Denig P, Zwaagstra R, Haaijer-Ruskamp FM. 2000. Improving drug treatment in general practice. J Clin Epidemiol 53:762–772. 10.1016/s0895-4356(00)00194-3. [DOI] [PubMed] [Google Scholar]
- 207.Lagerlov P, Loeb M, Andrew M, Hjortdahl P. 2000. Improving doctors’ prescribing behaviour through reflection on guidelines and prescription feedback: a randomised controlled study. Qual Health Care 9:159–165. 10.1136/qhc.9.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rivard K, Wu J, Lin E, Coccarelli A, Taylor R, Fertel BS. 2019. 182 Antimicrobial stewardship in the emergency department: leveraging the electronic health record to optimize discharge prescriptions. Ann Emerg Med 74:S72. 10.1016/j.annemergmed.2019.08.188. [DOI] [Google Scholar]
- 209.Giancola SE, Higginbotham JM, Sutter DE, Spencer SE, Aden JK, Barsoumian AE. 2020. Improvement in adherence to antibiotic duration of therapy recommendations for uncomplicated cystitis: a quasi-experimental study. Fam Pract 37:242–247. 10.1093/fampra/cmz068. [DOI] [PubMed] [Google Scholar]
- 210.Sadyrbaeva-Dolgova S, Aznarte-Padial P, Jimenez-Morales A, Expósito-Ruiz M, Calleja-Hernández M, Hidalgo-Tenorio C. 2020. Pharmacist recommendations for carbapenem de-escalation in urinary tract infection within an antimicrobial stewardship program. J Infect Public Health 13:558–563. 10.1016/j.jiph.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 211.Schulz L, Osterby K, Fox B. 2013. The use of best practice alerts with the development of an antimicrobial stewardship navigator to promote antibiotic de-escalation in the electronic medical record. Infect Control Hosp Epidemiol 34:1259–1265. 10.1086/673977. [DOI] [PubMed] [Google Scholar]
- 212.Abbo LM, Hooton TM. 2014. Antimicrobial stewardship and urinary tract infections. Antibiotics (Basel) 3:174–192. 10.3390/antibiotics3020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Langford BJ, Leung E, Haj R, McIntyre M, Taggart LR, Brown KA, Downing M, Matukas LM. 2019. Nudging In MicroBiology Laboratory Evaluation (NIMBLE): a scoping review. Infect Control Hosp Epidemiol 40:1400–1406. 10.1017/ice.2019.293. [DOI] [PubMed] [Google Scholar]
- 214.Keller SC, Tamma PD, Cosgrove SE, Miller MA, Sateia H, Szymczak J, Gurses AP, Linder JA. 2018. Ambulatory antibiotic stewardship through a human factors engineering approach: a systematic review. J Am Board Fam Med 31:417–430. 10.3122/jabfm.2018.03.170225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Patel MS, Volpp KG, Asch DA. 2018. Nudge units to improve the delivery of health care. N Engl J Med 378:214–216. 10.1056/NEJMp1712984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Buehrle DJ, Phulpoto RH, Wagener MM, Clancy CJ, Decker BK. 2020. Decreased overall and inappropriate antibiotic prescribing in a Veterans Affairs hospital emergency department following a peer comparison-based stewardship intervention. Antimicrob Agents Chemother 65:e01660-20. 10.1128/AAC.01660-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Meeker D, Knight TK, Friedberg MW, Linder JA, Goldstein NJ, Fox CR, Rothfeld A, Diaz G, Doctor JN. 2014. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med 174:425–431. 10.1001/jamainternmed.2013.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tannenbaum D, Doctor JN, Persell SD, Friedberg MW, Meeker D, Friesema EM, Goldstein NJ, Linder JA, Fox CR. 2015. Nudging physician prescription decisions by partitioning the order set: results of a vignette-based study. J Gen Intern Med 30:298–304. 10.1007/s11606-014-3051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Meeker D, Linder JA, Fox CR, Friedberg MW, Persell SD, Goldstein NJ, Knight TK, Hay JW, Doctor JN. 2016. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 315:562–570. 10.1001/jama.2016.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vaughn VM, Gandhi TN, Chopra V, Petty LA, Giesler DL, Malani AN, Bernstein SJ, Hsaiky LM, Pogue JM, Dumkow L, Ratz D, McLaughlin ES, Flanders SA. 11 September 2020. Antibiotic overuse after hospital discharge: a multi-hospital cohort study. Clin Infect Dis 10.1093/cid/ciaa1372. [DOI] [PMC free article] [PubMed] [Google Scholar]