Abstract
Background:
Most chimeric antigen receptor T (CAR-T) cells and other adoptive T cell therapies (ACT) are currently manufactured by ex-vivo expansion of patient lymphocytes in culture media supplemented with human plasma from group AB donors. As lymphocytes do not express A or B antigens, the isoagglutinins of non-AB plasmas are unlikely to cause deleterious effects on lymphocytes in culture.
Study Design and Methods:
Using peripheral blood mononuclear cell (PBMNC) concentrates from group A1 donors and a CAR-T culture protocol, parallel cultures were performed, each using unique donor plasmas as media supplements (including group O plasmas with high-titer anti-A and group AB plasmas as control). An additional variable, a 3% group A1 erythrocyte (RBC) spike, was added to simulate a RBC-contaminated PBMNC collection. Cultures were monitored by cell count, viability, flow cytometric phenotype, gene expression analysis, and supernatant chemokine analysis.
Results:
There was no difference in lymphocyte expansion or phenotype when cultured using AB plasma or O plasma with high-titer anti-A. Compared to controls, the presence of contaminating RBCs in lymphocyte culture led to poor lymphocyte expansion and a less-desirable phenotype—irrespective of the isoagglutinin titer of the plasma supplement used.
Conclusions:
This study suggests that ABO incompatible plasma may be used as a media supplement when culturing cell types that do not express ABO antigens—such as lymphocytes for CAR-T or other ACT. The presence of contaminating RBCs in culture was disadvantageous independent of isoagglutinin titer.
Keywords: Adoptive cellular immunotherapy, Chimeric Antigen Receptor T cells, Cell Culture, ABO Blood-Group System, ABO isoagglutinins, Plasma, Cell Culture Media Supplements
Introduction
Adoptive T cell therapies (ACT) are being used in numerous applications including cancer treatment,1–6 prevention of disease relapse following hematopoietic progenitor cell (HPC) transplantation,7,8 and prevention of viral infections following HPC transplantation.9–12 T-lymphocytes used in ACT, whether tumor-derived (tumor infiltrating lymphocytes) or peripherally-collected and modified—such as chimeric antigen receptor T cells (CAR-T) or tumor-specific high-affinity T-cell receptors—are traditionally cultured in media supplemented with human AB plasma or serum.13–15
Issues that arise from the use of human plasma include the infectious risk of a donated product, the unpredictable balance of cytokines and growth factors that may be present in a plasma donor at the time of donation, and the presence of immunoglobulins. While the variables inherent to human plasma are less than ideal, decades of research on serum-free media supplements have failed to produce a widely-adopted alternative.16–18
Plasma from group A, B, or O donors contains anti-B, anti-A, or both isoagglutinins, respectively. Plasma from group AB donors contains neither anti-A nor anti-B isoagglutinins. The paradigm for the exclusive use of group AB plasma or serum for the culture of cellular therapy products stems from concerns about the untoward effects of residual anti-A or anti-B isoagglutinins upon infusion to an ABO incompatible recipient. However, as ABO minor-incompatible HPC products are routinely infused safely after washing (to remove ABO isoagglutinins), the same process should be sufficient to remove anti-A and/or anti-B from ACT products cultured with minor-incompatible media supplements. Less clear is the potential effect of anti-A and anti-B agglutinins from minor-incompatible supplements on the cells in culture—where interactions of ABO agglutinins may have suppressive or lethal effects on cell types expressing the corresponding ABO antigens. However, as lymphocytes do not express ABO antigens,19,20 we postulated that anti-A or anti-B in ABO minor-incompatible media supplements would not compromise their expansion.
Using a culture method developed to expand lymphocytes for CAR-T cell manufacturing, we test the feasibility of using minor-incompatible group O plasma, including samples with high-titer anti-A, as a media supplement.
Methods
Donor lymphocytes.
Peripheral blood mononuclear cells (PBMNC) from consented ABO group A1 research donors were collected via two-armed apheresis (Fenwal Amicus; Fresenius Kabi, Bad Homburg vor der Höhe, Germany). The PBMNCs were further processed by counter-flow centrifugation elutriation procedure (Elutra Cell Separation System; TerumoBCT, Lakewood, CO) to yield a lymphocyte-enriched product. Residual red cells were removed from the elutriation product by ammonium-chloride-potassium (ACK)-lyse (Lonza, Walkersville, MD) procedure followed by two washes with phosphate-buffered saline. The cellular content of the product was then characterized using both the Cellometer (Nexcelom, Lawrence, MA) and Advia (Siemens, Munich, Germany). The PBMNC products were cryopreserved in aliquots, and stored in vapor phase liquid nitrogen for no more than 3 months before thaw and culture initiation. The ABO antigen A1 of the donors was confirmed by standard forward and reverse typing (tube method) as well as agglutination testing with anti-A1 lectin. Donor HLA types were determined (HLA-A, HLA-B, and HLA-C alleleSEQR Sequence-Based Typing kits; Atria Genetics, Hayward, CA; and ABI Prism* 3730xL DNA Analyser; Applied Biosystems, Waltham, MA). HLA high-resolution determination was performed by comparing sequence data to reference sequences for all known HLA alleles as noted in the IMGT/HLA database.
Donor plasma.
Plasma was collected from consented group O whole blood donors. After standard centrifugation and plasma extraction of whole blood, repeat centrifugation of plasma was performed to ensure removal of any contaminating platelets prior to freezing at −18°C. Donor plasma was screened for anti-A titer by tube method (ALBAcyte A1 cells; Quotient, Newtown, PA). This method detects anti-A and will, if present, also detect anti-A1; group O donors and a few donors with the A2 phenotype, lacking the antigen A1, may have anti-A1 in addition to anti-A.21 We did not specifically test for anti-A1, because the clinical relevance of anti-A1 is controversial and would be overwhelmed by the effect of anti-A.22,23 Selected units representing a range of anti-A titers were thawed, irradiated, heat-inactivated at 56 °C for 120 min, and filtered using standard blood filters (Fresenius Kabi, Lake Zurich, IL). The anti-A titer was repeated after the irradiation, heat-inactivation, and filtration (I/HI/F) to ensure anti-A persisted. Donor plasma was screened for HLA class I antibodies using a membrane-independent solid phase assay and a flow analyzer (LAB-Screen and LABScan 100 flow analyzer; One Lambda, Canoga Park, CA). Samples with a mean fluorescence intensity >500 were further tested for antibody specificity (LABScreen Single Antigen kits; One Lambda).
Heat-inactivated AB plasma was purchased (Valley Biomedical, Winchester, VA). Screening for HLA class I antibodies was performed as described above.
Cell culture.
Donor lymphocytes were cultured for nine days in media supplemented with either O plasma (with variable anti-A titers) or AB plasma. Culture media conformed to our current culturing procedure for CAR T-cells—consisting of AIM-V media (ThermoFisher Scientific, Waltham, MA) with 2mM GlutaMAX-I (ThermoFisher), 40IU/mL recombinant interleukin 2 (rIL-2) (Proleukin, Prometheus Therapeutics and Diagnostics, Vevey, Switzerland), anti-CD3/CD28 magnetic beads (Dynabeads, ThermoFisher), and donor plasma. The concentration of plasma (5%) in the culture media was kept consistent in all cultures—only the source of the plasma was changed. On day 3 of culture, the concentration of rIL-2 in each culture was increased to 300IU/mL and kept at that concentration through day 9. The increment in rIL-2 at day 3 coincides with CAR transduction in our clinical procedure—therefore it was paralleled in these cultures, although no transduction was performed in this study.
All cultures were initiated at a concentration of 1 million total nucleated cells (TNC) per mL. As flow cytometric results were not available immediately upon culture initiation, anti-CD3/28 beads were added at a ratio of 3 beads per cell based on a standard estimate of CD3 cells representing 50% of the TNC of our elutriated product.
Cell counts were performed using a Cellometer (Nexcelom, Lawrence, MA) and confirmed manually on days 0, 3, 5, 7, and 9 of culture. Additionally, media supplementation and culture splitting to retain adequate volume for culture expansion, if necessary, was performed on these days.
Effect of Anti-A Titer on the Culture of Lymphocytes of a Group A1 Donor:
Cultures were carried out as described above. For this experiment, cultures were initiated at a concentration of 1 million TNC per mL at a starting volume of 25 mL in a 30 mL culture bag (PermaLife PL30, OriGen Biomedical, Austin, TX). Cultures were run in parallel using Plasma Panel #1 (Table 1), which consisted of 5 group O plasmas with high-titer anti-A, 3 group O plasmas with low-titer anti-A, and 3 group AB plasmas as controls.
Table 1.
Characteristics of Plasma Supplements used in Cultures (in order of descending anti-A titers)
| Anti-A titer | ||||
|---|---|---|---|---|
| Plasma ID | Donor ABO | Pre-I/HI/F* | Post-I/HI/F* | HLA Antibody Screen |
| O (7) †‡ | O | 256 | 128 | Negative |
| O (8) †‡ | O | 256 | 64 | Negative |
| O (6) †‡ | O | 128 | 64 | Negative |
| O (9) ‡ | O | 128 | 32 | Negative |
| O (5) † | O | 128 | 32 | Negative |
| O (1) † | O | 64 | 32 | Negative |
| O (2) † | O | 16 | 16 | Negative |
| O (4) † | O | 8 | 8 | Negative |
| O (3) † | O | 4 | 2 | Negative |
| AB (1) †‡ | AB | n/a | n/a | Negative |
| AB (2) †‡ | AB | n/a | n/a | Negative |
| AB (3) † | AB | n/a | n/a | Strong anti-HLA A25 |
Irradiation, Heat-Inactivation, and Filtration (I/HI/F)
Sample included in Plasma Panel #1
Sample included in Plasma Panel #2
Effect of High Anti-A Titer on the Culture of Lymphocytes of Three Group A1 Donors with and without Contaminating Group A1 RBCs:
Cultures were carried out as described above. For this experiment, cultures were initiated at a concentration of 1 million TNC per mL at a starting volume of 2 mL in standard 6-well culture plates (Costar Cell Culture Plates, Corning, Corning, NY). Cultures were run in parallel using Plasma Panel #2 (Table 1), which consisted of 4 group O plasmas of high-titer anti-A and 2 group AB plasmas as controls. Additionally, in this experiment, each culture was performed with and without a 3% spike of group A1 donor RBCs. Figure 1 provides a visual reference for cultures performed.
Fig. 1. Diagram of cultures performed.
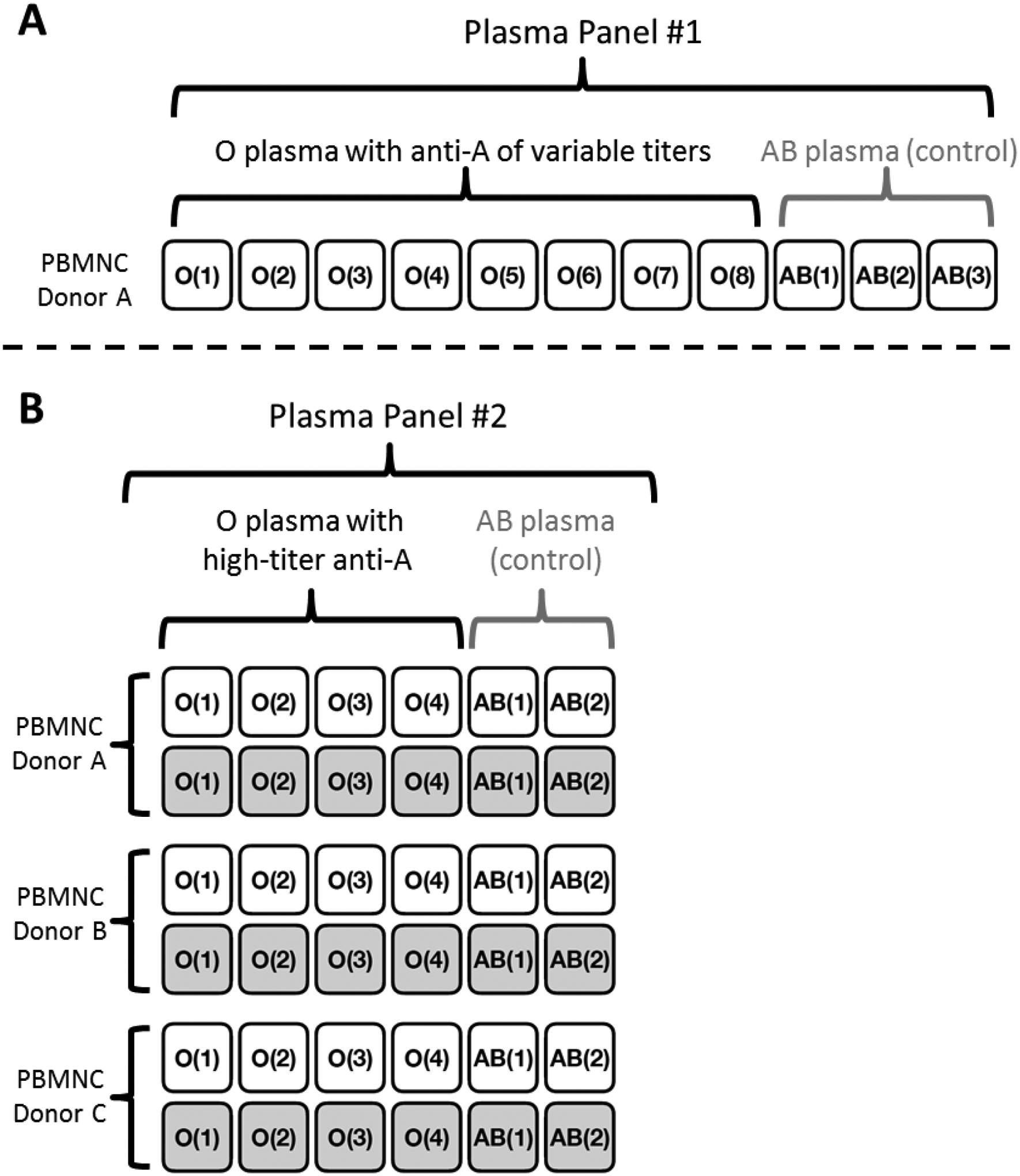
Each box represents a separate culture. Text within the box corresponds to the unique Plasma ID of the plasma supplement used in that culture (characteristics of plasma supplements are listed in Table 1). A) Cultures were performed using the elutriated PBMNCs of a single group A1 donor; each culture was supplemented with a unique plasma with variable titer anti-A or AB plasma as control. B) Cultures were performed using the elutriated PBMNCs of three group A1 donors; each culture was supplemented with a unique plasma with high-titer anti-A or AB plasma as control. Additionally, each donor/plasma combination was run without and with a 3% group A1 RBC spike (represented by white and gray boxes, respectively).
Flow cytometric analysis.
Flow cytometric analysis was performed on day 0 (post-thaw) and again on day 9 with a panel consisting of CD3, CD4, CD8, CD45RA, CCR7, CD45, CD19, CD56 (naïve/memory phenotype, as well as markers for B and NK cells) on FACSCanto II (BD Biosciences, San Jose, CA). Naïve/memory subsets defined as: naïve (CD45RA+, CCR7+), central memory (CD45RA−, CCR7+), and effector memory (CD45RA−, CCR7−)
Gene expression analysis.
At day 9 of culture, 10×10^6 cells were taken from culture, pelleted, supernatant removed, mixed in 700 uL of QIAzol, and immediately frozen at −80 °C for later RNA extraction using the miRNeasy Mini Kit (Qiagen, Germantown, MD,USA). The RNA concentration was determined using an spectrophotometer (ND-1000; Nano Drop Technologies, Wilmington, DE, USA) and RNA quality was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). RNA was amplified and labeled using an Agilent LowInput QuickAmp Labeling Kit and subsequently mixed with Universal Human Reference RNA (Stratagene, Santa Clara, CA, USA) and cohybridized to Agilent Chip Whole Human genome, 4 × 44 k slides according to the protocol provided by Agilent. The slides were incubated for 17 h at 65 °C and then the scanned using an Agilent B Scanner. Raw images were obtained by scanning the slides with the Agilent Scan G2505B and Agilent Scan Control software (version 9.5). The images were extracted using the Feature Extraction Software (Agilent Technologies).
Supernatant chemokine analysis.
At day 9 of culture, triplicate 1 mL supernatant samples from each culture were frozen at −80 °C for later analysis. The supernatants were later evaluated using a multiplex ELISA method. In total, 40 soluble factors were measured using the Bio-Plex Pro Human Chemokine Panel, 40-plex (Bio-Rad Laboratories, Hercules, CA, USA) and a Bio-Plex MAGPIX Multiplex Reader (Bio-Rad Laboratories). In brief, supernatant samples were incubated with microbeads coated with antibodies specific to the above-mentioned chemokines for 1 hour. After washing, the beads were incubated with the detection antibody cocktail, which included a biotinylated antibody specific to each cytokine. After another wash step, the beads were incubated with streptavidin-phycoerythrin for 10 minutes and washed again. Finally, the fluorescence was measured (Bio-Plex MAGPIX Multiplex Reader).
Statistical analysis.
Expansion, viability, and flow cytometric subsets of culture groups with variable anti-A titers were compared using two-tailed nonparametric Mann-Whitney U test. Comparison of expansion, viability, and flow cytometric subsets between paired cultures with and without 3% RBC was performed using two-tailed nonparametric Wilcoxon test. For each investigated parameter both a mixed-effects model (due to the repeat measures carried out on donor cells) and a linear model (for comparison, since the number of PBMNC donors was small) were built. Anti-A titer and RBC were set as fixed effects with PBMNC donor set as a random effect. Results of the two models were the same, thus, only linear model data is presented. Models were built for total expansion, fold-expansion, anti-A titer, viability, TCM, TEM, naïve, and CD4:CD8 ratio. Visual inspection of model residual plots did not reveal significant deviations from homoscedasticity or normality. Effect displays were plotted to better illustrate the relationship between model predictors and outcomes.24 The analysis was performed using R software (version 3.6.1).
Gene expression analysis used Partek Genomic Suite 6.4 (Partek, St. Louis, MO, USA) for data visualization, identification of differentially expressed transcripts, and hierarchical cluster analysis. We transformed the fluorescence intensity data to log2 ratios of each sample versus the universal human RNA reference (Stratagene, Santa Clara, CA, USA). T-tests were used to identify differentially expressed genes.
Supernatant chemokine analysis was evaluated using Partek Genomic Suite 6.4 for data visualization and hierarchical cluster analysis. The data was normalized using log(X + offset).
Results
Effect of Anti-A Titer on the Culture of Lymphocytes from a Group A1 Donor
Lymphocytes isolated from a single group A1 PBMNC donor were cultured in parallel using media which varied by plasma supplement (Fig. 1A). The 11 plasma supplements used included 5 group O plasma with high anti-A titer (32–128), 3 group O plasma with low anti-A titer (2–16), and 3 group AB plasma as controls (Table 1 – Plasma Panel #1).
T Cell Expansion, Purity, and Viability
There was no significant difference in T-lymphocyte expansion, purity, or viability when comparing group AB control cultures to either group O plasma with low anti-A titer or group O plasma with high anti-A titer cultures (Table 2). Though not a statistically significant difference, quantity of CD3+ cells, proportion of CD3+ cells, and TNC viability were highest among the cultures supplemented with group O plasma of high anti-A titer.
Table 2.
Quantity, Purity, Viability, and Phenotype of Lymphocytes by Media Supplement
| Plasma Supplement Group | p | ||||||
|---|---|---|---|---|---|---|---|
| AB (n=3) | O (low)* (n=3) | O (high) † (n=5) | O (all) ‡ (n=8) | AB vs. O (low) | AB vs. O (high) | AB vs. O (all) | |
| CD3+ Cells (×106) | 256±190 | 224±49 | 291±116 | 266±98 | >0.99 | 0.39 | 0.49 |
| CD3+ Cells (%) | 94.2±1.7 | 94.4±1.6 | 94.6±2.1 | 94.6±1.8 | 0.80 | 0.53 | 0.50 |
| TNC Viability (%) | 85.5±1.9 | 88.8±3.0 | 90.8±3.6 | 90.0±3.0 | 0.20 | 0.07 | 0.04 |
| CD4/CD8 ratio | 2.89±2.03 | 3.14±1.89 | 1.64±0.83 | 2.21±1.42 | 0.40 | 0.14 | 0.63 |
| Naïve (%) | 0.37±0.46 | 0.97±0.78 | 1.10±1.09 | 1.05±0.92 | 0.40 | 0.48 | 0.34 |
| TCM (%) | 13.70±16.01 | 22.57±19.64 | 29.96±18.69 | 27.19±18.01 | 0.70 | 0.26 | 0.29 |
| TEM (%) | 85.37±15.84 | 75.73±19.24 | 67.74±19.60 | 70.74±18.50 | 0.40 | 0.26 | 0.20 |
All values were determined at the end of the culture period (Day 9).
P determined by Mann-Whitney U test, two-tailed.
Cultures supplemented with group O plasma with anti-A titers between 2 and 16
Cultures supplemented with group O plasma with anti-A titers between 32 and 128
Cultures supplemented with group O plasma with anti-A titers between 2 and 128 (low and high-titer groups combined)
Comparison of group AB plasma control cultures to all group O plasma cultures (combining data of the low and high anti-A titer groups), revealed no difference in mean quantity of CD3+ cells 256±190 × 106 (n=3) vs. 266±98 × 106 (n=8) (p=0.49) or in proportion of CD3+ cells 94.2±1.7% (n=3) vs. 94.6±1.8% (n=8) (p=0.50) (Table 2). When comparing viability of the control group AB cultures to all group O plasmas, the O plasma cultures showed significantly higher viability, 85.5±1.9% vs. 90.0±3.0% (p=0.04). The reason for increased viability in this group is unclear, but is likely due to variables in human plasma other than the anti-A titer. Additionally, this finding suggests that higher anti-A titer did not hinder cell viability.
Phenotype Analysis
The mean CD4/CD8 ratio of the CD3+ cell changed, as expected, from 0.7 at the start of the culture to 2.2 at day 9, the end of the culture (data not shown). The differences between end-of-culture the CD4/CD8 ratio between AB plasma and low-titer O plasma or high-titer O plasma were not significant (Table 2).
Regardless of anti-A titer, group A1 lymphocytes cultured in O plasma showed similar phenotype to those grown in AB plasma—there was no significant difference in day 9 percentage of effector memory (TEM), central memory (TCM), or naïve T-cells (Table 2). As expected, by day 9 of culture, TEM (CD45RA−, CCR7−) predominated over TCM (CD45RA−, CCR7+), while naïve T-cells (CD45RA+, CCR7+) were few or absent. Anti-A in the media supplement did not appear to cause phenotypic changes of cultured lymphocytes.
Effect of High Anti-A Titer on the Culture of Lymphocytes of Three Group A1 Donors with and without Contaminating Group A1 RBCs
To determine if the presence of ABO-incompatible RBCs would affect T cell expansion in cultures using media supplemented with O plasma of high-titer anti-A, we added group A1 RBCs at a concentration of 3% to cultures of lymphocytes of 3 group A1 MNC donors and cultured the cells in group O plasma of high-titer anti-A from 4 donors and group AB plasma from 2 donors. In addition, the lymphocytes of the 3 group A1 donors were cultured with the same 4 group O and 2 group AB plasma supplements, but without 3% RBC spike (Table 1–Plasma Panel #2 and Fig. 1B).
T Cell Expansion, Purity, and Viability
There was no difference in expansion, purity, or viability when media supplemented with group O plasma or group AB plasma was used to expand lymphocytes in absence of group A1 RBCs (Table 3). Similarly, there was no difference in expansion, purity, or viability when media supplemented with group O plasma or group AB plasma was used to expand lymphocytes in presence of group A1 RBCs (Table 3).
Table 3.
Quantity, Purity, Viability, and Phenotype of Lymphocytes by Plasma Supplement and RBC Contamination
| RBC contamination | ||||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Group AB Plasma (n=6) | Group O Plasma* (n=12) | p | Group AB Plasma (n=6) | Group O Plasma* (n=12) | p | |
| CD3+ Cells (×106) | 129±47 | 145±56 | 0.61 | 92±27 | 111±44 | 0.29 |
| CD3+ Cells (%) | 91.6±0.9 | 91.9±1.8 | 0.61 | 91.7±2.4 | 92.5±1.8 | 0.24 |
| TNC Viability (%) | 91.6±2.5 | 92.8±2.5 | 0.53 | 91.2±2.6 | 91.2±1.6 | 0.63 |
| Naïve (%) | 0.01±0.01 | 0.01±0.01 | 0.82 | 0.07±0.03 | 0.07±0.05 | 0.44 |
| TCM (%) | 25.57±5.13 | 25.17±8.21 | 0.38 | 18.41±7.13 | 14.02±5.96 | 0.17 |
| TEM (%) | 74.39±5.13 | 74.76±8.18 | 0.38 | 81.51±7.13 | 85.89±5.94 | 0.17 |
All parameters were determined at the end of the culture period (Day 9).
P determined by Mann-Whitney U test, two-tailed.
with anti-A of high titer (titers between 32 and 128)
Phenotype Analysis
In cultures with and without RBCs present, phenotypes at the end of the 9 day culture period showed predominance of TEM over TCM, with almost no detectable naïve cells (Table 3). In the presence of RBCs, TEM represented 81.51±7.13% and 85.89±5.94% of day 9 cells in cultures supplemented with AB plasma or group O plasma of high-titer anti-A, respectively (p=0.17). In the absence of RBCs, TEM represented 74.39±5.13% and 74.76±8.18% of day 9 cells in cultures supplemented with AB plasma or group O plasma of high-titer anti-A, respectively (p=0.38). Thus, T cells cultured in a supplement of group O plasma with high-titer anti-A did not differ phenotypically from those with AB plasma as control.
Effect of RBC on Expansion, Purity, Viability, and Phenotype
When absolute CD3+ expansion in all 18 cultures without RBCs was compared to the 18 cultures with RBCs, the expansion was significantly greater in cultures without RBCs (Table 4). The reduced expansion in the presence of RBCs was seen in cultures with media supplemented with AB plasma as well as cultures with media supplemented with O plasma of high-titer anti-A, suggesting that the presence of the RBCs alone contributed to the poor growth. Fold-expansion was significantly greater in cultures without RBCs than with RBCs (90.1±33.4 and 67.3±24.8, respectively; p<0.005). Figure 2A shows the CD3+ cell fold-expansion for all 36 cultures plotted against the anti-A titer of the supplement. Most cultures without RBCs showed 20- to 40-fold higher expansion than the corresponding culture with RBCs (regardless of anti-A titer).
Table 4.
Effect of RBC Contamination on Lymphocyte Culture with regard to Quantity, Purity, Viability, and Phenotype
| RBC Absent (n=18) | RBC Present (n=18) | p | |
|---|---|---|---|
| CD3+ Cells (×106) | 139.7.1±52.6 | 104.8±39.7 | <0.005 |
| CD3+ Cells (%) | 91.8±1.6 | 92.3±2.0 | 0.70 |
| TNC Viability (%) | 92.4±2.5 | 91.9±2.0 | 0.28 |
| CD3+ fold-expansion* | 90.1±33.4 | 67.4±24.8 | <0.005 |
| Naïve (%) | 0.01±0.01 | 0.07±0.04 | <0.005 |
| TCM (%) | 25.30±7.17 | 15.48±6.52 | <0.005 |
| TEM (%) | 74.63±7.15 | 84.43±6.50 | <0.005 |
All values were determined at the end of the culture period (Day 9).
P determined by Wilcoxon test, two-tailed.
Fold Expansion = (number of CD3+ cells on day 9 harvest) / (number of CD3+ cells seeded on day 0)
Fig. 2. CD3+ fold-expansion without and with 3% RBC contamination by anti-A titer of media supplement.
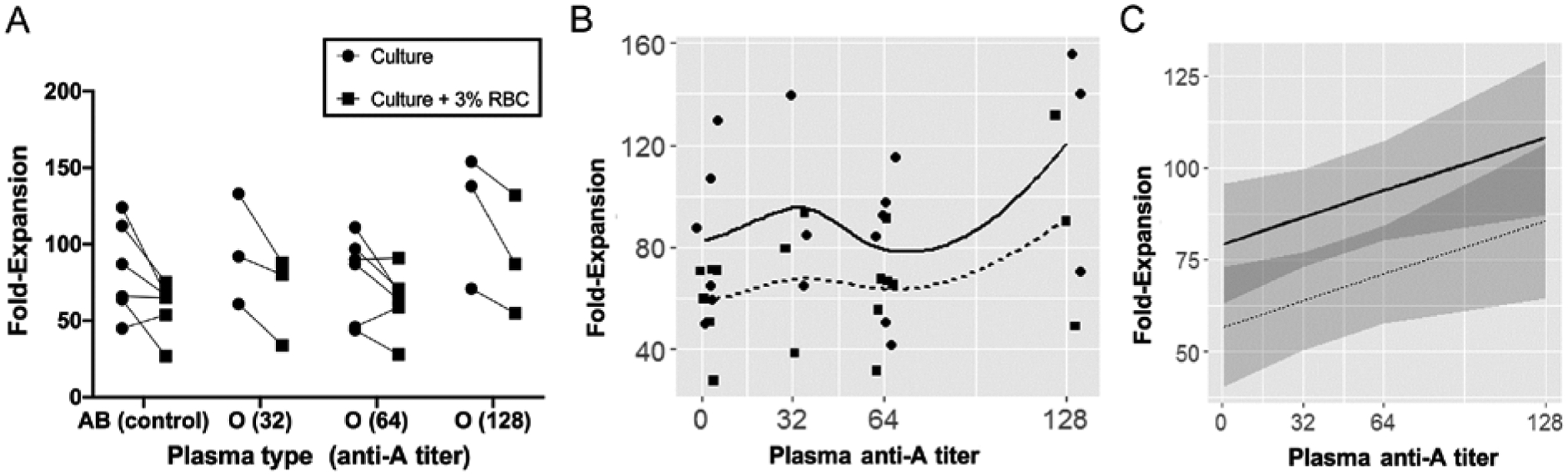
Circles and squares (in panels A and B) represent cultures without and with 3% RBC contamination, respectively. Solid and dashed lines (in panels B and C) represent trends of cultures without and with 3% RBC contamination, respectively. A) Corresponding cultures (i.e. cultures using the same MNC donor and plasma supplement—where the only variable is absence/presence of 3% RBC spike) are connected by a line. Most cultures with 3% RBC had a decreased fold-expansion compared to the corresponding culture without RBC (regardless of anti-A titer of plasma supplement). B) Scatterplot with LOESS curve fitting and C) Effects plot of cultures without and with 3% RBC spike.
A difference was also seen in the phenotype among cells cultured with and without added RBCs. When all cultures with added RBC were compared to those without RBCs (regardless of plasma supplement) the proportion of TEM at the end of the culture was greater in the cultures with added RBCs; 84.43±6.50% and 74.63±7.15%, respectively (p<0.005) (Table 4). Thus, the presence of contaminating RBCs in lymphocyte cultures appears to be an independent variable driving cells toward a TEM phenotype.
While both expansion and phenotype were negatively affected by the presence of RBCs in culture, purity and viability were similar between cultures grown in the presence or absence of RBCs (Table 4).
Linear Regression Model of Measured Parameters
The linear regression models corroborated the findings of the expansion, viability, and phenotypic parameters between cultures grown in the presence or absence of RBCs as discussed above. A scatterplot of CD3+ fold-expansion by titer, with LOESS curve fitting (Fig. 2B) and the respective effects plot (Fig. 2C) demonstrate that the effect of anti-A titer is uniform in cultures with and without contaminating RBCs. Scatterplots for phenotypic variables by anti-A titer are available online as supplementary material (Appendix S1). Pairwise comparisons of all 36 cultures showed that anti-A titer was not associated with changes in expansion, viability, or phenotype (Fig. 3). Predictably, a correlation is seen between CD3+ total expansion and CD3+ fold-expansion (p<0.001). Reciprocal negative and positive correlations (−0.37 and 0.37, p<0.05) are seen when comparing naïve to TEM cells and naïve to TCM cells, respectively. This is explained by the relatively small number of naïve cells in all cultures combined with the strong correlation between TCM and TEM cells (p<0.001). These findings are largely predictable based on the populations’ direct relationship.
Fig. 3. Correlation matrix plot.
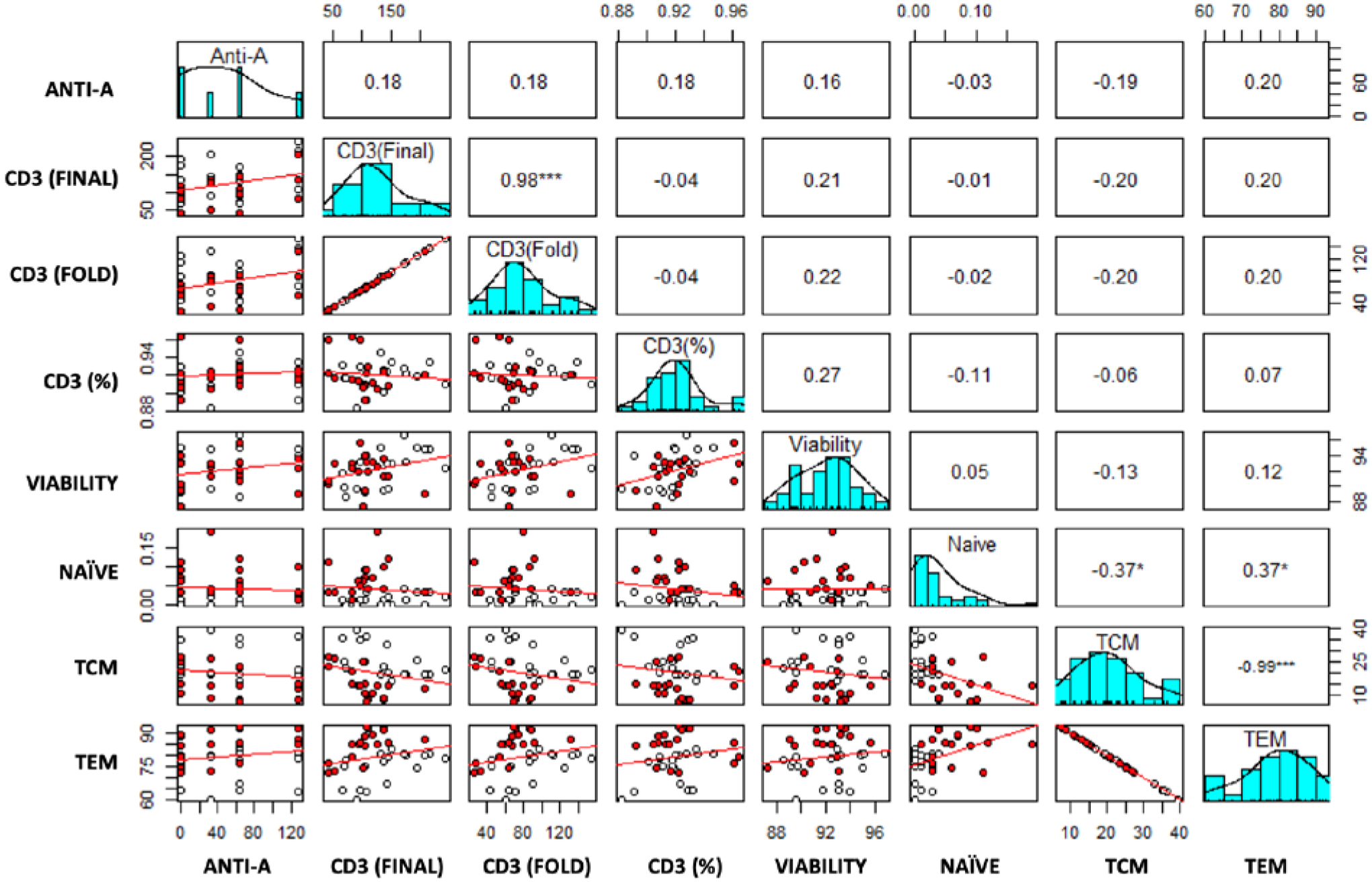
Pairwise comparisons of culture characteristics—anti-A titer of plasma supplement as well as final (day 9) cellular attributes (including CD3+ final expansion, CD3+ fold-expansion, % CD3, viability, % naïve T-cells, % TCM, and % TEM). The correlation matrix plot shows Kendall correlation coefficients (top and right) with asterisks representing the significance of each pairwise comparison (*p < 0.05 and ***p < 0.001) and corresponding bivariate scatter plots with red linear regression lines (bottom and left) where white and red dots represent cultures without and with 3% RBC, respectively. Diagonal blue bar graphs represent the distribution of each variable studied. No significant correlation is seen between anti-A titer and any of the expansion or phenotypic parameters (top row).
Gene Expression and Supernatant Chemokine Analysis
To further analyze the effects of anti-A on the cultured lymphocytes of group A1 donors, the 36 lymphocyte cultures were harvested at day 9, nucleic acid isolated, and subjected to gene expression analysis. Hierarchical clustering analysis of the transcripts of the 36 expanded T cell cultures separated the samples by PBMNC donor. After correcting for donor effect, principal component analysis (PCA) (Fig. 4A) and hierarchical clustering analysis (Fig. 4B) separated samples into two groups—those cultured in the presence and absence of RBCs. There was no clustering of samples based on ABO group or anti-A titer of the plasma media supplement used. A single outlier is seen in PCA—this represents a sample from MNC donor 3 cultured in AB plasma in the presence of RBC. Given that this outlier represents an AB plasma control, it is unclear how to interpret this result. However, cultures without RBC contamination form a tighter cluster than those with RBC contamination, which could indicate more variability and, therefore, more potential for outliers in cultures with RBC contamination. Hierarchical clustering showed 4077 genes differently expressed in cultures with and without RBC, yielding a p-value and false discovery rate of ≤0.01 (Fig. 4C). Transcripts that were upregulated in the presence of RBCs predominately represented immune-response pathways, while those which were downregulated in the presence of RBCs represented a variety of metabolic pathways (Appendix S2).
Fig. 4. Gene expression in cultures with and without RBC contamination.
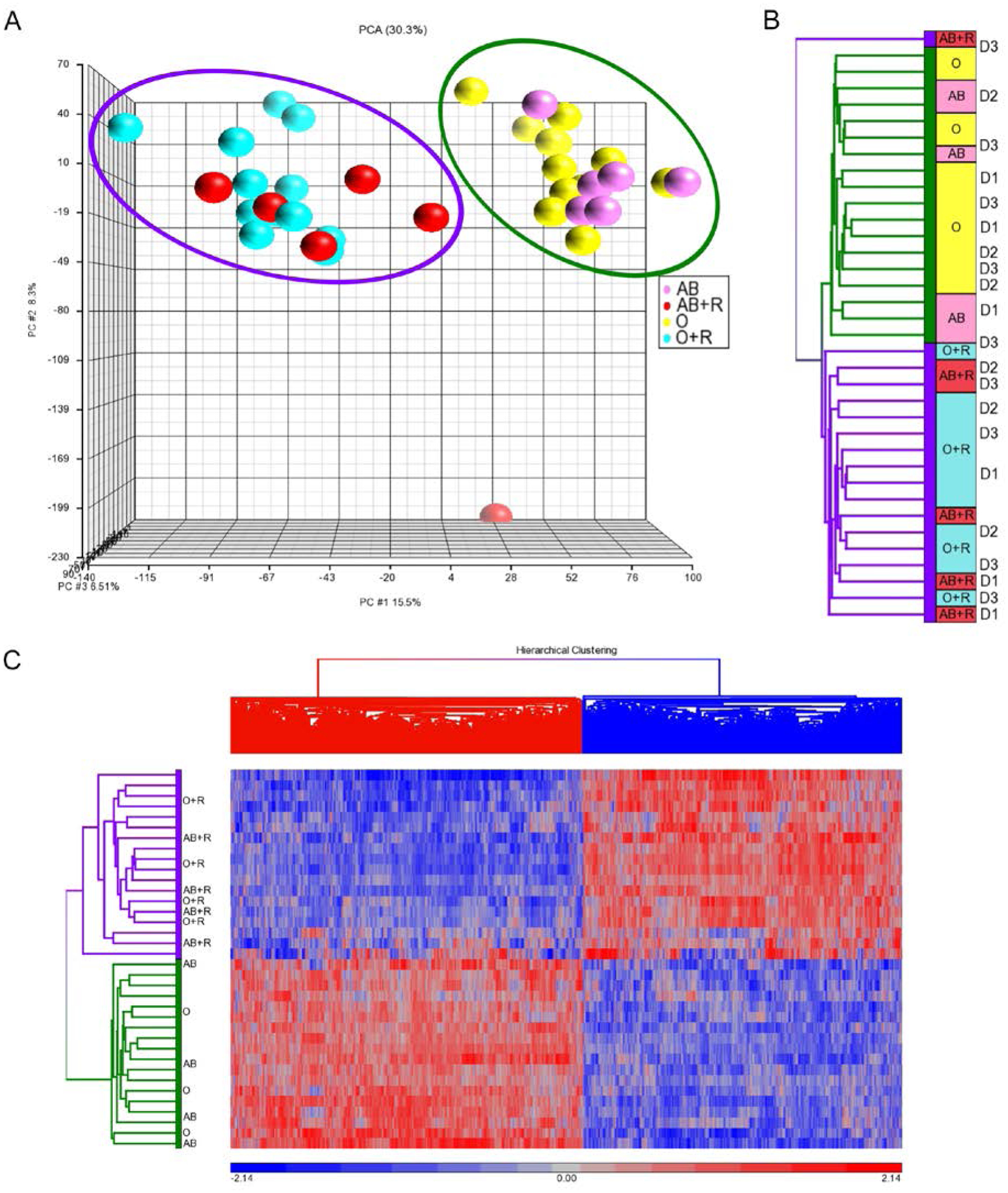
Labels indicate plasma group (“O” or “AB”) used in each culture with suffix of “+R” for any culture with 3% RBC spike. A) Principal component analysis (PCA) where individual dots represent each culture and are color-coded as follows: Pink (AB) are cultures with AB plasma without RBC; Red (AB+R) are cultures with AB plasma with RBC; Yellow (O) are cultures with group O plasma of high-titer anti-A without RBC; Cyan (O+R) are cultures with group O plasma of high-titer anti-A with RBC. Violet and green circles define the clusters—which group by presence or absence of 3% contaminating RBCs in the culture, respectively, with the exception of one outlier. B) Unsupervised hierarchical clustering analysis retains color-coding as listed above. Violet and green lines of the tree represent samples with and without 3% RBC, respectively. C) Heat-map of gene expression. Hierarchical clustering showing 4077 genes expressed differently in cultures with and without RBC contamination. Violet and green lines on the tree represent cultures with and without 3% RBC spike, respectively.
Finally, day 9 culture supernatants were analyzed for the levels of 40 cytokine/chemokines. We removed 6 of the 36 culture samples from the analysis due to data failure on the control supernatant sample. These 6 samples represented the cultures with and without RBC spike for all 3 PBMNC donors that were cultured in plasma supplement “O(9),” the sample with the lowest anti-A titer of Plasma Panel #2 (Table 1). Of the 30 samples analyzed, 3 of 40 factors were increased/decreased in AB vs. O cultures where RBC were absent (p=0.378), and only 1 of 40 factors were increased/decreased in AB vs. O cultures where RBC were present (p=0.505). A significant difference was seen when comparing all cultures without RBC to all cultures with RBC, where 10 of 40 factors were increased/decreased (p≤0.05). A heat-map analysis shows the differential expression of these 10 factors (IL-10, IL-2, CCL8, CCL21, TNF-a, CCL24, IFN-g, CCL23, CXCL16, CCL3) between cultures with and without RBCs (Appendix S3).
Discussion
Historic use of Group AB Plasma for ACT
Human AB plasma is currently the standard media supplement for culturing lymphocytes for CAR-T and other ACT—however, it is a limited resource. Only 4% of Caucasians are ABO group AB (with other populations having a similarly low prevalence), resulting in a small donor pool. Additionally, AB plasma is in critical demand by the general transfusion service, where it is required for life-saving, emergency transfusion in patients with group AB or with unknown ABO type. The difficulties of managing AB plasma inventory on the general transfusion service have been documented.25,26 While serum-free media supplements have been developed, they have not been widely-adopted.16–18 As the demand for ACT products increases, cell therapy services will create even greater competition for this already scarce resource—thus, finding alternatives to AB plasma is critical for patient care and safety. Human O plasma may represent a comparatively cheap and readily available alternative to AB plasma, but has not been historically used due to concerns regarding anti-A and/or anti-B isoagglutinins.
Both the AABB and FACT (the Foundation for Accreditation of Cellular Therapy) require that cellular therapy services address the issues of donor/recipient ABO incompatibility.27–29 Washing and red cell depletion of cellular therapy products for ABO minor and/or major incompatibility, respectively, have become standard. While these guidelines were developed to apply to the transfusion of HPC products, they should also be considered in products cultured in media supplemented with plasma from minor-incompatible donors. Risk of hemolytic reaction from residual ABO agglutinins to the recipient of a cultured ACT product, would be readily addressed by washing—which is already routinely performed on cultured cellular therapy products.
Potential for use of Group O Plasma for ACT
Given the ability to safely remove ABO isoagglutinins prior to infusion of ACT via washing, exclusive use of AB plasma as a media supplement is unnecessary as long as said isoagglutinins do not adversely affect the cell therapy cultures. Using a model consistent with our current culture practices for CAR-T, we demonstrated no significant difference in viability, expansion, or phenotype when substituting O plasma for AB plasma as a media supplement for culture. Our findings suggest that the practice of exclusive use of AB plasma for the culture of ACT products is likely unnecessary. In this study, we found no demonstrable negative change in lymphocyte expansion, purity, viability, phenotype, gene expression, or supernatant chemokine analysis when cultured using group O plasma with low or high-titer anti-A.
While most lymphocyte expansion protocols include steps to enrich for lymphocytes or to deplete RBCs prior to initiating culture, red cell contamination remains a problem with certain apheresis PBMNC collections. Thus, assuring that lymphocyte culture was not hindered by the potential interaction between isoagglutinins in minor-incompatible plasma supplements and contaminating donor RBCs expressing the cognate antigen was essential. For example, contaminating RBCs in an apheresis PBMNC product may be bound by ABO agglutinins in culture causing subsequent release of cytokines or RBC lysis—both of which may have a deleterious effect on culture conditions. Cultures with and without 3% group A1 RBCs showed no significant differences in expansion, purity, viability, gene expression, or supernatant chemokine analysis when grown in the presence of group O plasma with high-titer anti-A. Visually, we saw macroscopic and microscopic agglutination of RBCs in the cultures supplemented with plasma of high-titer anti-A (Appendix S4); however, we saw no signs of overt hemolysis on gross inspection. It should be noted that the heat-inactivation process used for all plasma used in cell culture, not only reduced the anti-A titer at least one-fold (Table 1), but is also known to inactivate complement. If active complement had been present in the cultures, there may have been in vitro hemolysis, which may have negatively affected culture expansion. Therefore, heat-inactivation of plasma supplements may be necessary to achieve similar results.
Early data on blood donor isoagluttinin titers suggest that 87% of group O blood donors have anti-A titers of 128 or below,30 suggesting that the vast majority of O plasma falls within the titer range tested in our study. A more recent study corroborates the early data suggesting that rates of high-titers in group O whole blood donors ranged between 6.4 and 20.7% (although the definition of high-titer at the sites in this study varied).31 Although we screened over 100 plasma products to obtain those with the highest titers available, the maximum post-I/HI/F anti-A titer tested herein was 128. While no dosage effect of anti-A was observed in the cultures tested, we cannot rule-out that titers above 128 may have adverse effects. Additional limitations include that the anti-A screening reagents used in this study primarily detect IgM—we did not screen donor plasma for rare, but potentially destructive, IgG isoagglutinins.23
This study represents a preliminary effort to demonstrate that lymphocytes for ACT may be cultured in O plasma. As anti-A titers in group O plasma tend to be higher than anti-B titers, we chose the combination of group A1 lymphocytes and group O plasma with high-titer anti-A to test our hypothesis regarding the use of minor-incompatible plasma supplements. While one would expect all ABO minor-incompatible plasma supplements to perform similarly, the effect of anti-B on group B lymphocytes was not tested.
Plasma factors other than ABO isoagglutinins are known to affect cellular expansion. For example, chemokine levels vary among donors and may be further altered by processing steps such as recalcification and heat-inactivation.32 While our cultures showed rather wide variability in CD3+ expansion, we have seen similar variability using this culture method in the past.
Effect of RBC contamination on Lymphocyte Culture
The RBC concentration of the starting materials has previously been shown to affect the final phenotype of CAR-T products (specifically increasing the CD4:CD8 ratio).33 In this study, we found that presence of contaminating RBCs in lymphocyte culture was independently associated with reduced lymphocyte expansion and phenotypic alteration of lymphocytes to a less-desirable (increased percentage of TEM) phenotype.34,35 Additionally, significant differences in transcriptional activity and protein expression were seen in cultures with contaminating RBCs. This underscores that removal of contaminating RBCs prior to lymphocyte culture is helpful to optimize lymphocyte growth and differentiation toward a TCM phenotype. While unrelated to the use of minor-incompatible plasma supplements, the adverse effect of contaminating RBCs represents an important finding for manufacturers of CAR-T and other ACT which, to our knowledge, has not been previously reported.
Summary
Our data suggest that group O plasma, regardless of isoagglutinin titer, can be used as a media supplement in the culture of lymphocytes for ACT without limiting expansion or causing changes in the viability, purity, quantity, or phenotype of the cultured lymphocytes. The ability to use O plasma as a media supplement would reduce cost for cellular therapy services as well as reducing the stress cellular therapy services have placed on transfusion services for access to a limited supply of AB plasma.
We also report the novel finding that contaminating RBCs in lymphocyte culture not only reduce the expansion of lymphocytes in culture, but also alter the balance of the final lymphocyte phenotype toward a higher percentage of TEM cells.
Supplementary Material
Acknowledgments
R.C.N. gratefully acknowledges the many laboratory technologists and staff of the NIH Clinical Center’s Department of Transfusion Medicine who made this research possible—the donor recruiting and nursing staff of the Blood Services Section, the Immunohematology and HLA staff of the Laboratory Services Section, and the staff of the Center for Cellular Engineering.
Supported by the Intramural Research Program (project ID Z99 CL999999) of the NIH Clinical Center at the National Institutes of Health.
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: Statement of Disclaimer: The views expressed are the authors’ own and do not represent the National Institutes of Health, Department of Health and Human Services, or U.S. Federal Government.
References
- 1.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385: 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 2018;378: 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, Mackall CL. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018;24: 20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, Kanakry JA, Ali SA, Mikkilineni L, Feldman SA, Stroncek DF, Hansen BG, Lawrence J, Patel R, Hakim F, Gress RE, Kochenderfer JN. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol 2018;36: 2267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011;17: 4550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Li YF, El-Gamil M, Rosenberg SA. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 2015;21: 1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, Gea-Banacloche JC, Pavletic SZ, Hickstein DD, Lu TL, Feldman SA, Iwamoto AT, Kurlander R, Maric I, Goy A, Hansen BG, Wilder JS, Blacklock-Schuver B, Hakim FT, Rosenberg SA, Gress RE, Kochenderfer JN. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol 2016;34: 1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, Diouf O, Liu E, Barrett AJ, Ito S, Shpall EJ, Krance RA, Kamble RT, Carrum G, Hosing CM, Gee AP, Mei Z, Grilley BJ, Heslop HE, Rooney CM, Brenner MK, Bollard CM, Dotti G. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 2013;122: 2965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, Burns D, McAulay K, Turner M, Bellamy C, Amlot PL, Kelly D, MacGilchrist A, Gandhi MK, Swerdlow AJ, Crawford DH. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 2007;110: 1123–31. [DOI] [PubMed] [Google Scholar]
- 10.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, Kapoor N, Pai SY, Rowley SD, Kebriaei P, Dey BR, Grilley BJ, Gee AP, Brenner MK, Rooney CM, Heslop HE. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013;121: 5113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, Carrum G, Sasa G, Lulla P, Watanabe A, Kuvalekar M, Gee AP, Wu MF, Liu H, Grilley BJ, Krance RA, Gottschalk S, Brenner MK, Rooney CM, Heslop HE, Leen AM, Omer B. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol 2017;35: 3547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, Bollard CM, Liu H, Wu MF, Rochester RJ, Amrolia PJ, Hurwitz JL, Brenner MK, Rooney CM. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010;115: 925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 2003;26: 332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin J, Sabatino M, Somerville R, Wilson JR, Dudley ME, Stroncek DF, Rosenberg SA. Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment. J Immunother 2012;35: 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumaini B, Lee DW, Lin T, Castiello L, Stroncek DF, Mackall C, Wayne A, Sabatino M. Simplified process for the production of anti-CD19-CAR-engineered T cells. Cytotherapy 2013;15: 1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannello F, Tonti GA. Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells 2007;25: 1603–9. [DOI] [PubMed] [Google Scholar]
- 17.Astori G, Amati E, Bambi F, Bernardi M, Chieregato K, Schafer R, Sella S, Rodeghiero F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther 2016;7: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schellekens PT, Eijsvoogel VP. Lymphocyte transformation in vitro. I. Tissue culture conditions and quantitative measurements. Clin Exp Immunol 1968;3: 571–84. [PMC free article] [PubMed] [Google Scholar]
- 19.Schafer R, Schnaidt M, Klaffschenkel RA, Siegel G, Schule M, Radlein MA, Hermanutz-Klein U, Ayturan M, Buadze M, Gassner C, Danielyan L, Kluba T, Northoff H, Flegel WA. Expression of blood group genes by mesenchymal stem cells. Br J Haematol 2011;153: 520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y, Merling A, Karsten U, Schwartz-Albiez R. The fucosylated histo-blood group antigens H type 2 (blood group O, CD173) and Lewis Y (CD174) are expressed on CD34+ hematopoietic progenitors but absent on mature lymphocytes. Glycobiology 2001;11: 677–83. [DOI] [PubMed] [Google Scholar]
- 21.Svensson L, Rydberg L, de Mattos LC, Henry SM. Blood group A(1) and A(2) revisited: an immunochemical analysis. Vox Sang 2009;96: 56–61. [DOI] [PubMed] [Google Scholar]
- 22.Flegel WA, Henry SM. Can anti-A1 cause hemolysis? Transfusion 2018;58: 3036–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flegel WA. Pathogenesis and mechanisms of antibody-mediated hemolysis. Transfusion 2015;55Suppl 2: S47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox J, Weisberg S. An R companion to applied regression. Thousand Oaks California: SAGE Publications, 2011. [Google Scholar]
- 25.Yazer M, Eder AF, Land KJ. How we manage AB plasma inventory in the blood center and transfusion service. Transfusion 2013;53: 1627–33. [DOI] [PubMed] [Google Scholar]
- 26.Zielinski MD, Schrager JJ, Johnson P, Stubbs JR, Polites S, Zietlow SP, Jenkins DH, Robinson BR. Multicenter comparison of emergency release group A versus AB plasma in blunt-injured trauma patients. Clin Transl Sci 2015;8: 43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AABB. Standards for Cellular Therapy Services 9th EditionBethesda, MD: AABB, 2019. [Google Scholar]
- 28.FACT-JACIE. International Standards for Hematopoietic Cellular Therapy: Seventh EditionProcessing Facility Standards: Policies and Standard Operating Procedures. Omaha, NE, 2018. [Google Scholar]
- 29.FACT-JACIE. Standards for Immune Effector Cells: 1st Edition. Omaha, NE, 2017. [Google Scholar]
- 30.Ebert RV, Emerson CP Jr. A Clinical Study Of Transfusion Reactions: The Hemolytic Effect Of Group-O Blood And Pooled Plasma Containing Incompatible Isoagglutinins. J Clin Invest 1946;25: 627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harm SK, Yazer MH, Bub CB, Cohn CS, Jacob EK, Kutner JM, Mair DC, Raval JS, Shaz BH, Ziman A, Dunbar NM. Seasonal variability is not observed in the rates of high anti-A and anti-B titers in plasma, apheresis platelet, and whole blood units tested by different methods. Transfusion 2019;59: 762–7. [DOI] [PubMed] [Google Scholar]
- 32.Ayache S, Panelli MC, Byrne KM, Slezak S, Leitman SF, Marincola FM, Stroncek DF. Comparison of proteomic profiles of serum, plasma, and modified media supplements used for cell culture and expansion. J Transl Med 2006;4: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elavia N, Panch SR, McManus A, Bikkani T, Szymanski J, Highfill SL, Jin P, Brudno J, Kochenderfer J, Stroncek DF. Effects of starting cellular material composition on chimeric antigen receptor T-cell expansion and characteristics. Transfusion 2019;59: 1755–64. [DOI] [PubMed] [Google Scholar]
- 34.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother 2012;35: 651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klebanoff CA, Scott CD, Leonardi AJ, Yamamoto TN, Cruz AC, Ouyang C, Ramaswamy M, Roychoudhuri R, Ji Y, Eil RL, Sukumar M, Crompton JG, Palmer DC, Borman ZA, Clever D, Thomas SK, Patel S, Yu Z, Muranski P, Liu H, Wang E, Marincola FM, Gros A, Gattinoni L, Rosenberg SA, Siegel RM, Restifo NP. Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Invest 2016;126: 318–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


