Abstract
Background
Severe coronavirus disease-2019 (COVID-19) can progress to an acute respiratory distress syndrome (ARDS), which involves alveolar infiltration by activated neutrophils. The beta-blocker metoprolol has been shown to ameliorate exacerbated inflammation in the myocardial infarction setting.
Objectives
The purpose of this study was to evaluate the effects of metoprolol on alveolar inflammation and on respiratory function in patients with COVID-19–associated ARDS.
Methods
A total of 20 COVID-19 patients with ARDS on invasive mechanical ventilation were randomized to metoprolol (15 mg daily for 3 days) or control (no treatment). All patients underwent bronchoalveolar lavage (BAL) before and after metoprolol/control. The safety of metoprolol administration was evaluated by invasive hemodynamic and electrocardiogram monitoring and echocardiography.
Results
Metoprolol administration was without side effects. At baseline, neutrophil content in BAL did not differ between groups. Conversely, patients randomized to metoprolol had significantly fewer neutrophils in BAL on day 4 (median: 14.3 neutrophils/µl [Q1, Q3: 4.63, 265 neutrophils/µl] vs median: 397 neutrophils/µl [Q1, Q3: 222, 1,346 neutrophils/µl] in the metoprolol and control groups, respectively; P = 0.016). Metoprolol also reduced neutrophil extracellular traps content and other markers of lung inflammation. Oxygenation (PaO2:FiO2) significantly improved after 3 days of metoprolol treatment (median: 130 [Q1, Q3: 110, 162] vs median: 267 [Q1, Q3: 199, 298] at baseline and day 4, respectively; P = 0.003), whereas it remained unchanged in control subjects. Metoprolol-treated patients spent fewer days on invasive mechanical ventilation than those in the control group (15.5 ± 7.6 vs 21.9 ± 12.6 days; P = 0.17).
Conclusions
In this pilot trial, intravenous metoprolol administration to patients with COVID-19–associated ARDS was safe, reduced exacerbated lung inflammation, and improved oxygenation. Repurposing metoprolol for COVID-19–associated ARDS appears to be a safe and inexpensive strategy that can alleviate the burden of the COVID-19 pandemic.
Key Words: acute care, ARDS, COVID, metoprolol
Abbreviations and Acronyms: ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage; COVID-19, coronavirus disease-2019; ICU, intensive care unit; IMV, invasive mechanical ventilation; NET, neutrophil extracellular trap
Central Illustration
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection, is an ongoing pandemic affecting more than 145 million people worldwide and responsible for more than 3 million deaths to date. An estimated 6%-18% of COVID-19 cases progress to an acute respiratory distress syndrome (ARDS) requiring intensive care unit (ICU) admission and invasive mechanical ventilation (IMV) (1). There is currently a lack of specific therapies for COVID-19–associated ARDS.
In the early stages of SARS-CoV-2 infection, the host immune system is activated to block disease progression. However, in some cases rapid replication of SARS-CoV-2 in the respiratory tract triggers an exacerbated inflammatory response and a cytokine storm (2). This situation leads to progression to ARDS together with other clinical complications, such as septic shock, microthrombi, coagulopathy, and multiple organ dysfunction (3).
ARDS of different etiologies (4), including SARS-CoV-2 infection (5,6), is highly dependent on the action of neutrophils. Activated neutrophils contribute to alveolar injury by releasing prestored inflammatory mediators (reactive oxygen species and myeloperoxidase [MPO]) and by interacting with other cells, such as platelets, to induce microthrombi. In addition, the formation of neutrophil extracellular traps (NETs) and highly injurious histones activates the inflammasome and triggers the release of pro-inflammatory cytokines (7). NETs released from alveolar-infiltrated activated neutrophils increase pulmonary inflammation and serum levels of proinflammatory cytokines, leading to extensive lung damage and microthrombotic events in COVID-19 patients (2,3,8,9).
Despite the massive worldwide impact of COVID-19, there is a shortage of effective therapies to prevent transition from moderate to severe disease and to improve prognosis. Given the intense pressure COVID-19 is placing on ICUs worldwide, there is an urgent need to identify therapies to reduce the number of days in the ICU. The most sought-after interventions are those able to mitigate COVID-19–associated immune dysregulation (10). An attractive candidate approach is to use host-directed therapies, which have emerged in recent years as an adjuvant strategy to limit damage during infectious or sterile exacerbated inflammation.
Beta-adrenergic receptor antagonists (β-blockers) have been used for many decades to treat cardiovascular conditions such as hypertension, arrhythmias, and myocardial infarction (11). Observational retrospective studies have established a link between β-blocker therapy and increased survival in critically ill patients caused by different conditions, such as sepsis (12, 13, 14), acute respiratory failure (15), severe traumatic brain injury (16,17), and others (18,19). Recent findings show that the β1-selective blocker metoprolol has a direct effect on neutrophils, dampening their deleterious effects during exacerbated inflammation (20). In the context of ischemia/reperfusion (acute myocardial infarction), metoprolol targeting of neutrophils has been shown to have a strong cardioprotective effect, both in animal models and in patients (20, 21, 22, 23). More recently, our group demonstrated that metoprolol (but not other clinically available intravenous β-blockers) abrogates neutrophil-driven exacerbated inflammation, neutrophil-platelet interaction, and NETs formation in a mouse model of LPS-induced acute lung injury (24). These experimental data prompted us to investigate whether treatment with intravenous (IV) metoprolol could ameliorate lung inflammation—and eventually improve prognosis—in patients with COVID-19–associated ARDS.
Methods
Study design and population
The MADRID-COVID (Intravenous Metoprolol in Respiratory Distress Due to COVID-19) pilot trial was approved by the Fundación Jiménez Díaz University Hospital ethics committee (Eudract registry number 2020-002310-41). All patients, or a close relative, gave written consent to participate. Inclusion criteria were age 18-80 years, rt-PCR–confirmed SARS-CoV-2 infection (in either nasal swab or bronchoalveolar lavage), invasive mechanical ventilation ≤72 hours, heart rate ≥60 beats/min, and invasive systolic blood pressure ≥120 mm Hg. Exclusion criteria included prolonged hospital admission (>5 days) before enrollment, concomitant acute heart failure, left ventricular ejection fraction <50%, right ventricular systolic dysfunction, concomitant pulmonary embolism, moderate-severe peripheral artery disease, moderate-severe valvular heart disease, moderate-severe COPD, or active treatment with β-blockers before enrollment. A total of 20 patients with ARDS secondary to SARS-CoV-2 infection under IMV were enrolled and randomized to IV metoprolol tartrate (Recordati) (3 × 5 mg boluses, 2 minutes apart, daily for 3 days; n = 12) or control (no treatment; n = 8). Two minutes after each bolus, blood pressure and heart rate were measured, and if they were above the limits set, the next bolus was injected.
Randomization was stratified by age (≤59 years vs >59 years), history of hypertension (yes/no), and circulating neutrophil counts (<6,000 vs ≥6,000). Bronchoalveolar lavage (BAL) fluid and blood samples were obtained from patients at randomization (baseline) and 24 hours after the third metoprolol dose/control (day 4). The main study goal was to assess the effect of metoprolol on inflammatory markers (mainly neutrophil infiltration and NETs). The main secondary goals were to assess the effect of metoprolol on days on invasive mechanical ventilation and days in the ICU after randomization, as well as pulmonary function. The main safety outcome measure was hemodynamic complications (cardiogenic shock, severe hypotension, or severe bradycardia/atrioventricular block).
Because this was a pilot trial, sample size was calculated based on the capacity of identifying changes in lung inflammation (neutrophil infiltration). Based on previous experimental studies, we speculated that 20 patients would be enough to detect a significant biological effect of metoprolol in this context.
Flow cytometry of BAL samples
For flow cytometry (FCM) studies, BAL samples (8 mL) were previously inactivated with 2 mL of a cellular antigen stabilization reagent containing formaldehyde (TransFix, Cytomark Ltd). Samples were then centrifuged (5 minutes at 540g), the supernatant discarded, and the cell pellet resuspended in 200 μL phosphate-buffered saline. Afterwards, 100 μL of cell suspension was stained for 15 minutes at room temperature with the following color combination: antihuman CD15-fluorescein isothiocyanate, CD33-phycoerythrin, and CD3-V-450 and CD45-V-500 (Becton Dickinson Biosciences). After staining, 2 mL of FACS lysing solution (Becton/Dickinson Biosciences) was added, and after 5 minutes incubation, the sample was centrifuged and resuspended in 100 μL phosphate-buffered saline. Before acquisition, the fluorescent dye DRAQ5 (Biostatus Limited) (25,26) and Perfect-COUNT microspheres (Cytognos SL) (27) were added for the selection of DNA-positive cells and cell count, respectively. Samples were run on a FACSCanto II flow cytometer (Becton Dickinson Biosciences) equipped with FACSDiva software (Becton Dickinson Biosciences), and information was acquired about all events corresponding to nucleated cells present in the stained sample aliquot.
Data were analyzed with INFINICYT software (Cytognos SL). FCM analysis included a first-step identification of nucleated cells by DRAQ5 staining. Leukocyte populations were identified with a gating strategy based on forward scatter, side scatter, and CD45 expression. Neutrophils and macrophages were identified from their relatively higher light-scattering properties, their unique pattern of CD45 expression, and the expression of CD15 (neutrophils) and CD33 (alveolar macrophages). Lymphocytes were also identified according to their CD45 expression and forward and side scatter properties. Neutrophil, macrophage, and lymphocyte populations were quantified as the percentage of total CD45 events.
Chemokine ELISA assays
Samples were inactivated by incubation in a final concentration of 0.2% SDS per 0.1% Tween-20 and heat treatment at 60°C for 15 minutes. Plasma and cell-free BAL samples were analyzed with human ELISA kits for von Willebrand factor (RAB0556-1KT, Sigma) and the chemokines monocyte chemoattractant protein (MCP)-1 (orb315028, Biorbyt), interleukin (IL)-6 (orb219452, Biorbyt), and IL-8 (orb315028, Biorbyt).
NETosis markers
A total of 3 NETosis biomarkers were measured: citrullinated histone-3 (Cit-H3), MPO-DNA complexes, and cell-free DNA. For Cit-H3 and MPO-DNA ELISA, samples were first inactivated by suspension in 0.2% SDS per 0.1% Tween-20 and heat treatment at 60°C for 15 minutes. For cell-free DNA measurement, samples were inactivated by heat treatment at 60°C for 1 hour.
Cit-H3 was measured with an ELISA kit (clone 11D3, Cayman, 501620). Quantification of MPO-DNA complexes was based on a previously described protocol (28,29) that uses several reagents from the Cell Death Detection ELISA Kit (Roche, 11544675001) but includes a high-binding EIA/RIA 96-well plate differently coated overnight at 4°C with antihuman MPO antibody (Bio-Rad, 0400-0002). Cell-free DNA was measured using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Thermo Fisher Scientific, P11496).
Neutrophil and NET visualization in BAL
NETs were visualized by Giemsa staining of BAL samples (30,31). BAL samples were centrifuged for 10 minutes at 2,500 revolutions/min. The pellet was resuspended and spread for staining with Giemsa solution. Samples were then inactivated and fixed for 10 minutes at room temperature with an alcohol-based spray fixative for cyto-diagnosis (M-Fix spray fixative). For image analysis, fixed samples were digitalized with a scanner (Nanozoomer-RS C110730, Hamamatsu) and analyzed using NDP view image analysis software (Hamamatsu).
Statistical analysis
Data were analyzed with Graphpad Prism version 8.4 and RStudio. Due to the small sample size, all distributions were considered non-normal, and nonparametric tests were applied for statistical analyses. Paired comparisons between pretreatment and post-treatment samples (basal and 4 days) were by Wilcoxon matched pairs signed rank test. Comparisons between treatment conditions (vehicle vs metoprolol) at baseline or after treatment were made by unpaired Mann-Whitney U test. For hemodynamics and functional parameters during metoprolol administration, differences at baseline or pre-post boluses were calculated by the nonparametric chi-square Friedman test with correction by the Durbin-Conover test for pairwise comparisons. For categorical data, percentages were compared by exact methods. Differences were deemed statistically significant at P values below 0.05.
Results
Patient characteristics
Between October 19, 2020, and January 19, 2021, a total of 20 patients were enrolled; 12 were randomized to metoprolol and 8 to control. There were no between-group differences in baseline characteristics (Table 1 ). All patients were treated during ICU admission with corticosteroids (dexamethasone 6 mg daily), anticoagulants, melatonin, and acetylcysteine. Before enrollment in the trial, all patients (except 1 in the metoprolol group) were treated with bolus and maintenance dose of corticosteroids (methylprednisolone and/or dexamethasone) in the ward before admission to the ICU without differences between groups.
Table 1.
Patient Characteristics at Randomization
| All | Metoprolol | Control | P Value | |
|---|---|---|---|---|
| Age, y | 60 (53.8, 68) | 60 (57.8, 68.5) | 58.5 (43.3, 65.8) | 0.354 |
| Male | 13 (65.0) | 8 (66.7) | 5 (62.5) | 1.000 |
| BMI, kg/m2 | 27.1 (25.3, 31.1) | 26.8 (25.1, 30.4) | 27.1 (26.2, 31.5) | 0.422 |
| Comorbidities | ||||
| Hypertension | 6 (30.0) | 4 (33.3) | 2 (25.0) | 1.000 |
| Diabetes | 2 (10.0) | 2 (16.7) | 0 (0.0) | 0.648 |
| Smokers | 3 (15.0) | 1 (8.3) | 2 (25.0) | 0.701 |
| Dyslipidemia | 6 (30.0) | 4 (33.3) | 2 (25.0) | 1.000 |
| Previous treatment | ||||
| RAS inhibitors | 5 (25.0) | 3 (25.0) | 2 (25.0) | 1.000 |
| Anticoagulants | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
Values are median (Q1, Q3) or n (%).
BMI = body mass index; RAS = renin-angiotensin system.
Of the patients randomized to metoprolol, 11 received all scheduled IV doses (15 mg daily for 3 days). The remaining patient received 15 mg of metoprolol on the first 2 days but not the third because the heart rate was <50 beats/min caused by intensified sedation (initiation of propofol). BAL was conducted without complications in all patients before and 24 hours after treatment. Clinical laboratory analyses at baseline and after treatment are presented in Supplemental Table 1.
Cardiovascular safety of intravenous metoprolol administration to ARDS patients on mechanical ventilation
Administration of IV β-blockers has largely been proven to be safe except for patients with acute pump failure. Given the cardiovascular effects of metoprolol, patients were monitored invasively and by echocardiography before and on every day after metoprolol injection/control. As expected, metoprolol significantly reduced heart rate (P < 0.01) and invasively measured systolic blood pressure (P < 0.05), although both remained within the physiological range (Supplemental Table 2). Echocardiography showed no deterioration of cardiac function parameters after metoprolol treatment (Supplemental Table 3). Overall, metoprolol intravenous administration was shown to be safe and without side effects in severe COVID-19 patients with ARDS on IMV.
Metoprolol administration attenuates neutrophil-driven lung exacerbated inflammation
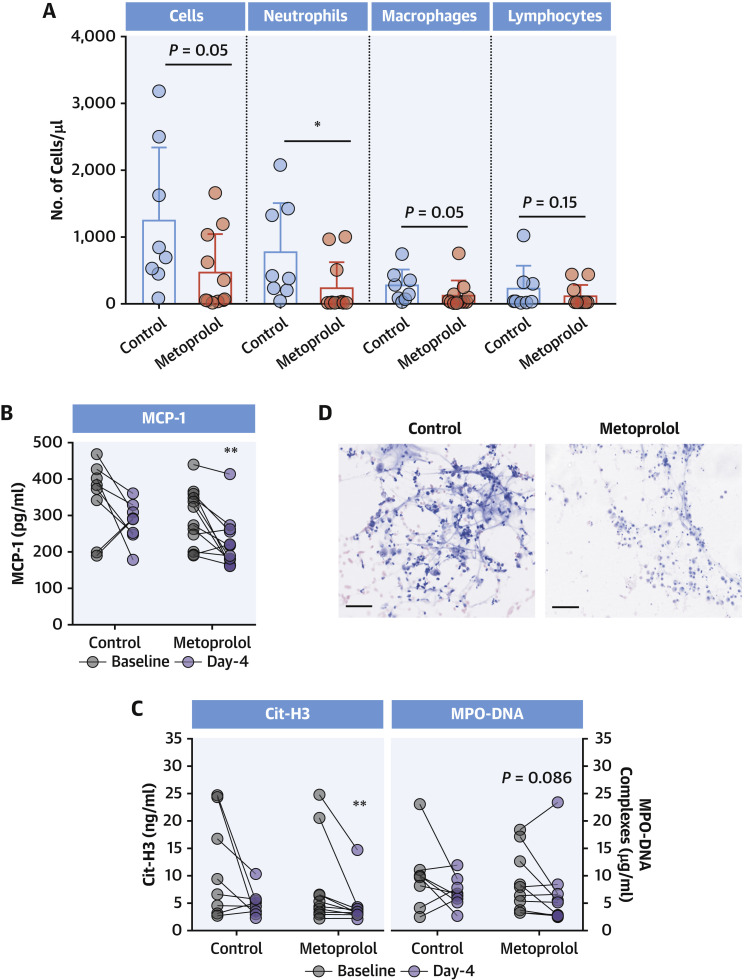
To assess the ability of metoprolol to ameliorate neutrophil-mediated exacerbated lung inflammation, we analyzed leukocyte populations in BAL samples by flow cytometry at baseline and on day 4. At baseline, the metoprolol and control groups showed no differences in BAL neutrophil content (Supplemental Figure 1). In contrast, on day 4 (after 3 days of metoprolol/control treatment), neutrophil content was significantly lower in BAL from patients in the metoprolol group than in those randomized to control (median: 14.3 neutrophils/μl [Q1, Q3: 4.63, 265 neutrophils/μl] vs median: 397 neutrophils/μl [Q1, Q3: 222, 1,346 neutrophils/μl]; P = 0.016). Day 4 BAL from metoprolol-treated patients also had lower total inflammatory-cell content and lower monocyte/macrophage content, whereas lymphocytes did not differ between groups (Figure 1A ). We further explored the impact of metoprolol on MCP-1 in BAL, because this chemokine has been shown to promote pulmonary fibrosis in late-stage ARDS (32,33). MCP-1 in cell-free BAL was significantly attenuated after 3 days of metoprolol treatment (median: 298 pg/mL [Q1, Q3: 236, 350 pg/mL] vs median: 203 pg/mL [Q1, Q3: 175, 258 pg/mL] for baseline and day 4, respectively; P = 0.009), whereas it remained unchanged in control patients (Figure 1B). Conversely, changes in IL-8 and -6 in cell-free BAL did not differ between treatment groups (Supplemental Figure 2).
Figure 1.
Metoprolol Disrupts COVID-19–Associated Exacerbated Lung Inflammation
(A) Day 4 inflammatory cell populations in BAL from control and metoprolol-treated severe COVID-19 patients. Dots represent individuals and bars and error bars show mean values (boxes) ± SD (error bars). ∗P < 0.05 by unpaired Student's t-test. (B) Attenuation of MCP-1 in cell-free BAL from metoprolol-treated patients. (C) Attenuation of neutrophil hyperactivation biomarkers (Cit-H3 and MPO-DNA complexes) in cell-free BAL from metoprolol-treated patients. Data are presented as individuals’ (dots) paired data between days 1 and 4. ∗∗P < 0.01 by paired Student's t-test. (D) Representative images of Giemsa-stained BAL samples from control and metoprolol-treated patients at day 4. Scale bar, 50 μm. Control, n = 8; metoprolol, n = 12. BAL = bronchoalveolar lavage; Cit-H3 = citrullinated histone-3; COVID-19 = coronavirus disease-2019; MCP = monocyte chemoattractant protein; MPO = myeloperoxidase.
Excessive neutrophil activation in the lungs is associated with NET formation and the release of reactive oxygen species and proteolytic enzymes, which can drive severe epithelial and endothelial injury (2,34). To study whether the inflammation-disrupting effect of metoprolol reduced the production of these neutrophil activation byproducts, we measured the NETosis markers Cit-H3 and MPO-DNA complexes. Levels of both markers were decreased in day 4 BAL from metoprolol-treated patients (P = 0.005 and P = 0.086 vs baseline, respectively), whereas no changes were observed in BAL from control patients (Figure 1C). Lower NET formation and inflammatory content in the metoprolol group was confirmed by Giemsa staining (Figure 1D). We found no differences in cell-free DNA content (Supplemental Figure 3), probably reflecting its nonspecific nature as a NETosis biomarker (3).
To determine if attenuated immune-cell infiltration in the lungs was associated with a systemic effect, we assessed changes in circulating levels of chemokines known to be markedly elevated in severe COVID-19 patients (34). The 3-day treatment with metoprolol was associated with a significant reduction in the circulating concentrations of the pro-inflammatory cytokine IL-8 (median: 94.4 pg/mL [Q1, Q3: 72.1, 168 pg/mL] vs median: 80.1 pg/mL [Q1, Q3: 69.5, 85.2 pg/mL] for baseline and day 4, respectively; P = 0.003), whereas no changes were observed in controls (Supplemental Figure 4). Metoprolol had no significant effect on circulating levels of IL-6 (Supplemental Figure 4) or NETosis markers (Supplemental Figure 5).
Metoprolol treatment improves respiratory function
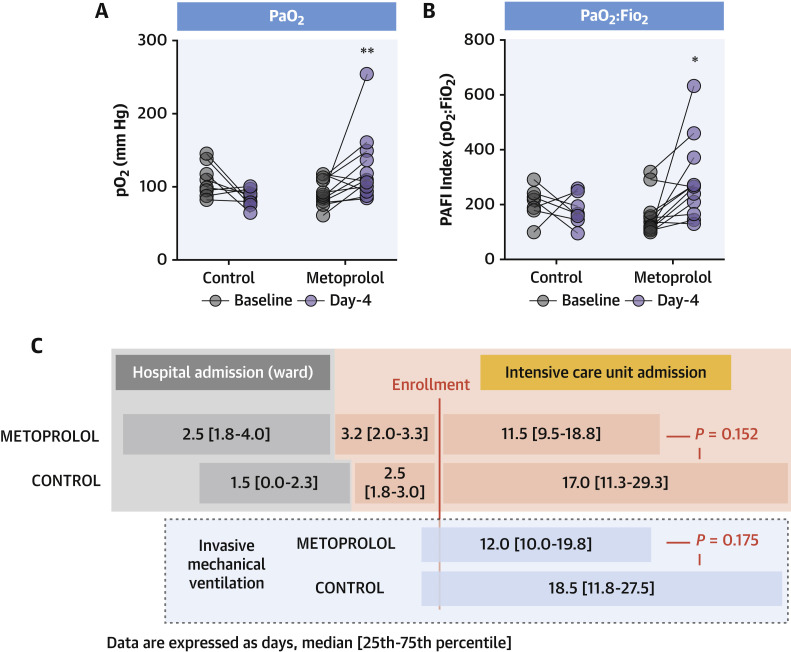
Oxygenation was measured as the ratio between arterial oxygen partial pressure and fractional inspired oxygen (PaO2:FiO2). Baseline and post-treatment oxygenation parameters are shown in Table 2 . At baseline, oxygenation was worse in patients randomized to metoprolol than in the control group, despite higher FiO2. After the 3-day metoprolol treatment, PaO2 significantly improved (median: 87.5 mm Hg [Q1, Q3: 78.8, 110 mm Hg] vs median: 108 mm Hg [Q1, Q3: 98.3, 139 mm Hg] for baseline and day 4, respectively; P = 0.017). Metoprolol treatment also significantly improved PaO2:FiO2 (median: 130 [Q1, Q3: 110, 162] vs median: 267 [Q1, Q3: 199, 298] at baseline and day 4, respectively; P = 0.007). Conversely, in control subjects, PaO2 and PaO2:FiO2 both deteriorated, although the change did not reach statistical significance (P = 0.107 and P = 0.363 vs baseline, respectively) (Figures 2A and 2B ).
Table 2.
Baseline and Post-Treatment Ventilation Parameters
| Baseline |
Day 4 |
|||||
|---|---|---|---|---|---|---|
| Metoprolol | Control | P Value | Metoprolol | Control | P Value | |
| PaO2, mm Hg | 87.5 (78.8, 110.0) | 104.0 (93.0, 122) | 0.105 | 108.0 (98.3, 139.0) | 83.5 (77.3, 92.5) | 0.004 |
| PaCO2, mm Hg | 48.5 (43.8, 52.5) | 47 (41.5, 48.8) | 0.562 | 51.0 (46.5, 53.3) | 47.0 (45.3, 50.5) | 0.353 |
| PEEP, cm H2O | 12.0 (10.0, 12.5) | 13.0 (10.0, 14.0) | 0.625 | 10.0 (9.00, 12.0) | 11.0 (10.0, 12.0) | 0.666 |
| FiO2 | 0.60 (0.5, 0.75) | 0.48 (0.44, 0.60) | 0.241 | 0.40 (0.39, 0.53) | 0.43 (0.40, 0.57) | 0.634 |
| PaO2/FiO2, | 130 (110, 162) | 223 (188, 242) | 0.076 | 267 (199, 298) | 163 (145, 209) | 0.037 |
| Lactic acid, mmol/L | 1.3 (1.2, 1.8) | 1.2 (0.98, 2.00) | 0.785 | 1.4 (1.20, 1.73) | 1.9 (1.50, 2.05) | 0.094 |
| pH | 7.41 (7.38, 7.42) | 7.42 (7.37, 7.45) | 0.485 | 7.43 (7.40, 7.46) | 7.41 (7.38, 7.44) | 0.461 |
Values are median (Q1, Q3). Bold indicates statistical significance.
FiO2 = fraction of inspired oxygen; PaCO2 = partial pressure of carbon dioxide; PaO2 = partial pressure of oxygen; PEEP = positive end-expiratory pressure.
Figure 2.
Metoprolol Rescues Pulmonary Function in ICU Patients With Severe COVID-19
(A and B) Improved oxygenation (PaO2 and PaO2:FiO2) in patients receiving metoprolol, but not those in the control group. Data are presented as individuals’ (dots) paired data between days 1 and 4. ∗P < 0.05, ∗∗P < 0.01 by paired Student's t-test. (C) Days spent by severe COVID-19 patients on IMV and in the ICU according to allocation to IV metoprolol or control (no treatment). Control, n = 8; metoprolol, n = 12. FiO2 = fraction of inspired oxygen; ICU = intensive care unit; IMV = invasive mechanical ventilation; PAFI = PaO2:FiO2 ratio; other abbreviations as in Figure 1.
Patients randomized to metoprolol spent fewer days on mechanical ventilation, although this difference did not reach statistical significance (15.5 ± 7.6 days vs 21.9 ± 12.6 days in the metoprolol and control groups, respectively; P = 0.17). A similar trend was observed for days of ICU admission after enrollment (14.5 ± 7.2 days vs 21.4 ± 13.4 days in the metoprolol and control groups, respectively; P = 0.15) (Figure 2C). All patients were discharged from the ICU, and 1 patient in each group died before hospital discharge.
Discussion
The COVID-19 pandemic and associated ARDS is placing an immense burden on health care systems. In addition to high mortality, COVID-19–associated ARDS results in prolonged ICU admission, contributing to morbidity among survivors and high hospital expenditure. The current approach with these patients is mainly based on protective IMV (35,36), which ensures sufficient gas exchange while causing minimal alveolar damage. With the exception of dexamethasone, which showed promising results in an early trial (37), there are no therapies specifically targeting exacerbated inflammation in ARDS (38).
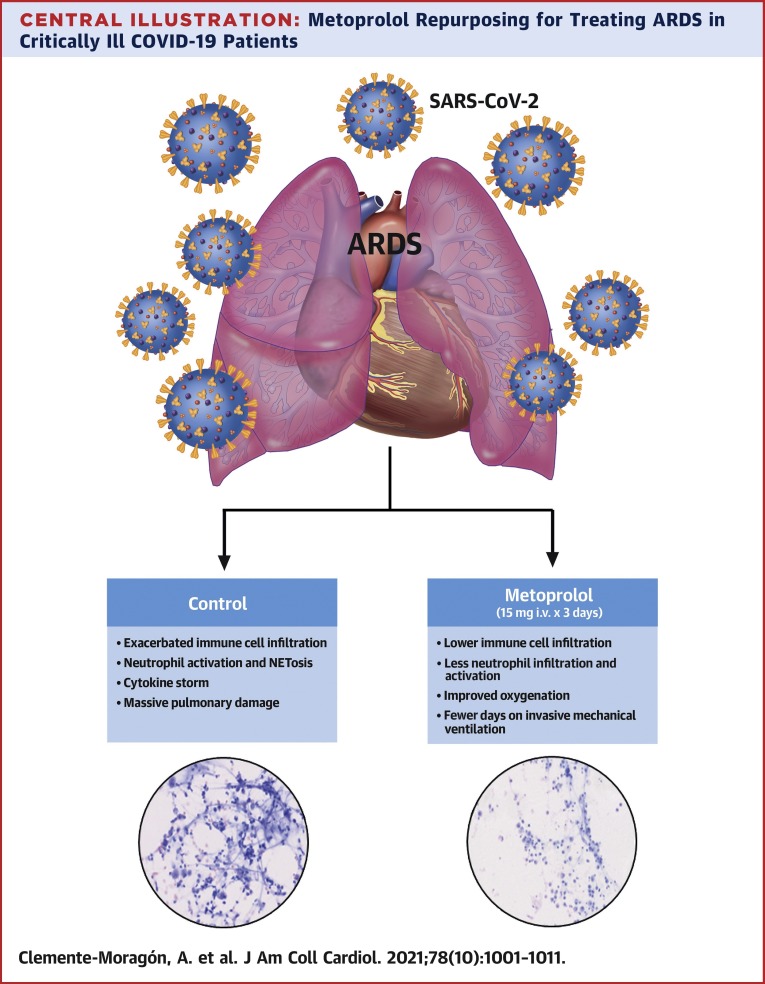
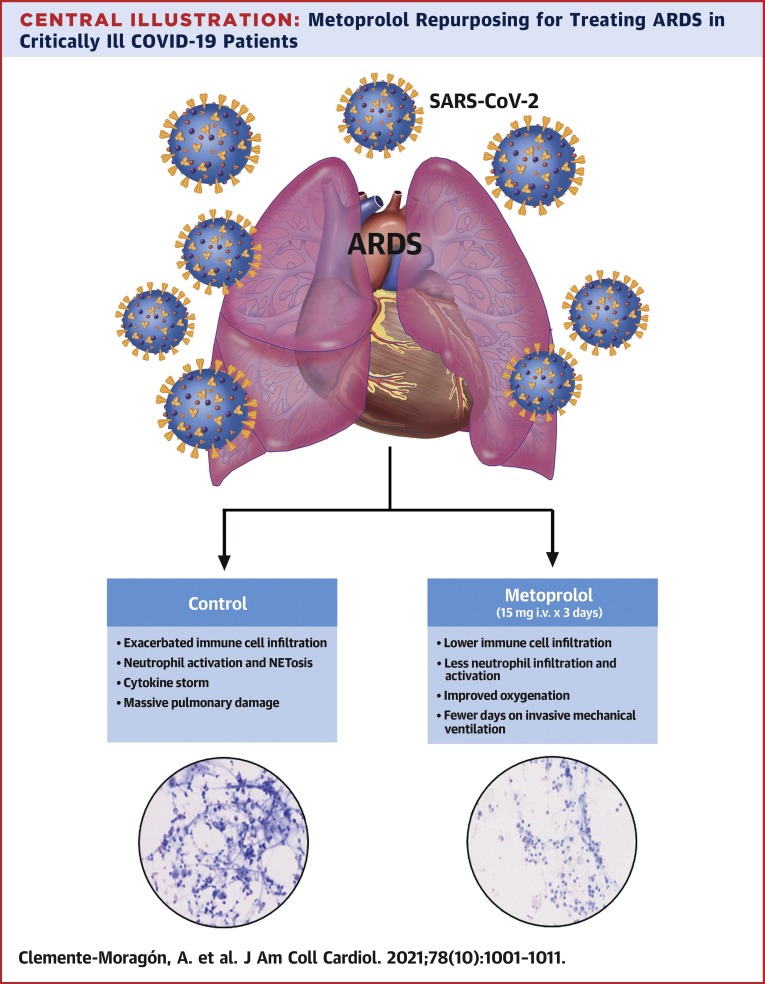
In this study, we present the effects of 3-day intravenous metoprolol administration on lung inflammation in COVID-19 patients with ARDS. The MADRID-COVID pilot trial shows the following: 1) IV administration of the clinically approved β-blocker metoprolol tartrate is safe in this clinical context; 2) metoprolol treatment abrogates the exacerbated lung inflammation associated with the disease; and 3) the disruptive effect on exacerbated inflammation is associated with better oxygenation and, consequently, fewer days on IMV and in the ICU (Central Illustration ). These data suggest that metoprolol repurposing for the treatment of ARDS in COVID-19 patients is a safe and inexpensive strategy with the potential to improve outcomes.
Central Illustration.
Metoprolol Repurposing for Treating ARDS in Critically Ill COVID-19 Patients
Reduced lung inflammation was associated with a significant improvement in oxygenation and fewer days on mechanical ventilation and of intensive care unit admission. Repurposing metoprolol for the treatment of acute respiratory distress syndrome associated with coronavirus disease-2019 (COVID-19) appears to be a safe and inexpensive strategy that can alleviate the burden of the COVID-19 pandemic.
The present study stems from our extensive experience in the field of myocardial ischemia/reperfusion injury. We previously demonstrated that metoprolol protects the heart during ongoing myocardial infarction by stunning neutrophils and abrogating exacerbated inflammation (20,24). The identification of this cardioprotective mechanism created an opportunity to repurpose metoprolol for other acute conditions in which exacerbated inflammation plays a role, as is the case for COVID-19–associated ARDS. The present study highlights the importance of knowing the mechanism of action of long-established drugs to identify other potential indications.
Patients with severe COVID-19 present with bilateral pneumonia that can lead to respiratory distress requiring IMV. COVID-19–associated ARDS is characterized by active neutrophil infiltration into the alveolar space, which perpetuates exacerbated inflammation, leading to a cytokine storm and hypoxemia (8,34). Neutrophil infiltration is thus a major contributing factor to the poor prognosis of these patients. Mitigation of immune dysregulation is therefore a major therapeutic avenue for the treatment and prevention of severe COVID-19.
Several studies have tested the potential benefits of β-blockers in sepsis/septic shock. Retrospective observational data have suggested that patients admitted with septic shock and previously on maintenance β-blocker therapy have a better vital prognosis than those who were not on β-blockers before admission (13). In addition, small prospective clinical trials have tested the benefits of IV β-blockers in sepsis patients (12,39, 40, 41). The conclusion of most of these trials is that β-blockers seem to offer a clinical benefit.
In an analysis of diverse experimental models of exacerbated inflammation, we very recently showed that not all β1-selective blockers exert the same effects on neutrophil biology. Of all tested β-blockers, only metoprolol significantly attenuated exacerbated inflammation and reduced neutrophil infiltration and interaction with other cell types (24). Those results position metoprolol as the β-blocker of choice in the context of exacerbated inflammation.
The present study shows that 3-day treatment with IV metoprolol reduces exacerbated inflammation in critically ill COVID-19 patients with associated ARDS. This was evidenced by the attenuation of infiltration by immune cells, especially neutrophils, and reduced levels of their related pro-inflammatory and NETosis byproducts (Figure 1), which are potential drivers of severe epithelial and endothelial injury. Lower neutrophil infiltration in metoprolol-treated patients was accompanied by a significant reduction in circulating levels of the pro-inflammatory IL8, which exerts chemotactic and activating functions on neutrophils, suggesting a systemic anti-inflammatory effect of this treatment (Supplemental Figure 4). Systemic markers of NETosis were unaffected at day 4; however, an effect over a longer time window after metoprolol treatment cannot be discarded (Supplemental Figure 5). The ameliorative effect of metoprolol on pulmonary inflammation of COVID-19 patients with ARDS was associated with strong indicators of clinical benefit, demonstrated by a significant improvement in oxygenation (PaO2:FiO2) not seen in control patients (Figure 2). These results are very encouraging, but further large-scale trials are needed to validate the clinical benefits of metoprolol in this context. Given that neutrophils play a major role in the pathophysiology of ARDS of many causes (not only COVID-19 related), further large validation studies might include a wide spectrum of patients with this condition.
The MADRID-COVID pilot trial has demonstrated that IV administration of the clinically approved β-blocker metoprolol to critically ill patients with ARDS caused by COVID-19 is safe and disrupts the exacerbated lung inflammation associated with the disease. The beneficial effects on exacerbated inflammation were associated with better oxygenation and a nonsignificant reduction in the number of days on mechanical ventilation and in the ICU. Intravenous metoprolol appears as a promising intervention that could improve the prognosis of critically ill COVID-19 patients. Although these data need to be corroborated in a larger sample, metoprolol is a clinically available and cheap drug (daily treatment costs <2€) that can improve outcomes in patients with severe COVID-19.
Study limitations
The main limitation of this study is the small sample size. The study was powered to detect differences in lung inflammation and not clinical events. Another limitation is the single-center nature of the study. This was an open-label study, and treating physicians were not blinded to treatment allocation. Finally, we cannot rule out a selection bias resulting in patients with very poor condition according to physicians not considered for inclusion.
Conclusions
Our results show that IV administration of metoprolol to patients with severe COVID-19–associated ARDS is safe and abrogates the exacerbated lung inflammation associated with the disease. Reduced lung inflammation was associated with a significant improvement in oxygenation and with a trend toward fewer days on mechanical ventilation and of ICU admission. Metoprolol repurposing for the treatment of ARDS associated with COVID-19 is a safe and cheap intervention that can help to alleviate the massive personal and health care burden associated with the pandemic.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL OUTCOMES: In critically ill patients with COVID-19 on mechanical ventilatory support, intravenous administration of metoprolol upon admission to the ICU is safe and improves pulmonary function and clinical outcome.
TRANSLATIONAL OUTLOOK: Future studies with larger sample sizes are needed to confirm the benefit of metoprolol in critically ill patients with COVID-19 and potentially other inflammatory etiologies of ARDS.
Funding Support and Author Disclosures
Mr Clemente-Moragón is supported by a fellowship from the Ministerio de Ciencia e Innovación (FPU2017/01932). The CNIC is supported by the ISCIII, the Ministerio de Ciencia e Innovación, and the Pro CNIC Foundation. Dr Ibáñez is supported by the European Commission (ERC-CoG grant No 819775) and by the Spanish Ministry of Science and Innovation (MCN; “RETOS 2019” grant No PID2019-107332RB-I00). Dr Oliver is supported by funds from the Comunidad de Madrid Programa de Atracción de Talento (2017-T1/BMD-5185). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the following for their important support during this study: Noemí Escalera, Rocío Escudero, and Antonio de Molina-Iracheta at the CNIC; and Luis Nieto, Ana María Venegas, Jose Tuñón, Ignacio Cornejo, Sandra Zazo, and Federico Rojo at the Fundación Jiménez Díaz. Simon Bartlett provided English editing.
Footnotes
Christie Ballantyne, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Ritter M., Ott D.V.M., Paul F., Haynes J.D., Ritter K. COVID-19: a simple statistical model for predicting intensive care unit load in exponential phases of the disease. Sci Rep. 2021;11:5018. doi: 10.1038/s41598-021-83853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middleton E.A., He X.Y., Denorme F. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuo Y., Yalavarthi S., Shi H. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 5.Cavalcante-Silva L.H.A., Carvalho D.C.M., Lima E.A. Neutrophils and COVID-19: the road so far. Int Immunopharmacol. 2021;90:107233. doi: 10.1016/j.intimp.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges L., Pithon-Curi T.C., Curi R., Hatanaka E. COVID-19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediators Inflamm. 2020;2020:8829674. doi: 10.1155/2020/8829674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Standiford T.J., Ward P.A. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res. 2016;167:183–191. doi: 10.1016/j.trsl.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang C.C., Korinek M., Cheng W.J., Hwang T.L. Targeting neutrophils to treat acute respiratory distress syndrome in coronavirus disease. Front Pharmacol. 2020;11:572009. doi: 10.3389/fphar.2020.572009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomar B., Anders H.J., Desai J., Mulay S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9:1383. doi: 10.3390/cells9061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulte-Schrepping J., Reusch N., Paclik D. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440.e23. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Milla J., Raposeiras-Roubin S., Pascual-Figal D.A., Ibanez B. Role of beta-blockers in cardiovascular disease in 2019. Rev Esp Cardiol (Engl Ed) 2019;72:844–852. doi: 10.1016/j.rec.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Chacko C.J., Gopal S. Systematic review of use of beta-blockers in sepsis. J Anaesthesiol Clin Pharmacol. 2015;31:460–465. doi: 10.4103/0970-9185.169063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan K., Harazim M., Tang B., McLean A., Nalos M. The association between premorbid beta blocker exposure and mortality in sepsis-a systematic review. Crit Care. 2019;23:298. doi: 10.1186/s13054-019-2562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliver E., Mayor F., Jr., D'Ocon P. Beta-blockers: historical perspective and mechanisms of action. Rev Esp Cardiol (Engl Ed) 2019;72(10):853–862. doi: 10.1016/j.rec.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Noveanu M., Breidthardt T., Reichlin T. Effect of oral beta-blocker on short and long-term mortality in patients with acute respiratory failure: results from the BASEL-II-ICU study. Crit Care. 2010;14:R198. doi: 10.1186/cc9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotton B.A., Snodgrass K.B., Fleming S.B. Beta-blocker exposure is associated with improved survival after severe traumatic brain injury. J Trauma. 2007;62:26–33. doi: 10.1097/TA.0b013e31802d02d0. discussion 33-5. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K., Teixeira P.G., David J.S. Beta-blockers in isolated blunt head injury. J Am Coll Surg. 2008;206:432–438. doi: 10.1016/j.jamcollsurg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Christensen S., Johansen M.B., Tonnesen E. Preadmission beta-blocker use and 30-day mortality among patients in intensive care: a cohort study. Crit Care. 2011;15:R87. doi: 10.1186/cc10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Jagt M., Miranda D.R. Beta-blockers in intensive care medicine: potential benefit in acute brain injury and acute respiratory distress syndrome. Recent Pat Cardiovasc Drug Discov. 2012;7:141–151. doi: 10.2174/157489012801227274. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Prieto J., Villena-Gutierrez R., Gomez M. Neutrophil stunning by metoprolol reduces infarct size. Nat Commun. 2017;8:14780. doi: 10.1038/ncomms14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Ruiz J.M., Fernandez-Jimenez R., Garcia-Alvarez A. Impact of the timing of metoprolol administration during STEMI on infarct size and ventricular function. J Am Coll Cardiol. 2016;67:2093–2104. doi: 10.1016/j.jacc.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 22.Pizarro G., Fernandez-Friera L., Fuster V. Long-term benefit of early pre-reperfusion metoprolol administration in patients with acute myocardial infarction: results from the METOCARD-CNIC Trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) J Am Coll Cardiol. 2014;63:2356–2362. doi: 10.1016/j.jacc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Ibanez B. Intravenous beta-blockers in STEMI: what you are about to do, do it quickly. Eur Heart J Acute Cardiovasc Care. 2020;9:459–461. doi: 10.1177/2048872620950205. [DOI] [PubMed] [Google Scholar]
- 24.Clemente-Moragon A., Gomez M., Villena-Gutierrez R. Metoprolol exerts a non-class effect against ischaemia-reperfusion injury by abrogating exacerbated inflammation. Eur Heart J. 2020;41:4425–4440. doi: 10.1093/eurheartj/ehaa733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith P.J., Wiltshire M., Errington R.J. DRAQ5 labeling of nuclear DNA in live and fixed cells. Curr Protoc Cytom. 2004 doi: 10.1002/0471142956.cy0725s28. Chapter 7:Unit 7.25. [DOI] [PubMed] [Google Scholar]
- 26.Martin R.M., Leonhardt H., Cardoso M.C. DNA labeling in living cells. Cytometry A. 2005;67:45–52. doi: 10.1002/cyto.a.20172. [DOI] [PubMed] [Google Scholar]
- 27.Mandy F., Brando B. Enumeration of Absolute Cell Counts Using Immunophenotypic Techniques. Curr Protoc Cytom. 2000;13:6.8.1–6.8.26. doi: 10.1002/0471142956.cy0608s13. [DOI] [PubMed] [Google Scholar]
- 28.Kessenbrock K., Krumbholz M., Schonermarck U. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo Y., Zuo M., Yalavarthi S. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis. 2021;51:446–453. doi: 10.1007/s11239-020-02324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malachowa N., Kobayashi S.D., Freedman B., Dorward D.W., DeLeo F.R. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J Immunol. 2013;191:6022–6029. doi: 10.4049/jimmunol.1301821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas A., Boivin R., Cano P., Murcia Y., Bazin I., Lavoie J.P. Neutrophil extracellular traps are downregulated by glucocorticosteroids in lungs in an equine model of asthma. Respir Res. 2017;18:207. doi: 10.1186/s12931-017-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartl D., Griese M., Nicolai T. A role for MCP-1/CCR2 in interstitial lung disease in children. Respir Res. 2005;6:93. doi: 10.1186/1465-9921-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He C., Carter A.B. C(C)Learing the Role of Chemokines in Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2020;62:546–547. doi: 10.1165/rcmb.2020-0017ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandolfi L., Fossali T., Frangipane V. Broncho-alveolar inflammation in COVID-19 patients: a correlation with clinical outcome. BMC Pulm Med. 2020;20:301. doi: 10.1186/s12890-020-01343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas C.F. Lung protective mechanical ventilation in acute respiratory distress syndrome. Respir Care Clin N Am. 2003;9:363–396. doi: 10.1016/s1078-5337(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 36.Gong M.N., Ferguson N.D. Lung-protective ventilation in acute respiratory distress syndrome. How soon is now? Am J Respir Crit Care Med. 2015;191:125–126. doi: 10.1164/rccm.201412-2250ED. [DOI] [PubMed] [Google Scholar]
- 37.Villar J., Ferrando C., Martinez D. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 38.Baudouin S.V. Manipulation of inflammation in ARDS: achievable goal or distant target? Thorax. 2006;61:464–465. doi: 10.1136/thx.2005.057265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmittinger C.A., Dunser M.W., Haller M. Combined milrinone and enteral metoprolol therapy in patients with septic myocardial depression. Crit Care. 2008;12:R99. doi: 10.1186/cc6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morelli A., Donati A., Ertmer C. Microvascular effects of heart rate control with esmolol in patients with septic shock: a pilot study. Crit Care Med. 2013;41:2162–2168. doi: 10.1097/CCM.0b013e31828a678d. [DOI] [PubMed] [Google Scholar]
- 41.Morelli A., Ertmer C., Westphal M. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–1691. doi: 10.1001/jama.2013.278477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.