Abstract
Background:
Computed tomography (CT) scans are the first-line imaging technique in acute stroke patients based on the argument of rapid feasibility. Using magnetic resonance imaging (MRI) as the first-line imaging technique is the exception to the rule, although it provides much more diagnostic information and avoids exposure to radiation. We evaluated whether an MRI-based acute stroke concept is fast, suitable, and useful to improve recanalization rates and patient outcomes.
Methods:
We performed a retrospective observational cohort study comparing patients treated at a comprehensive stroke center (Ulm/Germany) applying an MRI-based acute stroke concept with patients recorded in a large comprehensive stroke registry in Baden-Württemberg (Germany). We analyzed the quality indicators of acute stroke treatment, patient’s outcome, and the rate of transient ischemic attack (TIA) at discharge.
Results:
A total of 2182 patients from Ulm and 82,760 patients from the Baden-Württemberg (BW) stroke registry (including 29,575 patients of comprehensive stroke centers (BWc)) were included. Intravenous thrombolysis rate was higher in Ulm than in BW or the BWc stroke centers (Ulm 27.4% versus BW 20.9% versus BWc 26.1; p < 0.01), while a door-to-needle time <30 min could be achieved more frequently (Ulm 73.6% versus BW 44.1% versus BWc 47.1%; p < 0.01). Thrombectomy rate in patients with a proximal vascular occlusion was higher (Ulm 69.2% versus BW 50.7% versus BWc 59.3; p < 0.01). The number of TIA diagnoses was lower (Ulm 16.2% versus BW 24.6% versus BWc 19.9%; p < 0.01). More patients showed a shift to a favorable outcome (Ulm 21.1% versus BW 16.9% versus BWc 15.3; p < 0.01). Complication rates were similar.
Conclusions:
The MRI-based acute stroke concept is suitable, fast and seems to be beneficial. The time-dependent quality indicators were better both in comparison to all stroke units and to the comprehensive stroke units in the area. Based on the MRI concept, high rates of recanalization procedures and fewer TIA diagnoses could be observed. In addition, there was a clear trend towards an improved clinical outcome. A clinical trial comparing the effects of CT and MRI as the primary imaging technique in otherwise identical stroke unit settings is warranted.
Keywords: stroke, MRI, thrombolysis, thrombectomy, stroke imaging, DWI, FLAIR, stroke mimics, stroke chameleons
Introduction
Ischemic stroke is one of the most common diseases worldwide and a leading cause of morbidity and mortality.1 Alteplase (recombinant tissue plasminogen activator, rtPA) is the only approved drug for patients with acute ischemic stroke. It is recommended as an initial treatment within 4.5 hours after stroke onset and also shows therapeutic effects in a longer time window, if this is based on differentiated stroke imaging.1,2 Its safe routine use is documented by data analyses from large thrombolysis registers.3 In addition, since 2015, several studies have shown the efficacy of mechanical thrombectomy (MT) in strokes caused by occlusions of large vessels.4,5
Native noncontrast cerebral computed tomography (CT) imaging was used in the initial studies of intravenous thrombolysis (IVT) and is established as the standard in acute stroke care worldwide, in particular to exclude cerebral bleedings.6 A decision regarding IVT and MT is based on the patient’s symptoms and the clinical decision in the emergency room.7,8 Possible misdiagnoses are ‘stroke mimics’ (SMs) resulting in false-positives and ‘stroke chameleons’ (SCs) resulting in a false-negative stroke diagnoses.
The majority of SMs are conditions that simulate stroke, such as migraine and seizures. The proportion of patients with SMs who are misdiagnosed as stroke decreases with the use of magnetic resonance imaging (MRI) at baseline. Studies on the proportion of SMs treated as ischemic stroke vary from 0% (when MRI is utilized prior to thrombolysis) to 25%, if standard procedures are used.9 SM patients may receive IVT and are exposed to side effects of this therapy, in addition the underlying cause is recognized and treated late. Patients presenting with SCs are not identified as stroke patients and are not appropriately treated.10,11
Additional imaging modalities have been optimized in recent years and help to define potential tissue at risk according to the mismatch concept.12 Important tools are CT angiography (CT-A) for detection of larger arterial occlusions and CT perfusion (CT-P) analysis to define the penumbra and the infarct core. Advanced imaging technologies with MRI or CT-P can guide effective acute reperfusion treatment with MT and IVT.13
CT-based procedures are radiation-intensive. The radiation exposure of a single examination with CT-A and CT-P may be up to 1500 mGy surface dose. Compared with CT scans, the advantages of MRI include higher sensitivity for smaller lacunar or embolic lesions and ischemia in the posterior cranial fossa, easier assessment of the age of the infarction, and the reduction of SMs and SCs. These advantages add to the absence of radiation exposure.14 Nevertheless, CT is predominantly used in acute diagnostics and therapy in acute stroke patients based on the arguments of a faster imaging and shorter door-to-needle times (DNTs) as one of the most important quality parameters.15 To date, less than 20% of stroke units reportedly use MRI for acute diagnosis, although the number of available MRI scanners is constantly increasing worldwide.12 In Baden-Württemberg (BW), a federal state with 10.8 million inhabitants in southwest Germany, only 16% of all stroke patients received an MRI as their first imaging modality in 2017.16
It is our hypothesis that, within a high-standard-organization setting, MRI can be carried out quickly and easily, thereby resulting not only in an increased rate of acute reperfusion treatments using IVT and MT but also in an improved patient outcome.
Methods
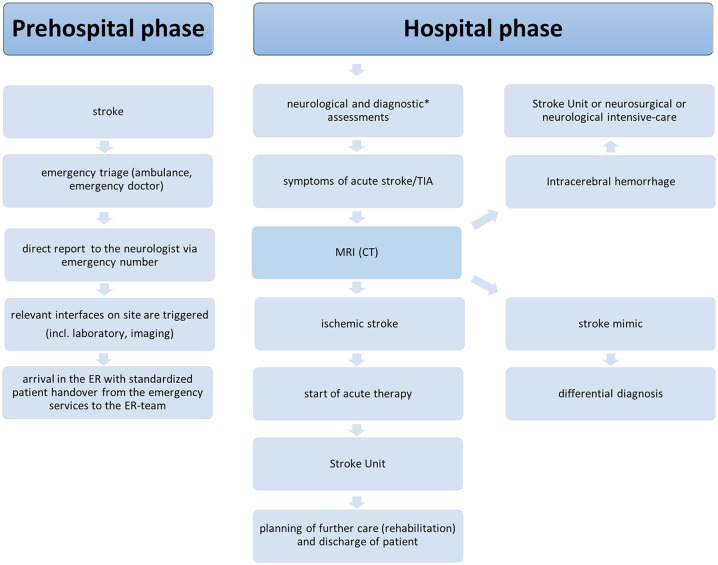
We developed an MRI-based Ulm Stroke Concept (Ulm’s concept) with the goal of establishing optimized treatment outcomes aiming at high diagnostic certainty and high reperfusion rates. (Figure 1)
Figure 1.
Ulm’s stroke concept pathway.
*Standard operating procedures include blood pressure and blood tests.
TIA, transient ischemic attack.
We first tested our hypothesis that MRI as first-line imaging technique can be performed quickly in the majority of all stroke patients with consecutive short DNTs. Second, we compared the rates of acute reperfusion treatments using IVT and MT, the outcome and safety parameters and the rate of patients diagnosed with a transient ischemic attack (TIA).
We performed a retrospective observational cohort study at the Department of Neurology at the University and Rehabilitation Clinic Ulm.
To this end, we compared the quality assurance data entered into the stroke registry of BW from January 2018 to December 2019, which includes patients with the diagnosis of an ischemic stroke and TIA, using the diagnostic codes International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) I 61 [intracerebral bleeding (ICB)], I 63 (ischemic stroke), and G 45 (TIA) in patients >18 years of age with an acute event (<1 week) for acute stroke with a complete dataset. Patients with a palliative situation at admission were excluded from most analyses. BW is a federal state with 10.8 million inhabitants in southwest Germany, in which the city of Ulm is located.
The stroke registry of BW contains comprehensive data from 133 hospitals in 2019 and 136 hospitals in 2018, describing procedures and outcomes of stroke medicine, such as demographics, stroke scores, treatment data, and outcome measures.17 BW operates a three-level system for the treatment of acute stroke, with local and regional stroke units and comprehensive stroke centers, all certified on a standardized and high level; only a few patients with stroke are treated in hospitals without a certified stroke unit. In order to reduce a possible bias due to differing care standards between the levels of the stroke units, we additionally compared the data exclusively with comprehensive stroke units, which are the maximum care providers for stroke patients. All hospitals in BW treating acute strokes, are obliged by law to participate in this comprehensive stroke registry. Details of the registry have been published elsewhere.18 The Department of Neurology in Ulm with its comprehensive stroke unit is the primary care center for all stroke patients in a region of more than 0.5 million inhabitants. Since its foundation in 1999, stroke diagnosis and therapy are based on MRI if no contraindications exist.
The Ulm’s concept includes the prehospital and the acute hospital phase: the prehospital phase includes a stroke allocation concept developed in cooperation with the emergency teams.
For acute admission, patients are allocated via the neurological emergency unit (ER), which has immediate access (24 h, 7 days a week) to both MRI and CT scanners. Every patient with an acute stroke within a defined time window <6 hours or an unclear time window is triaged as a potential lysis patient. The main distinguishing feature of Ulm’s concept is a structured and optimized treatment process based on advanced acute stroke imaging using MRI as the primary imaging technique.
An on-site multi-professional stroke-trained team is available. The patient is reported directly to the neurologist via an emergency phone number. Relevant information is already recorded here by telephone (see supplement for emergency service checklist), and an information cascade of the relevant interfaces on site (stroke care, imaging, laboratory, neuroradiologist) is triggered. When the patient arrives in the ER, a standardized procedure transfers the patient from the emergency services to the ER team.
A medical history, neurological examination, blood tests for coagulation abnormalities, and query for possible contraindications for MRI or thrombolysis follows before imaging is initiated. At the same time, the patient is provided with an intravenous line, vital signs are recorded, and the patient is transferred to the imaging table. In the meantime, the blood is examined, in particular with regards to coagulation parameters. A routine MRI stroke scan (Supplemental Data) has an acquisition time of 10′ 21″ and includes diffusion-weighted imaging (DWI), a T2*-weighted gradient echo sequence (sensitive to hemosiderin), a time-of-flight angiography (TOF-MRA), a fluid-attenuated inversion recovery (FLAIR) sequence, a T1-weighted sequence, and perfusion-weighted imaging (PWI).
In case of a time window <4.5 h, thrombolysis bolus and infusions are given in the scanner after hemorrhage has been excluded by T2* sequences. If the time window is unknown or >4.5 h, IVT is given according to the MRI paradigms after the FLAIR sequence or after the PWI. In addition to demographic data, we documented comorbidities, clinical scores, quality parameters such as DNT, as well as outcome parameters and complications.
Statistical analysis
Continuous variables were described as mean, median, and quartiles, or minimum/maximum. Categorical variables were presented as absolute and relative frequencies. The Chi-square test or Fisher’s exact test was used for comparison of categorical values as appropriate.
A two-sided p-value <0.05 was considered statistically significant. Because of the explorative nature of this study, all results from statistical tests have to be interpreted as hypothesis-generating and an adjustment for multiple testing was not made. Statistical analyses were conducted with SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 2182 acute patients with ICB, ischemic stroke, or TIA in Ulm and 82,760 in BW, including 29,575 patients treated in a comprehensive stroke unit (BWc) were admitted between 1 January 2018 and 31 December 2019. Of these, 1922 patients in Ulm, 77,667 patients in BW and 27,555 patients in BWc met the inclusion criteria and were included in the evaluation.
Patient baseline characteristics
Table 1 shows the characteristics of the study cohorts. The sex ratio and age were equally balanced between the groups with 46–47% female and a median age of 76–77 years [interquartile range (IQR) Ulm 18 to 100 versus BW 18 to 107 and BWc 18 to 105 years]. The mean National Institutes of Health stroke scale (NIHSS) score at admission was similar (Ulm 5.7, BW 5.1, BWc 6.3). The proportion of patients with a modified Rankin scale (mRS) 0–2 at admission (indicating functional independence) was lower (p < 0.01) in the cohort of Ulm (40.6%) versus BW (51.2%) and BWc (47.2%).
Table 1.
Patient characteristics of the study cohorts from Ulm versus BW versus BWc.
| Ulm | BW | BWc | p-value | |
|---|---|---|---|---|
| Patientsa, n | 2182 | 82,760 | 29,575 | |
| Stroke/TIA (meeting inclusion criteriab), n (%) | 1922 (88.1) | 77,667 (93.9) | 27,555 (93.2) | |
| Stroke/TIA (meeting inclusion criteriac), n (%) | 1921 (88.1) | 76,166 (92.0) | 27,457 (92.8) | |
| Age meanb (min–max) | 74.2 (18–100) | 73.7 (18–107) | 72.8 (18–105) | 0.99 |
| Femaleb, n (%) | 911 (47.4) | 36,497 (47.0) | 12,688 (46.1) | 0.73 |
| Maleb, n (%) | 1011 (52.6) | 41,167 (53.0) | 14,867 (54.0) | |
| NIHSS at admissionb (mean, median) | 5.65, 3.0 | 5.1, 2.0 | 6.3, 3.0 | <0.01 |
| mRS at admissionb, n (%) | <0.01 | |||
| 0–2 | 780 (40.6) | 39,789 (51.2) | 13,011 (47.2) | |
| 3–6 | 1142 (59.4) | 37,878 (49.8) | 14,544 (52.8) | |
| Onset to admission time <3 hb, n (%) | 703 (36.6) | 28,140 (36.5) | 9769 (36.5) | 0.91 |
| Comorbid conditionsc, (%) | ||||
| Diabetes mellitus | 23.6 | 23.9 | 22.8 | <0.01 |
| Atrial fibrillation | 30.2 | 26.7 | 27.3 | <0.01 |
| Hypertension | 88.5 | 80.3 | 76.7 | <0.01 |
Results are presented as absolute numbers (n), median (interquartile range) or percentage.
All cases.
Population: All treatment cases within the meaning of the inclusion criteria without patients with a palliative situation at admission.
Population: All treatment cases within the definition of the inclusion criteria minus patients with a palliative situation at admission and patients admitted after >48 h from symptom onset from a clinic with a stroke unit.
BW, Baden-Wuerttemberg; BWc, Baden-Wuerttemberg comprehensive stroke units (n = 12);
mRS, modified Rankin scale; NIHSS, National Institutes of Health stroke scale; TIA, transient ischemic attack.
Access to imaging and recanalization therapy
By establishing a fast-track service in the ER, we achieved significantly accelerated patient management processes. Overall, 90.7% of patients in Ulm, 88.1% of the BW patients and 81.9% of the BWc patients received acute cerebral imaging after admission, while 9.3% of the Ulm patients, 11.4% of BW patients and 17.8% of BWc patients had imaging before admission. In 89.5% of cases in Ulm, MRI was the first imaging modality (89.9% with PWI), an exact comparative figure from BW is not available for the period examined but was 16.45% in the period from 2016 to 2017 in BW and 23.5% in BWc. The processing times for MRI and CT in Ulm are almost the same with regard to the door-to-imaging time with a median door-to imaging time of 16 min for MRI and 17 min for CT (p = 0.35). When looking at the time-to-first-imaging after admission with MRI or CT in the hospital, a significantly higher proportion of patients received imaging within 30 min in the Ulm setting compared with the BW and BWc cohort (57.8% versus 41.4% versus 36.5%). Significantly more patients in Ulm (98.3%) had an intracranial vessel imaging than in BW (53.5%) and in BWc (62.8%). Overall, 56.5% of the patients in Ulm had intracranial vascular imaging for acute occlusion detection within 30 min after arrival compared with 39.6% of the BW and 43.6% of the BWc cohort (Table 2).
Table 2.
Recanalization characteristics and quality parameter of patients.
| Ulm | BW | BWc | p-value | |
|---|---|---|---|---|
| Imagingb, n | 1922 | 77,210 | 27,416 | |
| No imaging, % | 0.0 | 0.5 | 0.4 | <0.01 |
| Before, % | 9.7 | 11.4 | 17.8 | <0.01 |
| Time door-to-imagingb <30 min, % | 57.5 | 41.4 | 36.5 | <0.01 |
| Time door-to-imagingb ⩾30–40 min, % | 2.7 | 7.4 | 6.8 | <0.01 |
| Time door-to-imagingb >40–60 min, % | 3.8 | 9.9 | 9.0 | <0.01 |
| Time door-to-imagingb >60 min, % | 26.3 | 29.4 | 29.6 | <0.01 |
| Intracranial vessel imagingc, % | 98.3 | 53.5 | 62.8 | <0.01 |
| Time door-to-intracranial-vessel imaging in-house, n |
1755 | 41,201 | 17,167 | |
| ⩽30 min, % | 56.5 | 39.6 | 43.6 | <0.01 |
| >30–60 min, % | 10.9 | 17.12 | 15.5 | <0.01 |
| >60–120 min, % | 12.1 | 11.89 | 10.1 | =0.20 |
| >120 min, % | 20.5 | 31.3 | 30.9 | <0.01 |
| Eligible for IVTc n | 1921 | 77,109 | 27,457 | |
| IVT yes, % | 21.5 | 14.2 | 18.5 | <0.01 |
| IVT external, % | 2.3 | 1.9 | 4.4 | <0.01 |
| IVT internal, % | 19.1 | 12.3 | 14.0 | <0.01 |
| IVT no, % | 78.6 | 85.8 | 81.6 | <0.01 |
| IVT with discharge diagnosis I.63 and G.45, n |
1502 | 52,512 | 19,358 | |
| Yes, % | 27.4 | 20.9 | 26.1 | <0.01 |
| External, % | 3.0 | 2.8 | 6.3 | <0.01 |
| Internal, % | 24.4 | 18.1 | 19.8 | <0.01 |
| No, % | 72.6 | 79.1 | 73.9 | <0.01 |
| And STD ⩽4 h, NIHSS 4–25, age < 80 | 76.8 | 69.5 | 71.6 | <0.01 |
| And STD ⩽4 h, NIHSS 4–25, age > 80 (%) | 69.3 | 56.1 | 62.1 | <0.01 |
| Door-to-needle time of all intravenously treated patients, n | 367 | 9517 | 3834 | |
| <30 min, % | 73.6 | 44.1 | 47.1 | <0.01 |
| ⩾30–40 min, % | 15.5 | 20.0 | 20.2 | <0.01 |
| >40–60 min, % | 8.2 | 21.8 | 40.2 | <0.01 |
| >60 min, % | 2.7 | 14.1 | 6.2 | <0.01 |
| Patients with detected acute proximal vascular occlusion, n | 247 | 9419 | 5347 | |
| i.a. thrombolysis and/or mechanical recanalization, n | 171 | 4771 | 3168 | |
| Yes, % | 69.2 | 50.7 | 59.3 | <0.01 |
| No, % | 30.8 | 49.4 | 40.8 | <0.01 |
| External, % of all detected occlusions | 1.6 | 13.0 | 0.9 | <0.01 |
| Internal, % of all detected occlusions | 67.6 | 37.7 | 58.3 | <0.01 |
Door-to-imaging time, intracranial vessel imaging with door-to-intracranial vessel imaging in-house time in all patients, intravenous thrombolysis and door-to-needle time of all IVT-treated patients and MT in patients with detected acute proximal vascular occlusion.
Results are presented as absolute numbers (n) or percentage.
Population: All treatment cases within the meaning of the inclusion criteria without patients with a palliative situation at admission.
Population: All treatment cases within the definition of the inclusion criteria minus patients with a palliative situation at admission and patients admitted after >48 h later of symptom onset from a clinic with a stroke unit.
BW, Baden-Wuerttemberg; BWc, Baden-Wuerttemberg comprehensive stroke units; IVT, intravenous thrombolysis; MT, mechanical thrombectomy; NIHSS, National Institutes of Health stroke scale; STD, symptom to door; i.a, intra-arterial.
The proportion of patients receiving IVT was significantly higher (p < 0.01) in the cohort from Ulm (21.5%) compared with the BW cohort (14.2%) and also higher in comparison with the other comprehensive stroke units (18.5%). Regarding thrombolysis rates of patients with the discharge diagnosis of an ischemic stroke or TIA (excluding patients already palliative in advance of the stroke, or showing symptoms >48 h before admission), the thrombolysis rate was higher by one-third compared with BW with 27% versus 21% (p < 0.01) and also higher compared with BWc hospitals (26.1%, p < 0.01)
Ulm achieved a DNT < 30 min in 73.6% of the patients (BW 44.1%, BWc 47.1%) and a DNT < 40 min in 89.1% of the patients versus 64.1% in the BW and 67.3% in the BWc cohort. The DNT was <1 h in 97.3% of IVT patients in Ulm, whereas the corresponding number was 86% of patients in BW and 93.8% of BWc patients. When analyzing a subgroup of patients with an ischemic stroke and an age of 18 to 80 years with a symptom-to-door time (STD) ⩽ 4 h and with moderate to very severe symptoms (NIHSS 4–25), we see a significantly higher (p < 0.01) thrombolysis rate of 76.8% (Ulm) versus 69.5% (BW) and 71.6% (BWc). We consistently see higher thrombolysis rates in the group of patients aged 80 and older (Ulm 69.3% versus BW 56.1% versus BWc 62.1%, p < 0.01) (Table 3). The DNT in Ulm is 1.45 min longer for the CT compared to MRI, but also not statistically significant (p = 0.45)
Table 3.
Discharge diagnosis, reason for discharge, mortality, and complications in patients.
| Ulm | BW | BWc | p-value | |
|---|---|---|---|---|
| Discharge diagnosisa | 1921 | 76,166 | 27,555 | |
| TIA (G 45) % | 16.18 | 24.63 | 19.85 | <0.01 |
| Stroke (I 63) % | 77.94 | 68.13 | 70.44 | |
| ICB (I 61) % | 5.88 | 7.24 | 9.7 | |
| Discharge to:a | ||||
| Nursing home, % | 3.90 | 4.79 | 3.0 | <0.01 |
| Rehabilitation facility, % | 15.14 | 11.48 | 11.65 | |
| Patient home, % | 58.90 | 64.61 | 59.51 | |
| Other facility | 16.55 | 12.89 | 17.6 | |
| Mortality, % | 5.52 | 6.23 | 8.26 | |
| Mortality <80 years, without ICB, atrial fibrillation, diabetes, % | 1.30 | 1.60 | 2.44 | <0.01 |
| Complicationsa n (%) | 95 (4.95) | 3756 (4.93) | 1528 (5.55) | 0.98 |
| Nosocomial pneumonia, % | 2.80 | 2.58 | 3.29 | 0.57 |
| Secondary ICB, % | 1.87 | 1.13 | 1.66 | <0.01 |
| Cerebral artery embolism, % | 0.31 | 0.44 | 0.58 | 0.40 |
Results are presented as absolute numbers (n) or percentage.
Population: All treatment cases within the meaning of the inclusion criteria without patients with a palliative situation at admission.
BW, Baden-Wuerttemberg; BWc, Baden-Wuerttemberg comprehensive stroke units; ICB, intracerebral bleeding; TIA, transient ischemic attack.
In the subgroup of the patients with acute vascular occlusion, 69.2% of Ulm patients had MT. In comparison, 51% of the patients of the BW and 59.3% of the BWc cohort received MT after a large-vessel occlusion.
Effectiveness
A favorable outcome at discharge with an mRS of 0–2, regardless of therapy, was achieved in 61.7% of the patients in Ulm, in 68.1% of the BW and in 62.5% of the BWc patients [Figure 2(a)]. With respect to the shift of the mRS between admission and discharge, 21% of the Ulm patients shifted to a favorable mRS score at discharge compared to admission, which was significantly higher (p < 0.01) than the shift in BW with 17% and in the BWc patients with 15.3%, respectively [Figure 2(b)].
Figure 2.
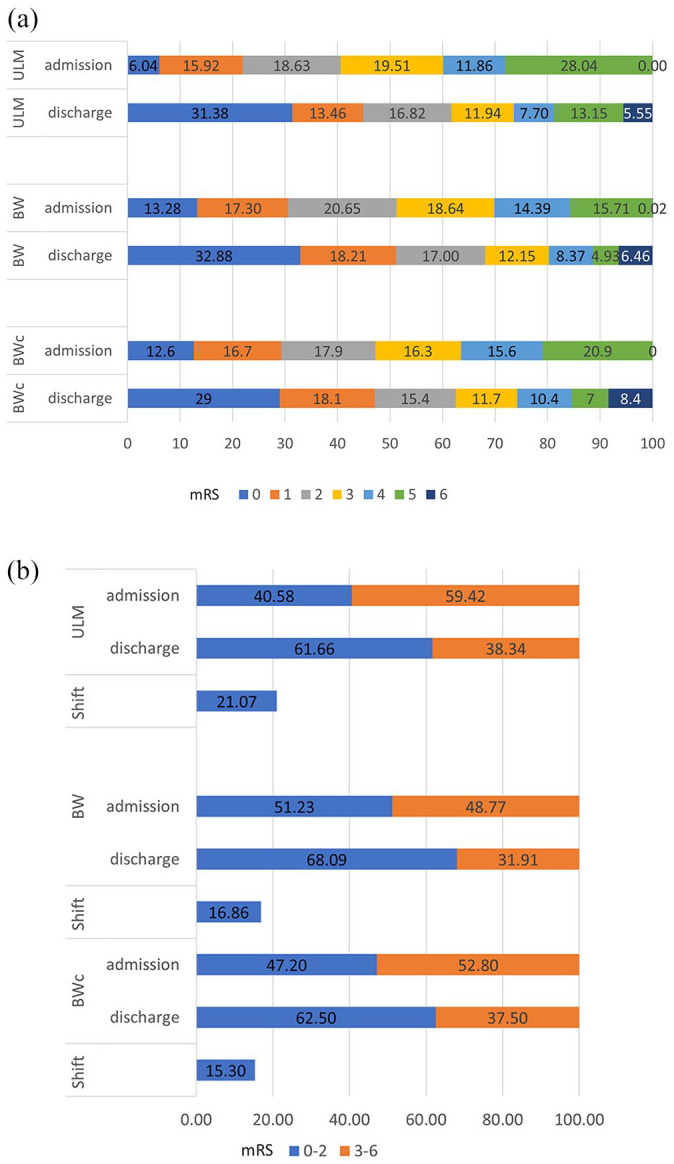
(a) mRS at admission versus mRS at discharge Ulm, BW and BWc. (b) Favorable mRS 0–2 versus unfavorable mRS 3–6, admission versus discharge, and the shift to a favorable mRS between Ulm, BW, BWc.
BW, Baden-Wuerttemberg; BWc, Baden-Wuerttemberg comprehensive; mRS, modified Rankin scale.
Discharge, complications, and mortality
At discharge, a significantly lower percentage of Ulm’s patients had the diagnosis of a TIA with 16.2% versus 24.6% in BW and 19.7% in BWc (p < 0.01). In contrast, Ulm had higher rates of ischemic stroke with 77.9% in comparison to other BW (68.1%) and BWc (70.4%) hospitals. The diagnosis of an ICB was higher in BW (7.2%) and BWc (9.7%) compared with Ulm (5.9%). (Table 3)
Most of the patients were discharged to their homes (58.9% versus 64.6% versus 59.5%) in all cohorts. 3.9% of the Ulm patients were discharged to a nursing home as were 4.9% of BW patients and 3.0% of BWc patients. Discharge to a rehabilitation facility took place in 15.1% of the cases in Ulm, in 11.5% of the BW patients and 11.7% of the BWc cohort.
In-hospital mortality of all patients included was lower in the cohort of Ulm with 5.5% compared to 6.2% in BW (p = 0.20) and 8.3 in BWc. In a subgroup analysis of all nonpalliative patients <80 years of age, without bleeding, without atrial fibrillation and without diabetes, 1.3% of the patients in Ulm did not survive, whereas the corresponding number in the BW cohort was 1.6% and 2.4% in BWc cohort.
If we consider the complication rates, rates of nosocomial pneumonia were similar (Ulm 2.8%, BW 2.6%, BWc 3.3%, p = 0.57). Ulm had more symptomatic ICBs [Ulm 1.9% versus BW 1.1% versus BWc 1.7%, (p < 0.01)], i.e. evidence of hemorrhage in the imaging combined with a secondary deterioration of the neurological status. On the other hand, the proportion of patients with secondary cerebral artery embolism was lower (Ulm 0.3% versus BW 0.4% and BWc 0.6%, p = 0.40).
Discussion
In this retrospective observational cohort study which contains data of patients from all stroke units in a region of about 11 million inhabitants in the southwest of Germany, we evaluated the Ulm Concept based on MRI as the first-line imaging tool. As a summary of the results, MRI was the first imaging procedure in 89.5% of the patients, while the MRI/CT ratio in BW and BWc was the opposite with more than 80% primary CT imaging.16 In the cohort of Ulm, the rate of cerebral imaging together with vessel imaging within 30 min after admission was significantly higher compared to both BW and the comprehensive stroke units.
Almost 25 years after the results of the first randomized controlled trial showing efficacy of IVT in patients with stroke, there is still a substantial discrepancy between the estimated 20–25% of patients potentially eligible and the proportion actually treated.19,20 Even in optimally organized stroke-treating regions in Europe such as Tyrol in Austria,21 regional networks such as TEMPIS in southeast Germany (17%)22 or stroke centers such as Bern in Switzerland (13%),23 these numbers are still not achieved. In our setting, both the IVT rate (27.4% in patients with an ischemic stroke was significantly higher than in BW (20.9%) or in the comprehensive stroke units (26.1%), and a DNT of <30 min could be achieved in the vast majority (74%) of the patients in Ulm, which is substantially better than in the BW group (44.1%) and the comprehensive stroke units (47.1%) and also worldwide.24 The American Heart Association sets as a primary goal that 75% of all patients be treated with IVT within 60 min after arrival, and as an advanced secondary goal the treatment of 50% and more of acute ischemic stroke patients within 45 min.25,26 It should be noted that this low DNT was achieved by use of an MRI-based diagnostic pathway in 80% of the cases. Of course, one can consider whether the differences in the DNT compared to our results are due to other factors than the imaging modality, for example in the ER organization or perhaps even due to the fact that it is necessary to start a large lumen intravenous line to perform CT-A and CT-P. However, the decision may also take longer because a relevant proportion of the patients require differentiated imaging. Differentiated CT-based imaging also takes around 10 min, including CT-A and CT-P. In addition, an experienced radiologist is usually required for the diagnosis since this is more difficult than the assessment of a stroke MRI.
In the past 5 years, multiple studies have shown that, in strokes caused by large-vessel occlusions, MT is superior compared with IVT.4 With the help of our concept, 70% of patients with acute vascular occlusion underwent MT in comparison with 51% in BW and 59.3% in comprehensive stroke units. This can be explained by the fact that an MR-TOF-angiography is part of the routine MRI protocol, thus the intracranial hemodynamics are already known from admission. More than 90% of these procedures could be completed with a successful recanalization TICI IIb or III. These rates are similar to those achieved by other hospitals in BW and show higher rates compared with the recanalization rates of other large case series or trials on endovascular treatment.27–30 In addition, these high IVT and MT rates clearly resulted in a shift to an improved and favorable outcome at discharge.31,32
Increased application of recanalization treatments seemed to result in more symptomatic intracerebral hemorrhage (1.9% versus 1.1% and 1.7% in the control cohort). This complication of IVT and MT is similar to that in other large series or trials on endovascular treatment,33,34 but did not result in worse outcome measures, given the higher shift to a favorable outcome at discharge. Furthermore, patients with a high load of cerebral microbleeds, who have associated with a higher risk of bleeding with IVT, can be detected using MRI, which is likely to result in safer IVT compared with CT.35
It is well known that SMs account for up to 25% of admissions for probable strokes, most usually misdiagnosed as TIAs. The use of MRI decreases this number compared with CT, and helps to discriminate between SMs and real stroke patients.9 The significantly lower TIA rate in Ulm (16.1% of the stroke events) compared with the control group reflects reduced numbers of misdiagnoses due to SMs. This could also be economically relevant due to potentially shorter hospital stays, as has already been discussed by some authors.36
Our study has several limitations. First, it is an observational study on routinely collected retrospective data from all stroke-treating hospitals in BW. The sources of the data could not be verified in each and every case, although they are regularly checked due to predefined logical and range checks.18,37,38 We assume that our favorable treatment results are mainly associated with the primary use of MRI as a core element of Ulm’s concept. However, treatment results are also fundamentally influenced by specific algorithms and procedures. There may probably be confounding factors that might have biased the present results, e.g. rural versus metropolitan environment of the included hospitals, the prehospital emergency triage, the organization and infrastructure of the emergency rooms, and the structure of the stroke units (e.g. tertiary stroke centers versus smaller stroke units). Nonetheless, since the vast majority of stroke units in BW are certified by the same organization at the identical very high standard, we believe that the general stroke unit treatment in Ulm is comparable to the quality of treatment algorithms of other stroke units in BW, especially to the other comprehensive stroke units. Furthermore, the organization of the rescue service is uniformly regulated in BW. In addition, it has already been shown in other studies that the functional outcome and mortality in patients selected by MRI were more favorable than in patients selected by CT.39 Our outcomes are not measured at a predefined time point but at hospital discharge, which limits the comparison of data compared to those from randomized controlled trials with standardized assessment of outcomes at 3 months.
Taken together, Ulm’s concept offers an optimized, time-efficient treatment process with a high degree of diagnostic certainty, a high reperfusion rate with improved clinical outcome. The reported data are from a German sample, but the underlying MRI-based concept could be transferred to all stroke-treating clinics that have immediate access to MRI imaging. Ultimately, we hypothesize that the primary use of MRI as an imaging technique is the major cause of our favorable outcome data. Therefore, we think that they call for prospective randomized clinical trials that address the question of potential benefits for public health of the primary use of MRI versus CT in stroke medicine. Such studies also should include the question of radiation exposure and potential additional economic aspects associated with an infrastructure for MRI-based stroke medicine.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864211030363 for MRI as a first-line imaging modality in acute ischemic stroke: a sustainable concept by Katharina Althaus, Jens Dreyhaupt, Sonja Hyrenbach, Elmar H. Pinkhardt, Jan Kassubek and Albert C. Ludolph in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-pdf-2-tan-10.1177_17562864211030363 for MRI as a first-line imaging modality in acute ischemic stroke: a sustainable concept by Katharina Althaus, Jens Dreyhaupt, Sonja Hyrenbach, Elmar H. Pinkhardt, Jan Kassubek and Albert C. Ludolph in Therapeutic Advances in Neurological Disorders
Footnotes
Author contributions: KA was responsible for drafting the manuscript, study concept and design, analysis and interpretation of data, acquisition of data and statistical analysis. JD was responsible for drafting the manuscript, analysis and interpretation of data and statistical analysis. SH was responsible for acquisition of data and revising the manuscript. EHP and JK were responsible for revising the manuscript and interpretation of data. ACL was responsible for revising the manuscript, study concept and design, interpretation of data and study supervision.
Conflict of interest statement: The Associate Editor of Therapeutic Advances in Neurological Disorders is an author of this paper, therefore, the peer review process was managed by alternative members of the Board and the submitting Editor was not involved in the decision-making process.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics: The study was approved by the Ethics Committee of the University of Ulm/Germany (reference 02/21), which waived the need for informed consent.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Katharina Althaus  https://orcid.org/0000-0002-9004-4335
https://orcid.org/0000-0002-9004-4335
Jan Kassubek  https://orcid.org/0000-0002-7106-9270
https://orcid.org/0000-0002-7106-9270
Contributor Information
Katharina Althaus, Department of Neurology, University of Ulm, Oberer Eselsberg 45, Ulm, Baden-Wuerttemberg 89075, Germany.
Jens Dreyhaupt, Institute of Epidemiology and Medical Biometry, University of Ulm, Germany.
Sonja Hyrenbach, Qualitätssicherung im Gesundheitswesen Baden-Württemberg, Stuttgart, Germany.
Elmar H. Pinkhardt, Department of Neurology, University of Ulm, Germany
Jan Kassubek, Department of Neurology, University of Ulm, Germany.
Albert C. Ludolph, Department of Neurology, University of Ulm, Germany
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell BCV, Ma H, Ringleb PA, et al. Extending thrombolysis to 4·5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet 2019; 394: 139–147. [DOI] [PubMed] [Google Scholar]
- 3.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Hill MD, Rubiera M, et al. Safety and efficacy of solitaire stent thrombectomy: individual patient data meta-analysis of randomized trials. Stroke 2016; 47: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 7.Saur D, Kucinski T, Grzyska U, et al. Sensitivity and interrater agreement of CT and diffusion-weighted MR imaging in hyperacute stroke. AJNR Am J Neuroradiol 2003; 24: 878–885. [PMC free article] [PubMed] [Google Scholar]
- 8.von Kummer R. Effect of training in reading CT scans on patient selection for ECASS II. Neurology 1998; 51: S50–S52. [DOI] [PubMed] [Google Scholar]
- 9.Moulin S, Leys D.Stroke mimics and chameleons. Curr Opin Neurol 2019; 32: 54–59. [DOI] [PubMed] [Google Scholar]
- 10.Long B, Koyfman A.Clinical mimics: an emergency medicine-focused review of stroke mimics. J Emerg Med 2017; 52: 176–183. [DOI] [PubMed] [Google Scholar]
- 11.Liberman AL, Prabhakaran S.Stroke chameleons and stroke mimics in the emergency department. Curr Neurol Neurosci Rep 2017; 17: 15. [DOI] [PubMed] [Google Scholar]
- 12.Campbell BC, Parsons MW.Imaging selection for acute stroke intervention. Int J Stroke 2018; 13: 554–567. [DOI] [PubMed] [Google Scholar]
- 13.Thomalla G, Gerloff C.Acute imaging for evidence-based treatment of ischemic stroke. Curr Opin Neurol 2019; 32: 521–529. [DOI] [PubMed] [Google Scholar]
- 14.Currie GM.Pharmacology, part 5: CT and MRI contrast media. J Nucl Med Technol 2019; 47: 189–202. [DOI] [PubMed] [Google Scholar]
- 15.Mair G, Wardlaw JM.Imaging of acute stroke prior to treatment: current practice and evolving techniques. Br J Radiol 2014; 87: 20140216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.QiG_BW. Baden-württembergisches Qualitätssicherungsverfahren gemäß Landesvertrag nach §112 Abs. 2 Nr. 3 SGB VModul 80/1. 2015. [Google Scholar]
- 17.QiG_BW. Baden-württembergisches Qualitätssicherungsverfahren gemäß Landesvertrag nach §112 Abs. 2 Nr. 3 SGB VModul 80/1. In: BW Q, (ed.). 2020. [Google Scholar]
- 18.Gumbinger C, Reuter B, Wietholter H, et al. A consecutive and prospective stroke database covers the state of Baden-Wuerttemberg with 10.8 million inhabitants in Germany. Neuroepidemiology 2013; 41: 161–168. [DOI] [PubMed] [Google Scholar]
- 19.Davalos A.Thrombolysis in acute ischemic stroke: successes, failures, and new hopes. Cerebrovasc Dis 2005; 20(Suppl. 2): 135–139. [DOI] [PubMed] [Google Scholar]
- 20.Boode B, Welzen V, Franke C, et al. Estimating the number of stroke patients eligible for thrombolytic treatment if delay could be avoided. Cerebrovasc Dis 2007; 23: 294–298. [DOI] [PubMed] [Google Scholar]
- 21.Willeit J, Geley T, Schoch J, et al. Thrombolysis and clinical outcome in patients with stroke after implementation of the tyrol stroke pathway: a retrospective observational study. Lancet Neurol 2015; 14: 48–56. [DOI] [PubMed] [Google Scholar]
- 22.Backhaus R.TEMPiS - ein Meilenstein in der Teleneurologie –. 1 Deutscher Teleneurologie Kongress. Erfurt2017. [Google Scholar]
- 23.Fischer U, Mono ML, Zwahlen M, et al. Impact of thrombolysis on stroke outcome at 12 months in a population: the Bern stroke project. Stroke 2012; 43: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 24.Kamal N, Smith EE, Jeerakathil T, et al. Thrombolysis: Improving door-to-needle times for ischemic stroke treatment - a narrative review. Int J Stroke 2018; 13: 268–276. [DOI] [PubMed] [Google Scholar]
- 25.Fonarow GC, Smith EE, Saver JL, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association’s target: stroke initiative. Stroke 2011; 42: 2983–2989. [DOI] [PubMed] [Google Scholar]
- 26.Association Herat Association. Target: stroke phase III, https://www.heart.org/-/media/files/professional/quality-improvement/target-stroke/target-stroke-phase-iii/ts-phase-iii-5-6-19/final5619-target-stroke-phase-3-brochure.pdf?la=en (2018, accessed 10 October 2020).
- 27.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 28.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 29.Truelsen T, Hansen K, Andersen G, et al. Acute endovascular reperfusion treatment in patients with ischaemic stroke and large-vessel occlusion (Denmark 2011-2017). Eur J Neurol 2019; 26: 1044–1050. [DOI] [PubMed] [Google Scholar]
- 30.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 31.Amiri H, Bluhmki E, Bendszus M, et al. European cooperative acute stroke study-4: extending the time for thrombolysis in emergency neurological deficits ECASS-4: ExTEND. Int J Stroke 2016; 11: 260–267. [DOI] [PubMed] [Google Scholar]
- 32.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 33.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med 2018; 379: 611–622. [DOI] [PubMed] [Google Scholar]
- 35.Tsivgoulis G, Zand R, Katsanos AH, et al. Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: a meta-analysis. JAMA Neurol 2016; 73: 675–683. [DOI] [PubMed] [Google Scholar]
- 36.Manwani B, Rath S, Lee NS, et al. Early magnetic resonance imaging decreases hospital length of stay in patients with ischemic stroke. J Stroke Cerebrovasc Dis 2019; 28: 425–429. [DOI] [PubMed] [Google Scholar]
- 37.Gumbinger C, Reuter B, Hacke W, et al. Restriction of therapy mainly explains lower thrombolysis rates in reduced stroke service levels. Neurology 2016; 86: 1975–1983. [DOI] [PubMed] [Google Scholar]
- 38.Gumbinger C, Ringleb P, Ippen F, et al. Outcomes of patients with stroke treated with thrombolysis according to prestroke Rankin Scale scores. Neurology 2019; 93: e1834–e1843. [DOI] [PubMed] [Google Scholar]
- 39.Meinel TR, Kaesmacher J, Mosimann PJ, et al. Association of initial imaging modality and futile recanalization after thrombectomy. Neurology 2020; 95: e2331–e2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864211030363 for MRI as a first-line imaging modality in acute ischemic stroke: a sustainable concept by Katharina Althaus, Jens Dreyhaupt, Sonja Hyrenbach, Elmar H. Pinkhardt, Jan Kassubek and Albert C. Ludolph in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-pdf-2-tan-10.1177_17562864211030363 for MRI as a first-line imaging modality in acute ischemic stroke: a sustainable concept by Katharina Althaus, Jens Dreyhaupt, Sonja Hyrenbach, Elmar H. Pinkhardt, Jan Kassubek and Albert C. Ludolph in Therapeutic Advances in Neurological Disorders