Abstract
Objectives:
To investigate factors associated with major adverse cardiovascular events (MACEs) in patients with rheumatoid arthritis (RA).
Methods:
We conducted a nationwide, population-based, case-control study using Taiwan’s National Health Insurance Research Database for 2003–2013. From 2004 to 2012, we identified 108,319 newly diagnosed RA patients without previous MACEs, of whom 7,580 patients (7.0%) developed MACEs during follow-up. From these incident RA patients, we included 5,994 MACE cases and 1:4 matched 23,976 non-MACE controls for analysis. The associations of MACEs with comorbidities and use of anti-rheumatic medications within 1 year before the index date were examined using conditional logistic regression analyses.
Results:
Using multivariable conditional logistic regression analysis, the risk of MACE in RA patients was associated with use of golimumab [odd’s ratio (OR), 0.09; 95% confidence interval (CI), 0.01-0.67], abatacept (OR, 0.13; 95% CI, 0.02–0.93), hydroxychloroquine (OR, 0.90; 95% CI, 0.82-0.99), methotrexate (OR, 0.72; 95% CI, 0.64–0.81), cyclosporin (OR, 1.43; 95% CI, 1.07–1.91), nonsteroidal anti-inflammation drugs (NSAIDs) (OR, 1.36; 95% CI, 1.27–1.46), antiplatelet agent (OR, 2.47; 95% CI, 2.31-2.63), hypertension (without anti-hypertensive agents: OR, 1.04; 95% CI, 0.96-1.12; with anti-hypertensive agents: OR, 1.47; 95% CI, 1.36-1.59), diabetes (OR, 1.27; 95% CI, 1.18-1.37), hyperlipidemia without lipid-lowering agents (OR, 1.09; 95% CI, 1.01-1.17), ischemic heart disease (OR, 1.20; 95% CI, 1.10-1.31), and chronic obstructive pulmonary disease (COPD) (OR, 1.12; 95% CI, 1.03-1.23) in the parsimonious model. The risk of MACE in RA patients also increased markedly in participants younger than 65 years with some comorbidities.
Conclusions:
This population-based case-control study revealed that the use of golimumab, abatacept, hydroxychloroquine, and methotrexate were associated with a decreased risk of MACE development in newly diagnosed RA patients, while the use of cyclosporin, NSAIDs, and antiplatelet agents, and comorbidities, including hypertension, diabetes, hyperlipidemia without lipid-lowering agent therapy, ischemic heart disease, and COPD, were associated with an increased risk of MACE development in RA patients.
Keywords: anti-rheumatic medications, case-control study, major adverse cardiovascular event, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disease, which is characterized by synovitis and extra-articular inflammation, leading to joint destruction, increased morbidity, major disability, and premature mortality.1 The annual incidence rate is 14.9 in 2017 globally and 15.8 per 100,000 in Taiwan (2002-2007), after adjustment for age.2 The prevalence of RA is estimated to be 0.5-1% globally, 0.53-0.55% in the United States in 2014, and is more prevalent in female and elderly patients.3,4 Since inflammation in RA involves not only articular parts but also extra-articular structures in up to 50% of RA patients, numerous complications including pulmonary, cardiovascular, and hematologic events develop.5 The most notable extra-articular manifestations are vascular inflammation and associated cardiovascular diseases (CVD), representing the leading cause of excess mortality in RA.6,7 A recent review indicated a 50% higher CVD mortality in RA patients than in the general population, with a 41-68% increase in ischemic heart disease, cerebrovascular accidents, and myocardial infarction (MI).8–10
Premature atherosclerosis and coronary artery diseases are the most significant causes associated with CVD in RA patients. Traditional risk factors for CVD, including hypertension, diabetes mellitus, hyperlipidemia, obesity, and smoking, as well as nontraditional factors associated with RA, increase the risk of CVD in RA patients.11 There is still controversy over which traditional or non-traditional risk factors have the greatest effect on cardiovascular risk in RA patients. A Trans-Atlantic Cardiovascular Consortium for Rheumatoid Arthritis (ATACC-RA) report suggests a larger effect attributable to traditional CVD risk factors on the development of adverse cardiovascular outcomes in RA patients compared with non-traditional factors,12 of which smoking and hypertension contribute most CVD risks, followed by RA disease activities, seropositivity and total cholesterol level (population attributable risk: 23.7%, 19.6%, 12.6%, 12.2%, 11.5%, respectively). However, some traditional CVD risk factors, such as diabetes mellitus (population attributable risk: 2.7%) and individual lipid profiles, including levels of low-density lipoprotein, high-density lipoprotein, and triglycerides, play little role in CVD risk in RA patients, which may imply there is discrepancy among the effect of traditional CVD risk factors in RA patients. Furthermore, some studies demonstrated less effect of traditional CVD risk factors in RA patients when compared with non-RA controls and competing RA-related factors may have a place in the disease process.13 The mechanism of RA-related factors in CVD has not been fully elucidated. Still, systemic inflammation burden is considered to represent the main association with CVD in RA.14–16 Several studies have demonstrated high RA activity, and seropositivity markers, including rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) were predictive factors for CVD in RA.17–19 Hansson20 also reported that pro-inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), interleukin (IL)-1, and IL-6, were associated with the pathogenesis of RA. Therefore, there is a need to identify the risks of major adverse cardiovascular events (MACEs) in RA and to implement therapeutic strategies to minimize the unfortunate CVD outcomes in order to enhance patient care and fulfil their unmet needs.21
Anti-inflammation therapy is considered a feasible way to diminish RA disease activity and related CVD risks. In the previous literature, few studies have investigated the effect of anti-rheumatic medications on the risk reduction of CVD in RA. Recently, Roubille et al.22 reported a meta-analysis demonstrating that TNF-α inhibitors [relative risk (RR), 0.70; 95% confidence interval (CI), 0.54-0.90; p = 0.005) and methotrexate (RR, 0.72; 95% CI, 0.57-0.91; p = 0.007) are beneficial for decreasing risks of all cardiovascular events in RA, but corticosteroids and nonsteroidal anti-inflammation drugs (NSAIDs) had the opposite effect. However, a diversity of cardiovascular outcome definitions and comparators exist across studies. Furthermore, biologics have not been evaluated except for TNF-α inhibitors, and no subgroup analyses have been performed due to insufficient data. Moreover, traditional and nontraditional factors have had different effects on adverse cardiovascular events in African white and black populations with RA, but few data exist for Asian populations.23 As a result, further study is needed to explore ethnic differences in CVD among RA patients. In the present study, we investigated the risk factors and the effects of individual anti-rheumatic drugs on MACE in Asian patients with RA.
Methods
Data source and identification of patients with RA
A nationwide, population-based, case-control study was conducted using Taiwan’s National Health Insurance Research Database (NHIRD). In Taiwan, a single-payer National Health Insurance program was launched on March 1, 1995, and as of 2014, 99.9% of Taiwan’s population has enrolled, around 23.69 million individuals.24 Claims data were collected from the NHIRD, including RA patients with MACE and non-MACE controls for the period January 2003 to December 2013. Newly diagnosed RA patients aged ⩾20 years without previous MACE during 2004-2012 were enrolled. RA was diagnosed according to the 1987 American College of Rheumatology (ACR) revised classification criteria or the ACR/European League Against Rheumatism (EULAR) 2010 RA classification criteria.25,26 RA subjects were identified if patients had a diagnosis of RA [the International Codes of Diseases-Ninth Revision-Clinical Modification (ICD-9-CM) code 714.0] at least three times during outpatient visits or at least once in a hospitalization. A sensitivity analysis was also performed in patients with the issuance of a catastrophic illness certification for RA (supplementary materials, supplementary Figure 1 and Supplementary Tables 1–5). Patients who had major illnesses, including cancers and some autoimmune diseases (such as systemic lupus erythematosus, RA, and systemic sclerosis), were registered in the NHI program. Patients with a catastrophic illness are exempt from copayment. A catastrophic illness certificate is issued after a comprehensive review of original medical records by at least two qualified specialists.
Identification of MACE cases and matched non-MACE controls
MACE was defined as a composite of MI, coronary revascularization, ischemic stroke, and cardiovascular death.27,28 MI was identified using ICD-9 code 410, except for 410.x2 for inpatients with a hospitalization for at least 3 days, unless mortality occurred. ICD-9 procedure codes 00.66, 36.03, 36.06, 36.07, and 36.09 (percutaneous coronary intervention, percutaneous transluminal coronary angioplasty) and ICD-9 procedure codes 36.1 and 36.2 (coronary artery bypass graft) were used for coronary revascularization, and ICD-9 code 433-436, except 433.x0 and 434.x0, for ischemic stroke. Cardiovascular death was defined by ICD-9 codes classifying death as heart disease or stroke. Patients were excluded if they developed MACE before diagnosis of RA.
MACE cases were defined as newly diagnosed RA patients who developed MACEs after a diagnosis of RA. Non-MACE controls were newly diagnosed RA patients without development of MACEs during the follow-up period, matching MACE cases for age, gender, and RA disease duration with a ratio of 1:4.
Covariates
Covariates included baseline characteristics, disease duration, selected comorbidities, and the use of anti-rheumatic medications. Baseline characteristics included age, gender, disease duration, and comorbidities by ICD-9 codes, including heart failure (ICD-9 code 428), hypertension (ICD-9 codes 401-405), diabetes mellitus (ICD-9 code 250), vascular disease (ICD-9 codes 410, 412, 440-445), hyperlipidemia (ICD-9 code 272), ischemic heart disease (ICD-9 codes 411, 413, 414), valvular heart disease (ICD-9 codes 093.2, 394-397, 424, 746.3-746.6), chronic obstructive pulmonary disease (COPD) (ICD-9 codes 490-493, 496), renal disease (ICD-9 codes 580-587), hyperthyroidism (ICD-9 code 242), hypothyroidism (ICD-9 code 243-244), depression (ICD-9 code 296.2, 296.3, 296.99, 298.0, 311), and anxiety (ICD-9 code 300.0, 300.2). The comorbidities were documented if the diagnostic ICD-9 code was recorded three times or more during outpatient visits or at least once in a hospitalization.
For case and control groups, information on the use of anti-rheumatic medications was collected, including biologic disease-modifying antirheumatic drugs (DMARDs) (etanercept, adalimumab, golimumab, tocilizumab, abatacept, rituximab), conventional DMARDs (methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, cyclosporin, and azathioprine), NSAIDs, corticosteroids, antiplatelet agents, anticoagulants, antihypertensive agents, and lipid-lowering agents. Drug exposure was defined as 1 year before the index date and during the observation period.
The associations of MACEs with comorbidities and the use of anti-rheumatic medications within 1 year before the index date were examined, using conditional logistic regression analyses with a full model and a parsimonious model, shown as ORs with 95% CIs. In addition, subgroup analysis with MACE risk stratification by age and gender was also performed.
Statistical analysis
The demographic data are shown as mean ± standard deviation (SD) for continuous variables and number (percent) for categorical variables. To compare variables between RA cases with MACE and non-MACE controls, the t-test and Chi-Square test were conducted for continuous parameters and categorical variables, respectively. Adjusted ORs of comorbidities and anti-rheumatic medications between RA participants with MACE and non-MACE controls were calculated by conditional logistic regression analysis. The significance of modification effect by age group and gender on covariate-associated MACE risk was examined by calculating the p-value of the coefficient associated with the product of the indicator of age group and gender and the indicator of each covariate using the Wald test. All data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 23.0. Significance was set at p < 0.05.
Ethics statement
The study was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taiwan (CE19038A). Because the NHIRD contains de-identified secondary data released to the public for research purposes, the requirement for informed consent from each participant was waived.
Results
Demographic characteristics and incidence of MACE
The flowchart of participant enrollment is shown in Figure 1. Between January 1, 2004, and December 31, 2012, there were 115,143 newly diagnosed RA patients aged ⩾20 years. Those who developed MACEs before diagnosis of RA were excluded (n = 6,824). In total, 108,319 participants were included, 7,580 of them (7.0%) developed MACEs and 100,739 patients did not during the follow-up period. The incidence rate of MACEs in RA patients was 145.78 per 10,000 person-years in total, 210.65 per 10,000 person-years in the male population, and 122.25 per 10,000 person-years in the female group (Table 1). In total, 5,994 RA patients with MACEs and 23,976 age-, gender-, and disease duration-matched (1:4) non-MACE controls were included in the final analysis.
Figure 1.
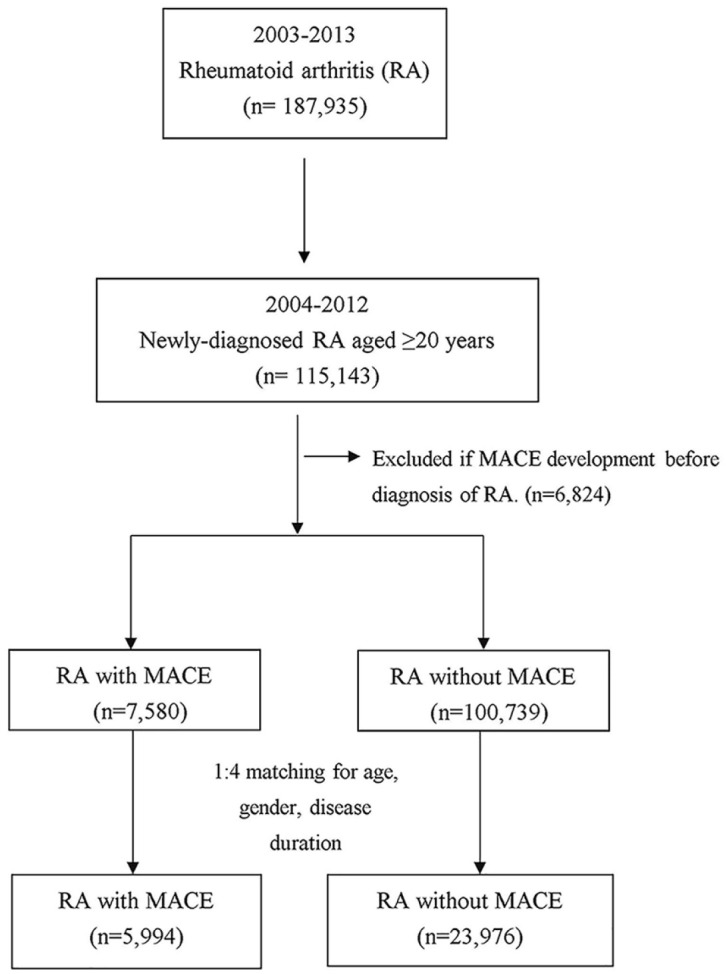
Flowchart of study subject’s enrollment.
MACE, major adverse cardiovascular events; RA, rheumatoid arthritis.
Table 1.
Incidences of MACE in patients with RA (n = 108,319, person-years = 519,971).
| Total events | Incidence rate (/10,000 PY) (95% CI) | |||||
|---|---|---|---|---|---|---|
| Total | Male | Female | <65 years | ⩾65 years | ||
| MACE | 7580 | 145.78 (142.61–148.94) | 210.65 (203.38–217.92) | 122.25 (118.85–125.65) | 73.92 (71.01–76.83) | 263.78 (257.08–270.49) |
| MI | 1159 | 22.29 (21.01–23.57) | 39.60 (36.32–42.89) | 16.01 (14.75–17.28) | 11.23 (10.08–12.38) | 40.45 (37.67–43.23) |
| Ischemic stroke | 5907 | 113.60 (110.79–116.42) | 150.10 (143.87–156.32) | 100.37 (97.27–103.47) | 55.08 (52.55–57.60 | 209.71 (203.65–215.78) |
| CABG | 179 | 3.44 (2.94–3.95) | 7.44 (6.01–8.88) | 1.99 (1.54–2.44) | 2.29 (1.77–2.81) | 5.34 (4.32–6.35) |
| PCI | 1446 | 27.81 (26.39–29.23) | 56.29 (52.39–60.20) | 17.48 (16.16–18.80) | 16.86 (15.45–18.27) | 45.78 (42.83–48.74) |
95% CI, 95% confidence intervals; CABG, coronary artery bypass grafting; MACE, major adverse cardiovascular events; MI, myocardial infarction; PCI, percutaneous coronary intervention; PY, person-years; RA, rheumatoid arthritis.
Risk factors of MACE in RA patients
Table 2 shows MACE cases exhibited higher percentages of comorbidities, as follows: heart failure (4.32% vs. 2.82%, p < 0.001), hypertension (52.70% vs. 41.47%, p < 0.001), diabetes mellitus (23.97% vs. 16.42%, p < 0.001), vascular disease (3.44% vs. 2.68%, p = 0.002), hyperlipidemia (35.37% vs. 27.43%, p < 0.001), ischemic heart disease (16.73% vs. 10.19%, p < 0.001), COPD (15.15% vs. 12.19%, p = 0.014), and depression or anxiety (10.53% vs. 9.02%, p < 0.001). MACE cases received lower percentages of biologic DMARDs, as follows: etanercept (0.82% vs. 1.37%, p < 0.001), golimumab (0.02% vs. 0.28%, p < 0.001), tocilizumab (0.05% vs. 0.16%, p = 0.034), and abatacept (0.02% vs. 0.20%, p = 0.001). Patients with MACE also received lower percentages of conventional DMARDs, as follows: methotrexate (8.43% vs. 13.15%, p < 0.001), sulfasalazine (10.33% vs. 12.31%, p < 0.001), leflunomide (2.32% vs. 2.91%, p = 0.012), and hydroxychloroquine (15.90% vs. 19.57%, p < 0.001). However, patients with MACE used NSAIDs (77.63% vs. 69.44%, p < 0.001), antiplatelet agents (47.98% vs. 25.04%, p < 0.001), anticoagulants (7.42% vs. 6.15%, p < 0.001), and lipid-lowering agents (24.51% vs. 18.79%, p < 0.001) more frequently.
Table 2.
Demographic data and clinical characteristics among RA patients.
| Total | Non-MACE | MACE | p-value | |
|---|---|---|---|---|
| (n = 29,970) | (n = 23,976) | (n = 5994) | ||
| Age, years (mean ± SD) | 69.19 ± 11.62 | 69.19 ± 11.62 | 69.19 ± 11.62 | 1.000 |
| Age group | 1.000 | |||
| <65 years | 9825 (32.78) | 7860 (32.78) | 1965 (32.78) | |
| ⩾65 years | 20,145 (67.22) | 16,116 (67.22) | 4029 (67.22) | |
| Gender | 1.000 | |||
| Female | 18,925 (63.15) | 15,140 (63.15) | 3785 (63.15) | |
| Male | 11,045 (36.85) | 8836 (36.85) | 2209 (36.85) | |
| Disease duration, years (mean ± SD) | 3.44 ± 2.21 | 3.44 ± 2.21 | 3.44 ± 2.21 | 1.000 |
| Co-morbidities | ||||
| Heart failure | 936 (3.12) | 677 (2.82) | 259 (4.32) | <0.001 |
| Hypertension | 13,101 (43.71) | 9942 (41.47) | 3159 (52.70) | <0.001 |
| Hypertension treatment group | <0.001 | |||
| Hypertension without anti-hypertensive agents | 6577 (21.95) | 5203 (21.70) | 1374 (22.92) | |
| Hypertension with anti-hypertensive agents | 6524 (21.77) | 4739 (19.77) | 1785 (29.78) | |
| Diabetes mellitus | 5375 (17.93) | 3938 (16.42) | 1437 (23.97) | <0.001 |
| Vascular disease | 849 (2.83) | 643 (2.68) | 206 (3.44) | 0.002 |
| Hyperlipidemia | 8696 (29.02) | 6576 (27.43) | 2120 (35.37) | <0.001 |
| Hyperlipidemia treatment group | <0.001 | |||
| Hyperlipidemia without lipid-lowering agents (statin or non-statin) | 2171 (7.25) | 1727 (7.20) | 445 (7.42) | |
| Hyperlipidemia with lipid-lowering agents | 6524 (21.77) | 4849 (20.22) | 1675 (27.94) | |
| Ischemic heart disease | 3446 (11.50) | 2443 (10.19) | 1003 (16.73) | <0.001 |
| Valvular heart disease | 802 (2.68) | 623 (2.60) | 179 (2.99) | 0.098 |
| COPD | 3830 (12.78) | 2922 (12.19) | 908 (15.15) | 0.014 |
| Renal disease | 1364 (4.55) | 1034 (4.31) | 330 (5.51) | 0.328 |
| Hyperthyroidism | 231 (0.77) | 182 (0.76) | 49 (0.82) | 0.621 |
| Hypothyroidism | 234 (0.78) | 187 (0.78) | 47 (0.78) | 0.974 |
| Depression or anxiety | 2794 (9.32) | 2163 (9.02) | 631 (10.53) | <0.001 |
| Biological agents | ||||
| Etanercept | 377 (1.26) | 328 (1.37) | 49 (0.82) | <0.001 |
| Adalimumab | 274 (0.91) | 229 (0.96) | 45 (0.75) | 0.149 |
| Golimumab | 68 (0.23) | 67 (0.28) | 1 (0.02) | <0.001 |
| Tocilizumab | 42 (0.14) | 39 (0.16) | 3 (0.05) | 0.034 |
| Abatacept | 49 (0.16) | 48 (0.20) | 1 (0.02) | 0.001 |
| Rituximab | 107 (0.36) | 93 (0.39) | 14 (0.23) | 0.089 |
| Concomitant DMARDs | ||||
| Methotrexate | 3658 (12.21) | 3153 (13.15) | 505 (8.43) | <0.001 |
| Sulfasalazine | 3571 (11.92) | 2952 (12.31) | 619 (10.33) | <0.001 |
| Leflunomide | 837 (2.79) | 698 (2.91) | 139 (2.32) | 0.012 |
| Hydroxychloroquine | 5646 (18.84) | 4693 (19.57) | 953 (15.90) | <0.001 |
| Cyclosporin | 322 (1.07) | 255 (1.06) | 67 (1.12) | 0.726 |
| Azathioprine | 316 (1.05) | 255 (1.06) | 61 (1.02) | 0.832 |
| NSAIDs | 21,301 (71.07) | 16,648 (69.44) | 4653 (77.63) | <0.001 |
| NSAIDs without naproxen | 20,464 (68.28) | 15,982 (66.66) | 4482 (74.77) | <0.001 |
| Naproxen | 1100 (3.67) | 895 (3.73) | 205 (3.42) | 0.249 |
| Steroid, mg Pd equivalent/day (mean ± SD) | 0.16 ± 0.41 | 0.16 ± 0.40 | 0.14 ± 0.43 | 0.713 |
| 0.277 | ||||
| not use | 15,810 (52.75) | 12,703 (52.98) | 3107 (51.84) | |
| ⩽5 mg/day | 14,142 (47.19) | 11,259 (46.96) | 2883 (48.10) | |
| >5 mg/day | 18 (0.06) | 14 (0.06) | 4 (0.07) | |
| Antiplatelet agents | 8880 (29.63) | 6004 (25.04) | 2876 (47.98) | <0.001 |
| Anticoagulants | 1919 (6.40) | 1474 (6.15) | 445 (7.42) | <0.001 |
| Lipid-lowering agents | ||||
| Statin | 5798 (19.35) | 4335 (18.08) | 1463 (24.41) | <0.001 |
| Non-statin | 1277 (4.26) | 859 (3.58) | 418 (6.97) | <0.001 |
Matched variables include age, gender, and disease duration.
COPD, chronic obstructive pulmonary disease; DMARDs, disease-modifying anti-rheumatic drugs; MACE, major adverse cardiovascular event; NSAIDs, nonsteroidal anti-inflammatory drugs; Pd, prednisolone; RA, rheumatoid arthritis.
After multivariable conditional logistic regression analysis, the risk of MACEs was associated with hypertension, regardless of antihypertensive agents use (without anti-hypertensive agents: OR, 1.04; 95% CI, 0.96-1.12; with anti-hypertensive agents: OR, 1.47; 95% CI, 1.36-1.59), diabetes (OR, 1.27; 95% CI, 1.18-1.37), hyperlipidemia without lipid-lowering agents (OR, 1.09; 95% CI, 1.01-1.17), ischemic heart disease (OR, 1.20; 95% CI, 1.10-1.31), and COPD (OR, 1.12; 95% CI, 1.03-1.23) (Table 3). The risk of MACEs decreased with the use of golimumab (OR, 0.09; 95% CI, 0.01-0.67), abatacept (OR, 0.13; 95% CI, 0.02–0.93), hydroxychloroquine (OR, 0.90; 95% CI, 0.82-0.99), and methotrexate (OR, 0.72; 95% CI, 0.64–0.81), and increased with the use of cyclosporin (OR, 1.43; 95% CI, 1.07–1.91), antiplatelet agents (OR, 2.47; 95% CI, 2.31-2.63), and NSAIDs (OR, 1.36; 95% CI, 1.27–1.46); but not naproxen.
Table 3.
Risk factors of MACEs in RA patients examined by conditional logistic regression analyses.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Co-morbidities | ||||||
| Heart failure | 1.57 | 1.35–1.82 | <0.001 | 1.13 | 0.97–1.32 | 0.131 |
| Hypertension | ||||||
| No | Ref | Ref | ||||
| Hypertension without anti-hypertensive agents | 1.37 | 1.27–1.47 | 0.411 | 1.04 | 0.96–1.12 | <0.001 |
| Hypertension with anti-hypertensive agents | 1.99 | 1.85–2.13 | <0.001 | 1.47 | 1.36–1.59 | <0.001 |
| Diabetes mellitus | 1.62 | 1.52–1.74 | <0.001 | 1.27 | 1.18–1.37 | <0.001 |
| Vascular disease | 1.29 | 1.10–1.52 | 0.002 | 1.04 | 0.88–1.23 | 0.621 |
| Hyperlipidemia | ||||||
| No | Ref | Ref | ||||
| Hyperlipidemia without lipid-lowering agents | 1.57 | 1.47–1.68 | <0.001 | 1.09 | 1.01–1.17 | 0.019 |
| Hyperlipidemia with lipid-lowering agents | 1.17 | 1.05–1.30 | 0.006 | 0.95 | 0.85–1.07 | 0.414 |
| Ischemic heart disease | 1.80 | 1.66–1.96 | <0.001 | 1.20 | 1.10–1.31 | <0.001 |
| Valvular heart disease | 1.16 | 0.98–1.37 | 0.095 | 0.92 | 0.77–1.10 | 0.356 |
| COPD | 1.30 | 1.20–1.41 | <0.001 | 1.12 | 1.03–1.23 | 0.008 |
| Renal disease | 1.30 | 1.14–1.47 | <0.001 | 1.06 | 0.92–1.21 | 0.431 |
| Hyperthyroidism | 1.08 | 0.79–1.48 | 0.643 | 0.98 | 0.71–1.36 | 0.923 |
| Hypothyroidism | 1.01 | 0.73–1.39 | 0.974 | 0.95 | 0.69–1.33 | 0.782 |
| Depression or Anxiety | 1.19 | 1.08–1.31 | <0.001 | 1.06 | 0.96–1.17 | 0.233 |
| Biological agents | ||||||
| Etanercept or Adalimumab or Golimumab | 0.61 | 0.49–0.76 | <0.001 | |||
| Etanercept | 0.59 | 0.44–0.80 | <0.001 | 0.85 | 0.62–1.17 | 0.310 |
| Adalimumab | 0.78 | 0.57–1.08 | 0.138 | 1.21 | 0.86–1.70 | 0.286 |
| Golimumab | 0.06 | 0.01–0.43 | 0.005 | 0.09 | 0.01–0.67 | 0.019 |
| Tocilizumab | 0.31 | 0.10–1.00 | 0.049 | 0.43 | 0.13–1.42 | 0.167 |
| Abatacept | 0.08 | 0.01–0.60 | 0.014 | 0.13 | 0.02–0.93 | 0.042 |
| Rituximab | 0.60 | 0.34–1.06 | 0.076 | 0.69 | 0.38–1.23 | 0.209 |
| Concomitant DMARDs | ||||||
| Methotrexate | 0.60 | 0.55–0.67 | <0.001 | 0.72 | 0.64–0.81 | <0.001 |
| Sulfasalazine | 0.82 | 0.75–0.90 | <0.001 | 1.06 | 0.95–1.18 | 0.326 |
| Leflunomide | 0.79 | 0.66–0.95 | 0.013 | 1.02 | 0.84–0.99 | 0.822 |
| Hydroxychloroquine | 0.77 | 0.71–0.83 | <0.001 | 0.90 | 0.82–0.99 | 0.025 |
| Cyclosporin | 1.05 | 0.80–1.38 | 0.716 | 1.43 | 1.07–1.91 | 0.016 |
| Azathioprine | 0.96 | 0.72–1.27 | 0.756 | 1.01 | 0.76–1.36 | 0.930 |
| NSAIDs | 1.54 | 1.44–1.65 | <0.001 | 1.36 | 1.27–1.46 | <0.001 |
| Naproxen | 0.91 | 0.78–1.07 | 0.250 | |||
| Steroid, mg Pd equivalent/day | 0.86 | 0.79–0.94 | <0.001 | |||
| Not use | Ref | Ref | ||||
| ⩽5 mg/day | 1.05 | 0.99–1.11 | 0.912 | 1.03 | 0.97–1.10 | 0.878 |
| >5 mg/day | 1.17 | 0.39–3.56 | 0.814 | 0.97 | 0.31–3.03 | 0.936 |
| Antiplatelet agents | 2.92 | 2.75–3.10 | <0.001 | 2.47 | 2.31–2.63 | <0.001 |
| Anticoagulants | 1.23 | 1.10–1.38 | <0.001 | 0.95 | 0.85–1.07 | 0.434 |
Matched variables include age, gender, and disease duration.
95% CI, 95% confidence intervals; COPD, chronic obstructive pulmonary disease; DMARDs, disease-modifying anti-rheumatic drugs; MACE, major adverse cardiovascular events; NSAIDs, nonsteroidal anti-inflammatory drugs; OR, odds ratios; RA, rheumatoid arthritis; ref, reference.
Predictors for MACE development stratified by age and gender
To examine the modification effects by age group and gender on the association of MACEs with other covariates in RA patients, conditional logistic regression analyses were performed, as shown in Tables 4 and 5, respectively. Table 4 revealed that age group (<65 years vs. ⩾65 years) significantly modified the association of MACEs with hypertension under anti-hypertensive agent treatment (OR, 1.86 vs. 1.30; p for interaction < 0.001), diabetes mellitus (OR, 1.47 vs. 1.18; p for interaction =0.014), hyperlipidemia without lipid-lowering agent (OR, 1.24 vs. 0.99; p for interaction =0.005), valvular heart disease (OR, 1.63 vs. 0.76; p for interaction < 0.001), COPD (OR, 1.36 vs. 1.06; p for interaction =0.025), renal disease (OR, 1.85 vs. 0.86; p for interaction < 0.001), or depression or anxiety (OR, 1.23 vs. 0.97; p for interaction =0.026), or concomitant use of NSAIDs (OR, 1.64 vs. 1.28; p for interaction =0.002), low dose corticosteroids (⩽5 mg/day prednisolone equivalent dose; OR, 1.16 vs. 0.97; p for interaction =0.009), antiplatelet agents (OR, 3.41 vs. 2.15; p for interaction < 0.001), or anticoagulants (OR, 1.18 vs. 0.85; p for interaction =0.018) (Table 4).
Table 4.
Conditional logistic regression analyses of predictors for MACEs in RA patients stratified by age group.
| <65 years | ⩾65 years | p for interaction | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Co-morbidities | |||||||
| Heart failure | 1.06 | 0.69–1.65 | 0.784 | 1.18 | 0.99–1.39 | 0.061 | 0.677 |
| Hypertension treatment group | |||||||
| Hypertension without anti-hypertensive agent | 0.99 | 0.85–1.15 | 0.899 | 0.98 | 0.89–1.07 | 0.626 | 0.882 |
| Hypertension with anti-hypertensive agent | 1.86 | 1.58–2.18 | <0.001 | 1.30 | 1.19–1.42 | <0.001 | <0.001 |
| Diabetes mellitus | 1.47 | 1.27–1.71 | <0.001 | 1.18 | 1.09–1.29 | <0.001 | 0.014 |
| Vascular disease | 1.14 | 0.80–1.62 | 0.459 | 1.03 | 0.85–1.25 | 0.740 | 0.621 |
| Hyperlipidemia | |||||||
| Hyperlipidemia without lipid-lowering agent | 1.24 | 1.09–1.41 | 0.001 | 0.99 | 0.90–1.08 | 0.759 | 0.005 |
| Hyperlipidemia with lipid-lowering agent | 0.88 | 0.70–1.10 | 0.248 | 0.93 | 0.81–1.07 | 0.301 | 0.649 |
| Ischemic heart disease | 1.16 | 0.94–1.42 | 0.163 | 1.21 | 1.10–1.33 | <0.001 | 0.694 |
| Valvular heart disease | 1.63 | 1.15–2.30 | 0.006 | 0.76 | 0.61–0.94 | <0.001 | <0.001 |
| COPD | 1.36 | 1.12–1.64 | 0.002 | 1.06 | 0.97–1.17 | 0.212 | 0.025 |
| Renal disease | 1.85 | 1.41–2.43 | <0.001 | 0.86 | 0.74–1.01 | 0.066 | <0.001 |
| Hyperthyroidism | 1.18 | 0.72–1.94 | 0.508 | 0.90 | 0.58–1.41 | 0.650 | 0.426 |
| Hypothyroidism | 0.90 | 0.50–1.61 | 0.719 | 1.04 | 0.69–1.55 | 0.858 | 0.691 |
| Depression or anxiety | 1.23 | 1.03–1.46 | 0.020 | 0.97 | 0.86–1.09 | 0.584 | 0.026 |
| Biological agents | |||||||
| Etanercept | 0.88 | 0.56–1.40 | 0.592 | 0.75 | 0.48–1.19 | 0.221 | 0.630 |
| Adalimumab | 1.35 | 0.82–2.22 | 0.239 | 1.13 | 0.71–1.82 | 0.604 | 0.620 |
| Golimumab | 0.55 | 0.13–2.27 | 0.411 | 0.42 | 0.11–1.63 | 0.208 | 0.775 |
| Tocilizumab | 0.80 | 0.18–3.44 | 0.760 | 0.73 | 0.18–2.95 | 0.662 | 0.937 |
| Abatacept | 0.63 | 0.16–2.46 | 0.508 | 0.49 | 0.10–2.42 | 0.381 | 0.813 |
| Rituximab | 0.81 | 0.25–2.57 | 0.716 | 0.71 | 0.36–1.39 | 0.314 | 0.847 |
| Concomitant DMARDs | |||||||
| Methotrexate | 0.74 | 0.61–0.89 | 0.002 | 0.72 | 0.61–0.85 | <0.001 | 0.850 |
| Sulfasalazine | 0.97 | 0.82–1.16 | 0.768 | 1.09 | 0.94–1.25 | 0.247 | 0.334 |
| Leflunomide | 1.24 | 0.91–1.68 | 0.165 | 0.82 | 0.62–1.09 | 0.174 | 0.052 |
| Hydroxychloroquine | 0.90 | 0.78–1.05 | 0.181 | 0.89 | 0.79–1.00 | 0.042 | 0.842 |
| Cyclosporin | 1.39 | 0.92–2.09 | 0.117 | 1.69 | 1.13–2.54 | 0.011 | 0.496 |
| Azathioprine | 0.99 | 0.66–1.48 | 0.954 | 0.89 | 0.56–1.41 | 0.613 | 0.731 |
| NSAIDs | 1.64 | 1.43–1.87 | <0.001 | 1.28 | 1.18–1.39 | <0.001 | 0.002 |
| Steroid, mg Pd equivalent/day | |||||||
| ⩽5 mg/day | 1.16 | 1.04–1.31 | 0.011 | 0.97 | 0.90–1.04 | 0.389 | 0.009 |
| >5 mg/day | 0.99 | 0.15–6.47 | 0.990 | 0.93 | 0.22–3.89 | 0.920 | 0.959 |
| Antiplatelet agents | 3.41 | 3.01–3.86 | <0.001 | 2.15 | 1.99–2.32 | <0.001 | <0.001 |
| Anticoagulants | 1.18 | 0.94–1.48 | 0.162 | 0.85 | 0.74–0.98 | 0.026 | 0.018 |
Matched variables include age, gender, and disease duration.
95% CI, 95% confidence intervals.; COPD, chronic obstructive pulmonary disease; DMARDs, disease-modifying anti-rheumatic drugs; MACE, major adverse cardiovascular events; NSAIDs, nonsteroidal anti-inflammatory drugs; OR, odd’s ratio; Pd, prednisolone; RA, rheumatoid arthritis.
Table 5.
Conditional logistic regression analyses of MACE risk factors in RA patients by gender.
| Male | Female | p for interaction | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Co-morbidities | |||||||
| Heart failure | 1.13 | 0.87–1.46 | 0.367 | 1.10 | 0.90–1.34 | 0.343 | 0.888 |
| Hypertension treatment group | |||||||
| Hypertension without anti-hypertensive agent | 0.96 | 0.85–1.09 | 0.547 | 1.04 | 0.94–1.15 | 0.422 | 0.332 |
| Hypertension with anti-hypertensive agent | 1.32 | 1.17–1.50 | <0.001 | 1.50 | 1.36–1.65 | <0.001 | 0.111 |
| Diabetes mellitus | 1.27 | 1.13–1.44 | <0.001 | 1.24 | 1.13–1.36 | <0.001 | 0.748 |
| Vascular disease | 1.10 | 0.85–1.43 | 0.472 | 1.01 | 0.82–1.25 | 0.932 | 0.614 |
| Hyperlipidemia | |||||||
| Hyperlipidemia without lipid-lowering agent | 1.11 | 0.98–1.25 | 0.112 | 1.08 | 0.99–1.18 | 0.079 | 0.792 |
| Hyperlipidemia with lipid-lowering agent | 0.91 | 0.75–1.11 | 0.347 | 0.96 | 0.83–1.11 | 0.573 | 0.679 |
| Ischemic heart disease | 1.34 | 1.16–1.54 | <0.001 | 1.12 | 1.01–1.26 | 0.037 | 0.063 |
| Valvular heart disease | 0.69 | 0.49–0.97 | 0.034 | 1.00 | 0.81–1.24 | 0.979 | 0.067 |
| COPD | 1.08 | 0.94–1.23 | 0.283 | 1.18 | 1.05–1.32 | 0.004 | 0.303 |
| Renal disease | 0.88 | 0.71–1.10 | 0.259 | 1.17 | 0.99–1.39 | 0.071 | 0.044 |
| Hyperthyroidism | 0.93 | 0.38–2.26 | 0.872 | 1.02 | 0.72–1.45 | 0.907 | 0.848 |
| Hypothyroidism | 0.87 | 0.36–2.10 | 0.751 | 0.97 | 0.68–1.38 | 0.853 | 0.822 |
| Depression or anxiety | 0.94 | 0.77–1.13 | 0.499 | 1.11 | 0.99–1.24 | 0.071 | 0.133 |
| Biological agents | |||||||
| Etanercept | 0.87 | 0.50–1.51 | 0.625 | 0.83 | 0.56–1.23 | 0.357 | 0.890 |
| Adalimumab | 0.91 | 0.51–1.62 | 0.737 | 1.51 | 1.00–2.30 | 0.052 | 0.159 |
| Golimumab | 0.36 | 0.03–3.83 | 0.397 | 0.17 | 0.03–0.93 | 0.041 | 0.619 |
| Tocilizumab | 0.64 | 0.10–4.15 | 0.636 | 0.58 | 0.16–2.18 | 0.421 | 0.938 |
| Abatacept | 0.47 | 0.03–8.24 | 0.603 | 0.21 | 0.04–1.19 | 0.078 | 0.649 |
| Rituximab | 0.55 | 0.20–1.49 | 0.240 | 0.73 | 0.35–1.53 | 0.399 | 0.660 |
| Concomitant DMARDs | |||||||
| Methotrexate | 0.93 | 0.76–1.13 | 0.471 | 0.62 | 0.53–0.73 | <0.001 | 0.002 |
| Sulfasalazine | 1.02 | 0.85–1.23 | 0.812 | 1.08 | 0.94–1.23 | 0.276 | 0.644 |
| Leflunomide | 0.89 | 0.61–1.29 | 0.531 | 1.06 | 0.83–1.35 | 0.665 | 0.446 |
| Hydroxychloroquine | 0.89 | 0.76–1.05 | 0.168 | 0.90 | 0.81–1.01 | 0.065 | 0.905 |
| Cyclosporin | 0.99 | 0.58–1.70 | 0.970 | 1.65 | 1.17–2.34 | 0.005 | 0.117 |
| Azathioprine | 1.05 | 0.63–1.76 | 0.848 | 0.99 | 0.70–1.42 | 0.977 | 0.862 |
| NSAIDs | 1.30 | 1.16–1.46 | <0.001 | 1.41 | 1.29–1.54 | <0.001 | 0.250 |
| Steroid, mg Pd equivalent/day | |||||||
| ⩽5 mg/day | 0.92 | 0.83–1.03 | 0.144 | 1.09 | 1.00–1.18 | 0.042 | 0.016 |
| >5 mg/day | 0.96 | 0.21–4.38 | 0.956 | 0.84 | 0.14–4.89 | 0.845 | 0.910 |
| Antiplatelet agents | 2.71 | 2.44–3.01 | <0.001 | 2.30 | 2.12–2.50 | <0.001 | 0.018 |
| Anticoagulants | 1.00 | 0.83–1.19 | 0.961 | 0.93 | 0.80–1.08 | 0.354 | 0.574 |
Matched variables include age, gender, and disease duration.
95% CI, 95% confidence intervals.; COPD, chronic obstructive pulmonary disease; DMARDs, disease-modifying anti-rheumatic drugs; MACE, major adverse cardiovascular events; NSAIDs, nonsteroidal anti-inflammatory drugs; OR, odds ratios; Pd, prednisolone; RA, rheumatoid arthritis.
As shown in Table 5, concomitant use of methotrexate in female patients (OR, 0.93 vs 0.62; p for interaction =0.002) was associated with a lower risk of MACEs. However, female participants who received low dose corticosteroids concomitantly (OR, 0.92 vs. 1.09; p for interaction =0.016) and male patients who received antiplatelet agents concomitantly (OR, 2.71 vs. 2.30; p for interaction =0.018) exhibited significant MACE risk, compared with their counterparts.
Discussion
This nationwide, population-based, case-control study demonstrated that, in newly diagnosed RA patients, the use of golimumab, abatacept, hydroxychloroquine, or methotrexate was associated with a decreased risk of MACE development, while the use of cyclosporin, NSAID, but not naproxen, and antiplatelet agents, and comorbidities, including hypertension, diabetes, hyperlipidemia without a lipid-lowering agent, ischemic heart disease, and COPD were associated with an increased risk of MACE development.
We found 7.0% of RA patients developed MACEs during the follow-up period among Asian population. However, the CARdiovascular in rheuMAtology (CARMA) project in Spanish patients revealed a lower rate of cardiovascular events and cardiovascular deaths in patients with chronic inflammatory rheumatic diseases, including RA.29 Different genetic components and biologics usage percentage in the two groups might be parts of the possible explanations. López-Mejías et al.30 summarized the genetic factors associated with the risk of cardiovascular disease in RA patients, including the HLA class II histocompatibility antigen, DRB1 beta chain (HLA-DRB1) gene, especially the HLA-DRB1*04 shared epitope (SE) alleles, and some polymorphisms inside and outside the HLA region such as the TNFA rs1800629 polymorphism (located at TNFA promoter), the C-C chemokine receptor type 5 (CCR5)Δ32 rs333 polymorphism (a 32-basepairs deletion), and the methylenetetrahydrofolate reductase (MTHFR) rs1801131 polymorphism. Turesson et al.31 found there was an increased risk of vasculitis (OR 2.44) and overall risk of extra-articular RA (OR 1.79) if patients had HLA-DRB1*04 SE genotypes, and an approximately 2-fold increase in CV mortality was observed in RA patients with 2 copies of the HLA-DRB1 SE.16 As we know, HLA-DRB1*04:01 is more common in Caucasian RA patients, while more HLA-DRB1*04:05 events are found in Asian and Taiwanese RA groups.32,33 Therefore, the main SE alleles might be differently associated with RA and the CVD development in RA patients in different populations. As a result, the incidence of MACEs in RA patients was distinct among each ethnic population. Moreover, the percentage of biologics usage in RA patients was different in our group and in the CARMA cohort (3.1% and > 40%, respectively), which inferred the cardiovascular protective effect of biologic therapy in RA patients.
We further demonstrated a markedly reduced risk of MACE in RA patients using abatacept and golimumab but not other TNF inhibitors. Conflicting results have been reported regarding the risk of CVD using TNF inhibitors. Reduced cardiovascular risk using TNF inhibitors in RA patients were reported in the Consortium of Rheumatology Researchers of North America (CORRONA) study (not included golimumab) and Nurmohamed et al.34,35 also reported longer durations treatment of TNF inhibitors were associated with decreasing risk of CVD risks in RA patients in a pooling effect (cumulative use of TNF inhibitors for 1, 2, and 3 years: reduces CVD risks by 21%, 38%, and 51%, respectively). However, Desai et al.36 revealed no cardiovascular protective role of TNF inhibitors in RA in a nested case-control study. If taking golimumab separately, the GO-BEFORE and GO-FORWARD studies on golimumab revealed stable atherogenic indices and significant improvement of inflammatory markers of CVD risk in RA patients if combining the use of golimumab and methotrexate versus methotrexate alone.37 Another study demonstrated improving diastolic left ventricular function in 67% ankylosing spondylitis patients after 1-year of golimumab treatment.38 Further research is necessary to address this issue and we should keep in mind that CVD risk reduction might be particularly observed in the responders among RA patients using TNF inhibitors.
There have been a number of studies exploring the effect of TNF inhibitors on the CVD risk reduction in patients with RA, but there was limited information on non-TNF biologics. One observational study revealed a higher risk of acute MI in RA patients using TNF inhibitor compared with abatacept [hazard ratio (HR) of 1.3, 95% CI 1.0-1.6 for TNF inhibitor; HR of 0.64, 95% CI 0.41-0.99 for abatacept).39 Other studies demonstrated similar results in two large United States insurance claims databases: Medicare (2008-2013, N = 12,204) and Truven MarketScan (2006–2015, N = 13,868), and suggested a 20% decreased CVD risk using abatacept versus TNF inhibitor in RA patients (combined HR of 0.79; 95% CI, 0.67–0.92), especially in those with baseline CVD (combined HR of 0.79; 95% CI, 0.64–0.98) and in those with DM (combined HR of 0.74; 95% CI, 0.57–0.96).40,41 The mechanism of CVD risk reduction by abatacept is still unclear, but the detection of costimulatory molecules CD80 and CD86 expression on macrophages in atherosclerotic lesions and on monocyte-derived dendritic cells in patients with CVD suggests that co-stimulation of T cells could play a role in atherosclerosis development.42,43 A previous study showed significantly higher expression levels of CD80 and CD86 in vulnerable atherosclerotic lesions compared to stable plaques in human carotid arteries.44 Moreover, ACPA was a pro-atherogenic factor associated with increased long-term mortality, and the combined endpoint of re-infarction and death was independently associated with the presence of ACPA in ST-elevation MI patients.45 Therefore, an early reduction in ACPA titer might also be associated with the protective effect of abatacept against CVD. Further studies are needed to explore the effect and possible mechanism of abatacept against CVD. In brief, abatacept is recommended as a priority treatment in RA patients with baseline comorbidities such as CVD or diabetes mellitus. In addition, there was a trend, though no statistical significance, on cardiovascular protective effects in RA patients using tofacitinib, which could significantly reduce pro-inflammatory markers together with pro-atherogenic factors, such as lipoprotein.46 Fewer participants using tofacitinib due to late Federal Drug Agency (FDA) approval for RA in comparison with other biologics might cause less statistic power in the tofacitinib group.
Our study found hydroxychloroquine and methotrexate was associated with a decreased risk of MACEs in newly diagnosed RA patients. Several studies indicated a reduced incidence of CVD in RA patients using hydroxychloroquine. One recent meta-analysis showed that hydroxychloroquine may benefit lipid profiles, the incidence of diabetes mellitus, and cardiovascular events (to a lesser extent) in patients with RA.47 Stronger evidence in favor of cardiovascular risk reduction in RA patients was shown in methotrexate, a mainstay therapy for RA.22,48 A recent meta-analysis demonstrated a reduced risk of all CVD events with methotrexate (OR, 0.72; 95% CI, 0.57-0.91; p = 0.007),22 and methotrexate was correlated with lower cardiovascular mortality as well, with a 15-85% reduction of fatal CV events.49 Interestingly, the protective effect of methotrexate was decreased and became non-significant in males in our study, which could be attributed to the male gender acting as a competitive risk factor for MACEs and the relatively small number of male cases.50 The protective effects of methotrexate might occur through the suppression of systemic inflammation, which in turn could mitigate the development of atherosclerosis by decreasing carotid intima-media thickness (IMT).51 Kim et al.52 showed a significantly lower carotid IMT in RA patients with methotrexate, compared with those without methotrexate (0.644 ± 0.136 mm, 0.767 ± 0.233 mm, respectively, p < 0.05), with a dosage-dependent effect (β = -0.029, p < 0.01). In addition, a reduction of CRP, TNFα, IL-6, and cytokines associated with atherosclerosis development, downregulation of foam cell production, and an increased expression of antiatherogenic reverse cholesterol-transport protein were all considered to contribute to the anti-inflammatory action and cardioprotective effects of methotrexate.53,54 As a result, methotrexate should be considered a priority for arthritis treatment as well as for RA-related CVD risk and mortality reduction.
In contrast, cyclosporin was associated with increased risk for MACEs in our study. Although there is less use of cyclosporin in RA managements nowadays, the reimbursement criteria for biologics in Taiwan was rigorous and a patient with RA could apply for biologics only if he/she received at least two DMARDs (including methotrexate) for at least 6 months with poor response. As a result, there were still 1.07% (322 out of 29970) patients who received cyclosporin for RA in Taiwan during the enrollment period. Cyclosporin is known to worsen hypertension, diabetes, and dyslipidemia. Cyclosporin is also associated with cardiovascular toxicities. The exact mechanisms of cyclosporin-associated hypertension (CAH) are uncertain, but animal model studies have shown that it might be related to the inhibition of calcineurin.55,56 Several potential mechanisms have been proposed in transplantation studies, including alterations in vascular reactivity, excess release of vasoconstrictors (prostaglandins, thromboxane, endothelin), and sodium reabsorption by renal tubules, which leads to volume expansion.57–59 The rise in blood pressure might contribute to cardiovascular events; as a result, a minimal effective dose of cyclosporin has been recommended for long-term use.60 Whether reformulating application requirements for biologics in RA patients was also worth thinking about for physicians and Taiwan’s National Health Insurance Administration.
The risk of MACEs in RA patients under a higher daily dose of corticosteroids (>5 mg/day prednisolone equivalent dose) was not statistically different from that in RA patients treated with a lower daily dose of corticosteroid (⩽5 mg/day prednisolone equivalent dose). It is well known, albeit without strong evidence, that corticosteroids could be associated with potential adverse cardiovascular events in the general population.61,62 Nevertheless, conflicting results on cardiovascular risk were found in RA patients in previous studies.22,63 Corticosteroids might increase the CVD risk by worsening hypertension, dyslipidemia, glucose intolerance, and central obesity,64 and it might decrease the risk via suppression of systemic inflammation, reduction of vascular wall proliferation, and modulation of antibody status, as well as improving mobility and physical activity due to alleviation of RA disease activities.65,66 Anti-atherosclerotic and anti-restenosis effects, along with myocardial protection of corticosteroids after an acute ischemic injury, have been demonstrated in experimental animal models.67,68 There were two actions of corticosteroids, classic genomic effects and non-genomic effects, and non-genomic effects were responsible for diminishing vascular inflammation via inhibition of leucocyte-endothelial interactions and the activation of endothelial nitric oxide synthase.69,70 Since RA is a disease related to systemic inflammation, including vascular inflammation, it should be borne in mind that corticosteroids in RA patients might lead to effects that are different from those seen in the general population, including a possible favorable cardiovascular effect. Moreover, it is worth noting that in the low dose corticosteroid group in our study, the beneficial effect of corticosteroids was observed in men and people over 65 years but not in women and younger patients. Given that estrogen in women, as well as younger age groups, are both competitive CVD protective factors with corticosteroids in RA patients,71 it might become statistically insignificant for the effect of corticosteroids on CVD risk in women and younger patients with RA.
Our study demonstrated an increased risk of MACEs among RA patients using NSAIDs but not naproxen. One landmark meta-analysis of randomized trials demonstrated increased major cardiovascular events in both non-selective NSAIDs and selective cyclooxygenase-2 (COX-2) inhibitors but not seen in naproxen (HR, 0.93; 95% CI, 0.69-1.27) in the general population.72 However, only a few studies have explored the cardiovascular effects of NSAIDs in RA patients. A population-based study in Korea suggested that both nonselective NSAIDs and Cox-2 inhibitors were risk factors for CVD in RA patients (OR 1.32 for nonselective NSAIDs, OR 1.31 for Cox-2 inhibitors), but there was no separate analysis of naproxen regarding the CVD risk.73 In addition, a nationwide cohort study suggested a neutral effect of naproxen on the cardiovascular risk in RA patients (OR 0.98; 95% CI, 0.47-2.06).74 It is necessary to perform a benefit-risk assessment regarding NSAIDs and naproxen use in RA patients with high cardiovascular risks in the future.
We found an increased risk of MACEs in RA patients with comorbidities including hypertension, DM, hyperlipidemia without a lipid-lowering agent, ischemic heart disease, and COPD. Consistent with previous studies in Caucasian populations, the CARMA cohort demonstrated an increased risk of CVD in RA patients with traditional CVD risk factors including hypertension (OR, 2.36; 95% CI, 1.12-4.98), diabetes (OR, 2.56; 95% CI, 1.06-6.18), and hypercholesterolemia (OR, 2.20; 95% CI, 1.09-4.45).75 It should be noted that our study revealed higher CVD risk in RA patients with hypertension, especially those under anti-hypertensive agent treatment, whose baseline blood pressure usually went higher than those without treatment; we might infer that blood pressure itself was an overwhelming risk factor regardless of antihypertensive agents use. In addition, we found an increased risk of MACEs in the RA population with COPD. A meta-analysis demonstrated an increased risk of COPD in RA patients,76 which might be due to the fact that systemic inflammation in RA could cause pulmonary alveoli damage, and a similar autoimmune pathogenesis and smoking play a role both in RA and COPD.77,78 Subsequent studies have revealed that the risk of MACEs increased especially after acute exacerbation of COPD in the general population (OR: 3.70; 95% CI: 3.60-3.80).79 There were no studies on RA patients before the present investigation. The association of activity status of COPD and MACE risk in RA patients warrants further study. As a result, we recommend that RA patients quit smoking and receive close follow-up by both rheumatologists and pulmonologists if comorbid with COPD.
In the subgroup analysis, there was higher risk of MACEs in RA patients with hypertension under anti-hypertensive agent treatment, DM, hyperlipidemia without lipid-lowering agent, and COPD in those younger than 65 years, compared with the elderly (> 65 years). A previous study revealed similar results for the general population, but no studies have been conducted on RA patients. The Framingham Heart Study cohort demonstrated higher CVD risk at 50 years than at advanced age, but the absence of CVD risk factors at 50 years was associated with low risk of cardiovascular events and longer survival.80 Possibly, a shorter time was left for the elderly to develop MACEs, and there were more competing risk factors for mortality in the elderly. In addition, young individuals with comorbidities might have genetic variants and adverse lifestyle factors that predispose them to cardiovascular events. Our results suggest that modification of risk factors and prevention of comorbidities in young individuals could alleviate risks of future cardiovascular events, although further studies are needed to elucidate these relationships.
The present study was the first to determine the cardiovascular effect of individual biologics in a nationwide RA population, to conduct risk stratification by age and gender, and to recommend treatment strategy in RA patients with high CVD risk. However, this study has some limitations. Firstly, RA patients were identified by ICD codes, and the accuracy of RA diagnosis is a possible concern. We performed sensitivity analyses in RA patients with catastrophic illness certificates, which required two or more qualified and experienced rheumatologists to confirm the diagnosis after comprehensive review of the original medical records. However, it might underestimate the number of RA patients, since numerous RA patients in Taiwan are not issued the catastrophic illness certificates if they already had a catastrophic illness certificate for another disease, a shorter follow-up period, no obvious radiographic bony erosions, or they can afford copayment. Secondly, RA disease activity data and laboratory results such as autoantibody status are also not available in the NHIRD, and we could not completely exclude these potential confounders in our study. These possible effects of these parameters should be explored in future studies. Thirdly, patients using antiplatelet therapy were included in the study, most of whom might have subclinical atherosclerotic disease, resulting in confounding by indication. However, we had minimized the bias via multivariable analyses adjusting for possible confounders. In addition, there are other aspects contributing to cardiovascular risk in patients with chronic inflammatory rheumatic diseases, including organic diseases, psychological illnesses, socioeconomic factors, and miscellany conditions such as low vitamin D levels and hyperhomocysteinem.81 Future study is needed to explore the association of more factors with the risk of MACEs in RA patients.
Conclusion
This population-based, case-controlled study revealed that, in newly diagnosed RA patients, the use of golimumab, abatacept, hydroxychloroquine, or methotrexate, was associated with a decreased risk of MACEs, while the use of cyclosporin, NSAIDs but not naproxen, or antiplatelet agents, and comorbidities, including hypertension, diabetes, hyperlipidemia without lipid-lowering agent, ischemic heart disease and COPD, were associated with an increased risk of MACEs. Further cohort studies are warranted to confirm our findings. Early intervention is recommended for RA patients at high risk for MACEs, including prevention of comorbidities, adopting a treatment strategy with steroids, using methotrexate as a priority, and consideration of abatacept or golimumab if biologics are needed. Cyclosporin should only be used at the minimally effective dose and NSAIDs. especially naproxen, can be considered after performing a benefit-risk assessment.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-3-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-4-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-5-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-6-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We would like to thank the Biostatistics Task Force of Taichung Veterans General Hospital for their assistance in performing the statistical analysis.
The authors sincerely appreciate the assistance of the Center for Translational Medicine of Taichung Veterans General Hospital.
Footnotes
Authorship Contributions: Hsin-Hua Chen conceived and designed the study. Yen-Ju Chen and Hsin-Hua Chen performed the literature search, interpretation of data and drafted the manuscript. Ching-Heng Lin, Donald F. Gotcher, Yu-Mei Chang, Chuang-Chun Chiou, Shih-Chia Liu, Shao-Jen Weng conducted data extraction, methodological quality assessments and performed the analysis. Hsin-Hua Chen, Kuo-Lung Lai, Tsu-Yi Hsieh, Yi-Ming Chen, Chih-Wei Tseng performed critical revision of the manuscript for important intellectual content. All authors read and approved the final version of submitted manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from Taichung Veterans General Hospital, Taiwan (TCVGH-T1087805).
ORCID iDs: Yen-Ju Chen  https://orcid.org/0000-0002-9450-9144
https://orcid.org/0000-0002-9450-9144
Yi-Ming Chen  https://orcid.org/0000-0001-7593-3065
https://orcid.org/0000-0001-7593-3065
Chih-Wei Tseng  https://orcid.org/0000-0002-5948-7306
https://orcid.org/0000-0002-5948-7306
Hsin-Hua Chen  https://orcid.org/0000-0002-7304-4587
https://orcid.org/0000-0002-7304-4587
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yen-Ju Chen, Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung; Institute of Clinical Medicine, National Yang-Ming University, Taipei; Department of Medical Research, Taichung Veterans General Hospital, Taichung.
Shih-Chia Liu, Department of Industrial Engineering and Enterprise Information, Tunghai University, Taichung.
Kuo-Lung Lai, Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung.
Kuo-Tung Tang, Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung; School of Medicine, National Yang-Ming University, Taipei.
Ching-Heng Lin, Department of Medical Research, Taichung Veterans General Hospital, Taichung; Department of Industrial Engineering and Enterprise Information, Tunghai University, Taichung; Department of Healthcare Management, National Taipei University of Nursing and Health Sciences, Taipei.
Yi-Ming Chen, Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung; Department of Medical Research, Taichung Veterans General Hospital, Taichung; School of Medicine, National Yang-Ming University, Taipei; Institute of Biomedical Science and Rong-Hsing Research Center for Translational Medicine, Chung-Hsing University, Taichung.
Chih-Wei Tseng, Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung.
Yu-Mei Chang, Department of Statistics, Tunghai University, Taichung.
Donald F. Gotcher, Department of International Business, Tunghai University, Taichung
Chuang-Chun Chiou, Department of Industrial Engineering and Enterprise Information, Tunghai University, Taichung.
Shao-Jen Weng, Department of Industrial Engineering and Enterprise Information, Tunghai University, Taichung.
Hsin-Hua Chen, Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung; Department of Industrial Engineering and Enterprise Information, Tunghai University, Taichung; School of Medicine, National Yang-Ming University, Taipei; Institute of Biomedical Science and Rong-Hsing Research Center for Translational Medicine, Chung-Hsing University, Taichung; Institute of Public Health and Community Medicine Research Center, National Yang-Ming University, Taipei; Department of Medical Research, Taichung Veterans General Hospital, 1650 Taiwan Boulevard Sect. 4, Taichung, 407.
References
- 1.Smolen JS, Aletaha D, McInnes IB.Rheumatoid arthritis. Lancet 2016; 388: 2023–2038. [DOI] [PubMed] [Google Scholar]
- 2.Kuo CF, Luo SF, See LC, et al. Rheumatoid arthritis prevalence, incidence, and mortality rates: a nationwide population study in Taiwan. Rheumatol Int 2013; 33: 355–360. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys JH, Verstappen SM, Hyrich KL, et al. The incidence of rheumatoid arthritis in the UK: comparisons using the 2010 ACR/EULAR classification criteria and the 1987 ACR classification criteria. Results from the Norfolk Arthritis Register. Ann Rheum Dis 2013; 72: 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter TM, Boytsov NN, Zhang X, et al. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int 2017; 37: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 5.Crowson CS, Matteson EL, Myasoedova E, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum 2011; 63: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radner H, Lesperance T, Accortt NA, et al. Incidence and prevalence of cardiovascular risk factors among patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis. Arthritis Care Res (Hoboken) 2017; 69: 1510–1518. [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003; 107: 1303–1307. [DOI] [PubMed] [Google Scholar]
- 8.Castañeda S, Nurmohamed MT, Gonzalez-Gay MA.Cardiovascular disease in inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol 2016; 30: 851–869. [DOI] [PubMed] [Google Scholar]
- 9.Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008; 59: 1690–1697. [DOI] [PubMed] [Google Scholar]
- 10.Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012; 71: 1524–1529. [DOI] [PubMed] [Google Scholar]
- 11.Jagpal A, Navarro-Millán I.Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol 2018; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowson CS, Rollefstad S, Ikdahl E, et al. ; A trans-atlantic cardiovascular consortium for rheumatoid arthritis (ATACC-RA). Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018; 77: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 2008; 67: 64–69. [DOI] [PubMed] [Google Scholar]
- 14.Choy E, Ganeshalingam K, Semb AG, et al. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford) 2014; 53: 2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Chen L, Delzell E, et al. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis 2014; 73: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Gay MA, Gonzalez-Juanatey C, Lopez-Diaz MJ, et al. HLA-DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum 2007; 57: 125–132. [DOI] [PubMed] [Google Scholar]
- 17.Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum 2005; 52: 2293–2299. [DOI] [PubMed] [Google Scholar]
- 18.Tomasson G, Aspelund T, Jonsson T, et al. Effect of rheumatoid factor on mortality and coronary heart disease. Ann Rheum Dis 2010; 69: 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Longo FJ, Oliver-Miñarro D, de la Torre I, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum 2009; 61: 419–424. [DOI] [PubMed] [Google Scholar]
- 20.Hansson GK.Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 21.Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010; 69: 325–331. [DOI] [PubMed] [Google Scholar]
- 22.Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon A, Tsang L, Woodiwiss AJ, et al. Cardiovascular disease risk amongst African black patients with rheumatoid arthritis: the need for population specific stratification. Biomed Res Int 2014; 2014: 826095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Health Insurance Annual Report 2014-2015. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan, R.O.C. [Google Scholar]
- 25.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–324. [DOI] [PubMed] [Google Scholar]
- 26.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 27.Hicks KA, Tcheng JE, Bozkurt B, et al. ; ACC/AHA Task Force on Clinical Data Standards Members. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Nucl Cardiol 2015; 22: 1041–1144. [DOI] [PubMed] [Google Scholar]
- 28.Dehmer GJ, Badhwar V, Bermudez EA, et al. 2020AHA/ACC key data elements and definitions for coronary revascularization: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (Writing Committee to Develop Clinical Data Standards for Coronary Revascularization). J Am Coll Cardiol 2020; 75: 1975–2088. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Martinez MA, Castañeda S, Sanchez-Alonso F, et al. ; CARMA Project Collaborative Group. Cardiovascular mortality and cardiovascular event rates in patients with inflammatory rheumatic diseases in the CARdiovascular in rheuMAtology (CARMA) prospective study-results at 5 years of follow-up. Rheumatology (Oxford). 2021; 60: 2906–2915. [DOI] [PubMed] [Google Scholar]
- 30.López-Mejías R, Castañeda S, González-Juanatey C, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis: the relevance of clinical, genetic and serological markers. Autoimmun Rev 2016; 15: 1013–1030. [DOI] [PubMed] [Google Scholar]
- 31.Turesson C, Schaid DJ, Weyand CM, et al. The impact of HLA-DRB1 genes on extra-articular disease manifestations in rheumatoid arthritis. Arthritis Res Ther 2005; 7: R1386–R1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu SC, Chang TY, Lee YJ, et al. Influence of HLA-DRB1 genes and the shared epitope on genetic susceptibility to rheumatoid arthritis in Taiwanese. J Rheumatol 2007; 34: 674–680. [PubMed] [Google Scholar]
- 33.Lee HS, Lee KW, Song GG, et al. Increased susceptibility to rheumatoid arthritis in Koreans heterozygous for HLA-DRB1*0405 and *0901. Arthritis Rheum 2004; 50: 3468–3475. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg JD, Kremer JM, Curtis JR, et al. ; CORRONA Investigators. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis 2011; 70: 576–582. [DOI] [PubMed] [Google Scholar]
- 35.Nurmohamed M, Bao Y, Signorovitch J, et al. Longer durations of antitumour necrosis factor treatment are associated with reduced risk of cardiovascular events in patients with rheumatoid arthritis. RMD Open 2015; 1: e000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai RJ, Rao JK, Hansen RA, et al. Tumor necrosis factor-α inhibitor treatment and the risk of incident cardiovascular events in patients with early rheumatoid arthritis: a nested case-control study. J Rheumatol 2014; 41: 2129–2136. [DOI] [PubMed] [Google Scholar]
- 37.Kirkham BW, Wasko MC, Hsia EC, et al. Effects of golimumab, an anti-tumour necrosis factor-α human monoclonal antibody, on lipids and markers of inflammation. Ann Rheum Dis 2014; 73: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heslinga SC, Konings TC, van der Horst-Bruinsma IE, et al. The effects of golimumab treatment on systolic and diastolic left ventricular function in ankylosing spondylitis. Biologics 2018; 12: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis 2016; 75: 1813–1818. [DOI] [PubMed] [Google Scholar]
- 40.Jin Y, Kang EH, Brill G, et al. Cardiovascular (CV) risk after initiation of abatacept versus TNF inhibitors in rheumatoid arthritis patients with and without baseline CV disease. J Rheumatol 2018; 45: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 41.Kang EH, Jin Y, Brill G, et al. Comparative cardiovascular risk of abatacept and tumor necrosis factor inhibitors in patients with rheumatoid arthritis with and without diabetes mellitus: a multidatabase cohort study. J Am Heart Assoc 2018; 7: e007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afek A, Harats D, Roth A, et al. Evidence for the involvement of T cell costimulation through the B-7/CD28 pathway in atherosclerotic plaques from apolipoprotein E knockout mice. Exp Mol Pathol 2004; 76: 219–223. [DOI] [PubMed] [Google Scholar]
- 43.Dopheide JF, Sester U, Schlitt A, et al. Monocyte-derived dendritic cells of patients with coronary artery disease show an increased expression of costimulatory molecules CD40, CD80 and CD86 in vitro. Coron Artery Dis 2007; 18: 523–531. [DOI] [PubMed] [Google Scholar]
- 44.Müller A, Mu L, Meletta R, et al. Towards non-invasive imaging of vulnerable atherosclerotic plaques by targeting costimulatory molecules. Int J Cardiol 2014; 174: 503–515. [DOI] [PubMed] [Google Scholar]
- 45.Hermans MPJ, van der Velden D, Montero Cabezas JM, et al. Long-term mortality in patients with ST-segment elevation myocardial infarction is associated with anti-citrullinated protein antibodies. Int J Cardiol 2017; 240: 20–24. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Gomez C, Martin-Martinez MA, Castañeda S, et al. ; CARMA Project Collaborative Group. Lipoprotein(a) concentrations in rheumatoid arthritis on biologic therapy: results from the CARdiovascular in rheuMAtology study project. J Clin Lipidol 2017; 11: 749–756.e3. [DOI] [PubMed] [Google Scholar]
- 47.Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2018; 77: 98–103. [DOI] [PubMed] [Google Scholar]
- 48.Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010; 49: 295–307. [DOI] [PubMed] [Google Scholar]
- 49.Wasko MC, Dasgupta A, Hubert H, et al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum 2013; 65: 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castañeda S, Gonzalez-Juanatey C, Gonzalez-Gay MA.Sex and cardiovascular involvement in inflammatory joint diseases. Clin Rev Allergy Immunol 2019; 56: 278–292. [DOI] [PubMed] [Google Scholar]
- 51.Pinto MRC, Kakehasi AM, Souza AJ, et al. Methotrexate use, not interleukin 33, is associated with lower carotid intima-media thickness in patients with rheumatoid arthritis. Adv Rheumatol 2019; 59: 15. [DOI] [PubMed] [Google Scholar]
- 52.Kim HJ, Kim MJ, Lee CK, et al. Effects of methotrexate on carotid intima-media thickness in patients with rheumatoid arthritis. J Korean Med Sci 2015; 30: 1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiss AB, Carsons SE, Anwar K, et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum 2008; 58: 3675–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aggarwal A, Misra R.Methotrexate inhibits interleukin-6 production in patients with juvenile rheumatoid arthritis. Rheumatol Int 2003; 23: 134–137. [DOI] [PubMed] [Google Scholar]
- 55.Sander M, Lyson T, Thomas GD, et al. Sympathetic neural mechanisms of cyclosporine-induced hypertension. Am J Hypertens 1996; 9: 121S–138S. [DOI] [PubMed] [Google Scholar]
- 56.Sander M, Victor RG.Hypertension after cardiac transplantation: pathophysiology and management. Curr Opin Nephrol Hypertens 1995; 4: 443–451. [DOI] [PubMed] [Google Scholar]
- 57.Textor SC, Taler SJ, Canzanello VJ, et al. Posttransplantation hypertension related to calcineurin inhibitors. Liver Transpl 2000; 6: 521–530. [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues-Diez R, González-Guerrero C, Ocaña-Salceda C, et al. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci Rep 2016; 6: 27915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hošková L, Málek I, Kopkan L, et al. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiol Res 2017; 66: 167–180. [DOI] [PubMed] [Google Scholar]
- 60.Robert N, Wong GW, Wright JM.Effect of cyclosporine on blood pressure. Cochrane Database Syst Rev 2010; 1: CD007893. [DOI] [PubMed] [Google Scholar]
- 61.Roubille C, Martel-Pelletier J, Davy JM, et al. Cardiovascular adverse effects of anti-inflammatory drugs. Antiinflamm Antiallergy Agents Med Chem 2013; 12: 55–67. [DOI] [PubMed] [Google Scholar]
- 62.Ng MK, Celermajer DS.Glucocorticoid treatment and cardiovascular disease. Heart 2004; 90: 829–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bijlsma JWJ, Buttgereit F. Adverse events of glucocorticoids during treatment of rheumatoid arthritis: lessons from cohort and registry studies. Rheumatology (Oxford) 2016; 55: ii3–ii5. [DOI] [PubMed] [Google Scholar]
- 64.Panoulas VF, Douglas KM, Stavropoulos-Kalinoglou A, et al. Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology (Oxford) 2008; 47: 72–75. [DOI] [PubMed] [Google Scholar]
- 65.Davis JM, 3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2007; 56: 820–830. [DOI] [PubMed] [Google Scholar]
- 66.Roubille C, Haraoui B.Important issues at heart: cardiovascular risk management in rheumatoid arthritis. Ther Adv Musculoskelet Dis 2013; 5: 163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hagihara H, Nomoto A, Mutoh S, et al. Role of inflammatory responses in initiation of atherosclerosis: effects of anti-inflammatory drugs on cuff-induced leukocyte accumulation and intimal thickening of rabbit carotid artery. Atherosclerosis 1991; 91: 107–116. [DOI] [PubMed] [Google Scholar]
- 68.Villa AE, Guzman LA, Chen W, et al. Local delivery of dexamethasone for prevention of neointimal proliferation in a rat model of balloon angioplasty. J Clin Invest 1994; 93: 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pitzalis C, Pipitone N, Perretti M.Regulation of leukocyte-endothelial interactions by glucocorticoids. Ann N Y Acad Sci 2002; 966: 108–118. [DOI] [PubMed] [Google Scholar]
- 70.Hafezi-Moghadam A, Simoncini T, Yang Z, et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med 2002; 8: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindsey SH, Chappell MC.Evidence that the G protein-coupled membrane receptor GPR30 contributes to the cardiovascular actions of estrogen. Gend Med 2011; 8: 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhala N, Emberson J, Merhi A, et al. ; Coxib and Traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013; 382: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho SK, Kim D, Won S, et al. Impact of anti-rheumatic treatment on cardiovascular risk in Asian patients with rheumatoid arthritis. Semin Arthritis Rheum 2018; 47: 501–506. [DOI] [PubMed] [Google Scholar]
- 74.Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis 2014; 73: 1515–1521. [DOI] [PubMed] [Google Scholar]
- 75.Castañeda S, Martin-Martinez MA, Gonzalez-Juanatey C, et al. ; CARMA Project Collaborative Group. Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: baseline data of the CARMA project. Semin Arthritis Rheum 2015; 44: 618–626. [DOI] [PubMed] [Google Scholar]
- 76.Ma Y, Tong H, Zhang X, et al. Chronic obstructive pulmonary disease in rheumatoid arthritis: a systematic review and meta-analysis. Respir Res 2019; 20: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen L, Krauss-Etschmann S, Petersen F, et al. Autoantibodies in chronic obstructive pulmonary disease. Front Immunol 2018; 9: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sze MA, Dimitriu PA, Suzuki M, et al. Host response to the lung microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reilev M, Pottegard A, Lykkegaard J, et al. Increased risk of major adverse cardiac events following the onset of acute exacerbations of COPD. Respirology 2019; 24: 1183–1190. [DOI] [PubMed] [Google Scholar]
- 80.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006; 113: 791–798. [DOI] [PubMed] [Google Scholar]
- 81.Castañeda S, Vicente-Rabaneda EF, García-Castañeda N, et al. Unmet needs in the management of cardiovascular risk in inflammatory joint diseases. Expert Rev Clin Immunol 2020; 16: 23–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-3-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-4-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-5-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-6-tab-10.1177_1759720X211030809 for Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study by Yen-Ju Chen, Shih-Chia Liu, Kuo-Lung Lai, Kuo-Tung Tang, Ching-Heng Lin, Yi-Ming Chen, Chih-Wei Tseng, Yu-Mei Chang, Donald F. Gotcher, Chuang-Chun Chiou, Shao-Jen Weng and Hsin-Hua Chen in Therapeutic Advances in Musculoskeletal Disease


